User login
The Alarm Burden of Excess Continuous Pulse Oximetry Monitoring Among Patients With Bronchiolitis
Practice guidelines discourage continuous pulse oximetry (SpO2) monitoring of patients with bronchiolitis who are not receiving supplemental oxygen.1,2 Overuse of SpO2 monitoring in this patient population has been associated with increased length of stay, unnecessary oxygen therapy, and excess hospital costs, without measurable patient benefit.3-5 In spite of this evidence base and expert guidance, nearly half of the more than 100,000 infants admitted for bronchiolitis each year receive excess SpO2 monitoring.6,7
Bronchiolitis guidelines suggest that guideline-discordant SpO2 monitoring may result in excess alarms, which disrupt families’ sleep and engender alarm fatigue among staff.1 Pediatric nurses receive up to 155 alarms per monitored patient per day.8,9 Frequent alarms are associated with slower nurse response times10,11 and increased nurse subjective workload.12
Methods
Cohort
We retrospectively evaluated SpO2 monitoring patterns and alarm rates of children 0 to 24 months old admitted to a general pediatrics service at a tertiary care children’s hospital. We included patients who had a discharge diagnosis of bronchiolitis (International Classification of Diseases, Tenth Revision codes J45x, T17.2x, T17.3x, T17.4x, T17.5x, T17.8x, T17.9x, A37xx, J04x, J05x, J05.1x, J69.0x, J69.1x, J69.8x) between November 24, 2019, and January 21, 2020, the period of time during which alarm data and monitor data were concurrently available for analysis. In order to conservatively assure applicability of clinical practice guidelines, we excluded patients with discharge diagnoses that included other respiratory conditions (eg, reactive airway disease), patients with complex chronic conditions (CCC) as defined by the CCC version 2 classification system,13 and patients with intensive care unit (ICU) stays during the admission.
Time
Flowsheet data detailing nursing respiratory assessments were extracted from the electronic health record (EHR) database (Clarity, Epic Systems). Using previously validated methodology,14 we identified minutes during which patients received supplemental oxygen or high-flow nasal cannula (supplemental oxygen) based on the documented fraction of inspired oxygen (FiO2), flow rate, and support devices. We then identified the final discontinuation of respiratory support during the hospital admission, and censored the 60 minutes after final discontinuation of supplemental oxygen based upon recent monitoring guidelines.2 Minutes up to an hour after supplemental oxygen discontinuation were classified as receiving supplemental oxygen and not included in our analysis. Only minutes between the end of the censored period and hospital discharge were used in the analysis. For patients who never received respiratory support during the admission, we censored the first 60 minutes of the admission and analyzed the remainder of their stay.
SpO2 Monitoring
We used device-integrated, physiologic-monitor, vital sign data sent each minute from the General Electric monitor network to the EHR to identify minutes during which patients were connected to physiologic monitors and transmitting signals from SpO2 sensors. We extracted minute-level SpO2 data from the hospital clinical data warehouse (CDW). Minutes in which SpO2 data were present were classified as “monitored,” an approach previously validated using in-person observation.14
To categorize time as “not receiving supplemental oxygen and continuously monitored (guideline-discordant monitoring),” or “not receiving supplemental oxygen and not continuously monitored (guideline-concordant intermittent measurement),” we evaluated the percent of minutes within an hour during which the patient received SpO2 monitoring and applied an a priori conservative rule. Hours during which ≥90% of minutes had SpO2 monitoring data were classified as “continuously monitored.” Hours during which ≤10% of minutes had SpO2 monitoring data were classified as “intermittently measured.” Hours during which 11% to 89% of minutes included monitor data were excluded from further analysis. The number of continuously monitored hours was tabulated for each patient. The median number of continuously monitored hours was computed; results were stratified by prior receipt of respiratory support.
Alarm Counts
Minute-level monitor alarm counts (the absolute number of abnormal vital signs that triggered a monitor to alarm) were extracted from the CDW. Alarm counts were tabulated for each patient hour. For each patient, the alarm rate (total number of alarms divided by time) was computed for continuously monitored and intermittently measured time. Results were stratified by prior receipt of respiratory support.
The study was reviewed by the institutional review board and determined to meet exemption criteria.
Results
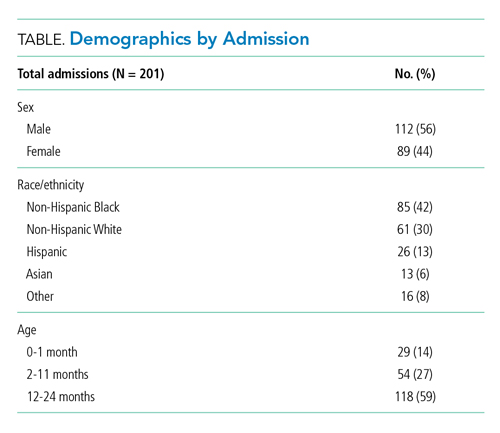
Our cohort included 201 admissions by 197 unique patients (Table). We evaluated 4402 hours that occurred ≥60 minutes following final discontinuation of supplemental oxygen, the time period during which guidelines discourage routine use of continuous SpO2 monitoring. This represented a median of 19 hours (interquartile range [IQR], 14-25) per admission. We excluded 474 hours (11%) that could not be classified as either continuously or intermittently measured.
During time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 1537 hours of guideline-discordant continuous monitoring, a median of 6 hours (IQR, 3-12) per admission. Patients experienced a median of 12 hours (IQR, 5-17) of intermittent measurement. Among patients who received supplemental oxygen, 91% experienced guideline-discordant continuous SpO2 monitoring, as compared to 68% of patients who did not receive supplemental oxygen. Among those who received guideline-discordant continuous SpO2 monitoring, the duration of this monitoring did not differ significantly between those who had received supplemental oxygen during the admission and those who had not.
During classifiable time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 14,371 alarms; 77% (11,101) of these alarms were generated during periods of guideline-discordant continuous monitoring. The median hourly alarm rate during these periods of guideline-discordant continuous monitoring was 6.7 alarms per hour (IQR, 2.1-12.3), representing a median of 35 (IQR, 10-81) additional alarms per patient. During periods of guideline-concordant intermittent measurement, the median hourly alarm rate was 0.5 (IQR, 0.1-0.8), with a median of 5 (IQR, 1-13) alarms per patient.
Those who received supplemental oxygen earlier in the admission had higher alarm rates during continuously monitored time (7.3 per hour [IQR, 2.7-12.7]) than patients who had not received supplemental oxygen (3.3 per hour [IQR, 0.6-11.8]), likely reflecting clinical differences between these patient populations. The most frequent alarm type among continuously monitored patients who had previously received supplemental oxygen was “SpO2 low.”
Discussion
Across 4402 patient hours, guideline-discordant continuous SpO2 monitoring of patients with bronchiolitis resulted in 11,101 alarms, at a rate of approximately 1 additional alarm every 9 minutes. Patients in our cohort received a median of 6 hours of guideline-discordant monitoring, which imposes a significant alarm burden that is potentially modifiable using targeted reduction strategies.15
Transient, self-resolved hypoxemia is a common feature of bronchiolitis and likely of little clinical consequence.16 Therefore, this rate of hypoxemia alarms is not unexpected. Though we evaluated only the period of time following final discontinuation of respiratory support, this finding is in keeping with the literature associating excess physiologic monitoring of patients with bronchiolitis with unnecessary oxygen therapy and increased length of stay,3-5 largely because clinicians may feel compelled to respond to hypoxemia alarms with either supplemental oxygen or longer monitoring.
Our findings must be contextualized in light of the limitations of our approach. We did not evaluate nurse workload associated with guideline-discordant continuous SpO2 monitoring. Prior work conducted by our lab has demonstrated that when nurses experience more than 40 alarms within a 2-hour period, their measured subjective workload increases to a degree associated with missing important tasks, threatening the quality and safety of the care they deliver.12,17 Given that nurses care for multiple patients, it is likely that the excess alarms introduced by guideline-discordant continuous monitoring contribute to increased nurse workload and alarm fatigue.
