User login
The Hospitalist and Stroke Prevention
Prevention has the greatest potential to reduce the societal burden from stroke.1 Several therapies that specifically target the underlying atherosclerotic disease process have been shown in clinical trials to markedly lower the risk of recurrent vascular events including stroke.2 However, there is great variability in how clinical trial data are implemented in clinical practice for ischemic stroke prevention.35 This has led to a knowledge‐implementation‐practice gap, possibly because of the limited awareness of the scientific evidence supporting various treatments, as well as the lack of a systematic approach to hospital stroke care.3 Our review discusses the evidence for reducing vascular risk after ischemic stroke and successful models of systematic interventions initiated during stroke hospitalization, with the goal of narrowing the stroke hospitalization evidencepractice gap.
Societal Burden
Stroke is the third‐leading cause of death in the United States and the leading cause of serious long‐term disability.6 Approximately 700,000 Americans have a new stroke or recurrent strokes every year, whereas nearly 5 million live with the consequences of stroke; nearly all stroke survivors (90%) have some residual functional deficit, and approximately 40% experience moderate to severe impairment.6 Stroke mortality is substantial, with a 30‐day case fatality rate after first stroke (of any cause) of about 25%.7, 8 Indeed, four‐fifths of patients do not survive for 10 years after stroke, and approximately one‐third of all case fatalities occur in the first year after a stroke.8 The estimated economic impact in 2006, US$57.9 billion, further underscores the substantial mortality and morbidity of stroke.6 Given the limited options for acute stroke therapies,9 stroke prevention remains an important therapeutic goal, especially because fewer than 5% of acute stroke patients in the United States currently receive the only Food and Drug Administrationapproved treatmentintravenous tissue plasminogen activator.10 It is obvious that additional strategies are urgently needed to reduce the devastating consequences of stroke.
Why Involve the Hospitalist?
The Hospitalist system in the United States is rapidly growing.11 Tthe Society of Hospital Medicine projects that by 2010 there will be approximately 30,000 hospitalists in the United States.11 A member census conducted by the American Academy of Neurology in 2000 found 13,500 practicing neurologists, most of whom are concentrated in urban and metropolitan areas.12 As such, with more than 700,000 strokes occurring each year,6 most stroke patients in the United States will not be seen or evaluated by a neurologist. Indeed, one study indicated that only 11.3% of stroke patients are attended exclusively by a neurologist.13 Furthermore, it is not uncommon for stroke patients to have numerous other medical issues that require attention and multidisciplinary care coordination during the hospital stay, an area where hospitalists excel. Conceivably, the ability to promptly identify and treat these non‐neurological comorbidities, which account for at least 30% of the deaths from acute ischemic stroke,14 could go a long way toward improving stroke outcomes.
Hospitalists are in the forefront of developing strategies for improving the quality of acute care and patient satisfaction, reducing medical errors, and focusing on efficient resource utilization. Translating evidence‐based strategies for acute stroke care into actual practice is a mechanism for improving the quality of care, ensuring that basic care does not deviate from provider to provider or from day to day (weekdays compared to weekend days/holidays) while at the same time allowing for the individualization of care appropriate to a patient's unique needs.15 After the acute treatment of stroke or TIA, additional measures must be initiated as soon as it is safe to do so in order to begin the process of limiting stroke progression and preventing recurrence. Secondary prevention measures require a coordinated transition in order to ensure continuation of care and follow‐up as needed. After a thorough risk assessment is complete, hospitalists will need to consider a 3‐pronged approach to secondary prevention that follows the national guidelines described above: pharmacotherapy, behavior modification, and, in some cases, surgical intervention.
Secondary Stroke
Secondary or recurrent strokes are strokes that occur after a first stroke or TIA,2 and the single biggest risk factor for having a stroke is already having had one.2 Because hospitalists generally see patients after ischemic cerebrovascular events have already happened, their opportunities to intervene are mostly geared toward reducing the risk of secondary stroke (beyond enhancing the prevention of complications from the index event). Recent community‐based data indicate that the short‐term risk of secondary stroke is high.16, 17 After a minor stroke or TIA, the risk of recurrent stroke or TIA increases over time8%‐12% within 7 days, 12%‐15% within 30 days, and 17%‐19% within 90 days.18 In the largest study of short‐term risk following TIA,19 there was an 11% risk of stroke (51% of which occurred in the 48 hours after TIA), an 13% risk of TIA, and a 25% risk of any adverse event within 90 days of the TIA.
Overall, the risk of a second cerebrovascular event is highest in the first year after a stroke/TIA (12%), declining to about 5% annually thereafter.7 The effects of secondary stroke are more devastating than those of the primary stroke: the 30‐day fatality rate after a first recurrent stroke is almost double that after the first‐ever stroke (41% versus 22%).20 The pathological factors that lead to TIA and stroke, such as platelet aggregation and subsequent thrombosis or the systolic stroke of blood against stenotic carotid plaques, are one and the same. As such, the short‐ and long‐term risks of recurrent events after both first stroke or first TIA necessitate investigation into a patient's vascular risk and early initiation of appropriate stroke prevention strategies.21
Cross Risk
Because the atherothrombotic disease process is systemic in nature with a variety of manifestations, stroke patients with atherosclerosis frequently have coexistent coronary artery disease and peripheral artery disease,22 and as such, are at risk for vascular events emanating from any of these beds in addition to that of the cervicocephalic arterial tree.23, 24 For instance, in a study of individuals in a long‐term care facility, among the patients with ischemic stroke, 56% had overlapping coronary artery disease, 28% had peripheral artery disease,25 and 38% of the patients had at least 2 manifestations of their atherosclerotic disease. The take‐home message here is that hospitalists also have the opportunity while treating patients hospitalized following stroke to prevent other vascular events by identifying and treating stroke patients who have systemic atherosclerosis.
Risk Factors
The first step in any approach to stroke prevention is the identification of predisposing risk factors. Several of the known biological and lifestyle risk factors associated with cerebrovascular disease were identified decades ago from large longitudinal studies.2 Certain stroke risk factors are nonmodifiable and therefore cannot be the target of intervention. 26 Treatment of the various stroke risk factors could have a substantial impact on reducing the burden of stroke. Table 1 shows the number needed to treat to prevent one stroke per year by modification of the individual stroke risk factor.
| Treatment | Relative risk reduction | Number needed to treat (1 stroke/year) |
|---|---|---|
| ||
| Antihypertensives | 28% | 51 |
| Statins | 25% | 57 |
| Aspirin | 28% | 77 |
| Smoking cessation | 33% | 43 |
| Carotid endarterectomy | 44% | 26 |
Guidelines for Secondary Stroke Prevention
Several organizations have published guidelines for the prevention of secondary stroke based on clinical evidence and expert consensus. Key guidelines include those published by the American Stroke Association (ASA),2 American College of Chest Physicians (ACCP),27 and the National Stroke Association. Although these guidelines are broadaddressing many components of stroke prevention and careeach contains recommendations specifically applicable to secondary prevention in most stroke patients who the hospitalist will encounter. Some provide hospital‐based guidelines that focus on care protocols and systems processes (ie, ASA Stroke Systems Guidelines), whereas others are therapy‐based guidelines (i.e, ACCP Guidelines on Antithrombotic Therapy for Ischemic Stroke). In the next few sections, we discuss common risk factors for and causes of secondary stroke and the prevailing guideline recommendations for modifying them. Discussion of the management of rare causes of ischemic stroke such as arterial dissection, vasculitis, patent foramen ovale, and so forth is beyond the scope of this article.
