User login
Serpentine Supravenous Hyperpigmentation Following Cisplatin and Pemetrexed Chemotherapy
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
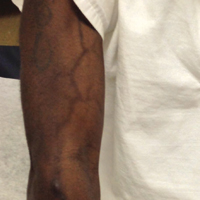
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
Practice Points
- A variety of dermatologic complications have been reported with cisplatin chemotherapy, including serpentine supravenous hyperpigmentation (SSH); however, pemetrexed has not been associated with SSH.
- Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.
An Intense Rash
A32-year-old white male with Down syndrome was initially admitted with fever, cough, and productive sputum. He was started on appropriate antibiotics, but soon became hypoxic and developed respiratory failure requiring mechanical ventilation. The patient developed acute respiratory distress syndrome (ARDS) and eventually had a tracheostomy. On day 10, while in the intensive care unit, the patient developed multiple 1-2 mL clear vesicular eruptions with no surrounding erythema or edema. These were primarily distributed on the chest; the patient’s face was clear. TH
In dealing with this new eruption, the physician should have:
- Lowered the ambient room temperature and otherwise continued the current management.
- Performed a Tzanck smear, isolated the patient, and started acyclovir IV for presumed disseminated herpes zoster.
- Discontinued current antibiotics due to the drug reaction.
- Performed a skin biopsy for hematoxylin and eosin (H&E) stain as well as immunofluorescence for suspected primary early bullous disease (i.e., bullous pemphigoid).
- Started amphotericin.
Discussion
The correct answer is A: The physician should have lowered the ambient room temperature and continued the current management.
This patient had developed miliaria crystallina, a transient disorder of occluded sweat glands that usually results from excessive exposure to heat and humidity. Miliaria, also known as sweat rash, defines a group of disorders exhibiting eccrine gland obstruction with leakage and retention of sweat at different levels in the skin. The clinical presentation of miliaria is related to the depth of this obstruction and occurs in three main forms: miliaria crystallina, miliaria rubra, and miliaria profunda.1
Of the three variants, eccrine obstruction in miliaria crystallina occurs most superficially, at either the distal duct or pore, and drives sweat vesiculation into the stratum corneum of the epidermis.1 It is characterized by diffuse eruptions of non-inflamed, translucent vesicles of one to two millimeters, typically forming in crops on the trunk of the body. The tiny blisters have been likened to beads of sweat and are extremely fragile, rupturing spontaneously or with light friction.
Clinically, miliaria crystallina is an asymptomatic and self-limited disorder. It often occurs during the summer months and in tropical climates after prolonged exposure to heat and humidity. It is thought that overhydration of corneocytes from excessive sweating predisposes the eccrine duct to obstruction. This may be compounded by any form of occlusion, like clothing or bedding, that traps moisture and impedes the evaporation of sweat.2 Other risk factors include persistent fevers, neonate age less than two weeks, secondary to eccrine duct immaturity, and drugs such as isotretinoin and bethanechol.3,4,5
In the case of this 32-year-old male with respiratory failure, the characteristic eruption of miliaria crystallina developed on day 10 of intensive care. After lowering the ambient temperature of the patient’s room and adding the benefit of a circulatory fan, the vesicles resolved within two to three days. As this example demonstrates, the treatment of miliaria crystallina is straightforward and consists of drying the skin and preventing sweating for several days by keeping the patient in a cool, air-conditioned environment. Eventually, the keratinous plug will be shed, and normal sweating will resume.
In the literature, there is only one published report documenting two distinct cases of miliaria crystallina in the intensive care setting. At the time of onset, these two patients had been in the ICU for slightly over two weeks, and both were receiving treatment with multiple neuroautonomic agents, including—but not limited to—clonidine, a beta blocker, and opiates for pain. The innervation of eccrine glands is under sympathetic control mostly through the postsynaptic release of acetylcholine but also via adrenergic stimulation of contractile myoepithelia. While temperature and humidity are carefully regulated in most intensive care facilities, significant sweating may result from eccrine stimulation by neuroautonomic medications with intrinsic sympathomimetic activity.6 Combined with prolonged immobility, this sweating may create the perfect environment for eccrine duct obstruction and the development of miliaria crystallina.