Similarly, we could not evaluate whether the alarms nurses experienced were actionable. Although our lab has previously reported that ≥99% of alarms occurring on non-ICU pediatric wards are nonactionable,10,11 it is possible that some of the alarms during guideline-discordant monitoring periods required action. However, it is unlikely that any life-sustaining actions were taken because (1) we only evaluated time >60 minutes after final discontinuation of supplemental oxygen, so by definition none of these alarms required treatment with supplemental oxygen, and (2) none of the patients we included received ICU care during their admission.
The avoidable alarm burden identified in our analysis suggests that eliminating continuous SpO2 monitoring overuse in bronchiolitis will likely reduce nurses’ workload and alarm fatigue in hospital settings that care for children with bronchiolitis.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Schondelmeyer AC, Dewan ML, Brady PW, et al. Cardiorespiratory and pulse oximetry monitoring in hospitalized children: a Delphi process. Pediatrics. 2020;146(2):e20193336. https://doi.org/10.1542/peds.2019-3336
3. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): A multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-xxiii, 1-172. https://doi.org/10.3310/hta19710
4. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
5. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
6. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
7. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
8. Schondelmeyer AC, Brady PW, Goel VV, et al. Physiologic monitor alarm rates at 5 children’s hospitals. J Hosp Med. 2018;13(6):396-398. https://doi.org/10.12788/jhm.2918
9. Schondelmeyer AC, Bonafide CP, Goel VV, et al. The frequency of physiologic monitor alarms in a children’s hospital. J Hosp Med. 2016;11(11):796-798. https://doi.org/10.1002/jhm.2612
10. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
11. Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524-531. https://doi.org/10.1001/jamapediatrics.2016.5123
12. Rasooly IR, Kern-Goldberger AS, Xiao R, et al. Physiologic monitor alarm burden and nurses’ subjective workload in a children’s hospital. Hosp Pediatr. 2021;11(7):703-710. https://doi.org/10.1542/hpeds.2020-003509
13. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
14. Kern-Goldberger AS, Rasooly IR, Luo B, et al. EHR-integrated monitor data to measure pulse oximetry use in bronchiolitis. Hosp Pediatr. 2021;11(10):1073-1082. https://doi.org/10.1542/hpeds.2021-005894
15. Schondelmeyer AC, Bettencourt AP, Xiao R, et al. Evaluation of an educational outreach and audit and feedback program to reduce continuous pulse oximetry use in hospitalized infants with stable bronchiolitis. JAMA Netw Open. 2021;4(9):e2122826. https://doi.org/10.1001/jamanetworkopen.2021.22826
16. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
17. Tubbs-Cooley HL, Mara CA, Carle AC, Mark BA, Pickler RH. Association of nurse workload with missed nursing care in the neonatal intensive care unit. JAMA Pediatr. 2019;173(1):44-51. https://doi.org/10.1001/jamapediatrics.2018.3619
Practice guidelines discourage continuous pulse oximetry (SpO2) monitoring of patients with bronchiolitis who are not receiving supplemental oxygen.1,2 Overuse of SpO2 monitoring in this patient population has been associated with increased length of stay, unnecessary oxygen therapy, and excess hospital costs, without measurable patient benefit.3-5 In spite of this evidence base and expert guidance, nearly half of the more than 100,000 infants admitted for bronchiolitis each year receive excess SpO2 monitoring.6,7
Bronchiolitis guidelines suggest that guideline-discordant SpO2 monitoring may result in excess alarms, which disrupt families’ sleep and engender alarm fatigue among staff.1 Pediatric nurses receive up to 155 alarms per monitored patient per day.8,9 Frequent alarms are associated with slower nurse response times10,11 and increased nurse subjective workload.12
Methods
Cohort
We retrospectively evaluated SpO2 monitoring patterns and alarm rates of children 0 to 24 months old admitted to a general pediatrics service at a tertiary care children’s hospital. We included patients who had a discharge diagnosis of bronchiolitis (International Classification of Diseases, Tenth Revision codes J45x, T17.2x, T17.3x, T17.4x, T17.5x, T17.8x, T17.9x, A37xx, J04x, J05x, J05.1x, J69.0x, J69.1x, J69.8x) between November 24, 2019, and January 21, 2020, the period of time during which alarm data and monitor data were concurrently available for analysis. In order to conservatively assure applicability of clinical practice guidelines, we excluded patients with discharge diagnoses that included other respiratory conditions (eg, reactive airway disease), patients with complex chronic conditions (CCC) as defined by the CCC version 2 classification system,13 and patients with intensive care unit (ICU) stays during the admission.
Time
Flowsheet data detailing nursing respiratory assessments were extracted from the electronic health record (EHR) database (Clarity, Epic Systems). Using previously validated methodology,14 we identified minutes during which patients received supplemental oxygen or high-flow nasal cannula (supplemental oxygen) based on the documented fraction of inspired oxygen (FiO2), flow rate, and support devices. We then identified the final discontinuation of respiratory support during the hospital admission, and censored the 60 minutes after final discontinuation of supplemental oxygen based upon recent monitoring guidelines.2 Minutes up to an hour after supplemental oxygen discontinuation were classified as receiving supplemental oxygen and not included in our analysis. Only minutes between the end of the censored period and hospital discharge were used in the analysis. For patients who never received respiratory support during the admission, we censored the first 60 minutes of the admission and analyzed the remainder of their stay.
SpO2 Monitoring
We used device-integrated, physiologic-monitor, vital sign data sent each minute from the General Electric monitor network to the EHR to identify minutes during which patients were connected to physiologic monitors and transmitting signals from SpO2 sensors. We extracted minute-level SpO2 data from the hospital clinical data warehouse (CDW). Minutes in which SpO2 data were present were classified as “monitored,” an approach previously validated using in-person observation.14
To categorize time as “not receiving supplemental oxygen and continuously monitored (guideline-discordant monitoring),” or “not receiving supplemental oxygen and not continuously monitored (guideline-concordant intermittent measurement),” we evaluated the percent of minutes within an hour during which the patient received SpO2 monitoring and applied an a priori conservative rule. Hours during which ≥90% of minutes had SpO2 monitoring data were classified as “continuously monitored.” Hours during which ≤10% of minutes had SpO2 monitoring data were classified as “intermittently measured.” Hours during which 11% to 89% of minutes included monitor data were excluded from further analysis. The number of continuously monitored hours was tabulated for each patient. The median number of continuously monitored hours was computed; results were stratified by prior receipt of respiratory support.
Alarm Counts
Minute-level monitor alarm counts (the absolute number of abnormal vital signs that triggered a monitor to alarm) were extracted from the CDW. Alarm counts were tabulated for each patient hour. For each patient, the alarm rate (total number of alarms divided by time) was computed for continuously monitored and intermittently measured time. Results were stratified by prior receipt of respiratory support.
The study was reviewed by the institutional review board and determined to meet exemption criteria.
Results
Our cohort included 201 admissions by 197 unique patients (Table). We evaluated 4402 hours that occurred ≥60 minutes following final discontinuation of supplemental oxygen, the time period during which guidelines discourage routine use of continuous SpO2 monitoring. This represented a median of 19 hours (interquartile range [IQR], 14-25) per admission. We excluded 474 hours (11%) that could not be classified as either continuously or intermittently measured.

During time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 1537 hours of guideline-discordant continuous monitoring, a median of 6 hours (IQR, 3-12) per admission. Patients experienced a median of 12 hours (IQR, 5-17) of intermittent measurement. Among patients who received supplemental oxygen, 91% experienced guideline-discordant continuous SpO2 monitoring, as compared to 68% of patients who did not receive supplemental oxygen. Among those who received guideline-discordant continuous SpO2 monitoring, the duration of this monitoring did not differ significantly between those who had received supplemental oxygen during the admission and those who had not.
