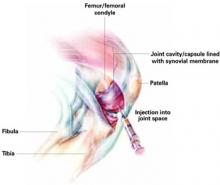
User login
Hyaluronic acid injections relieve knee pain
- Consider injections of hyaluronic acid only after conservative therapy has been tried for at least 3 months or the patient is unable to tolerate NSAIDs.
- Stress to patients that pain relief may not be fully experienced until 5 to 7 weeks following the last injection.
Hyaluronic acid injections can help relieve pain for carefully selected patients with knee osteoarthritis. But this option should be reserved for those whose pain has not responded to adequate trials of systemic therapeutic agents (acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs], cyclooxygenase-2 [COX-2] inhibitors), topical agents, or to lifestyle modifications such as weight reduction and exercise.
Hyaluronic acid injections may also be indicated when knee surgery must be delayed for middle-aged persons.1
In spite of the Food and Drug Administration’s approval of this therapy, uncertainty about its efficacy exists among the medical community. A recent meta-analysis of the effectiveness of intra-articular hyaluronic acid for knee osteoarthritis that included 22 published and unpublished, English and non-English, single or double-blinded, randomized controlled trials in humans showed that hyaluronic acid has only a small effect on pain relief when compared with placebo.2
We provide here a stringent test of the efficacy of viscosupplementation for relieving knee pain from osteoarthritis with a meta-analysis that includes only data from randomized, double-blinded, controlled trials of hyaluronic acid that measured pain using a visual analogue scale (VAS), the most widely accepted method for pain evaluation.
Long time coming
Balazs first proposed hyaluronic acid as a treatment for patients with arthritic diseases in 1942. In the early 1970s, therapeutic studies were begun to test the efficacy of hyaluronic acid on knee osteoarthritis. The results were encouraging and side effects were few.28 With the FDA’s approval in 1998, intra-articular “viscosupplementation” with hyaluronic acid—also called hyaluronan or hyaluronate, and the hylan derivatives of hyaluronic acid—is a welcome option for many of the 16 million older Americans with osteoarthritis of the knee.1
Methods
Selection of studies
We identified clinical trials of viscosupplementation with hyaluronic acid in humans published in English from 1965 through August 2004 through a computerized literature search of Medline. The keyword used was “hyaluronic acid,” which was combined with “trial” or “osteoarthritis knee” or “viscosupplementation.” We conducted an additional manual search of the reference lists of included articles and review articles. We also searched the Cochrane Library and websites of the Agency for Healthcare Research and Quality (AHRQ) for information on hyaluronic acid in knee osteoarthritis. We identified 1872 articles with this search process.
Of the 1872 articles, we identified by title and abstract 33 that might be pertinent to this study, including 17 randomized trials. We excluded reviews, meta-analyses, comparison trials, and trials reporting VAS as part of the WOMAC (Western Ontario McMaster Universities Index) scale. We attempted to contact authors of the studies that used a double-blind, randomized controlled design, to obtain any data that may not have been included in the publications. Three authors provided the details requested; the others did not respond or stated that additional data were not available. Of the 17 randomized trials we identified, 8 were excluded because they were open, single-blinded, or did not use the VAS to measure pain outcomes.3-10 The remaining 9 double-blinded, placebo controlled, randomized clinical trials of viscosupplementation with hyaluronic acid for knee osteoarthritis that did use a VAS to measure pain were included in this meta-analysis (TABLE 1). Because one of the studies (by Henderson) included 2 subgroups of pain severity, these were considered as 2 separate trials. The trial by Petrella had 2 treatment groups, one with only hyaluronic acid and the other with hyaluronic acid and NSAIDs. We considered them separately in the analysis, resulting in a total of 11 clinical trials for the meta-analysis.19 Henceforth in this report, we will refer to 11 rather than 9 clinical trials.
Extraction of data
Two investigators independently extracted the following data for each study: year of publication, study design, mean age, number of patients enrolled in each treatment group, number of doses of treatment used, and outcomes measured. When disagreements between investigators occurred, the point of disagreement was discussed until a consensus was reached. Since the treatment duration and the time post-treatment when pain was assessed varied among the trials, we grouped outcomes into four time intervals: at 1 week, 5 to 7 weeks, 8 to 12 weeks, and 15 to 22 weeks after the last hyaluronic acid injection.
Statistical analysis
The outcome was knee pain reported by patients on activity or at rest, measured using a VAS of 100 mm. The results of the clinical trials were recorded as the mean differences of change from baseline between the treatment and placebo groups. If not reported in the publication or provided by the authors, standard error was imputed using the method of Follman et al.20 We used the method of Chalmers for measuring the quality of randomized trials, with 2 of the authors rating the studies independently.21
This clinical trial grading system takes into account the following aspects of the trial to determine a quality score: evaluation of recruitment of subjects, rejection log, therapeutic regimen definition, randomization, blinding, prior estimates of numbers, testing compliance, statistical inference, use of appropriate statistical analysis, handling of withdrawal and side effects, dates of starting and ending, timing and tabulation of events. Because 4 of the trials had poor quality scores, an additional analysis excluding these 4 was performed. The DerSimonian and Laird random-effects model was used to obtain the summary estimates.22,23
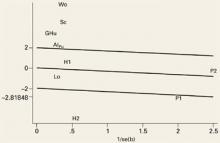
An important element of meta-analysis is exploration of the heterogeneity of the outcomes and the possible causes of heterogeneity if it exists. Heterogeneity is the degree to which results vary from study to study. If a test for heterogeneity is statistically significant, there is significant variability among the treatment effects observed in the trials. We explored heterogeneity using Galbraith plots (FIGURE 1).24 In the absence of heterogeneity, all points fall within the confidence limits. Because we did find heterogeneity among these trials, we developed random-effect regression models to explore 3 possible sources of heterogeneity in the efficacy of hyaluronic acid; pain (measured at rest or on activity), the form used (hyaluronan or hylan G-F20), and the quality of the study method (good or poor).
Publication bias was assessed by the Egger et al regression asymmetry test.25 Analyses were performed using the meta-analytic software program of STATA, Inc (College Station, Tex; available at www.stata.com).
FIGURE 1
Evidence of heterogeneity for the studies evaluated at outcome measurement time (week 1)
This Galbraith plot shows standardized effects (b/se=mean difference/standard error) as a function of study precision (1/standard error), slope of the line shows average effect over all the studies with upper and lower lines denoting an approximate 95% confidence interval for this common effect. Under the hypothesis of study heterogeneity, the common slope intersect zero at 1/se=0, and 95% of all study estimates (authors initials) will fall within the confidence interval of the regression line. Gr: Grecomoro; Pu: Puhl; H1: Henderson 1; H2: Henderson 2; Sc: Scale; Lo: Lohmander; Al: Altman; Wo: Wobig; Hu: Huskisson; P1: Petrella 1; P2: Petrella 2.
Results
The 11 randomized, placebo-controlled, double-blinded clinical trials that met our inclusion criteria are summarized in TABLE 1. Nine trials used hyaluronic acid, hyaluronan, or hyaluronate (all types will be referred to as hyaluronan in the text), and 2 studies used hylan GF-20. Only 3 hyaluronan trials have published outcome data at 15 to 22 weeks follow-up. Treatment was administered to patients as 3 to 5 weekly injections, with the exception of the Grecomoro study in which treatment was administered twice weekly. The control group in 10 trials received intra-articular saline injections as placebo. In the Puhl study the investigators added 0.25 mg of hyaluronic acid to the saline injections to impart viscosity to the solution. The mean age of the subjects for the 11 trials was 63 years.
Eight trials received support from pharmaceutical companies and 3 (the 2 by Henderson and the Grecomoro study) did not disclose any pharmaceutical support. One study was conducted in the United States, 3 in the UK, 3 in Germany, 1 in Sweden, 1 in Italy, and 2 in Canada. Five of the studies had scores over a cutoff quality score of >0.75,12,13,15,16,19 indicating they were good-quality randomized controlled trials; the remaining 4 had scores below 0.75.11,14,16,17
The outcomes of the 11 trials are summarized in TABLE 2. Patients’ pain ratings in both the active treatment and placebo groups improved in all the trials. Mean difference between improvements in treatment and placebo groups are shown in FIGURES 2A-2D for pain assessed at weeks 1, 5 to 7, 8 to 12 and 15 to 22, respectively. In each figure, we show the summary estimate of effect size with all the trials included and after excluding the 4 trials considered of poor quality, shown as “good quality studies.”
The mean difference in pain scores between treatment and placebo at week 1 was 4.4 (95% CI, +1.1, +7.2) and –1.0 (95% CI, -3.2, +1.2) for analysis restricted to the 7 good quality trials. The mean difference in pain scores at 5 to 7 weeks was 17.6 (95% CI, +7.5, +28.0) and 7.2 (95% CI, +2.4, +12.0) for the analysis restricted to the 2 good quality studies. At weeks 8 to 12 the mean difference in VAS between treatment and control was 18.1 (95% CI, +6.3, +29.9), and 7.1 (95% CI, +3.0, +11.3) in the analysis restricted to good quality trials. At weeks 15 to 22, the mean difference was 4.4 (95% CI, –15.3, +24.1). The Egger test was not statistically significant (2.3; P=.096; 95% CI, –0.5, +5.2) suggesting that there is no publication bias.
High heterogeneity was observed at all time intervals except 1 week (FIGURE 1). Of the 5 trials outside the confidence bounds (positioned 2 units above and below the regression line), 4 were poor-quality studies.
TABLE 3 shows the random-effect regression models we used to test the influence on the outcome of type of pain measured (pain with activity or pain at rest), type of medication (hyaluronan or hylan G-F 20), and study quality (good or poor). No significant association between treatment efficacy and type of pain used as outcome variable was observed. Clinical trials using hylan GF-20 showed statistically significant better results than those using hyaluronan at weeks 5 to 7 and 8 to 12. Poor-quality studies showed a larger treatment effect, but the difference was statistically significant only at week 1.
TABLE 3
Regression models to assess the sources of heterogeneity in the meta-analysis
| WEEK 1 | WEEKS 5–7 | WEEKS 8–12 | ||||
|---|---|---|---|---|---|---|
| Coef. (SE) [95% CI] | P value | Coef. (SE) [95% CI] | P value | Coef. (SE) [95% CI] | P value | |
| Pain | ||||||
| With activity | 1.7 (3.8) | 1.8 (4.3) | –0.5 (4.6) | |||
| At rest | [–5.8, +9.2] | 0.657 | [–6.6, +10.2] | 0.671 | [–9.5, +8.6] | 0.916 |
| Medication | ||||||
| Hyaluronan** | –3.4 (6.8) | 0.614 | 17.5 (4.9) | <0.001 | 14.8 (6.1) | 0.016 |
| Hylan G-F 20 | –16.7, +9.9] | [+7.8, +27.1] | [+2.8, +26.8] | |||
| Quality* | ||||||
| Poor (<0.75) | –19.9 (5.7) | 0.001 | –7.4 (4.9) | 0.131 | –11.7 (7.0) | 0.092 |
| Good (≥0.75) | [–31.1, –8.7] | [–17.0, +2.2] | [–25.3, +1.9] | |||
| Constant | 18.4 (5.6) | 0.001 | 13.5 (4.5) | 0.003 | 19.2 (5.8) | 0.001 |
| [+7.5, +29.3] | [+4.6, +22.3] | [+7.9, +30.5] | ||||
| *<.75 quality score: Grecomoro .439, Scale .570, Wobig .731, Huskisson .718. | ||||||
| ** 9 trials for hyaluronan (3 for Hyalgan®) and 2 trials for hylan G-F20 (Synvisc®). | ||||||
Discussion
This meta-analysis synthesized data from 9 randomized, double-blinded, placebo-controlled trials that evaluated the efficacy of intra-articular hyaluronic acid. Our findings show significantly decreased pain as measured by VAS at 5 to 7 weeks and at 8 to 12 weeks after the last injection. Intra-articular hyaluronic acid was not more effective than placebo in relieving pain at 1 week or at 15 to 22 weeks after the last injection. Because only 3 of the trials assessed patients after 12 weeks, however, the sample size is too small to definitively rule out a significant therapeutic effect after 12 weeks.
Reasons for the differences in efficacy among trials of hyaluronic acid in the treatment of knee osteoarthritis include dose, type, and frequency of administration, genetic or age differences among the study subjects, severity of osteoarthritis, time of follow-up, and quality of the studies. We confirmed that the treatment effect is time dependent. Although our meta-regression analysis (TABLE 2) suggests that hylan GF-20 is more effective than hyaluronan at 5 to 12 weeks, the number of clinical trials is relatively small and both of the hylan G-F20 studies were of poor quality. Therefore, we cannot say with confidence that one form is better than the other. Data in these trials were insufficient to assess the impact of body mass index, genetics, or severity of osteoarthritis.
