User login
Prescribing DOACs with specific patient populations in mind
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.
Overview of DOACs
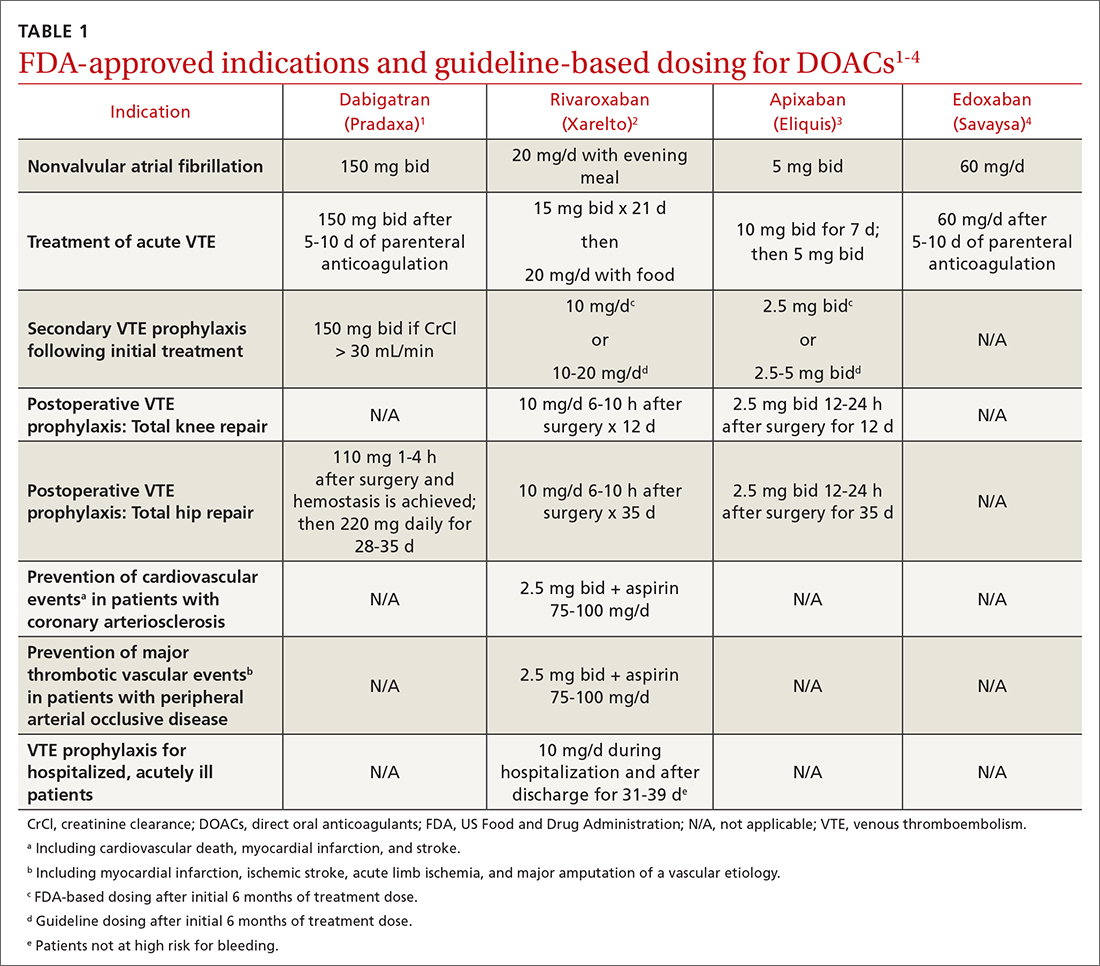
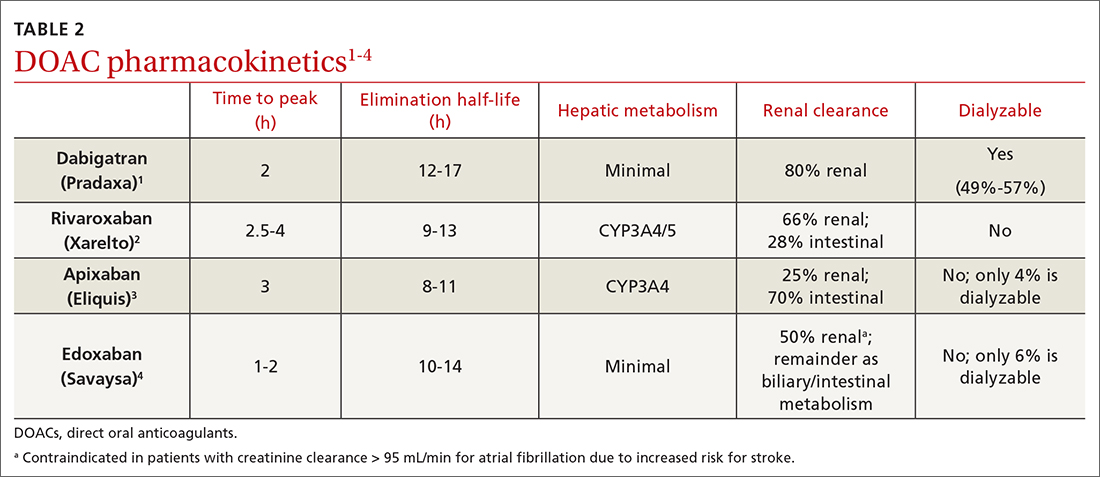
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4 TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7
Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3
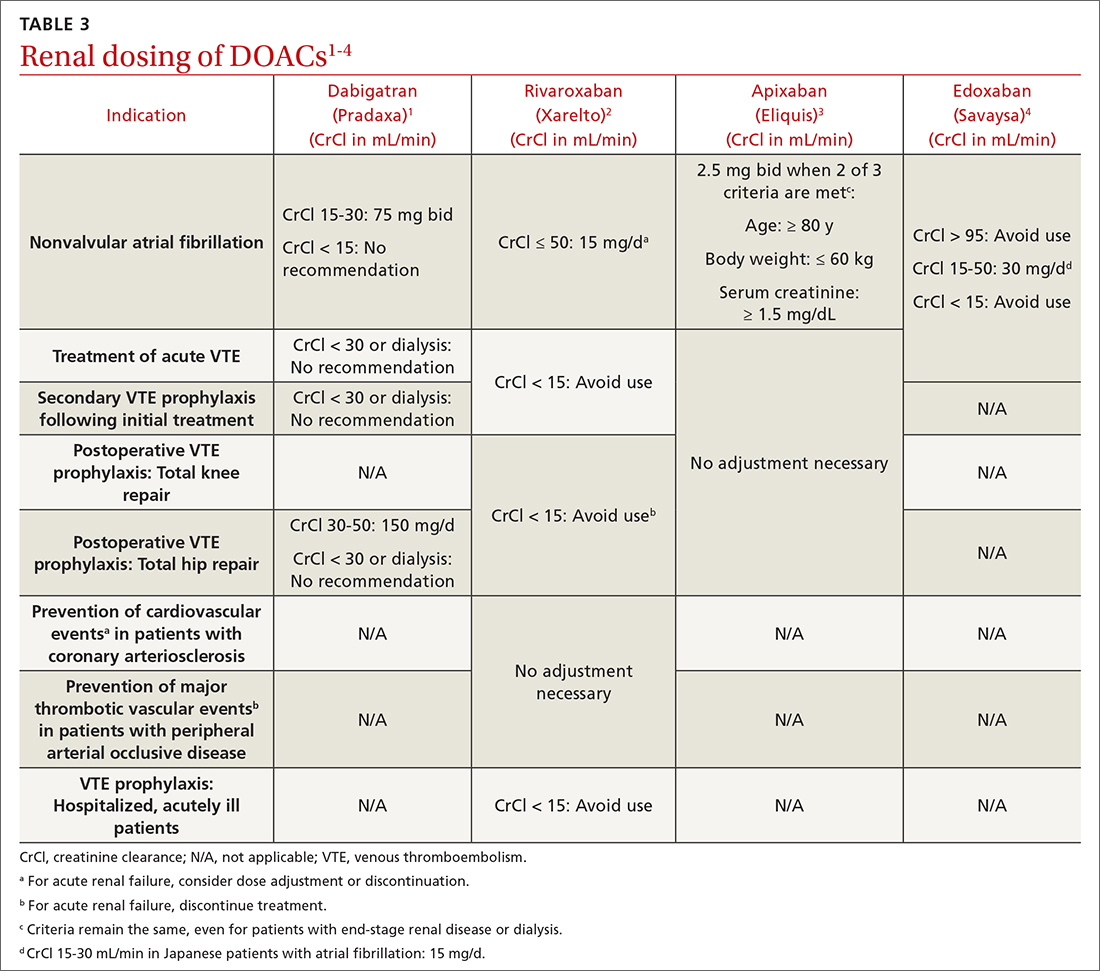
Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.

Overview of DOACs
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4 TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7

Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3

Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.

Overview of DOACs
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4 TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7

Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3

Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
PRACTICE RECOMMENDATIONS
› Consider a direct oral anticoagulant (DOAC) when treating venous thromboembolism (VTE) in patients with advanced chronic kidney disease or obesity. C
› Select apixaban for treatment of VTE or nonvalvular atrial fibrillation in patients with end-stage renal disease, due to its minimal renal clearance compared with other DOACs. B
› Consider DOACs such as dabigatran, rivaroxaban, or apixaban for treatment of VTE in the context of heparin-induced thrombocytopenia. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Make room for continuous glucose monitoring in type 2 diabetes management
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.