Incidence in the intensive care setting has not been studied, but miliaria crystallina probably occurs much more frequently than it is reported. The ability to recognize the characteristic eruptions may prevent the hospitalist who encounters it from ordering unnecessary consults or diagnostics, and knowledge of its risk factors will aid both in treatment and in prevention. TH
References
- Wenzel FG, Horn TD. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol. 1998 Jan;38(1):1–20.
- Sperling L. Chapter 3: Skin Diseases Associated with Excessive Heat, Humidity, and Sunlight. In: Textbook of Military Dermatology. Washington, D.C.: Office of The Surgeon General at TMM Publications; 1994:39-54. Available at: www.bordeninstitute.army.mil/derm/default_index.htm. Last accessed: September 8, 2006.
- Haas N, Henz BM, Weigel H. Congenital miliaria crystallina. J Am Acad Dermatol. 2002 Nov;47(5 Suppl):S270–272.
- Gupta AK, Ellis CN, Madison KC, et al. Miliaria crystallina occurring in a patient treated with isotretinoin. Cutis. 1986 Oct;38(4):275–276.
- Rochmis PG, Koplon BS. Iatrogenic miliaria crystallina due to bethanechol. Arch Dermatol. 1967 May;95(9):499–500.
- Haas N, Martens F, Henz BM. Miliaria crystallina in an intensive care setting. Clin Exp Dermatol. 2004 Jan;29 (1):32-34.
A32-year-old white male with Down syndrome was initially admitted with fever, cough, and productive sputum. He was started on appropriate antibiotics, but soon became hypoxic and developed respiratory failure requiring mechanical ventilation. The patient developed acute respiratory distress syndrome (ARDS) and eventually had a tracheostomy. On day 10, while in the intensive care unit, the patient developed multiple 1-2 mL clear vesicular eruptions with no surrounding erythema or edema. These were primarily distributed on the chest; the patient’s face was clear. TH
In dealing with this new eruption, the physician should have:
- Lowered the ambient room temperature and otherwise continued the current management.
- Performed a Tzanck smear, isolated the patient, and started acyclovir IV for presumed disseminated herpes zoster.
- Discontinued current antibiotics due to the drug reaction.
- Performed a skin biopsy for hematoxylin and eosin (H&E) stain as well as immunofluorescence for suspected primary early bullous disease (i.e., bullous pemphigoid).
- Started amphotericin.
Discussion
The correct answer is A: The physician should have lowered the ambient room temperature and continued the current management.
This patient had developed miliaria crystallina, a transient disorder of occluded sweat glands that usually results from excessive exposure to heat and humidity. Miliaria, also known as sweat rash, defines a group of disorders exhibiting eccrine gland obstruction with leakage and retention of sweat at different levels in the skin. The clinical presentation of miliaria is related to the depth of this obstruction and occurs in three main forms: miliaria crystallina, miliaria rubra, and miliaria profunda.1
Of the three variants, eccrine obstruction in miliaria crystallina occurs most superficially, at either the distal duct or pore, and drives sweat vesiculation into the stratum corneum of the epidermis.1 It is characterized by diffuse eruptions of non-inflamed, translucent vesicles of one to two millimeters, typically forming in crops on the trunk of the body. The tiny blisters have been likened to beads of sweat and are extremely fragile, rupturing spontaneously or with light friction.
Clinically, miliaria crystallina is an asymptomatic and self-limited disorder. It often occurs during the summer months and in tropical climates after prolonged exposure to heat and humidity. It is thought that overhydration of corneocytes from excessive sweating predisposes the eccrine duct to obstruction. This may be compounded by any form of occlusion, like clothing or bedding, that traps moisture and impedes the evaporation of sweat.2 Other risk factors include persistent fevers, neonate age less than two weeks, secondary to eccrine duct immaturity, and drugs such as isotretinoin and bethanechol.3,4,5
In the case of this 32-year-old male with respiratory failure, the characteristic eruption of miliaria crystallina developed on day 10 of intensive care. After lowering the ambient temperature of the patient’s room and adding the benefit of a circulatory fan, the vesicles resolved within two to three days. As this example demonstrates, the treatment of miliaria crystallina is straightforward and consists of drying the skin and preventing sweating for several days by keeping the patient in a cool, air-conditioned environment. Eventually, the keratinous plug will be shed, and normal sweating will resume.