We did not evaluate functional improvement in this meta-analysis because functional status was not measured in some trials and the assessment methods were too variable in the trials that did assess functional status. Publication bias, or the possibility that unpublished data would contradict the results of published studies, is always a potential source of bias in meta-analysis. However, the Egger test was not statistically significant (6.5; 95% CI, –0.5, +13.5) suggesting that there is no publication bias.25
Finally, the presence of heterogeneity of results indicates there were important differences among the studies. Exclusion of clinical trials considered of poor quality diminished this heterogeneity substantially. Subanalysis restricted to good-quality studies supports the efficacy of intra-articular hyaluronic acid in the treatment of knee osteoarthritis pain, although the effect size is smaller when one considers only the good quality studies.
There are 2 other potential limitations of this meta-analysis. Five studies allowed pain to be treated with analgesics such as acetaminophen or NSAIDs,12,13,16-18 and use of acetaminophen or NSAIDs may have altered the response to hyaluronic acid treatment. An intention-to-treat analysis was performed in only 2 studies (by Altman and Huskisson),16,18 wherein a post-hoc and “last observation carried forward” analysis showed a trend favoring hyaluronic acid. The treatment effects may have been smaller had the other trials used an intention to treat analysis.
This meta-analysis confirms that viscosupplementation with hyaluronic acid is modestly effective in short term relief of pain in knee osteoarthritis. Our meta-analysis included only double-blinded, randomized trials published in English language in humans using VAS as the pain outcome measure, and our conclusions are very similar to those of Lo.2
Indications for use. Hyaluronic acid is helpful in relieving pain for carefully selected patients with knee osteoarthritis who have not responded to adequate use of systemic therapeutic agents, including acetaminophen, NSAIDs and COX-2 inhibitors and topical agents, along with lifestyle modification such as weight reduction and exercise.
Patients should have a trial for at least three months of conservative therapy or be unable to tolerate NSAIDs before a decision to give 3- to 5-injection course with hyaluronic acid is made.
Hyaluronic acid may be an option when there is a need to delay knee surgery in middle-aged persons1 or for patients who have failed other treatments.
Time to pain relief. To improve adherence to treatment, tell patients receiving intra-articular hyaluronic acid that the benefits in pain reduction may not be noticeable until 5 to 10 weeks after the last injection.
Cost. Although the cost of hyaluronic acid treatment is covered by Medicare and most insurance plans for symptomatic osteoarthritis of knee, documentation in patient medical records should indicate the signs and symptoms supporting the diagnosis and functional impairment. Objective data to support a diagnosis of osteoarthritis such as x-ray, arthroscopy report, computed tomography scan, or magnetic resonance imaging should be available in the event of a review.
The cost of 1 hyaluronic acid (30 mg/mL) injection is approximately $230. Considering a course of 3 to 5 weekly knee injections, and adding other pharmacy, hospital, or clinic charges, the cost per treatment may exceed $1000 per knee.26,27 The cost-benefit of pain control with viscosupplementation must be carefully compared with other therapeutic agents and regimens currently available for knee osteoarthritis management.
FIGURE 3
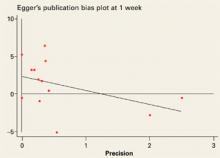
Studies evaluated at outcome measurement time (week 1)
Egger’s publication bias plot for the studies evaluated at outcome measurement time (week 1) not significant (+2.3, P=0.096, 95% CI: –0.5, +5.2).
Acknowledgments
Jeff Welge, PhD, University of Cincinnati Medical Center, Center for Bio-statistical Services, for comments on final data analysis and presentation, Marie Marley, PhD for editing the manuscript, and Charity Noble for help in preparation of this manuscript.
CORRESPONDING AUTHOR
Arvind Modawal, MD, MPH, MRCGP, University of Cincinnati Department of Family Medicine/Geriatrics, PO Box 670582, Cincinnati, OH 45267-0582. E-mail: [email protected]
1. Cefalu CA, Waddell DS. Viscosupplementation: Treatment alternative for osteoarthritis of the knee. Geriatrics 1999;54:51-57.
2. Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis. JAMA 2003;290:3115-3121.
3. Jones AC, Pattrick M, Doherty S, Doherty M. Intra-articular hyaluronic acid compared to intra-articular triamcinolone hexacetonide in inflammatory knee osteoarthritis. Osteoarthritis Cartilage 1995;3:269-273.
4. Graf J, Neusel E, Schneider E, Niethard FU. Intra-articular treatment with hyaluronic acid in osteoarthritis of the knee joint: a controlled clinical trial versus mucopolysaccharide polysulfuric acid ester. Clin Exp Rheumatol 1993;11:367-72.
5. Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: A one year placebo-controlled trial. Osteoarthritis Cartilage 1993;1:97-103.
6. Adams ME. An analysis of clinical studies of the use of cross-linked hyaluronan, hylan, in the treatment of osteoarthritis. J Rheumatol Supp 1993;39:16-18.
7. Grecomoro G, Piccione F, Letizia G. Therapeutic synergism between hyaluronic acid and dexamethasone in the intra-articular treatment of osteoarthritis of the knee: a preliminary open study. Curr Med Res Opin 1992;13:49-55.
8. Leardini G, Mattara L, Franceschini M, Perbellini A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and 6-methyl prednisolone acetate. Clin Exp Rheumatol 1991;9:375-381.
9. Wu J, Shih L, Hsu H, Chen T. The double-blind test of sodium hyaluronate (ARTZ) on osteoarthritis knee. Chin Med J (Taipei) 1997;59:99-106.
10. Dixon AS, Jacoby RK, Berry H, Hamilton EB. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin 1988;11:205-213.
11. Grecomoro G, Martorana U, Di Marco C. Intra-articular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmather 1987;5:137-141.
12. Puhl W, Bernau A, Greiling H, Kopcke W, Pforringer W, Steck KJ. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage 1993;1:233-241.
13. Henderson EB, Smith EC, Pegley F, Blake DR. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: a randomized single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann Rheum Dis 1994;53:529-554.
14. Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with Hylan: A treatment schedule study. Curr Ther Res 1994;55:220-232.
15. Lohmander LS, Dalen N, Englund G, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomized, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group [see comments]. Ann Rheum Dis 1996;55:424-431.
16. Altman RD, Moskowitz R. Intra-articular sodium hyaluronate (Hyalgan®) in the treatment of patients with osteoarthritis of the knee: A randomized clinical trial. J Rheumatol 1998;25:2203-2212.
17. Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with Hylan G-F-20: A 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther 1998;20:410-423.
18. Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology 1999;38:602-607.
19. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee. Arch Intern Med 2002;162:292-298.
20. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769-773.
21. Chalmers TC, Smith H, Jr, Blackburn B, et al. A Method for Assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49.
22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
23. Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med 1987;316:450-455.
24. Galbraith R. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988;7:889-894.
25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-634.
26. CIGNA Government Services. Not otherwise classified drug fee schedule. Available at: www.cignamedicare.com/partb/fsch/2004/q2/noc.html. Accessed on June 20, 2005.
27. Empire Medicare Services. Local coverage determination. Hyaluronate polymers (Synvisc, Hyalgan) (A02-0011-R3). Available at: www.empiremedicare.com/Nyorkpolicya/policy/A02-0011-R_FINAL.htm.
28. Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. Pathol Biol 1974;22:731-736.
- Consider injections of hyaluronic acid only after conservative therapy has been tried for at least 3 months or the patient is unable to tolerate NSAIDs.
- Stress to patients that pain relief may not be fully experienced until 5 to 7 weeks following the last injection.
Hyaluronic acid injections can help relieve pain for carefully selected patients with knee osteoarthritis. But this option should be reserved for those whose pain has not responded to adequate trials of systemic therapeutic agents (acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs], cyclooxygenase-2 [COX-2] inhibitors), topical agents, or to lifestyle modifications such as weight reduction and exercise.
Hyaluronic acid injections may also be indicated when knee surgery must be delayed for middle-aged persons.1
In spite of the Food and Drug Administration’s approval of this therapy, uncertainty about its efficacy exists among the medical community. A recent meta-analysis of the effectiveness of intra-articular hyaluronic acid for knee osteoarthritis that included 22 published and unpublished, English and non-English, single or double-blinded, randomized controlled trials in humans showed that hyaluronic acid has only a small effect on pain relief when compared with placebo.2
We provide here a stringent test of the efficacy of viscosupplementation for relieving knee pain from osteoarthritis with a meta-analysis that includes only data from randomized, double-blinded, controlled trials of hyaluronic acid that measured pain using a visual analogue scale (VAS), the most widely accepted method for pain evaluation.
Long time coming
Balazs first proposed hyaluronic acid as a treatment for patients with arthritic diseases in 1942. In the early 1970s, therapeutic studies were begun to test the efficacy of hyaluronic acid on knee osteoarthritis. The results were encouraging and side effects were few.28 With the FDA’s approval in 1998, intra-articular “viscosupplementation” with hyaluronic acid—also called hyaluronan or hyaluronate, and the hylan derivatives of hyaluronic acid—is a welcome option for many of the 16 million older Americans with osteoarthritis of the knee.1
Methods
Selection of studies
We identified clinical trials of viscosupplementation with hyaluronic acid in humans published in English from 1965 through August 2004 through a computerized literature search of Medline. The keyword used was “hyaluronic acid,” which was combined with “trial” or “osteoarthritis knee” or “viscosupplementation.” We conducted an additional manual search of the reference lists of included articles and review articles. We also searched the Cochrane Library and websites of the Agency for Healthcare Research and Quality (AHRQ) for information on hyaluronic acid in knee osteoarthritis. We identified 1872 articles with this search process.
Of the 1872 articles, we identified by title and abstract 33 that might be pertinent to this study, including 17 randomized trials. We excluded reviews, meta-analyses, comparison trials, and trials reporting VAS as part of the WOMAC (Western Ontario McMaster Universities Index) scale. We attempted to contact authors of the studies that used a double-blind, randomized controlled design, to obtain any data that may not have been included in the publications. Three authors provided the details requested; the others did not respond or stated that additional data were not available. Of the 17 randomized trials we identified, 8 were excluded because they were open, single-blinded, or did not use the VAS to measure pain outcomes.3-10 The remaining 9 double-blinded, placebo controlled, randomized clinical trials of viscosupplementation with hyaluronic acid for knee osteoarthritis that did use a VAS to measure pain were included in this meta-analysis (TABLE 1). Because one of the studies (by Henderson) included 2 subgroups of pain severity, these were considered as 2 separate trials. The trial by Petrella had 2 treatment groups, one with only hyaluronic acid and the other with hyaluronic acid and NSAIDs. We considered them separately in the analysis, resulting in a total of 11 clinical trials for the meta-analysis.19 Henceforth in this report, we will refer to 11 rather than 9 clinical trials.
Extraction of data
Two investigators independently extracted the following data for each study: year of publication, study design, mean age, number of patients enrolled in each treatment group, number of doses of treatment used, and outcomes measured. When disagreements between investigators occurred, the point of disagreement was discussed until a consensus was reached. Since the treatment duration and the time post-treatment when pain was assessed varied among the trials, we grouped outcomes into four time intervals: at 1 week, 5 to 7 weeks, 8 to 12 weeks, and 15 to 22 weeks after the last hyaluronic acid injection.
Statistical analysis
The outcome was knee pain reported by patients on activity or at rest, measured using a VAS of 100 mm. The results of the clinical trials were recorded as the mean differences of change from baseline between the treatment and placebo groups. If not reported in the publication or provided by the authors, standard error was imputed using the method of Follman et al.20 We used the method of Chalmers for measuring the quality of randomized trials, with 2 of the authors rating the studies independently.21
This clinical trial grading system takes into account the following aspects of the trial to determine a quality score: evaluation of recruitment of subjects, rejection log, therapeutic regimen definition, randomization, blinding, prior estimates of numbers, testing compliance, statistical inference, use of appropriate statistical analysis, handling of withdrawal and side effects, dates of starting and ending, timing and tabulation of events. Because 4 of the trials had poor quality scores, an additional analysis excluding these 4 was performed. The DerSimonian and Laird random-effects model was used to obtain the summary estimates.22,23
An important element of meta-analysis is exploration of the heterogeneity of the outcomes and the possible causes of heterogeneity if it exists. Heterogeneity is the degree to which results vary from study to study. If a test for heterogeneity is statistically significant, there is significant variability among the treatment effects observed in the trials. We explored heterogeneity using Galbraith plots (FIGURE 1).24 In the absence of heterogeneity, all points fall within the confidence limits. Because we did find heterogeneity among these trials, we developed random-effect regression models to explore 3 possible sources of heterogeneity in the efficacy of hyaluronic acid; pain (measured at rest or on activity), the form used (hyaluronan or hylan G-F20), and the quality of the study method (good or poor).
Publication bias was assessed by the Egger et al regression asymmetry test.25 Analyses were performed using the meta-analytic software program of STATA, Inc (College Station, Tex; available at www.stata.com).