Hypertension, Dyslipidemia, and Diabetes
Table 2 shows the current national guideline recommendations for the management of premier vascular risk factorshypertension, dyslipidemia, and diabetesin ischemic stroke and TIA patients.2 Antihypertensive therapy is recommended for the prevention of secondary stroke and other vascular events in patients who have experienced an ischemic stroke or TIA and are beyond the hyperacute period.28, 29 Such treatment should be considered for all ischemic stroke and TIA patients regardless of history of hypertension.28 Although available data support the use of diuretics and the combination of diuretics plus an angiotensin‐converting enzyme inhibitor,28, 30 selection of specific medications should be individualized according to a patient's comorbid conditions.29 It is also important to note that despite the proven benefit of beta blockers in the secondary prevention of recurrent cardiac events, current evidence shows no clear benefit from the use of beta blockers in the prevention of stroke.29, 31
| Risk Factor | Recommendation |
|---|---|
| |
| Hypertension | Antihypertensive beyond hyperacute stroke period60 |
| Data support diuretic or diuretic + ACEI,2830 but individualize based on patient characteristics | |
| Antihypertensive in all patients regardless of history of hypertension28 | |
| Aim for average reduction of 10/5 mm HG or blood pressure 120/80 mm Hg28 | |
| Encourage reduced intake of dietary salt | |
| Dyslipidemia | Statin for LDL‐C goal 100 mg/dL in those with CAD or symptomatic atherosclerosis33, 34 |
| Target LDL‐C 70 mg/dL for very high‐risk persons61 | |
| Statin for stroke or TIA because of atherosclerosis regardless of LDL‐C level33, 34 | |
| Niacin or gemfibrozil for patients with low HDL‐C62, 63 | |
| Diabetes | ACEIs and ARBs should be first‐choice blood pressure drugs37, 38a |
| Glucose control to near normoglycemic levels39 | |
| Target glycosylated hemoglobin 7%64 | |
For ischemic cerebrovascular disease patients with dyslipidemia or symptomatic atherosclerosis, cholesterol management should be according to the current Adult Treatment Panel (ATP) guidelines.32 Statins should be the first‐line treatment.33, 34 Ischemic stroke or TIA patients whose underlying stroke mechanism is presumed to be atherosclerosis should be considered for statin therapy even if they have normal cholesterol levels and no evidence of atherosclerosis.33, 34 The recent Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study was the first study to specifically investigate the effect of statins in patients with a prior stroke but with normal cholesterol levels and no evidence of coronary heart disease. It found that treatment with atorvastatin 80 mg/day (vs. placebo) was associated with a 16% reduction in relative risk of recurrent stroke.34
The care of an ischemic stroke or TIA patient who has diabetes warrants more rigorous control of blood pressure and lipids.35, 36 Such patients usually require more than one antihypertensive drug. ACEIs and angiotensin receptor blockers (ARBs) are more effective in reducing the progression of renal disease and are the recommended first‐choice medications for these patients.37, 38 The target for glucose control should be reaching near‐normoglycemic levels.39
Large‐Artery Atherosclerosis
In selected at‐risk stroke patients, surgical techniques (eg, carotid endarterectomy [CEA], carotid angioplasty and/or stenting [CAS]) may reduce the rate of recurrent stroke.4044 For patients who have had ischemic cerebrovascular events in the preceding 6 months and who have ipsilateral severe (70%‐99%) cervical carotid artery stenosis, CEA done by a surgeon is recommended; it has a perioperative morbidity and mortality of less than 6%.40 For those with ipsilateral moderate (50%‐69%) cervical carotid stenosis, CEA should be considered, and whether to operate should be decided on the basis of the patient's age, sex, comorbidities, and severity of initial symptoms.41 Analyses of endarterectomy trials indicated that the benefit from CEA is greatest if performed within 2 weeks of a patient's last ischemic event, the advantage it confers rapidly falling with increasing delay.45 From the hospitalist's standpoint, it is of prime importance to ensure that patients admitted to the hospital with a TIA or ischemic stroke are not discharged before it has been established whether have severe carotid stenosis that requires a revascularization procedure. If carotid stenosis is less than 50%, CEA is not recommended.41
A newer, less invasive form of carotid artery revascularization is CAS,46 which is performed by operators with established periprocedural morbidity and mortality rates of 4%‐6% and may be considered in those with:
-
Symptomatic severe stenosis (>70%) that is difficult to access surgically.2
-
Medical issues that greatly increase the risks of surgery, such as clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal nerve palsy, radiation‐induced stenosis or restenosis after carotid endarterectomy, and more than 80 years old.43
Angioplasty and/or stenting may also be considered when patients with symptomatic extracranial vertebral stenosis are having symptoms despite optimal medical risk factor treatments.2 Among those with hemodynamically significant stenosis of the major intracranial vasculature (basilar, middle cerebrals, distal carotids, and vertebrals) experiencing symptoms despite optimal medical risk factor treatments, angioplasty and/or stenting is considered experimental.2
The degree of arterial stenosis can be assessed by ultrasound, magnetic resonance angiogram (MRA), computed tomography angiogram (CTA), and conventional catheter angiogram, the last of which remains the gold standard. A carotid ultrasound performed at a certified vascular laboratory or by an experienced radiology technologist that shows less than 50% stenosis need not be followed up with another neuroimaging test. Generally, MRA tends to overestimate the degree of arterial stenosis but is a useful screening tool. In the event that an MRA reveals more than 50% stenosis, another diagnostic modality such as a carotid duplex, CTA, or conventional catheter angiogram should be performed to confirm this finding.
Antithrombotic Treatment
Cardioembolic Stroke Mechanism
Although it can sometimes be difficult to determine the precise mechanism underlying a patient's stroke or TIA, those who have a high‐risk source of cardiogenic embolism should generally be treated with anticoagulant medications to prevent recurrence.2 Among ischemic cerebrovascular event patients with persistent or paroxysmal atrial fibrillation, anticoagulation with adjusted‐dose warfarin (target international normalized ratio [INR] of 2.5; range, 2.0‐3.0) should be administered.47 The ASA recommends initiating oral anticoagulation within 2 weeks of an ischemic stroke or TIA but indicates that further delays may be appropriate for patients with large infarcts or uncontrolled hypertension.2 For patients unable to take oral anticoagulants, aspirin 325 mg/day should be given instead. Among patients who suffered an ischemic stroke or TIA because of an acute myocardial infarction in whom left ventricular mural thrombus is identified by echocardiography or another form of cardiac imaging, oral anticoagulation should be considered, aiming for an INR of 2.0‐3.0 for at least 3 months and up to 1 year.2 Patients receiving oral anticoagulation who also have ischemic coronary artery disease should be prescribed aspirin as well, in doses up to 162 mg/day.2
Noncardioembolic Stroke Mechanism
For ischemic stroke or TIA patients who have no high‐risk source of cardiogenic embolism, antiplatelet agents rather than oral anticoagulation are generally recommended to reduce the risk of recurrent stroke and other cardiovascular events.4850 Acceptable options for initial therapy include:
-
Aspirin (50 to 325 mg/day)48;
-
Combination of aspirin (50 mg) and extended‐release dipyridamole (400 mg) daily49, 51;
-
Clopidogrel (75 mg) daily.50
The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone, and clopidogrel may be considered instead of aspirin alone.49, 51 However, currently there is not enough data to make evidence‐based recommendations for choosing between antiplatelet drugs beyond aspirin.2 Furthermore, there is no evidence that increasing the dose of aspirin for patients who have had an ischemic stroke while taking aspirin provides additional benefit.2 The selection of an antiplatelet agent must be individualized, giving due consideration to a patient's presumed stroke mechanism, risk factor profile, and tolerance.
Other antiplatelet guidelines for noncardioembolic stroke/TIA patients include that:
Education for Behavior Modification
It is crucial to discharge patients with the tools they need to make important lifestyle changes. Patients can significantly reduce their stroke risk by making changes in their everyday patterns of behavior. As much education as possible about smoking cessation, exercise, diet, and the warning signs of stroke should be provided often as possible during hospitalization for a stroke and need not be left to nurses. Stroke education is extremely important so patients understand the need to call for emergency medical services immediately if they even suspect they are having stroke symptoms because of the very narrow window of opportunity for treatment of an acute stroke.54 All patients should be encouraged to make lifestyle adjustments such as ceasing smoking, reducing alcohol intake, and controlling weight. Smoking cessation appears to be effective in preventing secondary stroke (33% reduction in relative risk),44 and initiating smoking cessation counseling during hospitalization for stroke may result in a high rate of adherence to smoking cessation, at least in the short term.55 Table 3 displays current national guideline recommendations on lifestyle modification approaches.2
| Risk Factor | Recommendation |
|---|---|
| |
| Smoking | Smoking cessation |
| Avoid environmental smoke | |
| Counseling, nicotine products, and oral smoking cessation medications | |
| Alcohol | Eliminate or reduce alcohol consumption |
| Light to moderate levels2 drinks/day for men, 1 drink/day for nonpregnant women may be considered | |
| Obesity | Weight reduction goal: BMI 18.5‐24.9 kg/m2 and waist circumference 35 inches for women, 40 inches for men |
| Encourage weight management through balance of caloric intake, physical activity, behavioral counseling | |
| Physical Activity | At least 30 minutes of moderate‐intensity physical exercise most days of the week |
| Supervised therapeutic exercise regimen for those with residual disability | |
EvidencePractice Gap
There are now many secondary stroke prevention modalities, and there is a copious amount of data validating the efficacy of quite a few of them.2 Yet there is a large gap in implementing evidence‐based secondary prevention strategies.35 TIA and ischemic stroke patients are often discharged from the hospital without being prescribed any preventive medications, despite the data supporting the use of antiplatelet agents, anticoagulants, and antihypertensives for prevention of secondary stroke.4 In addition, several behavioral interventions could help patients to avoid stroke recurrence,2 but quite often stroke patients are not educated about them during the acute care period.4 Poor discharge treatment utilization limits the effectiveness of proven therapies, resulting in lost opportunities to reduce the burden of secondary stroke.
