User login
Reoperation Rates After Cartilage Restoration Procedures in the Knee: Analysis of a Large US Commercial Database
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).
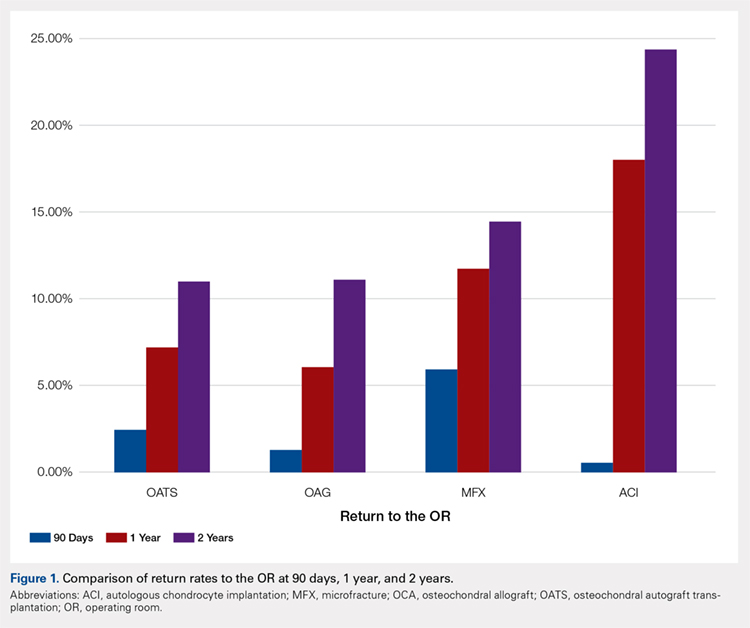
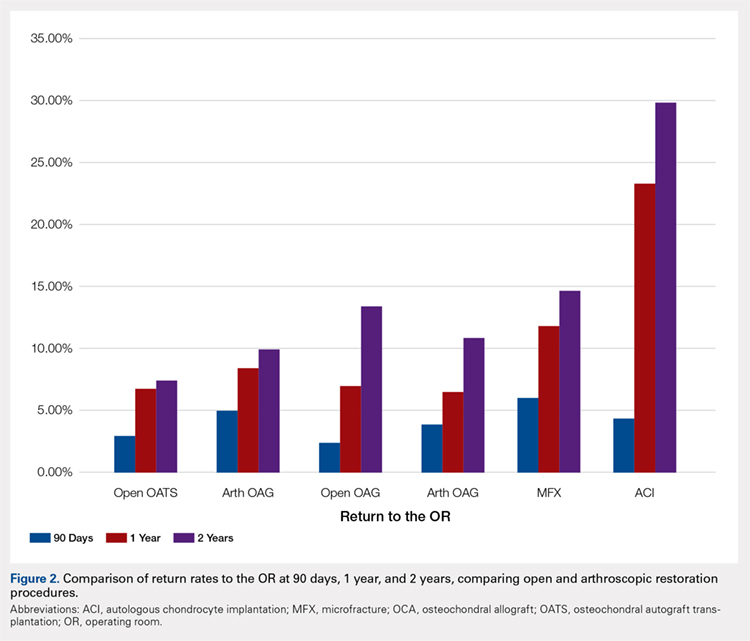
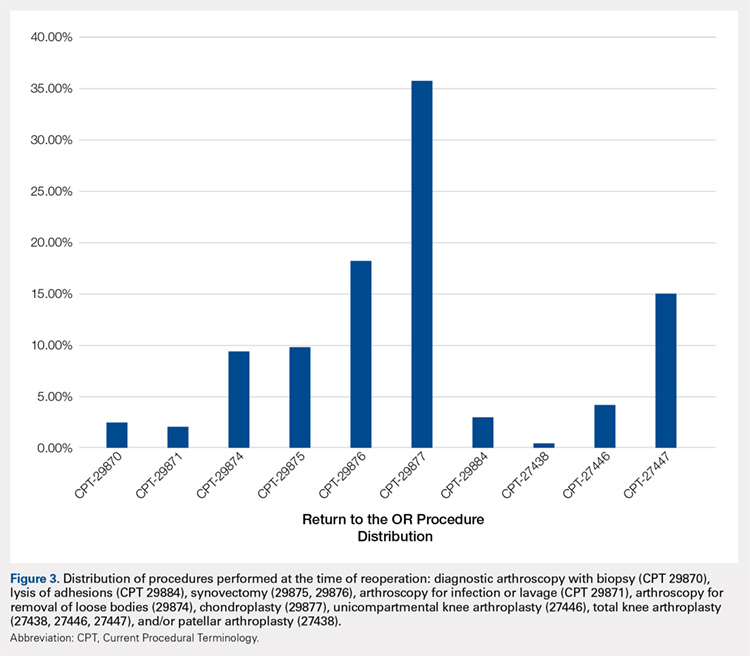
Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
ABSTRACT
The purpose of this study is to describe the rate of return to the operating room (OR) following microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and osteochondral allograft (OCA) procedures at 90 days, 1 year, and 2 years. Current Procedural Terminology codes for all patients undergoing MFX, ACI, OATS, and OCA were used to search a prospectively collected, commercially available private payer insurance company database from 2007 to 2011. Within 90 days, 1 year, and 2 years after surgery, the database was searched for the occurrence of these same patients undergoing knee diagnostic arthroscopy with biopsy, lysis of adhesions, synovectomy, arthroscopy for infection or lavage, arthroscopy for removal of loose bodies, chondroplasty, MFX, ACI, OATS, OCA, and/or knee arthroplasty. Descriptive statistical analysis and contingency table analysis were performed. A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX, 640 ACI, 386 open OATS, 997 arthroscopic OATS, 714 open OCA, and 894 arthroscopic OCA procedures. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. At 2 years, patients who underwent MFX, ACI, OATS, OCA had reoperation rates of 14.65%, 29.69%, 8.82%, and 12.22%, respectively. There was a statistically significantly increased risk for ACI return to OR within all intervals (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative treatment options. With a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference in failure/revision rates between the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
Continue to: Symptomatic, full-thickness articular cartilage
Symptomatic, full-thickness articular cartilage defects in the knee are difficult to manage, particularly in the young, athletic patient population. Fortunately, a variety of cartilage repair (direct repair of the cartilage or those procedures which attempt to generate fibrocartilage) and restoration (those aimed at restoring hyaline cartilage) procedures are available, with encouraging short- and long-term clinical outcomes. After failure of nonoperative management, several surgical options are available for treating symptomatic focal chondral defects, including microfracture (MFX), autologous chondrocyte implantation (ACI), osteochondral autograft transplantation (OATS), and open and arthroscopic osteochondral allograft (OCA) transplantation procedures.1,2 When appropriately indicated, each of these techniques has demonstrated good to excellent clinical outcomes with respect to reducing pain and improving function.3-5
While major complications following cartilage surgery are uncommon, the need for reoperation following an index articular cartilage operation is poorly understood. Recently, McCormick and colleagues6 found that reoperation within the first 2 years following meniscus allograft transplantation (MAT) is associated with an increased likelihood of revision MAT or future arthroplasty. Given the association between early reoperation following meniscus restoration surgery and subsequent failure, an improved understanding of the epidemiology and implications of reoperations following cartilage restoration surgery is warranted. Further, in deciding which treatment option is best suited to a particular patient, the rate of return to the operating room (OR) should be taken into consideration, as this could potentially influence surgical decision-making as to which procedure to perform, especially in value-based care decision-making environments.
