User login
Managing Glenoid Bone Deficiency—The Augment Experience in Anatomic and Reverse Shoulder Arthroplasty
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.
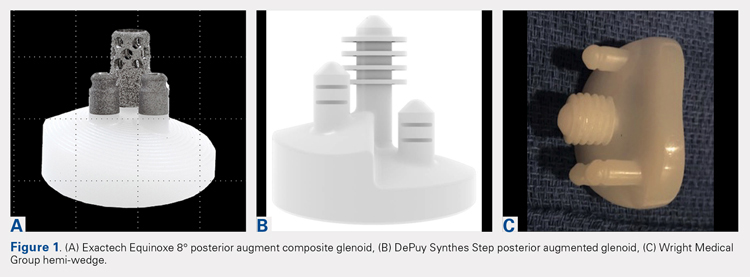
Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
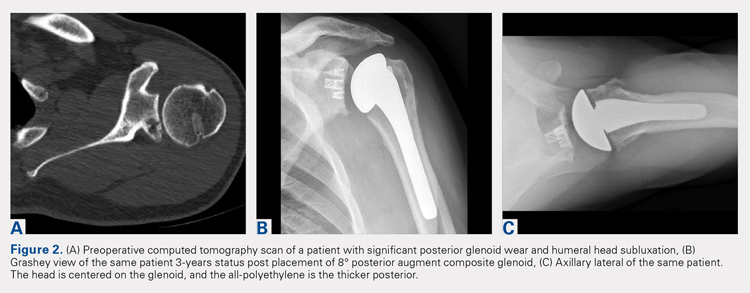
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.
Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
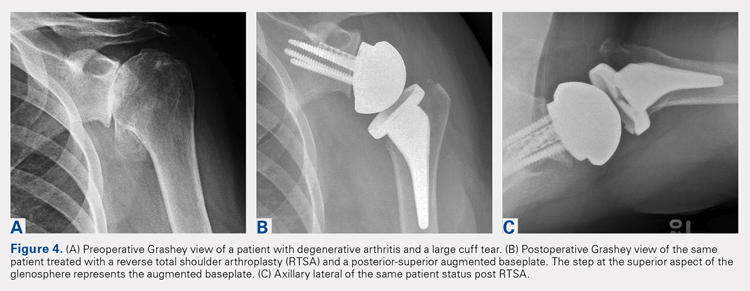
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.
Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
ABSTRACT
Glenoid bone deficiency in the setting of shoulder replacement surgery is far more common than originally reported. The frequency and severity of the glenoid defects are noted to be more common and severe with the advent of computer-assisted surgery. The results of an anatomic total shoulder arthroplasty (aTSA) with glenoid deficiency have been reported to be inferior to aTSA patients without a glenoid deficiency. Options for treating the glenoid deficiency include eccentric reaming, bone grafting, and the use of augmented glenoid components. The purpose of this article is to present the indications, technique, and results of augmented glenoids for both aTSA and reverse TSA (RTSA).
Augments for both aTSA and RTSA are viable options. They preserve subchondral bone at the same time as optimizing the joint line without the need for bone grafts. Complications, revisions and results are as good as compared to shoulder arthroplasties without glenoid wear.
Continue to: Glenoid bone deficiency...
Glenoid bone deficiency in arthritic or cuff-deficient shoulder has been reported in up to 50% of shoulder defect cases.1,2 The type and severity of glenoid deformities vary depending on the underlying pathology and time of manifestation. Osteoarthritis with bone loss typically results in posterior or posterior inferior glenoid wear and is commonly classified as Walch types B1 or B2 (biconcave). In cases of severe erosion, B3 classification has been proposed; in this classification, bone loss becomes extremely severe, progressing to resemble a type C glenoid. Unlike primary osteoarthritis, inflammatory arthropathy more commonly causes central loss of glenoid bone (Walch A2). With the rotator cuff insufficiency, superior migration of the humeral head occurs. As these conditions progress, cuff tear arthropathy (CTA) changes result in superior or posterior-superior bone loss.1 Anterior bone loss (type D) will be rarely encountered due to recurrent anterior instability.3
Classically, with anatomic total shoulder arthroplasty (aTSA), the surgeon considers several options for managing glenoid deficiencies. The most commonly employed technique involves eccentrically reaming the glenoid and correcting the deformity. This procedure is relatively easy but features significant drawbacks, such as sacrificing the subchondral bone, medializing the glenohumeral joint line, and secondarily shrinking the glenoid surface area. Other options include structural bone grafting behind the glenoid component. Most anatomic prosthetic glenoids prove to be unsuitable for fixation of structural bone graft. Therefore, the graft is first internally fixed, followed by placement of the glenoid component. Cement, which is commonly used for glenoid fixation, may potentially inhibit bone-graft healing. Reports using this technique documented high radiographic failure rate of up to 40% at midterm follow-up.4 Although leaving the glenoid component retroverted may be considered, surgeons should develop awareness of the possibility of peg penetration of the anterior glenoid neck. Additionally, retroversion in excess of 5°may increase the risk of recurrent posterior subluxation, resulting in early glenoid loosening.5-7 Results of aTSA under significant glenoid deficiency are inferior to those of aTSA patients without glenoid deficiency.8 Such findings have been extremely inferior in patients with significant glenoid wear, prompting numerous surgeons to abandon aTSA in this population in favor of reverse TSA (RTSA) due to improved bony fixation.
