User login
Staff/family meetings lower futile care
Futile. It’s a word not often heard in discussions with cancer patients or family members as test results are reviewed and next steps considered. But it may be key, as patients near the end of life, to debates about reducing costs and reducing suffering, dual objectives that so often go hand-in-hand.
As they weigh second-, third- or fourth-line options, should patients ask, "What is the likelihood this round of chemotherapy (or other aggressive treatment) will be futile? Would I gain function, time, or both? And at what physical and financial cost?"
All too often, the question isn’t even asked among colleagues during rounds, at hallway consults, or at the nursing station.
Then, when futile treatment fails and the inevitable family meeting transpires, often in the intensive care unit, the message to family members from the staff may be unclear.
Inevitably, the family member with the most assertive voice wins, more orders get written, and aggressive care continues.
At the City of Hope National Medical Center in Duarte, Calif., a novel project seeks to bring perspective to that process in the hopes of alleviating suffering, preserving patient dignity, and reducing the stress inherent in providing care that is "medically ineffective" through no fault of those ordering or administering that care.
Diane Morrison, licensed clinical social worker and manager of clinical social work, recently offered a snapshot of the Multidisciplinary ICU Team Family Meeting Project during a poster session at the annual meeting of the American Psychosocial Oncology Society.
Several years ago, a multidisciplinary steering committee was formed, made up of "physician champions" from the ICU and medical services, clinical social workers, nurses, and a chaplain. They designed a "shared accountability" strategy that brought goals and values to center stage in the ICU, hinging on structured family meetings.
Critically, a multidisciplinary premeeting is held, either in person or virtually, Ms. Morrison said in an interview after the meeting.
"The premeeting is used to make sure the medical staff is on the same page," she said.
Any disagreements about the course of care are aired and resolved. Details are organized, and expected "next steps" are considered. A primary spokesperson for the team is designated. Often, this will be the physician, but not always.
Perspectives of the family are shared to construct a culturally attuned scaffolding for the meeting to clarify that the patient’s life is ending.
During the meeting, speaking in a kind, professional, and unified voice, the team explains the patient’s medical condition, offers perspective on the role of supportive care and hospice, and provides support to the family or the patient as decisions are made.
As an added benefit, professionals extensively trained in family dynamics and end-of-life discussions lend help during the meetings to medical oncologists, hematologists, and surgeons.
Parents (24%), spouses (13%), siblings (16%), and offspring (26%) have attended the meetings, Ms. Morrison said. In just 5% of cases does the patient attend, a statistic that speaks to how far down the treatment road the case has progressed.
To administrators and budget-minded politicians, the results might as well be surrounded by flashing strobe lights. Among the roughly 100 patients who die in the ICU each year at the institution, the ICU stay was reduced by 4 days, from 14.2 days in 2010 to 10.3 days in 2012. (Approximately 1,850 patients are admitted each year to the ICU.)
But to Ms. Morrison, it’s not about money. It’s about better communication and more thoughtful attention to human values, dignity, and compassion.
Physician feedback has been strongly positive, with 19 of 22 participants agreeing or strongly agreeing that the family meetings have been useful in managing patients. Fully 18 of 22 felt that, by meeting the emotional and psychological needs of patients and caregivers, their time was protected to focus on duties related to their medical expertise.
Moreover, the program is believed to be reducing "moral distress" among nurses.
"We believe," concluded the poster, "that less time in futile care alleviates suffering for patients and family members. We also propose that less time spent providing medically ineffective care reduces stress on staff."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Futile. It’s a word not often heard in discussions with cancer patients or family members as test results are reviewed and next steps considered. But it may be key, as patients near the end of life, to debates about reducing costs and reducing suffering, dual objectives that so often go hand-in-hand.
As they weigh second-, third- or fourth-line options, should patients ask, "What is the likelihood this round of chemotherapy (or other aggressive treatment) will be futile? Would I gain function, time, or both? And at what physical and financial cost?"
All too often, the question isn’t even asked among colleagues during rounds, at hallway consults, or at the nursing station.
Then, when futile treatment fails and the inevitable family meeting transpires, often in the intensive care unit, the message to family members from the staff may be unclear.
Inevitably, the family member with the most assertive voice wins, more orders get written, and aggressive care continues.
At the City of Hope National Medical Center in Duarte, Calif., a novel project seeks to bring perspective to that process in the hopes of alleviating suffering, preserving patient dignity, and reducing the stress inherent in providing care that is "medically ineffective" through no fault of those ordering or administering that care.
Diane Morrison, licensed clinical social worker and manager of clinical social work, recently offered a snapshot of the Multidisciplinary ICU Team Family Meeting Project during a poster session at the annual meeting of the American Psychosocial Oncology Society.
Several years ago, a multidisciplinary steering committee was formed, made up of "physician champions" from the ICU and medical services, clinical social workers, nurses, and a chaplain. They designed a "shared accountability" strategy that brought goals and values to center stage in the ICU, hinging on structured family meetings.
Critically, a multidisciplinary premeeting is held, either in person or virtually, Ms. Morrison said in an interview after the meeting.
"The premeeting is used to make sure the medical staff is on the same page," she said.
Any disagreements about the course of care are aired and resolved. Details are organized, and expected "next steps" are considered. A primary spokesperson for the team is designated. Often, this will be the physician, but not always.
Perspectives of the family are shared to construct a culturally attuned scaffolding for the meeting to clarify that the patient’s life is ending.
During the meeting, speaking in a kind, professional, and unified voice, the team explains the patient’s medical condition, offers perspective on the role of supportive care and hospice, and provides support to the family or the patient as decisions are made.
As an added benefit, professionals extensively trained in family dynamics and end-of-life discussions lend help during the meetings to medical oncologists, hematologists, and surgeons.
Parents (24%), spouses (13%), siblings (16%), and offspring (26%) have attended the meetings, Ms. Morrison said. In just 5% of cases does the patient attend, a statistic that speaks to how far down the treatment road the case has progressed.
To administrators and budget-minded politicians, the results might as well be surrounded by flashing strobe lights. Among the roughly 100 patients who die in the ICU each year at the institution, the ICU stay was reduced by 4 days, from 14.2 days in 2010 to 10.3 days in 2012. (Approximately 1,850 patients are admitted each year to the ICU.)
But to Ms. Morrison, it’s not about money. It’s about better communication and more thoughtful attention to human values, dignity, and compassion.
Physician feedback has been strongly positive, with 19 of 22 participants agreeing or strongly agreeing that the family meetings have been useful in managing patients. Fully 18 of 22 felt that, by meeting the emotional and psychological needs of patients and caregivers, their time was protected to focus on duties related to their medical expertise.
Moreover, the program is believed to be reducing "moral distress" among nurses.
"We believe," concluded the poster, "that less time in futile care alleviates suffering for patients and family members. We also propose that less time spent providing medically ineffective care reduces stress on staff."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Futile. It’s a word not often heard in discussions with cancer patients or family members as test results are reviewed and next steps considered. But it may be key, as patients near the end of life, to debates about reducing costs and reducing suffering, dual objectives that so often go hand-in-hand.
As they weigh second-, third- or fourth-line options, should patients ask, "What is the likelihood this round of chemotherapy (or other aggressive treatment) will be futile? Would I gain function, time, or both? And at what physical and financial cost?"
All too often, the question isn’t even asked among colleagues during rounds, at hallway consults, or at the nursing station.
Then, when futile treatment fails and the inevitable family meeting transpires, often in the intensive care unit, the message to family members from the staff may be unclear.
Inevitably, the family member with the most assertive voice wins, more orders get written, and aggressive care continues.
At the City of Hope National Medical Center in Duarte, Calif., a novel project seeks to bring perspective to that process in the hopes of alleviating suffering, preserving patient dignity, and reducing the stress inherent in providing care that is "medically ineffective" through no fault of those ordering or administering that care.
Diane Morrison, licensed clinical social worker and manager of clinical social work, recently offered a snapshot of the Multidisciplinary ICU Team Family Meeting Project during a poster session at the annual meeting of the American Psychosocial Oncology Society.
Several years ago, a multidisciplinary steering committee was formed, made up of "physician champions" from the ICU and medical services, clinical social workers, nurses, and a chaplain. They designed a "shared accountability" strategy that brought goals and values to center stage in the ICU, hinging on structured family meetings.
Critically, a multidisciplinary premeeting is held, either in person or virtually, Ms. Morrison said in an interview after the meeting.
"The premeeting is used to make sure the medical staff is on the same page," she said.
Any disagreements about the course of care are aired and resolved. Details are organized, and expected "next steps" are considered. A primary spokesperson for the team is designated. Often, this will be the physician, but not always.
Perspectives of the family are shared to construct a culturally attuned scaffolding for the meeting to clarify that the patient’s life is ending.
During the meeting, speaking in a kind, professional, and unified voice, the team explains the patient’s medical condition, offers perspective on the role of supportive care and hospice, and provides support to the family or the patient as decisions are made.
As an added benefit, professionals extensively trained in family dynamics and end-of-life discussions lend help during the meetings to medical oncologists, hematologists, and surgeons.
Parents (24%), spouses (13%), siblings (16%), and offspring (26%) have attended the meetings, Ms. Morrison said. In just 5% of cases does the patient attend, a statistic that speaks to how far down the treatment road the case has progressed.
To administrators and budget-minded politicians, the results might as well be surrounded by flashing strobe lights. Among the roughly 100 patients who die in the ICU each year at the institution, the ICU stay was reduced by 4 days, from 14.2 days in 2010 to 10.3 days in 2012. (Approximately 1,850 patients are admitted each year to the ICU.)
But to Ms. Morrison, it’s not about money. It’s about better communication and more thoughtful attention to human values, dignity, and compassion.
Physician feedback has been strongly positive, with 19 of 22 participants agreeing or strongly agreeing that the family meetings have been useful in managing patients. Fully 18 of 22 felt that, by meeting the emotional and psychological needs of patients and caregivers, their time was protected to focus on duties related to their medical expertise.
Moreover, the program is believed to be reducing "moral distress" among nurses.
"We believe," concluded the poster, "that less time in futile care alleviates suffering for patients and family members. We also propose that less time spent providing medically ineffective care reduces stress on staff."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Passport to personality: Is it the patient who's 'difficult'?
You know that you’ve been at a valuable meeting when you can use on Monday morning the "take-home messages" you gathered over the weekend.
The annual conference of the American Psychosocial Oncology Society was just such a meeting, filled with valuable data and perspective on distress screening tools, survivorship guidance, and ways to integrate psychosocial care comfortably into the evolving medical model of cancer care.
But the lessons I put into play before I unpacked my briefcase were gleaned in a dynamic workshop led by Dr. John D. Wynn of the Swedish Cancer Institute in Seattle.
A psychiatrist, Dr. Wynn has long been regarded as an expert on the uneasy intersection of cancer and Axis II personality traits and disorders, having written the chapter on this topic in Dr. Jimmie Holland’s classic textbook Psycho-Oncology (New York: Oxford University Press, 2010).
Sorting through Axis II diagnoses, he frames Clusters A, B, and C as "weird," "wild," and "worried." Along the way, he challenged the accepted dogma that personality disorders are easily defined, enduring, and inflexible when research calls into question all of these notions.
To be sure, there are individuals whose ways of dealing with others and the world fall far outside the reassuring central band of "average." But labels – formal or informal –may be overused and unhelpful, he argued.
For example, "uncooperative" and "difficult" aren’t assigned codes in the DSM-IV or ICD-9, but they’re quasi-diagnoses used with great frequency in rounds, case conferences, and at the nursing station.
Dr. Wynn drove home the point that such terms are relative. Members of the treatment team are likely viewed as "difficult" by the very same patients who elicit the label from us. Consider the context, he counseled.
"Medical care encourages and reinforces dependency, passivity, and attention seeking," he explained.
While most people in society are able to adapt to the highly structured culture of Western medicine, grinning and bearing it in their new costumes (cotton gowns) and subservient patient roles, others may simply not possess that flexibility, particularly in the context of extreme fear, embarrassment, or mistrust.
Dr. Wynn argues that the people who are often diagnosed with personality disorders really have "adaptive failure" – a term he wishes would have made it into DSM-5. They have both a poor sense of self and a poor capacity for interpersonal functioning.
And this is a painful combination when it comes to coping with a life-threatening diagnosis in a strange new world, where amicability, conformity, and allegiance to the treatment plan are highly prized.
So how does all this play out in the clinic?
Dr. Wynn’s advice is to use our own reactions to such patients as diagnostic cues, tapping into our professionalism to guide our responses.
See each patient as unique and uniquely challenged by the circumstances of his or her disease, and respond based on what you see and feel, he advised.
Take extra time to listen to patients who fail to follow through with treatment, appear needy or overly-entitled, and even those who may "frighten or repel us."
Do fear, shame, or confusion lie at the heart of such behavior?