FIGURE 1
Evidence of heterogeneity for the studies evaluated at outcome measurement time (week 1)
This Galbraith plot shows standardized effects (b/se=mean difference/standard error) as a function of study precision (1/standard error), slope of the line shows average effect over all the studies with upper and lower lines denoting an approximate 95% confidence interval for this common effect. Under the hypothesis of study heterogeneity, the common slope intersect zero at 1/se=0, and 95% of all study estimates (authors initials) will fall within the confidence interval of the regression line. Gr: Grecomoro; Pu: Puhl; H1: Henderson 1; H2: Henderson 2; Sc: Scale; Lo: Lohmander; Al: Altman; Wo: Wobig; Hu: Huskisson; P1: Petrella 1; P2: Petrella 2.
Results
The 11 randomized, placebo-controlled, double-blinded clinical trials that met our inclusion criteria are summarized in TABLE 1. Nine trials used hyaluronic acid, hyaluronan, or hyaluronate (all types will be referred to as hyaluronan in the text), and 2 studies used hylan GF-20. Only 3 hyaluronan trials have published outcome data at 15 to 22 weeks follow-up. Treatment was administered to patients as 3 to 5 weekly injections, with the exception of the Grecomoro study in which treatment was administered twice weekly. The control group in 10 trials received intra-articular saline injections as placebo. In the Puhl study the investigators added 0.25 mg of hyaluronic acid to the saline injections to impart viscosity to the solution. The mean age of the subjects for the 11 trials was 63 years.
Eight trials received support from pharmaceutical companies and 3 (the 2 by Henderson and the Grecomoro study) did not disclose any pharmaceutical support. One study was conducted in the United States, 3 in the UK, 3 in Germany, 1 in Sweden, 1 in Italy, and 2 in Canada. Five of the studies had scores over a cutoff quality score of >0.75,12,13,15,16,19 indicating they were good-quality randomized controlled trials; the remaining 4 had scores below 0.75.11,14,16,17
The outcomes of the 11 trials are summarized in TABLE 2. Patients’ pain ratings in both the active treatment and placebo groups improved in all the trials. Mean difference between improvements in treatment and placebo groups are shown in FIGURES 2A-2D for pain assessed at weeks 1, 5 to 7, 8 to 12 and 15 to 22, respectively. In each figure, we show the summary estimate of effect size with all the trials included and after excluding the 4 trials considered of poor quality, shown as “good quality studies.”
The mean difference in pain scores between treatment and placebo at week 1 was 4.4 (95% CI, +1.1, +7.2) and –1.0 (95% CI, -3.2, +1.2) for analysis restricted to the 7 good quality trials. The mean difference in pain scores at 5 to 7 weeks was 17.6 (95% CI, +7.5, +28.0) and 7.2 (95% CI, +2.4, +12.0) for the analysis restricted to the 2 good quality studies. At weeks 8 to 12 the mean difference in VAS between treatment and control was 18.1 (95% CI, +6.3, +29.9), and 7.1 (95% CI, +3.0, +11.3) in the analysis restricted to good quality trials. At weeks 15 to 22, the mean difference was 4.4 (95% CI, –15.3, +24.1). The Egger test was not statistically significant (2.3; P=.096; 95% CI, –0.5, +5.2) suggesting that there is no publication bias.
High heterogeneity was observed at all time intervals except 1 week (FIGURE 1). Of the 5 trials outside the confidence bounds (positioned 2 units above and below the regression line), 4 were poor-quality studies.
TABLE 3 shows the random-effect regression models we used to test the influence on the outcome of type of pain measured (pain with activity or pain at rest), type of medication (hyaluronan or hylan G-F 20), and study quality (good or poor). No significant association between treatment efficacy and type of pain used as outcome variable was observed. Clinical trials using hylan GF-20 showed statistically significant better results than those using hyaluronan at weeks 5 to 7 and 8 to 12. Poor-quality studies showed a larger treatment effect, but the difference was statistically significant only at week 1.
TABLE 3
Regression models to assess the sources of heterogeneity in the meta-analysis
| WEEK 1 | WEEKS 5–7 | WEEKS 8–12 | ||||
|---|---|---|---|---|---|---|
| Coef. (SE) [95% CI] | P value | Coef. (SE) [95% CI] | P value | Coef. (SE) [95% CI] | P value | |
| Pain | ||||||
| With activity | 1.7 (3.8) | 1.8 (4.3) | –0.5 (4.6) | |||
| At rest | [–5.8, +9.2] | 0.657 | [–6.6, +10.2] | 0.671 | [–9.5, +8.6] | 0.916 |
| Medication | ||||||
| Hyaluronan** | –3.4 (6.8) | 0.614 | 17.5 (4.9) | <0.001 | 14.8 (6.1) | 0.016 |
| Hylan G-F 20 | –16.7, +9.9] | [+7.8, +27.1] | [+2.8, +26.8] | |||
| Quality* | ||||||
| Poor (<0.75) | –19.9 (5.7) | 0.001 | –7.4 (4.9) | 0.131 | –11.7 (7.0) | 0.092 |
| Good (≥0.75) | [–31.1, –8.7] | [–17.0, +2.2] | [–25.3, +1.9] | |||
| Constant | 18.4 (5.6) | 0.001 | 13.5 (4.5) | 0.003 | 19.2 (5.8) | 0.001 |
| [+7.5, +29.3] | [+4.6, +22.3] | [+7.9, +30.5] | ||||
| *<.75 quality score: Grecomoro .439, Scale .570, Wobig .731, Huskisson .718. | ||||||
| ** 9 trials for hyaluronan (3 for Hyalgan®) and 2 trials for hylan G-F20 (Synvisc®). | ||||||
Discussion
This meta-analysis synthesized data from 9 randomized, double-blinded, placebo-controlled trials that evaluated the efficacy of intra-articular hyaluronic acid. Our findings show significantly decreased pain as measured by VAS at 5 to 7 weeks and at 8 to 12 weeks after the last injection. Intra-articular hyaluronic acid was not more effective than placebo in relieving pain at 1 week or at 15 to 22 weeks after the last injection. Because only 3 of the trials assessed patients after 12 weeks, however, the sample size is too small to definitively rule out a significant therapeutic effect after 12 weeks.
Reasons for the differences in efficacy among trials of hyaluronic acid in the treatment of knee osteoarthritis include dose, type, and frequency of administration, genetic or age differences among the study subjects, severity of osteoarthritis, time of follow-up, and quality of the studies. We confirmed that the treatment effect is time dependent. Although our meta-regression analysis (TABLE 2) suggests that hylan GF-20 is more effective than hyaluronan at 5 to 12 weeks, the number of clinical trials is relatively small and both of the hylan G-F20 studies were of poor quality. Therefore, we cannot say with confidence that one form is better than the other. Data in these trials were insufficient to assess the impact of body mass index, genetics, or severity of osteoarthritis.
We did not evaluate functional improvement in this meta-analysis because functional status was not measured in some trials and the assessment methods were too variable in the trials that did assess functional status. Publication bias, or the possibility that unpublished data would contradict the results of published studies, is always a potential source of bias in meta-analysis. However, the Egger test was not statistically significant (6.5; 95% CI, –0.5, +13.5) suggesting that there is no publication bias.25
Finally, the presence of heterogeneity of results indicates there were important differences among the studies. Exclusion of clinical trials considered of poor quality diminished this heterogeneity substantially. Subanalysis restricted to good-quality studies supports the efficacy of intra-articular hyaluronic acid in the treatment of knee osteoarthritis pain, although the effect size is smaller when one considers only the good quality studies.
There are 2 other potential limitations of this meta-analysis. Five studies allowed pain to be treated with analgesics such as acetaminophen or NSAIDs,12,13,16-18 and use of acetaminophen or NSAIDs may have altered the response to hyaluronic acid treatment. An intention-to-treat analysis was performed in only 2 studies (by Altman and Huskisson),16,18 wherein a post-hoc and “last observation carried forward” analysis showed a trend favoring hyaluronic acid. The treatment effects may have been smaller had the other trials used an intention to treat analysis.
This meta-analysis confirms that viscosupplementation with hyaluronic acid is modestly effective in short term relief of pain in knee osteoarthritis. Our meta-analysis included only double-blinded, randomized trials published in English language in humans using VAS as the pain outcome measure, and our conclusions are very similar to those of Lo.2
Indications for use. Hyaluronic acid is helpful in relieving pain for carefully selected patients with knee osteoarthritis who have not responded to adequate use of systemic therapeutic agents, including acetaminophen, NSAIDs and COX-2 inhibitors and topical agents, along with lifestyle modification such as weight reduction and exercise.
Patients should have a trial for at least three months of conservative therapy or be unable to tolerate NSAIDs before a decision to give 3- to 5-injection course with hyaluronic acid is made.
Hyaluronic acid may be an option when there is a need to delay knee surgery in middle-aged persons1 or for patients who have failed other treatments.
Time to pain relief. To improve adherence to treatment, tell patients receiving intra-articular hyaluronic acid that the benefits in pain reduction may not be noticeable until 5 to 10 weeks after the last injection.
Cost. Although the cost of hyaluronic acid treatment is covered by Medicare and most insurance plans for symptomatic osteoarthritis of knee, documentation in patient medical records should indicate the signs and symptoms supporting the diagnosis and functional impairment. Objective data to support a diagnosis of osteoarthritis such as x-ray, arthroscopy report, computed tomography scan, or magnetic resonance imaging should be available in the event of a review.
The cost of 1 hyaluronic acid (30 mg/mL) injection is approximately $230. Considering a course of 3 to 5 weekly knee injections, and adding other pharmacy, hospital, or clinic charges, the cost per treatment may exceed $1000 per knee.26,27 The cost-benefit of pain control with viscosupplementation must be carefully compared with other therapeutic agents and regimens currently available for knee osteoarthritis management.
FIGURE 3
Studies evaluated at outcome measurement time (week 1)
Egger’s publication bias plot for the studies evaluated at outcome measurement time (week 1) not significant (+2.3, P=0.096, 95% CI: –0.5, +5.2).
Acknowledgments
Jeff Welge, PhD, University of Cincinnati Medical Center, Center for Bio-statistical Services, for comments on final data analysis and presentation, Marie Marley, PhD for editing the manuscript, and Charity Noble for help in preparation of this manuscript.
CORRESPONDING AUTHOR
Arvind Modawal, MD, MPH, MRCGP, University of Cincinnati Department of Family Medicine/Geriatrics, PO Box 670582, Cincinnati, OH 45267-0582. E-mail: [email protected]
- Consider injections of hyaluronic acid only after conservative therapy has been tried for at least 3 months or the patient is unable to tolerate NSAIDs.
- Stress to patients that pain relief may not be fully experienced until 5 to 7 weeks following the last injection.
Hyaluronic acid injections can help relieve pain for carefully selected patients with knee osteoarthritis. But this option should be reserved for those whose pain has not responded to adequate trials of systemic therapeutic agents (acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs], cyclooxygenase-2 [COX-2] inhibitors), topical agents, or to lifestyle modifications such as weight reduction and exercise.
Hyaluronic acid injections may also be indicated when knee surgery must be delayed for middle-aged persons.1
In spite of the Food and Drug Administration’s approval of this therapy, uncertainty about its efficacy exists among the medical community. A recent meta-analysis of the effectiveness of intra-articular hyaluronic acid for knee osteoarthritis that included 22 published and unpublished, English and non-English, single or double-blinded, randomized controlled trials in humans showed that hyaluronic acid has only a small effect on pain relief when compared with placebo.2
We provide here a stringent test of the efficacy of viscosupplementation for relieving knee pain from osteoarthritis with a meta-analysis that includes only data from randomized, double-blinded, controlled trials of hyaluronic acid that measured pain using a visual analogue scale (VAS), the most widely accepted method for pain evaluation.
Long time coming
Balazs first proposed hyaluronic acid as a treatment for patients with arthritic diseases in 1942. In the early 1970s, therapeutic studies were begun to test the efficacy of hyaluronic acid on knee osteoarthritis. The results were encouraging and side effects were few.28 With the FDA’s approval in 1998, intra-articular “viscosupplementation” with hyaluronic acid—also called hyaluronan or hyaluronate, and the hylan derivatives of hyaluronic acid—is a welcome option for many of the 16 million older Americans with osteoarthritis of the knee.1
Methods
Selection of studies
We identified clinical trials of viscosupplementation with hyaluronic acid in humans published in English from 1965 through August 2004 through a computerized literature search of Medline. The keyword used was “hyaluronic acid,” which was combined with “trial” or “osteoarthritis knee” or “viscosupplementation.” We conducted an additional manual search of the reference lists of included articles and review articles. We also searched the Cochrane Library and websites of the Agency for Healthcare Research and Quality (AHRQ) for information on hyaluronic acid in knee osteoarthritis. We identified 1872 articles with this search process.