In 2010, augmented anatomic glenoids were first introduced as a wedge (Exactech) and as a step shortly thereafter (DePuy Synthes; Figures 1A-1C). More recently, hemi-wedges have been introduced (Wright Medical Group). Augments have gained popularity due to improved range of motion vs reverse shoulder arthroplasty (RSA). However, debates remain regarding the use of posteriorly augmented components in the setting of posterior glenoid bone loss.8 Augments serve as another viable option for handling glenoid bone deficiency in aTSA.

Glenoid bone loss in RTSA presents similar options to aTSA. However, screw fixation of the glenoid component offers several distinct advantages. Baseplate fixation can readily be used with bone grafting and with a highly anticipated success rate. With multiple screw options, 100% support of the baseplate is not mandatory. Although bony increase offset RSAs (BIO-RSAs) have shown success, augmentation with allograft or autograft increases operative time and relies on osseous integration for long-term implant success.9 Metal augmented baseplates were first introduced in 2011 (Exactech) as a means of managing glenoid bone loss without structural grafting. Although initial results have been encouraging, additional studies are needed to assess the longevity of these implants (Figures 1A-1C).
aTSA AUGMENTS
aTSA augments were introduced as a means of correcting acquired glenoid bone deficiency, restoring native glenoid version, correcting humeral subluxation, and preserving the native subchondral bone. Compared with glenoid bone grafting, augmented glenoid components decrease operative time, allow for a technically easier operation, and require no bone healing for clinical success. Early and midterm results are encouraging, showing similar findings comparable to those of aTSA in non-glenoid deficient shoulders.10-12
Continue to: INDICATIONS
INDICATIONS
Indications and limitations for augmented aTSA glenoids remain incompletely defined. The most common indication for an augmented aTSA is osteoarthritis with a B2 glenoid. We recommend augments in the occurrence of any indication of significant eccentric glenoid wear. With the expertise of surgeons, deformities of up to 20° to 25° of deformity can be readily handled with good predictability. More severe deformities can be managed with augmented aTSA components, but early failure rates may be high. The most severe acquired deformities remain best managed with RTSA. Currently, we prefer RTSA when glenoid bone loss exceeds 25°. With the widespread availability of computed tomography (CT) scans with 3-dimensional (3-D) reconstruction, glenoid bone defects are increasingly recognized. When correcting deformity, surgeons should strive to limit residual retroversion to a maximum of 5°.13 Preoperative planning software and computer-assisted surgery (ExactechGPS) may allow surgeons to better define the limits of augmented glenoid fixation prior to the date of surgery. We routinely utilize computer-guided glenoid preparation to control glenoid version to within 5° of neutral position.
The differences between B3 and a true type C glenoid must be recognized. Although B3 glenoids may still be a candidate for an augmented anatomic glenoid component, type C glenoids are not. Developmental abnormalities of type C glenoid occur simultaneously with humeral deformities, including medialized posterior rotator cuff musculature. Correction of the joint line to neutral version may not replicate the non-diseased state of a dysplastic type shoulder. Davis and colleagues14 have proposed treating these patients by leaving both the humerus and glenoid in their native version without correction.