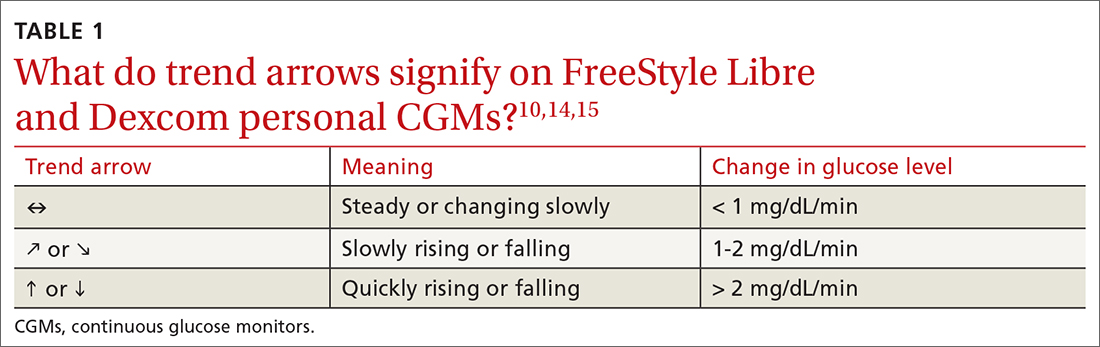
For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17
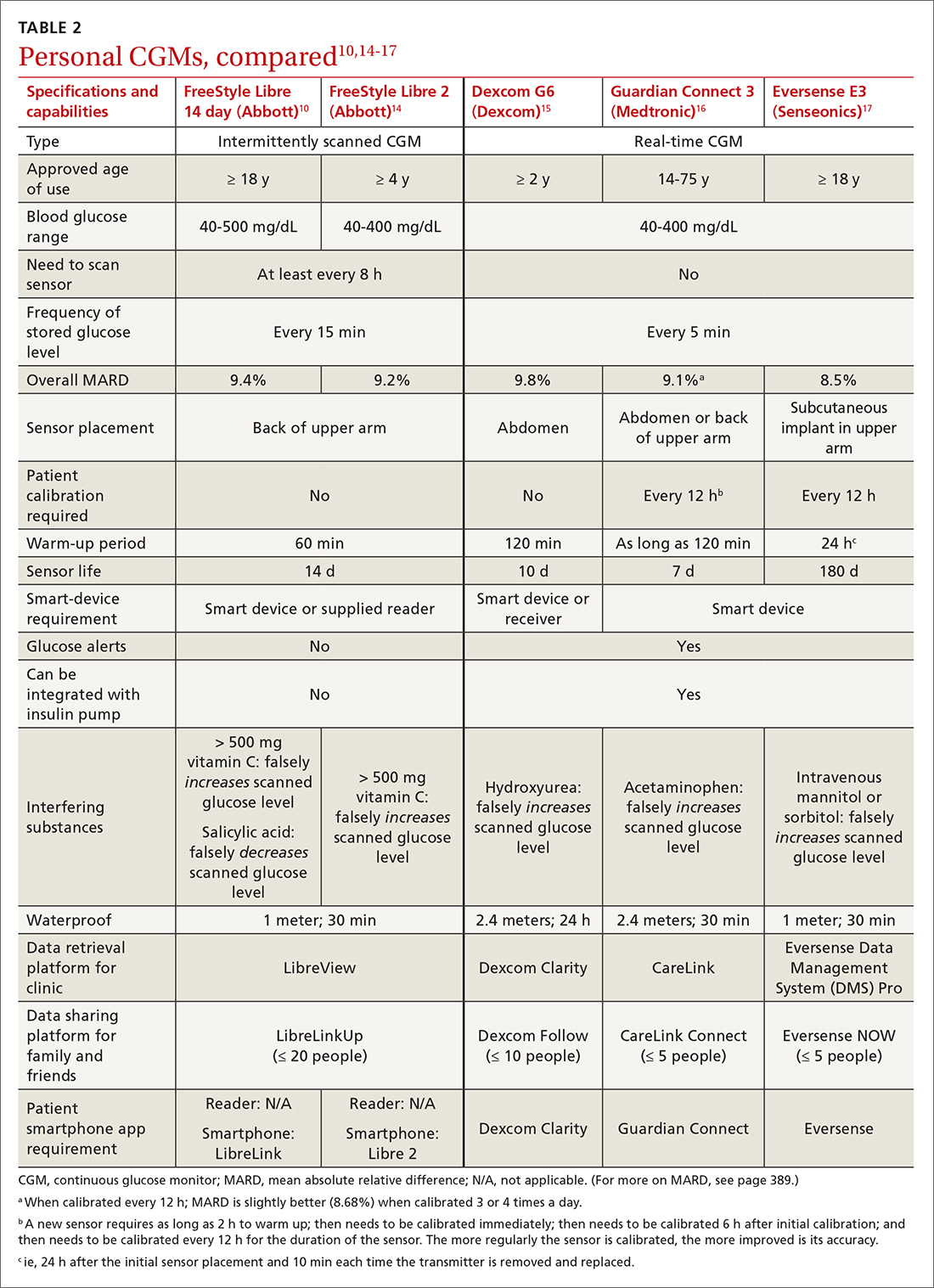
The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32
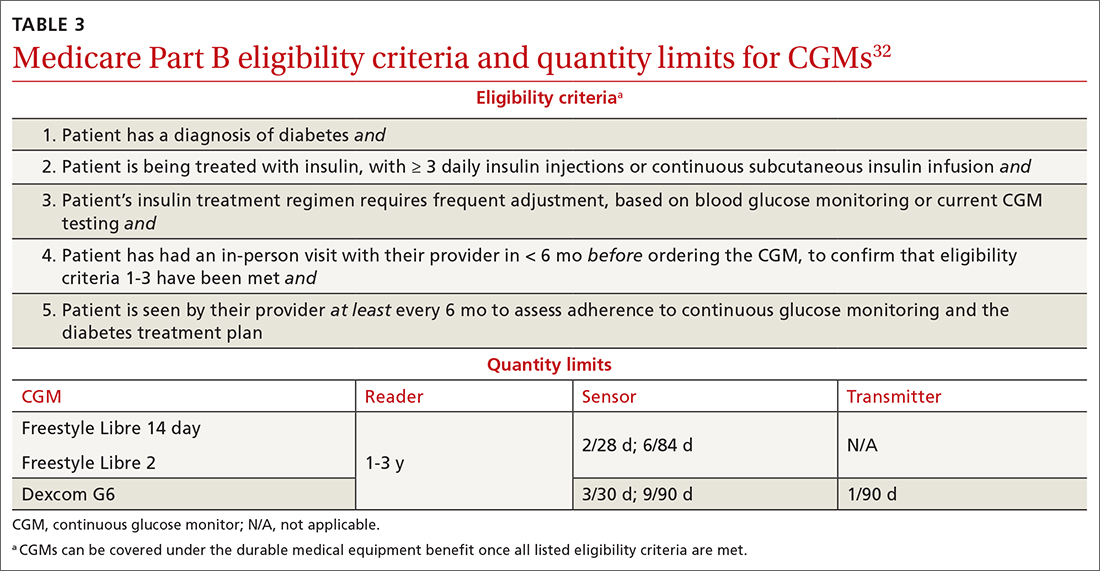
Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
A1C has been used to estimate 3-month glycemic control in patients with diabetes. However, A1C monitoring alone does not provide insight into daily glycemic variation, which is valuable in clinical management because tight glycemic control (defined as A1C < 7.0%) has been shown to reduce the risk of microvascular complications. Prior to the approval of glucagon-like peptide-1 receptor agonists and sodium-glucose co-transporter 2 inhibitors by the US Food and Drug Administration for the treatment of type 2 diabetes (T2D), reduction in the risk of macrovascular complications (aside from nonfatal myocardial infarction) was more difficult to achieve than it is now; some patients had a worse outcome with overly aggressive glycemic control.1
Previously, the use of a continuous glucose monitor (CGM) was limited to patients with type 1 diabetes who required basal and bolus insulin. However, technological advances have led to more patient-friendly and affordable devices, making CGMs more available. As such, the American Diabetes Association (ADA), in its 2022 Standards of Medical Care in Diabetes, recommends that clinicians offer continuous glucose monitoring to adults with T2D who require multiple daily injections, and based on a given patient’s ability, preferences, and needs.2
In this article, we discuss, first, the intricacies of CGMs and, second, what the evidence says about their use so that physicians can confidently recommend, and educate patients on, effective utilization of CGMs to obtain an individualized target of glycemic control.
Continuous glucose monitoring: A glossary