Could respect, collaboration, and limit setting put in check the most troubling interactions with such patients?
With Dr. Wynn’s conceptualization fresh in my mind, I was inspired to step back and put into context my own reactions to patients I find difficult.
I found myself more patient, more understanding, and more curious about the underpinnings of behaviors that play havoc with the treatment schedule and have the potential to bring out the worst in us all.
What particularly helps for me is to remember (and remind colleagues) that our shared enemy is cancer, not the patient. By seeing the patient as our ally in that fight, we are motivated to compassionately demystify our traditions and find creative bridges to connection with all types of people who need our care.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
You know that you’ve been at a valuable meeting when you can use on Monday morning the "take-home messages" you gathered over the weekend.
The annual conference of the American Psychosocial Oncology Society was just such a meeting, filled with valuable data and perspective on distress screening tools, survivorship guidance, and ways to integrate psychosocial care comfortably into the evolving medical model of cancer care.
But the lessons I put into play before I unpacked my briefcase were gleaned in a dynamic workshop led by Dr. John D. Wynn of the Swedish Cancer Institute in Seattle.
A psychiatrist, Dr. Wynn has long been regarded as an expert on the uneasy intersection of cancer and Axis II personality traits and disorders, having written the chapter on this topic in Dr. Jimmie Holland’s classic textbook Psycho-Oncology (New York: Oxford University Press, 2010).
Sorting through Axis II diagnoses, he frames Clusters A, B, and C as "weird," "wild," and "worried." Along the way, he challenged the accepted dogma that personality disorders are easily defined, enduring, and inflexible when research calls into question all of these notions.
To be sure, there are individuals whose ways of dealing with others and the world fall far outside the reassuring central band of "average." But labels – formal or informal –may be overused and unhelpful, he argued.
For example, "uncooperative" and "difficult" aren’t assigned codes in the DSM-IV or ICD-9, but they’re quasi-diagnoses used with great frequency in rounds, case conferences, and at the nursing station.
Dr. Wynn drove home the point that such terms are relative. Members of the treatment team are likely viewed as "difficult" by the very same patients who elicit the label from us. Consider the context, he counseled.
"Medical care encourages and reinforces dependency, passivity, and attention seeking," he explained.
While most people in society are able to adapt to the highly structured culture of Western medicine, grinning and bearing it in their new costumes (cotton gowns) and subservient patient roles, others may simply not possess that flexibility, particularly in the context of extreme fear, embarrassment, or mistrust.
Dr. Wynn argues that the people who are often diagnosed with personality disorders really have "adaptive failure" – a term he wishes would have made it into DSM-5. They have both a poor sense of self and a poor capacity for interpersonal functioning.
And this is a painful combination when it comes to coping with a life-threatening diagnosis in a strange new world, where amicability, conformity, and allegiance to the treatment plan are highly prized.
So how does all this play out in the clinic?
Dr. Wynn’s advice is to use our own reactions to such patients as diagnostic cues, tapping into our professionalism to guide our responses.
See each patient as unique and uniquely challenged by the circumstances of his or her disease, and respond based on what you see and feel, he advised.
Take extra time to listen to patients who fail to follow through with treatment, appear needy or overly-entitled, and even those who may "frighten or repel us."
Do fear, shame, or confusion lie at the heart of such behavior?
Could respect, collaboration, and limit setting put in check the most troubling interactions with such patients?
With Dr. Wynn’s conceptualization fresh in my mind, I was inspired to step back and put into context my own reactions to patients I find difficult.
I found myself more patient, more understanding, and more curious about the underpinnings of behaviors that play havoc with the treatment schedule and have the potential to bring out the worst in us all.
What particularly helps for me is to remember (and remind colleagues) that our shared enemy is cancer, not the patient. By seeing the patient as our ally in that fight, we are motivated to compassionately demystify our traditions and find creative bridges to connection with all types of people who need our care.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
You know that you’ve been at a valuable meeting when you can use on Monday morning the "take-home messages" you gathered over the weekend.
The annual conference of the American Psychosocial Oncology Society was just such a meeting, filled with valuable data and perspective on distress screening tools, survivorship guidance, and ways to integrate psychosocial care comfortably into the evolving medical model of cancer care.
But the lessons I put into play before I unpacked my briefcase were gleaned in a dynamic workshop led by Dr. John D. Wynn of the Swedish Cancer Institute in Seattle.
A psychiatrist, Dr. Wynn has long been regarded as an expert on the uneasy intersection of cancer and Axis II personality traits and disorders, having written the chapter on this topic in Dr. Jimmie Holland’s classic textbook Psycho-Oncology (New York: Oxford University Press, 2010).
Sorting through Axis II diagnoses, he frames Clusters A, B, and C as "weird," "wild," and "worried." Along the way, he challenged the accepted dogma that personality disorders are easily defined, enduring, and inflexible when research calls into question all of these notions.
To be sure, there are individuals whose ways of dealing with others and the world fall far outside the reassuring central band of "average." But labels – formal or informal –may be overused and unhelpful, he argued.
For example, "uncooperative" and "difficult" aren’t assigned codes in the DSM-IV or ICD-9, but they’re quasi-diagnoses used with great frequency in rounds, case conferences, and at the nursing station.
Dr. Wynn drove home the point that such terms are relative. Members of the treatment team are likely viewed as "difficult" by the very same patients who elicit the label from us. Consider the context, he counseled.
"Medical care encourages and reinforces dependency, passivity, and attention seeking," he explained.
While most people in society are able to adapt to the highly structured culture of Western medicine, grinning and bearing it in their new costumes (cotton gowns) and subservient patient roles, others may simply not possess that flexibility, particularly in the context of extreme fear, embarrassment, or mistrust.
Dr. Wynn argues that the people who are often diagnosed with personality disorders really have "adaptive failure" – a term he wishes would have made it into DSM-5. They have both a poor sense of self and a poor capacity for interpersonal functioning.
And this is a painful combination when it comes to coping with a life-threatening diagnosis in a strange new world, where amicability, conformity, and allegiance to the treatment plan are highly prized.
So how does all this play out in the clinic?
Dr. Wynn’s advice is to use our own reactions to such patients as diagnostic cues, tapping into our professionalism to guide our responses.
See each patient as unique and uniquely challenged by the circumstances of his or her disease, and respond based on what you see and feel, he advised.
Take extra time to listen to patients who fail to follow through with treatment, appear needy or overly-entitled, and even those who may "frighten or repel us."
Do fear, shame, or confusion lie at the heart of such behavior?
Could respect, collaboration, and limit setting put in check the most troubling interactions with such patients?
With Dr. Wynn’s conceptualization fresh in my mind, I was inspired to step back and put into context my own reactions to patients I find difficult.
I found myself more patient, more understanding, and more curious about the underpinnings of behaviors that play havoc with the treatment schedule and have the potential to bring out the worst in us all.
What particularly helps for me is to remember (and remind colleagues) that our shared enemy is cancer, not the patient. By seeing the patient as our ally in that fight, we are motivated to compassionately demystify our traditions and find creative bridges to connection with all types of people who need our care.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Online network offers 24/7 patient support
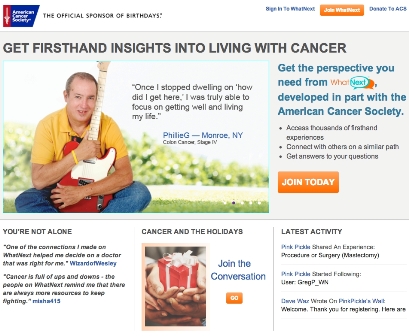
When a cancer patient from Menomonie, Wis., reached out to the American Cancer Society’s WhatNext online network seeking treatment reassurance, a ready-made support group jumped into action.
"Anyone familiar with 5-FU [5-fluorouracil], cetuximab (Erbitux), or carboplatin? Has anyone ever worn a pump?" asked Packerbacker. "New nodule revealed metastatic cancer. ... I had cisplatin in the past and tolerated it with minor side effects. I’m still nervous, though. Wish me luck?"
An optimistic response came from a user in New Jersey: "I received 5-FU via a pump. ... Not fun, more of a pain in the butt. It turned out to not be a big deal at all," wrote IKickedIt.
"Good luck!" wrote FreeBird in Tampa, Fla. "Dad had carboplatin and Taxol [paclitaxel] for his previous lung cancer. He did really well with it!"
Patients and family members of patients described how to wear the pump, how to shower with the pump (by using plastic wrap and a shower head from a home improvement store), and how to disguise the pump (in a "cute backpack," suggested one user). Another user wrote, "Just kept the pump on my desk."
The new network boasts more than 10,000 registered users who pose questions and offer suggestions on topics both weighty and mundane.
"For those of you who had both options available, how did you decide between a lumpectomy and a mastectomy?" asked a mom from Cincinnati.
"Best advice for a caregiver who gets a bad cold?" queried Iibcarolek from California. "First of all, take care of yourself," advised Fizztig, who recommended frequent hand washing, using hand sanitizer, protecting sneezes, and getting enough rest.
By negotiating the well-organized website, users will find categories where they can share their experience and journey. Categories include "Oh, No," "Celebration," "Decision Point," and "Procedure or Surgery."
The website is organized by diagnosis. There is also a Pinboard where users can search, browse, or post inspirational photos and videos (similar to Pinterest).
One photo on the Pinboard is of a young girl wearing a scarf on her head with the following note from bcncnabb: "People stare. You all know this. You’ve all felt it in restaurants, at the grocery store, or any other place where people don’t get it. ... But wow, what people don’t notice is the fight, the spirit, and the grace that it takes to live with cancer."
Dr. Len Lichtenfeld, deputy chief medical officer of the American Cancer Society (ACS), endorsed the new online tool as an "exciting" use of technology to extend the reach of both the ACS and the oncology community. "We know that people who connect with each other in times like this feel more empowered, less anxious, and can approach this crucial time in their lives feeling supported," he noted.
The website’s exchange of practical tips reminded me of a caregiver’s lament about the difficulties of managing her husband’s percutaneous endoscopic gastrostomy (PEG) tube, which even the visiting in-home nurses didn’t really understand. In the exhausting days following his discharge after surgery for esophageal cancer, she taught herself how to triple flush the tube to prevent middle-of-the-night clogs.
Today, I would refer her to WhatNext, where I’m confident she would receive (and offer) pep talks and helpful advice.
In some cases, connections formed on WhatNext have led to offline friendships. The ACS highlighted a bond formed between MGM48 and MichaelV, both of whom have prostate cancer. "We compare notes and discuss life with cancer," said MGM48. "I’m happy to have a live sounding board sometimes.
"Let’s face it, no one outside really completely gets it," he said.
Dr. Freed is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
When a cancer patient from Menomonie, Wis., reached out to the American Cancer Society’s WhatNext online network seeking treatment reassurance, a ready-made support group jumped into action.
"Anyone familiar with 5-FU [5-fluorouracil], cetuximab (Erbitux), or carboplatin? Has anyone ever worn a pump?" asked Packerbacker. "New nodule revealed metastatic cancer. ... I had cisplatin in the past and tolerated it with minor side effects. I’m still nervous, though. Wish me luck?"
An optimistic response came from a user in New Jersey: "I received 5-FU via a pump. ... Not fun, more of a pain in the butt. It turned out to not be a big deal at all," wrote IKickedIt.
"Good luck!" wrote FreeBird in Tampa, Fla. "Dad had carboplatin and Taxol [paclitaxel] for his previous lung cancer. He did really well with it!"
Patients and family members of patients described how to wear the pump, how to shower with the pump (by using plastic wrap and a shower head from a home improvement store), and how to disguise the pump (in a "cute backpack," suggested one user). Another user wrote, "Just kept the pump on my desk."
The new network boasts more than 10,000 registered users who pose questions and offer suggestions on topics both weighty and mundane.
"For those of you who had both options available, how did you decide between a lumpectomy and a mastectomy?" asked a mom from Cincinnati.
"Best advice for a caregiver who gets a bad cold?" queried Iibcarolek from California. "First of all, take care of yourself," advised Fizztig, who recommended frequent hand washing, using hand sanitizer, protecting sneezes, and getting enough rest.
By negotiating the well-organized website, users will find categories where they can share their experience and journey. Categories include "Oh, No," "Celebration," "Decision Point," and "Procedure or Surgery."
The website is organized by diagnosis. There is also a Pinboard where users can search, browse, or post inspirational photos and videos (similar to Pinterest).
One photo on the Pinboard is of a young girl wearing a scarf on her head with the following note from bcncnabb: "People stare. You all know this. You’ve all felt it in restaurants, at the grocery store, or any other place where people don’t get it. ... But wow, what people don’t notice is the fight, the spirit, and the grace that it takes to live with cancer."
