User login
Water woes: Recognizing and treating recreational water illness
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Most of our patients have been or will be exposed to water in a recreational setting this summer. As health care providers, we might not routinely consider illnesses associated with recreational water exposure or discuss preventive strategies; however, the Centers for Disease Control and Prevention has been actively promoting awareness about recreational water illnesses for years. May 18-24, 2015, was the 11th annual observance of Healthy and Safe Swimming Week, formerly known as Recreational Illness and Injury Prevention Week. The focus for 2015 was promoting the role of swimmers, residential pool owners, public health officials, and beach staff in the prevention of drownings, chemical injuries, and outbreaks of illness. One goal was for the swimmer to take a more active role in protecting themselves and preventing the spread of infections to others. For our colleagues, that means educating both parents and children.
To begin our discussion, let’s define recreational water illnesses (RWI). RWIs are caused by a variety of infectious pathogens transmitted by ingestion, inhalation of aerosols or mists, or having contact with contaminated water from both treated (swimming pools, hot tubs, water parks, and fountains) and untreated (lakes, rivers, and oceans) sources of water in recreational venues. RWIs also can be caused by chemicals that have evaporated from water leading to poor indoor air quality. However, I am focusing on the infectious etiologies.
A broad spectrum of infections are associated with RWIs, including infections of the gastrointestinal tract, ear, skin, eye, central nervous system, and wounds. Diarrhea is the most common infection. Implicated pathogens include Giardia, Shigella, norovirus, and Escherichia coli O157:H7, but it is Cryptosporidium that has emerged as the pathogen implicated most often in swimming pool–related outbreaks. Recently published data from the CDC revealed that in 2011-2012, there were 90 recreational-associated outbreaks reported from 32 states and Puerto Rico resulting in 1,788 infections, with 69 outbreaks occurring in treated water venues. Of these, 36 (51%) were caused by Cryptosporidium. Among 21 outbreaks occurring in untreated recreational water, E. coli was responsible for 7 (33%) (MMWR Morb. Mortal. Wkly Rep. 2015;64:668-72)
It’s no surprise diarrhea is the most common illness. Infection can easily occur after swallowing contaminated water. Many erroneously think chlorine kills all pathogens. Cryptosporidium is chlorine tolerant and can persist in treated water with the current recommended levels of chlorine for more than 10 days (J. Water Health 2008;6:513-20). For chlorine-sensitive pathogens, maintenance of the disinfection process must remain intact. What role do swimmers play? Most people have about 0.4 g of feces on their bottoms that can contaminate water when rinsed off. How many people enter a pool with a diarrheal illness? How many may go swimming after having recently recovered from a diarrheal illness and may have asymptomatic shedding? We all have cringed when we see a diapered child in the water. All of these are potential ways for the swimmer to contaminate an adequately treated pool. Additionally, while Cryptosporidium infections are usually self-limited, some individuals, including the immunocompromised host and especially those with advanced HIV and those who are solid organ transplant recipients, may have a protracted course of profuse diarrhea if infected.
While diarrhea maybe the most common RWI, it is not the only one. Acute otitis externa (AOE), more commonly known as “swimmer’s ear,” is one of the most frequent reasons for summer health care encounters. It has been estimated that in the United States in 2007, 2.4 million health care visits resulted in the diagnosis of AOE (MMWR Morb. Mortal. Wkly. Rep. 2011;60:605-9). Visits were highest among children aged 5-9 years; however, adults accounted for 53% of the encounters. Inflammation and infection of the external auditory canal is usually caused by bacteria. Pseudomonas aeruginosa or Staphylococcus aureus are the two most common etiologies. Water is easily introduced into the external auditory canal with recreational water activities, leading to maceration and subsequent infection of the canal. Simply reminding parents to thoroughly dry their child’s ears after water exposure can help prevent AOE.
P. aeruginosa also is the agent causing the self-limiting conditions hot tub folliculitis and hot-foot syndrome. Hot tub folliculitis is characterized by the development of tender, pruritic papules and papulopustules on the hips, buttocks, and axillae, usually developing 8-48 hours after exposure to water that has been contaminated because of inadequate chlorination. Hot-foot syndrome is characterized by painful planter nodules (N. Engl. J. Med. 2001;345:335).
Serious diseases are encountered infrequently, but there are some that require more urgent interventions. Primary amebic meningoencephalitis (PAM) is an extremely rare, progressive, and almost always fatal infection of the brain caused by Naegleria fowleri. The pathogen is found in warm freshwater including lakes, rivers, streams, and hot springs. It enters the body through the nose and travels via the olfactory nerve to the brain. Infection usually occurs when individuals swim or dive in warm freshwater. Most cases have been reported in children from Southern states. In 2010, the first case in a northern state was reported from Minnesota, and three additional cases have since been reported in Kansas and Indiana (J. Ped. Infect. Dis. 2014 [doi: 10.1093/jpids/piu103]). Cases also have been reported in two individuals who were regular users of neti pots for sinus irrigation because the irrigating solution was prepared with contaminated tap water (Clin. Infect. Dis. 2012;55:e79-85). Clinical presentation is similar to bacterial meningitis. Helpful diagnostic clues may come from obtaining a history of swimming in freshwater within the 2 weeks prior to presentation, especially during the summer, or the use of nasal or sinus irrigation with untreated tap water. Consultation with an infectious disease specialist is recommended.
Acanthamoeba keratitis is a potentially blinding infection of the cornea that primarily occurs in individuals who wear contact lenses. Risk factors for the infection include swimming, showering, and use of hot tubs while wearing contact lenses. Improper storage and cleansing contacts with tap water are other risk factors. Anyone with corneal trauma and similar water exposures also would be at risk. Clinically, the history combined with a foreign-body sensation, pain, and decreased visual acuity should make one include this infection in the differential diagnosis. Referral to an ophthalmologist is required.
Finally, swimming with an open wound is a portal of entry for Vibrio vulnificus. It usually is associated with consumption of contaminated seafood, especially oysters. In immunocompromised individuals, especially those with chronic liver disease, this bacteria can cause a life-threatening illness leading to bacteremia, septic shock, and development of blistering skin lesions. Infections are fatal in approximately 50% of cases.
The goal of this brief review was not to discourage swimming, but to make your patients and their families healthy swimmers. Here are a few things the CDC is recommending to help them achieve that goal:
• Shower prior to going swimming.
• Do not swallow or drink pool water.
• Take bathroom breaks every hour and rinse off before going back into the water.
• Do not swim if you have diarrhea.
• Wait at least 2 weeks to go swimming if you have had diarrhea.
• Change swim diapers frequently and away from the water.
• Suggest patients download the free CDC app Healthy Swimming for more detailed information and suggest they visit cdc.gov/healthywater/swimming.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Are your patients vaccinated for travel?
Graduation season is rapidly approaching, with high school graduations, followed by summer vacations. While searching for that unique gift and /or summer experience, many of your patients may choose an international destination. Not to be forgotten are those who might travel to resource-limited areas to visit relatives, volunteer, or have extended stays because of parental job relocation. More U.S. high school graduates are participating in gap year programs, many of which involve extensive travel while providing the participant the opportunity to immerse and to actively participate in other cultures. For many, it may be their first experience in a country with poor hygiene. This week alone, I’ve helped prepare travelers, including adolescents and children, for a safari and one for 4 weeks of volunteerism in Tanzania. Another young traveler’s destinations were Rwanda, Uganda, and Kenya, and a fourth is planning to explore and trek regions in the high elevations of Bolivia and Peru. The question is, Will you be ready to help prepare young travelers to stay healthy and return home without any unwanted souvenirs?
For many, health concerns often are not the top priority when they are planning vacations. However, the primary care physician will most likely will be their initial call and resource once they realize their potential to be exposed to diseases and/or conditions not routinely encountered in the United States. Even if you receive the call late, there are still interventions you can provide.
To avoid that last-minute call, develop strategies to identify international travelers in your practice. Many practices send out reminders yearly for influenza and well visits, so consider developing one for international travel. Text-message reminders have been shown to improve influenza vaccine administration rates and are another form of communication that can be considered. Frequently remind families that if planning international travel, they should seek pretravel advice in a timely manner: Ideally advice should be obtained 4-6 weeks in advance, and definitely at least 2 weeks prior to departure. Remind them that adequate time is needed for the vaccine to become effective. In addition, depending on the patients’ destination, trip duration, and type of activity, two vaccines (rabies and Japanese encephalitis) may be recommended and are administered over a 28-day period. Yellow fever vaccine, which is recommended or required for entry into some countries, can be obtained only at centers designated by each state health department. It should be administered at least 10 days prior to travel.
Vaccine interventions are based on the potential risk for disease exposure/acquisition. Factors to consider include the age of the travelers, their health and immunization status, in addition to their destination, duration of stay, accommodations, activities, and reason for travel (such as business or visiting friends and relatives). If you have a child with a chronic disorder or who is immunocompromised, comparable medical care may not be available at all international destinations. In addition, not everyone may be a candidate to receive some recommended or required vaccines. Involvement with a health professional prior to booking the trip would be advisable.
Identify a travel health specialist in your area as a local resource who can provide the most up-to-date information and recommendations. Ensure that individual is willing to see children of all ages.
Make sure routine immunizations are up to date for age. Measles is the one exception. I know you have heard it before, but outbreaks persist, even in the United States. Travelers 6- to 11-months-old should receive one MMR dose prior to international travel. This dose will not count, so these children should receive two additional doses of vaccine once they are at least 1 year old. Many children travel with adults. All travelers at least 12 months of age and born after 1956 should have two documented doses of MMR prior to international travel unless they have serologic evidence of immunity. The second dose can be given as early as 4 weeks after the first. If two doses at least 4 weeks apart are administered when a child is at least 12 months of age, no additional doses are necessary.
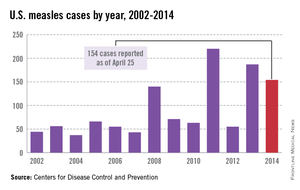
In 2014, there were 668 cases of measles from 27 states in the United States. The United States is still experiencing a multistate outbreak of measles at press time, which began December 2014. As of April 24, 2015, 166 cases have been reported from 19 states. The Centers for Disease Control and Prevention analyzed the virus type (B3). It is identical to the one responsible for the outbreak in the Philippines in 2014, and it has now been identified in 14 other countries.
Most U.S. measles cases occur in unvaccinated travelers who become ill after their return and spread the disease to susceptible individuals. Do you have patients who are unimmunized? Another point to consider when speaking with these parents about travel is the potential loss of the herd immunity afforded their children while living in the United States. This benefit may not exist when they are visiting and/or relocating to countries with lower immunization rates. Measles outbreaks are occurring in multiple countries and are not limited to underdeveloped countries. For the most up-to-date travel health-related information from the CDC, click here.
Travelers’ diarrhea (TD) occurs in up to 70 % of travelers to developing countries. The World Health Organization defines it as passage of at least three loose stools in a 24-hour period. Most often it is self-limited, with symptoms lasting a median of 3-4 days. Although TD can be caused by bacteria, protozoa, and viruses, bacteria are usually the etiology, with enterotoxigenic Escherichia coli being the most common pathogens. Other bacterial etiologies include Shigella and Campylobacter species. Two antimicrobials are frequently prescribed to travelers for self-treatment of TD: ciprofloxacin and azithromycin. Most young children are prescribed the latter; however, in older children, ciprofloxacin may be prescribed off label, as its use in persons younger than 18 years is not approved by the Food and Drug Administration.
In December 2014, PulseNet, the national molecular subtyping network for food-borne disease, detected a multistate cluster of ciprofloxacin-resistant Shigella sonnei. Between May 2014 and February 2015, 157 cases including 37 children were detected in 32 states and Puerto Rico. Nine of the cases identified by PulseNet, and an additional 76 cases, were associated with an outbreak of ciprofloxacin-resistant S. sonnei in San Francisco. Antibiotic susceptibility was available for 126 isolates, of which 109 (87%) were not ciprofloxacin susceptible. Travel history was available for 75 patients not associated with the San Francisco outbreak, and slightly more than half (40) were associated with international travel. The island of Hispaniola (Dominican Republic = 22 cases and Haiti = 4 cases) was the most common destination, followed by India (8 cases) and Morocco (3 cases). The remaining destinations were Asia and Europe (MMWR 2015;64:318-20) Travel history was available and positive for 23 of the 37 children (62%).
Why such a concern? International travelers are at risk of becoming colonized with drug-resistant bacteria and have the potential to spread them domestically. It has already begun. In 2012, the National Antimicrobial Resistance Monitoring System (NARMS) revealed that isolates of S. sonnei had the following resistance pattern: trimethoprim/sulfamethoxazole, 42%; ampicillin, 18%; and ciprofloxacin, 2.1%. During this outbreak, 19 of the 126 isolates were tested by NARMS with the following resistance patterns noted: trimethoprim/sulfamethoxazole, 84%; ampicillin, 5%; and ciprofloxacin, 32%.
More judicious use of antibiotics is necessary. As pediatricians, we are not immune to this issue. The challenge is when, if at all, antibiotics should be prescribed for TD, and under what conditions should patients be instructed to use them. I’m rethinking my own practice. TD is one of the most common illnesses travelers acquire and is easily treated, but at what cost? The one expression I keep hearing myself say is, First do no harm.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures.