In the literature, there is only one published report documenting two distinct cases of miliaria crystallina in the intensive care setting. At the time of onset, these two patients had been in the ICU for slightly over two weeks, and both were receiving treatment with multiple neuroautonomic agents, including—but not limited to—clonidine, a beta blocker, and opiates for pain. The innervation of eccrine glands is under sympathetic control mostly through the postsynaptic release of acetylcholine but also via adrenergic stimulation of contractile myoepithelia. While temperature and humidity are carefully regulated in most intensive care facilities, significant sweating may result from eccrine stimulation by neuroautonomic medications with intrinsic sympathomimetic activity.6 Combined with prolonged immobility, this sweating may create the perfect environment for eccrine duct obstruction and the development of miliaria crystallina.
Incidence in the intensive care setting has not been studied, but miliaria crystallina probably occurs much more frequently than it is reported. The ability to recognize the characteristic eruptions may prevent the hospitalist who encounters it from ordering unnecessary consults or diagnostics, and knowledge of its risk factors will aid both in treatment and in prevention. TH
References
- Wenzel FG, Horn TD. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol. 1998 Jan;38(1):1–20.
- Sperling L. Chapter 3: Skin Diseases Associated with Excessive Heat, Humidity, and Sunlight. In: Textbook of Military Dermatology. Washington, D.C.: Office of The Surgeon General at TMM Publications; 1994:39-54. Available at: www.bordeninstitute.army.mil/derm/default_index.htm. Last accessed: September 8, 2006.
- Haas N, Henz BM, Weigel H. Congenital miliaria crystallina. J Am Acad Dermatol. 2002 Nov;47(5 Suppl):S270–272.
- Gupta AK, Ellis CN, Madison KC, et al. Miliaria crystallina occurring in a patient treated with isotretinoin. Cutis. 1986 Oct;38(4):275–276.
- Rochmis PG, Koplon BS. Iatrogenic miliaria crystallina due to bethanechol. Arch Dermatol. 1967 May;95(9):499–500.
- Haas N, Martens F, Henz BM. Miliaria crystallina in an intensive care setting. Clin Exp Dermatol. 2004 Jan;29 (1):32-34.
A32-year-old white male with Down syndrome was initially admitted with fever, cough, and productive sputum. He was started on appropriate antibiotics, but soon became hypoxic and developed respiratory failure requiring mechanical ventilation. The patient developed acute respiratory distress syndrome (ARDS) and eventually had a tracheostomy. On day 10, while in the intensive care unit, the patient developed multiple 1-2 mL clear vesicular eruptions with no surrounding erythema or edema. These were primarily distributed on the chest; the patient’s face was clear. TH
In dealing with this new eruption, the physician should have:
- Lowered the ambient room temperature and otherwise continued the current management.
- Performed a Tzanck smear, isolated the patient, and started acyclovir IV for presumed disseminated herpes zoster.
- Discontinued current antibiotics due to the drug reaction.
- Performed a skin biopsy for hematoxylin and eosin (H&E) stain as well as immunofluorescence for suspected primary early bullous disease (i.e., bullous pemphigoid).
- Started amphotericin.
Discussion
The correct answer is A: The physician should have lowered the ambient room temperature and continued the current management.
This patient had developed miliaria crystallina, a transient disorder of occluded sweat glands that usually results from excessive exposure to heat and humidity. Miliaria, also known as sweat rash, defines a group of disorders exhibiting eccrine gland obstruction with leakage and retention of sweat at different levels in the skin. The clinical presentation of miliaria is related to the depth of this obstruction and occurs in three main forms: miliaria crystallina, miliaria rubra, and miliaria profunda.1
Of the three variants, eccrine obstruction in miliaria crystallina occurs most superficially, at either the distal duct or pore, and drives sweat vesiculation into the stratum corneum of the epidermis.1 It is characterized by diffuse eruptions of non-inflamed, translucent vesicles of one to two millimeters, typically forming in crops on the trunk of the body. The tiny blisters have been likened to beads of sweat and are extremely fragile, rupturing spontaneously or with light friction.