The reasons for these care gaps are multifactorial and can be traced to patient and provider issues as well as to health care delivery processes. Our understanding of the reasons for this gap is improving. Generally speaking, preventive services are used less frequently than those services or treatment modalities that provide immediate relief or economic benefit. The benefit of most preventive services is more readily seen at a population level than at a individual level and accrues slowly over time. It becomes more difficult to stress prevention in a health care system driven by technology‐based acute care.3
Current clinical management of acute stroke patients has stroke specialists and hospital physicians focusing on the acute management and diagnostic workup during hospitalization. Initiation of long‐term treatment is often deferred to after discharge, when the patient resumes long‐term primary care follow‐up.54 This deferred approach may result in therapy not being initiated or being initiated less efficiently and at a time (weeks or months after the initial presentation) when the stroke event and underlying atherosclerotic disease may no longer be the focus of either the patient or the primary care physician.54
Initiating medications during the acute stroke hospitalization phase sends the patient the message that these therapies are important for preventing recurrence and are an essential part of their treatment.54 More important, hospital initiation of secondary prevention therapies has been shown to be a strong predictor of these therapies continuing to be used after discharge56 and is associated with better clinical outcomes.5759 Table 4 shows some of the resources available to assist hospitalists in overcoming the evidencepractice gap in stroke treatment.
| Tool | Description |
|---|---|
| |
| AHA Get with the GuidelinesStroke ( |
Focuses on care team protocols to facilitate appropriate in‐hospital and discharge stroke treatment utilization |
| Identifies champions to lead, develop, and mobilize teams to optimally implement evidence‐based stroke treatment in acute care hospitals | |
| Utilizes standardized admission orders, patient educational materials, data monitoring | |
| Provides resources to help hospitals obtain JCAHO certification | |
| UCLA Stroke PROTECT (Preventing Recurrence of Thromboembolic Events through Coordinated Treatment) program ( |
Integrates 8 proven secondary stroke prevention measures into standard stroke care provided during hospitalization |
| Applies quality improvement measures through preprinted admission orders, care maps, discharge protocols, educational materials, patient self‐assessment logs, and data monitoring tools | |
| JCAHO Disease Specific Certification for acute stroke care ( |
Designates eligible hospitals as primary stroke centers |
| Promotes compliance with consensus‐based national standards | |
| Encourages effective use of established clinical practice guidelines to manage and optimize stroke care | |
| Fosters an organized approach to performance measurement and improvement activities | |
CONCLUSIONS
The acute stroke hospitalization setting provides the ideal opportunity for hospitalists to not only institute evidence‐based prevention therapies for recurrent stroke but also to have the undivided attention of patients and their families. Furthermore, it may be risky to assume that relevant therapy when deferred will be initiated in a timely fashion, if at all, after hospital discharge. As part of an effective continuum of care, hospitalists have an important role not just in the management of acute ischemic stroke, but also in long‐term reduction of vascular risk.
- .Stroke prevention: windows of opportunity and failed expectations? A discussion of modifiable cardiovascular risk factors and a prevention proposal.Neuroepidemiology.1997;16(4):163–173.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Stroke.2006;37:577–617.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.,.Lipid Assessment and treatment patterns in hospitalized TIA and ischemic stroke patients.J Hosp Med.2006;1:214–220.
- ,,, et al.Heart disease and stroke statistics—2006 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2006;113(6):e85–e151.
- .Long‐term outcome after ischaemic stroke/transient ischaemic attack.Cerebrovasc Dis.2003;16(Suppl 1):14–19.
- ,,,,.Ten‐year survival after first‐ever stroke in the perth community stroke study.Stroke.2003;34:1842–1846.
- NINDS rt‐PA Stroke Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,,,,.Assessing patterns of t‐PA use in acute stroke.Stroke.2002;33:354.
- Growth of hospital medicine nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_ Hospital_M.htm. Accessed April 12,2006.
- American Academy of Neurology. Available at: http://www.aan.com/students/medical/faq.cfm. Accessed June 25,2006.
- .The neurologist's role in stroke management.Stroke.1996;27:1935–1936.
- .Prevention and management of medical complications of the hospitalized elderly stroke patient.Clin Geriatr Med.1991;7:475–482.
- ,,, et al.The Stroke Prevention Policy Model: linking evidence and clinical decisions.Ann Intern Med.1997;127:704–711.
- ,,.Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services.BMJ.2004;328:326.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,.Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services.BMJ.2004;328:326.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .New strategies for prevention of ischemic stroke: the LIFE study.Curr Neurol Neurosci Rep.2003;3(1):46–51.
- ,,, et al.[Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis].Med Klin.2002;97(4):221–228.
- ,,,,,.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134(3):224–238.
- .Prevention of strokes and recurrent strokes.J Neurol Neurosurg Psychiatry.1998;64:716.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,.Risk factors and their management for stroke prevention: outlook for 1999 and beyond.Neurology.1999;53(7 Suppl 4):S15–S24.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest. Sep2004;126(3 Suppl):483S–512S.
- Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- Post‐stroke antihypertensive treatment study. A preliminary result.PATS Collaborating Group.Chin Med J (Engl).1995;108:710–717.
- ,,.Should beta blockers remain first choice in the treatment of primary hypertension? A meta‐analysis.Lancet.2005;366:1545–1553.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines.Circulation.2004;110(2):227–239.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with Simvastatin on stroke and other major vascular events in 20, 536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- , et al.The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study. Presented at the 15th European Stroke Conference, Brussels, Belgium, May 16‐19,2006.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38: UK Prospective Diabetes Study Group.BMJ.1998;317:703–713.
- ,,,,, for theHeart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial.Lancet.2003;361:2005–2016.
- ,,,.The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy: the Collaborative Study Group.N Engl J Med.1993;329:1456–1462.
- ,,, for theCollaborative Study Group.Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy.N Engl J Med.2001;345:861–869.
- ,,., et al.Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study.Diabetes Res Clin Pract.1995;28:103–117.
- Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis.North American Symptomatic Carotid Endarterectomy Trial Collaborators.N Engl J Med.1991;325:445–453.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.North American Symptomatic Carotid Endarterectomy Trial Collaborators.N Engl J Med.1998;339:1415–1425.
- Endarterectomy for asymptomatic carotid artery stenosis.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study.JAMA.1995;273:1421–1428.
- ,,, et al.Protected carotid‐artery stenting versus endarterectomy in high‐risk patients.N Engl J Med.2004;351:1493–1501.
- ,,.New evidence for stroke prevention: scientific review.JAMA.2002;288:1388–1395.
- ,,,,.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363:915–924.
- .Carotid artery surgery vs. stent: a cardiovascular perspective.Catheter Cardiovasc Interv.2004;63:377–384.
- ,,, et al.Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis.Chest.2004;126:1938–1945.
- Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143(1‐2):1–13.
- A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).CAPRIE Steering Committee.Lancet.1996;348:1329–1339.
- ,,,,.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med. Apr 202006;354(16):1706–1717.
- ,,, et al.PROTECT: A Coordinated Stroke Treatment Program to Prevent Recurrent Thromboembolic Events.Neurology. Vol63;2004:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow up.Stroke.2004;35:2879–2883.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- ,,, et al.Early effects of statins in patients with coronary artery disease and high C‐reactive protein.Am J Cardiol.2004;94:1107–1112.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol. Apr 12001;87(7):819–822.
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review.Am J Cardiol.2004;94:1155–1160.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:1024.
- ,,, et al.Intensive versus moderate lipid lowering with statins after acute coronary syndromes.N Engl J Med.2004;350:1495–1504.
- ,,.Niacin therapy in atherosclerosis.Curr Opin Lipidol.2004;15:659–665.
- ,,, et al.Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA‐HIT).Circulation.2001;103:2828–2833.
- ,,.The effect of long‐term intensified insulin treatment on the development of microvascular complications of diabetes mellitus.N Engl J Med.1993;329:304–309.
Prevention has the greatest potential to reduce the societal burden from stroke.1 Several therapies that specifically target the underlying atherosclerotic disease process have been shown in clinical trials to markedly lower the risk of recurrent vascular events including stroke.2 However, there is great variability in how clinical trial data are implemented in clinical practice for ischemic stroke prevention.35 This has led to a knowledge‐implementation‐practice gap, possibly because of the limited awareness of the scientific evidence supporting various treatments, as well as the lack of a systematic approach to hospital stroke care.3 Our review discusses the evidence for reducing vascular risk after ischemic stroke and successful models of systematic interventions initiated during stroke hospitalization, with the goal of narrowing the stroke hospitalization evidencepractice gap.