Graduation season is rapidly approaching, with high school graduations, followed by summer vacations. While searching for that unique gift and /or summer experience, many of your patients may choose an international destination. Not to be forgotten are those who might travel to resource-limited areas to visit relatives, volunteer, or have extended stays because of parental job relocation. More U.S. high school graduates are participating in gap year programs, many of which involve extensive travel while providing the participant the opportunity to immerse and to actively participate in other cultures. For many, it may be their first experience in a country with poor hygiene. This week alone, I’ve helped prepare travelers, including adolescents and children, for a safari and one for 4 weeks of volunteerism in Tanzania. Another young traveler’s destinations were Rwanda, Uganda, and Kenya, and a fourth is planning to explore and trek regions in the high elevations of Bolivia and Peru. The question is, Will you be ready to help prepare young travelers to stay healthy and return home without any unwanted souvenirs?
For many, health concerns often are not the top priority when they are planning vacations. However, the primary care physician will most likely will be their initial call and resource once they realize their potential to be exposed to diseases and/or conditions not routinely encountered in the United States. Even if you receive the call late, there are still interventions you can provide.
To avoid that last-minute call, develop strategies to identify international travelers in your practice. Many practices send out reminders yearly for influenza and well visits, so consider developing one for international travel. Text-message reminders have been shown to improve influenza vaccine administration rates and are another form of communication that can be considered. Frequently remind families that if planning international travel, they should seek pretravel advice in a timely manner: Ideally advice should be obtained 4-6 weeks in advance, and definitely at least 2 weeks prior to departure. Remind them that adequate time is needed for the vaccine to become effective. In addition, depending on the patients’ destination, trip duration, and type of activity, two vaccines (rabies and Japanese encephalitis) may be recommended and are administered over a 28-day period. Yellow fever vaccine, which is recommended or required for entry into some countries, can be obtained only at centers designated by each state health department. It should be administered at least 10 days prior to travel.
Vaccine interventions are based on the potential risk for disease exposure/acquisition. Factors to consider include the age of the travelers, their health and immunization status, in addition to their destination, duration of stay, accommodations, activities, and reason for travel (such as business or visiting friends and relatives). If you have a child with a chronic disorder or who is immunocompromised, comparable medical care may not be available at all international destinations. In addition, not everyone may be a candidate to receive some recommended or required vaccines. Involvement with a health professional prior to booking the trip would be advisable.
Identify a travel health specialist in your area as a local resource who can provide the most up-to-date information and recommendations. Ensure that individual is willing to see children of all ages.
Make sure routine immunizations are up to date for age. Measles is the one exception. I know you have heard it before, but outbreaks persist, even in the United States. Travelers 6- to 11-months-old should receive one MMR dose prior to international travel. This dose will not count, so these children should receive two additional doses of vaccine once they are at least 1 year old. Many children travel with adults. All travelers at least 12 months of age and born after 1956 should have two documented doses of MMR prior to international travel unless they have serologic evidence of immunity. The second dose can be given as early as 4 weeks after the first. If two doses at least 4 weeks apart are administered when a child is at least 12 months of age, no additional doses are necessary.
In 2014, there were 668 cases of measles from 27 states in the United States. The United States is still experiencing a multistate outbreak of measles at press time, which began December 2014. As of April 24, 2015, 166 cases have been reported from 19 states. The Centers for Disease Control and Prevention analyzed the virus type (B3). It is identical to the one responsible for the outbreak in the Philippines in 2014, and it has now been identified in 14 other countries.
Most U.S. measles cases occur in unvaccinated travelers who become ill after their return and spread the disease to susceptible individuals. Do you have patients who are unimmunized? Another point to consider when speaking with these parents about travel is the potential loss of the herd immunity afforded their children while living in the United States. This benefit may not exist when they are visiting and/or relocating to countries with lower immunization rates. Measles outbreaks are occurring in multiple countries and are not limited to underdeveloped countries. For the most up-to-date travel health-related information from the CDC, click here.
Travelers’ diarrhea (TD) occurs in up to 70 % of travelers to developing countries. The World Health Organization defines it as passage of at least three loose stools in a 24-hour period. Most often it is self-limited, with symptoms lasting a median of 3-4 days. Although TD can be caused by bacteria, protozoa, and viruses, bacteria are usually the etiology, with enterotoxigenic Escherichia coli being the most common pathogens. Other bacterial etiologies include Shigella and Campylobacter species. Two antimicrobials are frequently prescribed to travelers for self-treatment of TD: ciprofloxacin and azithromycin. Most young children are prescribed the latter; however, in older children, ciprofloxacin may be prescribed off label, as its use in persons younger than 18 years is not approved by the Food and Drug Administration.
In December 2014, PulseNet, the national molecular subtyping network for food-borne disease, detected a multistate cluster of ciprofloxacin-resistant Shigella sonnei. Between May 2014 and February 2015, 157 cases including 37 children were detected in 32 states and Puerto Rico. Nine of the cases identified by PulseNet, and an additional 76 cases, were associated with an outbreak of ciprofloxacin-resistant S. sonnei in San Francisco. Antibiotic susceptibility was available for 126 isolates, of which 109 (87%) were not ciprofloxacin susceptible. Travel history was available for 75 patients not associated with the San Francisco outbreak, and slightly more than half (40) were associated with international travel. The island of Hispaniola (Dominican Republic = 22 cases and Haiti = 4 cases) was the most common destination, followed by India (8 cases) and Morocco (3 cases). The remaining destinations were Asia and Europe (MMWR 2015;64:318-20) Travel history was available and positive for 23 of the 37 children (62%).
Why such a concern? International travelers are at risk of becoming colonized with drug-resistant bacteria and have the potential to spread them domestically. It has already begun. In 2012, the National Antimicrobial Resistance Monitoring System (NARMS) revealed that isolates of S. sonnei had the following resistance pattern: trimethoprim/sulfamethoxazole, 42%; ampicillin, 18%; and ciprofloxacin, 2.1%. During this outbreak, 19 of the 126 isolates were tested by NARMS with the following resistance patterns noted: trimethoprim/sulfamethoxazole, 84%; ampicillin, 5%; and ciprofloxacin, 32%.
More judicious use of antibiotics is necessary. As pediatricians, we are not immune to this issue. The challenge is when, if at all, antibiotics should be prescribed for TD, and under what conditions should patients be instructed to use them. I’m rethinking my own practice. TD is one of the most common illnesses travelers acquire and is easily treated, but at what cost? The one expression I keep hearing myself say is, First do no harm.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures.
Graduation season is rapidly approaching, with high school graduations, followed by summer vacations. While searching for that unique gift and /or summer experience, many of your patients may choose an international destination. Not to be forgotten are those who might travel to resource-limited areas to visit relatives, volunteer, or have extended stays because of parental job relocation. More U.S. high school graduates are participating in gap year programs, many of which involve extensive travel while providing the participant the opportunity to immerse and to actively participate in other cultures. For many, it may be their first experience in a country with poor hygiene. This week alone, I’ve helped prepare travelers, including adolescents and children, for a safari and one for 4 weeks of volunteerism in Tanzania. Another young traveler’s destinations were Rwanda, Uganda, and Kenya, and a fourth is planning to explore and trek regions in the high elevations of Bolivia and Peru. The question is, Will you be ready to help prepare young travelers to stay healthy and return home without any unwanted souvenirs?
For many, health concerns often are not the top priority when they are planning vacations. However, the primary care physician will most likely will be their initial call and resource once they realize their potential to be exposed to diseases and/or conditions not routinely encountered in the United States. Even if you receive the call late, there are still interventions you can provide.
To avoid that last-minute call, develop strategies to identify international travelers in your practice. Many practices send out reminders yearly for influenza and well visits, so consider developing one for international travel. Text-message reminders have been shown to improve influenza vaccine administration rates and are another form of communication that can be considered. Frequently remind families that if planning international travel, they should seek pretravel advice in a timely manner: Ideally advice should be obtained 4-6 weeks in advance, and definitely at least 2 weeks prior to departure. Remind them that adequate time is needed for the vaccine to become effective. In addition, depending on the patients’ destination, trip duration, and type of activity, two vaccines (rabies and Japanese encephalitis) may be recommended and are administered over a 28-day period. Yellow fever vaccine, which is recommended or required for entry into some countries, can be obtained only at centers designated by each state health department. It should be administered at least 10 days prior to travel.
Vaccine interventions are based on the potential risk for disease exposure/acquisition. Factors to consider include the age of the travelers, their health and immunization status, in addition to their destination, duration of stay, accommodations, activities, and reason for travel (such as business or visiting friends and relatives). If you have a child with a chronic disorder or who is immunocompromised, comparable medical care may not be available at all international destinations. In addition, not everyone may be a candidate to receive some recommended or required vaccines. Involvement with a health professional prior to booking the trip would be advisable.
Identify a travel health specialist in your area as a local resource who can provide the most up-to-date information and recommendations. Ensure that individual is willing to see children of all ages.
Make sure routine immunizations are up to date for age. Measles is the one exception. I know you have heard it before, but outbreaks persist, even in the United States. Travelers 6- to 11-months-old should receive one MMR dose prior to international travel. This dose will not count, so these children should receive two additional doses of vaccine once they are at least 1 year old. Many children travel with adults. All travelers at least 12 months of age and born after 1956 should have two documented doses of MMR prior to international travel unless they have serologic evidence of immunity. The second dose can be given as early as 4 weeks after the first. If two doses at least 4 weeks apart are administered when a child is at least 12 months of age, no additional doses are necessary.
In 2014, there were 668 cases of measles from 27 states in the United States. The United States is still experiencing a multistate outbreak of measles at press time, which began December 2014. As of April 24, 2015, 166 cases have been reported from 19 states. The Centers for Disease Control and Prevention analyzed the virus type (B3). It is identical to the one responsible for the outbreak in the Philippines in 2014, and it has now been identified in 14 other countries.
Most U.S. measles cases occur in unvaccinated travelers who become ill after their return and spread the disease to susceptible individuals. Do you have patients who are unimmunized? Another point to consider when speaking with these parents about travel is the potential loss of the herd immunity afforded their children while living in the United States. This benefit may not exist when they are visiting and/or relocating to countries with lower immunization rates. Measles outbreaks are occurring in multiple countries and are not limited to underdeveloped countries. For the most up-to-date travel health-related information from the CDC, click here.
Travelers’ diarrhea (TD) occurs in up to 70 % of travelers to developing countries. The World Health Organization defines it as passage of at least three loose stools in a 24-hour period. Most often it is self-limited, with symptoms lasting a median of 3-4 days. Although TD can be caused by bacteria, protozoa, and viruses, bacteria are usually the etiology, with enterotoxigenic Escherichia coli being the most common pathogens. Other bacterial etiologies include Shigella and Campylobacter species. Two antimicrobials are frequently prescribed to travelers for self-treatment of TD: ciprofloxacin and azithromycin. Most young children are prescribed the latter; however, in older children, ciprofloxacin may be prescribed off label, as its use in persons younger than 18 years is not approved by the Food and Drug Administration.
In December 2014, PulseNet, the national molecular subtyping network for food-borne disease, detected a multistate cluster of ciprofloxacin-resistant Shigella sonnei. Between May 2014 and February 2015, 157 cases including 37 children were detected in 32 states and Puerto Rico. Nine of the cases identified by PulseNet, and an additional 76 cases, were associated with an outbreak of ciprofloxacin-resistant S. sonnei in San Francisco. Antibiotic susceptibility was available for 126 isolates, of which 109 (87%) were not ciprofloxacin susceptible. Travel history was available for 75 patients not associated with the San Francisco outbreak, and slightly more than half (40) were associated with international travel. The island of Hispaniola (Dominican Republic = 22 cases and Haiti = 4 cases) was the most common destination, followed by India (8 cases) and Morocco (3 cases). The remaining destinations were Asia and Europe (MMWR 2015;64:318-20) Travel history was available and positive for 23 of the 37 children (62%).
Why such a concern? International travelers are at risk of becoming colonized with drug-resistant bacteria and have the potential to spread them domestically. It has already begun. In 2012, the National Antimicrobial Resistance Monitoring System (NARMS) revealed that isolates of S. sonnei had the following resistance pattern: trimethoprim/sulfamethoxazole, 42%; ampicillin, 18%; and ciprofloxacin, 2.1%. During this outbreak, 19 of the 126 isolates were tested by NARMS with the following resistance patterns noted: trimethoprim/sulfamethoxazole, 84%; ampicillin, 5%; and ciprofloxacin, 32%.
More judicious use of antibiotics is necessary. As pediatricians, we are not immune to this issue. The challenge is when, if at all, antibiotics should be prescribed for TD, and under what conditions should patients be instructed to use them. I’m rethinking my own practice. TD is one of the most common illnesses travelers acquire and is easily treated, but at what cost? The one expression I keep hearing myself say is, First do no harm.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures.
ID Consult: National immunization coverage and measles
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.
Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.
Measles
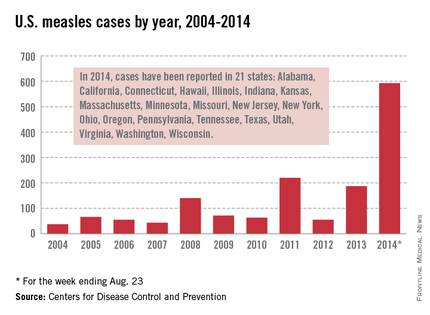
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
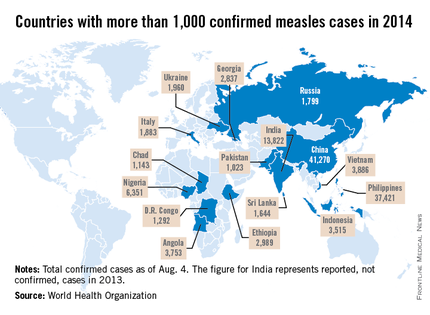
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.

Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.

Measles
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.

Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.

Measles
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Travel Medicine Tips and Updates
Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.
Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
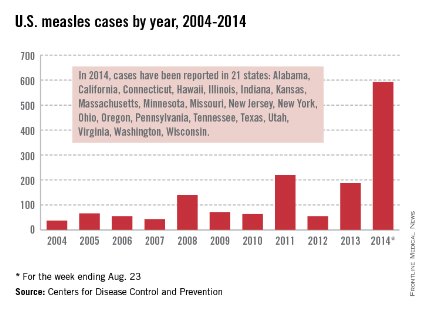
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.
• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
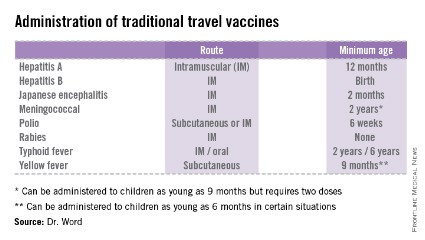
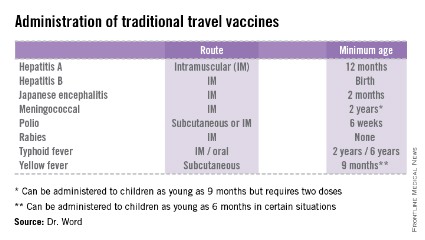
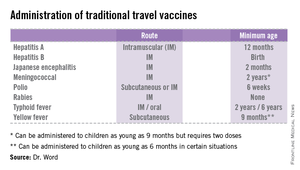
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.

Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.

• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.

Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.

• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Potpourri of travel medicine tips and updates
School’s out for the summer soon! Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.
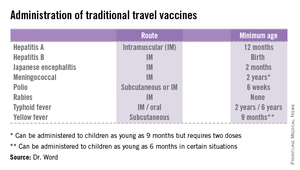
Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.
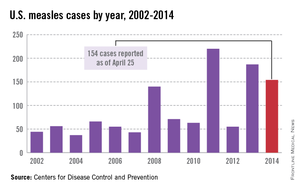
• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
School’s out for the summer soon! Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.

Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.

• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
School’s out for the summer soon! Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.

Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.

• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Adolescent immunizations – Focus on HPV vaccine
The U.S. immunization program has been one of the country’s most successful initiatives and best investments. Prior to 2005, vaccines were targeted for administration to infants and young children. Adolescence was a period for catch-up immunizations. All that changed in 2005 when the first meningococcal conjugate vaccine (MCV) was recommended for administration to preteens at 11-12 years and college freshmen residing in dormitories by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). Shortly thereafter in 2006, a new tetanus toxoid, diphtheria, and acellular pertussis vaccine (Tdap) was recommended, and in March 2007 the quadrivalent human papillomavirus vaccine (HPV4: types 6, 11, 16, and 18) was recommended for use in girls, starting at age 11-12 years, and young women up to 26 years of age. In 2009, a bivalent HPV vaccine (HPV2: types 16 and 18) was licensed, and in 2010, ACIP recommendations indicated that either HPV4 or HPV2 vaccine could be administered to girls and young women. In addition, the use of HPV4 vaccine in males was permitted. In 2011, ACIP recommended routine administration of HPV4 to boys and young adult males up to 21 years of age. Adolescents were the target population for these vaccines, and administration was recommended at the 11- to 12-year wellness visit. The primary role of the adolescent encounter was no longer to provide catch-up immunizations. A definitive adolescent immunization schedule had been established.
Why introduce the HPV vaccine so early?
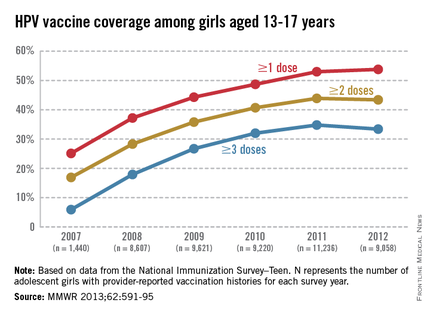
HPV is the most common sexually transmitted infection in both men and women. Recent data suggest that approximately 79 million individuals are infected (Sex. Transm. Dis. 2013;40:187-93). Annually, about 14 million, mostly young adults are infected. Most sexually active individuals will acquire HPV. It is most common in teens and young adults, and intercourse is not required for transmission. It can be transmitted with any type of intimate sexual contact, and it has been isolated from virgins. The majority of these infections are asymptomatic and self- limited. However, persistent infection is associated with cervical and other types of anogenital cancer, and genital warts in both men and women. Complications of these infections may take years to manifest.
HPV is categorized by its epidemiologic association with cervical cancer. High-risk types cause cervical cancer, and HPV types 16 and 18 account for the majority of cervical cancers (66%) These two types are also associated with vaginal (55%), anal (79%), and oropharyngeal (62%) cancer (MMWR 2014 Jan. 31;63;69-72). It is estimated that each year there are 26,000 HPV-related cancers including 8,800 cases in men and 17,000 in women, 4,000 of whom will die of cervical cancer, according to the CDC. Low-risk types including HPV types 6 and 11 cause benign/low-grade cervical cell changes, recurrent papillomatosis, and 90% of the cases of genital warts.
Once a person is infected, HPV usually clears. If not, cervical intraepithelial neoplasia (CIN) may occur. The infection may still resolve spontaneously. If it persists, the degree of dysplasia can progress. Several years may pass before progression to invasive cancer. HPV vaccines are prophylactic like other vaccines. They cannot prevent disease progression and need to be administered before exposure to the viruses.
Compared with the introduction of other vaccines, such as Haemophilus influenzae type b and Prevnar7, some pediatric care providers may feel we may not have the benefit of realizing our efforts as immediately as in the past. However, encouraging vaccine effectiveness data in U.S. teens has been published. In one study, the investigators compared HPV prevalence data from the pre- and postvaccine era collected during the National Health and Nutrition Examination Survey. Among females aged 14-19 years, HPV prevalence (HPV-6, -11, -16, or -18 ) decreased from 11.5% in 2003-2006 to 5.1% in 2007-2010. That is a 56% reduction in vaccine type HPV prevalence. This decrease in prevalence occurred within 4 years of vaccine introduction and low vaccine uptake. (J. Infect. Dis. 2013;208:385-93). Studies conducted in Denmark, Australia, Germany, and New Zealand also have shown significant declines in HPV4 vaccine type infection prevalence.
Vaccination coverage
The CDC tracks vaccination coverage annually in the National Immunization Survey–Teen (NIS-Teen), with data obtained from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR 2013;62:685-93). Vaccination coverage differed significantly although each vaccine is recommended to be routinely administered at the 11- to 12-year visit. Although an increase from 25% to 53% had been noted between 2007 and 2011, in 2012, coverage for receiving at least one dose of HPV among females was almost 54%, essentially unchanged since 2011. The number who had received the recommended three doses was also essentially unchanged from 2011 to 2012 (34.8% in 2011 and 33.4% in 2012). Receipt of a single dose of HPV in boys was 8.3% in 2011 and 20.8% in 2012, the first year after the vaccine was recommended. Completion of the series in boys was 6.3%, an increase from 1.3% in 2011.
In contrast, the 2012 coverage for Tdap increased to 85% and MCV4, to 74%. It has been suggested that the higher coverage of Tdap and MCV may be due to the 40 and 13 states, respectively, that require them for middle school entry.
The disparity in coverage between Tdap and other vaccines suggests there are numerous missed opportunities to vaccinate adolescents. Data revealed that missed opportunities for girls increased from 20.8% in 2007 to 84% in 2012. If all missed opportunities had been eliminated, HPV coverage for at least one dose could have reached 92.6%.Almost 25% of parents indicated that they had no plan to immunize their daughter. The top reasons parents stated for not immunizing their daughters included: not needed or necessary, 19.1%; not recommended by provider, 14.2%; safety concerns, 13.3%; lack of knowledge, 12.6%; and not sexually active, 10.1%. (MMWR 2013;62:591-5).
Vaccine safety also was addressed. All reported adverse events were consistent with prelicensure clinical trial data. Ninety two percent of all adverse events were nonserious and included syncope, dizziness, nausea, and fever. Reports peaked in 2008 and have declined each year thereafter.
Challenges for HPV prevention
Improving immunization coverage is critical. There are numerous strategies to increase coverage including reminder recall systems, standing orders, and educating parents, patients, health care providers, and office staff who interact with parents. Education should reemphasize why immunization is initiated at 11-12 years and that completion of the series is recommended by 13 years. School requirements have always led to an increase in vaccination coverage. Only the District of Columbia has one for HPV. In this case, eliminating missed opportunities is crucial. It is estimated that for every year coverage is delayed, an additional 4,400 women will develop cervical cancer. The reality is that the burden of HPV-related cancers will persist if coverage is not increased.
As Louis Pasteur once said, "When meditating over a disease, I never think of finding a remedy for it, but, instead a means of preventing it."
For additional resources to assist with discussions about HPV, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. E-mail her at [email protected]. Scan this QR code or visit pediatricnews.com.
The U.S. immunization program has been one of the country’s most successful initiatives and best investments. Prior to 2005, vaccines were targeted for administration to infants and young children. Adolescence was a period for catch-up immunizations. All that changed in 2005 when the first meningococcal conjugate vaccine (MCV) was recommended for administration to preteens at 11-12 years and college freshmen residing in dormitories by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). Shortly thereafter in 2006, a new tetanus toxoid, diphtheria, and acellular pertussis vaccine (Tdap) was recommended, and in March 2007 the quadrivalent human papillomavirus vaccine (HPV4: types 6, 11, 16, and 18) was recommended for use in girls, starting at age 11-12 years, and young women up to 26 years of age. In 2009, a bivalent HPV vaccine (HPV2: types 16 and 18) was licensed, and in 2010, ACIP recommendations indicated that either HPV4 or HPV2 vaccine could be administered to girls and young women. In addition, the use of HPV4 vaccine in males was permitted. In 2011, ACIP recommended routine administration of HPV4 to boys and young adult males up to 21 years of age. Adolescents were the target population for these vaccines, and administration was recommended at the 11- to 12-year wellness visit. The primary role of the adolescent encounter was no longer to provide catch-up immunizations. A definitive adolescent immunization schedule had been established.
Why introduce the HPV vaccine so early?