Clinically, miliaria crystallina is an asymptomatic and self-limited disorder. It often occurs during the summer months and in tropical climates after prolonged exposure to heat and humidity. It is thought that overhydration of corneocytes from excessive sweating predisposes the eccrine duct to obstruction. This may be compounded by any form of occlusion, like clothing or bedding, that traps moisture and impedes the evaporation of sweat.2 Other risk factors include persistent fevers, neonate age less than two weeks, secondary to eccrine duct immaturity, and drugs such as isotretinoin and bethanechol.3,4,5
In the case of this 32-year-old male with respiratory failure, the characteristic eruption of miliaria crystallina developed on day 10 of intensive care. After lowering the ambient temperature of the patient’s room and adding the benefit of a circulatory fan, the vesicles resolved within two to three days. As this example demonstrates, the treatment of miliaria crystallina is straightforward and consists of drying the skin and preventing sweating for several days by keeping the patient in a cool, air-conditioned environment. Eventually, the keratinous plug will be shed, and normal sweating will resume.
In the literature, there is only one published report documenting two distinct cases of miliaria crystallina in the intensive care setting. At the time of onset, these two patients had been in the ICU for slightly over two weeks, and both were receiving treatment with multiple neuroautonomic agents, including—but not limited to—clonidine, a beta blocker, and opiates for pain. The innervation of eccrine glands is under sympathetic control mostly through the postsynaptic release of acetylcholine but also via adrenergic stimulation of contractile myoepithelia. While temperature and humidity are carefully regulated in most intensive care facilities, significant sweating may result from eccrine stimulation by neuroautonomic medications with intrinsic sympathomimetic activity.6 Combined with prolonged immobility, this sweating may create the perfect environment for eccrine duct obstruction and the development of miliaria crystallina.
Incidence in the intensive care setting has not been studied, but miliaria crystallina probably occurs much more frequently than it is reported. The ability to recognize the characteristic eruptions may prevent the hospitalist who encounters it from ordering unnecessary consults or diagnostics, and knowledge of its risk factors will aid both in treatment and in prevention. TH
References
- Wenzel FG, Horn TD. Nonneoplastic disorders of the eccrine glands. J Am Acad Dermatol. 1998 Jan;38(1):1–20.
- Sperling L. Chapter 3: Skin Diseases Associated with Excessive Heat, Humidity, and Sunlight. In: Textbook of Military Dermatology. Washington, D.C.: Office of The Surgeon General at TMM Publications; 1994:39-54. Available at: www.bordeninstitute.army.mil/derm/default_index.htm. Last accessed: September 8, 2006.
- Haas N, Henz BM, Weigel H. Congenital miliaria crystallina. J Am Acad Dermatol. 2002 Nov;47(5 Suppl):S270–272.
- Gupta AK, Ellis CN, Madison KC, et al. Miliaria crystallina occurring in a patient treated with isotretinoin. Cutis. 1986 Oct;38(4):275–276.
- Rochmis PG, Koplon BS. Iatrogenic miliaria crystallina due to bethanechol. Arch Dermatol. 1967 May;95(9):499–500.
- Haas N, Martens F, Henz BM. Miliaria crystallina in an intensive care setting. Clin Exp Dermatol. 2004 Jan;29 (1):32-34.
Pacemaker Rash
A24-year-old white female is admitted directly to the hospital by her cardiologist for a wound infection. She is a medical technology student who underwent a pacemaker implantation three weeks prior for persistent symptomatic bradycardia. She now complains of pain, redness, and swelling at the site of her pacemaker incision site. She reports fevers, chills, night sweats, and multiple other systemic symptoms.
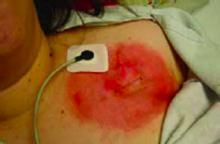
On physical exam, she appears quite pleasant, in no apparent distress, and without any abnormalities in vital signs. Her incision site reveals an erythematous, geometric, annular patch with no edema, warmth, induration or discharge. (See photo.)