The purpose of this study is to describe the rate of return to the OR for knee procedures following cartilage restoration at intervals of 90 days, 1 year, and 2 years across a large-scale US patient database. The authors hypothesize that the rate of return to the OR following knee cartilage repair or restoration procedures will be under 20% during the first post-operative year, with increasing reoperation rates over time. A secondary hypothesis is that there will be no difference in reoperation rates according to sex, but that younger patients (those younger than 40 years) will have higher reoperation rates than older patients.
METHODS
We performed a retrospective analysis of a prospectively collected, large-scale, and commercially available private payer insurance company database (PearlDiver) from 2007 to 2011. The PearlDiver database is a Health Insurance Portability and Accountability Act (HIPAA) compliant, publicly available national database consisting of a collection of private payer records, with United Health Group representing the contributing health plan. The database has more than 30 million patient records and contains Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision (ICD-9) codes related to orthopedic procedures. From 2007 to 2011, the private payer database captured between 5.9 million and 6.2 million patients per year.
Our search was based on the CPT codes for MFX (29879), ACI (27412), OATS (29866, 29867), and OCA (27415, 27416). Return to the OR for revision surgery for the above-mentioned procedures was classified as patients with a diagnosis of diagnostic arthroscopy with biopsy (CPT 29870), lysis of adhesions (CPT 29884), synovectomy (29875, 29876), arthroscopy for infection or lavage (CPT 29871), arthroscopy for removal of loose bodies (29874), chondroplasty (29877), unicompartmental knee arthroplasty (27446), total knee arthroplasty (27447), and/or patellar arthroplasty (27438). Patient records were followed for reoperations occurring within 90 days, 1 year, and 2 years after the index cartilage procedure. All data were compared based on patient age and sex.
Table 1. Breakdown of MFX, ACI, OATS, and OCA Procedures by Sex | ||||||
MFX | ACI | Open OATS | Arthroscopic OATS | Open OCA | Arthroscopic OCA | |
Females | 20,589 | 276 | 167 | 401 | 275 | 350 |
Males | 22,987 | 364 | 219 | 596 | 439 | 544 |
Total | 43,576 | 640 | 386 | 997 | 714 | 894 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analysis of this study was primarily descriptive to demonstrate the incidence for each code at each time interval. One-way analysis of variance, Chi-square analysis, and contingency tables were used to compare the incidence of each type of procedure throughout the various time intervals. A P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS v.20 (International Business Machines).
RESULTS
A total of 47,207 cartilage procedures were performed from 2007 to 2011, including 43,576 MFX (92.3%) 640 ACI (1.4%), 386 open OATS (0.82%), 997 arthroscopic OATS (2.11%), 714 open OCA (1.51%), and 894 arthroscopic OCA (1.89%) procedures. A summary of the procedures performed, broken down by age and sex, is provided in Tables 1 and 2. A total of 25,149 male patients (53.3%) underwent surgical procedures compared to 22,058 female patients (46.7%). For each category of procedure (MFX, ACI, OATS, OCA), there was a significantly higher proportion of males than females undergoing surgery (P < .0001 for all). Surgical treatment with MFX was consistently the most frequently performed surgery across all age groups (92.31%), while cell-based therapy with ACI was the least frequently performed procedure across all age ranges (1.36%). Restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not utilized in patients over 64 years of age (Table 2).
Table 2. Breakdown of MFX, ACI, OATS, and OCA Procedures by Age | ||||
Age (y) | MFX | ACI | OATS | OCA |
10 to 14 | 572 | 22 | 74 | 47 |
15 to 19 | 1984 | 83 | 254 | 235 |
20 to 24 | 1468 | 54 | 140 | 144 |
25 to 29 | 1787 | 74 | 152 | 176 |
30 to 34 | 2824 | 114 | 152 | 204 |
35 to 39 | 4237 | 96 | 153 | 210 |
40 to 44 | 5441 | 103 | 166 | 217 |
45 to 49 | 7126 | 57 | 149 | 180 |
50 to 54 | 7004 | 25 | 83 | 140 |
55 to 59 | 6410 | 12 | 40 | 40 |
60 to 64 | 4409 | 0 | 20 | 15 |
65 to 69 | 269 | 0 | 0 | 0 |
70 to 74 | 45 | 0 | 0 | 0 |
Total | 43,576 | 640 | 1383 | 1608 |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation.
A summary of all reoperation data is provided in Tables 3 to 7 and Figures 1 and 2. The weighted average reoperation rates for all procedures were 5.87% at 90 days, 11.94% at 1 year, and 14.90% at 2 years following the index cartilage surgery. Patients who underwent MFX had reoperation rates of 6.05% at 90 days, 11.80% at 1 year, and 14.65% at 2 years. Patients who underwent ACI had reoperation rates of 4.53% at 90 days, 23.28% at 1 year, and 29.69% at 2 years. Patients who had open and arthroscopic OATS had reoperation rates of 3.122% and 5.12% at 90 days, 6.74% and 8.53% at 1 year, and 7.51% and 10.13% at 2 years, respectively. Patients who underwent open and arthroscopic OCA had reoperation rates of 2.52% and 3.91% at 90 days, 7.14% and 6.60% at 1 year, and 13.59% and 10.85% at 2 years (Table 3). There was a statistically significantly increased risk for reoperation following ACI within all intervals compared to all other surgical techniques (P < .0001); however, MFX had a greater risk factor (P < .0001) for conversion to arthroplasty at 6.70%. There was no significant difference between failure rates (revision OATS/OCA or conversion to arthroplasty) between the restorative treatment options, with 14 failures for OATS (9.52% of reoperations at 2 years) compared to 22 failures for OCA (12.7% of reoperations at 2 years, P = .358). Among the entire cohort of cartilage surgery patients, arthroscopic chondroplasty was the most frequent procedure performed at the time of reoperation at all time points assessed, notably accounting for 33.08% of reoperations 2 years following microfracture, 51.58% of reoperations at 2 years following ACI, 53.06% of reoperations at 2 years following OATS, and 54.07% of reoperations at 2 years following OCA (Figure 3, Tables 4–7).