HPV is the most common sexually transmitted infection in both men and women. Recent data suggest that approximately 79 million individuals are infected (Sex. Transm. Dis. 2013;40:187-93). Annually, about 14 million, mostly young adults are infected. Most sexually active individuals will acquire HPV. It is most common in teens and young adults, and intercourse is not required for transmission. It can be transmitted with any type of intimate sexual contact, and it has been isolated from virgins. The majority of these infections are asymptomatic and self- limited. However, persistent infection is associated with cervical and other types of anogenital cancer, and genital warts in both men and women. Complications of these infections may take years to manifest.
HPV is categorized by its epidemiologic association with cervical cancer. High-risk types cause cervical cancer, and HPV types 16 and 18 account for the majority of cervical cancers (66%) These two types are also associated with vaginal (55%), anal (79%), and oropharyngeal (62%) cancer (MMWR 2014 Jan. 31;63;69-72). It is estimated that each year there are 26,000 HPV-related cancers including 8,800 cases in men and 17,000 in women, 4,000 of whom will die of cervical cancer, according to the CDC. Low-risk types including HPV types 6 and 11 cause benign/low-grade cervical cell changes, recurrent papillomatosis, and 90% of the cases of genital warts.
Once a person is infected, HPV usually clears. If not, cervical intraepithelial neoplasia (CIN) may occur. The infection may still resolve spontaneously. If it persists, the degree of dysplasia can progress. Several years may pass before progression to invasive cancer. HPV vaccines are prophylactic like other vaccines. They cannot prevent disease progression and need to be administered before exposure to the viruses.

Compared with the introduction of other vaccines, such as Haemophilus influenzae type b and Prevnar7, some pediatric care providers may feel we may not have the benefit of realizing our efforts as immediately as in the past. However, encouraging vaccine effectiveness data in U.S. teens has been published. In one study, the investigators compared HPV prevalence data from the pre- and postvaccine era collected during the National Health and Nutrition Examination Survey. Among females aged 14-19 years, HPV prevalence (HPV-6, -11, -16, or -18 ) decreased from 11.5% in 2003-2006 to 5.1% in 2007-2010. That is a 56% reduction in vaccine type HPV prevalence. This decrease in prevalence occurred within 4 years of vaccine introduction and low vaccine uptake. (J. Infect. Dis. 2013;208:385-93). Studies conducted in Denmark, Australia, Germany, and New Zealand also have shown significant declines in HPV4 vaccine type infection prevalence.
Vaccination coverage
The CDC tracks vaccination coverage annually in the National Immunization Survey–Teen (NIS-Teen), with data obtained from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR 2013;62:685-93). Vaccination coverage differed significantly although each vaccine is recommended to be routinely administered at the 11- to 12-year visit. Although an increase from 25% to 53% had been noted between 2007 and 2011, in 2012, coverage for receiving at least one dose of HPV among females was almost 54%, essentially unchanged since 2011. The number who had received the recommended three doses was also essentially unchanged from 2011 to 2012 (34.8% in 2011 and 33.4% in 2012). Receipt of a single dose of HPV in boys was 8.3% in 2011 and 20.8% in 2012, the first year after the vaccine was recommended. Completion of the series in boys was 6.3%, an increase from 1.3% in 2011.
In contrast, the 2012 coverage for Tdap increased to 85% and MCV4, to 74%. It has been suggested that the higher coverage of Tdap and MCV may be due to the 40 and 13 states, respectively, that require them for middle school entry.
The disparity in coverage between Tdap and other vaccines suggests there are numerous missed opportunities to vaccinate adolescents. Data revealed that missed opportunities for girls increased from 20.8% in 2007 to 84% in 2012. If all missed opportunities had been eliminated, HPV coverage for at least one dose could have reached 92.6%.Almost 25% of parents indicated that they had no plan to immunize their daughter. The top reasons parents stated for not immunizing their daughters included: not needed or necessary, 19.1%; not recommended by provider, 14.2%; safety concerns, 13.3%; lack of knowledge, 12.6%; and not sexually active, 10.1%. (MMWR 2013;62:591-5).
Vaccine safety also was addressed. All reported adverse events were consistent with prelicensure clinical trial data. Ninety two percent of all adverse events were nonserious and included syncope, dizziness, nausea, and fever. Reports peaked in 2008 and have declined each year thereafter.
Challenges for HPV prevention
Improving immunization coverage is critical. There are numerous strategies to increase coverage including reminder recall systems, standing orders, and educating parents, patients, health care providers, and office staff who interact with parents. Education should reemphasize why immunization is initiated at 11-12 years and that completion of the series is recommended by 13 years. School requirements have always led to an increase in vaccination coverage. Only the District of Columbia has one for HPV. In this case, eliminating missed opportunities is crucial. It is estimated that for every year coverage is delayed, an additional 4,400 women will develop cervical cancer. The reality is that the burden of HPV-related cancers will persist if coverage is not increased.
As Louis Pasteur once said, "When meditating over a disease, I never think of finding a remedy for it, but, instead a means of preventing it."
For additional resources to assist with discussions about HPV, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. E-mail her at [email protected]. Scan this QR code or visit pediatricnews.com.
The U.S. immunization program has been one of the country’s most successful initiatives and best investments. Prior to 2005, vaccines were targeted for administration to infants and young children. Adolescence was a period for catch-up immunizations. All that changed in 2005 when the first meningococcal conjugate vaccine (MCV) was recommended for administration to preteens at 11-12 years and college freshmen residing in dormitories by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP). Shortly thereafter in 2006, a new tetanus toxoid, diphtheria, and acellular pertussis vaccine (Tdap) was recommended, and in March 2007 the quadrivalent human papillomavirus vaccine (HPV4: types 6, 11, 16, and 18) was recommended for use in girls, starting at age 11-12 years, and young women up to 26 years of age. In 2009, a bivalent HPV vaccine (HPV2: types 16 and 18) was licensed, and in 2010, ACIP recommendations indicated that either HPV4 or HPV2 vaccine could be administered to girls and young women. In addition, the use of HPV4 vaccine in males was permitted. In 2011, ACIP recommended routine administration of HPV4 to boys and young adult males up to 21 years of age. Adolescents were the target population for these vaccines, and administration was recommended at the 11- to 12-year wellness visit. The primary role of the adolescent encounter was no longer to provide catch-up immunizations. A definitive adolescent immunization schedule had been established.
Why introduce the HPV vaccine so early?

HPV is the most common sexually transmitted infection in both men and women. Recent data suggest that approximately 79 million individuals are infected (Sex. Transm. Dis. 2013;40:187-93). Annually, about 14 million, mostly young adults are infected. Most sexually active individuals will acquire HPV. It is most common in teens and young adults, and intercourse is not required for transmission. It can be transmitted with any type of intimate sexual contact, and it has been isolated from virgins. The majority of these infections are asymptomatic and self- limited. However, persistent infection is associated with cervical and other types of anogenital cancer, and genital warts in both men and women. Complications of these infections may take years to manifest.
HPV is categorized by its epidemiologic association with cervical cancer. High-risk types cause cervical cancer, and HPV types 16 and 18 account for the majority of cervical cancers (66%) These two types are also associated with vaginal (55%), anal (79%), and oropharyngeal (62%) cancer (MMWR 2014 Jan. 31;63;69-72). It is estimated that each year there are 26,000 HPV-related cancers including 8,800 cases in men and 17,000 in women, 4,000 of whom will die of cervical cancer, according to the CDC. Low-risk types including HPV types 6 and 11 cause benign/low-grade cervical cell changes, recurrent papillomatosis, and 90% of the cases of genital warts.
Once a person is infected, HPV usually clears. If not, cervical intraepithelial neoplasia (CIN) may occur. The infection may still resolve spontaneously. If it persists, the degree of dysplasia can progress. Several years may pass before progression to invasive cancer. HPV vaccines are prophylactic like other vaccines. They cannot prevent disease progression and need to be administered before exposure to the viruses.