TECHNIQUE
The implant that we have the most experience with is an 8° full-wedge augmented glenoid component. Such an implant is typically utilized for B2 glenoids. We recommend that a high-quality CT scan be performed for preoperative planning. As a general rule, the starting point often lies close to the ridge of B2 glenoid and more anterior than the apparent glenoid center, which is viewed intraoperatively due to asymmetric posterior wear. Full-wedge component is utilized to ream the ridge separating the neo and paleoglenoids to create a flat surface. This condition is best achieved by drilling a pilot hole at the planned glenoid central peg position to prevent the reamer from sliding anteriorly during reaming. Glenoid preparation begins with the smallest reamer until the ridge has been flattened, and the reamer makes full contact with the glenoid. The reamer diameter is then increased based on glenoid size. Slightly downsizing the glenoid implant will require less reaming to achieve full backside support. Once the glenoid is properly reamed, the central and peripheral peg holes are drilled using the appropriate guides. Holes are then dried, and all-polyethylene or composite glenoid component (either partially or completely cemented) is installed using favored cementing techniques. The advantage of composite glenoid component is that the central cage allows for bone ingrowth and may potentially improve long-term implant survival. Press fit of the central cage requires no waiting time for glenoid cement hardening before proceeding to the humerus. When placing an augmented component, adequate glenoid exposure is imperative to allow in-line placement and appropriate seating of the component without impingement on adjacent retractors.
When using the step-augmented glenoid, the paleoglenoid is prepared in a similar fashion to a standard aTSA. Once the paleoglenoid has been reamed to a neutral position, a protector plate is placed onto the paleoglenoid. and a step-cut saw is used to prepare the posterior stepped bone cut. Peripheral pegs are then drilled, and the component is installed in routine fashion. When using hemi-wedge augments, the paleoglenoid is again prepared in a similar fashion as a standard glenoid component over a cannulated guidewire. The neoglenoid is subsequently prepared using a specialized angled reamer with a positive stop to prevent over-reaming. These glenoid implants improve rotational force neutralization given the absence of flat back against the glenoid. All 3 designs preserve bone when compared with eccentric reaming alone,15 with the half-augmented wedge preserving the most bone.
Table 1. Results of Various Augmented Glenoid Components in Anatomic Total Shoulder
Arthroplasty
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
8° cage (N = 21) |
|
|
|
|
| |||||||||||||||
All-polyethylene 8° (N = 45) |
|
|
|
|
| |||||||||||||||
All-polyethylene 16° (N = 7) |
|
|
|
|
|
RESULTS
In our institution, we first used all-polyethylene posteriorly augmented glenoid components in 2010. Between 2010 and 2015, 45 patients received an 8° all-polyethylene posterior augment, and 7 patients received a 16° augment. In 2015, we transitioned to the composite caged posterior augment. All patients in our database who received an augmented glenoid component experienced improvement in active forward elevation, external rotation, American Shoulder and Elbow Surgeons (ASES), and Constant scores (Table 1). Minimum follow-up was 1 year for patients receiving both an 8° cage (mean, 1.48 years) and an 8° all-polyethylene augment (mean, 3.18 years). Figures 2A-2C show a patient with significant posterior glenoid wear and humeral head subluxation treated with an 8° wedge composite posterior augment glenoid 3 years postoperative.

Continue to: COMPLICATIONS
COMPLICATIONS
Two complications developed in the group undergoing composite cage augment. One patient experienced glenoid loosening after a motor vehicle accident. Another patient sustained significant intraoperative tuberosity avulsion during implantation of the humeral component, requiring a change of implant and tuberosity fixation. Although no complications were noted in the 8° all-polyethylene group, 3 patients in the 16° augment group sustained complications. One of these patients suffered a cardiac event that was unrelated to the implant. Two complications in this group were both related to loosening of the glenoid component, requiring subsequent revision.
DISCUSSION
The first report on augmented aTSA was published in 2008, and it involved a 5° augmented, anatomic glenoid.12 One study was based on a small series of augments; the poor results led the reporting surgeons to subsequently abandon the implant.12 This early design produced a correction on the articular side of the implant rather than the pathologic bony side. By performing such correction, the component pegs remained anteriorly oriented, placing the component at risk of perforation through the anterior glenoid neck. All current augment designs feature pegs that are oriented down the glenoid vault, with corrections occurring on the bony surface. This condition requires 2 different axes for reaming the glenoid and drilling the pegs. This approach allows the pegs to be directed down the glenoid neck, and is a far superior solution to neutralizing shear forces when compared with the implants used in the 1990s.
Early to midterm results of modern aTSA augments have been extremely encouraging with low revision rates. The main concern of recurrent posterior subluxation has been rarely reported. The concerns over glenoid loosening due to high shear forces, similarly, have not been described to date. However, surgeons should remain cautious, as longer-term follow-up remains unavailable.
