User login
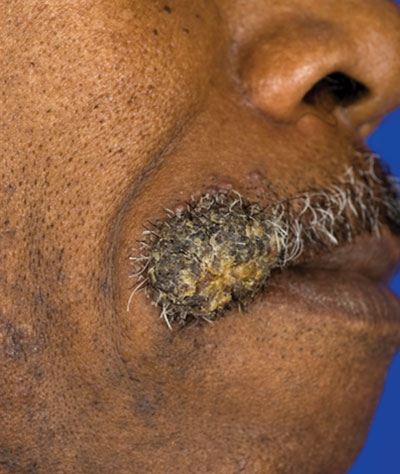
Verrucous Nodule on the Upper Lip
The Diagnosis: Disseminated Coccidioidomycosis
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
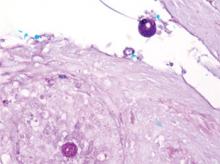
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
The Diagnosis: Disseminated Coccidioidomycosis
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
The Diagnosis: Disseminated Coccidioidomycosis
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
A 62-year-old black man presented to a dermatologist in the northeastern United States with a verrucous nontender nodule on the upper lip after traveling to southern California 1 month prior. The patient was not immunocompromised but reported a recent febrile upper respiratory illness.