Table 3. Comparison of Return to OR Following MFX, ACI, OCA, and OATS | |||||||
Procedure | Total No. of Cases in Study Period | No. of Reoperations at 90 Days | Return to OR Rate at 90 Days | No. of Reoperations at 1 Year | Return to OR Rate at 1 Year | No. of Reoperations at 2 Years | Return to OR Rate at 2 Years |
MFX | 43,576 | 2636 | 6.05% | 5142 | 11.80% | 6385 | 14.65% |
ACI | 640 | 29 | 4.53% | 149 | 23.28% | 190 | 29.69% |
Open OATS | 386 | 12 | 3.12% | 26 | 6.74% | 29 | 7.51% |
Arthroscopic OATS | 997 | 51 | 5.12% | 85 | 8.53% | 101 | 10.13% |
Open OCA | 714 | 18 | 2.52% | 51 | 7.14% | 97 | 13.59% |
Arthroscopic OCA | 894 | 161 | 3.91% | 59 | 6.60% | 97 | 10.85% |
Weighted average for all procedures |
| 5.87% |
| 11.94% |
| 14.90% | |
Abbreviations: ACI, autologous chondrocyte implantation; MFX, microfracture; OCA, osteochondral allograft; OATS, osteochondral autograft transplantation; OR, operating room.
Table 4. Rate of Return to OR Following MFX (n = 43,574) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 54 | 122 | 162 |
Knee arthroscopic drainage and lavage | 29871 | 84 | 102 | 104 |
Arthroscopic adhesions débridement | 29874 | 300 | 468 | 549 |
Arthroscopic synovectomy | 29875 | 324 | 528 | 611 |
Major arthroscopic synovectomy | 29876 | 557 | 926 | 1087 |
Knee arthroscopic chondroplasty | 29877 | 1063 | 1722 | 2112 |
Arthroscopic lysis of adhesions | 29884 | 61 | 129 | 171 |
Patellar arthroplasty | 27438 | 0 | 38 | 49 |
Medial or lateral knee arthroplasty | 27446 | 51 | 242 | 328 |
Medial and lateral knee arthroplasty | 27447 | 142 | 865 | 1212 |
Total | 2636 | 5142 | 6385 | |
Return to OR | 6.05% | 11.80% | 14.65% | |
Abbreviations: CPT, Current Procedural Terminology; MFX, microfracture; OR, operating room.
Table 5. Rate of Return to OR Following ACI (n = 640) | ||||
Procedure | CPT Code | 90 Daysa | 1 Yeara | 2 Yearsa |
Revision ACI | 27412 | 29 | 33 | 35 |
Knee arthroscopy | 29870 | -1 | -1 | -1 |
Knee arthroscopic drainage and lavage | 29871 | -1 | -1 | -1 |
Arthroscopic adhesions débridement | 29874 | 0 | -1 | -1 |
Arthroscopic synovectomy | 29875 | -1 | -1 | -1 |
Major arthroscopic synovectomy | 29876 | -1 | 12 | 20 |
Knee arthroscopic chondroplasty | 29877 | -1 | 71 | 98 |
Arthroscopic lysis of adhesions | 29884 | -1 | 33 | 37 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | -1 | -1 |
Medial and lateral knee arthroplasty | 27447 | 0 | -1 | -1 |
Total | 29 | 149 | 190 | |
Return to OR | 4.53% | 23.28% | 29.69% | |
aA -1 denotes No. <11 within the PearlDiver database, and exact numbers are not reported due to patient privacy considerations.
Abbreviations: ACI, autologous chondrocyte implantation; CPT, Current Procedural Terminology; OR, operating room.
Table 6. Rate of Return to OR Following OATS (n = 1320) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Years |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 12 | 13 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 14 |
Major arthroscopic synovectomy | 29876 | 16 | 25 | 28 |
Knee arthroscopic chondroplasty | 29877 | 17 | 58 | 78 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 14 |
Total | 33 | 95 | 147 | |
Return to OR | 2.50% | 7.20% | 11.14% | |
Abbreviations: CPT, Current Procedural Terminology; OATS, osteochondral autograft transplantation; OR, operating room.
Table 7. Rate of Return to OR Following OCA Transplantation (n = 1531) | ||||
Procedure | CPT Code | 90 Days | 1 Year | 2 Year |
Knee arthroscopy | 29870 | 0 | 0 | 0 |
Knee arthroscopic drainage and lavage | 29871 | 0 | 0 | 0 |
Arthroscopic adhesions débridement | 29874 | 0 | 15 | 19 |
Arthroscopic synovectomy | 29875 | 0 | 0 | 0 |
Major arthroscopic synovectomy | 29876 | 0 | 20 | 38 |
Knee arthroscopic chondroplasty | 29877 | 22 | 59 | 93 |
Arthroscopic lysis of adhesions | 29884 | 0 | 0 | 0 |
Patellar arthroplasty | 27438 | 0 | 0 | 0 |
Medial or lateral knee arthroplasty | 27446 | 0 | 0 | 0 |
Medial and lateral knee arthroplasty | 27447 | 0 | 0 | 22 |
Total | 22 | 94 | 172 | |
Return to OR | 1.44% | 6.14% | 11.23% | |
Abbreviations: CPT, Current Procedural Terminology; OCA, osteochondral allograft; OR, operating room.
Continue to: Discussion...
DISCUSSION
The principle findings of this study demonstrate that there is an overall reoperation rate of 14.90% at 2 years following cartilage repair/restoration surgery, with the highest reoperation rates following MFX at 90 days, and ACI at both 1 year and 2 years following the index procedure. Also, patients undergoing index MFX as the index procedure have the highest risk for conversion to arthroplasty, reoperation rates for all cartilage surgeries increase over time, and arthroscopic chondroplasty is the most frequent procedure performed at the time of reoperation.
The management of symptomatic articular cartilage knee pathology is extremely challenging. With improvements in surgical technique, instrumentation, and clinical decision-making, indications are constantly evolving. Techniques that may work for “small” defects, though there is some debate as to what constitutes a “small” defect, are not necessarily going to be successful for larger defects, and this certainly varies depending on where the defect is located within the knee joint (distal femur vs patella vs trochlea, etc.). Recently, in a 2015 analysis of 3 level I or II studies, Miller and colleagues7 demonstrated both MFX and OATS to be viable, cost-effective, first-line treatment options for articular cartilage injuries, with similar clinical outcomes at 8.7 years. The authors noted cumulative reoperation rates of 29% among patients undergoing MFX compared to 13% among patients undergoing OATS. While ACI and OCA procedures were not included in their study, the reported reoperation rates of 29% following MFX and 13% following OATS at nearly 10 years suggest a possible increased need for reoperation following MFX over time (approximately 15% at 2 years in our study) and a stable rate of reoperation following OATS (approximately 11% at 2 years in our study). This finding is significant, as one of the goals with these procedures is to deliver effective, long-lasting pain relief and restoration of function. Interestingly, in this study, restorative OATS and OCA techniques were performed with the greatest frequency in the 15-year-old to 19-year-old age group, but were not performed in patients older than 64 years. This may be explained by the higher prevalence of acute traumatic injuries and osteochondritis dissecans diagnoses in younger patients compared with older patients, as these diagnoses are more often indicated to undergo restorative procedures as opposed to marrow stimulation.