During classifiable time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 14,371 alarms; 77% (11,101) of these alarms were generated during periods of guideline-discordant continuous monitoring. The median hourly alarm rate during these periods of guideline-discordant continuous monitoring was 6.7 alarms per hour (IQR, 2.1-12.3), representing a median of 35 (IQR, 10-81) additional alarms per patient. During periods of guideline-concordant intermittent measurement, the median hourly alarm rate was 0.5 (IQR, 0.1-0.8), with a median of 5 (IQR, 1-13) alarms per patient.
Those who received supplemental oxygen earlier in the admission had higher alarm rates during continuously monitored time (7.3 per hour [IQR, 2.7-12.7]) than patients who had not received supplemental oxygen (3.3 per hour [IQR, 0.6-11.8]), likely reflecting clinical differences between these patient populations. The most frequent alarm type among continuously monitored patients who had previously received supplemental oxygen was “SpO2 low.”
Discussion
Across 4402 patient hours, guideline-discordant continuous SpO2 monitoring of patients with bronchiolitis resulted in 11,101 alarms, at a rate of approximately 1 additional alarm every 9 minutes. Patients in our cohort received a median of 6 hours of guideline-discordant monitoring, which imposes a significant alarm burden that is potentially modifiable using targeted reduction strategies.15
Transient, self-resolved hypoxemia is a common feature of bronchiolitis and likely of little clinical consequence.16 Therefore, this rate of hypoxemia alarms is not unexpected. Though we evaluated only the period of time following final discontinuation of respiratory support, this finding is in keeping with the literature associating excess physiologic monitoring of patients with bronchiolitis with unnecessary oxygen therapy and increased length of stay,3-5 largely because clinicians may feel compelled to respond to hypoxemia alarms with either supplemental oxygen or longer monitoring.
Our findings must be contextualized in light of the limitations of our approach. We did not evaluate nurse workload associated with guideline-discordant continuous SpO2 monitoring. Prior work conducted by our lab has demonstrated that when nurses experience more than 40 alarms within a 2-hour period, their measured subjective workload increases to a degree associated with missing important tasks, threatening the quality and safety of the care they deliver.12,17 Given that nurses care for multiple patients, it is likely that the excess alarms introduced by guideline-discordant continuous monitoring contribute to increased nurse workload and alarm fatigue.
Similarly, we could not evaluate whether the alarms nurses experienced were actionable. Although our lab has previously reported that ≥99% of alarms occurring on non-ICU pediatric wards are nonactionable,10,11 it is possible that some of the alarms during guideline-discordant monitoring periods required action. However, it is unlikely that any life-sustaining actions were taken because (1) we only evaluated time >60 minutes after final discontinuation of supplemental oxygen, so by definition none of these alarms required treatment with supplemental oxygen, and (2) none of the patients we included received ICU care during their admission.
The avoidable alarm burden identified in our analysis suggests that eliminating continuous SpO2 monitoring overuse in bronchiolitis will likely reduce nurses’ workload and alarm fatigue in hospital settings that care for children with bronchiolitis.
Practice guidelines discourage continuous pulse oximetry (SpO2) monitoring of patients with bronchiolitis who are not receiving supplemental oxygen.1,2 Overuse of SpO2 monitoring in this patient population has been associated with increased length of stay, unnecessary oxygen therapy, and excess hospital costs, without measurable patient benefit.3-5 In spite of this evidence base and expert guidance, nearly half of the more than 100,000 infants admitted for bronchiolitis each year receive excess SpO2 monitoring.6,7
Bronchiolitis guidelines suggest that guideline-discordant SpO2 monitoring may result in excess alarms, which disrupt families’ sleep and engender alarm fatigue among staff.1 Pediatric nurses receive up to 155 alarms per monitored patient per day.8,9 Frequent alarms are associated with slower nurse response times10,11 and increased nurse subjective workload.12
Methods
Cohort
We retrospectively evaluated SpO2 monitoring patterns and alarm rates of children 0 to 24 months old admitted to a general pediatrics service at a tertiary care children’s hospital. We included patients who had a discharge diagnosis of bronchiolitis (International Classification of Diseases, Tenth Revision codes J45x, T17.2x, T17.3x, T17.4x, T17.5x, T17.8x, T17.9x, A37xx, J04x, J05x, J05.1x, J69.0x, J69.1x, J69.8x) between November 24, 2019, and January 21, 2020, the period of time during which alarm data and monitor data were concurrently available for analysis. In order to conservatively assure applicability of clinical practice guidelines, we excluded patients with discharge diagnoses that included other respiratory conditions (eg, reactive airway disease), patients with complex chronic conditions (CCC) as defined by the CCC version 2 classification system,13 and patients with intensive care unit (ICU) stays during the admission.
Time
Flowsheet data detailing nursing respiratory assessments were extracted from the electronic health record (EHR) database (Clarity, Epic Systems). Using previously validated methodology,14 we identified minutes during which patients received supplemental oxygen or high-flow nasal cannula (supplemental oxygen) based on the documented fraction of inspired oxygen (FiO2), flow rate, and support devices. We then identified the final discontinuation of respiratory support during the hospital admission, and censored the 60 minutes after final discontinuation of supplemental oxygen based upon recent monitoring guidelines.2 Minutes up to an hour after supplemental oxygen discontinuation were classified as receiving supplemental oxygen and not included in our analysis. Only minutes between the end of the censored period and hospital discharge were used in the analysis. For patients who never received respiratory support during the admission, we censored the first 60 minutes of the admission and analyzed the remainder of their stay.
SpO2 Monitoring
We used device-integrated, physiologic-monitor, vital sign data sent each minute from the General Electric monitor network to the EHR to identify minutes during which patients were connected to physiologic monitors and transmitting signals from SpO2 sensors. We extracted minute-level SpO2 data from the hospital clinical data warehouse (CDW). Minutes in which SpO2 data were present were classified as “monitored,” an approach previously validated using in-person observation.14
To categorize time as “not receiving supplemental oxygen and continuously monitored (guideline-discordant monitoring),” or “not receiving supplemental oxygen and not continuously monitored (guideline-concordant intermittent measurement),” we evaluated the percent of minutes within an hour during which the patient received SpO2 monitoring and applied an a priori conservative rule. Hours during which ≥90% of minutes had SpO2 monitoring data were classified as “continuously monitored.” Hours during which ≤10% of minutes had SpO2 monitoring data were classified as “intermittently measured.” Hours during which 11% to 89% of minutes included monitor data were excluded from further analysis. The number of continuously monitored hours was tabulated for each patient. The median number of continuously monitored hours was computed; results were stratified by prior receipt of respiratory support.
Alarm Counts
Minute-level monitor alarm counts (the absolute number of abnormal vital signs that triggered a monitor to alarm) were extracted from the CDW. Alarm counts were tabulated for each patient hour. For each patient, the alarm rate (total number of alarms divided by time) was computed for continuously monitored and intermittently measured time. Results were stratified by prior receipt of respiratory support.
The study was reviewed by the institutional review board and determined to meet exemption criteria.
Results
Our cohort included 201 admissions by 197 unique patients (Table). We evaluated 4402 hours that occurred ≥60 minutes following final discontinuation of supplemental oxygen, the time period during which guidelines discourage routine use of continuous SpO2 monitoring. This represented a median of 19 hours (interquartile range [IQR], 14-25) per admission. We excluded 474 hours (11%) that could not be classified as either continuously or intermittently measured.

During time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 1537 hours of guideline-discordant continuous monitoring, a median of 6 hours (IQR, 3-12) per admission. Patients experienced a median of 12 hours (IQR, 5-17) of intermittent measurement. Among patients who received supplemental oxygen, 91% experienced guideline-discordant continuous SpO2 monitoring, as compared to 68% of patients who did not receive supplemental oxygen. Among those who received guideline-discordant continuous SpO2 monitoring, the duration of this monitoring did not differ significantly between those who had received supplemental oxygen during the admission and those who had not.
