User login
Bone remodeling associated with CTLA-4 inhibition: an unreported side effect
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
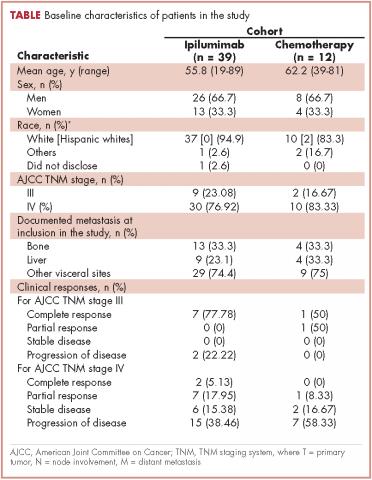
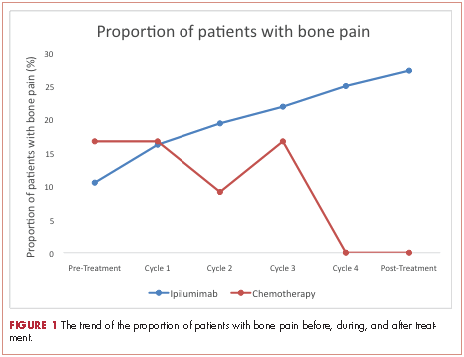
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
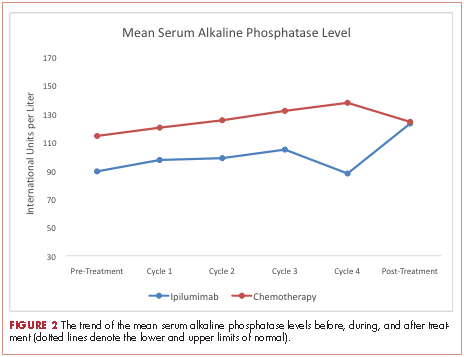
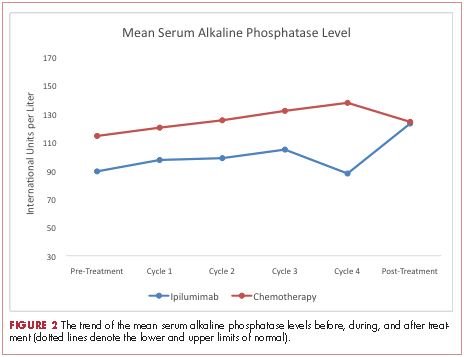
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is an important component of the immune checkpoint pathway. CTLA-4 inhibition causes T-cell activation and proliferation, increases T-cell responsiveness, and enhances the anti-tumor immune response. CTLA-4 inhibition also results in immune-related adverse reactions such as colitis, hepatitis, and endocrinopathies. Preclinical investigations have recently shown that CTLA-4 inhibition can cause cytokine-mediated increase in bone remodeling.1,2(p4) Ipilimumab, a recombinant IgG1 kappa antibody against human CTLA-4, has been approved for use in unresectable or metastatic melanoma. We hypothesize that ipilumumab results in increase in bone remodeling manifesting as an autoimmune reaction.
Methods
We conducted a retrospective case-control study of patients with stage III/IV melanoma treated at the University of New Mexico Comprehensive Cancer Center during April 2009-July 2014. The university’s Institutional Review Board approved the study.
Two cohorts were compared: an ipilumimab cohort receiving ipilumimab at 3 mg/kg every 3 weeks, and a chemotherapy cohort receiving an investigational chemotherapy regimen: carboplatin IV at an area under curve of 5 on day 1, paclitaxel IV at 175 mg/m2 on day 1, and temozolomide orally at 125 mg/m2 daily on days 2 to 6 every 21 days. Patients receiving at least 1 cycle of treatment were included. Those with known hepatic disease or concurrent malignancy were excluded from the study.
Serum ALP level (normal range, 38-150 international units per liter [IU/L]) and patient-reported bone pain measured by the 11-point numeric rating scale (NRS) for pain assessment were recorded before treatment initiation, on each cycle, and upon treatment completion.3 Clinical response was assessed per RECIST guidelines.4 Bone pain was dichotomized into Absent (pain intensity of 0 on the NRS, meaning no pain) or Present (pain intensity of 1-10 on the NRS, with 1 = mild pain and 10 = worst imaginable pain). Patients with a complete or partial response to the therapy were categorized as responders, and those with progressive or stable disease were categorized as nonresponders.
Descriptive statistics were generated for demographic and clinical characteristics. The primary outcome variables of interest were bone pain and mean ALP levels. Generalized linear mixed-effect models for proportion of patients with bone pain (with logit link function) and mean ALP levels (with identify link function) were used to evaluate for a difference in trends between the two cohorts over time. We used the Kenward-Roger approach to adjust for the small size of the degrees of freedom. To assess the significance of difference of the proportions of patients with bone pain and the mean ALP levels between responders and nonresponders in the ipilumimab cohort, the Fisher exact test and Wilcoxon rank-sum test were used, respectively. Statistical analyses were performed with statistical packages R (v3.1.3) and SAS (v9.4).