Compared with the introduction of other vaccines, such as Haemophilus influenzae type b and Prevnar7, some pediatric care providers may feel we may not have the benefit of realizing our efforts as immediately as in the past. However, encouraging vaccine effectiveness data in U.S. teens has been published. In one study, the investigators compared HPV prevalence data from the pre- and postvaccine era collected during the National Health and Nutrition Examination Survey. Among females aged 14-19 years, HPV prevalence (HPV-6, -11, -16, or -18 ) decreased from 11.5% in 2003-2006 to 5.1% in 2007-2010. That is a 56% reduction in vaccine type HPV prevalence. This decrease in prevalence occurred within 4 years of vaccine introduction and low vaccine uptake. (J. Infect. Dis. 2013;208:385-93). Studies conducted in Denmark, Australia, Germany, and New Zealand also have shown significant declines in HPV4 vaccine type infection prevalence.
Vaccination coverage
The CDC tracks vaccination coverage annually in the National Immunization Survey–Teen (NIS-Teen), with data obtained from the 50 states, the District of Columbia, the U.S. Virgin Islands, and six major urban areas (MMWR 2013;62:685-93). Vaccination coverage differed significantly although each vaccine is recommended to be routinely administered at the 11- to 12-year visit. Although an increase from 25% to 53% had been noted between 2007 and 2011, in 2012, coverage for receiving at least one dose of HPV among females was almost 54%, essentially unchanged since 2011. The number who had received the recommended three doses was also essentially unchanged from 2011 to 2012 (34.8% in 2011 and 33.4% in 2012). Receipt of a single dose of HPV in boys was 8.3% in 2011 and 20.8% in 2012, the first year after the vaccine was recommended. Completion of the series in boys was 6.3%, an increase from 1.3% in 2011.
In contrast, the 2012 coverage for Tdap increased to 85% and MCV4, to 74%. It has been suggested that the higher coverage of Tdap and MCV may be due to the 40 and 13 states, respectively, that require them for middle school entry.
The disparity in coverage between Tdap and other vaccines suggests there are numerous missed opportunities to vaccinate adolescents. Data revealed that missed opportunities for girls increased from 20.8% in 2007 to 84% in 2012. If all missed opportunities had been eliminated, HPV coverage for at least one dose could have reached 92.6%.Almost 25% of parents indicated that they had no plan to immunize their daughter. The top reasons parents stated for not immunizing their daughters included: not needed or necessary, 19.1%; not recommended by provider, 14.2%; safety concerns, 13.3%; lack of knowledge, 12.6%; and not sexually active, 10.1%. (MMWR 2013;62:591-5).
Vaccine safety also was addressed. All reported adverse events were consistent with prelicensure clinical trial data. Ninety two percent of all adverse events were nonserious and included syncope, dizziness, nausea, and fever. Reports peaked in 2008 and have declined each year thereafter.
Challenges for HPV prevention
Improving immunization coverage is critical. There are numerous strategies to increase coverage including reminder recall systems, standing orders, and educating parents, patients, health care providers, and office staff who interact with parents. Education should reemphasize why immunization is initiated at 11-12 years and that completion of the series is recommended by 13 years. School requirements have always led to an increase in vaccination coverage. Only the District of Columbia has one for HPV. In this case, eliminating missed opportunities is crucial. It is estimated that for every year coverage is delayed, an additional 4,400 women will develop cervical cancer. The reality is that the burden of HPV-related cancers will persist if coverage is not increased.
As Louis Pasteur once said, "When meditating over a disease, I never think of finding a remedy for it, but, instead a means of preventing it."
For additional resources to assist with discussions about HPV, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. E-mail her at [email protected]. Scan this QR code or visit pediatricnews.com.
International travel - Focus on timely intervention
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Many of your patients will head for international destinations this summer, where they may be exposed to infectious diseases and other health risks they normally do not encounter in the United States.
For the majority of patients, these exposures will be brief; however, several may be extended due to study abroad or parental job relocation. More and more adolescents also are traveling to resource-limited areas doing volunteer work or adventure travel, and many are residing with host families. Children with chronic diseases pose concerns directly related to their underlying conditions, susceptibility, and availability of medical care in the host country. While most international travel plans are made at least 3 months in advance, health precautions such as immunizations and preventive medication often are not considered as travel plans are being finalized. If you are lucky, your patients will have mentioned their plans to you prior to finalizing their trips. You may receive a call at the last minute for assistance in helping to prepare them for a safe and healthy journey.
The U.S. Office of Travel & Tourism reports that slightly more than 60 million Americans traveled outside of the United States in 2012, with 28.5 million of the final destinations being overseas. Children accounted for approximately 2.4 million travelers. While tourism was the most common reason for travel, children were more likely to be visiting friends and relatives (VFR). Studies have revealed significantly increased health risks among VFR travelers, who often stay in private homes and in less-developed areas, compared with vacationers or business travelers who are more likely to be staying in hotels and in urban areas (Pediatrics 2010;125:e1072-80).
Is it really necessary to seek pretravel advice? Some travelers are not convinced. To facilitate this discussion, I thought I would share a recent call.
You are informed via voicemail that a 3-year-old is traveling with his family to Madras, India, for 8 weeks. He is visiting relatives, and the family may visit rural areas. The accommodations are air conditioned and the family is departing in 5 days! They would like to schedule an appointment immediately. What can you do?
Vital information has already been provided. The destination, type of accommodations, activities, duration of stay, and that the patient is a VFR are all important details when making vaccine and other recommendations. First, determine if the child’s routine immunizations are up to date. Next, determine the potential exposures for this patient, and identify vaccine-preventable and nonpreventable diseases. If there is a travel medicine specialist in your area who also sees children, you can refer the patient. If one is not readily available or you prefer to manage the patient, a great resource is the Centers for Disease Control and Prevention Traveler's Health site.
Vaccine preventable diseases include hepatitis A, hepatitis B, Japanese encephalitis, polio, rabies, typhoid, and influenza. Nonvaccine preventable diseases include chikungunya and dengue fevers. Avian influenza, malaria, tuberculosis, and traveler’s diarrhea are also cause for concern.
If you determine the routine immunizations are up to date, remember that measles is still a concern in many countries, and current U.S. recommendations state that all children at least 12 months of age should have two doses prior to leaving the United States. Although routinely administered at 4 years of age, the second dose of MMR can be administered as early as 4 weeks after the first dose. Those aged 6-11 months should have one dose prior to leaving the country. The remaining two doses should be administered at the usual time. Therefore, a total of three doses will be required to complete the series. Since the immunizations are up to date, this patient will also be protected against hepatitis A and B in addition to polio. Hepatitis A is the most common vaccine preventable disease acquired by travelers.
Rabies is prevalent in India, and all animal bites should be taken seriously. Because the patient is in a major urban area, access to both rabies vaccine and immunoglobulin should not be a concern. Japanese encephalitis will be circulating (May-October), but is usually found in rural agricultural areas. Mosquito precautions utilizing DEET (30%) on exposed areas or Permethrine-containing sprays on clothes to repel mosquitoes and ticks should be emphasized if travel to rural areas occurs. Vaccines for rabies and Japanese encephalitis would not be recommended for this patient. If the itinerary were different, they may be considered. Ixiaro, an inactivated Japanese encephalitis (JE) vaccine was approved for use in children as young as 2 months of age in May 2013. Previously, it was approved for use only in those at least 17 years of age in the United States. Both rabies and JE require a minimum of 21 and 28 days, respectively, to complete, and JE should be completed at least 1 week prior to exposure.
Typhoid fever (enteric fever) occurs worldwide, with an estimated 22 million cases annually. In 2012, 343 cases were reported in the United States, most of which were in recent travelers. The risk for typhoid fever is highest for travelers to southern Asia (6-30 times higher) than for all other destinations (Centers for Disease Control and Prevention. CDC Health Information for International Travel 2012. New York: Oxford University Press; 2012). Two types of vaccine are available: an oral, live attenuated vaccine for those at least 6 years of age and an injectable polysaccharide vaccine for those at least 2 years of age. In this case there is only one option, the injectable vaccine. Ideally, it should be administered at least 2 weeks prior to travel. Although this patient will not have optimal benefit of vaccine for at least 2 weeks, he will be there an additional 6 weeks, staying with friends and relatives, and is traveling to a high-risk country. Vaccine administration is recommended, and the parent should be fully informed when maximum benefit will occur. Food and water precautions are essential, especially during the first 2 weeks.
Precautions such as consumption of only boiled or bottled water, avoidance of undercooked or raw meat and seafood, and avoidance of raw fruit and vegetables to minimize acquisition of traveler’s diarrhea should be discussed. Antimicrobials also can be provided.
Options for malaria prophylaxis are limited due to the ensuing departure date and the child’s age. Atovaquone-Proguanil can be prescribed because it can be initiated 1-2 days prior to departure. It is taken daily while in India and for 1 week after return. He is too young for doxycycline. Mefloquine, administered weekly, should begin at least 2 weeks prior to exposure, so it is not an option. There is no role for chloroquine because chloroquine-resistant malaria is present in this country. In contrast to malaria, where mosquitoes usually feed dusk to dawn, chikungunya and dengue fever are transmitted by mosquitoes during the daytime.
No specific prevention for tuberculosis is available. Avoidance of persons with chronic cough or known disease is recommended.
It can be challenging for a busy practitioner to stay abreast of the latest developments in non–routinely administered vaccines, disease outbreaks, or country-specific entry requirements. Many vaccines, such as those against typhoid or rabies, are not routinely available in the patient’s medical home.
Ideally, patients planning international travel should be referred to a travel medicine clinic 1 month prior to travel. Some vaccines take up to 2 weeks to become effective, while others – such as yellow fever – should be administered at least 10 days prior to travel. However, interventions are still available for the last-minute patient, as in this case. Counseling for a variety of issues is provided. It’s not just about the vaccines.
International travel among children and adolescents will continue to rise. It behooves every primary care practitioner to develop a system to determine the summertime plans/needs of their patients. Not all travel medicine clinics provide services to children. It’s a good idea to find out which ones do in your area. You can always locate a clinic through the International Society of Travel Medicine and the Centers for Disease Control and Prevention.
While this call is not the norm, it occurs frequently. In contrast, another call for a 2-month photography trip to Uganda was received the same day. Departure was 6 weeks later!
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Cold weather and diarrhea: Don't forget yersiniosis
The genus Yersinia includes 11 species. Three species are generally associated with human disease; Y. enterocolitica, Y. pestis, and Y. pseudotuberculosis. Yersinia pestis is the causative agent of plague. Yersinia pseudotuberculosis can manifest with fever, abdominal pain, and scarlatiniform rash. Additional symptoms include diarrhea, sterile joint effusions, erythema nodosum, and septicemia; these symptoms can be indistinguishable from Kawasaki Disease. By report, almost 10 % of cases of Kawasaki Disease in Japan have serologic or bacteriologic evidence of Y. pseudotuberculosis infection [Redbook: 2012 Report of the Committee on Infectious Diseases, 795-7]. Y. enterocolitica is most often associated with yersiniosis.
Although Y. enterocolitica is not the most common cause of diarrheal illness in the United States, it is one of the nine pathogens that have been monitored by the Foodborne Diseases Active Surveillance Network (FoodNet) since 1996. In the United States, it is estimated that Y. enterocolitica causes slightly over 115,000 infections annually (Emerg. Infect. Dis. 2011;17:7-15). The disease is more common in cooler months. It is transmitted by consumption of contaminated food, especially raw or undercooked pork products.
Only a few outbreaks have been reported in the United States, and these were usually associated with consumption of pork, specifically chitterlings (pig intestines), a winter holiday dish prepared most frequently in black households in the South (MMWR 1990;39:819-20). Transmission to infants and young children is thought to occur from caretakers preparing chitterlings who have not adequately cleaned their hands prior to touching objects subsequently handled by the child.
The incubation period is usually 4-6 days (range, 1-14 days). The duration of diarrhea is variable and can persist up to 3 weeks. Organisms can be excreted an average of 6 weeks. Clinical manifestations vary by age. Younger children usually present with fever and diarrhea. Stools frequently contain blood and leucocytes. Vomiting is also reported in most series. In contrast, older children and adults often present with a pseudoappendicitis syndrome with right-sided abdominal pain and fever. Leukocytosis is often present. At surgery, mesenteric adenitis is observed, and the appendix generally is normal.
Bacteremia can occur and is usually associated with infection in children less than 1 year of age and in those with iron-overloaded states, including persons with sickle cell disease, beta-thalassemia, and those receiving deferoxamine therapy. While uncommon, focal manifestations including pharyngitis, osteomyelitis, pyomyositis, pneumonia, empyema, and meningitis may occur.
Diagnosis is confirmed by isolation of the organism from stool, blood, peritoneal fluid, lymph nodes, and throat cultures. Most laboratories do not routinely test for Yersinia in stool cultures. If Y. enterocolitica is suspected, you should notify the laboratory so the stool can be plated on appropriate media (CIN agar). Serologic tests to detect a rise in serum antibody titers to confirm infection are available in reference and research laboratories, but are not generally used for diagnosis. Cross reactivity with Brucella, Salmonella, Vibrio, and Rickettsia may lead to false positive titer results. Y. enterocolitica antibodies also have antigenic similarity with thyroid tissue. You may see persistent elevation of titers in patients with thyroid disease.
Benefit of antimicrobial therapy for isolated Y. enterocolitica gastrointestinal disease and Y. pseudotuberculosis has not been established. Therapy may decrease the duration of fecal shedding. Treatment is indicated for immunocompromised hosts and persons with septicemia and focal infections. Y. enterocolitica and Y. pseudotuberculosis are usually sensitive to trimethoprim-sulfamethoxazole, aminoglycosides, cefotaxime, fluoroquinolones (persons greater than 18 years of age or older), and tetracycline or doxycycline (for children at least 8 years of age and older).