During classifiable time ≥60 minutes following discontinuation of supplemental oxygen, our cohort experienced 14,371 alarms; 77% (11,101) of these alarms were generated during periods of guideline-discordant continuous monitoring. The median hourly alarm rate during these periods of guideline-discordant continuous monitoring was 6.7 alarms per hour (IQR, 2.1-12.3), representing a median of 35 (IQR, 10-81) additional alarms per patient. During periods of guideline-concordant intermittent measurement, the median hourly alarm rate was 0.5 (IQR, 0.1-0.8), with a median of 5 (IQR, 1-13) alarms per patient.
Those who received supplemental oxygen earlier in the admission had higher alarm rates during continuously monitored time (7.3 per hour [IQR, 2.7-12.7]) than patients who had not received supplemental oxygen (3.3 per hour [IQR, 0.6-11.8]), likely reflecting clinical differences between these patient populations. The most frequent alarm type among continuously monitored patients who had previously received supplemental oxygen was “SpO2 low.”
Discussion
Across 4402 patient hours, guideline-discordant continuous SpO2 monitoring of patients with bronchiolitis resulted in 11,101 alarms, at a rate of approximately 1 additional alarm every 9 minutes. Patients in our cohort received a median of 6 hours of guideline-discordant monitoring, which imposes a significant alarm burden that is potentially modifiable using targeted reduction strategies.15
Transient, self-resolved hypoxemia is a common feature of bronchiolitis and likely of little clinical consequence.16 Therefore, this rate of hypoxemia alarms is not unexpected. Though we evaluated only the period of time following final discontinuation of respiratory support, this finding is in keeping with the literature associating excess physiologic monitoring of patients with bronchiolitis with unnecessary oxygen therapy and increased length of stay,3-5 largely because clinicians may feel compelled to respond to hypoxemia alarms with either supplemental oxygen or longer monitoring.
Our findings must be contextualized in light of the limitations of our approach. We did not evaluate nurse workload associated with guideline-discordant continuous SpO2 monitoring. Prior work conducted by our lab has demonstrated that when nurses experience more than 40 alarms within a 2-hour period, their measured subjective workload increases to a degree associated with missing important tasks, threatening the quality and safety of the care they deliver.12,17 Given that nurses care for multiple patients, it is likely that the excess alarms introduced by guideline-discordant continuous monitoring contribute to increased nurse workload and alarm fatigue.
Similarly, we could not evaluate whether the alarms nurses experienced were actionable. Although our lab has previously reported that ≥99% of alarms occurring on non-ICU pediatric wards are nonactionable,10,11 it is possible that some of the alarms during guideline-discordant monitoring periods required action. However, it is unlikely that any life-sustaining actions were taken because (1) we only evaluated time >60 minutes after final discontinuation of supplemental oxygen, so by definition none of these alarms required treatment with supplemental oxygen, and (2) none of the patients we included received ICU care during their admission.
The avoidable alarm burden identified in our analysis suggests that eliminating continuous SpO2 monitoring overuse in bronchiolitis will likely reduce nurses’ workload and alarm fatigue in hospital settings that care for children with bronchiolitis.
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Schondelmeyer AC, Dewan ML, Brady PW, et al. Cardiorespiratory and pulse oximetry monitoring in hospitalized children: a Delphi process. Pediatrics. 2020;146(2):e20193336. https://doi.org/10.1542/peds.2019-3336
3. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): A multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-xxiii, 1-172. https://doi.org/10.3310/hta19710
4. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
5. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
6. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
7. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
8. Schondelmeyer AC, Brady PW, Goel VV, et al. Physiologic monitor alarm rates at 5 children’s hospitals. J Hosp Med. 2018;13(6):396-398. https://doi.org/10.12788/jhm.2918
9. Schondelmeyer AC, Bonafide CP, Goel VV, et al. The frequency of physiologic monitor alarms in a children’s hospital. J Hosp Med. 2016;11(11):796-798. https://doi.org/10.1002/jhm.2612
10. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
11. Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524-531. https://doi.org/10.1001/jamapediatrics.2016.5123
12. Rasooly IR, Kern-Goldberger AS, Xiao R, et al. Physiologic monitor alarm burden and nurses’ subjective workload in a children’s hospital. Hosp Pediatr. 2021;11(7):703-710. https://doi.org/10.1542/hpeds.2020-003509
13. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
14. Kern-Goldberger AS, Rasooly IR, Luo B, et al. EHR-integrated monitor data to measure pulse oximetry use in bronchiolitis. Hosp Pediatr. 2021;11(10):1073-1082. https://doi.org/10.1542/hpeds.2021-005894
15. Schondelmeyer AC, Bettencourt AP, Xiao R, et al. Evaluation of an educational outreach and audit and feedback program to reduce continuous pulse oximetry use in hospitalized infants with stable bronchiolitis. JAMA Netw Open. 2021;4(9):e2122826. https://doi.org/10.1001/jamanetworkopen.2021.22826
16. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
17. Tubbs-Cooley HL, Mara CA, Carle AC, Mark BA, Pickler RH. Association of nurse workload with missed nursing care in the neonatal intensive care unit. JAMA Pediatr. 2019;173(1):44-51. https://doi.org/10.1001/jamapediatrics.2018.3619
1. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742
2. Schondelmeyer AC, Dewan ML, Brady PW, et al. Cardiorespiratory and pulse oximetry monitoring in hospitalized children: a Delphi process. Pediatrics. 2020;146(2):e20193336. https://doi.org/10.1542/peds.2019-3336
3. Cunningham S, Rodriguez A, Boyd KA, McIntosh E, Lewis SC, BIDS Collaborators Group. Bronchiolitis of Infancy Discharge Study (BIDS): A multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation. Health Technol Assess. 2015;19(71):i-xxiii, 1-172. https://doi.org/10.3310/hta19710
4. McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis: a randomized clinical trial. JAMA Pediatr. 2015;169(10):898-904. https://doi.org/10.1001/jamapediatrics.2015.1746
5. Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: a randomized clinical trial. JAMA. 2014;312(7):712-718. https://doi.org/10.1001/jama.2014.8637
6. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
7. Bonafide CP, Xiao R, Brady PW, et al. Prevalence of continuous pulse oximetry monitoring in hospitalized children with bronchiolitis not requiring supplemental oxygen. JAMA. 2020;323(15):1467-1477. https://doi.org/10.1001/jama.2020.2998
8. Schondelmeyer AC, Brady PW, Goel VV, et al. Physiologic monitor alarm rates at 5 children’s hospitals. J Hosp Med. 2018;13(6):396-398. https://doi.org/10.12788/jhm.2918
9. Schondelmeyer AC, Bonafide CP, Goel VV, et al. The frequency of physiologic monitor alarms in a children’s hospital. J Hosp Med. 2016;11(11):796-798. https://doi.org/10.1002/jhm.2612
10. Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345-351. https://doi.org/10.1002/jhm.2331
11. Bonafide CP, Localio AR, Holmes JH, et al. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524-531. https://doi.org/10.1001/jamapediatrics.2016.5123
12. Rasooly IR, Kern-Goldberger AS, Xiao R, et al. Physiologic monitor alarm burden and nurses’ subjective workload in a children’s hospital. Hosp Pediatr. 2021;11(7):703-710. https://doi.org/10.1542/hpeds.2020-003509
13. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199
14. Kern-Goldberger AS, Rasooly IR, Luo B, et al. EHR-integrated monitor data to measure pulse oximetry use in bronchiolitis. Hosp Pediatr. 2021;11(10):1073-1082. https://doi.org/10.1542/hpeds.2021-005894
15. Schondelmeyer AC, Bettencourt AP, Xiao R, et al. Evaluation of an educational outreach and audit and feedback program to reduce continuous pulse oximetry use in hospitalized infants with stable bronchiolitis. JAMA Netw Open. 2021;4(9):e2122826. https://doi.org/10.1001/jamanetworkopen.2021.22826
16. Principi T, Coates AL, Parkin PC, Stephens D, DaSilva Z, Schuh S. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr. 2016;170(6):602-608. https://doi.org/10.1001/jamapediatrics.2016.0114
17. Tubbs-Cooley HL, Mara CA, Carle AC, Mark BA, Pickler RH. Association of nurse workload with missed nursing care in the neonatal intensive care unit. JAMA Pediatr. 2019;173(1):44-51. https://doi.org/10.1001/jamapediatrics.2018.3619
© 2021 Society of Hospital Medicine
Secure Text Messaging in Healthcare: Latent Threats and Opportunities to Improve Patient Safety
UNINTENDED CONSEQUENCES
Over the past two decades, physicians and nurses practicing in hospital settings have faced an onslaught of challenges in communication, an area frequently cited as critical to providing safe and effective care to patients.1-3 Communication needs have increased significantly as hospitalized patients have become more acute, complex, and technology-dependent, requiring larger healthcare teams comprising subspecialists across multiple disciplines spread across increasingly larger inpatient facilities.4 During this same period, the evolution of mobile phones has led to dramatic shifts in personal communication patterns, with asynchronous text messaging replacing verbal communication.5-7
In response to both the changing communication needs of clinicians and shifting cultural conventions, healthcare systems and providers alike have viewed text messaging as a solution to these growing communication problems. In fact, an entire industry has developed around “secure” and “Health Insurance Portability and Accountability Act (HIPAA)-compliant” text messaging platforms, which we will refer to below as secure text messaging systems (STMS). These systems offer benefits over carrier-based text messaging given their focus on the healthcare environment and HIPAA compliance. However, hospitals’ rapid adoption of these systems has outpaced our abilities to surveil, recognize, and understand the unintended consequences of transitioning to STMS communication in the hospital setting where failures in communication can be catastrophic. Below, we highlight three critical areas of concern encountered at our institutions and offer five potential mitigating strategies (Table).