Of the 1872 articles, we identified by title and abstract 33 that might be pertinent to this study, including 17 randomized trials. We excluded reviews, meta-analyses, comparison trials, and trials reporting VAS as part of the WOMAC (Western Ontario McMaster Universities Index) scale. We attempted to contact authors of the studies that used a double-blind, randomized controlled design, to obtain any data that may not have been included in the publications. Three authors provided the details requested; the others did not respond or stated that additional data were not available. Of the 17 randomized trials we identified, 8 were excluded because they were open, single-blinded, or did not use the VAS to measure pain outcomes.3-10 The remaining 9 double-blinded, placebo controlled, randomized clinical trials of viscosupplementation with hyaluronic acid for knee osteoarthritis that did use a VAS to measure pain were included in this meta-analysis (TABLE 1). Because one of the studies (by Henderson) included 2 subgroups of pain severity, these were considered as 2 separate trials. The trial by Petrella had 2 treatment groups, one with only hyaluronic acid and the other with hyaluronic acid and NSAIDs. We considered them separately in the analysis, resulting in a total of 11 clinical trials for the meta-analysis.19 Henceforth in this report, we will refer to 11 rather than 9 clinical trials.
Extraction of data
Two investigators independently extracted the following data for each study: year of publication, study design, mean age, number of patients enrolled in each treatment group, number of doses of treatment used, and outcomes measured. When disagreements between investigators occurred, the point of disagreement was discussed until a consensus was reached. Since the treatment duration and the time post-treatment when pain was assessed varied among the trials, we grouped outcomes into four time intervals: at 1 week, 5 to 7 weeks, 8 to 12 weeks, and 15 to 22 weeks after the last hyaluronic acid injection.
Statistical analysis
The outcome was knee pain reported by patients on activity or at rest, measured using a VAS of 100 mm. The results of the clinical trials were recorded as the mean differences of change from baseline between the treatment and placebo groups. If not reported in the publication or provided by the authors, standard error was imputed using the method of Follman et al.20 We used the method of Chalmers for measuring the quality of randomized trials, with 2 of the authors rating the studies independently.21
This clinical trial grading system takes into account the following aspects of the trial to determine a quality score: evaluation of recruitment of subjects, rejection log, therapeutic regimen definition, randomization, blinding, prior estimates of numbers, testing compliance, statistical inference, use of appropriate statistical analysis, handling of withdrawal and side effects, dates of starting and ending, timing and tabulation of events. Because 4 of the trials had poor quality scores, an additional analysis excluding these 4 was performed. The DerSimonian and Laird random-effects model was used to obtain the summary estimates.22,23
An important element of meta-analysis is exploration of the heterogeneity of the outcomes and the possible causes of heterogeneity if it exists. Heterogeneity is the degree to which results vary from study to study. If a test for heterogeneity is statistically significant, there is significant variability among the treatment effects observed in the trials. We explored heterogeneity using Galbraith plots (FIGURE 1).24 In the absence of heterogeneity, all points fall within the confidence limits. Because we did find heterogeneity among these trials, we developed random-effect regression models to explore 3 possible sources of heterogeneity in the efficacy of hyaluronic acid; pain (measured at rest or on activity), the form used (hyaluronan or hylan G-F20), and the quality of the study method (good or poor).
Publication bias was assessed by the Egger et al regression asymmetry test.25 Analyses were performed using the meta-analytic software program of STATA, Inc (College Station, Tex; available at www.stata.com).
FIGURE 1
Evidence of heterogeneity for the studies evaluated at outcome measurement time (week 1)
This Galbraith plot shows standardized effects (b/se=mean difference/standard error) as a function of study precision (1/standard error), slope of the line shows average effect over all the studies with upper and lower lines denoting an approximate 95% confidence interval for this common effect. Under the hypothesis of study heterogeneity, the common slope intersect zero at 1/se=0, and 95% of all study estimates (authors initials) will fall within the confidence interval of the regression line. Gr: Grecomoro; Pu: Puhl; H1: Henderson 1; H2: Henderson 2; Sc: Scale; Lo: Lohmander; Al: Altman; Wo: Wobig; Hu: Huskisson; P1: Petrella 1; P2: Petrella 2.
Results
The 11 randomized, placebo-controlled, double-blinded clinical trials that met our inclusion criteria are summarized in TABLE 1. Nine trials used hyaluronic acid, hyaluronan, or hyaluronate (all types will be referred to as hyaluronan in the text), and 2 studies used hylan GF-20. Only 3 hyaluronan trials have published outcome data at 15 to 22 weeks follow-up. Treatment was administered to patients as 3 to 5 weekly injections, with the exception of the Grecomoro study in which treatment was administered twice weekly. The control group in 10 trials received intra-articular saline injections as placebo. In the Puhl study the investigators added 0.25 mg of hyaluronic acid to the saline injections to impart viscosity to the solution. The mean age of the subjects for the 11 trials was 63 years.
Eight trials received support from pharmaceutical companies and 3 (the 2 by Henderson and the Grecomoro study) did not disclose any pharmaceutical support. One study was conducted in the United States, 3 in the UK, 3 in Germany, 1 in Sweden, 1 in Italy, and 2 in Canada. Five of the studies had scores over a cutoff quality score of >0.75,12,13,15,16,19 indicating they were good-quality randomized controlled trials; the remaining 4 had scores below 0.75.11,14,16,17
The outcomes of the 11 trials are summarized in TABLE 2. Patients’ pain ratings in both the active treatment and placebo groups improved in all the trials. Mean difference between improvements in treatment and placebo groups are shown in FIGURES 2A-2D for pain assessed at weeks 1, 5 to 7, 8 to 12 and 15 to 22, respectively. In each figure, we show the summary estimate of effect size with all the trials included and after excluding the 4 trials considered of poor quality, shown as “good quality studies.”
The mean difference in pain scores between treatment and placebo at week 1 was 4.4 (95% CI, +1.1, +7.2) and –1.0 (95% CI, -3.2, +1.2) for analysis restricted to the 7 good quality trials. The mean difference in pain scores at 5 to 7 weeks was 17.6 (95% CI, +7.5, +28.0) and 7.2 (95% CI, +2.4, +12.0) for the analysis restricted to the 2 good quality studies. At weeks 8 to 12 the mean difference in VAS between treatment and control was 18.1 (95% CI, +6.3, +29.9), and 7.1 (95% CI, +3.0, +11.3) in the analysis restricted to good quality trials. At weeks 15 to 22, the mean difference was 4.4 (95% CI, –15.3, +24.1). The Egger test was not statistically significant (2.3; P=.096; 95% CI, –0.5, +5.2) suggesting that there is no publication bias.
High heterogeneity was observed at all time intervals except 1 week (FIGURE 1). Of the 5 trials outside the confidence bounds (positioned 2 units above and below the regression line), 4 were poor-quality studies.
TABLE 3 shows the random-effect regression models we used to test the influence on the outcome of type of pain measured (pain with activity or pain at rest), type of medication (hyaluronan or hylan G-F 20), and study quality (good or poor). No significant association between treatment efficacy and type of pain used as outcome variable was observed. Clinical trials using hylan GF-20 showed statistically significant better results than those using hyaluronan at weeks 5 to 7 and 8 to 12. Poor-quality studies showed a larger treatment effect, but the difference was statistically significant only at week 1.
TABLE 3
Regression models to assess the sources of heterogeneity in the meta-analysis
| WEEK 1 | WEEKS 5–7 | WEEKS 8–12 | ||||
|---|---|---|---|---|---|---|
| Coef. (SE) [95% CI] | P value | Coef. (SE) [95% CI] | P value | Coef. (SE) [95% CI] | P value | |
| Pain | ||||||
| With activity | 1.7 (3.8) | 1.8 (4.3) | –0.5 (4.6) | |||
| At rest | [–5.8, +9.2] | 0.657 | [–6.6, +10.2] | 0.671 | [–9.5, +8.6] | 0.916 |
| Medication | ||||||
| Hyaluronan** | –3.4 (6.8) | 0.614 | 17.5 (4.9) | <0.001 | 14.8 (6.1) | 0.016 |
| Hylan G-F 20 | –16.7, +9.9] | [+7.8, +27.1] | [+2.8, +26.8] | |||
| Quality* | ||||||
| Poor (<0.75) | –19.9 (5.7) | 0.001 | –7.4 (4.9) | 0.131 | –11.7 (7.0) | 0.092 |
| Good (≥0.75) | [–31.1, –8.7] | [–17.0, +2.2] | [–25.3, +1.9] | |||
| Constant | 18.4 (5.6) | 0.001 | 13.5 (4.5) | 0.003 | 19.2 (5.8) | 0.001 |
| [+7.5, +29.3] | [+4.6, +22.3] | [+7.9, +30.5] | ||||
| *<.75 quality score: Grecomoro .439, Scale .570, Wobig .731, Huskisson .718. | ||||||
| ** 9 trials for hyaluronan (3 for Hyalgan®) and 2 trials for hylan G-F20 (Synvisc®). | ||||||
Discussion
This meta-analysis synthesized data from 9 randomized, double-blinded, placebo-controlled trials that evaluated the efficacy of intra-articular hyaluronic acid. Our findings show significantly decreased pain as measured by VAS at 5 to 7 weeks and at 8 to 12 weeks after the last injection. Intra-articular hyaluronic acid was not more effective than placebo in relieving pain at 1 week or at 15 to 22 weeks after the last injection. Because only 3 of the trials assessed patients after 12 weeks, however, the sample size is too small to definitively rule out a significant therapeutic effect after 12 weeks.
Reasons for the differences in efficacy among trials of hyaluronic acid in the treatment of knee osteoarthritis include dose, type, and frequency of administration, genetic or age differences among the study subjects, severity of osteoarthritis, time of follow-up, and quality of the studies. We confirmed that the treatment effect is time dependent. Although our meta-regression analysis (TABLE 2) suggests that hylan GF-20 is more effective than hyaluronan at 5 to 12 weeks, the number of clinical trials is relatively small and both of the hylan G-F20 studies were of poor quality. Therefore, we cannot say with confidence that one form is better than the other. Data in these trials were insufficient to assess the impact of body mass index, genetics, or severity of osteoarthritis.
We did not evaluate functional improvement in this meta-analysis because functional status was not measured in some trials and the assessment methods were too variable in the trials that did assess functional status. Publication bias, or the possibility that unpublished data would contradict the results of published studies, is always a potential source of bias in meta-analysis. However, the Egger test was not statistically significant (6.5; 95% CI, –0.5, +13.5) suggesting that there is no publication bias.25
Finally, the presence of heterogeneity of results indicates there were important differences among the studies. Exclusion of clinical trials considered of poor quality diminished this heterogeneity substantially. Subanalysis restricted to good-quality studies supports the efficacy of intra-articular hyaluronic acid in the treatment of knee osteoarthritis pain, although the effect size is smaller when one considers only the good quality studies.
There are 2 other potential limitations of this meta-analysis. Five studies allowed pain to be treated with analgesics such as acetaminophen or NSAIDs,12,13,16-18 and use of acetaminophen or NSAIDs may have altered the response to hyaluronic acid treatment. An intention-to-treat analysis was performed in only 2 studies (by Altman and Huskisson),16,18 wherein a post-hoc and “last observation carried forward” analysis showed a trend favoring hyaluronic acid. The treatment effects may have been smaller had the other trials used an intention to treat analysis.
This meta-analysis confirms that viscosupplementation with hyaluronic acid is modestly effective in short term relief of pain in knee osteoarthritis. Our meta-analysis included only double-blinded, randomized trials published in English language in humans using VAS as the pain outcome measure, and our conclusions are very similar to those of Lo.2
Indications for use. Hyaluronic acid is helpful in relieving pain for carefully selected patients with knee osteoarthritis who have not responded to adequate use of systemic therapeutic agents, including acetaminophen, NSAIDs and COX-2 inhibitors and topical agents, along with lifestyle modification such as weight reduction and exercise.
Patients should have a trial for at least three months of conservative therapy or be unable to tolerate NSAIDs before a decision to give 3- to 5-injection course with hyaluronic acid is made.
Hyaluronic acid may be an option when there is a need to delay knee surgery in middle-aged persons1 or for patients who have failed other treatments.
Time to pain relief. To improve adherence to treatment, tell patients receiving intra-articular hyaluronic acid that the benefits in pain reduction may not be noticeable until 5 to 10 weeks after the last injection.
Cost. Although the cost of hyaluronic acid treatment is covered by Medicare and most insurance plans for symptomatic osteoarthritis of knee, documentation in patient medical records should indicate the signs and symptoms supporting the diagnosis and functional impairment. Objective data to support a diagnosis of osteoarthritis such as x-ray, arthroscopy report, computed tomography scan, or magnetic resonance imaging should be available in the event of a review.
The cost of 1 hyaluronic acid (30 mg/mL) injection is approximately $230. Considering a course of 3 to 5 weekly knee injections, and adding other pharmacy, hospital, or clinic charges, the cost per treatment may exceed $1000 per knee.26,27 The cost-benefit of pain control with viscosupplementation must be carefully compared with other therapeutic agents and regimens currently available for knee osteoarthritis management.
