User login
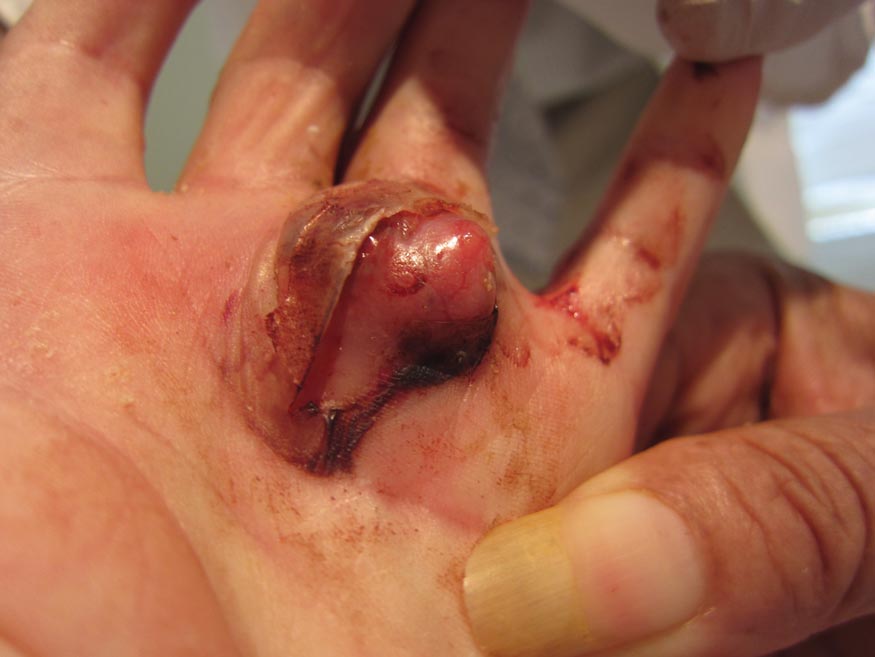
Cystic Nodule on the Palm
The Diagnosis: Nodular Hidradenoma
Nodular hidradenomas (NHs) are rare benign cutaneous adnexal neoplasms first described in 1949 as clear cell papillary carcinomas.1 Since then, various terms have been used to describe this entity, such as eccrine acrospiroma, solid-cystic hidradenoma, and clear cell hidradenoma.2 Review of the literature revealed a female predominance (2:1 ratio) and a mean age at presentation of 37.2 years.3,4 Nodular hidradenoma presents as an asymptomatic, solitary, mobile, firm nodule with intact overlying skin. Rarely, multiple nodules may occur.3 Some tumors display ulceration and serous fluid leakage.5 They occur most commonly on the scalp, face, and upper extremities with an average size of 2 cm.3 Rapid growth of the tumor may signal a malignant change.6
Histopathology reveals a lobulated, circumscribed, symmetrical tumor with dermal nests of epithelial cells that are polygonal with eosinophilic cytoplasm forming ductlike spaces (Figure). However, clear cell changes and squamous differentiation may be prominent features. Cystic spaces may result from tumor cell degeneration. Most tumors are encased by collagenous fibrous tissue and rarely have epidermal attachments.3
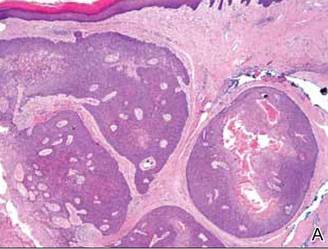
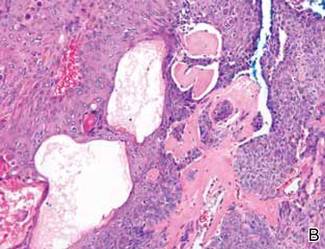
Anastomosing aggregates of squamous cells forming ductlike spaces were viewed on low-power magnification (A)(H&E, original magnification ×10). On higher power there were ductlike spaces and eosinophilic hyalinized stroma entrapped by the bland-appearing squamous proliferation (B)(H&E, original magnification ×20). |
Nodular hidradenoma traditionally has been considered to be of eccrine origin, but more recent literature indicates that the majority of NHs are of apocrine origin. Histologically, apocrine tumors display eosinophilic secretion, mucinous epithelium, squamous or sebaceous differentiation, and decapitation secretion, whereas eccrine tumors are identified by their lack of specific features.3
Nodular hidradenoma may recur after excision. Malignant transformation is rare. In one review, 6.7% (6/89) of NHs were malignant, characterized by abnormal mitoses, nuclear atypia, and necrosis.4 Malignant NH or nodular hidradenocarcinoma behaves aggressively with up to an 86% local recurrence and 60% rate of metastasis within 2 years.6 Survival time is inversely proportional to the size of the tumor and is generally poor, with a 5-year disease-free survival of less than 30%.6,7
Treatment of NH is achieved through primary excision or Mohs micrographic surgery; however, treatment of nodular hidradenocarcinoma is controversial and typically begins with wide local excision but may involve lymph node dissection if necessary. Use of adjuvant chemotherapy and radiation therapy for metastases warrants more clinical studies, as it is a rare occurrence.6 Our patient planned to undergo a total excision of the benign nodule once she healed from the biopsy; however, she was lost to follow-up, as she moved out of state.
1. Lui Y. The histogenesis of clear cell papillary carcinoma of the skin. Am J Pathol. 1949;25:93-103.
2. Obaidat NA, Khaled OA, Ghazarian D. Skin adnexal neoplasms–part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159.
3. Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470.
4. Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985;12:15-20.
5. Sirinoglu H, Celebiler O. Benign nodular hidradenoma of the face. J Craniofac Surg. 2011;22:750-751.
6. Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554.
7. Ko CJ, Cochran AJ, Eng W, et al. Hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:726-730.
The Diagnosis: Nodular Hidradenoma
Nodular hidradenomas (NHs) are rare benign cutaneous adnexal neoplasms first described in 1949 as clear cell papillary carcinomas.1 Since then, various terms have been used to describe this entity, such as eccrine acrospiroma, solid-cystic hidradenoma, and clear cell hidradenoma.2 Review of the literature revealed a female predominance (2:1 ratio) and a mean age at presentation of 37.2 years.3,4 Nodular hidradenoma presents as an asymptomatic, solitary, mobile, firm nodule with intact overlying skin. Rarely, multiple nodules may occur.3 Some tumors display ulceration and serous fluid leakage.5 They occur most commonly on the scalp, face, and upper extremities with an average size of 2 cm.3 Rapid growth of the tumor may signal a malignant change.6
Histopathology reveals a lobulated, circumscribed, symmetrical tumor with dermal nests of epithelial cells that are polygonal with eosinophilic cytoplasm forming ductlike spaces (Figure). However, clear cell changes and squamous differentiation may be prominent features. Cystic spaces may result from tumor cell degeneration. Most tumors are encased by collagenous fibrous tissue and rarely have epidermal attachments.3


Anastomosing aggregates of squamous cells forming ductlike spaces were viewed on low-power magnification (A)(H&E, original magnification ×10). On higher power there were ductlike spaces and eosinophilic hyalinized stroma entrapped by the bland-appearing squamous proliferation (B)(H&E, original magnification ×20). |
Nodular hidradenoma traditionally has been considered to be of eccrine origin, but more recent literature indicates that the majority of NHs are of apocrine origin. Histologically, apocrine tumors display eosinophilic secretion, mucinous epithelium, squamous or sebaceous differentiation, and decapitation secretion, whereas eccrine tumors are identified by their lack of specific features.3
Nodular hidradenoma may recur after excision. Malignant transformation is rare. In one review, 6.7% (6/89) of NHs were malignant, characterized by abnormal mitoses, nuclear atypia, and necrosis.4 Malignant NH or nodular hidradenocarcinoma behaves aggressively with up to an 86% local recurrence and 60% rate of metastasis within 2 years.6 Survival time is inversely proportional to the size of the tumor and is generally poor, with a 5-year disease-free survival of less than 30%.6,7
Treatment of NH is achieved through primary excision or Mohs micrographic surgery; however, treatment of nodular hidradenocarcinoma is controversial and typically begins with wide local excision but may involve lymph node dissection if necessary. Use of adjuvant chemotherapy and radiation therapy for metastases warrants more clinical studies, as it is a rare occurrence.6 Our patient planned to undergo a total excision of the benign nodule once she healed from the biopsy; however, she was lost to follow-up, as she moved out of state.