Dr. Len Lichtenfeld, deputy chief medical officer of the American Cancer Society (ACS), endorsed the new online tool as an "exciting" use of technology to extend the reach of both the ACS and the oncology community. "We know that people who connect with each other in times like this feel more empowered, less anxious, and can approach this crucial time in their lives feeling supported," he noted.
The website’s exchange of practical tips reminded me of a caregiver’s lament about the difficulties of managing her husband’s percutaneous endoscopic gastrostomy (PEG) tube, which even the visiting in-home nurses didn’t really understand. In the exhausting days following his discharge after surgery for esophageal cancer, she taught herself how to triple flush the tube to prevent middle-of-the-night clogs.
Today, I would refer her to WhatNext, where I’m confident she would receive (and offer) pep talks and helpful advice.
In some cases, connections formed on WhatNext have led to offline friendships. The ACS highlighted a bond formed between MGM48 and MichaelV, both of whom have prostate cancer. "We compare notes and discuss life with cancer," said MGM48. "I’m happy to have a live sounding board sometimes.
"Let’s face it, no one outside really completely gets it," he said.
Dr. Freed is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
When a cancer patient from Menomonie, Wis., reached out to the American Cancer Society’s WhatNext online network seeking treatment reassurance, a ready-made support group jumped into action.
"Anyone familiar with 5-FU [5-fluorouracil], cetuximab (Erbitux), or carboplatin? Has anyone ever worn a pump?" asked Packerbacker. "New nodule revealed metastatic cancer. ... I had cisplatin in the past and tolerated it with minor side effects. I’m still nervous, though. Wish me luck?"
An optimistic response came from a user in New Jersey: "I received 5-FU via a pump. ... Not fun, more of a pain in the butt. It turned out to not be a big deal at all," wrote IKickedIt.
"Good luck!" wrote FreeBird in Tampa, Fla. "Dad had carboplatin and Taxol [paclitaxel] for his previous lung cancer. He did really well with it!"
Patients and family members of patients described how to wear the pump, how to shower with the pump (by using plastic wrap and a shower head from a home improvement store), and how to disguise the pump (in a "cute backpack," suggested one user). Another user wrote, "Just kept the pump on my desk."
The new network boasts more than 10,000 registered users who pose questions and offer suggestions on topics both weighty and mundane.
"For those of you who had both options available, how did you decide between a lumpectomy and a mastectomy?" asked a mom from Cincinnati.
"Best advice for a caregiver who gets a bad cold?" queried Iibcarolek from California. "First of all, take care of yourself," advised Fizztig, who recommended frequent hand washing, using hand sanitizer, protecting sneezes, and getting enough rest.
By negotiating the well-organized website, users will find categories where they can share their experience and journey. Categories include "Oh, No," "Celebration," "Decision Point," and "Procedure or Surgery."
The website is organized by diagnosis. There is also a Pinboard where users can search, browse, or post inspirational photos and videos (similar to Pinterest).
One photo on the Pinboard is of a young girl wearing a scarf on her head with the following note from bcncnabb: "People stare. You all know this. You’ve all felt it in restaurants, at the grocery store, or any other place where people don’t get it. ... But wow, what people don’t notice is the fight, the spirit, and the grace that it takes to live with cancer."
Dr. Len Lichtenfeld, deputy chief medical officer of the American Cancer Society (ACS), endorsed the new online tool as an "exciting" use of technology to extend the reach of both the ACS and the oncology community. "We know that people who connect with each other in times like this feel more empowered, less anxious, and can approach this crucial time in their lives feeling supported," he noted.
The website’s exchange of practical tips reminded me of a caregiver’s lament about the difficulties of managing her husband’s percutaneous endoscopic gastrostomy (PEG) tube, which even the visiting in-home nurses didn’t really understand. In the exhausting days following his discharge after surgery for esophageal cancer, she taught herself how to triple flush the tube to prevent middle-of-the-night clogs.
Today, I would refer her to WhatNext, where I’m confident she would receive (and offer) pep talks and helpful advice.
In some cases, connections formed on WhatNext have led to offline friendships. The ACS highlighted a bond formed between MGM48 and MichaelV, both of whom have prostate cancer. "We compare notes and discuss life with cancer," said MGM48. "I’m happy to have a live sounding board sometimes.
"Let’s face it, no one outside really completely gets it," he said.
Dr. Freed is a clinical psychologist in Santa Barbara, Calif., and a medical journalist.
Thoughts on tragedy, beauty, and perspective
Writing the "Vitality Signs" column is part of my routine, and naturally offers me the opportunity to ponder and put forth reassuringly straightforward numbers. Statistical significances, clinically meaningful results. Each potentially offers insight, in some small way, about how the oncology community might ease the distress or minimize the suffering or sorrow of an individual or a family stricken by cancer.
It is the sort of task that psychologists like myself recommend to those in the aftermath of a tragedy such as the inexplicable shooting of 20 first-graders and their heroic protectors in a bucolic Connecticut town.
Grieve, yes. Pull your family close.
But do not spend hours watching network coverage, as minuscule facts or fallacies emerge, the latter to be discounted later in the interminable cycle of televised despair.
Minimize exposure to the event unless there is something tangible you can do.
Focus on your routine.
Concentrate on positive ways that can make a difference in your world.
I heard the heartbreaking news, as you likely did, in the middle of a busy workday filled with the day-to-day challenges, personal tragedies, and yes, small joys, that cancer leaves in its wake.
I noticed that my colleagues and coworkers spoke of the horror unfolding in hushed tones, behind closed doors, tears brimming. I later wondered if we were trying to protect some of our patients, even for a brief time, from having to hear of sadnesses beyond the ones they bravely faced that day. For others, perhaps celebrating a last day of chemotherapy or radiation treatment, we may have felt compelled to preserve their relief: Freezing in time a moment of silent peace in the village snow globe before the storm swirls once again.
Tragedy spares no one who works in the field of oncology.
It is a specialty infused by existential questions; why heartache touched this family, why so young, why so poor a prognosis or response.
Yet we have in common with each other, our patients – even feuding family members – a simple enemy, cancer. It’s the bad guy who rallies us in solidarity. When it wins, the ending at least is in accordance with life’s rhythm of beginnings and endings, birth followed by life and then a natural, if not always easy, death. Ideally, peace comes at last to the family, who may have been there to offer comfort, perhaps to a 6-year-old.
I spoke recently with a gifted pediatric hospice nurse who described moments of beauty, relief, and spiritual connection in such exquisitely painful bedside moments.
The memory of that conversation only deepens my sorrow about the 6-year-olds who died in horror at Sandy Hook Elementary School.
I’m sure that people ask you, not infrequently, how you could have chosen oncology as your specialty, when so many specialties would keep you far from the angst that is a part of that world. Of course, such questioners do not consider the joy of an unexpected remission; the deep connections reforged between family members once estranged; the bravery, grace, and abiding loyalty unfolding before us every day.
As the days have passed since the Connecticut tragedy, I hope you have been especially attuned to the indescribable beauty in the world of your chosen calling. I hope you are gratefully holding your family close. I hope you see clearly now the difference you make to the world in ways great and small.
Dr. Freed is a clinical psychologist in Santa Barbara, California, and a medical journalist. This column "Vitality Signs," appears regularly in The Oncology Report. Visit www.oncologyreport.com to see what is new in Vitality Signs.
Writing the "Vitality Signs" column is part of my routine, and naturally offers me the opportunity to ponder and put forth reassuringly straightforward numbers. Statistical significances, clinically meaningful results. Each potentially offers insight, in some small way, about how the oncology community might ease the distress or minimize the suffering or sorrow of an individual or a family stricken by cancer.
It is the sort of task that psychologists like myself recommend to those in the aftermath of a tragedy such as the inexplicable shooting of 20 first-graders and their heroic protectors in a bucolic Connecticut town.
Grieve, yes. Pull your family close.
But do not spend hours watching network coverage, as minuscule facts or fallacies emerge, the latter to be discounted later in the interminable cycle of televised despair.
Minimize exposure to the event unless there is something tangible you can do.
Focus on your routine.
Concentrate on positive ways that can make a difference in your world.
I heard the heartbreaking news, as you likely did, in the middle of a busy workday filled with the day-to-day challenges, personal tragedies, and yes, small joys, that cancer leaves in its wake.
I noticed that my colleagues and coworkers spoke of the horror unfolding in hushed tones, behind closed doors, tears brimming. I later wondered if we were trying to protect some of our patients, even for a brief time, from having to hear of sadnesses beyond the ones they bravely faced that day. For others, perhaps celebrating a last day of chemotherapy or radiation treatment, we may have felt compelled to preserve their relief: Freezing in time a moment of silent peace in the village snow globe before the storm swirls once again.
Tragedy spares no one who works in the field of oncology.
It is a specialty infused by existential questions; why heartache touched this family, why so young, why so poor a prognosis or response.
Yet we have in common with each other, our patients – even feuding family members – a simple enemy, cancer. It’s the bad guy who rallies us in solidarity. When it wins, the ending at least is in accordance with life’s rhythm of beginnings and endings, birth followed by life and then a natural, if not always easy, death. Ideally, peace comes at last to the family, who may have been there to offer comfort, perhaps to a 6-year-old.
I spoke recently with a gifted pediatric hospice nurse who described moments of beauty, relief, and spiritual connection in such exquisitely painful bedside moments.
The memory of that conversation only deepens my sorrow about the 6-year-olds who died in horror at Sandy Hook Elementary School.
I’m sure that people ask you, not infrequently, how you could have chosen oncology as your specialty, when so many specialties would keep you far from the angst that is a part of that world. Of course, such questioners do not consider the joy of an unexpected remission; the deep connections reforged between family members once estranged; the bravery, grace, and abiding loyalty unfolding before us every day.
As the days have passed since the Connecticut tragedy, I hope you have been especially attuned to the indescribable beauty in the world of your chosen calling. I hope you are gratefully holding your family close. I hope you see clearly now the difference you make to the world in ways great and small.
Dr. Freed is a clinical psychologist in Santa Barbara, California, and a medical journalist. This column "Vitality Signs," appears regularly in The Oncology Report. Visit www.oncologyreport.com to see what is new in Vitality Signs.
Writing the "Vitality Signs" column is part of my routine, and naturally offers me the opportunity to ponder and put forth reassuringly straightforward numbers. Statistical significances, clinically meaningful results. Each potentially offers insight, in some small way, about how the oncology community might ease the distress or minimize the suffering or sorrow of an individual or a family stricken by cancer.
It is the sort of task that psychologists like myself recommend to those in the aftermath of a tragedy such as the inexplicable shooting of 20 first-graders and their heroic protectors in a bucolic Connecticut town.
Grieve, yes. Pull your family close.
But do not spend hours watching network coverage, as minuscule facts or fallacies emerge, the latter to be discounted later in the interminable cycle of televised despair.
Minimize exposure to the event unless there is something tangible you can do.
Focus on your routine.
Concentrate on positive ways that can make a difference in your world.
I heard the heartbreaking news, as you likely did, in the middle of a busy workday filled with the day-to-day challenges, personal tragedies, and yes, small joys, that cancer leaves in its wake.
I noticed that my colleagues and coworkers spoke of the horror unfolding in hushed tones, behind closed doors, tears brimming. I later wondered if we were trying to protect some of our patients, even for a brief time, from having to hear of sadnesses beyond the ones they bravely faced that day. For others, perhaps celebrating a last day of chemotherapy or radiation treatment, we may have felt compelled to preserve their relief: Freezing in time a moment of silent peace in the village snow globe before the storm swirls once again.
Tragedy spares no one who works in the field of oncology.
It is a specialty infused by existential questions; why heartache touched this family, why so young, why so poor a prognosis or response.
Yet we have in common with each other, our patients – even feuding family members – a simple enemy, cancer. It’s the bad guy who rallies us in solidarity. When it wins, the ending at least is in accordance with life’s rhythm of beginnings and endings, birth followed by life and then a natural, if not always easy, death. Ideally, peace comes at last to the family, who may have been there to offer comfort, perhaps to a 6-year-old.
I spoke recently with a gifted pediatric hospice nurse who described moments of beauty, relief, and spiritual connection in such exquisitely painful bedside moments.
The memory of that conversation only deepens my sorrow about the 6-year-olds who died in horror at Sandy Hook Elementary School.
I’m sure that people ask you, not infrequently, how you could have chosen oncology as your specialty, when so many specialties would keep you far from the angst that is a part of that world. Of course, such questioners do not consider the joy of an unexpected remission; the deep connections reforged between family members once estranged; the bravery, grace, and abiding loyalty unfolding before us every day.
As the days have passed since the Connecticut tragedy, I hope you have been especially attuned to the indescribable beauty in the world of your chosen calling. I hope you are gratefully holding your family close. I hope you see clearly now the difference you make to the world in ways great and small.