The main advantage of aTSA augments is their capacity to preserve bone compared with eccentric reaming and better long-term stability. Each of the augment designs requires varying amounts of bone removal. Through biomechanics and using finite element analysis, the 3 augment types act differently, with no design demonstrating remarkable biomechanical superiority.6 Favorito and colleagues16 performed a retrospective review of 22 patients who underwent aTSA using an all-polyethylene, posteriorly augmented, and stepped glenoid component for posterior bone loss. At an average follow-up of 36 months, all patients experienced improvements in active forward elevation, external rotation, visual analog scale, Short Form-36 Physical Component Summary, and Western Ontario Osteoarthritis of the Shoulder scores. The authors noted that 2 patients (9%) experienced complications: 1 with an anterior dislocation and the other with recurrent posterior instability requiring revision. Sandow and Schutz17 reported the preliminary results of 10 patients who underwent aTSA using trabecular metal augment with a minimum of 2-year follow-up. All patients received either a 15° or 30° posterior, metal-backed augment for severe glenoid bone loss (Walch grade B2 or C). At a minimum of 2-year follow-up, all patients received correction to within 10° of neutral glenoid version, without any complications nor implant failures.
Regardless of augment design, all current components restore the native glenoid version, improving the length and subsequent tension of rotator cuff musculature. Similarly, re-centering the humeral head decreases the forces on the glenoid and allows for optimal function with decreasing loss of vital subchondral bone.
Continue to: RTSA AUGMENTS
RTSA AUGMENTS
Similar to anatomic augments, metal augments were introduced for use with RTSA in 2011. Unlike anatomic augments, those for RTSA were manufactured with metal. Given the difference in bony wear patterns in patients requiring RTSA, augments were available in a number of configurations. With CTA, wear is most commonly superior. Leaving a superiorly inclined baseplate must be avoided due to risks of notching, loosening, and early failure. However, correcting this tilt will require significant reaming of the inferior glenoid. A superior augment is ideally suited for this bone-loss pattern. If the glenoid is retroverted significantly, difficulty can also arise during glenoid preparation and baseplate placement. Posterior augments may ease this aspect of the procedure. Posterior augments feature the additional benefits of tensioning any remaining posterior rotator cuff, minimizing posterior inferior impingement, and technically easing the operation.18 As we improve our awareness of glenoid orientation using computer navigation, a posterior-superior augmented implant is commonly needed to simultaneously optimize the baseplate position and to minimize reaming (Figure 3). The posterior-superior augmented baseplate has become the most commonly used baseplate augment of choice in 90% of our RTSA cases that require an augment.

INDICATIONS
Augmented RTSA baseplates are indicated when adequate backside contact cannot be achieved with eccentric reaming, thus compromising potential fixation. In our practice, we preferably use augments at <50% contact with the backside of the baseplate. Excessive superior inclination is observed in a CTA setting, commonly indicating the use of superior augments. Similarly, severe primary osteoarthritis may contain elements of posterior bone loss, leading to increased retroversion, which is where we use posterior augments. When patients exhibit combined deformities, or when the surgeon wishes to tension the posterior rotator cuff, a posterior-superior augmented glenoid baseplate is used. For extremely severe defects, we have combined bone grafting and augments. In patients with a highly deficient glenoid but good quality of the remaining bone stock, an augment allows for better contact with less reaming although it is not fully supported when compared with a non-augmented baseplate. Bone grafts can function similarly, but the autograft humeral head is not constantly present in revision situations and requires increased operative time to allow for precision carpentry. Additionally, the success of BIO-RSA requires healing of bone graft on the native glenoid to support the baseplate.19 Jones and colleagues9 compared metal augmented RTSA with BIO-RSA and presented equivalent results.
To minimize reaming and to obtain appropriately inferior inclination, we have discovered preoperative templating and intraoperative, computer-guided glenoid preparation to be extremely valuable (ExactechGPS). These tools allow appropriate assessment of augments and for minimal bone removal when preparing the glenoid.