The Diagnosis: Nodular Hidradenoma
Nodular hidradenomas (NHs) are rare benign cutaneous adnexal neoplasms first described in 1949 as clear cell papillary carcinomas.1 Since then, various terms have been used to describe this entity, such as eccrine acrospiroma, solid-cystic hidradenoma, and clear cell hidradenoma.2 Review of the literature revealed a female predominance (2:1 ratio) and a mean age at presentation of 37.2 years.3,4 Nodular hidradenoma presents as an asymptomatic, solitary, mobile, firm nodule with intact overlying skin. Rarely, multiple nodules may occur.3 Some tumors display ulceration and serous fluid leakage.5 They occur most commonly on the scalp, face, and upper extremities with an average size of 2 cm.3 Rapid growth of the tumor may signal a malignant change.6
Histopathology reveals a lobulated, circumscribed, symmetrical tumor with dermal nests of epithelial cells that are polygonal with eosinophilic cytoplasm forming ductlike spaces (Figure). However, clear cell changes and squamous differentiation may be prominent features. Cystic spaces may result from tumor cell degeneration. Most tumors are encased by collagenous fibrous tissue and rarely have epidermal attachments.3


Anastomosing aggregates of squamous cells forming ductlike spaces were viewed on low-power magnification (A)(H&E, original magnification ×10). On higher power there were ductlike spaces and eosinophilic hyalinized stroma entrapped by the bland-appearing squamous proliferation (B)(H&E, original magnification ×20). |
Nodular hidradenoma traditionally has been considered to be of eccrine origin, but more recent literature indicates that the majority of NHs are of apocrine origin. Histologically, apocrine tumors display eosinophilic secretion, mucinous epithelium, squamous or sebaceous differentiation, and decapitation secretion, whereas eccrine tumors are identified by their lack of specific features.3
Nodular hidradenoma may recur after excision. Malignant transformation is rare. In one review, 6.7% (6/89) of NHs were malignant, characterized by abnormal mitoses, nuclear atypia, and necrosis.4 Malignant NH or nodular hidradenocarcinoma behaves aggressively with up to an 86% local recurrence and 60% rate of metastasis within 2 years.6 Survival time is inversely proportional to the size of the tumor and is generally poor, with a 5-year disease-free survival of less than 30%.6,7
Treatment of NH is achieved through primary excision or Mohs micrographic surgery; however, treatment of nodular hidradenocarcinoma is controversial and typically begins with wide local excision but may involve lymph node dissection if necessary. Use of adjuvant chemotherapy and radiation therapy for metastases warrants more clinical studies, as it is a rare occurrence.6 Our patient planned to undergo a total excision of the benign nodule once she healed from the biopsy; however, she was lost to follow-up, as she moved out of state.
1. Lui Y. The histogenesis of clear cell papillary carcinoma of the skin. Am J Pathol. 1949;25:93-103.
2. Obaidat NA, Khaled OA, Ghazarian D. Skin adnexal neoplasms–part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159.
3. Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470.
4. Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985;12:15-20.
5. Sirinoglu H, Celebiler O. Benign nodular hidradenoma of the face. J Craniofac Surg. 2011;22:750-751.
6. Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554.