Dr. Freed is a clinical psychologist in Santa Barbara, California, and a medical journalist. This column "Vitality Signs," appears regularly in The Oncology Report. Visit www.oncologyreport.com to see what is new in Vitality Signs.
Lung Cancer Adjustment
They’re the guilt cancers: lung cancer in smokers, oral cancers originating at the very spot where a wad of tobacco nested for years, advanced-stage colorectal cancer in patients who long neglected ominous but obvious signs such as hematochezia.
The festering emotional consequences of self-blame play out in the clinic in many forms: denial, displaced anger at staff, and passive self-harm through noncompliance.
But the question lingers: How can we help these guilt-ridden patients refocus their gaze from the rearview mirror to the path ahead?
Although it may be necessary and cathartic for a patient to acknowledge and process the role that behavior may have played in the development of his or her cancer, the ultimate goal, it seems to me, must be to help such patients muster the energy to cope with challenges here and now.
A recent study may help the oncology community toward that end (Ann. Behav. Med. 2012;44:331-40).
Dr. Kathrin Milbury of the University of Texas MD Anderson Cancer Center, Houston, and her associates studied 158 couples, illuminating not only the potent negative impact of guilt on adjustment to lung cancer but offering a fresh and revealing perspective on inward self-blame’s evil twin – reproach from a spouse.
Among the participants, 85.4% acknowledged a smoking history, with 46.7% blaming themselves "very much" or "completely" for engaging in behaviors that may have contributed to their cancer. Spouses were less likely to blame patients at that early stage of treatment, but when it was present, blame was far more powerfully correlated with distress (P less than .0001), versus P less than .05 for patients who blamed themselves for their disease.
Nonsmoking spouses were more likely to blame patients for their cancer; current or recent smokers were more likely to be blamed than former smokers.
Among patients, the researchers found that a good marriage was protective, mediating the link between blame and distress. (Ratings of marital adjustment were based on measures of consensus, satisfaction, cohesion, and affectionate expression.)
Not so for spouses. Regardless of dyadic adjustment, high levels of blame were associated with sharply increased distress. Only having a good support network helped to reduce distress, but only marginally.
"Prospectively, there was no buffering effect for patients or spouses," the researchers said. "In fact, initial blame predicted later distress above and beyond initial distress emphasizing the lingering harmful effects of blame in couples."
The authors offered a number of important insights into their findings.
"Blaming one’s loved one for developing a life-threatening disease may be particularly psychologically taxing because spouses may experience conflicting cognition and emotions (e.g. anger, guilt, fear, worry, compassion, and affection)," they noted.
Moreover, previous research (Oncol. Nurs. Forum 2008;35:681-9) demonstrates that "caregivers who blamed the patient for developing cancer were more likely to experience negative affect, which in turn was associated with fewer helping behaviors," Dr. Milbury and her associates wrote.
The importance of early, couple-based interventions, especially among current or recent smokers, was emphasized by the authors to hopefully prevent "a snowballing effect of distress."
Cognitive restructuring, helping patients and spouses to connect with supportive networks, and bolstering marital communication all were seen as important and viable interventions to help couples acknowledge and work through the toxic issue of blame.
"It is possible that in well-functioning marriages, couples have discussions about the cancer cause in a factual, non-accusatory manner, as opposed to displaying derogatory or punitive behaviors," the investigators said.
Helping to foster such communication might be seen as a key goal of interventions with couples.
"Rather than holding back distressing thoughts and emotions, teaching couples how to mutually engage and openly exchange thoughts, concerns, and feelings with the goal of providing and receiving support may be particularly effective components of such programs," they suggested.
Certainly, this new study points to potential benefits for the patient with such an approach. For spouses, whose blame-linked distress persisted despite the health of their marriages, better links to supportive networks may offer relief.
As with so many aspects of emotional adjustment in cancer patients and their families, more research is needed.
But it is valuable for all in the oncology community to know that blame – whether self-directed or coming from a spouse – may seriously complicate emotional perspective and care in the "guilt cancers" well beyond their physical toll.
Dr. Freed is a clinical psychologist in Santa Barbara, Calif., and a medical journalist. This column "Vitality Signs," appears regularly in The Oncology Report. Visit www.oncologyreport.com to see what is new in Vitality Signs.
They’re the guilt cancers: lung cancer in smokers, oral cancers originating at the very spot where a wad of tobacco nested for years, advanced-stage colorectal cancer in patients who long neglected ominous but obvious signs such as hematochezia.
The festering emotional consequences of self-blame play out in the clinic in many forms: denial, displaced anger at staff, and passive self-harm through noncompliance.
But the question lingers: How can we help these guilt-ridden patients refocus their gaze from the rearview mirror to the path ahead?
Although it may be necessary and cathartic for a patient to acknowledge and process the role that behavior may have played in the development of his or her cancer, the ultimate goal, it seems to me, must be to help such patients muster the energy to cope with challenges here and now.
A recent study may help the oncology community toward that end (Ann. Behav. Med. 2012;44:331-40).
Dr. Kathrin Milbury of the University of Texas MD Anderson Cancer Center, Houston, and her associates studied 158 couples, illuminating not only the potent negative impact of guilt on adjustment to lung cancer but offering a fresh and revealing perspective on inward self-blame’s evil twin – reproach from a spouse.
Among the participants, 85.4% acknowledged a smoking history, with 46.7% blaming themselves "very much" or "completely" for engaging in behaviors that may have contributed to their cancer. Spouses were less likely to blame patients at that early stage of treatment, but when it was present, blame was far more powerfully correlated with distress (P less than .0001), versus P less than .05 for patients who blamed themselves for their disease.
Nonsmoking spouses were more likely to blame patients for their cancer; current or recent smokers were more likely to be blamed than former smokers.
Among patients, the researchers found that a good marriage was protective, mediating the link between blame and distress. (Ratings of marital adjustment were based on measures of consensus, satisfaction, cohesion, and affectionate expression.)
Not so for spouses. Regardless of dyadic adjustment, high levels of blame were associated with sharply increased distress. Only having a good support network helped to reduce distress, but only marginally.
"Prospectively, there was no buffering effect for patients or spouses," the researchers said. "In fact, initial blame predicted later distress above and beyond initial distress emphasizing the lingering harmful effects of blame in couples."
The authors offered a number of important insights into their findings.
"Blaming one’s loved one for developing a life-threatening disease may be particularly psychologically taxing because spouses may experience conflicting cognition and emotions (e.g. anger, guilt, fear, worry, compassion, and affection)," they noted.
Moreover, previous research (Oncol. Nurs. Forum 2008;35:681-9) demonstrates that "caregivers who blamed the patient for developing cancer were more likely to experience negative affect, which in turn was associated with fewer helping behaviors," Dr. Milbury and her associates wrote.
The importance of early, couple-based interventions, especially among current or recent smokers, was emphasized by the authors to hopefully prevent "a snowballing effect of distress."
Cognitive restructuring, helping patients and spouses to connect with supportive networks, and bolstering marital communication all were seen as important and viable interventions to help couples acknowledge and work through the toxic issue of blame.
"It is possible that in well-functioning marriages, couples have discussions about the cancer cause in a factual, non-accusatory manner, as opposed to displaying derogatory or punitive behaviors," the investigators said.
Helping to foster such communication might be seen as a key goal of interventions with couples.
"Rather than holding back distressing thoughts and emotions, teaching couples how to mutually engage and openly exchange thoughts, concerns, and feelings with the goal of providing and receiving support may be particularly effective components of such programs," they suggested.
Certainly, this new study points to potential benefits for the patient with such an approach. For spouses, whose blame-linked distress persisted despite the health of their marriages, better links to supportive networks may offer relief.
As with so many aspects of emotional adjustment in cancer patients and their families, more research is needed.
But it is valuable for all in the oncology community to know that blame – whether self-directed or coming from a spouse – may seriously complicate emotional perspective and care in the "guilt cancers" well beyond their physical toll.
Dr. Freed is a clinical psychologist in Santa Barbara, Calif., and a medical journalist. This column "Vitality Signs," appears regularly in The Oncology Report. Visit www.oncologyreport.com to see what is new in Vitality Signs.
They’re the guilt cancers: lung cancer in smokers, oral cancers originating at the very spot where a wad of tobacco nested for years, advanced-stage colorectal cancer in patients who long neglected ominous but obvious signs such as hematochezia.
The festering emotional consequences of self-blame play out in the clinic in many forms: denial, displaced anger at staff, and passive self-harm through noncompliance.
But the question lingers: How can we help these guilt-ridden patients refocus their gaze from the rearview mirror to the path ahead?
Although it may be necessary and cathartic for a patient to acknowledge and process the role that behavior may have played in the development of his or her cancer, the ultimate goal, it seems to me, must be to help such patients muster the energy to cope with challenges here and now.
A recent study may help the oncology community toward that end (Ann. Behav. Med. 2012;44:331-40).
Dr. Kathrin Milbury of the University of Texas MD Anderson Cancer Center, Houston, and her associates studied 158 couples, illuminating not only the potent negative impact of guilt on adjustment to lung cancer but offering a fresh and revealing perspective on inward self-blame’s evil twin – reproach from a spouse.
Among the participants, 85.4% acknowledged a smoking history, with 46.7% blaming themselves "very much" or "completely" for engaging in behaviors that may have contributed to their cancer. Spouses were less likely to blame patients at that early stage of treatment, but when it was present, blame was far more powerfully correlated with distress (P less than .0001), versus P less than .05 for patients who blamed themselves for their disease.
Nonsmoking spouses were more likely to blame patients for their cancer; current or recent smokers were more likely to be blamed than former smokers.
Among patients, the researchers found that a good marriage was protective, mediating the link between blame and distress. (Ratings of marital adjustment were based on measures of consensus, satisfaction, cohesion, and affectionate expression.)
Not so for spouses. Regardless of dyadic adjustment, high levels of blame were associated with sharply increased distress. Only having a good support network helped to reduce distress, but only marginally.
"Prospectively, there was no buffering effect for patients or spouses," the researchers said. "In fact, initial blame predicted later distress above and beyond initial distress emphasizing the lingering harmful effects of blame in couples."
The authors offered a number of important insights into their findings.
"Blaming one’s loved one for developing a life-threatening disease may be particularly psychologically taxing because spouses may experience conflicting cognition and emotions (e.g. anger, guilt, fear, worry, compassion, and affection)," they noted.
Moreover, previous research (Oncol. Nurs. Forum 2008;35:681-9) demonstrates that "caregivers who blamed the patient for developing cancer were more likely to experience negative affect, which in turn was associated with fewer helping behaviors," Dr. Milbury and her associates wrote.
The importance of early, couple-based interventions, especially among current or recent smokers, was emphasized by the authors to hopefully prevent "a snowballing effect of distress."
Cognitive restructuring, helping patients and spouses to connect with supportive networks, and bolstering marital communication all were seen as important and viable interventions to help couples acknowledge and work through the toxic issue of blame.
"It is possible that in well-functioning marriages, couples have discussions about the cancer cause in a factual, non-accusatory manner, as opposed to displaying derogatory or punitive behaviors," the investigators said.
Helping to foster such communication might be seen as a key goal of interventions with couples.
"Rather than holding back distressing thoughts and emotions, teaching couples how to mutually engage and openly exchange thoughts, concerns, and feelings with the goal of providing and receiving support may be particularly effective components of such programs," they suggested.
Certainly, this new study points to potential benefits for the patient with such an approach. For spouses, whose blame-linked distress persisted despite the health of their marriages, better links to supportive networks may offer relief.
As with so many aspects of emotional adjustment in cancer patients and their families, more research is needed.
But it is valuable for all in the oncology community to know that blame – whether self-directed or coming from a spouse – may seriously complicate emotional perspective and care in the "guilt cancers" well beyond their physical toll.
Dr. Freed is a clinical psychologist in Santa Barbara, Calif., and a medical journalist. This column "Vitality Signs," appears regularly in The Oncology Report. Visit www.oncologyreport.com to see what is new in Vitality Signs.
Snapshot after diagnosis: prevalence of distress varies by cancer, gender, and age
Meaningful research on interventions for distress among cancer patients has long been hamstrung by the dearth of solid science about the true prevalence of anxiety and depression in this population.
Reported prevalence rates for depression in cancer patients range wildly and utterly unhelpfully from 0 to 38% for major depression in one study and from 0 to 46% in another. Trying to pin down the prevalence for anxiety is nerve-rackingly vague as well, with reported rates between 1% and 49%.
How many cancer patients cope well – and how many suffer the toxic toll of anxiety and depression, which have the potential to compromise compliance, undermine quality of life, and drive excess mortality?
A refreshingly comprehensive look at distress prevalence shortly after diagnosis has recently been published by a group of researchers at the University of British Columbia and the BC [British Columbia] Cancer Agency, (J. Affect. Disord. 2012;141:343-51 [doi:10.1016/j.jad.2012.03.025]).