CGMs are characterized by who possesses the device and how data are recorded. This review is not about professional CGMs, which are owned by the health care provider and consist of a sensor that is applied in the clinic and returned to clinic for downloading of data1; rather, we focus on the novel category of nonprofessional, or personal, CGMs.
Three words to remember. Every CGM has 3 common components:
- The reader (also known as a receiver) is a handheld device that allows a patient to scan a sensor (see definition below) and instantaneously collect a glucose reading. The patient can use a standalone reader; a smartphone or other smart device with an associated app that serves as a reader; or both.
- A sensor is inserted subcutaneously to measure interstitial glucose. The lifespan of a sensor is 10 to 14 days.
- A transmitter relays information from the sensor to the reader.
The technology behind a CGM
CGM sensors measure interstitial glucose by means of a chemical reaction involving glucose oxidase and an oxidation-reduction cofactor, measuring the generation of hydrogen peroxide.3 Interstitial glucose readings lag behind plasma blood glucose readings by 2 to 21 minutes.4,5 Although this lag time is often not clinically significant, situations such as aerobic exercise and a rapidly changing glucose level might warrant confirmation by means of fingerstick measurement.5 It is common for CGM readings to vary slightly from venipuncture or fingerstick glucose readings.
What CGMs are availableto your patients?
Intermittently scanned CGMs (isCGMs) measure the glucose level continuously; the patient must scan a sensor to display and record the glucose level.6 Prolonged periods without scanning result in gaps in glycemic data.7,8
Continue to: Two isCGM systems...
Two isCGM systems are available: the FreeStyle Libre 14 day and the FreeStyle Libre 2 (both from Abbott).a Both consist of a reader and a disposable sensor, applied to the back of the arm, that is worn for 14 days. If the patient has a compatible smartphone or other smart device, the reader can be replaced by the smart device with the downloaded FreeStyle Libre or FreeStyle Libre 2 app.