In a 2016 systematic review of 20 studies incorporating 1117 patients, Campbell and colleagues8 assessed return-to-play rates following MFX, ACI, OATS, and OCA. The authors noted that return to sport (RTS) rates were greatest following OATS (89%), followed by OCA (88%), ACI (84%), and MFX (75%). Positive prognostic factors for RTS included younger age, shorter duration of preoperative symptoms, no history of prior ipsilateral knee surgery, and smaller chondral defects. Reoperation rates between the 4 techniques were not statistically compared in their study. Interestingly, in 2013, Chalmers and colleagues9 conducted a separate systematic review of 20 studies comprising 1375 patients undergoing MFX, ACI, or OATS. In their study, the authors found significant advantages following ACI and OATS compared to MFX with respect to patient-reported outcome scores but noted significantly faster RTS rates with MFX. Reoperation rates were noted to be similar between the 3 procedures (25% for ACI, 21% for MFX, and 28% for OATS) at an average 3.7 years following the index procedure. When considering these 2 systematic reviews together, despite a faster RTS rate following MFX, a greater proportion of patients seem to be able to RTS over time following other procedures such as OATS, OCA, and ACI. Unfortunately, these reviews do not provide insight as to the role, if any, of reoperation on return to play rates nor on overall clinical outcome scores on patients undergoing articular cartilage surgery. However, this information is valuable when counseling athletes who are in season and would like to RTS as soon as possible as opposed to those who do not have tight time constraints for when they need to RTS.
Regardless of the cartilage technique chosen, the goals of surgery remain similar—to reduce pain and improve function. For athletes, the ultimate goal is to return to the same level of play that the athlete was able to achieve prior to injury. Certainly, the need for reoperation following a cartilage surgery has implications on pain, function, and ability to RTS. Our review of nearly 50,000 cartilage surgeries demonstrates that reoperations following cartilage repair surgery are not uncommon, with a rate of 14.90% at 2 years, and that while reoperation rates are the highest following ACI, the rate of conversion to knee arthroplasty is highest following MFX. Due to the limitations of the PearlDiver database, it is not possible to determine the clinical outcomes of patients undergoing reoperation following cartilage surgery, but certainly, given these data, reoperation is clearly not necessarily indicative of clinical failure. This is highlighted by the fact that the most common procedure performed at the time of reoperation is arthroscopic chondroplasty, which, despite being an additional surgical procedure, may be acceptable for patients who wish to RTS, particularly in the setting of an index ACI in which there may be graft hypertrophy. Ideally, additional studies incorporating a cost-effectiveness analysis of each of the procedures, incorporating reoperation rates as well as patient-reported clinical outcomes, would be helpful to truly determine the patient and societal implications of reoperation following cartilage repair/restoration.
Many of the advantages and disadvantages of the described cartilage repair/restoration procedures have been well described.10-17 Microfracture is the most commonly utilized first-line repair/restoration option for small articular cartilage lesions, mainly due to its low cost, low morbidity, and relatively low level of difficulty.18 Despite these advantages, MFX is not without limitations, and the need for revision cartilage restoration and/or conversion to arthroplasty is concerning. In 2013, Salzmann and colleagues19 evaluated a cohort of 454 patients undergoing MFX for a symptomatic knee defect and noted a reoperation rate of 26.9% (n = 123) within 2 years of the index surgery, with risk factors for reoperation noted to include an increased number of pre-MFX ipsilateral knee surgeries, patellofemoral lesions, smoking, and lower preoperative numeric analog scale scores. The definition of reoperation in their study is unfortunately not described, and thus the extent of reoperation (arthroscopy to arthroplasty) is unclear. In a 2009 systematic review of 3122 patients (28 studies) undergoing MFX conducted by Mithoefer and colleagues,20 revision rates were noted to range from 2% to 31% depending on the study analyzed, with increasing revision rates after 2 years. Unfortunately, the heterogeneity of the included studies makes it difficult to determine which patients tend to fail over time.
Continue to: OATS...
OATS is a promising cartilage restoration technique indicated for treatment of patients with large, uncontained chondral lesions, and/or lesions with both bone and cartilage loss.1 OCA is similar to OATS but uses allograft tissue instead of autograft tissue and is typically considered a viable treatment option in larger lesions (>2 cm2).21 Cell-based ACI therapy has evolved substantially over the past decade and is now available as a third-generation model utilizing biodegradable 3-dimensional scaffolds seeded with chondrocytes. Reoperation rates following ACI can often be higher than those following other cartilage treatments, particularly given the known complication of graft hypertrophy and/or delamination. Harris and colleagues22 conducted a systematic review of 5276 subjects undergoing ACI (all generations), noting an overall reoperation rate of 33%, but a failure rate of 5.8% at an average of 22 months following ACI. Risk factors for reoperation included periosteal-based ACI as well as open (vs arthroscopic) ACI. In this study, we found a modestly lower return to OR rate of 29.69% at 2 years.
When the outcomes of patients undergoing OATS or OCA are compared to those of patients undergoing MFX or ACI, it can be difficult to interpret the results, as the indications for performing these procedures tend to be very different. Further, the reasons for reoperation, as well as the procedures performed at the time of reoperation, are often poorly described, making it difficult to truly quantify the risk of reoperation and the implications of reoperation for patients undergoing any of these index cartilage procedures.
Overall, in this database, the return to the OR rate approaches 15% at 2 years following cartilage surgery, with cell-based therapy demonstrating higher reoperation rates at 2 years, without the risk of conversion to arthroplasty. Reoperation rates appear to stabilize at 1 year following surgery and consist mostly of minor arthroscopic procedures. These findings can help surgeons counsel patients as to the rate and type of reoperations that can be expected following cartilage surgery. Additional research incorporating patient-reported outcomes and patient-specific risk factors are needed to complement these data as to the impact of reoperations on overall clinical outcomes. Further, studies incorporating 90-day, 1-year, and 2-year costs associated with cartilage surgery will help to determine which index procedure is the most cost effective over the short- and long-term.
LIMITATIONS
This study is not without limitations. The PearlDiver database is reliant upon accurate CPT and ICD-9 coding, which creates a potential for a reporting bias. The overall reliability of the analyses is dependent on the quality of the available data, which, as noted in previous PearlDiver studies,18,23-28 may include inaccurate billing codes, miscoding, and/or non-coding by physicians as potential sources of error. At the time of this study, the PearlDiver database did not provide consistent data points on laterality, and thus it is possible that the reported rates of reoperation overestimate the true reoperation rate following a given procedure. Fortunately, the reoperation rates for each procedure analyzed in this database study are consistent with those previously presented in the literature. In addition, it is not uncommon for patients receiving one of these procedures to have previously been treated with one of the others. Due to the inherent limitations of the PearlDiver database, this study did not investigate concomitant procedures performed along with the index procedure, nor did it investigate confounding factors such as comorbidities. The PearlDiver database does not provide data on defect size, location within the knee, concomitant pathologies (eg, meniscus tear), prior surgeries, or patient comorbidities, and while important, these factors cannot be accounted for in our analysis. The inability to account for these important factors, particularly concomitant diagnoses, procedures, and lesion size/location, represents an important limitation of this study, as this is a source of selection bias and may influence the need for reoperation in a given patient. Despite these limitations, the results of this study are supported by previous and current literature. In addition, the PearlDiver database, as a HIPAA-compliant database, does not report exact numbers when the value of the outcome of interest is between 0 and 10, which prohibits analysis of any cartilage procedure performed in a cohort of patients greater than 1 and less than 11. Finally, while not necessarily a limitation, it should be noted that CPT 29879 is not specific for microfracture, as the code also includes abrasion arthroplasty and drilling. Due to the limitations of the methodology of searching the database for this code, it is unclear as to how many patients underwent actual microfracture vs abrasion arthroplasty.