A study of 10,153 consecutive cancer patients found that shortly after diagnosis, approximately 19% met diagnostic criteria for an anxiety disorder and nearly 13% for clinical depression, with an additional 22.6% and 16.5% demonstrating symptoms of anxiety and depression, respectively.
The numbers offer just a snapshot in time, capturing the period in which individuals are coping with the fresh news of a diagnosis, but before the rigors of treatment or recurrence have begun to weigh upon them.
For patients with diagnoses facing brutal but promising treatments (head and neck patients come to mind), this portrait may appear misleadingly rosy. Indeed, compared with the "grand mean," head and neck cancer patients were less depressed at the time of the study than patients with other forms of cancer.
Had the patients been examined weeks or months into treatment, when some could no longer eat or speak normally, the rates of depression might have been far different.
And yet, how valuable to have a foothold into the prevalence conundrum. I highly recommend reading the study itself for insight into differences among patients by age, cancer type, and gender.
But here’s the Cliff Notes version:
Patients with lung, gynecologic, and hematologic cancer had the highest levels of distress at the time of diagnosis.
Women were significantly more distressed than were men across all cancer types, paralleling findings among adults who do not have cancer. As the authors note, "This gender difference may reflect a ... difference in willingness to report distress but could also arise because women tend to use emotional approach coping."
In some forms of cancer, the gender disparity was especially pronounced.
For example, women with hematologic cancers were the most distressed of all patients, with rates of anxiety two standard deviations above the \"grand mean\" and rates of depression far higher than average newly diagnosed patients in the study. Men with hematologic cancers, on the other hand, were far closer to the average in terms of depression, while their anxiety rates were actually lower than grand mean.
Age also mattered.
In a finding that has become increasingly consistent in research populations, younger adults with cancer reported more distress than did older adults. Although little difference was seen among patients with poor-prognosis cancers, in most cases, a diagnosis in patients younger than age 50 years revealed higher rates of both anxiety and depression.
Finally, the authors offered a caveat that their overall findings reflect emotional well-being in Canada’s economically protective vacuum, suggesting that in the United States, where a diagnosis can have catastrophic economical consequences, rates of depression and anxiety may be higher.
In their words, "We also need to sensitize readers to the fact that the data reflect the population of one limited geographical area in which all patients receive a similar quality of universal, third-party paid health care. Cancer may be perceived as an even greater threat when patients worry about medical bills that may exceed their resources, or have reason to fear loss of a job if their country of residence does not provide illness leaves and disability pensions."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Meaningful research on interventions for distress among cancer patients has long been hamstrung by the dearth of solid science about the true prevalence of anxiety and depression in this population.
Reported prevalence rates for depression in cancer patients range wildly and utterly unhelpfully from 0 to 38% for major depression in one study and from 0 to 46% in another. Trying to pin down the prevalence for anxiety is nerve-rackingly vague as well, with reported rates between 1% and 49%.
How many cancer patients cope well – and how many suffer the toxic toll of anxiety and depression, which have the potential to compromise compliance, undermine quality of life, and drive excess mortality?
A refreshingly comprehensive look at distress prevalence shortly after diagnosis has recently been published by a group of researchers at the University of British Columbia and the BC [British Columbia] Cancer Agency, (J. Affect. Disord. 2012;141:343-51 [doi:10.1016/j.jad.2012.03.025]).
A study of 10,153 consecutive cancer patients found that shortly after diagnosis, approximately 19% met diagnostic criteria for an anxiety disorder and nearly 13% for clinical depression, with an additional 22.6% and 16.5% demonstrating symptoms of anxiety and depression, respectively.
The numbers offer just a snapshot in time, capturing the period in which individuals are coping with the fresh news of a diagnosis, but before the rigors of treatment or recurrence have begun to weigh upon them.
For patients with diagnoses facing brutal but promising treatments (head and neck patients come to mind), this portrait may appear misleadingly rosy. Indeed, compared with the "grand mean," head and neck cancer patients were less depressed at the time of the study than patients with other forms of cancer.
Had the patients been examined weeks or months into treatment, when some could no longer eat or speak normally, the rates of depression might have been far different.
And yet, how valuable to have a foothold into the prevalence conundrum. I highly recommend reading the study itself for insight into differences among patients by age, cancer type, and gender.
But here’s the Cliff Notes version:
Patients with lung, gynecologic, and hematologic cancer had the highest levels of distress at the time of diagnosis.
Women were significantly more distressed than were men across all cancer types, paralleling findings among adults who do not have cancer. As the authors note, "This gender difference may reflect a ... difference in willingness to report distress but could also arise because women tend to use emotional approach coping."
In some forms of cancer, the gender disparity was especially pronounced.
For example, women with hematologic cancers were the most distressed of all patients, with rates of anxiety two standard deviations above the \"grand mean\" and rates of depression far higher than average newly diagnosed patients in the study. Men with hematologic cancers, on the other hand, were far closer to the average in terms of depression, while their anxiety rates were actually lower than grand mean.
Age also mattered.
In a finding that has become increasingly consistent in research populations, younger adults with cancer reported more distress than did older adults. Although little difference was seen among patients with poor-prognosis cancers, in most cases, a diagnosis in patients younger than age 50 years revealed higher rates of both anxiety and depression.
Finally, the authors offered a caveat that their overall findings reflect emotional well-being in Canada’s economically protective vacuum, suggesting that in the United States, where a diagnosis can have catastrophic economical consequences, rates of depression and anxiety may be higher.
In their words, "We also need to sensitize readers to the fact that the data reflect the population of one limited geographical area in which all patients receive a similar quality of universal, third-party paid health care. Cancer may be perceived as an even greater threat when patients worry about medical bills that may exceed their resources, or have reason to fear loss of a job if their country of residence does not provide illness leaves and disability pensions."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Meaningful research on interventions for distress among cancer patients has long been hamstrung by the dearth of solid science about the true prevalence of anxiety and depression in this population.
Reported prevalence rates for depression in cancer patients range wildly and utterly unhelpfully from 0 to 38% for major depression in one study and from 0 to 46% in another. Trying to pin down the prevalence for anxiety is nerve-rackingly vague as well, with reported rates between 1% and 49%.
How many cancer patients cope well – and how many suffer the toxic toll of anxiety and depression, which have the potential to compromise compliance, undermine quality of life, and drive excess mortality?
A refreshingly comprehensive look at distress prevalence shortly after diagnosis has recently been published by a group of researchers at the University of British Columbia and the BC [British Columbia] Cancer Agency, (J. Affect. Disord. 2012;141:343-51 [doi:10.1016/j.jad.2012.03.025]).
A study of 10,153 consecutive cancer patients found that shortly after diagnosis, approximately 19% met diagnostic criteria for an anxiety disorder and nearly 13% for clinical depression, with an additional 22.6% and 16.5% demonstrating symptoms of anxiety and depression, respectively.
The numbers offer just a snapshot in time, capturing the period in which individuals are coping with the fresh news of a diagnosis, but before the rigors of treatment or recurrence have begun to weigh upon them.
For patients with diagnoses facing brutal but promising treatments (head and neck patients come to mind), this portrait may appear misleadingly rosy. Indeed, compared with the "grand mean," head and neck cancer patients were less depressed at the time of the study than patients with other forms of cancer.
Had the patients been examined weeks or months into treatment, when some could no longer eat or speak normally, the rates of depression might have been far different.
And yet, how valuable to have a foothold into the prevalence conundrum. I highly recommend reading the study itself for insight into differences among patients by age, cancer type, and gender.
But here’s the Cliff Notes version:
Patients with lung, gynecologic, and hematologic cancer had the highest levels of distress at the time of diagnosis.
Women were significantly more distressed than were men across all cancer types, paralleling findings among adults who do not have cancer. As the authors note, "This gender difference may reflect a ... difference in willingness to report distress but could also arise because women tend to use emotional approach coping."
In some forms of cancer, the gender disparity was especially pronounced.
For example, women with hematologic cancers were the most distressed of all patients, with rates of anxiety two standard deviations above the \"grand mean\" and rates of depression far higher than average newly diagnosed patients in the study. Men with hematologic cancers, on the other hand, were far closer to the average in terms of depression, while their anxiety rates were actually lower than grand mean.
Age also mattered.
In a finding that has become increasingly consistent in research populations, younger adults with cancer reported more distress than did older adults. Although little difference was seen among patients with poor-prognosis cancers, in most cases, a diagnosis in patients younger than age 50 years revealed higher rates of both anxiety and depression.
Finally, the authors offered a caveat that their overall findings reflect emotional well-being in Canada’s economically protective vacuum, suggesting that in the United States, where a diagnosis can have catastrophic economical consequences, rates of depression and anxiety may be higher.
In their words, "We also need to sensitize readers to the fact that the data reflect the population of one limited geographical area in which all patients receive a similar quality of universal, third-party paid health care. Cancer may be perceived as an even greater threat when patients worry about medical bills that may exceed their resources, or have reason to fear loss of a job if their country of residence does not provide illness leaves and disability pensions."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Predicting QoL in Colorectal Cancer Survivors
In history, economics, and psychology, it’s a fundamental truth: Past events/performance/behavior predicts future events/performance/behavior. And that’s the lesson we can take from an important Australian study of colorectal cancer survivors in the journal Quality of Life Research (2012;21:1551-64).
As noted by the authors, well-designed prospective studies about the long-term adjustment of colorectal cancer survivors are few and far between. That’s why they set about prospectively following a population-based cohort of colorectal cancer patients from 5 months post diagnosis to 5 years post diagnosis, determining the trajectory of their adjustment and quality of life (QoL) from a sociodemographic, medical, and psychological perspective.
What did they find?
"Importantly," they wrote, "the strongest predictor for long-term outcomes was the baseline values of health-related and global quality of life soon after diagnosis."
In other words, patients who at 5 months post diagnosis were optimistic, euthymic, and secure financially and in their relationships, continued to do well at 5 years.
It makes sense.
But the data also held key lessons about identifying people most likely to take their diagnosis in stride, seek out and receive good care, and then rebound – versus individuals at the other end of the spectrum, who started out not coping well and failed to rally.
First, some details about the study. Led by Professor Suzanne K. Chambers, a health psychologist at Griffith University’s Gold Coast campus in Queensland, Australia, the research team mined the Queensland cancer registry to locate 1,825 adults who were diagnosed with colorectal cancer during 2003-2004 and had signed up for a longitudinal study shortly after diagnosis.
Five months into their treatment, participants filled out an extensive battery designed to measure demographic variables, disease stage, treatment, physical function, cancer threat appraisal (how the disease affected their sense of identity, relationships, and perceptions of the future), perceived social support, quality of life, and psychological distress (depression, anxiety, and somatization).
Five years later, 259 (21.2%) had died.
Others were not included in the study because they failed to complete follow-up paperwork; notably, these nonresponders tended to be male, younger, uninsured, and smokers, and tended to have more advanced cancer, a more negative view of the threat of their cancer, higher somatization, and lower FACT-C (Functional Assessment of Cancer Therapy – Colorectal) scores. In other words, this group was fairly reflective of those patients who had not fared well on follow-up – possibly suggesting that the study’s findings were underestimates of the impact of identified risk factors on future distress.
Extreme contrast in survival rates among patients with stage 0 disease (100% of whom survived 5 years) and stage IV disease (5-year survival rate, 18.5%) led the researchers to focus only on patients with stage I-III disease, a total of 763 patients.
Five years following diagnosis, the group overall showed significantly improved health-related and global QoL, leading the researchers to conclude, reassuringly, that in general, colorectal cancer patients "adjust well to their cancer experience."
Psychological distress, however, did not show the same healing trajectory.
Given a relatively low distress rate initially, a statistical "floor effect" might account for this. Nonetheless, the study revealed subgroups of patients who were quite distressed initially and did not improve over time.
What risk factors at 5 months post diagnosis predicted poorer quality of life and/or greater psychological distress 5 years post diagnosis? Here’s the breakdown:
| Protective Factors | Elevated Risk for Distress |
|---|---|
| Being female | Being single |
| Good social support | Low social support |
| Positive outlook on cancer’s threat | Negative appraisal of cancer’s threat |
| Having a pet | Lower initial QoL |
| Optimism | Low optimism |
| Private health insurance | Rectal cancer |
| Surgery and adjuvant therapy | Permanent stoma |
| Later-stage disease | |
| Being fatigued | |
| Being a smoker |
The lists paint a picture, do they not?
On one side, we have single, pessimistic people who were not surrounded by, and shored up by, a network of close friends and family during their initial cancer treatment. On the other, we have women who were predisposed to view life optimistically and graced as well by strong social networks, good insurance and treatment options, and, not trivially, the love of a pet.
Certainly, it would not be difficult to identify, early on, those on the elevated-risk list, and customize support aimed at building their comfort networks and personal resilience.