To activate a new sensor, the patient applies the sensor, then scans it. Once activated, scanning the sensor provides the current glucose reading and recalls the last 8 hours of data. In addition to providing an instantaneous glucose reading, the display also provides a trend arrow indicating the direction and degree to which the glucose level is changing (TABLE 110,14,15). This feature helps patients avoid hypoglycemic episodes by allowing them to preemptively correct if the arrow indicates a rapidly declining glucose level.

For the first 12 hours after a new sensor is activated, and when a glucose reading is < 70 mg/dL, patients should be instructed to avoid making treatment decisions and encouraged to utilize fingerstick glucose readings. FreeStyle Libre 14 day does not allow a glucose level alarm to be set; the system cannot detect these events without scanning the sensor.10 Bluetooth connectivity does allow FreeStyle Libre 2 users to set a glucose alarm if the reader or smart device is within 20 feet of the sensor. A default alarm is set to activate at 70 mg/dL (“low”) and 240 mg/dL (“high”); low and high alarm settings are also customizable. Because both FreeStyle Libre devices store 8 hours of data, patients must scan the sensor every 8 hours for a comprehensive glycemic report.14
FreeStyle Libre CGMs allow patients to add therapy notes, including time and amount of insulin administered and carbohydrates ingested. Readers for both devices function as a glucometer that is compatible with Abbott FreeStyle Precision Neo test strips.
Real-time CGMs (rtCGMs) measure and display glucose levels continuously for the duration of the life of the sensor, without the need to scan. Three rtCGM systems are available: Dexcom G6, Medtronic Guardian 3, and Senseonics Eversense E3.
Continue to: Dexcom G6...
Dexcom G6 is the first Dexcom CGM that does not require fingerstick calibration and the only rtCGM available in the United States that does not require patient calibration. This system comprises a single-use sensor replaced every 10 days; a transmitter that is transferred to each new sensor and replaced every 3 months; and an optional receiver that can be omitted if the patient prefers to utilize a smart device.
Dexcom G6 never requires a patient to scan a sensor. Instead, the receiver (or smart device) utilizes Bluetooth technology to obtain blood glucose readings if it is positioned within 20 feet of the transmitter. Patients can set both hypoglycemic and hyperglycemic alarms to predict events within 20 minutes. Similar to the functionality of the FreeStyle Libre systems, Dexcom G6 provides the opportunity to log lifestyle events, including insulin dosing, carbohydrate ingestion, exercise, and sick days.15
Medtronic Guardian 3 comprises the multi-use Guardian Connect Transmitter that is replaced annually and a single-use Guardian Sensor that is replaced every 7 days. Guardian 3 requires twice-daily fingerstick glucose calibration, which reduces the convenience of a CGM.
Guardian 3 allows the user to set alarm levels, providing predictive alerts 10 to 60 minutes before set glucose levels are reached. Patients must utilize a smart device to connect through Bluetooth to the CareLink Connect app and remain within 20 feet of the transmitter to provide continuous glucose readings. The CareLink Connect app allows patients to document exercise, calibration of fingerstick readings, meals, and insulin administration.16
Senseonics Eversense E3 consists of a 3.5 mm × 18.3 mm sensor inserted subcutaneously in the upper arm once every 180 days; a removable transmitter that attaches to an adhesive patch placed over the sensor; and a smart device with the Eversense app. The transmitter has a 1-year rechargeable battery and provides the patient with on-body vibration alerts even when they are not near their smart device.
Continue to: The Eversense E3 transmitter...
The Eversense E3 transmitter can be removed and reapplied without affecting the life of the sensor; however, no glucose data will be collected during this time. Once the transmitter is reapplied, it takes 10 minutes for the sensor to begin communicating with the transmitter. Eversense provides predictive alerts as long as 30 minutes before hyperglycemic or hypoglycemic events. The device requires twice-daily fingerstick calibrations.17
A comparison of the specifications and capabilities of the personal CGMs discussed here is provided in TABLE 2.10,14-17

The evidence, reviewed
Clinical outcomes evidence with CGMs in patients with T2D is sparse. Most studies that support improved clinical outcomes enrolled patients with type 1 diabetes who were treated with intensive insulin regimens. Many studies utilized rtCGMs that are capable of incorporating a hypoglycemic alarm, and results might not be generalizable to isCGMs.18,19 In this article, we review only the continuous glucose monitoring literature in which subjects had T2D.