CONCLUSION
Within a large US commercial insurance database from 2007 to 2011, reparative procedures were favored for chondral injuries, but yielded an increased risk for conversion to arthroplasty. There was no difference between failure/revision rates among the restorative approaches, yet cell-based approaches yielded a significantly increased risk for a return to the OR.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
- Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469(10):2696-2705. doi:10.1007/s11999-010-1764-z.
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295-306. doi:10.1177/03635465004273510.
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-460. doi:10.1177/0363546505274578.
- Gudas R, Gudaitė A, Pocius A, et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40(11):2499-2508. doi:10.1177/0363546512458763.
- Saris DBF, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(suppl 1):10-19. doi:10.1177/0363546509350694.
- McCormick F, Harris JD, Abrams GD, et al. Survival and reoperation rates after meniscal allograft transplantation: analysis of failures for 172 consecutive transplants at a minimum 2-year follow-up. Am J Sports Med. 2014;42(4):892-897. doi:10.1177/0363546513520115.
- Miller DJ, Smith MV, Matava MJ, Wright RW, Brophy RH. Microfracture and osteochondral autograft transplantation are cost-effective treatments for articular cartilage lesions of the distal femur. Am J Sports Med. 2015;43(9):2175-2181. doi:10.1177/0363546515591261.
- Campbell AB, Pineda M, Harris JD, Flanigan DC. Return to sport after articular cartilage repair in athletes' knees: a systematic review. Arthroscopy. 2016;32(4):651-668.
- Chalmers PN, Vigneswaran H, Harris JD, Cole BJ. Activity-related outcomes of articular cartilage surgery: a systematic review. Cartilage. 2013;4(3):193-203.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, Carrington RW. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. JBone Joint Surg Br. 2012;94(4):504-509. doi:10.1177/1947603513481603.
- Beris AE, Lykissas MG, Kostas-Agnantis I, Manoudis GN. Treatment of full-thickness chondral defects of the knee with autologous chondrocyte implantation: a functional evaluation with long-term follow-up. Am J Sports Med. 2012;40(3):562-567.
- Chahal J, Gross AE, Gross C, et al. Outcomes of osteochondral allograft transplantation in the knee. Arthroscopy. 2013;29(3):575-588. doi:10.1177/0363546511428778.
- Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907-914. doi:10.1177/0363546507299932.
- Gudas R, Stankevičius E, Monastyreckienė E, Pranys D, Kalesinskas R. Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):834-842. doi:10.1007/s00167-006-0067-0.
- Lynch TS, Patel RM, Benedick A, Amin NH, Jones MH, Miniaci A. Systematic review of autogenous osteochondral transplant outcomes. Arthroscopy. 2015;31(4):746-754. doi:10.1016/j.arthro.2014.11.018.
- Niemeyer P, Porichis S, Steinwachs M, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-157. doi:10.1177/0363546513506593.
- Ulstein S, Årøen A, Røtterud J, Løken S, Engebretsen L, Heir S. Microfracture technique versus osteochondral autologous transplantation mosaicplasty in patients with articular chondral lesions of the knee: a prospective randomized trial with long-term follow-up. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1207-1215. doi:10.1007/s00167-014-2843-6.
- Montgomery S, Foster B, Ngo S, et al. Trends in the surgical treatment of articular cartilage defects of the knee in the United States. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2070-2075. doi:10.1007/s00167-013-2614-9.
- Salzmann GM, Sah B, Südkamp NP, Niemeyer P. Reoperative characteristics after microfracture of knee cartilage lesions in 454 patients. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):365-371. doi:10.1007/s00167-012-1973-y.
- Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-2063. doi:10.1177/0363546508328414.
- Wajsfisz A, Makridis KG, Djian P. Arthroscopic retrograde osteochondral autograft transplantation for cartilage lesions of the tibial plateau: a prospective study. Am J Sports Med. 2013;41(2):411-415. doi:10.1177/0363546512469091.
- Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation–a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791. doi:10.1016/j.joca.2011.02.010.
- Abrams GD, Frank RM, Gupta AK, Harris JD, McCormick FM, Cole BJ. Trends in meniscus repair and meniscectomy in the United States, 2005-2011. Am J Sports Med. 2013;41(10):2333-2339. doi:10.1177/0363546513495641.
- Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. doi:10.1016/j.arthro.2012.11.005.
- Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA. Incidence of acute postoperative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med. 2014;42(2):437-441. doi:10.1177/0363546513510686.
- Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the United States. Arthroscopy. 2014;30(4):436-443. doi:10.1016/j.arthro.2013.12.013.
- Werner BC, Carr JB, Wiggins JC, Gwathmey FW, Browne JA. Manipulation under anesthesia after total knee arthroplasty is associated with an increased incidence of subsequent revision surgery. J Arthroplasty. 2015;30(suppl 9):72-75. doi:10.1016/j.arth.2015.01.061.
- Carr JB 2nd, Werner BC, Browne JA. Trends and outcomes in the treatment of failed septic total knee arthroplasty: comparing arthrodesis and above-knee amputation. J Arthroplasty. 2016;31(7):1574-1577. doi:10.1016/j.arth.2016.01.010.
TAKE-HOME POINTS
- With a large US commercial insurance database analyzing techniques for cartilage restoration, reparative procedures were favored for chondral injuries compared to restorative approaches.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 90-day reoperation rate is 6%.
- Among patients undergoing microfracture, autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation, the average 2-year reoperation rate is 15%.
- Patients undergoing autologous chondrocyte implantation are more likely to experience reoperation at 90 days, 1 year, and 2 years compared to other cartilage restoration techniques including microfracture, osteochondral autograft transfer, and osteochondral allograft transplantation.
- Patients undergoing microfracture are more likely to experience an ultimate conversion to arthroplasty compared to other cartilage restoration techniques including autologous chondrocyte implantation, osteochondral autograft transfer, and osteochondral allograft transplantation.
Management of Isolated Greater Tuberosity Fractures: A Systematic Review
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Subscapularis Tenotomy Versus Lesser Tuberosity Osteotomy for Total Shoulder Arthroplasty: A Systematic Review
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- According to the orthopedic literature, ST and LTO for a TSA produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
- Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions.
- ST and LTO approaches for a TSA result in similar Constant scores, pain scores, radiographic outcomes, and complication rates.