"Consistent with response shift theory, the antecedents of QoL after colorectal cancer are multifactorial and include predisposing sociodemographic, medical, and psychosocial variables," the authors concluded.
"Psychosocial interventions that target both social support and threat appraisal may be effective for this patient group."
Such interventions, they suggest, would include maximizing of social support, as well as cognitive behavioral therapy designed "to challenge unhelpful or unrealistically negative cognitions about cancer."
I couldn’t agree more.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
In history, economics, and psychology, it’s a fundamental truth: Past events/performance/behavior predicts future events/performance/behavior. And that’s the lesson we can take from an important Australian study of colorectal cancer survivors in the journal Quality of Life Research (2012;21:1551-64).
As noted by the authors, well-designed prospective studies about the long-term adjustment of colorectal cancer survivors are few and far between. That’s why they set about prospectively following a population-based cohort of colorectal cancer patients from 5 months post diagnosis to 5 years post diagnosis, determining the trajectory of their adjustment and quality of life (QoL) from a sociodemographic, medical, and psychological perspective.
What did they find?
"Importantly," they wrote, "the strongest predictor for long-term outcomes was the baseline values of health-related and global quality of life soon after diagnosis."
In other words, patients who at 5 months post diagnosis were optimistic, euthymic, and secure financially and in their relationships, continued to do well at 5 years.
It makes sense.
But the data also held key lessons about identifying people most likely to take their diagnosis in stride, seek out and receive good care, and then rebound – versus individuals at the other end of the spectrum, who started out not coping well and failed to rally.
First, some details about the study. Led by Professor Suzanne K. Chambers, a health psychologist at Griffith University’s Gold Coast campus in Queensland, Australia, the research team mined the Queensland cancer registry to locate 1,825 adults who were diagnosed with colorectal cancer during 2003-2004 and had signed up for a longitudinal study shortly after diagnosis.
Five months into their treatment, participants filled out an extensive battery designed to measure demographic variables, disease stage, treatment, physical function, cancer threat appraisal (how the disease affected their sense of identity, relationships, and perceptions of the future), perceived social support, quality of life, and psychological distress (depression, anxiety, and somatization).
Five years later, 259 (21.2%) had died.
Others were not included in the study because they failed to complete follow-up paperwork; notably, these nonresponders tended to be male, younger, uninsured, and smokers, and tended to have more advanced cancer, a more negative view of the threat of their cancer, higher somatization, and lower FACT-C (Functional Assessment of Cancer Therapy – Colorectal) scores. In other words, this group was fairly reflective of those patients who had not fared well on follow-up – possibly suggesting that the study’s findings were underestimates of the impact of identified risk factors on future distress.
Extreme contrast in survival rates among patients with stage 0 disease (100% of whom survived 5 years) and stage IV disease (5-year survival rate, 18.5%) led the researchers to focus only on patients with stage I-III disease, a total of 763 patients.
Five years following diagnosis, the group overall showed significantly improved health-related and global QoL, leading the researchers to conclude, reassuringly, that in general, colorectal cancer patients "adjust well to their cancer experience."
Psychological distress, however, did not show the same healing trajectory.
Given a relatively low distress rate initially, a statistical "floor effect" might account for this. Nonetheless, the study revealed subgroups of patients who were quite distressed initially and did not improve over time.
What risk factors at 5 months post diagnosis predicted poorer quality of life and/or greater psychological distress 5 years post diagnosis? Here’s the breakdown:
| Protective Factors | Elevated Risk for Distress |
|---|---|
| Being female | Being single |
| Good social support | Low social support |
| Positive outlook on cancer’s threat | Negative appraisal of cancer’s threat |
| Having a pet | Lower initial QoL |
| Optimism | Low optimism |
| Private health insurance | Rectal cancer |
| Surgery and adjuvant therapy | Permanent stoma |
| Later-stage disease | |
| Being fatigued | |
| Being a smoker |
The lists paint a picture, do they not?
On one side, we have single, pessimistic people who were not surrounded by, and shored up by, a network of close friends and family during their initial cancer treatment. On the other, we have women who were predisposed to view life optimistically and graced as well by strong social networks, good insurance and treatment options, and, not trivially, the love of a pet.
Certainly, it would not be difficult to identify, early on, those on the elevated-risk list, and customize support aimed at building their comfort networks and personal resilience.
"Consistent with response shift theory, the antecedents of QoL after colorectal cancer are multifactorial and include predisposing sociodemographic, medical, and psychosocial variables," the authors concluded.
"Psychosocial interventions that target both social support and threat appraisal may be effective for this patient group."
Such interventions, they suggest, would include maximizing of social support, as well as cognitive behavioral therapy designed "to challenge unhelpful or unrealistically negative cognitions about cancer."
I couldn’t agree more.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
In history, economics, and psychology, it’s a fundamental truth: Past events/performance/behavior predicts future events/performance/behavior. And that’s the lesson we can take from an important Australian study of colorectal cancer survivors in the journal Quality of Life Research (2012;21:1551-64).
As noted by the authors, well-designed prospective studies about the long-term adjustment of colorectal cancer survivors are few and far between. That’s why they set about prospectively following a population-based cohort of colorectal cancer patients from 5 months post diagnosis to 5 years post diagnosis, determining the trajectory of their adjustment and quality of life (QoL) from a sociodemographic, medical, and psychological perspective.
What did they find?
"Importantly," they wrote, "the strongest predictor for long-term outcomes was the baseline values of health-related and global quality of life soon after diagnosis."
In other words, patients who at 5 months post diagnosis were optimistic, euthymic, and secure financially and in their relationships, continued to do well at 5 years.
It makes sense.
But the data also held key lessons about identifying people most likely to take their diagnosis in stride, seek out and receive good care, and then rebound – versus individuals at the other end of the spectrum, who started out not coping well and failed to rally.
First, some details about the study. Led by Professor Suzanne K. Chambers, a health psychologist at Griffith University’s Gold Coast campus in Queensland, Australia, the research team mined the Queensland cancer registry to locate 1,825 adults who were diagnosed with colorectal cancer during 2003-2004 and had signed up for a longitudinal study shortly after diagnosis.
Five months into their treatment, participants filled out an extensive battery designed to measure demographic variables, disease stage, treatment, physical function, cancer threat appraisal (how the disease affected their sense of identity, relationships, and perceptions of the future), perceived social support, quality of life, and psychological distress (depression, anxiety, and somatization).
Five years later, 259 (21.2%) had died.
Others were not included in the study because they failed to complete follow-up paperwork; notably, these nonresponders tended to be male, younger, uninsured, and smokers, and tended to have more advanced cancer, a more negative view of the threat of their cancer, higher somatization, and lower FACT-C (Functional Assessment of Cancer Therapy – Colorectal) scores. In other words, this group was fairly reflective of those patients who had not fared well on follow-up – possibly suggesting that the study’s findings were underestimates of the impact of identified risk factors on future distress.
Extreme contrast in survival rates among patients with stage 0 disease (100% of whom survived 5 years) and stage IV disease (5-year survival rate, 18.5%) led the researchers to focus only on patients with stage I-III disease, a total of 763 patients.
Five years following diagnosis, the group overall showed significantly improved health-related and global QoL, leading the researchers to conclude, reassuringly, that in general, colorectal cancer patients "adjust well to their cancer experience."
Psychological distress, however, did not show the same healing trajectory.
Given a relatively low distress rate initially, a statistical "floor effect" might account for this. Nonetheless, the study revealed subgroups of patients who were quite distressed initially and did not improve over time.
What risk factors at 5 months post diagnosis predicted poorer quality of life and/or greater psychological distress 5 years post diagnosis? Here’s the breakdown:
| Protective Factors | Elevated Risk for Distress |
|---|---|
| Being female | Being single |
| Good social support | Low social support |
| Positive outlook on cancer’s threat | Negative appraisal of cancer’s threat |
| Having a pet | Lower initial QoL |
| Optimism | Low optimism |
| Private health insurance | Rectal cancer |
| Surgery and adjuvant therapy | Permanent stoma |
| Later-stage disease | |
| Being fatigued | |
| Being a smoker |
The lists paint a picture, do they not?
On one side, we have single, pessimistic people who were not surrounded by, and shored up by, a network of close friends and family during their initial cancer treatment. On the other, we have women who were predisposed to view life optimistically and graced as well by strong social networks, good insurance and treatment options, and, not trivially, the love of a pet.
Certainly, it would not be difficult to identify, early on, those on the elevated-risk list, and customize support aimed at building their comfort networks and personal resilience.
"Consistent with response shift theory, the antecedents of QoL after colorectal cancer are multifactorial and include predisposing sociodemographic, medical, and psychosocial variables," the authors concluded.
"Psychosocial interventions that target both social support and threat appraisal may be effective for this patient group."
Such interventions, they suggest, would include maximizing of social support, as well as cognitive behavioral therapy designed "to challenge unhelpful or unrealistically negative cognitions about cancer."
I couldn’t agree more.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
The Long and the Short of Bladder Cancer Survival
Is your patient with bladder cancer blessed with long telomeres? If so, you can expect him to live many years with the disease – as long as he is not depressed, that is.
An intriguing observational study from the department of epidemiology at the University of Texas M.D. Anderson Cancer Center in Houston found that a magic combination of euthymia and telomere length translated into a staggering sixfold increase in survival in 464 patients with bladder cancer.
To put actual numbers to that, it means that depressed patients with short telomeres lived an average of 31.3 months – about 2.5 years – while those with no depression and long telomeres lived an astonishing 199.8 months, or 16.6 years.
Patients with short telomeres and high levels of depression had a threefold risk of mortality.
"This is the first study of its kind that analyzes bladder cancer outcomes," said Meng Chen, Ph.D., a coinvestigator on the study presented during the annual American Association for Cancer Research international conference on frontiers in cancer prevention research.
She continued, "Psychological factors are not usually included in epidemiologic studies," which, given the study results, begs the question, "Why not?"
Short telomeres have been previously identified as likely suspects in several biomarker studies of aging and diseases, including cancer. A press release from M.D. Anderson explained, "As people grow older, telomeres on the tips of chromosomes, which protect chromosomes from unraveling as cells replicate, shorten, and eventually fail, leading to cell death."
Are short telomeres the egg, presaging a bad outcome in bladder cancer patients, or the chicken, telegraphing damage already done? No one knows for sure. The jury isn’t in on the precise role telomeres play in disease progression, much less on how to alter the picture.
Stress has also been associated with shortened telomeres, prompting coauthor Jie Lin, Ph.D., to recommend that enhanced stress management be incorporated into cancer treatment.
It certainly makes sense.
Until telomere enhancement procedures become the rage, it seems prudent to me to identify the risk we now know exists – depression – and treat it well, in patients with bladder cancer and other forms of the disease.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Is your patient with bladder cancer blessed with long telomeres? If so, you can expect him to live many years with the disease – as long as he is not depressed, that is.
An intriguing observational study from the department of epidemiology at the University of Texas M.D. Anderson Cancer Center in Houston found that a magic combination of euthymia and telomere length translated into a staggering sixfold increase in survival in 464 patients with bladder cancer.
To put actual numbers to that, it means that depressed patients with short telomeres lived an average of 31.3 months – about 2.5 years – while those with no depression and long telomeres lived an astonishing 199.8 months, or 16.6 years.
Patients with short telomeres and high levels of depression had a threefold risk of mortality.
"This is the first study of its kind that analyzes bladder cancer outcomes," said Meng Chen, Ph.D., a coinvestigator on the study presented during the annual American Association for Cancer Research international conference on frontiers in cancer prevention research.
She continued, "Psychological factors are not usually included in epidemiologic studies," which, given the study results, begs the question, "Why not?"
Short telomeres have been previously identified as likely suspects in several biomarker studies of aging and diseases, including cancer. A press release from M.D. Anderson explained, "As people grow older, telomeres on the tips of chromosomes, which protect chromosomes from unraveling as cells replicate, shorten, and eventually fail, leading to cell death."
Are short telomeres the egg, presaging a bad outcome in bladder cancer patients, or the chicken, telegraphing damage already done? No one knows for sure. The jury isn’t in on the precise role telomeres play in disease progression, much less on how to alter the picture.
Stress has also been associated with shortened telomeres, prompting coauthor Jie Lin, Ph.D., to recommend that enhanced stress management be incorporated into cancer treatment.
It certainly makes sense.
Until telomere enhancement procedures become the rage, it seems prudent to me to identify the risk we now know exists – depression – and treat it well, in patients with bladder cancer and other forms of the disease.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Is your patient with bladder cancer blessed with long telomeres? If so, you can expect him to live many years with the disease – as long as he is not depressed, that is.
An intriguing observational study from the department of epidemiology at the University of Texas M.D. Anderson Cancer Center in Houston found that a magic combination of euthymia and telomere length translated into a staggering sixfold increase in survival in 464 patients with bladder cancer.