Evidence for is CGMs
The REPLACE trial compared outcomes in patients with T2D who used an isCGM vs those who self-monitored blood glucose (SMBG); both groups were being treated with intensive insulin regimens. Both groups had similar glucose reductions, but the time in the hypoglycemic range (see “Clinical targets,” in the text that follows) was significantly shorter in the isCGM group.20
A randomized controlled trial (RCT) that compared intermittently scanned continuous glucose monitoring and SMBG in patients with T2D who received multiple doses of insulin daily demonstrated a significant A1C reduction of 0.82% with an isCGM and 0.33% with SMBG, with no difference in the rate of hypoglycemic events, over 10 weeks.21
Continue to: Chart review
Chart review. Data extracted from chart reviews in Austria, France, and Germany demonstrated a mean improvement in A1C of 0.9% among patients when using a CGM after using SMBG previously.22
A retrospective review of patients with T2D who were not taking bolus insulin and who used a CGM had a reduction in A1C from 10.1% to 8.6% over 60 to 300 days.23
Evidence for rtCGMs
The DIAMOND study included a subset of patients with T2D who used an rtCGM and were compared to a subset who received usual care. The primary outcome was the change in A1C. A 0.3% greater reduction was observed in the CGM group at 24 weeks. There was no difference in hypoglycemic events between the 2 groups; there were few events in either group.24
An RCT demonstrated a similar reduction in A1C in rtCGM users and in nonusers over 1 year.25 However, patients who used the rtCGM by protocol demonstrated the greatest reduction in A1C. The CGM utilized in this trial required regular fingerstick calibration, which likely led to poorer adherence to protocol than would have been the case had the trial utilized a CGM that did not require calibration.
A prospective trial demonstrated that utilization of an rtCGM only 3 days per month for 3 consecutive months was associated with (1) significant improvement in A1C (a decrease of 1.1% in the CGM group, compared to a decrease of 0.4% in the SMBG group) and (2) numerous lifestyle modifications, including reduction in total caloric intake, weight loss, decreased body mass index, and an increase in total weekly exercise.26 This trial demonstrated that CGMs might be beneficial earlier in the course of disease by reinforcing lifestyle changes.
Continue to: The MOBILE trial
The MOBILE trial demonstrated that use of an rtCGM reduced baseline A1C from 9.1% to 8.0% in the CGM group, compared to 9.0% to 8.4% in the non-CGM group.27
Practical utilization of CGMs
Patient education
Detailed patient education resources—for initial setup, sensor application, methods to ensure appropriate sensor adhesion, and app and platform assistance—are available on each manufacturer’s website.
Clinical targets
In 2019, the Advanced Technologies & Treatments for Diabetes Congress determined that what is known as the time in range metric yields the most practical data to help clinicians manage glycemic control.28 The time in range metric comprises:
- time in the target glucose range (TIR)
- time below the target glucose range (TBR)
- time above the target glucose range (TAR).
TIR glucose ranges are modifiable and based on the A1C goal. For example, if the A1C goal is < 7.0%, the TIR glucose range is 70-180 mg/dL. If a patient maintains TIR > 70% for 3 months, the measured A1C will correlate well with the goal. Each 10% fluctuation in TIR from the goal of 70% corresponds to a difference of approximately 0.5% in A1C. Therefore, TIR of approximately 50% predicts an A1C of 8.0%.28
A retrospective review of 1440 patients with CGM data demonstrated that progression of retinopathy and development of microalbuminuria increased 64% and 40%, respectively, over 10 years for each 10% reduction in TIR—highlighting the importance of TIR and consistent glycemic control.29 Importantly, the CGM sensor must be active ≥ 70% of the wearable time to provide adequate TIR data.30
Continue to: Concerns about accuracy
Concerns about accuracy
There is no universally accepted standard for determining the accuracy of a CGM; however, the mean absolute relative difference (MARD) is the most common statistic referenced. MARD is calculated as the average of the absolute error between all CGM values and matched reference values that are usually obtained from SMBG.31 The lower the MARD percentage, the better the accuracy of the CGM. A MARD of ≤ 10% is considered acceptable for making therapeutic decisions.30
Package labeling for all CGMs recommends that patients have access to a fingerstick glucometer to verify CGM readings when concerns about accuracy exist. If a sensor becomes dislodged, it can malfunction or lose accuracy. Patients should not try to re-apply the sensor; instead, they should remove and discard the sensor and apply a new one. TABLE 210,14-17 compares MARD for CGMs and lists substances that might affect the accuracy of a CGM.
Patient–provider data-sharing platforms
FreeStyle Libre and Libre 2. Providers create a LibreView Practice ID at www.Libre View.com. Patient data-sharing depends on whether they are using a smart device, a reader, or both. Patients can utilize both the smart device and the reader but must upload data from the reader at regular intervals to provide a comprehensive report that will merge data from the smart device (ie, data that have been uploaded automatically) and the reader.7
Dexcom G6. Clinicians create a Dexcom CLARITY account at https://clarity.dexcom.com and add patients to a practice list or gain access to a share code generated by the patient. Patients must download the Dexcom CLARITY app to create an account; once the account is established, readings will be transmitted to the clinic automatically.15 A patient who is utilizing a nonsmart-device reader must upload data manually to their web-based CLARITY account.