FIGURE 3
Studies evaluated at outcome measurement time (week 1)
Egger’s publication bias plot for the studies evaluated at outcome measurement time (week 1) not significant (+2.3, P=0.096, 95% CI: –0.5, +5.2).
Acknowledgments
Jeff Welge, PhD, University of Cincinnati Medical Center, Center for Bio-statistical Services, for comments on final data analysis and presentation, Marie Marley, PhD for editing the manuscript, and Charity Noble for help in preparation of this manuscript.
CORRESPONDING AUTHOR
Arvind Modawal, MD, MPH, MRCGP, University of Cincinnati Department of Family Medicine/Geriatrics, PO Box 670582, Cincinnati, OH 45267-0582. E-mail: [email protected]
1. Cefalu CA, Waddell DS. Viscosupplementation: Treatment alternative for osteoarthritis of the knee. Geriatrics 1999;54:51-57.
2. Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis. JAMA 2003;290:3115-3121.
3. Jones AC, Pattrick M, Doherty S, Doherty M. Intra-articular hyaluronic acid compared to intra-articular triamcinolone hexacetonide in inflammatory knee osteoarthritis. Osteoarthritis Cartilage 1995;3:269-273.
4. Graf J, Neusel E, Schneider E, Niethard FU. Intra-articular treatment with hyaluronic acid in osteoarthritis of the knee joint: a controlled clinical trial versus mucopolysaccharide polysulfuric acid ester. Clin Exp Rheumatol 1993;11:367-72.
5. Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: A one year placebo-controlled trial. Osteoarthritis Cartilage 1993;1:97-103.
6. Adams ME. An analysis of clinical studies of the use of cross-linked hyaluronan, hylan, in the treatment of osteoarthritis. J Rheumatol Supp 1993;39:16-18.
7. Grecomoro G, Piccione F, Letizia G. Therapeutic synergism between hyaluronic acid and dexamethasone in the intra-articular treatment of osteoarthritis of the knee: a preliminary open study. Curr Med Res Opin 1992;13:49-55.
8. Leardini G, Mattara L, Franceschini M, Perbellini A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and 6-methyl prednisolone acetate. Clin Exp Rheumatol 1991;9:375-381.
9. Wu J, Shih L, Hsu H, Chen T. The double-blind test of sodium hyaluronate (ARTZ) on osteoarthritis knee. Chin Med J (Taipei) 1997;59:99-106.
10. Dixon AS, Jacoby RK, Berry H, Hamilton EB. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin 1988;11:205-213.
11. Grecomoro G, Martorana U, Di Marco C. Intra-articular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmather 1987;5:137-141.
12. Puhl W, Bernau A, Greiling H, Kopcke W, Pforringer W, Steck KJ. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage 1993;1:233-241.
13. Henderson EB, Smith EC, Pegley F, Blake DR. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: a randomized single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann Rheum Dis 1994;53:529-554.
14. Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with Hylan: A treatment schedule study. Curr Ther Res 1994;55:220-232.
15. Lohmander LS, Dalen N, Englund G, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomized, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group [see comments]. Ann Rheum Dis 1996;55:424-431.
16. Altman RD, Moskowitz R. Intra-articular sodium hyaluronate (Hyalgan®) in the treatment of patients with osteoarthritis of the knee: A randomized clinical trial. J Rheumatol 1998;25:2203-2212.
17. Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with Hylan G-F-20: A 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther 1998;20:410-423.
18. Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology 1999;38:602-607.
19. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee. Arch Intern Med 2002;162:292-298.
20. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769-773.
21. Chalmers TC, Smith H, Jr, Blackburn B, et al. A Method for Assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49.
22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
23. Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med 1987;316:450-455.
24. Galbraith R. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988;7:889-894.
25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-634.
26. CIGNA Government Services. Not otherwise classified drug fee schedule. Available at: www.cignamedicare.com/partb/fsch/2004/q2/noc.html. Accessed on June 20, 2005.
27. Empire Medicare Services. Local coverage determination. Hyaluronate polymers (Synvisc, Hyalgan) (A02-0011-R3). Available at: www.empiremedicare.com/Nyorkpolicya/policy/A02-0011-R_FINAL.htm.
28. Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. Pathol Biol 1974;22:731-736.
1. Cefalu CA, Waddell DS. Viscosupplementation: Treatment alternative for osteoarthritis of the knee. Geriatrics 1999;54:51-57.
2. Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis. JAMA 2003;290:3115-3121.
3. Jones AC, Pattrick M, Doherty S, Doherty M. Intra-articular hyaluronic acid compared to intra-articular triamcinolone hexacetonide in inflammatory knee osteoarthritis. Osteoarthritis Cartilage 1995;3:269-273.
4. Graf J, Neusel E, Schneider E, Niethard FU. Intra-articular treatment with hyaluronic acid in osteoarthritis of the knee joint: a controlled clinical trial versus mucopolysaccharide polysulfuric acid ester. Clin Exp Rheumatol 1993;11:367-72.
5. Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: A one year placebo-controlled trial. Osteoarthritis Cartilage 1993;1:97-103.
6. Adams ME. An analysis of clinical studies of the use of cross-linked hyaluronan, hylan, in the treatment of osteoarthritis. J Rheumatol Supp 1993;39:16-18.
7. Grecomoro G, Piccione F, Letizia G. Therapeutic synergism between hyaluronic acid and dexamethasone in the intra-articular treatment of osteoarthritis of the knee: a preliminary open study. Curr Med Res Opin 1992;13:49-55.
8. Leardini G, Mattara L, Franceschini M, Perbellini A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and 6-methyl prednisolone acetate. Clin Exp Rheumatol 1991;9:375-381.
9. Wu J, Shih L, Hsu H, Chen T. The double-blind test of sodium hyaluronate (ARTZ) on osteoarthritis knee. Chin Med J (Taipei) 1997;59:99-106.
10. Dixon AS, Jacoby RK, Berry H, Hamilton EB. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin 1988;11:205-213.
11. Grecomoro G, Martorana U, Di Marco C. Intra-articular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmather 1987;5:137-141.
12. Puhl W, Bernau A, Greiling H, Kopcke W, Pforringer W, Steck KJ. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage 1993;1:233-241.
13. Henderson EB, Smith EC, Pegley F, Blake DR. Intra-articular injections of 750 kD hyaluronan in the treatment of osteoarthritis: a randomized single centre double-blind placebo-controlled trial of 91 patients demonstrating lack of efficacy. Ann Rheum Dis 1994;53:529-554.
14. Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with Hylan: A treatment schedule study. Curr Ther Res 1994;55:220-232.
15. Lohmander LS, Dalen N, Englund G, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomized, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group [see comments]. Ann Rheum Dis 1996;55:424-431.
16. Altman RD, Moskowitz R. Intra-articular sodium hyaluronate (Hyalgan®) in the treatment of patients with osteoarthritis of the knee: A randomized clinical trial. J Rheumatol 1998;25:2203-2212.
17. Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with Hylan G-F-20: A 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther 1998;20:410-423.
18. Huskisson EC, Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology 1999;38:602-607.
19. Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee. Arch Intern Med 2002;162:292-298.
20. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769-773.
21. Chalmers TC, Smith H, Jr, Blackburn B, et al. A Method for Assessing the quality of a randomized control trial. Control Clin Trials 1981;2:31-49.
22. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
23. Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analyses of randomized controlled trials. N Engl J Med 1987;316:450-455.
24. Galbraith R. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med 1988;7:889-894.
25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-634.
26. CIGNA Government Services. Not otherwise classified drug fee schedule. Available at: www.cignamedicare.com/partb/fsch/2004/q2/noc.html. Accessed on June 20, 2005.
27. Empire Medicare Services. Local coverage determination. Hyaluronate polymers (Synvisc, Hyalgan) (A02-0011-R3). Available at: www.empiremedicare.com/Nyorkpolicya/policy/A02-0011-R_FINAL.htm.
28. Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. Pathol Biol 1974;22:731-736.
Is your patient’s dizziness psychogenic?
Dizziness is common among patients age 65 and older, and more than one-third have a psychiatric disorder that is caused by or is causing their dizziness.1
When older patients present with dizziness, psychiatrists may be asked to alleviate the psychological symptoms and help identify the underlying disease state.2
More than 60 medical and psychiatric disorders and many medications can cause dizziness. To help you sort through the possibilities, we offer:
- six diagnostic questions to rule out underlying medical problems
- lists of commonly used psychotropics and other drugs that may cause dizziness
- advice on treating depression, anxiety, and panic disorder in an older patient with dizziness while avoiding side effects and drug interactions.
Table 1
Four types of dizziness and their usual causes
| Vertigo Benign positional vertigo CNS cause—tumor, demyelination, neurodegenerative disorders Labyrinthitis Meniere’s disease Peripheral vestibulopathy (in 50% of cases) Vestibular neuronitis |
| Presyncope Arrhythmias Carotid sinus disease Hypoglycemia Neurocardiogenic syncope Organic heart disease Orthostatic hypotension Seizures Situational Transient ischemic attacks |
| Disequilibrium Balance and gait disorder Mixed CNS diseases (ischemic, degenerative) Neurodegenerative CNS disorders Presbystasis Sensorimotor dysfunction |
| Psychogenic lightheadedness Agoraphobia Anxiety Depression Panic disorder Hyperventilation |
| Source: Adapted from reference 6 |
Many causes of dizziness
The term “dizziness” is hard to define because of its nonspecific and variable symptom description, multiple causes, and lack of clear diagnostic and management guidelines. In clinical use, dizziness encompasses abnormal sensations relating to perception of the body’s relationship to space.
Some researchers believe dizziness is a distinct geriatric syndrome because numerous factors related to aging cause dizziness,2 including physiologic changes (presbystasis), accumulated impairment, disease states, and interactions between multiple medications.
Anxiety, somatization, panic disorder, and depression cause dizziness in the elderly, as do:
- peripheral vestibular disorders
- brainstem cerebrovascular accident
- diabetes mellitus
- neurologic disorders such as Parkinson’s disease
- and cardiovascular disorders.
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants also have been shown to cause dizziness, as have numerous nonpsychotropic agents.
Recognizing patterns, testing hypotheses, and extending the diagnostic process over time can help you differentiate psychogenic from medicationinduced or neurologic dizziness.3 Because the presentation is so complex and the differential diagnosis so broad, algorithmic diagnosis is less effective than a flexible clinical approach that allows for uncertainty in evaluating initial symptoms.
Determining the cause
A thorough patient history and physical examination can uncover a cause of dizziness in 75% of cases.4 Look for duration of dizziness symptoms; history of heart disease, diabetes or other illnesses; family history of psychiatric disorders; and other illnesses among family members.
Ask the following six complaint-specific questions to help you narrow the differential diagnosis and rule out nonpsychiatric causes.5
1. WHAT TYPE OF DIZZINESS DOES THE PATIENT HAVE?
Four categories—vertigo, presyncope, disequilibrium, and lightheadedness—are used to classify dizziness (Table 1).6
Vertigo is a sense that the body or environment is Patients may feel as if the floor is tilting, sinking, rising or veering sideways, or they may feel pulled to one side.
Vertigo is commonly caused by peripheral vestibular disorders—including benign positional vertigo, Meniere’s disease, labyrinthitis, and vestibular neuronitis—and central vestibular disorders associated with cerebrovascular disease, tumors, demyelinating diseases, migraines, seizures, multiple sclerosis and other CNS diseases. Acute-onset vertigo and neurologic signs suggest brainstem infarction.
Nystagmus is usually present, horizontal, and may be rotational at times. A vertical-beating nystagmus points to a probable CNS cause and requires urgent neuroimaging and referral to a neurologist or otolaryngologist.
Presyncope describes near-fainting. A dimming of vision and roaring in the ears may precede presyncope.
Depending on its cause, presyncope may occur regardless of position or only when upright. Common causes include orthostatic hypotension, neurocardiogenic syncope, organic heart disease, arrhythmias, carotid sinus disease, seizures, hypoglycemia, and transient ischemic attacks.
Abrupt presyncopal attacks that occur regardless of position suggest a cardiovascular cause. If onset is gradual and not improved by lying down, suspect a cerebral metabolic cause such as hypoglycemia.
Syncope, like presyncope, often is traced to an underlying cardiovascular disease. Dizziness and syncope often coexist, and both can be multifactorial. Dizziness may precede or follow syncopal episodes.
Differentiating syncope and dizziness is important because many underlying causes of syncope can be fatal. By contrast, dizziness symptoms are usually benign and self-limiting.7
A thorough history is critical to distinguishing dizziness from presyncope. Assess medication effects—especially CNS-acting medications, cardiovascular drugs, antihypertensives, antibiotics, and over-the-counter medications such as dextromethorphan and acetaminophen compounds. Also check for dehydration.
Disequilibrium disorder signifies unsteadiness or a loss of balance primarily involving the lower extremities. Symptoms are evoked by walking or standing and relieved by sitting or lying down. Gait is abnormal and balance is compromised without abnormal head sensations.