Societal Burden
Stroke is the third‐leading cause of death in the United States and the leading cause of serious long‐term disability.6 Approximately 700,000 Americans have a new stroke or recurrent strokes every year, whereas nearly 5 million live with the consequences of stroke; nearly all stroke survivors (90%) have some residual functional deficit, and approximately 40% experience moderate to severe impairment.6 Stroke mortality is substantial, with a 30‐day case fatality rate after first stroke (of any cause) of about 25%.7, 8 Indeed, four‐fifths of patients do not survive for 10 years after stroke, and approximately one‐third of all case fatalities occur in the first year after a stroke.8 The estimated economic impact in 2006, US$57.9 billion, further underscores the substantial mortality and morbidity of stroke.6 Given the limited options for acute stroke therapies,9 stroke prevention remains an important therapeutic goal, especially because fewer than 5% of acute stroke patients in the United States currently receive the only Food and Drug Administrationapproved treatmentintravenous tissue plasminogen activator.10 It is obvious that additional strategies are urgently needed to reduce the devastating consequences of stroke.
Why Involve the Hospitalist?
The Hospitalist system in the United States is rapidly growing.11 Tthe Society of Hospital Medicine projects that by 2010 there will be approximately 30,000 hospitalists in the United States.11 A member census conducted by the American Academy of Neurology in 2000 found 13,500 practicing neurologists, most of whom are concentrated in urban and metropolitan areas.12 As such, with more than 700,000 strokes occurring each year,6 most stroke patients in the United States will not be seen or evaluated by a neurologist. Indeed, one study indicated that only 11.3% of stroke patients are attended exclusively by a neurologist.13 Furthermore, it is not uncommon for stroke patients to have numerous other medical issues that require attention and multidisciplinary care coordination during the hospital stay, an area where hospitalists excel. Conceivably, the ability to promptly identify and treat these non‐neurological comorbidities, which account for at least 30% of the deaths from acute ischemic stroke,14 could go a long way toward improving stroke outcomes.
Hospitalists are in the forefront of developing strategies for improving the quality of acute care and patient satisfaction, reducing medical errors, and focusing on efficient resource utilization. Translating evidence‐based strategies for acute stroke care into actual practice is a mechanism for improving the quality of care, ensuring that basic care does not deviate from provider to provider or from day to day (weekdays compared to weekend days/holidays) while at the same time allowing for the individualization of care appropriate to a patient's unique needs.15 After the acute treatment of stroke or TIA, additional measures must be initiated as soon as it is safe to do so in order to begin the process of limiting stroke progression and preventing recurrence. Secondary prevention measures require a coordinated transition in order to ensure continuation of care and follow‐up as needed. After a thorough risk assessment is complete, hospitalists will need to consider a 3‐pronged approach to secondary prevention that follows the national guidelines described above: pharmacotherapy, behavior modification, and, in some cases, surgical intervention.
Secondary Stroke
Secondary or recurrent strokes are strokes that occur after a first stroke or TIA,2 and the single biggest risk factor for having a stroke is already having had one.2 Because hospitalists generally see patients after ischemic cerebrovascular events have already happened, their opportunities to intervene are mostly geared toward reducing the risk of secondary stroke (beyond enhancing the prevention of complications from the index event). Recent community‐based data indicate that the short‐term risk of secondary stroke is high.16, 17 After a minor stroke or TIA, the risk of recurrent stroke or TIA increases over time8%‐12% within 7 days, 12%‐15% within 30 days, and 17%‐19% within 90 days.18 In the largest study of short‐term risk following TIA,19 there was an 11% risk of stroke (51% of which occurred in the 48 hours after TIA), an 13% risk of TIA, and a 25% risk of any adverse event within 90 days of the TIA.
Overall, the risk of a second cerebrovascular event is highest in the first year after a stroke/TIA (12%), declining to about 5% annually thereafter.7 The effects of secondary stroke are more devastating than those of the primary stroke: the 30‐day fatality rate after a first recurrent stroke is almost double that after the first‐ever stroke (41% versus 22%).20 The pathological factors that lead to TIA and stroke, such as platelet aggregation and subsequent thrombosis or the systolic stroke of blood against stenotic carotid plaques, are one and the same. As such, the short‐ and long‐term risks of recurrent events after both first stroke or first TIA necessitate investigation into a patient's vascular risk and early initiation of appropriate stroke prevention strategies.21
Cross Risk
Because the atherothrombotic disease process is systemic in nature with a variety of manifestations, stroke patients with atherosclerosis frequently have coexistent coronary artery disease and peripheral artery disease,22 and as such, are at risk for vascular events emanating from any of these beds in addition to that of the cervicocephalic arterial tree.23, 24 For instance, in a study of individuals in a long‐term care facility, among the patients with ischemic stroke, 56% had overlapping coronary artery disease, 28% had peripheral artery disease,25 and 38% of the patients had at least 2 manifestations of their atherosclerotic disease. The take‐home message here is that hospitalists also have the opportunity while treating patients hospitalized following stroke to prevent other vascular events by identifying and treating stroke patients who have systemic atherosclerosis.
Risk Factors
The first step in any approach to stroke prevention is the identification of predisposing risk factors. Several of the known biological and lifestyle risk factors associated with cerebrovascular disease were identified decades ago from large longitudinal studies.2 Certain stroke risk factors are nonmodifiable and therefore cannot be the target of intervention. 26 Treatment of the various stroke risk factors could have a substantial impact on reducing the burden of stroke. Table 1 shows the number needed to treat to prevent one stroke per year by modification of the individual stroke risk factor.
| Treatment | Relative risk reduction | Number needed to treat (1 stroke/year) |
|---|---|---|
| ||
| Antihypertensives | 28% | 51 |
| Statins | 25% | 57 |
| Aspirin | 28% | 77 |
| Smoking cessation | 33% | 43 |
| Carotid endarterectomy | 44% | 26 |
Guidelines for Secondary Stroke Prevention
Several organizations have published guidelines for the prevention of secondary stroke based on clinical evidence and expert consensus. Key guidelines include those published by the American Stroke Association (ASA),2 American College of Chest Physicians (ACCP),27 and the National Stroke Association. Although these guidelines are broadaddressing many components of stroke prevention and careeach contains recommendations specifically applicable to secondary prevention in most stroke patients who the hospitalist will encounter. Some provide hospital‐based guidelines that focus on care protocols and systems processes (ie, ASA Stroke Systems Guidelines), whereas others are therapy‐based guidelines (i.e, ACCP Guidelines on Antithrombotic Therapy for Ischemic Stroke). In the next few sections, we discuss common risk factors for and causes of secondary stroke and the prevailing guideline recommendations for modifying them. Discussion of the management of rare causes of ischemic stroke such as arterial dissection, vasculitis, patent foramen ovale, and so forth is beyond the scope of this article.