What is the most appropriate treatment for this patient?
- Draw blood cultures, place central line, and begin broad-spectrum antibiotics.
- Draw blood cultures, place central line, and begin broad-spectrum antibiotics and also itraconazole to cover atypical mycobacteria infection.
- Schedule surgery to remove pacemaker.
- Gently approach patient about stressors and possible underlying psychiatric issues and consult psychiatry.
- Obtain wound cultures and apply mupirocin ointment twice daily.
Discussion
The correct answer is D: Gently approach patient about stressors and possible underlying psychiatric issues and consult psychiatry.
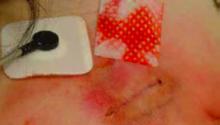
This patient had been applying an external agent (most likely makeup) to her wound site. On questioning, the patient and her family continued to deny any manipulation of the wound even when the substance was wiped away with an alcohol pad. (See photo above.) Additionally, a half-empty bottle of clonidine was found under her pillow. The clonidine was apparently used as an attempt to feign hypotension. She denied taking the medication, but the medical team suspected that the use of these antihypertensives led to her previous symptomatic bradycardia and eventual pacemaker implantation.
Despite gentle questioning and evaluation for stressors and signs of depression, the patient left the hospital against medical advice before psychiatric consult could be obtained. The patient eventually returned to the cardiology clinic complaining again of wound infection. A wound culture revealed Enterococcus faecalis consistent with fecal contamination of her incision site. Eventually, her pacemaker was removed. She did continue to see different physicians and visit different hospitals before being permanently lost to follow up.
This case of Munchausen syndrome demonstrates many of its defining characteristics. Munchausen syndrome was originally described by Asher in 1951 in Lancet. Its name is derived from Baron von Munchausen, a German nobleman who told humorous but outlandish tales about his travels, including riding on cannonballs, traveling to the moon, and discovering an island made of cheese. Munchausen syndrome is a factitious disorder (symptoms are intentionally produced), but unlike malingering there is no apparent secondary gain except to satisfy the psychological need to receive attention or support. These patients often undergo medical evaluations and multiple, invasive, surgical procedures simply to have them. The DSM-IV points out that the motivation for the behavior is only to assume the sick role.
Patients tend to be young adults and are more often male. They may describe and have physical findings of any number of illnesses. They may have undergone many prior surgical procedures and have several scars on physical exam. Classically, they have seen several different physicians and often have some medical knowledge including medical terminology.
Treatment is often difficult. It is appropriate to address any possible underlying organic disease by systemic approach to avoid overlooking any dangerous conditions. If none is found, the patient should be assessed for stressors, signs of psychosis or depression, and any possible financial or other secondary gains. It is important to recognize Munchausen syndrome as a factitious disorder as opposed to a somatoform disorder (somatization, conversion, hypochondriasis). A factitious disorder is produced artificially by the patient, whereas symptoms of a somatoform disorder are not under the patient’s control. TH
Bibliography
- Asher R. Munchausen’s syndrome. Lancet. 1951;1:339-341.
- Hammerschmidt DE. The adventures of Freiherr von Munchausen. J Lab Clin Med. 2004 Dec;144(6):320-321.
- Park TA, Borsch MA, Dyer AR, et al. Cardiopathia fantastica: the cardiac variant of Munchausen syndrome. South Med J. 2004;97(1):48-52;quiz 53.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med. 1983;99:240–247.
- Lad SP, Jobe KW, Polley J, et al. Munchausen’s syndrome in neurosurgery: report of two cases and review of the literature. Neurosurgery. 2004 Dec;55(6):1436.
- Huffman JC, Stern TA. The diagnosis and treatment of Munchausen’s syndrome. General Hospital Psychiatry. 2003 Sept-Oct;25(5):358-363.
A24-year-old white female is admitted directly to the hospital by her cardiologist for a wound infection. She is a medical technology student who underwent a pacemaker implantation three weeks prior for persistent symptomatic bradycardia. She now complains of pain, redness, and swelling at the site of her pacemaker incision site. She reports fevers, chills, night sweats, and multiple other systemic symptoms.