So what is the actual incidence and when should the practitioner be concerned? Initial population based surveillance data for Y. enterocolitica infections in FoodNet sites between 1996 and 1999 reported an overall incidence of 0.9 cases per 100,000 population. The highest incidence was among black and Asian individuals and was 3.2 cases and 1.5 cases per 100,000 population, respectively. The incidence in Hispanics and whites was 0.6 and 0.4 cases per 100,000 respectively. Incidence increased with decreasing age in all racial/ethnic groups. Blacks infants had the highest incidence, 141.9 cases/100,000 population, and the highest incidence in infants was reported from Georgia (207 cases/100,000). Seasonal variation in incidence was noted only in black individuals with peak activity occurring in December (Clin. Infect. Dis. 2004;38[Suppl 3]:S181-9).
The most recent data from FoodNet (1996-2009) reveals an overall incidence of 0.5/100,000. There was a decline in incidence in all racial and ethnic groups. The highest incidence is still observed in black and Asians (0.9 and 0.7 per 100,000). The most dramatic decline occurred in black individuals (3.2 vs. 0.9 per 100,000). In 1998, an educational campaign was initiated in Georgia that targeted high-risk individuals and provided information on the safe handling and preparation of chitterlings. The state of Georgia reported the greatest decline to 0.4/100,000, which has almost eliminated the racial disparity reported in 2009. It is unclear if this campaign was the only reason for the decline in Georgia. The incidence in whites is 0.2/100,000. Since 2007, the incidence in Asian children less than 5 years of ages has been the highest amongst all racial and ethnic groups. Pork consumption is still assumed to be the major source. Seasonal variability persists amongst Black children less 5 years of age, implying that chitterlings may still be the source of infection for individuals in this group (Clin. Infect. Dis. 2012:54 [Suppl 5]:S385-S90).
In general, yersiniosis should be included in the differential of a febrile diarrheal illness, particularly during the cooler months and holiday season. It is prudent to determine if consumption and/or preparation of chitterlings or other pork products by the patient or caretakers has occurred. This will enable you to alert the laboratory so stool specimens can be cultured on the appropriate medium (CIN agar). Consumption of chitterlings is not limited to any specific racial or ethnic group. Individuals from rural and farming areas may also consume this product.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The genus Yersinia includes 11 species. Three species are generally associated with human disease; Y. enterocolitica, Y. pestis, and Y. pseudotuberculosis. Yersinia pestis is the causative agent of plague. Yersinia pseudotuberculosis can manifest with fever, abdominal pain, and scarlatiniform rash. Additional symptoms include diarrhea, sterile joint effusions, erythema nodosum, and septicemia; these symptoms can be indistinguishable from Kawasaki Disease. By report, almost 10 % of cases of Kawasaki Disease in Japan have serologic or bacteriologic evidence of Y. pseudotuberculosis infection [Redbook: 2012 Report of the Committee on Infectious Diseases, 795-7]. Y. enterocolitica is most often associated with yersiniosis.
Although Y. enterocolitica is not the most common cause of diarrheal illness in the United States, it is one of the nine pathogens that have been monitored by the Foodborne Diseases Active Surveillance Network (FoodNet) since 1996. In the United States, it is estimated that Y. enterocolitica causes slightly over 115,000 infections annually (Emerg. Infect. Dis. 2011;17:7-15). The disease is more common in cooler months. It is transmitted by consumption of contaminated food, especially raw or undercooked pork products.
Only a few outbreaks have been reported in the United States, and these were usually associated with consumption of pork, specifically chitterlings (pig intestines), a winter holiday dish prepared most frequently in black households in the South (MMWR 1990;39:819-20). Transmission to infants and young children is thought to occur from caretakers preparing chitterlings who have not adequately cleaned their hands prior to touching objects subsequently handled by the child.
The incubation period is usually 4-6 days (range, 1-14 days). The duration of diarrhea is variable and can persist up to 3 weeks. Organisms can be excreted an average of 6 weeks. Clinical manifestations vary by age. Younger children usually present with fever and diarrhea. Stools frequently contain blood and leucocytes. Vomiting is also reported in most series. In contrast, older children and adults often present with a pseudoappendicitis syndrome with right-sided abdominal pain and fever. Leukocytosis is often present. At surgery, mesenteric adenitis is observed, and the appendix generally is normal.
Bacteremia can occur and is usually associated with infection in children less than 1 year of age and in those with iron-overloaded states, including persons with sickle cell disease, beta-thalassemia, and those receiving deferoxamine therapy. While uncommon, focal manifestations including pharyngitis, osteomyelitis, pyomyositis, pneumonia, empyema, and meningitis may occur.
Diagnosis is confirmed by isolation of the organism from stool, blood, peritoneal fluid, lymph nodes, and throat cultures. Most laboratories do not routinely test for Yersinia in stool cultures. If Y. enterocolitica is suspected, you should notify the laboratory so the stool can be plated on appropriate media (CIN agar). Serologic tests to detect a rise in serum antibody titers to confirm infection are available in reference and research laboratories, but are not generally used for diagnosis. Cross reactivity with Brucella, Salmonella, Vibrio, and Rickettsia may lead to false positive titer results. Y. enterocolitica antibodies also have antigenic similarity with thyroid tissue. You may see persistent elevation of titers in patients with thyroid disease.
Benefit of antimicrobial therapy for isolated Y. enterocolitica gastrointestinal disease and Y. pseudotuberculosis has not been established. Therapy may decrease the duration of fecal shedding. Treatment is indicated for immunocompromised hosts and persons with septicemia and focal infections. Y. enterocolitica and Y. pseudotuberculosis are usually sensitive to trimethoprim-sulfamethoxazole, aminoglycosides, cefotaxime, fluoroquinolones (persons greater than 18 years of age or older), and tetracycline or doxycycline (for children at least 8 years of age and older).
So what is the actual incidence and when should the practitioner be concerned? Initial population based surveillance data for Y. enterocolitica infections in FoodNet sites between 1996 and 1999 reported an overall incidence of 0.9 cases per 100,000 population. The highest incidence was among black and Asian individuals and was 3.2 cases and 1.5 cases per 100,000 population, respectively. The incidence in Hispanics and whites was 0.6 and 0.4 cases per 100,000 respectively. Incidence increased with decreasing age in all racial/ethnic groups. Blacks infants had the highest incidence, 141.9 cases/100,000 population, and the highest incidence in infants was reported from Georgia (207 cases/100,000). Seasonal variation in incidence was noted only in black individuals with peak activity occurring in December (Clin. Infect. Dis. 2004;38[Suppl 3]:S181-9).
The most recent data from FoodNet (1996-2009) reveals an overall incidence of 0.5/100,000. There was a decline in incidence in all racial and ethnic groups. The highest incidence is still observed in black and Asians (0.9 and 0.7 per 100,000). The most dramatic decline occurred in black individuals (3.2 vs. 0.9 per 100,000). In 1998, an educational campaign was initiated in Georgia that targeted high-risk individuals and provided information on the safe handling and preparation of chitterlings. The state of Georgia reported the greatest decline to 0.4/100,000, which has almost eliminated the racial disparity reported in 2009. It is unclear if this campaign was the only reason for the decline in Georgia. The incidence in whites is 0.2/100,000. Since 2007, the incidence in Asian children less than 5 years of ages has been the highest amongst all racial and ethnic groups. Pork consumption is still assumed to be the major source. Seasonal variability persists amongst Black children less 5 years of age, implying that chitterlings may still be the source of infection for individuals in this group (Clin. Infect. Dis. 2012:54 [Suppl 5]:S385-S90).
In general, yersiniosis should be included in the differential of a febrile diarrheal illness, particularly during the cooler months and holiday season. It is prudent to determine if consumption and/or preparation of chitterlings or other pork products by the patient or caretakers has occurred. This will enable you to alert the laboratory so stool specimens can be cultured on the appropriate medium (CIN agar). Consumption of chitterlings is not limited to any specific racial or ethnic group. Individuals from rural and farming areas may also consume this product.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The genus Yersinia includes 11 species. Three species are generally associated with human disease; Y. enterocolitica, Y. pestis, and Y. pseudotuberculosis. Yersinia pestis is the causative agent of plague. Yersinia pseudotuberculosis can manifest with fever, abdominal pain, and scarlatiniform rash. Additional symptoms include diarrhea, sterile joint effusions, erythema nodosum, and septicemia; these symptoms can be indistinguishable from Kawasaki Disease. By report, almost 10 % of cases of Kawasaki Disease in Japan have serologic or bacteriologic evidence of Y. pseudotuberculosis infection [Redbook: 2012 Report of the Committee on Infectious Diseases, 795-7]. Y. enterocolitica is most often associated with yersiniosis.
Although Y. enterocolitica is not the most common cause of diarrheal illness in the United States, it is one of the nine pathogens that have been monitored by the Foodborne Diseases Active Surveillance Network (FoodNet) since 1996. In the United States, it is estimated that Y. enterocolitica causes slightly over 115,000 infections annually (Emerg. Infect. Dis. 2011;17:7-15). The disease is more common in cooler months. It is transmitted by consumption of contaminated food, especially raw or undercooked pork products.
Only a few outbreaks have been reported in the United States, and these were usually associated with consumption of pork, specifically chitterlings (pig intestines), a winter holiday dish prepared most frequently in black households in the South (MMWR 1990;39:819-20). Transmission to infants and young children is thought to occur from caretakers preparing chitterlings who have not adequately cleaned their hands prior to touching objects subsequently handled by the child.
The incubation period is usually 4-6 days (range, 1-14 days). The duration of diarrhea is variable and can persist up to 3 weeks. Organisms can be excreted an average of 6 weeks. Clinical manifestations vary by age. Younger children usually present with fever and diarrhea. Stools frequently contain blood and leucocytes. Vomiting is also reported in most series. In contrast, older children and adults often present with a pseudoappendicitis syndrome with right-sided abdominal pain and fever. Leukocytosis is often present. At surgery, mesenteric adenitis is observed, and the appendix generally is normal.
Bacteremia can occur and is usually associated with infection in children less than 1 year of age and in those with iron-overloaded states, including persons with sickle cell disease, beta-thalassemia, and those receiving deferoxamine therapy. While uncommon, focal manifestations including pharyngitis, osteomyelitis, pyomyositis, pneumonia, empyema, and meningitis may occur.
Diagnosis is confirmed by isolation of the organism from stool, blood, peritoneal fluid, lymph nodes, and throat cultures. Most laboratories do not routinely test for Yersinia in stool cultures. If Y. enterocolitica is suspected, you should notify the laboratory so the stool can be plated on appropriate media (CIN agar). Serologic tests to detect a rise in serum antibody titers to confirm infection are available in reference and research laboratories, but are not generally used for diagnosis. Cross reactivity with Brucella, Salmonella, Vibrio, and Rickettsia may lead to false positive titer results. Y. enterocolitica antibodies also have antigenic similarity with thyroid tissue. You may see persistent elevation of titers in patients with thyroid disease.
Benefit of antimicrobial therapy for isolated Y. enterocolitica gastrointestinal disease and Y. pseudotuberculosis has not been established. Therapy may decrease the duration of fecal shedding. Treatment is indicated for immunocompromised hosts and persons with septicemia and focal infections. Y. enterocolitica and Y. pseudotuberculosis are usually sensitive to trimethoprim-sulfamethoxazole, aminoglycosides, cefotaxime, fluoroquinolones (persons greater than 18 years of age or older), and tetracycline or doxycycline (for children at least 8 years of age and older).
So what is the actual incidence and when should the practitioner be concerned? Initial population based surveillance data for Y. enterocolitica infections in FoodNet sites between 1996 and 1999 reported an overall incidence of 0.9 cases per 100,000 population. The highest incidence was among black and Asian individuals and was 3.2 cases and 1.5 cases per 100,000 population, respectively. The incidence in Hispanics and whites was 0.6 and 0.4 cases per 100,000 respectively. Incidence increased with decreasing age in all racial/ethnic groups. Blacks infants had the highest incidence, 141.9 cases/100,000 population, and the highest incidence in infants was reported from Georgia (207 cases/100,000). Seasonal variation in incidence was noted only in black individuals with peak activity occurring in December (Clin. Infect. Dis. 2004;38[Suppl 3]:S181-9).
The most recent data from FoodNet (1996-2009) reveals an overall incidence of 0.5/100,000. There was a decline in incidence in all racial and ethnic groups. The highest incidence is still observed in black and Asians (0.9 and 0.7 per 100,000). The most dramatic decline occurred in black individuals (3.2 vs. 0.9 per 100,000). In 1998, an educational campaign was initiated in Georgia that targeted high-risk individuals and provided information on the safe handling and preparation of chitterlings. The state of Georgia reported the greatest decline to 0.4/100,000, which has almost eliminated the racial disparity reported in 2009. It is unclear if this campaign was the only reason for the decline in Georgia. The incidence in whites is 0.2/100,000. Since 2007, the incidence in Asian children less than 5 years of ages has been the highest amongst all racial and ethnic groups. Pork consumption is still assumed to be the major source. Seasonal variability persists amongst Black children less 5 years of age, implying that chitterlings may still be the source of infection for individuals in this group (Clin. Infect. Dis. 2012:54 [Suppl 5]:S385-S90).
In general, yersiniosis should be included in the differential of a febrile diarrheal illness, particularly during the cooler months and holiday season. It is prudent to determine if consumption and/or preparation of chitterlings or other pork products by the patient or caretakers has occurred. This will enable you to alert the laboratory so stool specimens can be cultured on the appropriate medium (CIN agar). Consumption of chitterlings is not limited to any specific racial or ethnic group. Individuals from rural and farming areas may also consume this product.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
To Combat West Nile Virus, Emphasize Prevention
The best way to deal with the recent resurgence in West Nile virus is to emphasize prevention.
West Nile virus (WNV) is back with a vengeance this year. As of Sept. 4, 2012, there were 1,993 reported cases of WNV disease in people, including 1,069 with neuroinvasive disease and 87 deaths. My state, Texas, is leading the pack with a total of 888 cases, 443 neuroinvasive disease cases, and 35 deaths. Texas’ West Nile problem is clearly the worst in the country. The next-highest number of total cases is only 119, in South Dakota.
According to the Centers for Disease Control and Prevention, the total of 1,993 cases is the highest number of WNV disease cases reported to the CDC through the first week in September since the virus was first detected in the United States in 1999. It’s not clear why this resurgence is happening now, or why Texas has been so hard hit. Some say that rising temperatures have resulted in an increased mosquito population, but here in Texas there is also a drought and mosquitos need moisture, so I’m not sure about that.