To put actual numbers to that, it means that depressed patients with short telomeres lived an average of 31.3 months – about 2.5 years – while those with no depression and long telomeres lived an astonishing 199.8 months, or 16.6 years.
Patients with short telomeres and high levels of depression had a threefold risk of mortality.
"This is the first study of its kind that analyzes bladder cancer outcomes," said Meng Chen, Ph.D., a coinvestigator on the study presented during the annual American Association for Cancer Research international conference on frontiers in cancer prevention research.
She continued, "Psychological factors are not usually included in epidemiologic studies," which, given the study results, begs the question, "Why not?"
Short telomeres have been previously identified as likely suspects in several biomarker studies of aging and diseases, including cancer. A press release from M.D. Anderson explained, "As people grow older, telomeres on the tips of chromosomes, which protect chromosomes from unraveling as cells replicate, shorten, and eventually fail, leading to cell death."
Are short telomeres the egg, presaging a bad outcome in bladder cancer patients, or the chicken, telegraphing damage already done? No one knows for sure. The jury isn’t in on the precise role telomeres play in disease progression, much less on how to alter the picture.
Stress has also been associated with shortened telomeres, prompting coauthor Jie Lin, Ph.D., to recommend that enhanced stress management be incorporated into cancer treatment.
It certainly makes sense.
Until telomere enhancement procedures become the rage, it seems prudent to me to identify the risk we now know exists – depression – and treat it well, in patients with bladder cancer and other forms of the disease.
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
When Terminal Cancer Patients Don't Get the Message
Oh, how disheartening.
I was troubled, but not too surprised, to read recently published conclusions of two large, population-based studies that found many patients with terminal cancer erroneously believe that palliative treatment (chemotherapy or radiation therapy) will likely cure their disease.
What left me truly dismayed was the first study’s powerful association (P = .002) between patients’ inaccurate perceptions of their prognoses and endorsements of their physicians’ communication skills.
That study, published Oct. 25 in the New England Journal of Medicine (2012;367:1616-25), documented beliefs of 1,193 patients who had received palliative chemotherapy for newly diagnosed stage IV lung or colorectal cancer. As oncologists well know, in this context, chemotherapy might extend life a bit (by weeks or perhaps months) and may alleviate pain or other symptoms of the disease. But it is not curative. And that’s the secret that wasn’t conveyed to patients – at least in a way that they could hear or accept.
Fully 81% of patients with metastatic colorectal cancer didn’t get the message. Nearly 70% of patients with metastatic lung cancer didn’t get the message. By disproportionately high percentages, minority patients didn’t get the message.
Most discouragingly, though, was this fact: The higher patients rated the quality of their communication with their physicians, the less likely they were to know the truth.
"This suggests that patients perceive physicians as better communicators when they convey a more optimistic view of chemotherapy," wrote the authors, led by first author Dr. Jane C. Weeks, a medical oncologist at the Dana-Farber Cancer Institute in Boston.
Denial is a familiar companion to anyone who works with patients with cancer, their families, and friends. It was the grief reaction cited first and foremost by Elisabeth Kübler Ross. As noted in an editorial accompanying one New England Journal of Medicine study (N. Engl. J Med. 2012;367:1651-2), "Self-deception [can be] a valuable coping tool."
But, at a certain point for cancer patients, denial has the opposite effect, prolonging exposure to side effects, draining a family’s (and society’s) resources, and wasting precious life moments while someone sits in a chemo chair, hoping for and believing in a cure that will not come.
In their editorial, Dr. Thomas J. Smith and Dr. Dan L. Longo of the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore rightfully pointed out that the study’s design does not allow us to retrospectively tune into those exam rooms to set the record straight. Did the doctors perceived as great communicators simply not state the prognosis? Or did their patients "choose not to believe"?
"These are not trivial issues," they wrote. "Chemotherapy near the end of life is still common, does not improve survival, and is one preventable reason why 25% of all Medicare funds are spent in the last year of life.
"Patients need truthful information in order to make truthful choices."
A second study with strikingly similar results from Dana-Farber Cancer Institute in Boston was presented by radiation oncologist Dr. Aileen B. Chen at a plenary session during the annual meeting of the American Society for Radiation Oncology (ASTRO). In her study, Dr. Chen found that 64% of 384 patients with stage IIIB (wet) or IV lung cancer did not realize that palliative radiation therapy was not likely to cure their disease. In other words, same story, different treatment modality.
Together, these findings raise profoundly serious questions about the role that oncologists play in conveying information that they may not want to share and patients may not seem to want to hear. The implications are vast, both on an individual and societal level.
Dr. Smith and Dr. Longo, fortunately, offered specific guidance in their editorial, based on experience at their institution that has doubled the length of time patients benefit from hospice, kept survival rates steady, and reduced costs.
Here’s their formula for honestly conveying prognostic information, along with use of the "essential" communication skill "ask, tell, ask," that assesses patients’ understanding of a message by asking them to restate it in their own words.
• Clearly state the prognosis at the first visit (and whenever the prognosis changes).
• Assign someone in the office to consistently address advance directives.
• Facilitate a hospice information visit within the first three visits following conveyance of a terminal prognosis.
• Discuss the prognosis and coping ("What is important to you?") at each transition.
"If patients are offered truthful information – repeatedly – on what is going to happen to them. They can choose wisely," concluded the Johns Hopkins physicians. "We have the tools to help patients make these difficult decisions. We just need the gumption and incentives to use them."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Oh, how disheartening.
I was troubled, but not too surprised, to read recently published conclusions of two large, population-based studies that found many patients with terminal cancer erroneously believe that palliative treatment (chemotherapy or radiation therapy) will likely cure their disease.
What left me truly dismayed was the first study’s powerful association (P = .002) between patients’ inaccurate perceptions of their prognoses and endorsements of their physicians’ communication skills.
That study, published Oct. 25 in the New England Journal of Medicine (2012;367:1616-25), documented beliefs of 1,193 patients who had received palliative chemotherapy for newly diagnosed stage IV lung or colorectal cancer. As oncologists well know, in this context, chemotherapy might extend life a bit (by weeks or perhaps months) and may alleviate pain or other symptoms of the disease. But it is not curative. And that’s the secret that wasn’t conveyed to patients – at least in a way that they could hear or accept.
Fully 81% of patients with metastatic colorectal cancer didn’t get the message. Nearly 70% of patients with metastatic lung cancer didn’t get the message. By disproportionately high percentages, minority patients didn’t get the message.
Most discouragingly, though, was this fact: The higher patients rated the quality of their communication with their physicians, the less likely they were to know the truth.
"This suggests that patients perceive physicians as better communicators when they convey a more optimistic view of chemotherapy," wrote the authors, led by first author Dr. Jane C. Weeks, a medical oncologist at the Dana-Farber Cancer Institute in Boston.
Denial is a familiar companion to anyone who works with patients with cancer, their families, and friends. It was the grief reaction cited first and foremost by Elisabeth Kübler Ross. As noted in an editorial accompanying one New England Journal of Medicine study (N. Engl. J Med. 2012;367:1651-2), "Self-deception [can be] a valuable coping tool."
But, at a certain point for cancer patients, denial has the opposite effect, prolonging exposure to side effects, draining a family’s (and society’s) resources, and wasting precious life moments while someone sits in a chemo chair, hoping for and believing in a cure that will not come.
In their editorial, Dr. Thomas J. Smith and Dr. Dan L. Longo of the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore rightfully pointed out that the study’s design does not allow us to retrospectively tune into those exam rooms to set the record straight. Did the doctors perceived as great communicators simply not state the prognosis? Or did their patients "choose not to believe"?
"These are not trivial issues," they wrote. "Chemotherapy near the end of life is still common, does not improve survival, and is one preventable reason why 25% of all Medicare funds are spent in the last year of life.
"Patients need truthful information in order to make truthful choices."
A second study with strikingly similar results from Dana-Farber Cancer Institute in Boston was presented by radiation oncologist Dr. Aileen B. Chen at a plenary session during the annual meeting of the American Society for Radiation Oncology (ASTRO). In her study, Dr. Chen found that 64% of 384 patients with stage IIIB (wet) or IV lung cancer did not realize that palliative radiation therapy was not likely to cure their disease. In other words, same story, different treatment modality.
Together, these findings raise profoundly serious questions about the role that oncologists play in conveying information that they may not want to share and patients may not seem to want to hear. The implications are vast, both on an individual and societal level.
Dr. Smith and Dr. Longo, fortunately, offered specific guidance in their editorial, based on experience at their institution that has doubled the length of time patients benefit from hospice, kept survival rates steady, and reduced costs.
Here’s their formula for honestly conveying prognostic information, along with use of the "essential" communication skill "ask, tell, ask," that assesses patients’ understanding of a message by asking them to restate it in their own words.
• Clearly state the prognosis at the first visit (and whenever the prognosis changes).
• Assign someone in the office to consistently address advance directives.
• Facilitate a hospice information visit within the first three visits following conveyance of a terminal prognosis.
• Discuss the prognosis and coping ("What is important to you?") at each transition.
"If patients are offered truthful information – repeatedly – on what is going to happen to them. They can choose wisely," concluded the Johns Hopkins physicians. "We have the tools to help patients make these difficult decisions. We just need the gumption and incentives to use them."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Oh, how disheartening.
I was troubled, but not too surprised, to read recently published conclusions of two large, population-based studies that found many patients with terminal cancer erroneously believe that palliative treatment (chemotherapy or radiation therapy) will likely cure their disease.
What left me truly dismayed was the first study’s powerful association (P = .002) between patients’ inaccurate perceptions of their prognoses and endorsements of their physicians’ communication skills.
That study, published Oct. 25 in the New England Journal of Medicine (2012;367:1616-25), documented beliefs of 1,193 patients who had received palliative chemotherapy for newly diagnosed stage IV lung or colorectal cancer. As oncologists well know, in this context, chemotherapy might extend life a bit (by weeks or perhaps months) and may alleviate pain or other symptoms of the disease. But it is not curative. And that’s the secret that wasn’t conveyed to patients – at least in a way that they could hear or accept.
Fully 81% of patients with metastatic colorectal cancer didn’t get the message. Nearly 70% of patients with metastatic lung cancer didn’t get the message. By disproportionately high percentages, minority patients didn’t get the message.
Most discouragingly, though, was this fact: The higher patients rated the quality of their communication with their physicians, the less likely they were to know the truth.
"This suggests that patients perceive physicians as better communicators when they convey a more optimistic view of chemotherapy," wrote the authors, led by first author Dr. Jane C. Weeks, a medical oncologist at the Dana-Farber Cancer Institute in Boston.
Denial is a familiar companion to anyone who works with patients with cancer, their families, and friends. It was the grief reaction cited first and foremost by Elisabeth Kübler Ross. As noted in an editorial accompanying one New England Journal of Medicine study (N. Engl. J Med. 2012;367:1651-2), "Self-deception [can be] a valuable coping tool."
But, at a certain point for cancer patients, denial has the opposite effect, prolonging exposure to side effects, draining a family’s (and society’s) resources, and wasting precious life moments while someone sits in a chemo chair, hoping for and believing in a cure that will not come.
In their editorial, Dr. Thomas J. Smith and Dr. Dan L. Longo of the Johns Hopkins Sidney Kimmel Cancer Center in Baltimore rightfully pointed out that the study’s design does not allow us to retrospectively tune into those exam rooms to set the record straight. Did the doctors perceived as great communicators simply not state the prognosis? Or did their patients "choose not to believe"?
"These are not trivial issues," they wrote. "Chemotherapy near the end of life is still common, does not improve survival, and is one preventable reason why 25% of all Medicare funds are spent in the last year of life.
"Patients need truthful information in order to make truthful choices."
A second study with strikingly similar results from Dana-Farber Cancer Institute in Boston was presented by radiation oncologist Dr. Aileen B. Chen at a plenary session during the annual meeting of the American Society for Radiation Oncology (ASTRO). In her study, Dr. Chen found that 64% of 384 patients with stage IIIB (wet) or IV lung cancer did not realize that palliative radiation therapy was not likely to cure their disease. In other words, same story, different treatment modality.
Together, these findings raise profoundly serious questions about the role that oncologists play in conveying information that they may not want to share and patients may not seem to want to hear. The implications are vast, both on an individual and societal level.
Dr. Smith and Dr. Longo, fortunately, offered specific guidance in their editorial, based on experience at their institution that has doubled the length of time patients benefit from hospice, kept survival rates steady, and reduced costs.
Here’s their formula for honestly conveying prognostic information, along with use of the "essential" communication skill "ask, tell, ask," that assesses patients’ understanding of a message by asking them to restate it in their own words.
• Clearly state the prognosis at the first visit (and whenever the prognosis changes).
• Assign someone in the office to consistently address advance directives.
• Facilitate a hospice information visit within the first three visits following conveyance of a terminal prognosis.