On physical exam, she appears quite pleasant, in no apparent distress, and without any abnormalities in vital signs. Her incision site reveals an erythematous, geometric, annular patch with no edema, warmth, induration or discharge. (See photo.)
What is the most appropriate treatment for this patient?
- Draw blood cultures, place central line, and begin broad-spectrum antibiotics.
- Draw blood cultures, place central line, and begin broad-spectrum antibiotics and also itraconazole to cover atypical mycobacteria infection.
- Schedule surgery to remove pacemaker.
- Gently approach patient about stressors and possible underlying psychiatric issues and consult psychiatry.
- Obtain wound cultures and apply mupirocin ointment twice daily.
Discussion
The correct answer is D: Gently approach patient about stressors and possible underlying psychiatric issues and consult psychiatry.
This patient had been applying an external agent (most likely makeup) to her wound site. On questioning, the patient and her family continued to deny any manipulation of the wound even when the substance was wiped away with an alcohol pad. (See photo above.) Additionally, a half-empty bottle of clonidine was found under her pillow. The clonidine was apparently used as an attempt to feign hypotension. She denied taking the medication, but the medical team suspected that the use of these antihypertensives led to her previous symptomatic bradycardia and eventual pacemaker implantation.
Despite gentle questioning and evaluation for stressors and signs of depression, the patient left the hospital against medical advice before psychiatric consult could be obtained. The patient eventually returned to the cardiology clinic complaining again of wound infection. A wound culture revealed Enterococcus faecalis consistent with fecal contamination of her incision site. Eventually, her pacemaker was removed. She did continue to see different physicians and visit different hospitals before being permanently lost to follow up.
This case of Munchausen syndrome demonstrates many of its defining characteristics. Munchausen syndrome was originally described by Asher in 1951 in Lancet. Its name is derived from Baron von Munchausen, a German nobleman who told humorous but outlandish tales about his travels, including riding on cannonballs, traveling to the moon, and discovering an island made of cheese. Munchausen syndrome is a factitious disorder (symptoms are intentionally produced), but unlike malingering there is no apparent secondary gain except to satisfy the psychological need to receive attention or support. These patients often undergo medical evaluations and multiple, invasive, surgical procedures simply to have them. The DSM-IV points out that the motivation for the behavior is only to assume the sick role.
Patients tend to be young adults and are more often male. They may describe and have physical findings of any number of illnesses. They may have undergone many prior surgical procedures and have several scars on physical exam. Classically, they have seen several different physicians and often have some medical knowledge including medical terminology.
Treatment is often difficult. It is appropriate to address any possible underlying organic disease by systemic approach to avoid overlooking any dangerous conditions. If none is found, the patient should be assessed for stressors, signs of psychosis or depression, and any possible financial or other secondary gains. It is important to recognize Munchausen syndrome as a factitious disorder as opposed to a somatoform disorder (somatization, conversion, hypochondriasis). A factitious disorder is produced artificially by the patient, whereas symptoms of a somatoform disorder are not under the patient’s control. TH
Bibliography
- Asher R. Munchausen’s syndrome. Lancet. 1951;1:339-341.
- Hammerschmidt DE. The adventures of Freiherr von Munchausen. J Lab Clin Med. 2004 Dec;144(6):320-321.
- Park TA, Borsch MA, Dyer AR, et al. Cardiopathia fantastica: the cardiac variant of Munchausen syndrome. South Med J. 2004;97(1):48-52;quiz 53.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med. 1983;99:240–247.
- Lad SP, Jobe KW, Polley J, et al. Munchausen’s syndrome in neurosurgery: report of two cases and review of the literature. Neurosurgery. 2004 Dec;55(6):1436.
- Huffman JC, Stern TA. The diagnosis and treatment of Munchausen’s syndrome. General Hospital Psychiatry. 2003 Sept-Oct;25(5):358-363.