Common causes include balance and gait disorders, sensorimotor dysfunction, presbystasis, neurodegenerative CNS disorders, and mixed ischemic and degenerative CNS diseases.
Vague lightheadedness is often associated with somatic symptoms such as headache. Some patients describe a floating sensation.
Lightheadedness is frequently associated with anxiety, panic disorder, depression, and somatization. Hyperventilation and agoraphobia are other common causes.
Multiple symptoms, multiple types. Classifying an older patient’s dizziness can be challenging because many patients report symptoms that suggest two or more subtypes.2 Also, patients often have trouble describing their dizziness symptoms, sometimes using terms such as “giddiness,” “wooziness,” or “confusion.”
To help patients explain dizziness symptoms more accurately, ask specific questions such as:
- Do you at times feel like you’re about to faint?
- Do you feel as if the room is moving?
- Do you sometimes feel as though you’re going to fall?
Table 2
Psychotropics that may cause dizziness
| Anti-Alzheimer’s medications Memantine, rivastigmine, tacrine |
| Anticonvulsants Phenytoin |
| Antidepressants Monoamine oxidase inhibitors (phenelzine, selegiline) Selective serotonin reuptake inhibitors (all) Tricyclics (amitriptyline, imipramine, nortriptyline, trazodone) Others (bupropion, buspirone, mirtazapine, nefazodone, venlafaxine) |
| Antipsychotics Typicals (chlorpromazine, fluphenazine, perphenazine, prochlorperazine, thioridazine, trifluoperazine) Atypicals (all except olanzapine) |
| Anxiolytics Alprazolam, chlordiazepoxide, clonazepam, diazepam, lorazepam, oxazepam |
| Hypnotics Estazolam, flurazepam, quazepam, temazepam, triazolam, zolpidem |
| Mood stabilizers Carbamazepine, divalproex/valproic acid, gabapentin, lamotrigine, oxcarbazepine |
| Source: Clinical Pharmacology version 2.11. Tampa, FL: Gold Standard MultiMedia, 2004. |
2. HOW DO DIZZINESS SYMPTOMS RELATE TO POSITION OR MOTION?
By reproducing dizziness symptoms, some quick-maneuver tests can help patients describe their symptoms and may reveal a medical cause.
Dix-Hallpike maneuver.3 Move the patient rapidly from a seated to prone position with the head below the horizontal plane and turned 45 degrees for 10 seconds; then have the patient sit up. Repeat with the head turned to the other side. If dizziness does not occur within a few seconds after each test, rule out benign positional vertigo.
Seated head turn, or head-thrust test, measures qualitative vestibular function.8 Move the head rapidly by 45 degrees in a brief, small-amplitude thrust to one side while the patient focuses on your nose; this gauges vestibularocular control. Repeat the test in the other direction. A refixation corrective saccade, occurring as the patient tries to fixate on the target, indicates a possible vestibular disorder.
‘Get-Up and Go’ test, which takes less than 10 seconds, measures balance in older patents.9 Have the patient stand up, walk 10 feet, turn around, walk back, and sit down. Watch for staggering, unsteadiness, and use of hands to balance. Onset of symptoms suggests dizziness brought on during activities of daily living and provides information on how dizziness is affecting the patient’s ability to function.
Romberg test. Have the patient stand with heels together, first with eyes open and then closed. Vision and proprioceptive signals are used to compensate for vestibular loss. Thus, a balance disturbance with eyes closed suggests vestibular or spinal proprioceptive problems and may predict risk of falls caused by inability to compensate.8
3. WHAT IS THE COURSE OF DIZZINESS?
Differentiating acute, sudden-onset dizziness from chronic, gradual-onset dizziness can help uncover the problem’s cause and seriousness. The latter often has a psychological cause or may point to vestibular or minor cardiovascular problems. Tinetti et al2 identified anxiety or depressive symptoms as risk factors among community-based older persons who reported dizziness episodes lasting 1 month.
Table 3
Recommended SSRI starting dosages for older patients
| SSRI | Starting dosage (mg/d) | Maximum dosage (mg/d) |
|---|---|---|
| Citalopram | 10 to 20 | 30 |
| Escitalopram | 10 | 10 |
| Fluoxetine* | 5 to 10 | 60 |
| Paroxetine | 5 | 40 |
| Sertraline | 25 to 50 | 200 |
| * Most patients will not need more than 20 mg/d. Dosages 40 mg/d should be divided into twice-daily doses. | ||
| Source: Adapted from Reuben DB, Herr K, Pacala JT, et al. Geriatrics at your fingertips (5th ed). Malden, MA: Blackwell Publishing, 2003:47. | ||
An acute presentation can suggest a panic disorder or acute anxiety state, but first rule out serious conditions such as acute myocardial infarction, arrhythmias, acute infections, GI bleeding, and carbon monoxide poisoning.
Also ask about:
- exacerbating and relieving factors. For example, positional changes, exercise or other physical activity, eating, or missing a meal can trigger presyncope. Also find out about situations that may bring on anxiety, panic, or phobia. Onset of dizziness following these situations may suggest psychogenesis.
- recent falls and injuries. Recurrent falls with presyncope suggest a probable orthostatic or cardiovascular diagnosis in older adults.
4. ANY PAST MEDICAL PROBLEMS?
Ask disease-specific questions. For example:
- Tinnitus or hearing loss could point to a vestibular disorder.
- Metabolic and cardiovascular disorders such as diabetes, ischemic heart disease, postural hypotension, and seizures can result in presyncope.
- Orthostasis, coronary ischemic events, hypoglycemia, and transient ischemic attacks may cause dizziness.
5. IS DIZZINESS RECURRENT?
Panic disorder, anxiety disorders, phobia, and psychogenic hyperventilation are commonly associated with chronic, recurrent dizziness episodes.
6. WHAT MEDICATIONS IS THE PATIENT TAKING?
All psychotropics are suspect when a patient presents with dizziness. When dizziness occurs after a dose or start of therapy, evaluate response to the medication and consider reducing the dosage or changing the medication. If symptoms persist, refer the patient back to the primary care physician to investigate for other causes of dizziness.
Psychotropics that may cause dizziness are listed in Table 2, For a list of other medications associated with dizziness, see this article at www.currentpsychiatry.com.
If the above strategies do not reveal a physical cause of dizziness despite multiple physical complaints, consider examining the patient for depression, anxiety, or panic disorder.
Treating a psychiatric cause
If dizziness is found to be psychogenic and the symptoms impede daily activities or contribute to functional decline, treat the psychiatric disorder but carefully weigh the risks and benefits of drug treatment.
Although SSRIs may cause dizziness, these agents are recommended first-line treatment for depression, anxiety, and/or phobia in older patients with dizziness because of their relative lack of anticholinergic action and side effects compared with other antidepressants or anxiolytics.
Coexisting medical symptoms may dictate choice of agent. For example, consider a sedating SSRI for a patient with sleep disturbances caused by dizziness or the psychiatric disorder; choose a nonsedating SSRI if the patient is sleeping normally.
Because SSRIs may cause weight loss, avoid giving them to patients with weight loss associated with dizziness or an underlying psychiatric illness. Mirtazapine, which is associated with weight gain, may offset weight loss. Start mirtazapine at 15 mg at bedtime for older patients.
Start low and go slow when prescribing an SSRI to an older patient. Dosing strategies applicable to younger patients should not be extrapolated to older patients, especially those with dizziness.
We have found that older patients respond well to minimum or below-normal SSRI dosages (Table 3). Titrate very slowly and instruct patients to report dizziness. Reduce the dosage if dizziness emerges.
If the patient does not respond to an SSRI or mirtazapine, consider a serotonin and norepinephrine reuptake inhibitor, which also has favorable anticholinergic and side-effect profiles.
Related resources
- WebMD Health—Dizziness: lightheadedness and vertigo. http://my.webMD.com/hw/health_guide_atoz/hw88500.asp.
- Sloane PD. Clinical research and geriatric dizziness: The blind men and the elephant. J Am Geriatr Soc 1999;47:113-14.
- Kroenke K, Hoffman RM, Einstadter D. How common are various forms of dizziness? A critical review. South Med J 2000;93:160-7.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Carbamazepine • Tegretol
- Chlordiazepoxide • Librium
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Divalproex/valproic acid • Depakote
- Escitalopram • Lexapro
- Estazolam • ProSom
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Memantine • Namenda
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Phenytoin • Dilantin
- Prochlorperazine • Compazine
- Quazepam • Doral
- Rivastigmine • Exelon
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Tacrine • Cognex
- Temazepam • Restoril
- Thioridazine • Mellaril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trifluoperazine • Vesprin
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Robert Cluxton, PharmD, University of Cincinnati College of Pharmacy, for helping to prepare this manuscript for publication.
1. Sloane PD, Hartman M, Mitchell CM. Psychological factors associated with chronic dizziness in patients aged 60 and older. J Am Geriatr Soc 1994;42:847-52.
2. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med 2000;132:337-44.
3. Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med 2001;134(9 pt 2):823-32.
4. Hoffman RM, Einstadter D, Kroenke K. Evaluating dizziness. Am J Med 1999;107:468-78.
5. Drachman DA. A 69-year-old man with chronic dizziness. JAMA 1998;280:2111-18.
6. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology 1972;22:323-34.
7. Kapoor WN. Syncope. N Engl J Med 2000;343:1856-62.
8. Baloh RW. Hearing and equilibrium. In: Goldman L, Ansiello D (eds). Cecil textbook of medicine (22nd ed). Philadelphia: Saunders 2004;2436-42.
9. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387-9.
Dizziness is common among patients age 65 and older, and more than one-third have a psychiatric disorder that is caused by or is causing their dizziness.1
When older patients present with dizziness, psychiatrists may be asked to alleviate the psychological symptoms and help identify the underlying disease state.2
More than 60 medical and psychiatric disorders and many medications can cause dizziness. To help you sort through the possibilities, we offer:
- six diagnostic questions to rule out underlying medical problems
- lists of commonly used psychotropics and other drugs that may cause dizziness
- advice on treating depression, anxiety, and panic disorder in an older patient with dizziness while avoiding side effects and drug interactions.
Table 1
Four types of dizziness and their usual causes
| Vertigo Benign positional vertigo CNS cause—tumor, demyelination, neurodegenerative disorders Labyrinthitis Meniere’s disease Peripheral vestibulopathy (in 50% of cases) Vestibular neuronitis |
| Presyncope Arrhythmias Carotid sinus disease Hypoglycemia Neurocardiogenic syncope Organic heart disease Orthostatic hypotension Seizures Situational Transient ischemic attacks |
| Disequilibrium Balance and gait disorder Mixed CNS diseases (ischemic, degenerative) Neurodegenerative CNS disorders Presbystasis Sensorimotor dysfunction |
| Psychogenic lightheadedness Agoraphobia Anxiety Depression Panic disorder Hyperventilation |
| Source: Adapted from reference 6 |
Many causes of dizziness
The term “dizziness” is hard to define because of its nonspecific and variable symptom description, multiple causes, and lack of clear diagnostic and management guidelines. In clinical use, dizziness encompasses abnormal sensations relating to perception of the body’s relationship to space.
Some researchers believe dizziness is a distinct geriatric syndrome because numerous factors related to aging cause dizziness,2 including physiologic changes (presbystasis), accumulated impairment, disease states, and interactions between multiple medications.
Anxiety, somatization, panic disorder, and depression cause dizziness in the elderly, as do:
- peripheral vestibular disorders
- brainstem cerebrovascular accident
- diabetes mellitus
- neurologic disorders such as Parkinson’s disease
- and cardiovascular disorders.
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants also have been shown to cause dizziness, as have numerous nonpsychotropic agents.
Recognizing patterns, testing hypotheses, and extending the diagnostic process over time can help you differentiate psychogenic from medicationinduced or neurologic dizziness.3 Because the presentation is so complex and the differential diagnosis so broad, algorithmic diagnosis is less effective than a flexible clinical approach that allows for uncertainty in evaluating initial symptoms.
Determining the cause
A thorough patient history and physical examination can uncover a cause of dizziness in 75% of cases.4 Look for duration of dizziness symptoms; history of heart disease, diabetes or other illnesses; family history of psychiatric disorders; and other illnesses among family members.
Ask the following six complaint-specific questions to help you narrow the differential diagnosis and rule out nonpsychiatric causes.5
1. WHAT TYPE OF DIZZINESS DOES THE PATIENT HAVE?
Four categories—vertigo, presyncope, disequilibrium, and lightheadedness—are used to classify dizziness (Table 1).6
Vertigo is a sense that the body or environment is Patients may feel as if the floor is tilting, sinking, rising or veering sideways, or they may feel pulled to one side.
Vertigo is commonly caused by peripheral vestibular disorders—including benign positional vertigo, Meniere’s disease, labyrinthitis, and vestibular neuronitis—and central vestibular disorders associated with cerebrovascular disease, tumors, demyelinating diseases, migraines, seizures, multiple sclerosis and other CNS diseases. Acute-onset vertigo and neurologic signs suggest brainstem infarction.
