User login
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.
Overview of DOACs
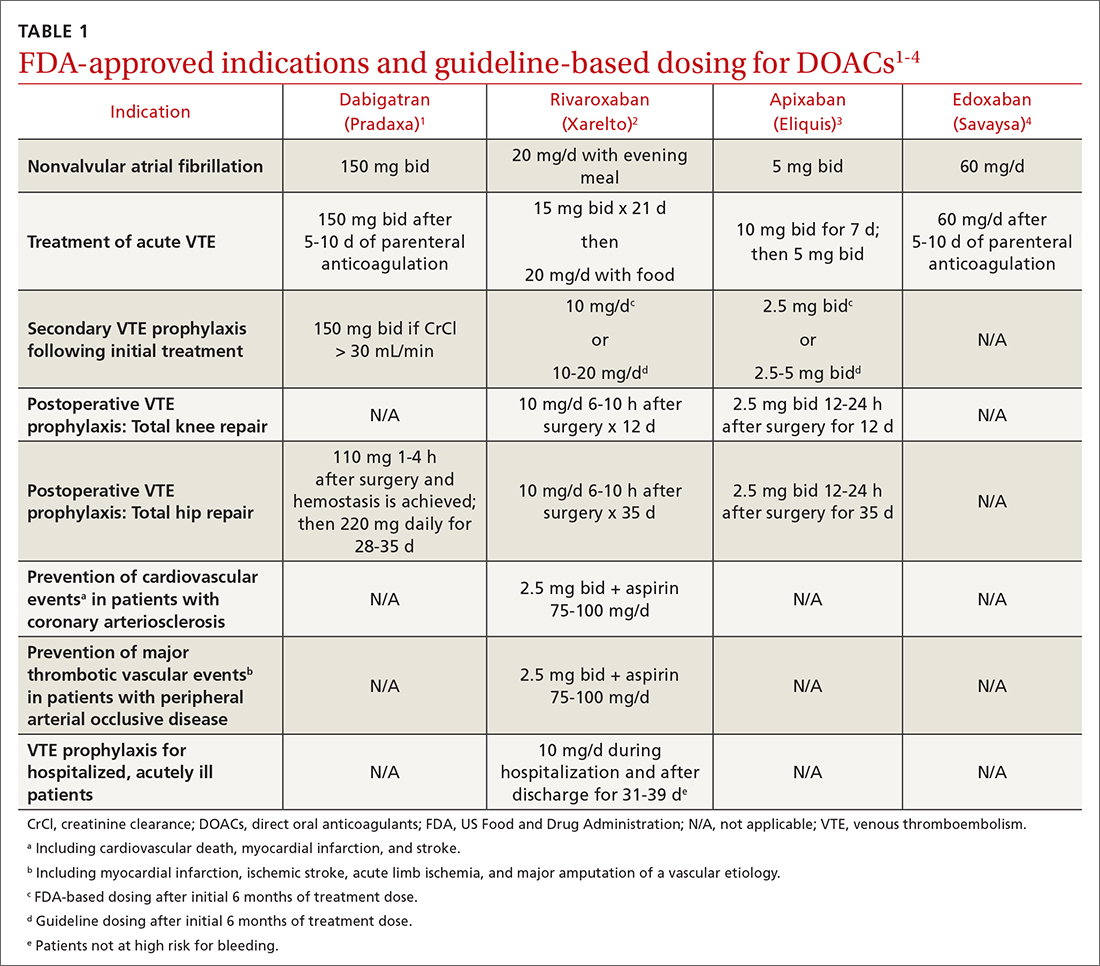
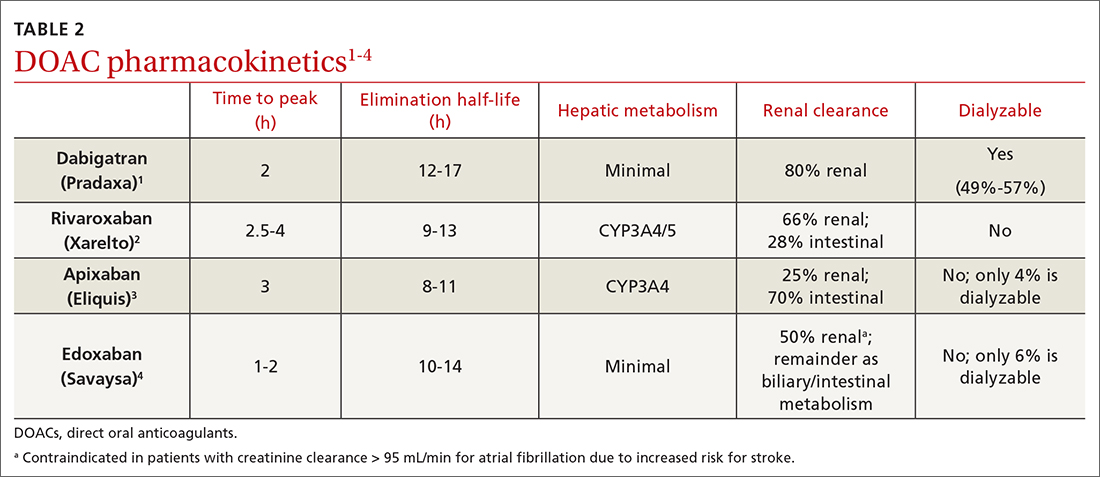
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4 TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7
Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
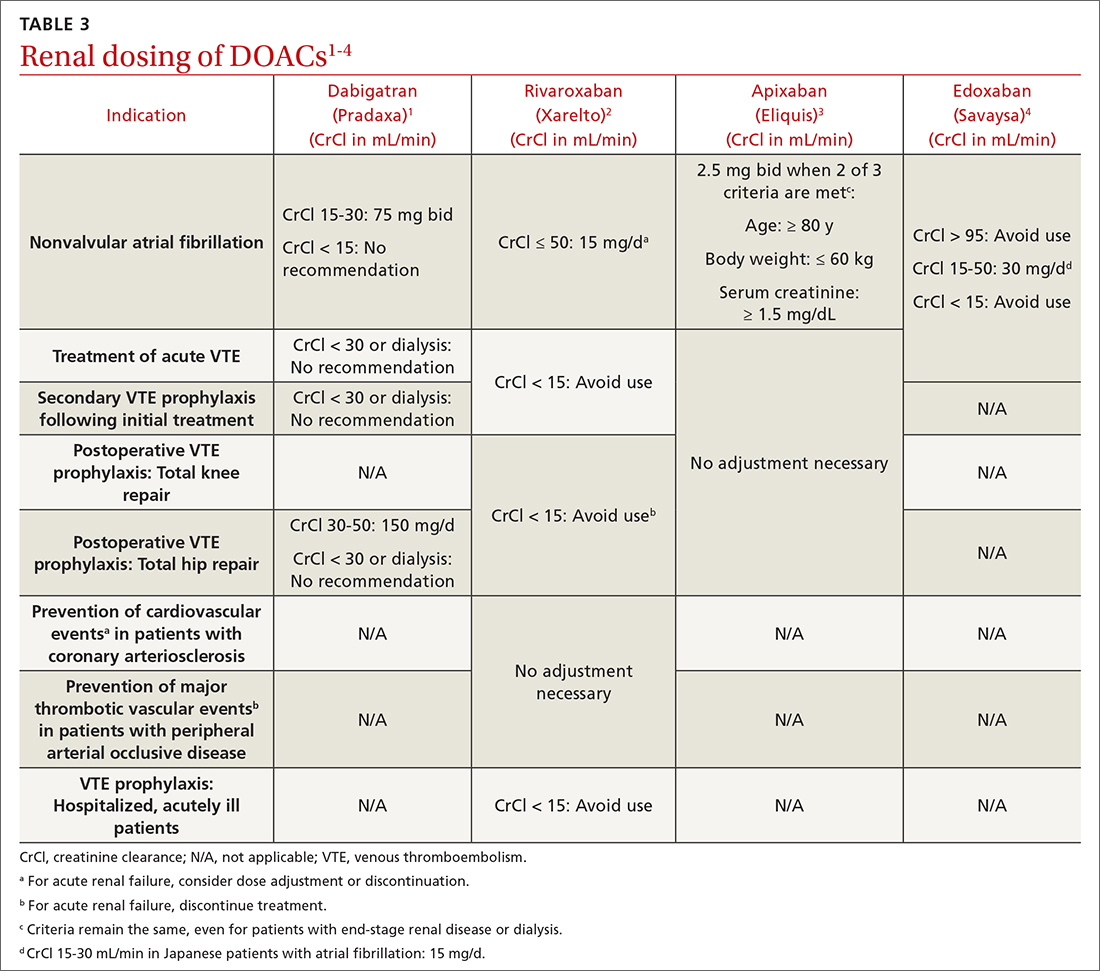
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3
Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.

Overview of DOACs
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4 TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7

Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3

Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.

Overview of DOACs
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4 TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7

Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3

Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; [email protected]
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
PRACTICE RECOMMENDATIONS
› Consider a direct oral anticoagulant (DOAC) when treating venous thromboembolism (VTE) in patients with advanced chronic kidney disease or obesity. C
› Select apixaban for treatment of VTE or nonvalvular atrial fibrillation in patients with end-stage renal disease, due to its minimal renal clearance compared with other DOACs. B
› Consider DOACs such as dabigatran, rivaroxaban, or apixaban for treatment of VTE in the context of heparin-induced thrombocytopenia. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series