Results
A total of 281 patients were screened, and 51 met the inclusion criteria (39 in the ipilumimab and 12 in chemotherapy cohorts). Baseline parameters were well matched between the cohorts (Table). Of the 39 patients in the ipilimumab cohort, 14 (35.9%) had bone pain during at least one of the treatment cycles, compared with 3 of the 12 patients (25%) in the chemotherapy cohort. At baseline, 4 of 38 ipilimumab patients (10.5%; 95% confidence interval [CI], 2.9-24.8) and 2 of 12 chemotherapy patients (16.7%; 95% CI, 2.1-48.4) had bone pain. Upon treatment completion, 9 of 33 ipilimumab patients (27.3%; 95% CI, 13.3-45.5) and 0 of 12 chemotherapy patients (0%; 95% CI, 0-26.5) had bone pain. The trend of proportion of patients with bone pain over time was statistically significant between the two cohorts (P = .023, Figure 1). The trends of proportion of patients with bone pain were not statistically significant when stratified by the presence of bone metastasis at inclusion in the study (P = .418) or disease progression at treatment completion (P = .500).
At baseline, the mean ALP level was 89.39 IU/L (95% CI, 81.03-97.75) in the ipilumimab cohort and 114.33 IU/L (95% CI, 69.48-159.19) in the chemotherapy cohort. Upon treatment completion, the mean ALP level was 123.09 IU/L (95% C.I. 80.78-165.41) in the ipilumimab cohort and 124.24 IU/L (95% C.I. 90.88-157.62) in the chemotherapy cohort. The trend of mean ALP level over time was not statistically significant between the 2 cohorts (P = .653, Figure 2).
Discussion
Immune checkpoints are inhibitory pathways that are critical for maintenance of self-tolerance and regulation of appropriate immune response. CTLA-4 is present exclusively on T cells and interacts with its ligands B7.1 and B7.2. CTLA-4 competes with CD28 in binding with B7, leading to dampening of T-cell activation and function.5,6 Development of checkpoint inhibitors such as ipilumimab have heralded a new era of immune targeted therapies for various malignancies including malignant melanoma.
Bone remodeling involves 4 distinct but overlapping phases. The first phase involves detection of loss of bone continuity by osteocytes and activation of osteoclast precursors derived from progenitors of the monocyte-macrophage lineage. The second phase involves osteoclast-medicated bone resorption and concurrent recruitment of mesenchymal stem cells and osteoprogenitors. The third phase involves osteoblast differentiation and osteoid synthesis, and the fourth phase results in mineralization of osteoid and termination of bone remodeling.7,8
The role of T-lymphocytes and cytokines, such as IL-1 and TNF-α, and receptor activator of NF-κB ligand (RANK-L) in osteoclastogenesis is well studied. RANK-L is considered to be the final downstream effector of this process.9 T-lymphocytes have also been shown to promote osteoblast maturation and function.9,10 These findings suggest a significant interaction between immune system activation and bone remodeling.
The search for a reliable biomarker for immune therapy is ongoing. Although ipilumimab-associated immune-related adverse events have been suggested to predict response to therapy,11 there is considerable debate on the subject. Ipilumimab’s impact on bone remodeling could offer a solution.
In the current study, there was a statistically significant difference in proportion of patients with bone pain in the 2 cohorts. This was preserved with stratification based on bone metastasis at inclusion and disease progression on treatment completion making new or worsening skeletal metastasis. Furthermore, the proportion of patients with bone pain increased with each cycle for ipilumimab cohort. However, we were unable to detect an association between bone pain and response to ipilimumab.
We were not able to detect a difference in trend of mean ALP level with treatment in the two cohorts. Although it is possible that no such association exists, we believe our study was not powered to detect it. Finally, we were not able to study markers for osteoblast (bone-specific ALP) and osteoclasts (N- and C-telopeptides of type 1 collagen, deoxypyridinoline, etc) to better assess this interaction because they are not commonly clinically used.
Regarding the limitations of our study, we chose to dichotomize the patient-reported bone pain because it is a subjective measure and there is a significant variability of the perceived pain intensity among patients. We also excluded patients with hepatitis from receiving the ipilumimab therapy and those with known hepatic disease from the study to reduce the impact of hepatic ALP on total serum ALP levels.
In conclusion, as far as we know, this is the first clinical report suggesting a possible relationship between CTLA-4 inhibition and bone remodeling. Supported by a strong preclinical rationale, this side effect remains under-studied and under-recognized by clinicians. A prospective assessment of this interaction using bone specific markers is planned.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.
1. Bozec A, Zaiss MM, Kagwiria R, et al. T-cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med. 2014;6(235):235ra60.
2. Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F. EphB4 promotes osteogenesis of CTLA 4-modified bone marrow-derived mesenchymal stem cells through cross talk with wnt pathway in xenotransplantation. Tissue Eng Part A. 2015;21(17-18):2404-2416.
3. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149-158.
4. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
6. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205-214.
7. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(suppl 3):S131-S139.
8. Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121-145.
9. Gillespie MT. Impact of cytokines and T lymphocytes upon osteoclast differentiation and function. Arthritis Res Ther. 2007;9(2):103.
10. Sims NA, Walsh NC. Intercellular cross-talk among bone cells: new factors and pathways. Curr Osteoporos Rep. 2012;10(2):109-117.
11. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681-6688.