A24-year-old white female is admitted directly to the hospital by her cardiologist for a wound infection. She is a medical technology student who underwent a pacemaker implantation three weeks prior for persistent symptomatic bradycardia. She now complains of pain, redness, and swelling at the site of her pacemaker incision site. She reports fevers, chills, night sweats, and multiple other systemic symptoms.
On physical exam, she appears quite pleasant, in no apparent distress, and without any abnormalities in vital signs. Her incision site reveals an erythematous, geometric, annular patch with no edema, warmth, induration or discharge. (See photo.)
What is the most appropriate treatment for this patient?
- Draw blood cultures, place central line, and begin broad-spectrum antibiotics.
- Draw blood cultures, place central line, and begin broad-spectrum antibiotics and also itraconazole to cover atypical mycobacteria infection.
- Schedule surgery to remove pacemaker.
- Gently approach patient about stressors and possible underlying psychiatric issues and consult psychiatry.
- Obtain wound cultures and apply mupirocin ointment twice daily.
Discussion
The correct answer is D: Gently approach patient about stressors and possible underlying psychiatric issues and consult psychiatry.
This patient had been applying an external agent (most likely makeup) to her wound site. On questioning, the patient and her family continued to deny any manipulation of the wound even when the substance was wiped away with an alcohol pad. (See photo above.) Additionally, a half-empty bottle of clonidine was found under her pillow. The clonidine was apparently used as an attempt to feign hypotension. She denied taking the medication, but the medical team suspected that the use of these antihypertensives led to her previous symptomatic bradycardia and eventual pacemaker implantation.
Despite gentle questioning and evaluation for stressors and signs of depression, the patient left the hospital against medical advice before psychiatric consult could be obtained. The patient eventually returned to the cardiology clinic complaining again of wound infection. A wound culture revealed Enterococcus faecalis consistent with fecal contamination of her incision site. Eventually, her pacemaker was removed. She did continue to see different physicians and visit different hospitals before being permanently lost to follow up.
This case of Munchausen syndrome demonstrates many of its defining characteristics. Munchausen syndrome was originally described by Asher in 1951 in Lancet. Its name is derived from Baron von Munchausen, a German nobleman who told humorous but outlandish tales about his travels, including riding on cannonballs, traveling to the moon, and discovering an island made of cheese. Munchausen syndrome is a factitious disorder (symptoms are intentionally produced), but unlike malingering there is no apparent secondary gain except to satisfy the psychological need to receive attention or support. These patients often undergo medical evaluations and multiple, invasive, surgical procedures simply to have them. The DSM-IV points out that the motivation for the behavior is only to assume the sick role.
Patients tend to be young adults and are more often male. They may describe and have physical findings of any number of illnesses. They may have undergone many prior surgical procedures and have several scars on physical exam. Classically, they have seen several different physicians and often have some medical knowledge including medical terminology.
Treatment is often difficult. It is appropriate to address any possible underlying organic disease by systemic approach to avoid overlooking any dangerous conditions. If none is found, the patient should be assessed for stressors, signs of psychosis or depression, and any possible financial or other secondary gains. It is important to recognize Munchausen syndrome as a factitious disorder as opposed to a somatoform disorder (somatization, conversion, hypochondriasis). A factitious disorder is produced artificially by the patient, whereas symptoms of a somatoform disorder are not under the patient’s control. TH
Bibliography
- Asher R. Munchausen’s syndrome. Lancet. 1951;1:339-341.
- Hammerschmidt DE. The adventures of Freiherr von Munchausen. J Lab Clin Med. 2004 Dec;144(6):320-321.
- Park TA, Borsch MA, Dyer AR, et al. Cardiopathia fantastica: the cardiac variant of Munchausen syndrome. South Med J. 2004;97(1):48-52;quiz 53.
- Reich P, Gottfried LA. Factitious disorders in a teaching hospital. Ann Intern Med. 1983;99:240–247.
- Lad SP, Jobe KW, Polley J, et al. Munchausen’s syndrome in neurosurgery: report of two cases and review of the literature. Neurosurgery. 2004 Dec;55(6):1436.
- Huffman JC, Stern TA. The diagnosis and treatment of Munchausen’s syndrome. General Hospital Psychiatry. 2003 Sept-Oct;25(5):358-363.