Nystagmus is usually present, horizontal, and may be rotational at times. A vertical-beating nystagmus points to a probable CNS cause and requires urgent neuroimaging and referral to a neurologist or otolaryngologist.
Presyncope describes near-fainting. A dimming of vision and roaring in the ears may precede presyncope.
Depending on its cause, presyncope may occur regardless of position or only when upright. Common causes include orthostatic hypotension, neurocardiogenic syncope, organic heart disease, arrhythmias, carotid sinus disease, seizures, hypoglycemia, and transient ischemic attacks.
Abrupt presyncopal attacks that occur regardless of position suggest a cardiovascular cause. If onset is gradual and not improved by lying down, suspect a cerebral metabolic cause such as hypoglycemia.
Syncope, like presyncope, often is traced to an underlying cardiovascular disease. Dizziness and syncope often coexist, and both can be multifactorial. Dizziness may precede or follow syncopal episodes.
Differentiating syncope and dizziness is important because many underlying causes of syncope can be fatal. By contrast, dizziness symptoms are usually benign and self-limiting.7
A thorough history is critical to distinguishing dizziness from presyncope. Assess medication effects—especially CNS-acting medications, cardiovascular drugs, antihypertensives, antibiotics, and over-the-counter medications such as dextromethorphan and acetaminophen compounds. Also check for dehydration.
Disequilibrium disorder signifies unsteadiness or a loss of balance primarily involving the lower extremities. Symptoms are evoked by walking or standing and relieved by sitting or lying down. Gait is abnormal and balance is compromised without abnormal head sensations.
Common causes include balance and gait disorders, sensorimotor dysfunction, presbystasis, neurodegenerative CNS disorders, and mixed ischemic and degenerative CNS diseases.
Vague lightheadedness is often associated with somatic symptoms such as headache. Some patients describe a floating sensation.
Lightheadedness is frequently associated with anxiety, panic disorder, depression, and somatization. Hyperventilation and agoraphobia are other common causes.
Multiple symptoms, multiple types. Classifying an older patient’s dizziness can be challenging because many patients report symptoms that suggest two or more subtypes.2 Also, patients often have trouble describing their dizziness symptoms, sometimes using terms such as “giddiness,” “wooziness,” or “confusion.”
To help patients explain dizziness symptoms more accurately, ask specific questions such as:
- Do you at times feel like you’re about to faint?
- Do you feel as if the room is moving?
- Do you sometimes feel as though you’re going to fall?
Table 2
Psychotropics that may cause dizziness
| Anti-Alzheimer’s medications Memantine, rivastigmine, tacrine |
| Anticonvulsants Phenytoin |
| Antidepressants Monoamine oxidase inhibitors (phenelzine, selegiline) Selective serotonin reuptake inhibitors (all) Tricyclics (amitriptyline, imipramine, nortriptyline, trazodone) Others (bupropion, buspirone, mirtazapine, nefazodone, venlafaxine) |
| Antipsychotics Typicals (chlorpromazine, fluphenazine, perphenazine, prochlorperazine, thioridazine, trifluoperazine) Atypicals (all except olanzapine) |
| Anxiolytics Alprazolam, chlordiazepoxide, clonazepam, diazepam, lorazepam, oxazepam |
| Hypnotics Estazolam, flurazepam, quazepam, temazepam, triazolam, zolpidem |
| Mood stabilizers Carbamazepine, divalproex/valproic acid, gabapentin, lamotrigine, oxcarbazepine |
| Source: Clinical Pharmacology version 2.11. Tampa, FL: Gold Standard MultiMedia, 2004. |
2. HOW DO DIZZINESS SYMPTOMS RELATE TO POSITION OR MOTION?
By reproducing dizziness symptoms, some quick-maneuver tests can help patients describe their symptoms and may reveal a medical cause.
Dix-Hallpike maneuver.3 Move the patient rapidly from a seated to prone position with the head below the horizontal plane and turned 45 degrees for 10 seconds; then have the patient sit up. Repeat with the head turned to the other side. If dizziness does not occur within a few seconds after each test, rule out benign positional vertigo.
Seated head turn, or head-thrust test, measures qualitative vestibular function.8 Move the head rapidly by 45 degrees in a brief, small-amplitude thrust to one side while the patient focuses on your nose; this gauges vestibularocular control. Repeat the test in the other direction. A refixation corrective saccade, occurring as the patient tries to fixate on the target, indicates a possible vestibular disorder.
‘Get-Up and Go’ test, which takes less than 10 seconds, measures balance in older patents.9 Have the patient stand up, walk 10 feet, turn around, walk back, and sit down. Watch for staggering, unsteadiness, and use of hands to balance. Onset of symptoms suggests dizziness brought on during activities of daily living and provides information on how dizziness is affecting the patient’s ability to function.
Romberg test. Have the patient stand with heels together, first with eyes open and then closed. Vision and proprioceptive signals are used to compensate for vestibular loss. Thus, a balance disturbance with eyes closed suggests vestibular or spinal proprioceptive problems and may predict risk of falls caused by inability to compensate.8
3. WHAT IS THE COURSE OF DIZZINESS?
Differentiating acute, sudden-onset dizziness from chronic, gradual-onset dizziness can help uncover the problem’s cause and seriousness. The latter often has a psychological cause or may point to vestibular or minor cardiovascular problems. Tinetti et al2 identified anxiety or depressive symptoms as risk factors among community-based older persons who reported dizziness episodes lasting 1 month.
Table 3
Recommended SSRI starting dosages for older patients
| SSRI | Starting dosage (mg/d) | Maximum dosage (mg/d) |
|---|---|---|
| Citalopram | 10 to 20 | 30 |
| Escitalopram | 10 | 10 |
| Fluoxetine* | 5 to 10 | 60 |
| Paroxetine | 5 | 40 |
| Sertraline | 25 to 50 | 200 |
| * Most patients will not need more than 20 mg/d. Dosages 40 mg/d should be divided into twice-daily doses. | ||
| Source: Adapted from Reuben DB, Herr K, Pacala JT, et al. Geriatrics at your fingertips (5th ed). Malden, MA: Blackwell Publishing, 2003:47. | ||
An acute presentation can suggest a panic disorder or acute anxiety state, but first rule out serious conditions such as acute myocardial infarction, arrhythmias, acute infections, GI bleeding, and carbon monoxide poisoning.
Also ask about:
- exacerbating and relieving factors. For example, positional changes, exercise or other physical activity, eating, or missing a meal can trigger presyncope. Also find out about situations that may bring on anxiety, panic, or phobia. Onset of dizziness following these situations may suggest psychogenesis.
- recent falls and injuries. Recurrent falls with presyncope suggest a probable orthostatic or cardiovascular diagnosis in older adults.
4. ANY PAST MEDICAL PROBLEMS?
Ask disease-specific questions. For example:
- Tinnitus or hearing loss could point to a vestibular disorder.
- Metabolic and cardiovascular disorders such as diabetes, ischemic heart disease, postural hypotension, and seizures can result in presyncope.
- Orthostasis, coronary ischemic events, hypoglycemia, and transient ischemic attacks may cause dizziness.
5. IS DIZZINESS RECURRENT?
Panic disorder, anxiety disorders, phobia, and psychogenic hyperventilation are commonly associated with chronic, recurrent dizziness episodes.
6. WHAT MEDICATIONS IS THE PATIENT TAKING?
All psychotropics are suspect when a patient presents with dizziness. When dizziness occurs after a dose or start of therapy, evaluate response to the medication and consider reducing the dosage or changing the medication. If symptoms persist, refer the patient back to the primary care physician to investigate for other causes of dizziness.
Psychotropics that may cause dizziness are listed in Table 2, For a list of other medications associated with dizziness, see this article at www.currentpsychiatry.com.
If the above strategies do not reveal a physical cause of dizziness despite multiple physical complaints, consider examining the patient for depression, anxiety, or panic disorder.
Treating a psychiatric cause
If dizziness is found to be psychogenic and the symptoms impede daily activities or contribute to functional decline, treat the psychiatric disorder but carefully weigh the risks and benefits of drug treatment.
Although SSRIs may cause dizziness, these agents are recommended first-line treatment for depression, anxiety, and/or phobia in older patients with dizziness because of their relative lack of anticholinergic action and side effects compared with other antidepressants or anxiolytics.
Coexisting medical symptoms may dictate choice of agent. For example, consider a sedating SSRI for a patient with sleep disturbances caused by dizziness or the psychiatric disorder; choose a nonsedating SSRI if the patient is sleeping normally.
Because SSRIs may cause weight loss, avoid giving them to patients with weight loss associated with dizziness or an underlying psychiatric illness. Mirtazapine, which is associated with weight gain, may offset weight loss. Start mirtazapine at 15 mg at bedtime for older patients.
Start low and go slow when prescribing an SSRI to an older patient. Dosing strategies applicable to younger patients should not be extrapolated to older patients, especially those with dizziness.
We have found that older patients respond well to minimum or below-normal SSRI dosages (Table 3). Titrate very slowly and instruct patients to report dizziness. Reduce the dosage if dizziness emerges.
If the patient does not respond to an SSRI or mirtazapine, consider a serotonin and norepinephrine reuptake inhibitor, which also has favorable anticholinergic and side-effect profiles.
Related resources
- WebMD Health—Dizziness: lightheadedness and vertigo. http://my.webMD.com/hw/health_guide_atoz/hw88500.asp.
- Sloane PD. Clinical research and geriatric dizziness: The blind men and the elephant. J Am Geriatr Soc 1999;47:113-14.
- Kroenke K, Hoffman RM, Einstadter D. How common are various forms of dizziness? A critical review. South Med J 2000;93:160-7.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Carbamazepine • Tegretol
- Chlordiazepoxide • Librium
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Divalproex/valproic acid • Depakote
- Escitalopram • Lexapro
- Estazolam • ProSom
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Memantine • Namenda
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Phenytoin • Dilantin
- Prochlorperazine • Compazine
- Quazepam • Doral
- Rivastigmine • Exelon
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Tacrine • Cognex
- Temazepam • Restoril
- Thioridazine • Mellaril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trifluoperazine • Vesprin
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Robert Cluxton, PharmD, University of Cincinnati College of Pharmacy, for helping to prepare this manuscript for publication.
Dizziness is common among patients age 65 and older, and more than one-third have a psychiatric disorder that is caused by or is causing their dizziness.1
When older patients present with dizziness, psychiatrists may be asked to alleviate the psychological symptoms and help identify the underlying disease state.2
More than 60 medical and psychiatric disorders and many medications can cause dizziness. To help you sort through the possibilities, we offer:
- six diagnostic questions to rule out underlying medical problems
- lists of commonly used psychotropics and other drugs that may cause dizziness
- advice on treating depression, anxiety, and panic disorder in an older patient with dizziness while avoiding side effects and drug interactions.
Table 1
Four types of dizziness and their usual causes
| Vertigo Benign positional vertigo CNS cause—tumor, demyelination, neurodegenerative disorders Labyrinthitis Meniere’s disease Peripheral vestibulopathy (in 50% of cases) Vestibular neuronitis |
| Presyncope Arrhythmias Carotid sinus disease Hypoglycemia Neurocardiogenic syncope Organic heart disease Orthostatic hypotension Seizures Situational Transient ischemic attacks |
| Disequilibrium Balance and gait disorder Mixed CNS diseases (ischemic, degenerative) Neurodegenerative CNS disorders Presbystasis Sensorimotor dysfunction |
| Psychogenic lightheadedness Agoraphobia Anxiety Depression Panic disorder Hyperventilation |
| Source: Adapted from reference 6 |
Many causes of dizziness
The term “dizziness” is hard to define because of its nonspecific and variable symptom description, multiple causes, and lack of clear diagnostic and management guidelines. In clinical use, dizziness encompasses abnormal sensations relating to perception of the body’s relationship to space.
Some researchers believe dizziness is a distinct geriatric syndrome because numerous factors related to aging cause dizziness,2 including physiologic changes (presbystasis), accumulated impairment, disease states, and interactions between multiple medications.
Anxiety, somatization, panic disorder, and depression cause dizziness in the elderly, as do:
- peripheral vestibular disorders
- brainstem cerebrovascular accident
- diabetes mellitus
- neurologic disorders such as Parkinson’s disease
- and cardiovascular disorders.
Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants also have been shown to cause dizziness, as have numerous nonpsychotropic agents.
Recognizing patterns, testing hypotheses, and extending the diagnostic process over time can help you differentiate psychogenic from medicationinduced or neurologic dizziness.3 Because the presentation is so complex and the differential diagnosis so broad, algorithmic diagnosis is less effective than a flexible clinical approach that allows for uncertainty in evaluating initial symptoms.
Determining the cause
A thorough patient history and physical examination can uncover a cause of dizziness in 75% of cases.4 Look for duration of dizziness symptoms; history of heart disease, diabetes or other illnesses; family history of psychiatric disorders; and other illnesses among family members.