During total shoulder arthroplasty (TSA) exposure, the subscapularis muscle must be mobilized; its repair is crucial to the stability of the arthroplasty. The subscapularis is the largest rotator cuff muscle and has a contractile force equal to that of the other 3 muscles combined.1,2 Traditionally it is mobilized with a tenotomy just medial to the tendon’s insertion onto the lesser tuberosity. Over the past 15 years, however, numerous authors have reported dysfunction after subscapularis tenotomy (ST). In 2003, Miller and colleagues3 reported that, at 2-year follow-up, almost 70% of patients had abnormal belly-press and liftoff tests, surrogate markers of subscapularis function. Other authors have found increased rates of anterior instability after subscapularis rupture.4,5
In 2005, Gerber and colleagues6 introduced a technique for circumventing surgical division of the subscapularis. They described a lesser tuberosity osteotomy (LTO), in which the subscapularis tendon is detached with a bone fragment 5 mm to 10 mm in thickness and 3 cm to 4 cm in length. This approach was based on the premise that bone-to-bone healing is more reliable than tendon-to-tendon healing. Initial studies reported successful osteotomy healing, improved clinical outcome scores, and fewer abnormalities with belly-press and liftoff tests.2,6 More recent literature, however, has questioned the necessity of LTO.2,4,7-9We performed a systematic review to evaluate the literature, describe ST and LTO, and summarize the radiographic and clinical outcomes of both techniques. We hypothesized there would be no significant clinical differences between these approaches.
Methods
Search Strategy and Study Selection
Using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, we systematically reviewed the literature.10 Searches were completed in September 2014 using the PubMed Medline database and the Cochrane Central Register of Clinical Trials. Two reviewers (Dr. Louie, Dr. Levy) independently performed the search and assessed eligibility of all relevant studies based on predetermined inclusion criteria. Disagreements between reviewers were resolved by discussion. Key word selection was designed to capture all English-language studies with clinical and/or radiographic outcomes and level I to IV evidence. We used an electronic search algorithm with key words and a series of NOT phrases to match certain exclusion criteria:
(((((((((((((((((((((((((((((((((((((total[Text Word]) AND shoulder[Title]) AND arthroplasty[Title] AND (English[lang]))) NOT reverse[Title/Abstract]) NOT hemiarthroplasty[Title]) NOT nonoperative[Title]) NOT nonsurgical[Title] AND (English[lang]))) NOT rheumatoid[Title/Abstract]) NOT inflammatory[Title/Abstract]) NOT elbow[Title/Abstract]) NOT wrist[Title/Abstract]) NOT hip[Title/Abstract]) NOT knee[Title/Abstract]) NOT ankle[Title/Abstract] AND (English[lang]))) NOT biomechanic[Title/Abstract]) NOT biomechanics[Title/Abstract]) NOT biomechanical [Title/Abstract]) NOT cadaveric[Title/Abstract]) NOT revision[Title]) NOT resurfacing[Title/Abstract]) NOT surface[Title/Abstract]) NOT interphalangeal[Title/Abstract] AND (English[lang]))) NOT radiostereometric[Title/Abstract] AND (English[lang]))) NOT cmc[Title/Abstract]) NOT carpometacarpal[Title/Abstract]) NOT cervical[Title/Abstract]) NOT histology[Title/Abstract]) NOT histological[Title/Abstract]) NOT collagen[Title/Abstract] AND (English[lang]))) NOT kinematic[Title/Abstract]) NOT kinematics[Title/Abstract] AND (English[lang]))) NOT vitro[Title/Abstract] AND (English[lang]))) NOT inverted[Title/Abstract]) NOT grammont[Title/Abstract]) NOT arthrodesis[Title/Abstract]) NOT fusion[Title/Abstract]) NOT reverse[Title/Abstract] AND (English[lang]))
Study exclusion criteria consisted of cadaveric, biomechanical, histologic, and kinematic results as well as analyses of nonoperative management, hemiarthroplasty, or reverse TSA. Studies were excluded if they did not report clinical and/or radiographic data. Minimum mean follow-up was 2 years. To discount the effect of other TSA technical innovations, we evaluated the same period for the 2 surgical approaches. The first study with clinical outcomes after LTO was published in early 2005,6 so all studies published before 2005 were excluded.
We reviewed all references within the studies included by the initial search algorithm: randomized control trials, retrospective and prospective cohort designs, case series, and treatment studies. Technical notes, review papers, letters to the editor, and level V evidence reviews were excluded. To avoid counting patients twice, we compared each study’s authors and data collection period with those of the other studies. If there was overlap in authorship, period, and place, only the study with the longer follow-up or more comprehensive data was included. All trials comparing ST and LTO were included. If the authors of a TSA study did not describe the approach used, that study was excluded from our review.
Data Extraction
We collected details of study design, sample size, and patient demographics (sex, age, hand dominance, primary diagnosis). We also abstracted surgical factors about the glenoid component (cemented vs uncemented; pegged vs keeled; all-polyethylene vs metal-backed) and the humeral component (cemented vs press-fit; stemmed vs stemless). Clinical outcomes included pain scores, functional scores, number of revisions, range of motion (ROM), and subscapularis-specific tests (eg, belly-press, liftoff). As pain scales varied between studies, all values were converted to a 10-point scoring scale (0 = no pain; 10 = maximum pain) for comparisons. Numerous functional outcome scores were reported, but the Constant score was the only one consistently used across studies, making it a good choice for comparisons. One study used Penn Shoulder Scores (PSSs) and directly compared ST and LTO groups, so its data were included. In addition, radiographic data were compiled: radiolucencies around the humeral stem and glenoid component, humeral head subluxation/migration, and osteotomy healing. The only consistent radiographic parameter available for comparisons between groups was the presence of radiolucencies.
The Modified Coleman Methodology Score (MCMS), described by Cowan and colleagues,11 was used to evaluate the methodologic quality of each study. The MCMS is a 15-item instrument that has been used to assess both randomized and nonrandomized trials.12,13 It has a scaled score ranging from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor). Study quality was not factored into the data synthesis analysis.
Statistical Analysis
Data are reported as weighted means and standard deviations. A mean was calculated for each study reporting on a respective data point and was then weighed according to the study sample size. The result was that the nonweighted means from studies with smaller samples did not carry as much weight as those from studies with larger samples. Student t tests and 2-way analysis of variance were used to compare the ST and LTO groups and assess differences over time (SPSS Version 18; IBM). An α of 0.05 was set as statistically significant.
Results
Twenty studies (1420 shoulders, 1392 patients) were included in the final dataset (Figure).2,6,8,14-30
The youngest patients in the ST and LTO groups were 22 years and 19 years of age, respectively.
Table 2 lists the details regarding the surgical components. For glenoid components, the ST and LTO groups’ fixation types and material used were not significantly different.