Hypertension, Dyslipidemia, and Diabetes
Table 2 shows the current national guideline recommendations for the management of premier vascular risk factorshypertension, dyslipidemia, and diabetesin ischemic stroke and TIA patients.2 Antihypertensive therapy is recommended for the prevention of secondary stroke and other vascular events in patients who have experienced an ischemic stroke or TIA and are beyond the hyperacute period.28, 29 Such treatment should be considered for all ischemic stroke and TIA patients regardless of history of hypertension.28 Although available data support the use of diuretics and the combination of diuretics plus an angiotensin‐converting enzyme inhibitor,28, 30 selection of specific medications should be individualized according to a patient's comorbid conditions.29 It is also important to note that despite the proven benefit of beta blockers in the secondary prevention of recurrent cardiac events, current evidence shows no clear benefit from the use of beta blockers in the prevention of stroke.29, 31
| Risk Factor | Recommendation |
|---|---|
| |
| Hypertension | Antihypertensive beyond hyperacute stroke period60 |
| Data support diuretic or diuretic + ACEI,2830 but individualize based on patient characteristics | |
| Antihypertensive in all patients regardless of history of hypertension28 | |
| Aim for average reduction of 10/5 mm HG or blood pressure 120/80 mm Hg28 | |
| Encourage reduced intake of dietary salt | |
| Dyslipidemia | Statin for LDL‐C goal 100 mg/dL in those with CAD or symptomatic atherosclerosis33, 34 |
| Target LDL‐C 70 mg/dL for very high‐risk persons61 | |
| Statin for stroke or TIA because of atherosclerosis regardless of LDL‐C level33, 34 | |
| Niacin or gemfibrozil for patients with low HDL‐C62, 63 | |
| Diabetes | ACEIs and ARBs should be first‐choice blood pressure drugs37, 38a |
| Glucose control to near normoglycemic levels39 | |
| Target glycosylated hemoglobin 7%64 | |
For ischemic cerebrovascular disease patients with dyslipidemia or symptomatic atherosclerosis, cholesterol management should be according to the current Adult Treatment Panel (ATP) guidelines.32 Statins should be the first‐line treatment.33, 34 Ischemic stroke or TIA patients whose underlying stroke mechanism is presumed to be atherosclerosis should be considered for statin therapy even if they have normal cholesterol levels and no evidence of atherosclerosis.33, 34 The recent Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study was the first study to specifically investigate the effect of statins in patients with a prior stroke but with normal cholesterol levels and no evidence of coronary heart disease. It found that treatment with atorvastatin 80 mg/day (vs. placebo) was associated with a 16% reduction in relative risk of recurrent stroke.34
The care of an ischemic stroke or TIA patient who has diabetes warrants more rigorous control of blood pressure and lipids.35, 36 Such patients usually require more than one antihypertensive drug. ACEIs and angiotensin receptor blockers (ARBs) are more effective in reducing the progression of renal disease and are the recommended first‐choice medications for these patients.37, 38 The target for glucose control should be reaching near‐normoglycemic levels.39
Large‐Artery Atherosclerosis
In selected at‐risk stroke patients, surgical techniques (eg, carotid endarterectomy [CEA], carotid angioplasty and/or stenting [CAS]) may reduce the rate of recurrent stroke.4044 For patients who have had ischemic cerebrovascular events in the preceding 6 months and who have ipsilateral severe (70%‐99%) cervical carotid artery stenosis, CEA done by a surgeon is recommended; it has a perioperative morbidity and mortality of less than 6%.40 For those with ipsilateral moderate (50%‐69%) cervical carotid stenosis, CEA should be considered, and whether to operate should be decided on the basis of the patient's age, sex, comorbidities, and severity of initial symptoms.41 Analyses of endarterectomy trials indicated that the benefit from CEA is greatest if performed within 2 weeks of a patient's last ischemic event, the advantage it confers rapidly falling with increasing delay.45 From the hospitalist's standpoint, it is of prime importance to ensure that patients admitted to the hospital with a TIA or ischemic stroke are not discharged before it has been established whether have severe carotid stenosis that requires a revascularization procedure. If carotid stenosis is less than 50%, CEA is not recommended.41
A newer, less invasive form of carotid artery revascularization is CAS,46 which is performed by operators with established periprocedural morbidity and mortality rates of 4%‐6% and may be considered in those with:
-
Symptomatic severe stenosis (>70%) that is difficult to access surgically.2
-
Medical issues that greatly increase the risks of surgery, such as clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal nerve palsy, radiation‐induced stenosis or restenosis after carotid endarterectomy, and more than 80 years old.43
Angioplasty and/or stenting may also be considered when patients with symptomatic extracranial vertebral stenosis are having symptoms despite optimal medical risk factor treatments.2 Among those with hemodynamically significant stenosis of the major intracranial vasculature (basilar, middle cerebrals, distal carotids, and vertebrals) experiencing symptoms despite optimal medical risk factor treatments, angioplasty and/or stenting is considered experimental.2
The degree of arterial stenosis can be assessed by ultrasound, magnetic resonance angiogram (MRA), computed tomography angiogram (CTA), and conventional catheter angiogram, the last of which remains the gold standard. A carotid ultrasound performed at a certified vascular laboratory or by an experienced radiology technologist that shows less than 50% stenosis need not be followed up with another neuroimaging test. Generally, MRA tends to overestimate the degree of arterial stenosis but is a useful screening tool. In the event that an MRA reveals more than 50% stenosis, another diagnostic modality such as a carotid duplex, CTA, or conventional catheter angiogram should be performed to confirm this finding.
Antithrombotic Treatment
Cardioembolic Stroke Mechanism
Although it can sometimes be difficult to determine the precise mechanism underlying a patient's stroke or TIA, those who have a high‐risk source of cardiogenic embolism should generally be treated with anticoagulant medications to prevent recurrence.2 Among ischemic cerebrovascular event patients with persistent or paroxysmal atrial fibrillation, anticoagulation with adjusted‐dose warfarin (target international normalized ratio [INR] of 2.5; range, 2.0‐3.0) should be administered.47 The ASA recommends initiating oral anticoagulation within 2 weeks of an ischemic stroke or TIA but indicates that further delays may be appropriate for patients with large infarcts or uncontrolled hypertension.2 For patients unable to take oral anticoagulants, aspirin 325 mg/day should be given instead. Among patients who suffered an ischemic stroke or TIA because of an acute myocardial infarction in whom left ventricular mural thrombus is identified by echocardiography or another form of cardiac imaging, oral anticoagulation should be considered, aiming for an INR of 2.0‐3.0 for at least 3 months and up to 1 year.2 Patients receiving oral anticoagulation who also have ischemic coronary artery disease should be prescribed aspirin as well, in doses up to 162 mg/day.2
Noncardioembolic Stroke Mechanism
For ischemic stroke or TIA patients who have no high‐risk source of cardiogenic embolism, antiplatelet agents rather than oral anticoagulation are generally recommended to reduce the risk of recurrent stroke and other cardiovascular events.4850 Acceptable options for initial therapy include:
-
Aspirin (50 to 325 mg/day)48;
-
Combination of aspirin (50 mg) and extended‐release dipyridamole (400 mg) daily49, 51;
-
Clopidogrel (75 mg) daily.50
The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone, and clopidogrel may be considered instead of aspirin alone.49, 51 However, currently there is not enough data to make evidence‐based recommendations for choosing between antiplatelet drugs beyond aspirin.2 Furthermore, there is no evidence that increasing the dose of aspirin for patients who have had an ischemic stroke while taking aspirin provides additional benefit.2 The selection of an antiplatelet agent must be individualized, giving due consideration to a patient's presumed stroke mechanism, risk factor profile, and tolerance.
Other antiplatelet guidelines for noncardioembolic stroke/TIA patients include that:
Education for Behavior Modification
It is crucial to discharge patients with the tools they need to make important lifestyle changes. Patients can significantly reduce their stroke risk by making changes in their everyday patterns of behavior. As much education as possible about smoking cessation, exercise, diet, and the warning signs of stroke should be provided often as possible during hospitalization for a stroke and need not be left to nurses. Stroke education is extremely important so patients understand the need to call for emergency medical services immediately if they even suspect they are having stroke symptoms because of the very narrow window of opportunity for treatment of an acute stroke.54 All patients should be encouraged to make lifestyle adjustments such as ceasing smoking, reducing alcohol intake, and controlling weight. Smoking cessation appears to be effective in preventing secondary stroke (33% reduction in relative risk),44 and initiating smoking cessation counseling during hospitalization for stroke may result in a high rate of adherence to smoking cessation, at least in the short term.55 Table 3 displays current national guideline recommendations on lifestyle modification approaches.2
| Risk Factor | Recommendation |
|---|---|
| |
| Smoking | Smoking cessation |
| Avoid environmental smoke | |
| Counseling, nicotine products, and oral smoking cessation medications | |
| Alcohol | Eliminate or reduce alcohol consumption |
| Light to moderate levels2 drinks/day for men, 1 drink/day for nonpregnant women may be considered | |
| Obesity | Weight reduction goal: BMI 18.5‐24.9 kg/m2 and waist circumference 35 inches for women, 40 inches for men |
| Encourage weight management through balance of caloric intake, physical activity, behavioral counseling | |
| Physical Activity | At least 30 minutes of moderate‐intensity physical exercise most days of the week |
| Supervised therapeutic exercise regimen for those with residual disability | |
EvidencePractice Gap
There are now many secondary stroke prevention modalities, and there is a copious amount of data validating the efficacy of quite a few of them.2 Yet there is a large gap in implementing evidence‐based secondary prevention strategies.35 TIA and ischemic stroke patients are often discharged from the hospital without being prescribed any preventive medications, despite the data supporting the use of antiplatelet agents, anticoagulants, and antihypertensives for prevention of secondary stroke.4 In addition, several behavioral interventions could help patients to avoid stroke recurrence,2 but quite often stroke patients are not educated about them during the acute care period.4 Poor discharge treatment utilization limits the effectiveness of proven therapies, resulting in lost opportunities to reduce the burden of secondary stroke.