Family and caregiver access
Beyond sharing CGM data with clinic staff, an important feature available with FreeStyle Libre and Dexcom systems is the ability to share data with friends and caregivers. The relevant platforms and apps are listed in TABLE 2.10,14-17
Continue to: Insurance coverage, cost, and accessibility
Insurance coverage, cost, and accessibility
Medicare Part B has established criteria by which patients with T2D qualify for a CGM (TABLE 332). A Medicare patient who has been determined to be eligible is responsible for 20% of the out-of-pocket expense of the CGM and supplies once their deductible is met. Once Medicare covers a CGM, the patient is no longer able to obtain fingerstick glucose supplies through Medicare; they must pay the cash price for any fingerstick supplies that are determined to be necessary.32

Patients with private insurance can obtain CGM supplies through their preferred pharmacy when the order is written as a prescription (the same as for fingerstick glucometers). That is not the case for patients with Medicare because not all US distributors and pharmacies are contracted to bill Medicare Part B for CGM supplies. A list of distributors and eligible pharmacies can be found on each manufacturer’s website.
Risk–benefit analysis
CGMs are associated with few risks overall. The predominant adverse effect is contact dermatitis; the prevalence of CGM-associated contact dermatitis is difficult to quantify and differs from device to device.
FreeStyle Libre. In a retrospective review of records of patients with diabetes, researchers determined that a cutaneous adverse event occurred in approximately 5.5% of 1036 patients who utilized a FreeStyle Libre sensor.33 Of that percentage, 3.8% of dermatitis cases were determined to be allergic in nature and related to isobornyl acrylate (IBOA), a chemical constituent of the sensor’s adhesive that is not used in the FreeStyle Libre 2. Among patients who wore a sensor and developed allergic contact dermatitis, interventions such as a barrier film were of limited utility in alleviating or preventing further cutaneous eruption.33
Dexcom G6. The prevalence of Dexcom G6–associated allergic contact dermatitis is more difficult to ascertain (the IBOA adhesive was replaced in October 2019) but has been reported to be less common than with FreeStyle Libre,34 a finding that corroborates our anecdotal clinical experience. Although Dexcom sensors no longer contain IBOA, cases of allergic contact dermatitis are still reported.35 We propose that the lower incidence of cutaneous reactions associated with the Dexcom G6 sensor might be due to the absence of IBOA and shorter contact time with skin.
Continue to: In general, patients should be...
In general, patients should be counseled to rotate the location of the sensor and to use only specific barrier products that are recommended on each manufacturer’s website. The use of other barriers that are not specifically recommended might compromise the accuracy of the sensor.
Summing up
As CGM technology improves, it is likely that more and more of your patients will utilize one of these devices. The value of CGMs has been documented, but any endorsement of their use is qualified:
- Data from many older RCTs of patients with T2D who utilize a CGM did not demonstrate a significant reduction in A1C20,24,36; however, real-world observational data do show a greater reduction in A1C.
- From a safety standpoint, contact dermatitis is the primary drawback of CGMs.
- CGMs can provide patients and clinicians with a comprehensive picture of daily glucose trends, which can help patients make lifestyle changes and serve as a positive reinforcement for the effects of diet and exercise. Analysis of glucose trends can also help clinicians confidently make decisions about when to intensify or taper a medication regimen, based on data that is reported more often than 90-day A1C changes.
Health insurance coverage will continue to dictate access to CGM technology for many patients. When a CGM is reimbursable by the patient’s insurance, consider offering it as an option—even for patients who do not require an intensive insulin regimen.
a The US Food and Drug Administration cleared a new Abbott CGM, FreeStyle Libre 3, earlier this year; however, the device is not yet available for purchase. With advances in monitoring technology, several other manufacturers also anticipate introducing novel CGMs. (See “Continuous glucose monitors: The next generation.” )
SIDEBAR
Continuous glucose monitors: The next generation9-13
Expect new continuous glucose monitoring devices to be introduced to US and European health care markets in the near future.
FreeStyle Libre 3 (Abbott) was cleared by the US Food and Drug Administration in May 2022, although it is not yet available for purchase. The manufacturer promotes the device as having the smallest sensor of any continuous glucose monitor (the diameter and thickness of 2 stacked pennies); improved mean absolute relative difference; the ability to provide real-time glucose level readings; and 50% greater range of Bluetooth connectivity (about 10 extra feet).9,10
Dexcom G7 (Dexcom) has a sensor that is 60% smaller than the Dexcom G6 sensor and a 30-minute warm-up time, compared to 120 minutes for the G6.11 The device has received European Union CE mark approval.
Guardian 4 Sensor (Medtronic) does not require fingerstick calibration. The device has also received European Union CE mark approval12 but is available only for investigational use in the United States.
Eversense XL technology is similar to that of the Eversense E3, including a 180-day sensor.13 The device, which has received European Union CE mark approval, includes a removable smart transmitter.
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
1. Rodríguez-Gutiérrez R, Montori VM. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circ Cardiovasc Qual Outcomes. 2016;9:504-512. doi: 10.1161/CIRCOUTCOMES.116.002901
2. Draznin B, Aroda VR, Bakris G, et al; . 7. Diabetes technology: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
3. Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181-187. doi: 10.1016/j.dsx.2017.09.005
4. Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. 2022;16:70-77. doi: 10.1177/1932296820958754
5. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21:313-321. doi: 10.1089/dia.2018.0364
6. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. doi: 10.2337/dc21-S002
7. FreeStyle Libre systems: The #1 CGM used in the US. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyleprovider.abbott/us-en/home.html
8. Rowland K. Choosing Wisely: 10 practices to stop—or adopt—to reduce overuse in health care. J Fam Pract. 2020;69:396-400.
