User login
Multinucleate Cell Angiohistiocytoma
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
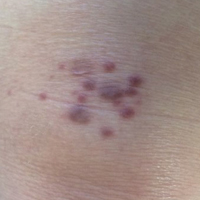
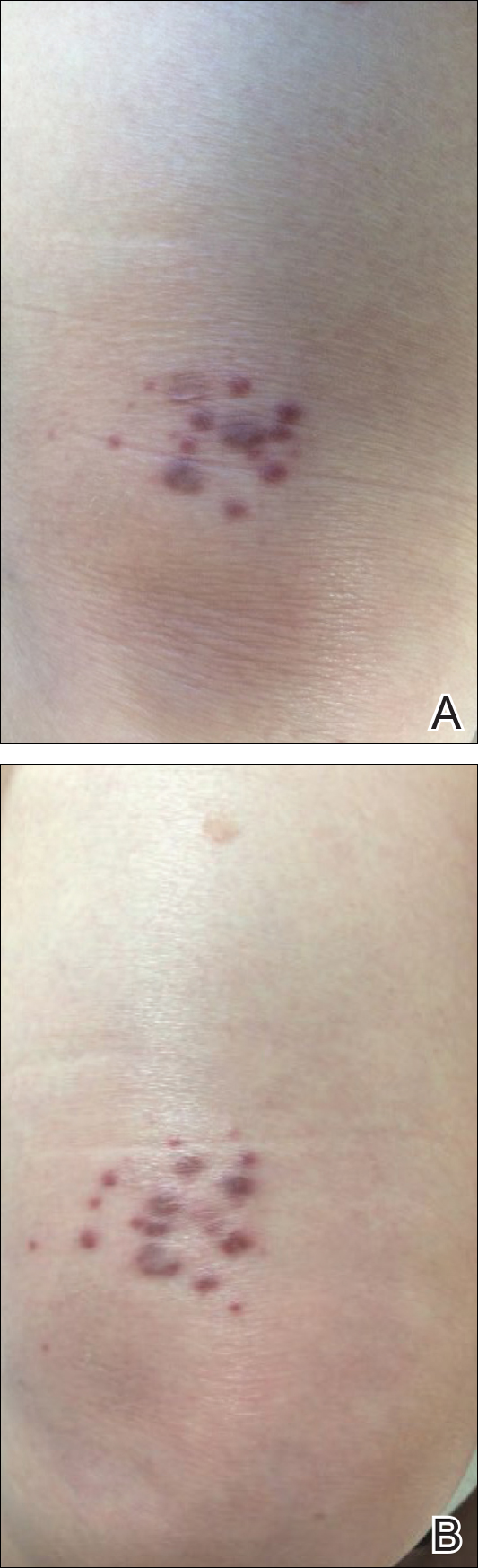
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
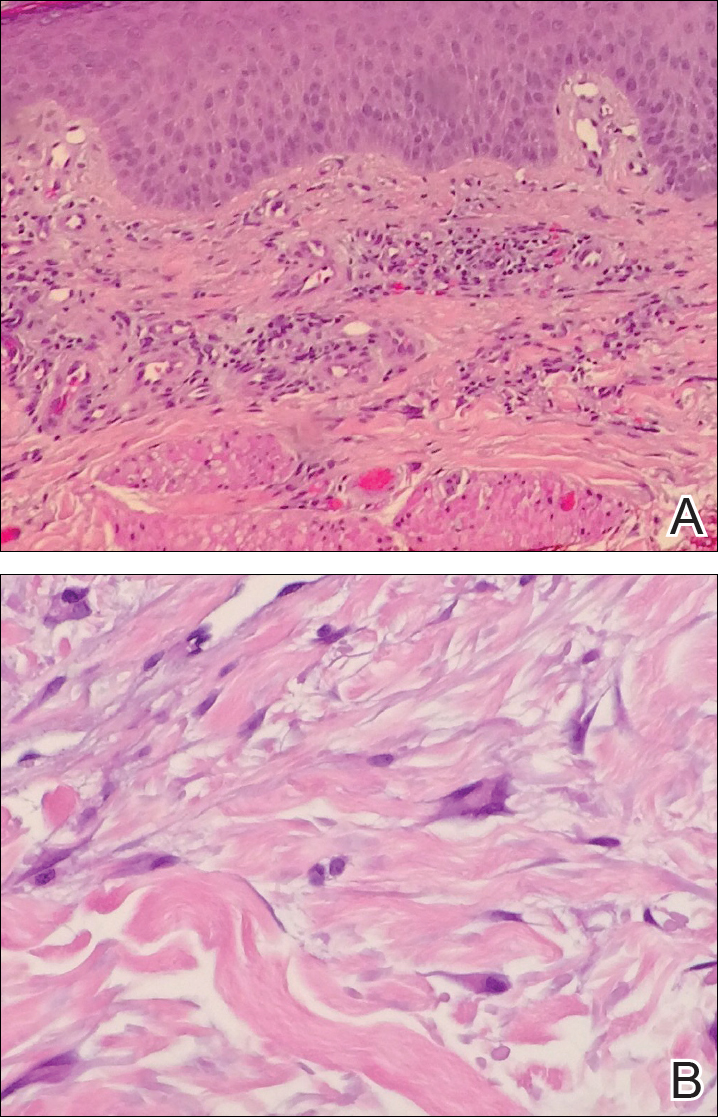
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.
At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Practice Points
- Multinucleate cell angiohistiocytoma (MCAH) is a rare underrecognized cutaneous tumor presenting as erythematous to violaceous papules.
- Although it clinically mimics Kaposi sarcoma, MCAH may be distinguished histopathologically by negative immunostaining for human herpesvirus 8.
- Surgical excision and laser therapies are definitive treatments for MCAH, which is a benign lesion.