The reasons for these care gaps are multifactorial and can be traced to patient and provider issues as well as to health care delivery processes. Our understanding of the reasons for this gap is improving. Generally speaking, preventive services are used less frequently than those services or treatment modalities that provide immediate relief or economic benefit. The benefit of most preventive services is more readily seen at a population level than at a individual level and accrues slowly over time. It becomes more difficult to stress prevention in a health care system driven by technology‐based acute care.3
Current clinical management of acute stroke patients has stroke specialists and hospital physicians focusing on the acute management and diagnostic workup during hospitalization. Initiation of long‐term treatment is often deferred to after discharge, when the patient resumes long‐term primary care follow‐up.54 This deferred approach may result in therapy not being initiated or being initiated less efficiently and at a time (weeks or months after the initial presentation) when the stroke event and underlying atherosclerotic disease may no longer be the focus of either the patient or the primary care physician.54
Initiating medications during the acute stroke hospitalization phase sends the patient the message that these therapies are important for preventing recurrence and are an essential part of their treatment.54 More important, hospital initiation of secondary prevention therapies has been shown to be a strong predictor of these therapies continuing to be used after discharge56 and is associated with better clinical outcomes.5759 Table 4 shows some of the resources available to assist hospitalists in overcoming the evidencepractice gap in stroke treatment.
| Tool | Description |
|---|---|
| |
| AHA Get with the GuidelinesStroke ( |
Focuses on care team protocols to facilitate appropriate in‐hospital and discharge stroke treatment utilization |
| Identifies champions to lead, develop, and mobilize teams to optimally implement evidence‐based stroke treatment in acute care hospitals | |
| Utilizes standardized admission orders, patient educational materials, data monitoring | |
| Provides resources to help hospitals obtain JCAHO certification | |
| UCLA Stroke PROTECT (Preventing Recurrence of Thromboembolic Events through Coordinated Treatment) program ( |
Integrates 8 proven secondary stroke prevention measures into standard stroke care provided during hospitalization |
| Applies quality improvement measures through preprinted admission orders, care maps, discharge protocols, educational materials, patient self‐assessment logs, and data monitoring tools | |
| JCAHO Disease Specific Certification for acute stroke care ( |
Designates eligible hospitals as primary stroke centers |
| Promotes compliance with consensus‐based national standards | |
| Encourages effective use of established clinical practice guidelines to manage and optimize stroke care | |
| Fosters an organized approach to performance measurement and improvement activities | |
CONCLUSIONS
The acute stroke hospitalization setting provides the ideal opportunity for hospitalists to not only institute evidence‐based prevention therapies for recurrent stroke but also to have the undivided attention of patients and their families. Furthermore, it may be risky to assume that relevant therapy when deferred will be initiated in a timely fashion, if at all, after hospital discharge. As part of an effective continuum of care, hospitalists have an important role not just in the management of acute ischemic stroke, but also in long‐term reduction of vascular risk.
Prevention has the greatest potential to reduce the societal burden from stroke.1 Several therapies that specifically target the underlying atherosclerotic disease process have been shown in clinical trials to markedly lower the risk of recurrent vascular events including stroke.2 However, there is great variability in how clinical trial data are implemented in clinical practice for ischemic stroke prevention.35 This has led to a knowledge‐implementation‐practice gap, possibly because of the limited awareness of the scientific evidence supporting various treatments, as well as the lack of a systematic approach to hospital stroke care.3 Our review discusses the evidence for reducing vascular risk after ischemic stroke and successful models of systematic interventions initiated during stroke hospitalization, with the goal of narrowing the stroke hospitalization evidencepractice gap.
Societal Burden
Stroke is the third‐leading cause of death in the United States and the leading cause of serious long‐term disability.6 Approximately 700,000 Americans have a new stroke or recurrent strokes every year, whereas nearly 5 million live with the consequences of stroke; nearly all stroke survivors (90%) have some residual functional deficit, and approximately 40% experience moderate to severe impairment.6 Stroke mortality is substantial, with a 30‐day case fatality rate after first stroke (of any cause) of about 25%.7, 8 Indeed, four‐fifths of patients do not survive for 10 years after stroke, and approximately one‐third of all case fatalities occur in the first year after a stroke.8 The estimated economic impact in 2006, US$57.9 billion, further underscores the substantial mortality and morbidity of stroke.6 Given the limited options for acute stroke therapies,9 stroke prevention remains an important therapeutic goal, especially because fewer than 5% of acute stroke patients in the United States currently receive the only Food and Drug Administrationapproved treatmentintravenous tissue plasminogen activator.10 It is obvious that additional strategies are urgently needed to reduce the devastating consequences of stroke.
Why Involve the Hospitalist?
The Hospitalist system in the United States is rapidly growing.11 Tthe Society of Hospital Medicine projects that by 2010 there will be approximately 30,000 hospitalists in the United States.11 A member census conducted by the American Academy of Neurology in 2000 found 13,500 practicing neurologists, most of whom are concentrated in urban and metropolitan areas.12 As such, with more than 700,000 strokes occurring each year,6 most stroke patients in the United States will not be seen or evaluated by a neurologist. Indeed, one study indicated that only 11.3% of stroke patients are attended exclusively by a neurologist.13 Furthermore, it is not uncommon for stroke patients to have numerous other medical issues that require attention and multidisciplinary care coordination during the hospital stay, an area where hospitalists excel. Conceivably, the ability to promptly identify and treat these non‐neurological comorbidities, which account for at least 30% of the deaths from acute ischemic stroke,14 could go a long way toward improving stroke outcomes.
Hospitalists are in the forefront of developing strategies for improving the quality of acute care and patient satisfaction, reducing medical errors, and focusing on efficient resource utilization. Translating evidence‐based strategies for acute stroke care into actual practice is a mechanism for improving the quality of care, ensuring that basic care does not deviate from provider to provider or from day to day (weekdays compared to weekend days/holidays) while at the same time allowing for the individualization of care appropriate to a patient's unique needs.15 After the acute treatment of stroke or TIA, additional measures must be initiated as soon as it is safe to do so in order to begin the process of limiting stroke progression and preventing recurrence. Secondary prevention measures require a coordinated transition in order to ensure continuation of care and follow‐up as needed. After a thorough risk assessment is complete, hospitalists will need to consider a 3‐pronged approach to secondary prevention that follows the national guidelines described above: pharmacotherapy, behavior modification, and, in some cases, surgical intervention.
Secondary Stroke
Secondary or recurrent strokes are strokes that occur after a first stroke or TIA,2 and the single biggest risk factor for having a stroke is already having had one.2 Because hospitalists generally see patients after ischemic cerebrovascular events have already happened, their opportunities to intervene are mostly geared toward reducing the risk of secondary stroke (beyond enhancing the prevention of complications from the index event). Recent community‐based data indicate that the short‐term risk of secondary stroke is high.16, 17 After a minor stroke or TIA, the risk of recurrent stroke or TIA increases over time8%‐12% within 7 days, 12%‐15% within 30 days, and 17%‐19% within 90 days.18 In the largest study of short‐term risk following TIA,19 there was an 11% risk of stroke (51% of which occurred in the 48 hours after TIA), an 13% risk of TIA, and a 25% risk of any adverse event within 90 days of the TIA.
Overall, the risk of a second cerebrovascular event is highest in the first year after a stroke/TIA (12%), declining to about 5% annually thereafter.7 The effects of secondary stroke are more devastating than those of the primary stroke: the 30‐day fatality rate after a first recurrent stroke is almost double that after the first‐ever stroke (41% versus 22%).20 The pathological factors that lead to TIA and stroke, such as platelet aggregation and subsequent thrombosis or the systolic stroke of blood against stenotic carotid plaques, are one and the same. As such, the short‐ and long‐term risks of recurrent events after both first stroke or first TIA necessitate investigation into a patient's vascular risk and early initiation of appropriate stroke prevention strategies.21
Cross Risk
Because the atherothrombotic disease process is systemic in nature with a variety of manifestations, stroke patients with atherosclerosis frequently have coexistent coronary artery disease and peripheral artery disease,22 and as such, are at risk for vascular events emanating from any of these beds in addition to that of the cervicocephalic arterial tree.23, 24 For instance, in a study of individuals in a long‐term care facility, among the patients with ischemic stroke, 56% had overlapping coronary artery disease, 28% had peripheral artery disease,25 and 38% of the patients had at least 2 manifestations of their atherosclerotic disease. The take‐home message here is that hospitalists also have the opportunity while treating patients hospitalized following stroke to prevent other vascular events by identifying and treating stroke patients who have systemic atherosclerosis.