Table 3 lists the clinical and radiographic outcomes most consistently reported in the literature. Physical examination data were reported in 18 ST populations8,14-16,21-30 and 11 LTO populations.2,6,14-20
Constant scores were reported in 4 ST studies14,22,24,27 and 3 LTO studies14,17,18 (Table 3). There was no significant difference (P = .37) in post-TSA Constant score improvement between the 2 groups. In the one study that performed direct comparisons, PSS improved on average from 29 to 81 in the ST group and from 29 to 92 in the LTO group.15 Several ST studies reported improved scores on various indices: WOOS (Western Ontario Osteoarthritis of the Shoulder), ASES (American Shoulder and Elbow Surgeons), SST (Simple Shoulder Test), DASH (Disabilities of the Arm, Shoulder, and Hand), SF-12 (Short Form 12-Item Health Survey), MACTAR (McMaster Toronto Arthritis Patient Preference Disability Questionnaire), and Neer shoulder impingement test.8,14,15,21,23-25,27-30 However, these outcomes were not reported in LTO cohorts for comparison. Similarly, 2 LTO cohorts reported improvements in SSV (subjective shoulder value) scores, but this measure was not used in the ST cohorts.6,17 Five ST studies recorded patients’ subjective satisfaction: 58% of patients indicated an excellent outcome, 35% a satisfactory outcome, and 7% a less than satisfactory outcome.21,23,25,26,29 Only 1 LTO study reported patient satisfaction: 69% excellent, 31% satisfactory, 0% dissatisfied.17
Complications were reported in 16 ST studies8,15,21-30 and 6 LTO studies.15,17-19 There were 117 complications (17.8%) and 58 revisions (10.0%) in the ST group and 52 complications (17.2%) and 49 revisions (16.2%) in the LTO group. In the ST group, aseptic loosening (6.2%) was the most common complication, followed by subscapularis tear or attenuation (5.2%), dislocation (2.1%), and deep infection (0.5%). In the LTO group, aseptic loosening was again the most common (9.0%), followed by dislocation (4.0%), subscapularis tear or attenuation (2.2%), and deep infection (0.7%). There were no significant differences in the incidence of individual complications between groups. The difference in revision rates was not statistically significant (P = .31).
Radiolucency data were reported in 12 ST studies19,21-26,28,30 and 2 LTO studies.17,18 There were no discussions of humeral component radiolucencies in the LTO studies. At final follow-up, radiolucencies of the glenoid component were detected in 42.3% of patients in the ST group and 40.7% of patients in the LTO group (P = .76).
Discussion
Our goal in this systematic review was to analyze outcomes associated with ST and LTO in a heterogenous TSA population. We hypothesized TSA with ST or LTO would produce similar clinical and radiographic outcomes. There were no significant differences in Constant scores, pain scores, radiolucencies, or complications between the 2 groups. The ST group showed trends toward wider ROM improvements and fewer revisions, but only the change in forward elevation was significant. The components used in the 2 groups were similar with the exception of a lack of keeled glenoids and cemented humeral stems in the LTO group; data stratification controlling for these differences revealed no change in outcomes.
The optimal method of subscapularis mobilization for TSA remains a source of debate. Jackson and colleagues23 found significant improvements in Neer and DASH scores after ST. However, 7 of 15 patients ruptured the subscapularis after 6 months and had significantly lower DASH scores. In 2005, Gerber and colleagues6 first described the LTO technique as an alternative to ST. After a mean of 39 months, 89% of their patients had a negative belly-press test, and 75% had a normal liftoff test. Radiographic evaluation revealed that the osteotomized fragment had healed in an anatomical position in all shoulders. In a large case series, Small and colleagues20 used radiographs and computed tomography to further investigate LTO healing rates and found that 89% of patients had bony union by 6 months and that smoking was a significant risk factor for nonunion.
Biomechanical studies comparing ST and LTO approaches have shown mixed results. Ponce and colleagues2 found decreased cyclic displacement and increased maximum load to failure with LTO, but Giuseffi and colleagues32 showed less cyclic displacement with ST and no difference in load to failure. Others authors have found no significant differences in stiffness or maximum load to failure.33 Van den Berghe and colleagues7 reported a higher failure rate in bone-to-bone repairs compared with tendon-to-tendon constructs. Moreover, they found that suture cut-out through bone tunnels is the primary mode of LTO failure, so many LTO surgeons now pass sutures around the humeral stem instead.
Three TSA studies directly compared ST and LTO approaches. Buckley and colleagues14 analyzed 60 TSAs and found no significant differences in WOOS, DASH, or Constant scores between groups. The authors described an ST subgroup with subscapularis attenuation on ultrasound but did not report the group as having any inferior functional outcome. Scalise and colleagues15 showed improved strength and PSSs in both groups after 2 years. However, the LTO group had a lower rate of subscapularis tears and significantly higher PSSs. Finally, Jandhyala and colleagues16 reported more favorable outcomes with LTO, which trended toward wider ROM and significantly higher belly-press test grades. Lapner and colleagues34 conducted a randomized, controlled trial (often referenced) and found no significant differences between the 2 groups in terms of strength or functional outcome at 2-year follow-up. Their study, however, included hemiarthroplasties and did not substratify the TSA population, so we did not include it in our review.
Our systematic review found significantly more forward elevation improvement for the ST group than the LTO group, which may suggest improved ROM with a soft-tissue approach than a bony approach. At the same time, the ST group trended toward better passive external rotation relative to the LTO group. This trend indicates fewer constraints to external rotation in the ST group, possibly attributable to a more attenuated subscapularis after tenotomy. Subscapularis tear or attenuation was more commonly reported in the ST group than in the LTO group, though not significantly so. This may indicate that more ST studies than LTO studies specially emphasized postoperative subscapularis function, but these data also highlight some authors’ concerns regarding subscapularis dysfunction after tenotomy.6,15,16The study populations’ complication rates were similar, just over 17%. The LTO group trended toward a higher revision rate, but it was not statistically significant. The LTO group also had significantly fewer patients with osteoarthritis and more patients with posttraumatic arthritis, so this group may have had more complex patients predisposed to a higher likelihood of revision surgery. Revisions were most commonly performed for aseptic loosening; theoretically, if osteotomies heal less effectively than tenotomies, the LTO approach could produce component instability and aseptic loosening. However, no prior studies or other clinical findings from this review suggest LTO predisposes to aseptic loosening. Overall, the uneven revision rates represent a clinical concern that should be monitored as larger samples of patients undergo ST and LTO procedures.
Glenoid radiolucencies were the only radiographic parameter consistently reported in the included studies. Twelve ST studies had radiolucency data—compared with only 2 LTO studies. Thus, our ability to compare radiographic outcomes was limited. Our data revealed similar rates of glenoid radiolucencies between the 2 approaches. The clinical relevance of radiolucencies is questioned by some authors, and, indeed, Razmjou and colleagues25 found no correlation of radiolucencies with patient satisfaction. Nevertheless, early presence of radiolucencies may raise concerns about progressive loss of fixation,35,36 so this should be monitored.