An arbovirus, WNV is usually transmitted to humans after a bite from an infected Culex mosquito. The transmission cycle is maintained between mosquito and vertebrate hosts, usually birds. Humans are actually an incidental and dead-end host. Though rare, person-to-person transmission has been documented through both blood transfusion and solid organ transplantation (N. Engl. J. Med. 2003;348:2196-203).
When speaking with your patients, it’s worth reemphasizing the methods for prevention. Parents should be instructed to apply one of the Environmental Protection Agency–registered insect repellents to their children before they go outside, using just enough to protect exposed skin. Products containing up to 30% DEET – but not higher – can be used in children older than 2 months of age. Products containing both DEET and a sunscreen should be avoided, since sunscreen needs to be applied more frequently.
Children should be covered up with clothing as much as possible when outside, and netting should be used over infant carriers. Outdoor exposure should be limited at dusk and dawn, when mosquitos are most active. Holes in screen doors should be repaired. Standing water, which attracts mosquitos, is a major hazard and should be avoided. Birdba ths and blow-up pools need to be emptied out often, and children should steer clear of puddles.
Here in Texas, the state health department has issued a statement preparing people for aerial spraying of chemicals to control the mosquitos. Each state most likely will develop its own recommendations, which should be available on the state health department website.
Routine screening of the U.S. blood supply was initiated in 2003, and no cases have been identified in donated blood since then. A single case of congenital infection also has been reported (MMWR 2002;51:1135-6). There are specific management guidelines for mother, fetus, and newborn if women are diagnosed with WNV during pregnancy (MMWR 2004;53:154-7).
Most people who become infected with WNV will be asymptomatic. About 1 in 5 who are infected will develop symptoms such as fever, headache, body aches, joint pains, vomiting, diarrhea, or rash after a 2-14 day incubation period. Less than 1% will develop neuroinvasive disease, but of those who do, about 10% are fatal.
It’s important to include WNV in your differential diagnosis for children who present with a febrile illness, meningitis, encephalitis, or acute flaccid paralysis, particularly if they’ve had exposure to mosquitos during the summer and early fall in endemic areas. The clinical presentation of neuroinvasive WNV is indistinguishable from those of other causes of viral meningitis and/or encephalitis. While the epidemiological characteristics of WNV disease in children are similar to those in adults, neuroinvasive disease due to WNV is more likely to manifest as meningitis in children than in older adults, who are more likely to develop encephalitis (Pediatrics 2009;123:e1084-9).
Because there is no specific treatment for WNV, and the majority of patients have a self-limited course, the diagnosis need not be made in every febrile patient. A definitive diagnosis should be sought in individuals with fever and acute neurologic symptoms who have recently been exposed to mosquitos, are solid organ transplant recipients, or are pregnant.
The presence of WNV-reactive IgM antibody in serum or cerebrospinal fluid supports a recent infection. However, anti-WNV IgM can persist up to a year in some people, so its presence may represent a prior infection. Moreover, if serum is tested within the first 10 days of illness, IgM antibody may not always be detected. Convalescent titers should be obtained 2-3 weeks following the onset of illness.
Treatment is supportive care. Standard precautions are recommended for hospitalized patients in the American Academy of Pediatrics Red Book on pages 792-5 (Red Book: 2012 Report of the Committee on Infectious Diseases. L.K. Pickering, Ed. 29th ed. Elk Grove Village, Ill.: American Academy of Pediatrics). Most people will recover from even the neuroinvasive manifestations of WNV, although symptoms may last for several weeks and those with severe cases may require hospitalization for supportive treatment. Serious sequelae can occur among those with underlying immune deficiencies.
For the most up-to-date information on WNV statistics from the CDC, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The best way to deal with the recent resurgence in West Nile virus is to emphasize prevention.
West Nile virus (WNV) is back with a vengeance this year. As of Sept. 4, 2012, there were 1,993 reported cases of WNV disease in people, including 1,069 with neuroinvasive disease and 87 deaths. My state, Texas, is leading the pack with a total of 888 cases, 443 neuroinvasive disease cases, and 35 deaths. Texas’ West Nile problem is clearly the worst in the country. The next-highest number of total cases is only 119, in South Dakota.
According to the Centers for Disease Control and Prevention, the total of 1,993 cases is the highest number of WNV disease cases reported to the CDC through the first week in September since the virus was first detected in the United States in 1999. It’s not clear why this resurgence is happening now, or why Texas has been so hard hit. Some say that rising temperatures have resulted in an increased mosquito population, but here in Texas there is also a drought and mosquitos need moisture, so I’m not sure about that.
An arbovirus, WNV is usually transmitted to humans after a bite from an infected Culex mosquito. The transmission cycle is maintained between mosquito and vertebrate hosts, usually birds. Humans are actually an incidental and dead-end host. Though rare, person-to-person transmission has been documented through both blood transfusion and solid organ transplantation (N. Engl. J. Med. 2003;348:2196-203).
When speaking with your patients, it’s worth reemphasizing the methods for prevention. Parents should be instructed to apply one of the Environmental Protection Agency–registered insect repellents to their children before they go outside, using just enough to protect exposed skin. Products containing up to 30% DEET – but not higher – can be used in children older than 2 months of age. Products containing both DEET and a sunscreen should be avoided, since sunscreen needs to be applied more frequently.
Children should be covered up with clothing as much as possible when outside, and netting should be used over infant carriers. Outdoor exposure should be limited at dusk and dawn, when mosquitos are most active. Holes in screen doors should be repaired. Standing water, which attracts mosquitos, is a major hazard and should be avoided. Birdba ths and blow-up pools need to be emptied out often, and children should steer clear of puddles.
Here in Texas, the state health department has issued a statement preparing people for aerial spraying of chemicals to control the mosquitos. Each state most likely will develop its own recommendations, which should be available on the state health department website.
Routine screening of the U.S. blood supply was initiated in 2003, and no cases have been identified in donated blood since then. A single case of congenital infection also has been reported (MMWR 2002;51:1135-6). There are specific management guidelines for mother, fetus, and newborn if women are diagnosed with WNV during pregnancy (MMWR 2004;53:154-7).
Most people who become infected with WNV will be asymptomatic. About 1 in 5 who are infected will develop symptoms such as fever, headache, body aches, joint pains, vomiting, diarrhea, or rash after a 2-14 day incubation period. Less than 1% will develop neuroinvasive disease, but of those who do, about 10% are fatal.
It’s important to include WNV in your differential diagnosis for children who present with a febrile illness, meningitis, encephalitis, or acute flaccid paralysis, particularly if they’ve had exposure to mosquitos during the summer and early fall in endemic areas. The clinical presentation of neuroinvasive WNV is indistinguishable from those of other causes of viral meningitis and/or encephalitis. While the epidemiological characteristics of WNV disease in children are similar to those in adults, neuroinvasive disease due to WNV is more likely to manifest as meningitis in children than in older adults, who are more likely to develop encephalitis (Pediatrics 2009;123:e1084-9).
Because there is no specific treatment for WNV, and the majority of patients have a self-limited course, the diagnosis need not be made in every febrile patient. A definitive diagnosis should be sought in individuals with fever and acute neurologic symptoms who have recently been exposed to mosquitos, are solid organ transplant recipients, or are pregnant.
The presence of WNV-reactive IgM antibody in serum or cerebrospinal fluid supports a recent infection. However, anti-WNV IgM can persist up to a year in some people, so its presence may represent a prior infection. Moreover, if serum is tested within the first 10 days of illness, IgM antibody may not always be detected. Convalescent titers should be obtained 2-3 weeks following the onset of illness.
Treatment is supportive care. Standard precautions are recommended for hospitalized patients in the American Academy of Pediatrics Red Book on pages 792-5 (Red Book: 2012 Report of the Committee on Infectious Diseases. L.K. Pickering, Ed. 29th ed. Elk Grove Village, Ill.: American Academy of Pediatrics). Most people will recover from even the neuroinvasive manifestations of WNV, although symptoms may last for several weeks and those with severe cases may require hospitalization for supportive treatment. Serious sequelae can occur among those with underlying immune deficiencies.
For the most up-to-date information on WNV statistics from the CDC, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
The best way to deal with the recent resurgence in West Nile virus is to emphasize prevention.
West Nile virus (WNV) is back with a vengeance this year. As of Sept. 4, 2012, there were 1,993 reported cases of WNV disease in people, including 1,069 with neuroinvasive disease and 87 deaths. My state, Texas, is leading the pack with a total of 888 cases, 443 neuroinvasive disease cases, and 35 deaths. Texas’ West Nile problem is clearly the worst in the country. The next-highest number of total cases is only 119, in South Dakota.
According to the Centers for Disease Control and Prevention, the total of 1,993 cases is the highest number of WNV disease cases reported to the CDC through the first week in September since the virus was first detected in the United States in 1999. It’s not clear why this resurgence is happening now, or why Texas has been so hard hit. Some say that rising temperatures have resulted in an increased mosquito population, but here in Texas there is also a drought and mosquitos need moisture, so I’m not sure about that.
An arbovirus, WNV is usually transmitted to humans after a bite from an infected Culex mosquito. The transmission cycle is maintained between mosquito and vertebrate hosts, usually birds. Humans are actually an incidental and dead-end host. Though rare, person-to-person transmission has been documented through both blood transfusion and solid organ transplantation (N. Engl. J. Med. 2003;348:2196-203).
When speaking with your patients, it’s worth reemphasizing the methods for prevention. Parents should be instructed to apply one of the Environmental Protection Agency–registered insect repellents to their children before they go outside, using just enough to protect exposed skin. Products containing up to 30% DEET – but not higher – can be used in children older than 2 months of age. Products containing both DEET and a sunscreen should be avoided, since sunscreen needs to be applied more frequently.
Children should be covered up with clothing as much as possible when outside, and netting should be used over infant carriers. Outdoor exposure should be limited at dusk and dawn, when mosquitos are most active. Holes in screen doors should be repaired. Standing water, which attracts mosquitos, is a major hazard and should be avoided. Birdba ths and blow-up pools need to be emptied out often, and children should steer clear of puddles.
Here in Texas, the state health department has issued a statement preparing people for aerial spraying of chemicals to control the mosquitos. Each state most likely will develop its own recommendations, which should be available on the state health department website.
Routine screening of the U.S. blood supply was initiated in 2003, and no cases have been identified in donated blood since then. A single case of congenital infection also has been reported (MMWR 2002;51:1135-6). There are specific management guidelines for mother, fetus, and newborn if women are diagnosed with WNV during pregnancy (MMWR 2004;53:154-7).
Most people who become infected with WNV will be asymptomatic. About 1 in 5 who are infected will develop symptoms such as fever, headache, body aches, joint pains, vomiting, diarrhea, or rash after a 2-14 day incubation period. Less than 1% will develop neuroinvasive disease, but of those who do, about 10% are fatal.
It’s important to include WNV in your differential diagnosis for children who present with a febrile illness, meningitis, encephalitis, or acute flaccid paralysis, particularly if they’ve had exposure to mosquitos during the summer and early fall in endemic areas. The clinical presentation of neuroinvasive WNV is indistinguishable from those of other causes of viral meningitis and/or encephalitis. While the epidemiological characteristics of WNV disease in children are similar to those in adults, neuroinvasive disease due to WNV is more likely to manifest as meningitis in children than in older adults, who are more likely to develop encephalitis (Pediatrics 2009;123:e1084-9).
Because there is no specific treatment for WNV, and the majority of patients have a self-limited course, the diagnosis need not be made in every febrile patient. A definitive diagnosis should be sought in individuals with fever and acute neurologic symptoms who have recently been exposed to mosquitos, are solid organ transplant recipients, or are pregnant.
The presence of WNV-reactive IgM antibody in serum or cerebrospinal fluid supports a recent infection. However, anti-WNV IgM can persist up to a year in some people, so its presence may represent a prior infection. Moreover, if serum is tested within the first 10 days of illness, IgM antibody may not always be detected. Convalescent titers should be obtained 2-3 weeks following the onset of illness.
Treatment is supportive care. Standard precautions are recommended for hospitalized patients in the American Academy of Pediatrics Red Book on pages 792-5 (Red Book: 2012 Report of the Committee on Infectious Diseases. L.K. Pickering, Ed. 29th ed. Elk Grove Village, Ill.: American Academy of Pediatrics). Most people will recover from even the neuroinvasive manifestations of WNV, although symptoms may last for several weeks and those with severe cases may require hospitalization for supportive treatment. Serious sequelae can occur among those with underlying immune deficiencies.
For the most up-to-date information on WNV statistics from the CDC, click here.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
Increasing Temperature: Watch for Dengue
Warmer temperatures in the United States are leading to the earlier presence of mosquitoes and the potential for mosquito-borne illnesses.
There are a number of arboviral diseases that occur in the United States, including St. Louis and La Cross encephalitis. Since its emergence in 1999, clinicians in the United States have been familiar with West Nile virus. Dengue is one you may not know as much about, and it has the potential to reestablish itself in the United States. Here’s a quick review.
An emerging infection, dengue is transmitted by the mosquitoes Aedes aegypti and A. albopictus, which are found worldwide. The dengue virus complex consists of four related, but distinct, serotypes. Infection with one confers lifelong immunity, but there is no cross-protection against the other serotypes. Dengue fever (DF) is endemic in at least 100 countries in Asia, the Pacific, the Americas, Africa, and the Caribbean. It often peaks during seasons when rainfall is optimal for mosquito breeding. The Aedes mosquito is common in the southern United States, and dengue is endemic in northern Mexico. Most cases in the United States are seen in returning travelers or immigrants (MMWR 2005;54;556-8), including children (Am. J. Trop. Med. Hyg. 2012;86:474-6).
Since 1980 there have been seven outbreaks along the Texas-Mexico border. However, dengue can be acquired locally. In 2010, 28 cases were reported from Key West, Fla. They were the first cases of locally acquired dengue outside the Texas-Mexico border since 1995. The initial case was diagnosed by an astute physician in a patient from New York who had visited Key West (MMWR 2010;59:577-81).