TECHNIQUE
When using an augment, a fine-cut CT scan is highly recommended to aid in surgery planning. We also find 3-D reconstructions to be helpful. Preoperative planning software also allows surgeons to maximize fixation of implant within the glenoid vault. The starting point for reaming is planned based on CT. Some surgeons using augments perform minimal or no reaming at all, electing to remove the remaining cartilage with a Cobb elevator. Different reaming and drilling axes are used when using augments. In cases of severe glenoid deformity and unavailability of computer assistance, a guide wire with inferior inclination can be installed based on CT scan. Penetration of this wire down the glenoid neck can be palpated and compared with the preoperative plan. We generally prefer at least 24 mm of bone containment for the central cage. Once the surgeon is satisfied with the placement of the wire, the appropriate augment guide is placed, followed by a second guide wire. This second wire acts as the reaming axis. The first wire is removed, and the glenoid is reamed with a cannulated reamer. Once reaming is completed, the original wire is replaced in the same hole and trajectory, and the reaming wire is removed. The first wire is then drilled with a cannulated drill for the central cage. The augmented baseplate is then impacted into place, and screw fixation is performed. Again, intraoperative computer guidance allows for precision screw placement with maximal bone attachment.
Table 2. Results of Reverse Total Shoulder Arthroplasty Augmented Baseplates
| Augment | American Shoulder and Elbow Surgeons Score | Constant Score | Active Forward Flexion | Active External Rotation | ||||||||||||||||
Superior (N = 22) |
|
|
|
|
| |||||||||||||||
Posterior (N = 50) |
|
|
|
|
| |||||||||||||||
Posterosuperior (N = 67) |
|
|
|
|
|
RESULTS
Based on our experience, glenoid augments for RTSA have performed well at short- and mid-term follow-up. From October 2011 to July 2016, 139 patients undergoing RTSA received a posterior, superior, or posterior-superior augmented glenoid baseplate. All groups demonstrated improvements in functional outcome measures, including Constant, ASES, Shoulder Pain and Disability Index, and Simple Shoulder Test scores compared with baseline values (Table 2). The posterior-superior augment group experienced the most significant improvement in active forward flexion and external rotation, whereas the posterior augment group experienced the most significant improvement in ASES and Constant scores. Figures 4A-4C displays the radiographs of a patient with significant glenoid wear treated with a posterior-superior augment RTSA.

Continue to: COMPLICATIONS
COMPLICATIONS
In the superior augment group, 3 patients (13%) sustained 5 complications. One patient sustained 3 separate episodes of instability, eventually requiring revision of prosthesis. In the posterior augment group, 4 patients (8%) sustained complications. Two of the 4 patients presented postoperative humeral fractures related to traumatic events, whereas another patient sustained an intraoperative tuberosity fracture. The last complication in this group involved a postoperative draining wound that was treated with oral antibiotics.
Nine complications developed in the posterior-superior augment group (13%); these complications included aseptic baseplate loosening (5), glenoid fracture (1), humeral fracture (1), acromial stress fracture (1), and cerebrovascular accident (1).
DISCUSSION
As the use of augments in RTSA is relatively new, significantly scarce data exist regarding their outcomes and longevity. A few studies have focused on the short-term outcomes of these augments. Jones and colleagues9 performed a retrospective review of 80 patients who underwent RTSA and required either a structural bone graft or an augmented glenoid baseplate.9 They observed that although all patients showed improvements in pain, range of motion, and functional scores, the structural bone graft group incurred a 14.6% complication rate compared with none observed in the augment group. Additionally, Jones and colleagues9 noted that the augmented baseplate group exhibited a significantly lower rate of scapular notching compared with the bone-graft group (10% vs 18.5%) at similar follow-up intervals. A separate study by Wright and colleagues18 compared posterior vs superior augmented baseplates in RTSA. The posterior augment group demonstrated lower rates of scapular notching (6.3% vs 14.3%) and showed more significant improvements in Constant, ASES, and active forward elevation measures, compared with the superior augment group.
As more manufacturers develop augments for RTSA, and as ExactechGPS uses become more widespread, the use of RTSA baseplate augments will continually grow. Custom implants using massive metal augments are now also being introduced. Although currently too expensive for most cases, as technology drives the cost down, every patient may receive customized augmented implants in the future.
The advantages of augmented baseplate designs include minimized reaming and notching, improved tension of the remaining rotator cuff, and decreased operating room time. The disadvantages include increased cost and lack of mid- or long-term clinical data. The concerns with baseplate loosening with augments in RTSA are much less than those with augments for aTSA due to the outstanding baseplate fixation that can be achieved in RTSA.