CRITICAL AREAS OF CONCERN
Text Messaging is a Form of Alarm Fatigue
Text messaging renders clinicians vulnerable to a unique form of alarm fatigue. The burden of alarm fatigue has been well described in the literature and applies to interruptions to workflow in the electronic medical record and sensory alerts in clinical settings.8,9 Text messaging serves as yet another interruption for healthcare providers. Without a framework to triage urgent versus nonurgent messages, a clinician can become inundated with information and miss critical messages. This can lead to delayed or incorrect responses and impede patient care. System design and implementation can also contribute to this phenomenon. For example, a text message analysis at one center identified how system and workflow design resulted in all messages to an intensive care unit team being routed to a single physician’s phone.10 This design left the singular physician at risk of information and task overload and at the mercy of endless interruptive alerts. Although this can occur with any communication system, it has been well demonstrated that adopting STMS correlates with an increased frequency of messaging, leading to an increase in interruptive alerts, which may have implications for patient safety.11 This type of systems failure is silent unless proactively identified or revealed through a retrospective review of a resulting safety event.
Text Messaging Inappropriately Replaces Critical Communications that Should Happen in Person or by Phone
Text messaging has de-emphasized interpersonal communication skills and behaviors critical for quality and safety in hospital-based care. This concern emerges alongside evidence suggesting that new generations of physician trainees have profoundly different communication habits, preferences, and skillsets based on their experience in a text-heavy, asynchronous world of communication.12 There is reason to worry that reliance on text messaging in healthcare leads to similar alterations in relationships and collaboration as it has in our broader cultural context.13 Academic medical centers in particular should attempt to mitigate the loss of profound and formative learning that occurs during face-to-face encounters between providers of different disciplines, experience levels, and specialties.
Text Messaging Increases the Risk of Communication Error
Finally, text messaging appears to be highly vulnerable to communication errors in the healthcare setting. Prior work emphasizes the importance of nonverbal communication in face-to-face and even voice-to-voice interactions, highlighting the loss of fidelity when using text-only methods to communicate.1 Furthermore, the asynchronous nature of text messaging grants little room for clarification of minor misunderstandings that often arise in text-only communication through minor alterations in punctuation or automatic spelling corrections, a frequent occurrence when using medical terminology. Although a seasoned physician may be able to piece together the issues that deserve further clarification, young residents may be more hesitant to ask clarifying questions and determine the right course of action due to clinical inexperience.
PROPOSED SOLUTIONS
Deliberate Design and Implementation
A recent systematic review identified a lack of high-quality evidence evaluating the impact of mobile technologies on communication and teamwork in hospital settings.14 This paucity of understanding renders communication via STMS in the healthcare setting uniquely vulnerable to latent safety threats unless the design and implementation of these systems are purposeful and proactive.
These concerns led us to postulate that deliberate and proactive implementation of these systems, rather than passive adoption, is needed in the healthcare environment. We propose a number of approaches and interventions that may guide institutions as they seek to implement STMS or redesign communication in the inpatient setting. At the core of these proposals lies an important tension: can implementation of STMS occur in isolation or should the arrival of these systems prompt an overhaul of an institution’s clinical communication system and culture?15
Proactive Surveillance
Surveillance is one proactive method for healthcare systems to understand where and how the implementation of STMS might lead to safety threats. From a quantitative standpoint, understanding the burden of messaging for each user across the system can reveal the clinical roles in the system that are particularly vulnerable to alert fatigue or information overload. Quality assurance monitoring of critical roles in the hospital (ie, airway emergency team, rapid response teams) could be conducted to ensure accurate directory listings at all times. Associating conversations with events, from serious safety events to near misses, could help leaders understand when and how text messaging contributes to safety events and create actionable learnings for safety learning systems.
Standardized Communication
A standardized language eliminates the burden of individuals to parse and translate each individual text message. A standardized algorithm for language, urgency, and expectations (ie, response before escalation) would help define the interaction in the clinical setting.16 Moving toward standardized, meaningful “quick messages,” one of our centers has implemented a campaign to “stick to the FACS,” where the following four standard quick messages are available for users: (1) “FYI no response needed,” (2) “ACTION needed within X min,” (3) “CONCERN can we talk or meet,” and (4) “STAT immediate response required.” These quick messages, developed with frontline stakeholders, represent the majority of requests exchanged by providers, and help standardize expectations and task prioritization.
Targeted Training
Targeted training and culture change efforts might help institutions counteract the broader impact of asynchronous messaging on communication skills and behaviors. Highlighting the contrast between clinical and casual communication with an emphasis on examples, scenarios, or role-playing has the potential to emphasize why and how clinical communication with STMS requires a careful, deliberate approach. For instance, safety culture training at one of our institutions features a scenario that illustrates the potential for miscommunication and missed connection between a nurse and a physician on the wards. The scenario gives way to discussion between participants about the shortcomings of text messaging and allows the facilitator to segue into the “dos and don’ts” of text messaging and when a phone call might be more appropriate.
Innovate
Finally, creatively harnessing the technology and data underlying these STMS may uncover methods to identify and mitigate communication errors in real time. For instance, using trigger methods to create a “ripple in the pond,” whereby a floor nurse reaching out with an urgent text automatically loops in the charge nurse of the unit. Building a chatbot or a virtual assistant functionality by leveraging user behavior patterns and natural language processing to provide text-based guidance to users might help busy clinicians connect to the key decision-makers on their team. For example, in response to an unanswered text, a virtual assistant might reach out to the waiting provider as follows: “you texted the resident 20 minutes ago and they haven’t replied, would you like to call the fellow instead?” The data-rich nature of these systems implies that they are ripe for automated solutions that can respond to behavioral- or text-based patterns to augment the existing operation and safety infrastructure.
CONCLUSION
The transition of healthcare communication systems toward STMS is already well underway. These systems, despite their flaws, are undoubtedly an improvement over legacy paging systems and, if properly implemented, offer several benefits to large healthcare systems. However, the communication needs in the healthcare setting are vastly different from the personal communication needs in everyday text messaging. As clinicians at the forefront of these transitions, we have the opportunity to critically assess the unique communication requirements in our hospital settings and help shape the way STMS are implemented in our hospitals. Pausing to deliberate about the limitations and the vulnerabilities of the current messaging systems for our acute clinical needs, including how they impact training and education, will allow us to proactively design and implement better communication systems that improve patient safety.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019.
2. Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: The nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11(2):104-112. https://doi.org/10.1197/jamia.M1471.
3. Coiera E. When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277-286. https://doi.org/10.1136/jamia.2000.0070277.
4. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. https://doi.org/10.1542/peds.2009-3266.
5. The Nielsen Company. In U.S., SMS Text Messaging Tops Mobile Phone Calling. https://www.nielsen.com/us/en/insights/article/2008/in-us-text-messaging-tops-mobile-phone-calling/. Accessed July 22, 2019.
6. The Nielsen Company. New Mobile Obsession in U.S. Teens Triple Data Usage. The Nielsen Company. Published 2011. Accessed July 22, 2019.
7. The Nielsen Company. U.S. Teen Mobile Report Calling Yesterday, Texting Today, Using Apps Tomorrow. The Nielsen Company. https://www.nielsen.com/us/en/insights/article/2010/u-s-teen-mobile-report-calling-yesterday-texting-today-using-apps-tomorrow/. Accessed July 22, 2019.
8. Sendelbach S, Funk M. Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378-386; quiz 387-378.
9. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136-144. https://doi.org/10.1002/jhm.2520.
10. Hagedorn PA, Kirkendall ES, Spooner SA, Mohan V. Inpatient communication networks: leveraging secure text-messaging platforms to gain insight into inpatient communication systems. Appl Clin Inform. 2019;10(3):471-478. https://doi.org/10.1055/s-0039-1692401.
11. Westbrook JI, Coiera E, Dunsmuir WT, et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284-289. https://doi.org/10.1136/qshc.2009.039255.
12. Castells M. The Rise of the Network Society. 2nd ed. Malden, MA: Wiley-Blackwell; 2010.
13. Lo V, Wu RC, Morra D, Lee L, Reeves S. The use of smartphones in general and internal medicine units: A boon or a bane to the promotion of interprofessional collaboration? J Interprof Care. 2012;26(4):276-282. https://doi.org/10.3109/13561820.2012.663013.
14. Martin G, Khajuria A, Arora S, King D, Ashrafian H, Darzi A. The impact of mobile technology on teamwork and communication in hospitals: a systematic review. J Am Med Inform Assn. 2019;26(4):339-355. https://doi.org/10.1093/jamia/ocy175.
15. Liu X, Sutton PR, McKenna R, et al. Evaluation of secure messaging applications for a health care system: a case study. Appl Clin Inform. 2019;10(1):140-150. https://doi.org/10.1055/s-0039-1678607.
16. Weigert RM, Schmitz AH, Soung PJ, Porada K, Weisgerber MC. Improving standardization of paging communication using quality improvement methodology. Pediatrics. 2019;143(4). https://doi.org/10.1542/peds.2018-1362.
UNINTENDED CONSEQUENCES
Over the past two decades, physicians and nurses practicing in hospital settings have faced an onslaught of challenges in communication, an area frequently cited as critical to providing safe and effective care to patients.1-3 Communication needs have increased significantly as hospitalized patients have become more acute, complex, and technology-dependent, requiring larger healthcare teams comprising subspecialists across multiple disciplines spread across increasingly larger inpatient facilities.4 During this same period, the evolution of mobile phones has led to dramatic shifts in personal communication patterns, with asynchronous text messaging replacing verbal communication.5-7
In response to both the changing communication needs of clinicians and shifting cultural conventions, healthcare systems and providers alike have viewed text messaging as a solution to these growing communication problems. In fact, an entire industry has developed around “secure” and “Health Insurance Portability and Accountability Act (HIPAA)-compliant” text messaging platforms, which we will refer to below as secure text messaging systems (STMS). These systems offer benefits over carrier-based text messaging given their focus on the healthcare environment and HIPAA compliance. However, hospitals’ rapid adoption of these systems has outpaced our abilities to surveil, recognize, and understand the unintended consequences of transitioning to STMS communication in the hospital setting where failures in communication can be catastrophic. Below, we highlight three critical areas of concern encountered at our institutions and offer five potential mitigating strategies (Table).
CRITICAL AREAS OF CONCERN
Text Messaging is a Form of Alarm Fatigue
Text messaging renders clinicians vulnerable to a unique form of alarm fatigue. The burden of alarm fatigue has been well described in the literature and applies to interruptions to workflow in the electronic medical record and sensory alerts in clinical settings.8,9 Text messaging serves as yet another interruption for healthcare providers. Without a framework to triage urgent versus nonurgent messages, a clinician can become inundated with information and miss critical messages. This can lead to delayed or incorrect responses and impede patient care. System design and implementation can also contribute to this phenomenon. For example, a text message analysis at one center identified how system and workflow design resulted in all messages to an intensive care unit team being routed to a single physician’s phone.10 This design left the singular physician at risk of information and task overload and at the mercy of endless interruptive alerts. Although this can occur with any communication system, it has been well demonstrated that adopting STMS correlates with an increased frequency of messaging, leading to an increase in interruptive alerts, which may have implications for patient safety.11 This type of systems failure is silent unless proactively identified or revealed through a retrospective review of a resulting safety event.
Text Messaging Inappropriately Replaces Critical Communications that Should Happen in Person or by Phone
Text messaging has de-emphasized interpersonal communication skills and behaviors critical for quality and safety in hospital-based care. This concern emerges alongside evidence suggesting that new generations of physician trainees have profoundly different communication habits, preferences, and skillsets based on their experience in a text-heavy, asynchronous world of communication.12 There is reason to worry that reliance on text messaging in healthcare leads to similar alterations in relationships and collaboration as it has in our broader cultural context.13 Academic medical centers in particular should attempt to mitigate the loss of profound and formative learning that occurs during face-to-face encounters between providers of different disciplines, experience levels, and specialties.
Text Messaging Increases the Risk of Communication Error
Finally, text messaging appears to be highly vulnerable to communication errors in the healthcare setting. Prior work emphasizes the importance of nonverbal communication in face-to-face and even voice-to-voice interactions, highlighting the loss of fidelity when using text-only methods to communicate.1 Furthermore, the asynchronous nature of text messaging grants little room for clarification of minor misunderstandings that often arise in text-only communication through minor alterations in punctuation or automatic spelling corrections, a frequent occurrence when using medical terminology. Although a seasoned physician may be able to piece together the issues that deserve further clarification, young residents may be more hesitant to ask clarifying questions and determine the right course of action due to clinical inexperience.
PROPOSED SOLUTIONS
Deliberate Design and Implementation
A recent systematic review identified a lack of high-quality evidence evaluating the impact of mobile technologies on communication and teamwork in hospital settings.14 This paucity of understanding renders communication via STMS in the healthcare setting uniquely vulnerable to latent safety threats unless the design and implementation of these systems are purposeful and proactive.
These concerns led us to postulate that deliberate and proactive implementation of these systems, rather than passive adoption, is needed in the healthcare environment. We propose a number of approaches and interventions that may guide institutions as they seek to implement STMS or redesign communication in the inpatient setting. At the core of these proposals lies an important tension: can implementation of STMS occur in isolation or should the arrival of these systems prompt an overhaul of an institution’s clinical communication system and culture?15
Proactive Surveillance
Surveillance is one proactive method for healthcare systems to understand where and how the implementation of STMS might lead to safety threats. From a quantitative standpoint, understanding the burden of messaging for each user across the system can reveal the clinical roles in the system that are particularly vulnerable to alert fatigue or information overload. Quality assurance monitoring of critical roles in the hospital (ie, airway emergency team, rapid response teams) could be conducted to ensure accurate directory listings at all times. Associating conversations with events, from serious safety events to near misses, could help leaders understand when and how text messaging contributes to safety events and create actionable learnings for safety learning systems.