Risk Factors
The first step in any approach to stroke prevention is the identification of predisposing risk factors. Several of the known biological and lifestyle risk factors associated with cerebrovascular disease were identified decades ago from large longitudinal studies.2 Certain stroke risk factors are nonmodifiable and therefore cannot be the target of intervention. 26 Treatment of the various stroke risk factors could have a substantial impact on reducing the burden of stroke. Table 1 shows the number needed to treat to prevent one stroke per year by modification of the individual stroke risk factor.
| Treatment | Relative risk reduction | Number needed to treat (1 stroke/year) |
|---|---|---|
| ||
| Antihypertensives | 28% | 51 |
| Statins | 25% | 57 |
| Aspirin | 28% | 77 |
| Smoking cessation | 33% | 43 |
| Carotid endarterectomy | 44% | 26 |
Guidelines for Secondary Stroke Prevention
Several organizations have published guidelines for the prevention of secondary stroke based on clinical evidence and expert consensus. Key guidelines include those published by the American Stroke Association (ASA),2 American College of Chest Physicians (ACCP),27 and the National Stroke Association. Although these guidelines are broadaddressing many components of stroke prevention and careeach contains recommendations specifically applicable to secondary prevention in most stroke patients who the hospitalist will encounter. Some provide hospital‐based guidelines that focus on care protocols and systems processes (ie, ASA Stroke Systems Guidelines), whereas others are therapy‐based guidelines (i.e, ACCP Guidelines on Antithrombotic Therapy for Ischemic Stroke). In the next few sections, we discuss common risk factors for and causes of secondary stroke and the prevailing guideline recommendations for modifying them. Discussion of the management of rare causes of ischemic stroke such as arterial dissection, vasculitis, patent foramen ovale, and so forth is beyond the scope of this article.
Hypertension, Dyslipidemia, and Diabetes
Table 2 shows the current national guideline recommendations for the management of premier vascular risk factorshypertension, dyslipidemia, and diabetesin ischemic stroke and TIA patients.2 Antihypertensive therapy is recommended for the prevention of secondary stroke and other vascular events in patients who have experienced an ischemic stroke or TIA and are beyond the hyperacute period.28, 29 Such treatment should be considered for all ischemic stroke and TIA patients regardless of history of hypertension.28 Although available data support the use of diuretics and the combination of diuretics plus an angiotensin‐converting enzyme inhibitor,28, 30 selection of specific medications should be individualized according to a patient's comorbid conditions.29 It is also important to note that despite the proven benefit of beta blockers in the secondary prevention of recurrent cardiac events, current evidence shows no clear benefit from the use of beta blockers in the prevention of stroke.29, 31
| Risk Factor | Recommendation |
|---|---|
| |
| Hypertension | Antihypertensive beyond hyperacute stroke period60 |
| Data support diuretic or diuretic + ACEI,2830 but individualize based on patient characteristics | |
| Antihypertensive in all patients regardless of history of hypertension28 | |
| Aim for average reduction of 10/5 mm HG or blood pressure 120/80 mm Hg28 | |
| Encourage reduced intake of dietary salt | |
| Dyslipidemia | Statin for LDL‐C goal 100 mg/dL in those with CAD or symptomatic atherosclerosis33, 34 |
| Target LDL‐C 70 mg/dL for very high‐risk persons61 | |
| Statin for stroke or TIA because of atherosclerosis regardless of LDL‐C level33, 34 | |
| Niacin or gemfibrozil for patients with low HDL‐C62, 63 | |
| Diabetes | ACEIs and ARBs should be first‐choice blood pressure drugs37, 38a |
| Glucose control to near normoglycemic levels39 | |
| Target glycosylated hemoglobin 7%64 | |
For ischemic cerebrovascular disease patients with dyslipidemia or symptomatic atherosclerosis, cholesterol management should be according to the current Adult Treatment Panel (ATP) guidelines.32 Statins should be the first‐line treatment.33, 34 Ischemic stroke or TIA patients whose underlying stroke mechanism is presumed to be atherosclerosis should be considered for statin therapy even if they have normal cholesterol levels and no evidence of atherosclerosis.33, 34 The recent Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study was the first study to specifically investigate the effect of statins in patients with a prior stroke but with normal cholesterol levels and no evidence of coronary heart disease. It found that treatment with atorvastatin 80 mg/day (vs. placebo) was associated with a 16% reduction in relative risk of recurrent stroke.34
The care of an ischemic stroke or TIA patient who has diabetes warrants more rigorous control of blood pressure and lipids.35, 36 Such patients usually require more than one antihypertensive drug. ACEIs and angiotensin receptor blockers (ARBs) are more effective in reducing the progression of renal disease and are the recommended first‐choice medications for these patients.37, 38 The target for glucose control should be reaching near‐normoglycemic levels.39
Large‐Artery Atherosclerosis
In selected at‐risk stroke patients, surgical techniques (eg, carotid endarterectomy [CEA], carotid angioplasty and/or stenting [CAS]) may reduce the rate of recurrent stroke.4044 For patients who have had ischemic cerebrovascular events in the preceding 6 months and who have ipsilateral severe (70%‐99%) cervical carotid artery stenosis, CEA done by a surgeon is recommended; it has a perioperative morbidity and mortality of less than 6%.40 For those with ipsilateral moderate (50%‐69%) cervical carotid stenosis, CEA should be considered, and whether to operate should be decided on the basis of the patient's age, sex, comorbidities, and severity of initial symptoms.41 Analyses of endarterectomy trials indicated that the benefit from CEA is greatest if performed within 2 weeks of a patient's last ischemic event, the advantage it confers rapidly falling with increasing delay.45 From the hospitalist's standpoint, it is of prime importance to ensure that patients admitted to the hospital with a TIA or ischemic stroke are not discharged before it has been established whether have severe carotid stenosis that requires a revascularization procedure. If carotid stenosis is less than 50%, CEA is not recommended.41
A newer, less invasive form of carotid artery revascularization is CAS,46 which is performed by operators with established periprocedural morbidity and mortality rates of 4%‐6% and may be considered in those with:
-
Symptomatic severe stenosis (>70%) that is difficult to access surgically.2
-
Medical issues that greatly increase the risks of surgery, such as clinically significant cardiac disease, severe pulmonary disease, contralateral carotid occlusion, contralateral laryngeal nerve palsy, radiation‐induced stenosis or restenosis after carotid endarterectomy, and more than 80 years old.43
Angioplasty and/or stenting may also be considered when patients with symptomatic extracranial vertebral stenosis are having symptoms despite optimal medical risk factor treatments.2 Among those with hemodynamically significant stenosis of the major intracranial vasculature (basilar, middle cerebrals, distal carotids, and vertebrals) experiencing symptoms despite optimal medical risk factor treatments, angioplasty and/or stenting is considered experimental.2
The degree of arterial stenosis can be assessed by ultrasound, magnetic resonance angiogram (MRA), computed tomography angiogram (CTA), and conventional catheter angiogram, the last of which remains the gold standard. A carotid ultrasound performed at a certified vascular laboratory or by an experienced radiology technologist that shows less than 50% stenosis need not be followed up with another neuroimaging test. Generally, MRA tends to overestimate the degree of arterial stenosis but is a useful screening tool. In the event that an MRA reveals more than 50% stenosis, another diagnostic modality such as a carotid duplex, CTA, or conventional catheter angiogram should be performed to confirm this finding.
Antithrombotic Treatment
Cardioembolic Stroke Mechanism
Although it can sometimes be difficult to determine the precise mechanism underlying a patient's stroke or TIA, those who have a high‐risk source of cardiogenic embolism should generally be treated with anticoagulant medications to prevent recurrence.2 Among ischemic cerebrovascular event patients with persistent or paroxysmal atrial fibrillation, anticoagulation with adjusted‐dose warfarin (target international normalized ratio [INR] of 2.5; range, 2.0‐3.0) should be administered.47 The ASA recommends initiating oral anticoagulation within 2 weeks of an ischemic stroke or TIA but indicates that further delays may be appropriate for patients with large infarcts or uncontrolled hypertension.2 For patients unable to take oral anticoagulants, aspirin 325 mg/day should be given instead. Among patients who suffered an ischemic stroke or TIA because of an acute myocardial infarction in whom left ventricular mural thrombus is identified by echocardiography or another form of cardiac imaging, oral anticoagulation should be considered, aiming for an INR of 2.0‐3.0 for at least 3 months and up to 1 year.2 Patients receiving oral anticoagulation who also have ischemic coronary artery disease should be prescribed aspirin as well, in doses up to 162 mg/day.2
Noncardioembolic Stroke Mechanism
For ischemic stroke or TIA patients who have no high‐risk source of cardiogenic embolism, antiplatelet agents rather than oral anticoagulation are generally recommended to reduce the risk of recurrent stroke and other cardiovascular events.4850 Acceptable options for initial therapy include:
-
Aspirin (50 to 325 mg/day)48;
-
Combination of aspirin (50 mg) and extended‐release dipyridamole (400 mg) daily49, 51;
-
Clopidogrel (75 mg) daily.50
The combination of aspirin and extended‐release dipyridamole is suggested instead of aspirin alone, and clopidogrel may be considered instead of aspirin alone.49, 51 However, currently there is not enough data to make evidence‐based recommendations for choosing between antiplatelet drugs beyond aspirin.2 Furthermore, there is no evidence that increasing the dose of aspirin for patients who have had an ischemic stroke while taking aspirin provides additional benefit.2 The selection of an antiplatelet agent must be individualized, giving due consideration to a patient's presumed stroke mechanism, risk factor profile, and tolerance.