Continue to: CONLCLUSION
CONCLUSION
Augments offer an excellent tool for surgeons performing both aTSA and RTSA with glenoid bone loss. Use of augments will become more common as more manufacturers develop them. Although clinical results fall short in full midterm, they have been positive for both augmented RTSA and aTSA. Concerns arise when performing augmented aTSA, as an upper limit of correction has not been defined with regard to component failure. Currently, no data support the maximum amount of correction that can be achieved. In our current practice, we face difficulty in correcting more than 25° of version in young active patients with aTSA augment. Beyond this point, we perform a RTSA with an augment. In older patients or low-demand patients, we only correct minor deformities (<20°) with an aTSA augment, opting instead for an augmented RTSA due to the lower midterm failure rates observed with this implant.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
1. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. J Bone Joint Surg Br. 2004;86(3):388-395. doi:10.1302/0301-620X.86B3.
2. Churchill RS, Spencer Jr EE, Fehringer EV. Quantification of B2 glenoid morphology in total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(8):1212-1217. doi:10.1016/j.jse.2015.01.007.
3. Bercik MJ, Kruse K, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606. doi:10.1016/j.jse.2016.03.010.
4. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072. doi:10.1016/j.jse.2013.09.017.
5. Franklin JL, Barrett WP, Jackins SE, Matsen FA 3rd. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3(1):39-46.
6. Hermida JC, Flores-Hernandez C, Hoenecke HR, D’Lima DD. Augmented wedge-shaped glenoid component for the correction of glenoid retroversion: a finite element analysis. J Shoulder Elbow Surg. 2014;23(3):347-354. doi:10.1016/j.jse.2013.06.008.
7. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82. doi:10.2106/JBJS.L.00336.
8. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598. doi:10.1016/j.jse.2013.06.017.
9. Jones RB, Wright TW, Roche CP. Bone grafting the glenoid versus use of augmented glenoid baseplates with reverse shoulder arthroplasty. Bull Hosp Jt Dis (2013). 2015;73(suppl 1):S129-S135.
10. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymmetric posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308. doi:10.1016/j.jse.2013.04.014.
11. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157. doi:10.1016/j.jse.2013.09.023.
12. Rice RS, Sperling JW, Miletti J, Schleck C, Cofield RH. Augmented glenoid component for bone deficiency in shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):579-583. doi:10.1007/s11999-007-0104-4.
13. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973. doi:10.1016/j.jse.2013.09.019.
14. Davis DE, Acevedo D, Williams A, Williams G. Total shoulder arthroplasty using an inlay mini-glenoid component for glenoid deficiency: a 2-year follow-up of 9 shoulders in 7 patients. J Shoulder Elbow Surg. 2016;25(8):1354-1361. doi:10.1016/j.jse.2015.12.010.
15. Kersten AD, Flores-Hernandez C, Hoenecke HR, D'Lima DD. Posterior augmented glenoid designs preserve more bone in biconcave glenoids. J Shoulder Elbow Surg. 2015;24(7):1135-1141. doi:10.1016/j.jse.2014.12.007.
16. Favorito PJ, Freed RJ, Passanise AM, Brown MJ. Total shoulder arthroplasty for glenohumeral arthritis associated with posterior glenoid bone loss: results of an all-polyethylene, posteriorly augmented glenoid component. J Shoulder Elbow Surg. 2016;25(10):1681-1689. doi:10.1016/j.jse.2016.02.020.
17. Sandow M, Schutz C. Total shoulder arthroplasty using trabecular metal augments to address glenoid retroversion: the preliminary result of 10 patients with minimum 2-year follow-up. J Shoulder Elbow Surg. 2016;25(4):598-607. doi:10.1016/j.jse.2016.01.001.
18. Wright TW, Roche CP, Wright L, Flurin PH, Crosby LA, Zuckerman JD. Reverse shoulder arthroplasty augments for glenoid wear: A comparison of posterior augments to superior augments. Bull Hosp Jt Dis. 2015;73(suppl 1):S124-S128.
19. Boileau P, Morin-Salvo N, Gauci MO, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26(12):2133-2142. doi:10.1016/j.jse.2017.05.024.
TAKE-HOME POINTS
- Glenoid defects are very common.
- Options for treating glenoid defects include eccentric reaming, bone grafting, and augmented glenoids.
- As computer-assisted surgery use becomes more widespread the use of augments in both TSA and RTSA will become very common.
- Subchondral bone is precious and cannot be replaced once reamed away. Eccentric glenoids introduce a mechanism to minimize reaming and preserve this precious bone.
- On short-term to midterm follow-up augments perform at least as well if not better than non-augmented glenoid components with complication rate and revisions likewise similar.