Ask the following six complaint-specific questions to help you narrow the differential diagnosis and rule out nonpsychiatric causes.5
1. WHAT TYPE OF DIZZINESS DOES THE PATIENT HAVE?
Four categories—vertigo, presyncope, disequilibrium, and lightheadedness—are used to classify dizziness (Table 1).6
Vertigo is a sense that the body or environment is Patients may feel as if the floor is tilting, sinking, rising or veering sideways, or they may feel pulled to one side.
Vertigo is commonly caused by peripheral vestibular disorders—including benign positional vertigo, Meniere’s disease, labyrinthitis, and vestibular neuronitis—and central vestibular disorders associated with cerebrovascular disease, tumors, demyelinating diseases, migraines, seizures, multiple sclerosis and other CNS diseases. Acute-onset vertigo and neurologic signs suggest brainstem infarction.
Nystagmus is usually present, horizontal, and may be rotational at times. A vertical-beating nystagmus points to a probable CNS cause and requires urgent neuroimaging and referral to a neurologist or otolaryngologist.
Presyncope describes near-fainting. A dimming of vision and roaring in the ears may precede presyncope.
Depending on its cause, presyncope may occur regardless of position or only when upright. Common causes include orthostatic hypotension, neurocardiogenic syncope, organic heart disease, arrhythmias, carotid sinus disease, seizures, hypoglycemia, and transient ischemic attacks.
Abrupt presyncopal attacks that occur regardless of position suggest a cardiovascular cause. If onset is gradual and not improved by lying down, suspect a cerebral metabolic cause such as hypoglycemia.
Syncope, like presyncope, often is traced to an underlying cardiovascular disease. Dizziness and syncope often coexist, and both can be multifactorial. Dizziness may precede or follow syncopal episodes.
Differentiating syncope and dizziness is important because many underlying causes of syncope can be fatal. By contrast, dizziness symptoms are usually benign and self-limiting.7
A thorough history is critical to distinguishing dizziness from presyncope. Assess medication effects—especially CNS-acting medications, cardiovascular drugs, antihypertensives, antibiotics, and over-the-counter medications such as dextromethorphan and acetaminophen compounds. Also check for dehydration.
Disequilibrium disorder signifies unsteadiness or a loss of balance primarily involving the lower extremities. Symptoms are evoked by walking or standing and relieved by sitting or lying down. Gait is abnormal and balance is compromised without abnormal head sensations.
Common causes include balance and gait disorders, sensorimotor dysfunction, presbystasis, neurodegenerative CNS disorders, and mixed ischemic and degenerative CNS diseases.
Vague lightheadedness is often associated with somatic symptoms such as headache. Some patients describe a floating sensation.
Lightheadedness is frequently associated with anxiety, panic disorder, depression, and somatization. Hyperventilation and agoraphobia are other common causes.
Multiple symptoms, multiple types. Classifying an older patient’s dizziness can be challenging because many patients report symptoms that suggest two or more subtypes.2 Also, patients often have trouble describing their dizziness symptoms, sometimes using terms such as “giddiness,” “wooziness,” or “confusion.”
To help patients explain dizziness symptoms more accurately, ask specific questions such as:
- Do you at times feel like you’re about to faint?
- Do you feel as if the room is moving?
- Do you sometimes feel as though you’re going to fall?
Table 2
Psychotropics that may cause dizziness
| Anti-Alzheimer’s medications Memantine, rivastigmine, tacrine |
| Anticonvulsants Phenytoin |
| Antidepressants Monoamine oxidase inhibitors (phenelzine, selegiline) Selective serotonin reuptake inhibitors (all) Tricyclics (amitriptyline, imipramine, nortriptyline, trazodone) Others (bupropion, buspirone, mirtazapine, nefazodone, venlafaxine) |
| Antipsychotics Typicals (chlorpromazine, fluphenazine, perphenazine, prochlorperazine, thioridazine, trifluoperazine) Atypicals (all except olanzapine) |
| Anxiolytics Alprazolam, chlordiazepoxide, clonazepam, diazepam, lorazepam, oxazepam |
| Hypnotics Estazolam, flurazepam, quazepam, temazepam, triazolam, zolpidem |
| Mood stabilizers Carbamazepine, divalproex/valproic acid, gabapentin, lamotrigine, oxcarbazepine |
| Source: Clinical Pharmacology version 2.11. Tampa, FL: Gold Standard MultiMedia, 2004. |
2. HOW DO DIZZINESS SYMPTOMS RELATE TO POSITION OR MOTION?
By reproducing dizziness symptoms, some quick-maneuver tests can help patients describe their symptoms and may reveal a medical cause.
Dix-Hallpike maneuver.3 Move the patient rapidly from a seated to prone position with the head below the horizontal plane and turned 45 degrees for 10 seconds; then have the patient sit up. Repeat with the head turned to the other side. If dizziness does not occur within a few seconds after each test, rule out benign positional vertigo.
Seated head turn, or head-thrust test, measures qualitative vestibular function.8 Move the head rapidly by 45 degrees in a brief, small-amplitude thrust to one side while the patient focuses on your nose; this gauges vestibularocular control. Repeat the test in the other direction. A refixation corrective saccade, occurring as the patient tries to fixate on the target, indicates a possible vestibular disorder.
‘Get-Up and Go’ test, which takes less than 10 seconds, measures balance in older patents.9 Have the patient stand up, walk 10 feet, turn around, walk back, and sit down. Watch for staggering, unsteadiness, and use of hands to balance. Onset of symptoms suggests dizziness brought on during activities of daily living and provides information on how dizziness is affecting the patient’s ability to function.
Romberg test. Have the patient stand with heels together, first with eyes open and then closed. Vision and proprioceptive signals are used to compensate for vestibular loss. Thus, a balance disturbance with eyes closed suggests vestibular or spinal proprioceptive problems and may predict risk of falls caused by inability to compensate.8
3. WHAT IS THE COURSE OF DIZZINESS?
Differentiating acute, sudden-onset dizziness from chronic, gradual-onset dizziness can help uncover the problem’s cause and seriousness. The latter often has a psychological cause or may point to vestibular or minor cardiovascular problems. Tinetti et al2 identified anxiety or depressive symptoms as risk factors among community-based older persons who reported dizziness episodes lasting 1 month.
Table 3
Recommended SSRI starting dosages for older patients
| SSRI | Starting dosage (mg/d) | Maximum dosage (mg/d) |
|---|---|---|
| Citalopram | 10 to 20 | 30 |
| Escitalopram | 10 | 10 |
| Fluoxetine* | 5 to 10 | 60 |
| Paroxetine | 5 | 40 |
| Sertraline | 25 to 50 | 200 |
| * Most patients will not need more than 20 mg/d. Dosages 40 mg/d should be divided into twice-daily doses. | ||
| Source: Adapted from Reuben DB, Herr K, Pacala JT, et al. Geriatrics at your fingertips (5th ed). Malden, MA: Blackwell Publishing, 2003:47. | ||
An acute presentation can suggest a panic disorder or acute anxiety state, but first rule out serious conditions such as acute myocardial infarction, arrhythmias, acute infections, GI bleeding, and carbon monoxide poisoning.
Also ask about:
- exacerbating and relieving factors. For example, positional changes, exercise or other physical activity, eating, or missing a meal can trigger presyncope. Also find out about situations that may bring on anxiety, panic, or phobia. Onset of dizziness following these situations may suggest psychogenesis.
- recent falls and injuries. Recurrent falls with presyncope suggest a probable orthostatic or cardiovascular diagnosis in older adults.
4. ANY PAST MEDICAL PROBLEMS?
Ask disease-specific questions. For example:
- Tinnitus or hearing loss could point to a vestibular disorder.
- Metabolic and cardiovascular disorders such as diabetes, ischemic heart disease, postural hypotension, and seizures can result in presyncope.
- Orthostasis, coronary ischemic events, hypoglycemia, and transient ischemic attacks may cause dizziness.
5. IS DIZZINESS RECURRENT?
Panic disorder, anxiety disorders, phobia, and psychogenic hyperventilation are commonly associated with chronic, recurrent dizziness episodes.
6. WHAT MEDICATIONS IS THE PATIENT TAKING?
All psychotropics are suspect when a patient presents with dizziness. When dizziness occurs after a dose or start of therapy, evaluate response to the medication and consider reducing the dosage or changing the medication. If symptoms persist, refer the patient back to the primary care physician to investigate for other causes of dizziness.
Psychotropics that may cause dizziness are listed in Table 2, For a list of other medications associated with dizziness, see this article at www.currentpsychiatry.com.
If the above strategies do not reveal a physical cause of dizziness despite multiple physical complaints, consider examining the patient for depression, anxiety, or panic disorder.
Treating a psychiatric cause
If dizziness is found to be psychogenic and the symptoms impede daily activities or contribute to functional decline, treat the psychiatric disorder but carefully weigh the risks and benefits of drug treatment.
Although SSRIs may cause dizziness, these agents are recommended first-line treatment for depression, anxiety, and/or phobia in older patients with dizziness because of their relative lack of anticholinergic action and side effects compared with other antidepressants or anxiolytics.
Coexisting medical symptoms may dictate choice of agent. For example, consider a sedating SSRI for a patient with sleep disturbances caused by dizziness or the psychiatric disorder; choose a nonsedating SSRI if the patient is sleeping normally.
Because SSRIs may cause weight loss, avoid giving them to patients with weight loss associated with dizziness or an underlying psychiatric illness. Mirtazapine, which is associated with weight gain, may offset weight loss. Start mirtazapine at 15 mg at bedtime for older patients.
Start low and go slow when prescribing an SSRI to an older patient. Dosing strategies applicable to younger patients should not be extrapolated to older patients, especially those with dizziness.
We have found that older patients respond well to minimum or below-normal SSRI dosages (Table 3). Titrate very slowly and instruct patients to report dizziness. Reduce the dosage if dizziness emerges.
If the patient does not respond to an SSRI or mirtazapine, consider a serotonin and norepinephrine reuptake inhibitor, which also has favorable anticholinergic and side-effect profiles.
Related resources
- WebMD Health—Dizziness: lightheadedness and vertigo. http://my.webMD.com/hw/health_guide_atoz/hw88500.asp.
- Sloane PD. Clinical research and geriatric dizziness: The blind men and the elephant. J Am Geriatr Soc 1999;47:113-14.
- Kroenke K, Hoffman RM, Einstadter D. How common are various forms of dizziness? A critical review. South Med J 2000;93:160-7.
Drug brand names
- Alprazolam • Xanax
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Carbamazepine • Tegretol
- Chlordiazepoxide • Librium
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Divalproex/valproic acid • Depakote
- Escitalopram • Lexapro
- Estazolam • ProSom
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Imipramine • Tofranil
- Lamotrigine • Lamictal
- Lorazepam • Ativan
- Memantine • Namenda
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Oxazepam • Serax
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Phenelzine • Nardil
- Phenytoin • Dilantin
- Prochlorperazine • Compazine
- Quazepam • Doral
- Rivastigmine • Exelon
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Tacrine • Cognex
- Temazepam • Restoril
- Thioridazine • Mellaril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trifluoperazine • Vesprin
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Robert Cluxton, PharmD, University of Cincinnati College of Pharmacy, for helping to prepare this manuscript for publication.
1. Sloane PD, Hartman M, Mitchell CM. Psychological factors associated with chronic dizziness in patients aged 60 and older. J Am Geriatr Soc 1994;42:847-52.
2. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med 2000;132:337-44.
3. Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med 2001;134(9 pt 2):823-32.
4. Hoffman RM, Einstadter D, Kroenke K. Evaluating dizziness. Am J Med 1999;107:468-78.
5. Drachman DA. A 69-year-old man with chronic dizziness. JAMA 1998;280:2111-18.
6. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology 1972;22:323-34.
7. Kapoor WN. Syncope. N Engl J Med 2000;343:1856-62.
8. Baloh RW. Hearing and equilibrium. In: Goldman L, Ansiello D (eds). Cecil textbook of medicine (22nd ed). Philadelphia: Saunders 2004;2436-42.
9. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387-9.
1. Sloane PD, Hartman M, Mitchell CM. Psychological factors associated with chronic dizziness in patients aged 60 and older. J Am Geriatr Soc 1994;42:847-52.
2. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med 2000;132:337-44.
3. Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med 2001;134(9 pt 2):823-32.
4. Hoffman RM, Einstadter D, Kroenke K. Evaluating dizziness. Am J Med 1999;107:468-78.
5. Drachman DA. A 69-year-old man with chronic dizziness. JAMA 1998;280:2111-18.
6. Drachman DA, Hart CW. An approach to the dizzy patient. Neurology 1972;22:323-34.
7. Kapoor WN. Syncope. N Engl J Med 2000;343:1856-62.
8. Baloh RW. Hearing and equilibrium. In: Goldman L, Ansiello D (eds). Cecil textbook of medicine (22nd ed). Philadelphia: Saunders 2004;2436-42.
9. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil 1986;67:387-9.