Limitations of this systematic review reflect the studies analyzed. We minimized selection bias by including level I to IV evidence, but most studies were level IV, and only 1 was level I. As such, there was a relative paucity of consistent clinical and radiographic data. For instance, although many ST studies reported patient satisfaction as an outcomes measure, only 1 LTO study commented on it. Perhaps the relative novelty of the LTO approach has prompted some authors to focus more on technical details and less on reporting a variety of outcome measures. As mentioned earlier, the significance of radiolucency data is controversial, and determination of their presence or absence depends on the observer. A radiolucency found in one study may not qualify as one in a study that uses different criteria. However, lucency data were the most frequently and reliably reported radiographic parameter, so we deemed it the most appropriate method for comparing radiographic outcomes. Finally, the baseline differences in diagnosis between the ST and LTO groups complicated comparisons. We stratified the groups by component design because use of keeled or pegged implants or humeral cemented or press-fit stems was usually a uniform feature of each study—enabling removal of certain studies for data stratification. However, we were unable to stratify by original diagnosis because these groups were not stratified within the individual studies.
Conclusion
Our systematic review found similar Constant scores, pain scores, radiographic outcomes, and complication rates for the ST and LTO approaches. Compared with the LTO approach, the ST approach produced significantly more forward elevation improvement and trended toward more external rotation and abduction and fewer revisions. Although not definitive, these data suggest the ST approach may provide more stability over the long term, but additional comprehensive studies are needed to increase the sample size and the power of the trends elucidated in this review. According to the orthopedic literature, both techniques produce excellent clinical outcomes, and technique selection should be based on surgeon discretion and expertise.
Am J Orthop. 2017;46(2):E131-E138. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
1. Keating JF, Waterworth P, Shaw-Dunn J, Crossan J. The relative strengths of the rotator cuff muscles. A cadaver study. J Bone Joint Surg Br. 1993;75(1):137-140.
2. Ponce BA, Ahluwalia RS, Mazzocca AD, Gobezie RG, Warner JJ, Millett PJ. Biomechanical and clinical evaluation of a novel lesser tuberosity repair technique in total shoulder arthroplasty. J Bone Joint Surg Am. 2005;87(suppl 2):1-8.
3. Miller SL, Hazrati Y, Klepps S, Chiang A, Flatow EL. Loss of subscapularis function after total shoulder replacement: a seldom recognized problem. J Shoulder Elbow Surg. 2003;12(1):29-34.
4. Gerber A, Ghalambor N, Warner JJ. Instability of shoulder arthroplasty: balancing mobility and stability. Orthop Clin North Am. 2001;32(4):661-670, ix.
5. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg Am. 1993;75(4):492-497.
6. Gerber C, Yian EH, Pfirrmann CA, Zumstein MA, Werner CM. Subscapularis muscle function and structure after total shoulder replacement with lesser tuberosity osteotomy and repair. J Bone Joint Surg Am. 2005;87(8):1739-1745.
7. Van den Berghe GR, Nguyen B, Patil S, et al. A biomechanical evaluation of three surgical techniques for subscapularis repair. J Shoulder Elbow Surg. 2008;17(1):156-161.
8. Caplan JL, Whitfield B, Neviaser RJ. Subscapularis function after primary tendon to tendon repair in patients after replacement arthroplasty of the shoulder. J Shoulder Elbow Surg. 2009;18(2):193-196.
9. Armstrong A, Lashgari C, Teefey S, Menendez J, Yamaguchi K, Galatz LM. Ultrasound evaluation and clinical correlation of subscapularis repair after total shoulder arthroplasty. J Shoulder Elbow Surg. 2006;15(5):541-548.
10. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
11. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
12. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
13. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
14. Buckley T, Miller R, Nicandri G, Lewis R, Voloshin I. Analysis of subscapularis integrity and function after lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty using ultrasound and validated clinical outcome measures. J Shoulder Elbow Surg. 2014;23(9):1309-1317.
15. Scalise JJ, Ciccone J, Iannotti JP. Clinical, radiographic, and ultrasonographic comparison of subscapularis tenotomy and lesser tuberosity osteotomy for total shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(7):1627-1634.
16. Jandhyala S, Unnithan A, Hughes S, Hong T. Subscapularis tenotomy versus lesser tuberosity osteotomy during total shoulder replacement: a comparison of patient outcomes. J Shoulder Elbow Surg. 2011;20(7):1102-1107.
17. Fucentese SF, Costouros JG, Kühnel SP, Gerber C. Total shoulder arthroplasty with an uncemented soft-metal-backed glenoid component. J Shoulder Elbow Surg. 2010;19(4):624-631.
18. Clement ND, Duckworth AD, Colling RC, Stirrat AN. An uncemented metal-backed glenoid component in total shoulder arthroplasty for osteoarthritis: factors affecting survival and outcome. J Orthop Sci. 2013;18(1):22-28.
19. Rosenberg N, Neumann L, Modi A, Mersich IJ, Wallace AW. Improvements in survival of the uncemented Nottingham Total Shoulder prosthesis: a prospective comparative study. BMC Musculoskelet Disord. 2007;8(1):76.
20. Small KM, Siegel EJ, Miller LR, Higgins LD. Imaging characteristics of lesser tuberosity osteotomy after total shoulder replacement: a study of 220 patients. J Shoulder Elbow Surg. 2014;23(9):1318-1326.
21. Mileti J, Sperling JW, Cofield RH, Harrington JR, Hoskin TL. Monoblock and modular total shoulder arthroplasty for osteoarthritis. J Bone Joint Surg Br. 2005;87(4):496-500.
22. Merolla G, Paladini P, Campi F, Porcellini G. Efficacy of anatomical prostheses in primary glenohumeral osteoarthritis. Chir Organi Mov. 2008;91(2):109-115.
23. Jackson JD, Cil A, Smith J, Steinmann SP. Integrity and function of the subscapularis after total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(7):1085-1090.
24. Jost PW, Dines JS, Griffith MH, Angel M, Altchek DW, Dines DM. Total shoulder arthroplasty utilizing mini-stem humeral components: technique and short-term results. HSS J. 2011;7(3):213-217.
25. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
26. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-1710.
27. Litchfied RB, McKee MD, Balyk R, et al. Cemented versus uncemented fixation of humeral components in total shoulder arthroplasty for osteoarthritis of the shoulder: a prospective, randomized, double-blind clinical trial—a JOINTs Canada Project. J Shoulder Elbow Surg. 2011;20(4):529-536.
28. Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty. Survivorship and outcomes. J Bone Joint Surg Am. 2005;87(6):1284-1292.
29. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188.
30. Budge MD, Nolan EM, Heisey MH, Baker K, Wiater JM. Results of total shoulder arthroplasty with a monoblock porous tantalum glenoid component: a prospective minimum 2-year follow-up study. J Shoulder Elbow Surg. 2013;22(4):535-541.
31. Gerber C, Costouros JG, Sukthankar A, Fucentese SF. Static posterior humeral head subluxation and total shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):505-510.
32. Giuseffi SA, Wongtriratanachai P, Omae H, et al. Biomechanical comparison of lesser tuberosity osteotomy versus subscapularis tenotomy in total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(8):1087-1095.
33. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(5):657-663.
34. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94(24):2239-2246.
35. Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66(6):899-906.
36. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.