• Discuss the prognosis and coping ("What is important to you?") at each transition.
"If patients are offered truthful information – repeatedly – on what is going to happen to them. They can choose wisely," concluded the Johns Hopkins physicians. "We have the tools to help patients make these difficult decisions. We just need the gumption and incentives to use them."
Dr. Freed is a psychologist in Santa Barbara, Calif., and a medical journalist.
Got a minute to extend a life?
Depression, the all-too-often unacknowledged wallflower of cancer comorbidities, powerfully impacts outcomes. (One 2009 meta-analysis in the journal Cancer, for example, found all-cause mortality was a staggering 39% higher among depressed cancer patients (Cancer 2009;115:5349-61).
If depression were easy to spot and referral resources were readily available, it seems likely that modifying this potent risk factor for negative outcomes could significantly improve patients’ life spans, as well as the quality of their lives.
Unfortunately, though, abundant research raises serious questions about oncologists’ acumen at detecting a lurking threat to their patients, that doesn’t show up on a lab slip or physical examination. A 2001 study of 143 physicians found that their rate of detecting depression in 2,297 cancer patients had a sensitivity of 29% and specificity of 85% (Br. J. Cancer 2001;84:1011-15).
So what to do?
Oncologists need the equivalent of a flagged level on a laboratory slip to raise their suspicion that a patient may warrant a closer look to rule out depression, or, if it is confirmed, to prescribe appropriate medication and/or psychotherapeutic interventions.
An impressive recent study may shine light on a number of ways to improve detection of depression in a clinically realistic way, without adding undue time to clinical visits or burdening patients and oncologists with lengthy questionnaires (J. Affect. Disord 2012;140:149-60).
Dr. Alex J. Mitchell of Leicester Royal Infirmary led a multidisciplinary, multinational team of researchers exploring myriad depression screening tools to identify those that not only passed muster in terms of sensitivity and specificity, but would also pass the sniff test in real oncology clinics and practices because of the ease of their delivery and interpretation.
Among 19 screening tools evaluated in 63 studies, the researchers narrowed the field to include only those that had been validated by at least two independent studies, leaving 8 tools for comparison in their meta-analysis. Those tools had been subject to 56 diagnostic validity studies in more than 10,000 patients with all stages of cancer. Studies assessing depression in palliative care settings were less common, but 16 met the stringent criteria established by Dr. Mitchell and his team.
In nonpalliative settings, the weighted prevalence of depression was 17.6%, quite in line with the 15% point prevalence of depression in the first 2 years after a cancer diagnosis. For this population, two diagnostic tools were classified as offering level 2 evidence for case-finding in the meta-analysis: the 21-item Beck Depression Inventory II (BDI-II) and the use of a single-stem question, as simple as, "Are you feeling depressed?"
Not surprisingly, the more comprehensive BDI-II was more accurate and better at ruling out false positives. But it had lower acceptability, the team concluded.
For screening of all cancer patients, asking two stem questions elicited better results than just one, and this approach received a rating of Level 1b evidence with high acceptability. These questions can vary, but usually address mood in question one and interest in question two. For example, a common two-stem query might be:
– During the past 2 weeks (or since your last visit), have you felt sad, blue, or depressed?
– During the past 2 weeks (or since your last visit), have you lost interest in most things that you normally enjoy, such as hobbies, work, or other activities?
According to the study authors, "For every 100 people screened in advanced cancer, the two questions would accurately detect 18 cases, while missing only 1 and correctly reassuring 74, with 7 falsely identified [as having depression when they did not].
For every 100 people screened in nonpalliative settings, the BDI-II would accurately detect 17 cases, missing 2 and correctly reassure 70, with 11 falsely identified as cases."
As with any study’s conclusions, there were limitations to this one, among them that the two-stem question yields only a "modest" positive predictive value among cancer patients.
"These limitations," the authors concluded, "are not so great as to completely preclude clinical usefulness and it nevertheless is likely to outperform oncologists’ unassisted clinical ratings."
They added that it is unlikely that a one-size-fits-all tool will ever be found to address every oncologist’s needs in every oncology setting for patients of all ages with all stages of disease. Perhaps a quick screen (like the two-question approach), followed by a more elaborate diagnostic tool for those who answer "yes," would be best to ensure that cases have not been inappropriately included.
Moreover, no screen is worth its salt unless treatment is available that can change the course of the illness and its impact on outcomes.
Still, it would only take an oncologist a minute to ask two questions about depression. And if the answers could chip away at a very large, very powerful hidden elephant in the room, it seems to me it would be worth a try.
Next Week: Depression and Telomeres: Why Both Matter in Bladder Cancer.
Depression, the all-too-often unacknowledged wallflower of cancer comorbidities, powerfully impacts outcomes. (One 2009 meta-analysis in the journal Cancer, for example, found all-cause mortality was a staggering 39% higher among depressed cancer patients (Cancer 2009;115:5349-61).
If depression were easy to spot and referral resources were readily available, it seems likely that modifying this potent risk factor for negative outcomes could significantly improve patients’ life spans, as well as the quality of their lives.
Unfortunately, though, abundant research raises serious questions about oncologists’ acumen at detecting a lurking threat to their patients, that doesn’t show up on a lab slip or physical examination. A 2001 study of 143 physicians found that their rate of detecting depression in 2,297 cancer patients had a sensitivity of 29% and specificity of 85% (Br. J. Cancer 2001;84:1011-15).
So what to do?
Oncologists need the equivalent of a flagged level on a laboratory slip to raise their suspicion that a patient may warrant a closer look to rule out depression, or, if it is confirmed, to prescribe appropriate medication and/or psychotherapeutic interventions.
An impressive recent study may shine light on a number of ways to improve detection of depression in a clinically realistic way, without adding undue time to clinical visits or burdening patients and oncologists with lengthy questionnaires (J. Affect. Disord 2012;140:149-60).
Dr. Alex J. Mitchell of Leicester Royal Infirmary led a multidisciplinary, multinational team of researchers exploring myriad depression screening tools to identify those that not only passed muster in terms of sensitivity and specificity, but would also pass the sniff test in real oncology clinics and practices because of the ease of their delivery and interpretation.
Among 19 screening tools evaluated in 63 studies, the researchers narrowed the field to include only those that had been validated by at least two independent studies, leaving 8 tools for comparison in their meta-analysis. Those tools had been subject to 56 diagnostic validity studies in more than 10,000 patients with all stages of cancer. Studies assessing depression in palliative care settings were less common, but 16 met the stringent criteria established by Dr. Mitchell and his team.
In nonpalliative settings, the weighted prevalence of depression was 17.6%, quite in line with the 15% point prevalence of depression in the first 2 years after a cancer diagnosis. For this population, two diagnostic tools were classified as offering level 2 evidence for case-finding in the meta-analysis: the 21-item Beck Depression Inventory II (BDI-II) and the use of a single-stem question, as simple as, "Are you feeling depressed?"
Not surprisingly, the more comprehensive BDI-II was more accurate and better at ruling out false positives. But it had lower acceptability, the team concluded.
For screening of all cancer patients, asking two stem questions elicited better results than just one, and this approach received a rating of Level 1b evidence with high acceptability. These questions can vary, but usually address mood in question one and interest in question two. For example, a common two-stem query might be:
– During the past 2 weeks (or since your last visit), have you felt sad, blue, or depressed?
– During the past 2 weeks (or since your last visit), have you lost interest in most things that you normally enjoy, such as hobbies, work, or other activities?
According to the study authors, "For every 100 people screened in advanced cancer, the two questions would accurately detect 18 cases, while missing only 1 and correctly reassuring 74, with 7 falsely identified [as having depression when they did not].
For every 100 people screened in nonpalliative settings, the BDI-II would accurately detect 17 cases, missing 2 and correctly reassure 70, with 11 falsely identified as cases."
As with any study’s conclusions, there were limitations to this one, among them that the two-stem question yields only a "modest" positive predictive value among cancer patients.
"These limitations," the authors concluded, "are not so great as to completely preclude clinical usefulness and it nevertheless is likely to outperform oncologists’ unassisted clinical ratings."
They added that it is unlikely that a one-size-fits-all tool will ever be found to address every oncologist’s needs in every oncology setting for patients of all ages with all stages of disease. Perhaps a quick screen (like the two-question approach), followed by a more elaborate diagnostic tool for those who answer "yes," would be best to ensure that cases have not been inappropriately included.
Moreover, no screen is worth its salt unless treatment is available that can change the course of the illness and its impact on outcomes.
Still, it would only take an oncologist a minute to ask two questions about depression. And if the answers could chip away at a very large, very powerful hidden elephant in the room, it seems to me it would be worth a try.
Next Week: Depression and Telomeres: Why Both Matter in Bladder Cancer.
Depression, the all-too-often unacknowledged wallflower of cancer comorbidities, powerfully impacts outcomes. (One 2009 meta-analysis in the journal Cancer, for example, found all-cause mortality was a staggering 39% higher among depressed cancer patients (Cancer 2009;115:5349-61).
If depression were easy to spot and referral resources were readily available, it seems likely that modifying this potent risk factor for negative outcomes could significantly improve patients’ life spans, as well as the quality of their lives.
Unfortunately, though, abundant research raises serious questions about oncologists’ acumen at detecting a lurking threat to their patients, that doesn’t show up on a lab slip or physical examination. A 2001 study of 143 physicians found that their rate of detecting depression in 2,297 cancer patients had a sensitivity of 29% and specificity of 85% (Br. J. Cancer 2001;84:1011-15).
So what to do?
Oncologists need the equivalent of a flagged level on a laboratory slip to raise their suspicion that a patient may warrant a closer look to rule out depression, or, if it is confirmed, to prescribe appropriate medication and/or psychotherapeutic interventions.
An impressive recent study may shine light on a number of ways to improve detection of depression in a clinically realistic way, without adding undue time to clinical visits or burdening patients and oncologists with lengthy questionnaires (J. Affect. Disord 2012;140:149-60).
Dr. Alex J. Mitchell of Leicester Royal Infirmary led a multidisciplinary, multinational team of researchers exploring myriad depression screening tools to identify those that not only passed muster in terms of sensitivity and specificity, but would also pass the sniff test in real oncology clinics and practices because of the ease of their delivery and interpretation.
Among 19 screening tools evaluated in 63 studies, the researchers narrowed the field to include only those that had been validated by at least two independent studies, leaving 8 tools for comparison in their meta-analysis. Those tools had been subject to 56 diagnostic validity studies in more than 10,000 patients with all stages of cancer. Studies assessing depression in palliative care settings were less common, but 16 met the stringent criteria established by Dr. Mitchell and his team.
In nonpalliative settings, the weighted prevalence of depression was 17.6%, quite in line with the 15% point prevalence of depression in the first 2 years after a cancer diagnosis. For this population, two diagnostic tools were classified as offering level 2 evidence for case-finding in the meta-analysis: the 21-item Beck Depression Inventory II (BDI-II) and the use of a single-stem question, as simple as, "Are you feeling depressed?"
Not surprisingly, the more comprehensive BDI-II was more accurate and better at ruling out false positives. But it had lower acceptability, the team concluded.
For screening of all cancer patients, asking two stem questions elicited better results than just one, and this approach received a rating of Level 1b evidence with high acceptability. These questions can vary, but usually address mood in question one and interest in question two. For example, a common two-stem query might be:
– During the past 2 weeks (or since your last visit), have you felt sad, blue, or depressed?
– During the past 2 weeks (or since your last visit), have you lost interest in most things that you normally enjoy, such as hobbies, work, or other activities?
According to the study authors, "For every 100 people screened in advanced cancer, the two questions would accurately detect 18 cases, while missing only 1 and correctly reassuring 74, with 7 falsely identified [as having depression when they did not].
For every 100 people screened in nonpalliative settings, the BDI-II would accurately detect 17 cases, missing 2 and correctly reassure 70, with 11 falsely identified as cases."
As with any study’s conclusions, there were limitations to this one, among them that the two-stem question yields only a "modest" positive predictive value among cancer patients.
"These limitations," the authors concluded, "are not so great as to completely preclude clinical usefulness and it nevertheless is likely to outperform oncologists’ unassisted clinical ratings."
They added that it is unlikely that a one-size-fits-all tool will ever be found to address every oncologist’s needs in every oncology setting for patients of all ages with all stages of disease. Perhaps a quick screen (like the two-question approach), followed by a more elaborate diagnostic tool for those who answer "yes," would be best to ensure that cases have not been inappropriately included.
Moreover, no screen is worth its salt unless treatment is available that can change the course of the illness and its impact on outcomes.
Still, it would only take an oncologist a minute to ask two questions about depression. And if the answers could chip away at a very large, very powerful hidden elephant in the room, it seems to me it would be worth a try.
Next Week: Depression and Telomeres: Why Both Matter in Bladder Cancer.