Why is this virus reemerging in the United States? One thought is that the mosquito vector has optimal breeding conditions. It prefers to breed close to or inside a home, and it can lay eggs in natural or man-made water containers. It also is a daytime feeder with a preference for humans. Often, the mosquito has multiple feeds before a breeding cycle, thus exposing several persons in the same household.
Dengue should be considered in the differential diagnosis of all febrile patients who reside in the tropics or subtropics, including areas with subtropical climates in the United States, or in febrile patients who have a history of travel to such places in the 2 weeks before symptom onset. It is now the leading cause of a febrile illness in U.S. travelers returning from the Caribbean, South America, and Asia (N. Eng. J. Med. 2006;354:119-30). Because of the increasing number of cases in returning travelers, many of whom may still be viremic and capable of introducing the virus into the community, combined with the presence of an efficient mosquito vector, dengue became a nationally notifiable disease in 2009.
Symptoms typically begin 4-7 days after the mosquito bite and last about 3-10 days. Individuals, especially children, infected with dengue for the first time may be asymptomatic or have a nonspecific febrile illness, but subsequent infections are usually more severe. Classic DF is primarily a disease of older children and adults.
The World Health Organization defines DF as an acute febrile illness with two or more of the following: headache, retro-orbital pain, muscle aches, joint pain, rash, hemorrhagic manifestation, or leukopenia. The rash is either macular or maculopapular and generalized, is often confluent with small patches of normal skin, and may become scaly and pruritic. It usually appears as the fever subsides and lasts 2-4 days. Other signs and symptoms include flushed skin (usually during the first 24-48 hours), nausea, and vomiting. DF is usually a self-limited illness and is rarely fatal. Approximately 1% of patients with DF develop dengue hemorrhagic fever about 3-8 days after the onset of fever. There is evidence of vascular leakage, hemoconcentration, and thrombocytopenia.
The primary serologic test for dengue virus (DENV) in patients with acute illness is IgM anti-DENV, which becomes positive more than 5 days after symptom onset. This, in combination with a compatible travel history and symptom profile, suggests a probable recent DENV infection. There are several commercially available diagnostic tests for dengue, although none have been approved by the Food and Drug Administration. Testing is available at some state laboratories, and through the Centers for Disease Control and Prevention (see "Requesting Dengue Laboratory Testing and Reporting" at www.cdc.gov/Dengue/clinicalLab/index.html). For additional information, contact the CDC Dengue Branch (787-706-2399) or visit http://www.cdc.gov/dengue/.
There is no specific treatment for DENV infections. Management involves bed rest and fluid maintenance during the fever, which can be controlled with acetaminophen. Headache, eye pain, joint pain, and muscle ache may require narcotics, but aspirin and nonsteroidal anti-inflammatory agents should be avoided. Patients should be instructed that, as the fever subsides, they should go to the hospital if they develop abrupt change to hypothermia, severe abdominal pain, persistent vomiting, bleeding, difficulties breathing, or altered mental status such as irritability, confusion, and lethargy. These may be signs of dengue hemorrhagic fever, which can prove fatal.
Prevention is the best bet. Travelers should be advised to use insecticides to get rid of mosquitoes in these areas and to select accommodations with well-screened windows or air conditioning when possible. Additionally, travelers should take measures to avoid being bitten by mosquitoes during the daytime. Children at least 3 months of age can use a repellant containing not more than 30% DEET. Younger infants should have permethrin-treated nets placed over carriers. Everyone can benefit from permethrin-treated clothing, as well as from eliminating or avoiding standing water.
Currently, there is no licensed vaccine available. However, Sanofi Pasteur has a tetravalent vaccine in phase III clinical trials.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
This column, "ID Consult," appears regularly in Pediatric News, a publication of Elsevier.
Warmer temperatures in the United States are leading to the earlier presence of mosquitoes and the potential for mosquito-borne illnesses.
There are a number of arboviral diseases that occur in the United States, including St. Louis and La Cross encephalitis. Since its emergence in 1999, clinicians in the United States have been familiar with West Nile virus. Dengue is one you may not know as much about, and it has the potential to reestablish itself in the United States. Here’s a quick review.
An emerging infection, dengue is transmitted by the mosquitoes Aedes aegypti and A. albopictus, which are found worldwide. The dengue virus complex consists of four related, but distinct, serotypes. Infection with one confers lifelong immunity, but there is no cross-protection against the other serotypes. Dengue fever (DF) is endemic in at least 100 countries in Asia, the Pacific, the Americas, Africa, and the Caribbean. It often peaks during seasons when rainfall is optimal for mosquito breeding. The Aedes mosquito is common in the southern United States, and dengue is endemic in northern Mexico. Most cases in the United States are seen in returning travelers or immigrants (MMWR 2005;54;556-8), including children (Am. J. Trop. Med. Hyg. 2012;86:474-6).
Since 1980 there have been seven outbreaks along the Texas-Mexico border. However, dengue can be acquired locally. In 2010, 28 cases were reported from Key West, Fla. They were the first cases of locally acquired dengue outside the Texas-Mexico border since 1995. The initial case was diagnosed by an astute physician in a patient from New York who had visited Key West (MMWR 2010;59:577-81).
Why is this virus reemerging in the United States? One thought is that the mosquito vector has optimal breeding conditions. It prefers to breed close to or inside a home, and it can lay eggs in natural or man-made water containers. It also is a daytime feeder with a preference for humans. Often, the mosquito has multiple feeds before a breeding cycle, thus exposing several persons in the same household.
Dengue should be considered in the differential diagnosis of all febrile patients who reside in the tropics or subtropics, including areas with subtropical climates in the United States, or in febrile patients who have a history of travel to such places in the 2 weeks before symptom onset. It is now the leading cause of a febrile illness in U.S. travelers returning from the Caribbean, South America, and Asia (N. Eng. J. Med. 2006;354:119-30). Because of the increasing number of cases in returning travelers, many of whom may still be viremic and capable of introducing the virus into the community, combined with the presence of an efficient mosquito vector, dengue became a nationally notifiable disease in 2009.
Symptoms typically begin 4-7 days after the mosquito bite and last about 3-10 days. Individuals, especially children, infected with dengue for the first time may be asymptomatic or have a nonspecific febrile illness, but subsequent infections are usually more severe. Classic DF is primarily a disease of older children and adults.
The World Health Organization defines DF as an acute febrile illness with two or more of the following: headache, retro-orbital pain, muscle aches, joint pain, rash, hemorrhagic manifestation, or leukopenia. The rash is either macular or maculopapular and generalized, is often confluent with small patches of normal skin, and may become scaly and pruritic. It usually appears as the fever subsides and lasts 2-4 days. Other signs and symptoms include flushed skin (usually during the first 24-48 hours), nausea, and vomiting. DF is usually a self-limited illness and is rarely fatal. Approximately 1% of patients with DF develop dengue hemorrhagic fever about 3-8 days after the onset of fever. There is evidence of vascular leakage, hemoconcentration, and thrombocytopenia.
The primary serologic test for dengue virus (DENV) in patients with acute illness is IgM anti-DENV, which becomes positive more than 5 days after symptom onset. This, in combination with a compatible travel history and symptom profile, suggests a probable recent DENV infection. There are several commercially available diagnostic tests for dengue, although none have been approved by the Food and Drug Administration. Testing is available at some state laboratories, and through the Centers for Disease Control and Prevention (see "Requesting Dengue Laboratory Testing and Reporting" at www.cdc.gov/Dengue/clinicalLab/index.html). For additional information, contact the CDC Dengue Branch (787-706-2399) or visit http://www.cdc.gov/dengue/.
There is no specific treatment for DENV infections. Management involves bed rest and fluid maintenance during the fever, which can be controlled with acetaminophen. Headache, eye pain, joint pain, and muscle ache may require narcotics, but aspirin and nonsteroidal anti-inflammatory agents should be avoided. Patients should be instructed that, as the fever subsides, they should go to the hospital if they develop abrupt change to hypothermia, severe abdominal pain, persistent vomiting, bleeding, difficulties breathing, or altered mental status such as irritability, confusion, and lethargy. These may be signs of dengue hemorrhagic fever, which can prove fatal.
Prevention is the best bet. Travelers should be advised to use insecticides to get rid of mosquitoes in these areas and to select accommodations with well-screened windows or air conditioning when possible. Additionally, travelers should take measures to avoid being bitten by mosquitoes during the daytime. Children at least 3 months of age can use a repellant containing not more than 30% DEET. Younger infants should have permethrin-treated nets placed over carriers. Everyone can benefit from permethrin-treated clothing, as well as from eliminating or avoiding standing water.
Currently, there is no licensed vaccine available. However, Sanofi Pasteur has a tetravalent vaccine in phase III clinical trials.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
This column, "ID Consult," appears regularly in Pediatric News, a publication of Elsevier.
Warmer temperatures in the United States are leading to the earlier presence of mosquitoes and the potential for mosquito-borne illnesses.
There are a number of arboviral diseases that occur in the United States, including St. Louis and La Cross encephalitis. Since its emergence in 1999, clinicians in the United States have been familiar with West Nile virus. Dengue is one you may not know as much about, and it has the potential to reestablish itself in the United States. Here’s a quick review.
An emerging infection, dengue is transmitted by the mosquitoes Aedes aegypti and A. albopictus, which are found worldwide. The dengue virus complex consists of four related, but distinct, serotypes. Infection with one confers lifelong immunity, but there is no cross-protection against the other serotypes. Dengue fever (DF) is endemic in at least 100 countries in Asia, the Pacific, the Americas, Africa, and the Caribbean. It often peaks during seasons when rainfall is optimal for mosquito breeding. The Aedes mosquito is common in the southern United States, and dengue is endemic in northern Mexico. Most cases in the United States are seen in returning travelers or immigrants (MMWR 2005;54;556-8), including children (Am. J. Trop. Med. Hyg. 2012;86:474-6).
Since 1980 there have been seven outbreaks along the Texas-Mexico border. However, dengue can be acquired locally. In 2010, 28 cases were reported from Key West, Fla. They were the first cases of locally acquired dengue outside the Texas-Mexico border since 1995. The initial case was diagnosed by an astute physician in a patient from New York who had visited Key West (MMWR 2010;59:577-81).
Why is this virus reemerging in the United States? One thought is that the mosquito vector has optimal breeding conditions. It prefers to breed close to or inside a home, and it can lay eggs in natural or man-made water containers. It also is a daytime feeder with a preference for humans. Often, the mosquito has multiple feeds before a breeding cycle, thus exposing several persons in the same household.
Dengue should be considered in the differential diagnosis of all febrile patients who reside in the tropics or subtropics, including areas with subtropical climates in the United States, or in febrile patients who have a history of travel to such places in the 2 weeks before symptom onset. It is now the leading cause of a febrile illness in U.S. travelers returning from the Caribbean, South America, and Asia (N. Eng. J. Med. 2006;354:119-30). Because of the increasing number of cases in returning travelers, many of whom may still be viremic and capable of introducing the virus into the community, combined with the presence of an efficient mosquito vector, dengue became a nationally notifiable disease in 2009.
Symptoms typically begin 4-7 days after the mosquito bite and last about 3-10 days. Individuals, especially children, infected with dengue for the first time may be asymptomatic or have a nonspecific febrile illness, but subsequent infections are usually more severe. Classic DF is primarily a disease of older children and adults.
The World Health Organization defines DF as an acute febrile illness with two or more of the following: headache, retro-orbital pain, muscle aches, joint pain, rash, hemorrhagic manifestation, or leukopenia. The rash is either macular or maculopapular and generalized, is often confluent with small patches of normal skin, and may become scaly and pruritic. It usually appears as the fever subsides and lasts 2-4 days. Other signs and symptoms include flushed skin (usually during the first 24-48 hours), nausea, and vomiting. DF is usually a self-limited illness and is rarely fatal. Approximately 1% of patients with DF develop dengue hemorrhagic fever about 3-8 days after the onset of fever. There is evidence of vascular leakage, hemoconcentration, and thrombocytopenia.
The primary serologic test for dengue virus (DENV) in patients with acute illness is IgM anti-DENV, which becomes positive more than 5 days after symptom onset. This, in combination with a compatible travel history and symptom profile, suggests a probable recent DENV infection. There are several commercially available diagnostic tests for dengue, although none have been approved by the Food and Drug Administration. Testing is available at some state laboratories, and through the Centers for Disease Control and Prevention (see "Requesting Dengue Laboratory Testing and Reporting" at www.cdc.gov/Dengue/clinicalLab/index.html). For additional information, contact the CDC Dengue Branch (787-706-2399) or visit http://www.cdc.gov/dengue/.
There is no specific treatment for DENV infections. Management involves bed rest and fluid maintenance during the fever, which can be controlled with acetaminophen. Headache, eye pain, joint pain, and muscle ache may require narcotics, but aspirin and nonsteroidal anti-inflammatory agents should be avoided. Patients should be instructed that, as the fever subsides, they should go to the hospital if they develop abrupt change to hypothermia, severe abdominal pain, persistent vomiting, bleeding, difficulties breathing, or altered mental status such as irritability, confusion, and lethargy. These may be signs of dengue hemorrhagic fever, which can prove fatal.
Prevention is the best bet. Travelers should be advised to use insecticides to get rid of mosquitoes in these areas and to select accommodations with well-screened windows or air conditioning when possible. Additionally, travelers should take measures to avoid being bitten by mosquitoes during the daytime. Children at least 3 months of age can use a repellant containing not more than 30% DEET. Younger infants should have permethrin-treated nets placed over carriers. Everyone can benefit from permethrin-treated clothing, as well as from eliminating or avoiding standing water.
Currently, there is no licensed vaccine available. However, Sanofi Pasteur has a tetravalent vaccine in phase III clinical trials.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Write to Dr. Word at [email protected].
This column, "ID Consult," appears regularly in Pediatric News, a publication of Elsevier.