7. Ko CJ, Cochran AJ, Eng W, et al. Hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:726-730.
1. Lui Y. The histogenesis of clear cell papillary carcinoma of the skin. Am J Pathol. 1949;25:93-103.
2. Obaidat NA, Khaled OA, Ghazarian D. Skin adnexal neoplasms–part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol. 2007;60:145-159.
3. Nandeesh BN, Rajalakshmi T. A study of histopathologic spectrum of nodular hidradenoma. Am J Dermatopathol. 2012;34:461-470.
4. Hernández-Pérez E, Cestoni-Parducci R. Nodular hidradenoma and hidradenocarcinoma: a 10-year review. J Am Acad Dermatol. 1985;12:15-20.
5. Sirinoglu H, Celebiler O. Benign nodular hidradenoma of the face. J Craniofac Surg. 2011;22:750-751.
6. Souvatzidis P, Sbano P, Mandato F, et al. Malignant nodular hidradenoma of the skin: report of seven cases. J Eur Acad Dermatol Venereol. 2008;22:549-554.
7. Ko CJ, Cochran AJ, Eng W, et al. Hidradenocarcinoma: a histological and immunohistochemical study. J Cutan Pathol. 2006;33:726-730.
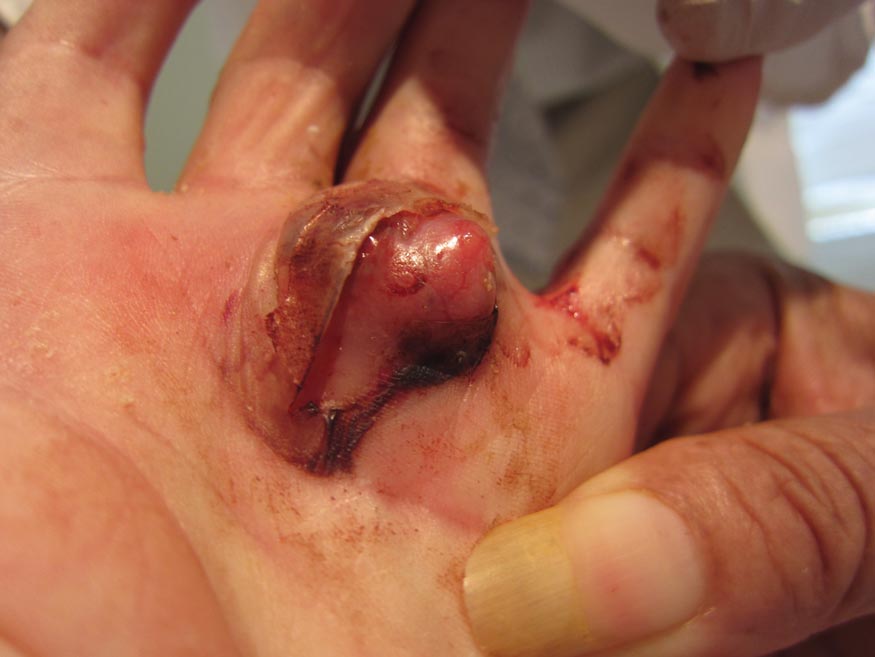
A 73-year-old woman with a history of multiple strokes with residual left-sided motor deficits and resultant left-hand contracture, type 2 diabetes mellitus, hypertension, and a remote history of treated colon cancer and breast cancer presented with hypertensive urgency and neck pain. Upon admission, the nursing staff found an “unusual growth” on the patient’s left hand. Dermatology was consulted and a 2×1.5×1.5-cm multilobulated, malodorous, slightly tender, nonfluctuant, gelatinous, mobile, cystic nodule overlying the fourth metacarpal palmar head was examined. The patient reported the lesion was present for more than a year. Imaging was pursued, but radiography, ultrasonography, and magnetic resonance imaging could not be performed adequately due to the patient’s severe contracture. Given the extensive differential diagnoses, an orthopedic hand surgeon performed a large incisional biopsy to obtain tissue diagnosis.