9. Tucker ME. FDA clears Abbott Freestyle Libre 3 glucose sensor. MDedge. June 1, 2022. Accessed October 21, 2022. www.mdedge.com/endocrinology/article/255095/diabetes/fda-clears-abbott-freestyle-libre-3-glucose-sensor
10. Manage your diabetes with more confidence. Abbott. Updated May 2022. Accessed October 22, 2022. www.freestyle.abbott/us-en/home.html
11. Whooley S. Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’. Drug Delivery Business News. July 19, 2021. Accessed October 21, 2022. www.drugdeliverybusiness.com/dexcom-ceo-kevin-sayer-says-g7-will-be-wonderful
12. Medtronic secures two CE mark approvals for Guardian 4 Sensor & for InPen MDI Smart Insulin Pen. Medtronic. Press release. May 26, 2021. Accessed October 22, 2022. https://news.medtronic.com/2021-05-26-Medtronic-Secures-Two-CE-Mark-Approvals-for-Guardian-4-Sensor-for-InPen-MDI-Smart-Insulin-Pen
13. Eversense—up to 180 days of freedom [XL CGM System]. Senseonics. Accessed September 14, 2022. https://global.eversensediabetes.com
14. FreeStyle Libre 2 User’s Manual. Abbott. Revised August 24, 2022. Accessed October 2, 2022. https://freestyleserver.com/Payloads/IFU/2022/q3/ART46983-001_rev-A.pdf
15. Dexcom G6 Continuous Glucose Monitoring System user guide. Dexcom. Revised March 2022. Accessed October 21, 2022. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf
16. Guardian Connect System user guide. Medtronic. 2020. Accessed October 21, 2022. www.medtronicdiabetes.com/sites/default/files/library/download-library/user-guides/Guardian-Connect-System-User-Guide.pdf
17. Eversense E3 user guides. Senseonics. 2022. Accessed October 22, 2022. www.ascensiadiabetes.com/eversense/user-guides/
18. Battelino T, Conget I, Olsen B, et al; SWITCH Study Group. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. doi: 10.1007/s00125-012-2708-9
19. Weinzimer S, Miller K, Beck R, et al; Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. doi: 10.2337/dc09-1502
20. Haak T, Hanaire H, Ajjan R, et al. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55-73. doi: 10.1007/s13300-016-0223-6
21. Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42:1178-1184. doi: 10.2337/dc18-0166
22. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11:279-291. doi: 10.1007/s13300-019-00741-9
23. Wright EE, Jr, Kerr MSD, Reyes IJ, et al. Use of flash continuous glucose monitoring is associated with A1C reduction in people with type 2 diabetes treated with basal insulin or noninsulin therapy. Diabetes Spectr. 2021;34:184-189. doi: 10.2337/ds20-0069
24. Beck RW, Riddlesworth TD, Ruedy K, et al; DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167:365-374. doi: 10.7326/M16-2855
25. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35:32-38. doi: 10.2337/dc11-1438
26. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008;82:73-79. doi: 10.1016/j.diabres.2008.06.015
27. Martens T, Beck RW, Bailey R, et al; MOBILE Study Group. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021;325:2262-2272. doi: 10.1001/jama.2021.7444
28. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. doi: 10.2337/dci19-0028
29. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. doi: 10.2337/dc18-1444
30. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. Journal of Laboratory Medicine. 2020;44:71-79. doi: 10.1515/labmed-2019-0189
31. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. doi: 10.2337/dc17-1600
32. Glucose monitors. Centers for Medicare & Medicaid Services. April 22, 2022. Accessed October 22, 2022. www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822
33. Pyl J, Dendooven E, Van Eekelen I, et al. Prevalence and prevention of contact dermatitis caused by FreeStyle Libre: a monocentric experience. Diabetes Care. 2020;43:918-920. doi: 10.2337/dc19-1354
34. Smith J, Bleiker T, Narang I. Cutaneous reactions to glucose sensors: a sticky problem [Abstract 677]. Arch Dis Child. 2021;106 (suppl 1):A80.
35. MAUDE Adverse event report: Dexcom, Inc G6 Sensor. U.S. Food & Drug Administration. Updated September 30, 2022. Accessed October 21, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/detail.cfm?mdrfoi__id=11064819&pc=MDS
36. New JP, Ajjan R, Pfeiffer AFH, et al. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med. 2015;32:609-617. doi: 10.1111/dme.12713
PRACTICE RECOMMENDATIONS
› Initiate continuous glucose monitoring early in the disease process, based on a patient’s needs or preferences. C
› Interpret a continuous glucose monitor (CGM) report with the understanding that time within target range is the most important factor to evaluate. Minimizing or eliminating time below range is of paramount importance. B
› Advise patients who use a CGM to continue to have access to a glucometer and instruct them on appropriate times when such confirmation might be necessary. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series