Standardized Communication
A standardized language eliminates the burden of individuals to parse and translate each individual text message. A standardized algorithm for language, urgency, and expectations (ie, response before escalation) would help define the interaction in the clinical setting.16 Moving toward standardized, meaningful “quick messages,” one of our centers has implemented a campaign to “stick to the FACS,” where the following four standard quick messages are available for users: (1) “FYI no response needed,” (2) “ACTION needed within X min,” (3) “CONCERN can we talk or meet,” and (4) “STAT immediate response required.” These quick messages, developed with frontline stakeholders, represent the majority of requests exchanged by providers, and help standardize expectations and task prioritization.
Targeted Training
Targeted training and culture change efforts might help institutions counteract the broader impact of asynchronous messaging on communication skills and behaviors. Highlighting the contrast between clinical and casual communication with an emphasis on examples, scenarios, or role-playing has the potential to emphasize why and how clinical communication with STMS requires a careful, deliberate approach. For instance, safety culture training at one of our institutions features a scenario that illustrates the potential for miscommunication and missed connection between a nurse and a physician on the wards. The scenario gives way to discussion between participants about the shortcomings of text messaging and allows the facilitator to segue into the “dos and don’ts” of text messaging and when a phone call might be more appropriate.
Innovate
Finally, creatively harnessing the technology and data underlying these STMS may uncover methods to identify and mitigate communication errors in real time. For instance, using trigger methods to create a “ripple in the pond,” whereby a floor nurse reaching out with an urgent text automatically loops in the charge nurse of the unit. Building a chatbot or a virtual assistant functionality by leveraging user behavior patterns and natural language processing to provide text-based guidance to users might help busy clinicians connect to the key decision-makers on their team. For example, in response to an unanswered text, a virtual assistant might reach out to the waiting provider as follows: “you texted the resident 20 minutes ago and they haven’t replied, would you like to call the fellow instead?” The data-rich nature of these systems implies that they are ripe for automated solutions that can respond to behavioral- or text-based patterns to augment the existing operation and safety infrastructure.
CONCLUSION
The transition of healthcare communication systems toward STMS is already well underway. These systems, despite their flaws, are undoubtedly an improvement over legacy paging systems and, if properly implemented, offer several benefits to large healthcare systems. However, the communication needs in the healthcare setting are vastly different from the personal communication needs in everyday text messaging. As clinicians at the forefront of these transitions, we have the opportunity to critically assess the unique communication requirements in our hospital settings and help shape the way STMS are implemented in our hospitals. Pausing to deliberate about the limitations and the vulnerabilities of the current messaging systems for our acute clinical needs, including how they impact training and education, will allow us to proactively design and implement better communication systems that improve patient safety.
UNINTENDED CONSEQUENCES
Over the past two decades, physicians and nurses practicing in hospital settings have faced an onslaught of challenges in communication, an area frequently cited as critical to providing safe and effective care to patients.1-3 Communication needs have increased significantly as hospitalized patients have become more acute, complex, and technology-dependent, requiring larger healthcare teams comprising subspecialists across multiple disciplines spread across increasingly larger inpatient facilities.4 During this same period, the evolution of mobile phones has led to dramatic shifts in personal communication patterns, with asynchronous text messaging replacing verbal communication.5-7
In response to both the changing communication needs of clinicians and shifting cultural conventions, healthcare systems and providers alike have viewed text messaging as a solution to these growing communication problems. In fact, an entire industry has developed around “secure” and “Health Insurance Portability and Accountability Act (HIPAA)-compliant” text messaging platforms, which we will refer to below as secure text messaging systems (STMS). These systems offer benefits over carrier-based text messaging given their focus on the healthcare environment and HIPAA compliance. However, hospitals’ rapid adoption of these systems has outpaced our abilities to surveil, recognize, and understand the unintended consequences of transitioning to STMS communication in the hospital setting where failures in communication can be catastrophic. Below, we highlight three critical areas of concern encountered at our institutions and offer five potential mitigating strategies (Table).
CRITICAL AREAS OF CONCERN
Text Messaging is a Form of Alarm Fatigue
Text messaging renders clinicians vulnerable to a unique form of alarm fatigue. The burden of alarm fatigue has been well described in the literature and applies to interruptions to workflow in the electronic medical record and sensory alerts in clinical settings.8,9 Text messaging serves as yet another interruption for healthcare providers. Without a framework to triage urgent versus nonurgent messages, a clinician can become inundated with information and miss critical messages. This can lead to delayed or incorrect responses and impede patient care. System design and implementation can also contribute to this phenomenon. For example, a text message analysis at one center identified how system and workflow design resulted in all messages to an intensive care unit team being routed to a single physician’s phone.10 This design left the singular physician at risk of information and task overload and at the mercy of endless interruptive alerts. Although this can occur with any communication system, it has been well demonstrated that adopting STMS correlates with an increased frequency of messaging, leading to an increase in interruptive alerts, which may have implications for patient safety.11 This type of systems failure is silent unless proactively identified or revealed through a retrospective review of a resulting safety event.
Text Messaging Inappropriately Replaces Critical Communications that Should Happen in Person or by Phone
Text messaging has de-emphasized interpersonal communication skills and behaviors critical for quality and safety in hospital-based care. This concern emerges alongside evidence suggesting that new generations of physician trainees have profoundly different communication habits, preferences, and skillsets based on their experience in a text-heavy, asynchronous world of communication.12 There is reason to worry that reliance on text messaging in healthcare leads to similar alterations in relationships and collaboration as it has in our broader cultural context.13 Academic medical centers in particular should attempt to mitigate the loss of profound and formative learning that occurs during face-to-face encounters between providers of different disciplines, experience levels, and specialties.
Text Messaging Increases the Risk of Communication Error
Finally, text messaging appears to be highly vulnerable to communication errors in the healthcare setting. Prior work emphasizes the importance of nonverbal communication in face-to-face and even voice-to-voice interactions, highlighting the loss of fidelity when using text-only methods to communicate.1 Furthermore, the asynchronous nature of text messaging grants little room for clarification of minor misunderstandings that often arise in text-only communication through minor alterations in punctuation or automatic spelling corrections, a frequent occurrence when using medical terminology. Although a seasoned physician may be able to piece together the issues that deserve further clarification, young residents may be more hesitant to ask clarifying questions and determine the right course of action due to clinical inexperience.
PROPOSED SOLUTIONS
Deliberate Design and Implementation
A recent systematic review identified a lack of high-quality evidence evaluating the impact of mobile technologies on communication and teamwork in hospital settings.14 This paucity of understanding renders communication via STMS in the healthcare setting uniquely vulnerable to latent safety threats unless the design and implementation of these systems are purposeful and proactive.
These concerns led us to postulate that deliberate and proactive implementation of these systems, rather than passive adoption, is needed in the healthcare environment. We propose a number of approaches and interventions that may guide institutions as they seek to implement STMS or redesign communication in the inpatient setting. At the core of these proposals lies an important tension: can implementation of STMS occur in isolation or should the arrival of these systems prompt an overhaul of an institution’s clinical communication system and culture?15
Proactive Surveillance
Surveillance is one proactive method for healthcare systems to understand where and how the implementation of STMS might lead to safety threats. From a quantitative standpoint, understanding the burden of messaging for each user across the system can reveal the clinical roles in the system that are particularly vulnerable to alert fatigue or information overload. Quality assurance monitoring of critical roles in the hospital (ie, airway emergency team, rapid response teams) could be conducted to ensure accurate directory listings at all times. Associating conversations with events, from serious safety events to near misses, could help leaders understand when and how text messaging contributes to safety events and create actionable learnings for safety learning systems.
Standardized Communication
A standardized language eliminates the burden of individuals to parse and translate each individual text message. A standardized algorithm for language, urgency, and expectations (ie, response before escalation) would help define the interaction in the clinical setting.16 Moving toward standardized, meaningful “quick messages,” one of our centers has implemented a campaign to “stick to the FACS,” where the following four standard quick messages are available for users: (1) “FYI no response needed,” (2) “ACTION needed within X min,” (3) “CONCERN can we talk or meet,” and (4) “STAT immediate response required.” These quick messages, developed with frontline stakeholders, represent the majority of requests exchanged by providers, and help standardize expectations and task prioritization.
Targeted Training
Targeted training and culture change efforts might help institutions counteract the broader impact of asynchronous messaging on communication skills and behaviors. Highlighting the contrast between clinical and casual communication with an emphasis on examples, scenarios, or role-playing has the potential to emphasize why and how clinical communication with STMS requires a careful, deliberate approach. For instance, safety culture training at one of our institutions features a scenario that illustrates the potential for miscommunication and missed connection between a nurse and a physician on the wards. The scenario gives way to discussion between participants about the shortcomings of text messaging and allows the facilitator to segue into the “dos and don’ts” of text messaging and when a phone call might be more appropriate.
Innovate
Finally, creatively harnessing the technology and data underlying these STMS may uncover methods to identify and mitigate communication errors in real time. For instance, using trigger methods to create a “ripple in the pond,” whereby a floor nurse reaching out with an urgent text automatically loops in the charge nurse of the unit. Building a chatbot or a virtual assistant functionality by leveraging user behavior patterns and natural language processing to provide text-based guidance to users might help busy clinicians connect to the key decision-makers on their team. For example, in response to an unanswered text, a virtual assistant might reach out to the waiting provider as follows: “you texted the resident 20 minutes ago and they haven’t replied, would you like to call the fellow instead?” The data-rich nature of these systems implies that they are ripe for automated solutions that can respond to behavioral- or text-based patterns to augment the existing operation and safety infrastructure.
CONCLUSION
The transition of healthcare communication systems toward STMS is already well underway. These systems, despite their flaws, are undoubtedly an improvement over legacy paging systems and, if properly implemented, offer several benefits to large healthcare systems. However, the communication needs in the healthcare setting are vastly different from the personal communication needs in everyday text messaging. As clinicians at the forefront of these transitions, we have the opportunity to critically assess the unique communication requirements in our hospital settings and help shape the way STMS are implemented in our hospitals. Pausing to deliberate about the limitations and the vulnerabilities of the current messaging systems for our acute clinical needs, including how they impact training and education, will allow us to proactively design and implement better communication systems that improve patient safety.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019.
2. Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: The nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11(2):104-112. https://doi.org/10.1197/jamia.M1471.
3. Coiera E. When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277-286. https://doi.org/10.1136/jamia.2000.0070277.
4. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. https://doi.org/10.1542/peds.2009-3266.
5. The Nielsen Company. In U.S., SMS Text Messaging Tops Mobile Phone Calling. https://www.nielsen.com/us/en/insights/article/2008/in-us-text-messaging-tops-mobile-phone-calling/. Accessed July 22, 2019.
6. The Nielsen Company. New Mobile Obsession in U.S. Teens Triple Data Usage. The Nielsen Company. Published 2011. Accessed July 22, 2019.
7. The Nielsen Company. U.S. Teen Mobile Report Calling Yesterday, Texting Today, Using Apps Tomorrow. The Nielsen Company. https://www.nielsen.com/us/en/insights/article/2010/u-s-teen-mobile-report-calling-yesterday-texting-today-using-apps-tomorrow/. Accessed July 22, 2019.
8. Sendelbach S, Funk M. Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378-386; quiz 387-378.
9. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136-144. https://doi.org/10.1002/jhm.2520.
10. Hagedorn PA, Kirkendall ES, Spooner SA, Mohan V. Inpatient communication networks: leveraging secure text-messaging platforms to gain insight into inpatient communication systems. Appl Clin Inform. 2019;10(3):471-478. https://doi.org/10.1055/s-0039-1692401.
11. Westbrook JI, Coiera E, Dunsmuir WT, et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284-289. https://doi.org/10.1136/qshc.2009.039255.
12. Castells M. The Rise of the Network Society. 2nd ed. Malden, MA: Wiley-Blackwell; 2010.
13. Lo V, Wu RC, Morra D, Lee L, Reeves S. The use of smartphones in general and internal medicine units: A boon or a bane to the promotion of interprofessional collaboration? J Interprof Care. 2012;26(4):276-282. https://doi.org/10.3109/13561820.2012.663013.
14. Martin G, Khajuria A, Arora S, King D, Ashrafian H, Darzi A. The impact of mobile technology on teamwork and communication in hospitals: a systematic review. J Am Med Inform Assn. 2019;26(4):339-355. https://doi.org/10.1093/jamia/ocy175.
15. Liu X, Sutton PR, McKenna R, et al. Evaluation of secure messaging applications for a health care system: a case study. Appl Clin Inform. 2019;10(1):140-150. https://doi.org/10.1055/s-0039-1678607.
16. Weigert RM, Schmitz AH, Soung PJ, Porada K, Weisgerber MC. Improving standardization of paging communication using quality improvement methodology. Pediatrics. 2019;143(4). https://doi.org/10.1542/peds.2018-1362.
1. Sutcliffe KM, Lewton E, Rosenthal MM. Communication failures: an insidious contributor to medical mishaps. Acad Med. 2004;79(2):186-194. https://doi.org/10.1097/00001888-200402000-00019.
2. Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: The nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11(2):104-112. https://doi.org/10.1197/jamia.M1471.
3. Coiera E. When conversation is better than computation. J Am Med Inform Assoc. 2000;7(3):277-286. https://doi.org/10.1136/jamia.2000.0070277.
4. Simon TD, Berry J, Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126(4):647-655. https://doi.org/10.1542/peds.2009-3266.
5. The Nielsen Company. In U.S., SMS Text Messaging Tops Mobile Phone Calling. https://www.nielsen.com/us/en/insights/article/2008/in-us-text-messaging-tops-mobile-phone-calling/. Accessed July 22, 2019.
6. The Nielsen Company. New Mobile Obsession in U.S. Teens Triple Data Usage. The Nielsen Company. Published 2011. Accessed July 22, 2019.
7. The Nielsen Company. U.S. Teen Mobile Report Calling Yesterday, Texting Today, Using Apps Tomorrow. The Nielsen Company. https://www.nielsen.com/us/en/insights/article/2010/u-s-teen-mobile-report-calling-yesterday-texting-today-using-apps-tomorrow/. Accessed July 22, 2019.
8. Sendelbach S, Funk M. Alarm fatigue: a patient safety concern. AACN Adv Crit Care. 2013;24(4):378-386; quiz 387-378.
9. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136-144. https://doi.org/10.1002/jhm.2520.
10. Hagedorn PA, Kirkendall ES, Spooner SA, Mohan V. Inpatient communication networks: leveraging secure text-messaging platforms to gain insight into inpatient communication systems. Appl Clin Inform. 2019;10(3):471-478. https://doi.org/10.1055/s-0039-1692401.
11. Westbrook JI, Coiera E, Dunsmuir WT, et al. The impact of interruptions on clinical task completion. Qual Saf Health Care. 2010;19(4):284-289. https://doi.org/10.1136/qshc.2009.039255.
12. Castells M. The Rise of the Network Society. 2nd ed. Malden, MA: Wiley-Blackwell; 2010.
13. Lo V, Wu RC, Morra D, Lee L, Reeves S. The use of smartphones in general and internal medicine units: A boon or a bane to the promotion of interprofessional collaboration? J Interprof Care. 2012;26(4):276-282. https://doi.org/10.3109/13561820.2012.663013.
14. Martin G, Khajuria A, Arora S, King D, Ashrafian H, Darzi A. The impact of mobile technology on teamwork and communication in hospitals: a systematic review. J Am Med Inform Assn. 2019;26(4):339-355. https://doi.org/10.1093/jamia/ocy175.
15. Liu X, Sutton PR, McKenna R, et al. Evaluation of secure messaging applications for a health care system: a case study. Appl Clin Inform. 2019;10(1):140-150. https://doi.org/10.1055/s-0039-1678607.
16. Weigert RM, Schmitz AH, Soung PJ, Porada K, Weisgerber MC. Improving standardization of paging communication using quality improvement methodology. Pediatrics. 2019;143(4). https://doi.org/10.1542/peds.2018-1362.
© 2019 Society of Hospital Medicine