Other antiplatelet guidelines for noncardioembolic stroke/TIA patients include that:
Education for Behavior Modification
It is crucial to discharge patients with the tools they need to make important lifestyle changes. Patients can significantly reduce their stroke risk by making changes in their everyday patterns of behavior. As much education as possible about smoking cessation, exercise, diet, and the warning signs of stroke should be provided often as possible during hospitalization for a stroke and need not be left to nurses. Stroke education is extremely important so patients understand the need to call for emergency medical services immediately if they even suspect they are having stroke symptoms because of the very narrow window of opportunity for treatment of an acute stroke.54 All patients should be encouraged to make lifestyle adjustments such as ceasing smoking, reducing alcohol intake, and controlling weight. Smoking cessation appears to be effective in preventing secondary stroke (33% reduction in relative risk),44 and initiating smoking cessation counseling during hospitalization for stroke may result in a high rate of adherence to smoking cessation, at least in the short term.55 Table 3 displays current national guideline recommendations on lifestyle modification approaches.2
| Risk Factor | Recommendation |
|---|---|
| |
| Smoking | Smoking cessation |
| Avoid environmental smoke | |
| Counseling, nicotine products, and oral smoking cessation medications | |
| Alcohol | Eliminate or reduce alcohol consumption |
| Light to moderate levels2 drinks/day for men, 1 drink/day for nonpregnant women may be considered | |
| Obesity | Weight reduction goal: BMI 18.5‐24.9 kg/m2 and waist circumference 35 inches for women, 40 inches for men |
| Encourage weight management through balance of caloric intake, physical activity, behavioral counseling | |
| Physical Activity | At least 30 minutes of moderate‐intensity physical exercise most days of the week |
| Supervised therapeutic exercise regimen for those with residual disability | |
EvidencePractice Gap
There are now many secondary stroke prevention modalities, and there is a copious amount of data validating the efficacy of quite a few of them.2 Yet there is a large gap in implementing evidence‐based secondary prevention strategies.35 TIA and ischemic stroke patients are often discharged from the hospital without being prescribed any preventive medications, despite the data supporting the use of antiplatelet agents, anticoagulants, and antihypertensives for prevention of secondary stroke.4 In addition, several behavioral interventions could help patients to avoid stroke recurrence,2 but quite often stroke patients are not educated about them during the acute care period.4 Poor discharge treatment utilization limits the effectiveness of proven therapies, resulting in lost opportunities to reduce the burden of secondary stroke.
The reasons for these care gaps are multifactorial and can be traced to patient and provider issues as well as to health care delivery processes. Our understanding of the reasons for this gap is improving. Generally speaking, preventive services are used less frequently than those services or treatment modalities that provide immediate relief or economic benefit. The benefit of most preventive services is more readily seen at a population level than at a individual level and accrues slowly over time. It becomes more difficult to stress prevention in a health care system driven by technology‐based acute care.3
Current clinical management of acute stroke patients has stroke specialists and hospital physicians focusing on the acute management and diagnostic workup during hospitalization. Initiation of long‐term treatment is often deferred to after discharge, when the patient resumes long‐term primary care follow‐up.54 This deferred approach may result in therapy not being initiated or being initiated less efficiently and at a time (weeks or months after the initial presentation) when the stroke event and underlying atherosclerotic disease may no longer be the focus of either the patient or the primary care physician.54
Initiating medications during the acute stroke hospitalization phase sends the patient the message that these therapies are important for preventing recurrence and are an essential part of their treatment.54 More important, hospital initiation of secondary prevention therapies has been shown to be a strong predictor of these therapies continuing to be used after discharge56 and is associated with better clinical outcomes.5759 Table 4 shows some of the resources available to assist hospitalists in overcoming the evidencepractice gap in stroke treatment.
| Tool | Description |
|---|---|
| |
| AHA Get with the GuidelinesStroke ( |
Focuses on care team protocols to facilitate appropriate in‐hospital and discharge stroke treatment utilization |
| Identifies champions to lead, develop, and mobilize teams to optimally implement evidence‐based stroke treatment in acute care hospitals | |
| Utilizes standardized admission orders, patient educational materials, data monitoring | |
| Provides resources to help hospitals obtain JCAHO certification | |
| UCLA Stroke PROTECT (Preventing Recurrence of Thromboembolic Events through Coordinated Treatment) program ( |
Integrates 8 proven secondary stroke prevention measures into standard stroke care provided during hospitalization |
| Applies quality improvement measures through preprinted admission orders, care maps, discharge protocols, educational materials, patient self‐assessment logs, and data monitoring tools | |
| JCAHO Disease Specific Certification for acute stroke care ( |
Designates eligible hospitals as primary stroke centers |
| Promotes compliance with consensus‐based national standards | |
| Encourages effective use of established clinical practice guidelines to manage and optimize stroke care | |
| Fosters an organized approach to performance measurement and improvement activities | |
CONCLUSIONS
The acute stroke hospitalization setting provides the ideal opportunity for hospitalists to not only institute evidence‐based prevention therapies for recurrent stroke but also to have the undivided attention of patients and their families. Furthermore, it may be risky to assume that relevant therapy when deferred will be initiated in a timely fashion, if at all, after hospital discharge. As part of an effective continuum of care, hospitalists have an important role not just in the management of acute ischemic stroke, but also in long‐term reduction of vascular risk.
- .Stroke prevention: windows of opportunity and failed expectations? A discussion of modifiable cardiovascular risk factors and a prevention proposal.Neuroepidemiology.1997;16(4):163–173.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Stroke.2006;37:577–617.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.,.Lipid Assessment and treatment patterns in hospitalized TIA and ischemic stroke patients.J Hosp Med.2006;1:214–220.
- ,,, et al.Heart disease and stroke statistics—2006 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2006;113(6):e85–e151.
- .Long‐term outcome after ischaemic stroke/transient ischaemic attack.Cerebrovasc Dis.2003;16(Suppl 1):14–19.
- ,,,,.Ten‐year survival after first‐ever stroke in the perth community stroke study.Stroke.2003;34:1842–1846.
- NINDS rt‐PA Stroke Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,,,,.Assessing patterns of t‐PA use in acute stroke.Stroke.2002;33:354.
- Growth of hospital medicine nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_ Hospital_M.htm. Accessed April 12,2006.
- American Academy of Neurology. Available at: http://www.aan.com/students/medical/faq.cfm. Accessed June 25,2006.
- .The neurologist's role in stroke management.Stroke.1996;27:1935–1936.
- .Prevention and management of medical complications of the hospitalized elderly stroke patient.Clin Geriatr Med.1991;7:475–482.
- ,,, et al.The Stroke Prevention Policy Model: linking evidence and clinical decisions.Ann Intern Med.1997;127:704–711.
- ,,.Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services.BMJ.2004;328:326.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,.Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services.BMJ.2004;328:326.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .New strategies for prevention of ischemic stroke: the LIFE study.Curr Neurol Neurosci Rep.2003;3(1):46–51.
- ,,, et al.[Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis].Med Klin.2002;97(4):221–228.
- ,,,,,.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134(3):224–238.
- .Prevention of strokes and recurrent strokes.J Neurol Neurosurg Psychiatry.1998;64:716.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,.Risk factors and their management for stroke prevention: outlook for 1999 and beyond.Neurology.1999;53(7 Suppl 4):S15–S24.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest. Sep2004;126(3 Suppl):483S–512S.
- Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- Post‐stroke antihypertensive treatment study. A preliminary result.PATS Collaborating Group.Chin Med J (Engl).1995;108:710–717.
- ,,.Should beta blockers remain first choice in the treatment of primary hypertension? A meta‐analysis.Lancet.2005;366:1545–1553.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines.Circulation.2004;110(2):227–239.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with Simvastatin on stroke and other major vascular events in 20, 536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- , et al.The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study. Presented at the 15th European Stroke Conference, Brussels, Belgium, May 16‐19,2006.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38: UK Prospective Diabetes Study Group.BMJ.1998;317:703–713.
- ,,,,, for theHeart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial.Lancet.2003;361:2005–2016.
- ,,,.The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy: the Collaborative Study Group.N Engl J Med.1993;329:1456–1462.
- ,,, for theCollaborative Study Group.Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy.N Engl J Med.2001;345:861–869.
- ,,., et al.Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study.Diabetes Res Clin Pract.1995;28:103–117.
- Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis.North American Symptomatic Carotid Endarterectomy Trial Collaborators.N Engl J Med.1991;325:445–453.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.North American Symptomatic Carotid Endarterectomy Trial Collaborators.N Engl J Med.1998;339:1415–1425.
- Endarterectomy for asymptomatic carotid artery stenosis.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study.JAMA.1995;273:1421–1428.
- ,,, et al.Protected carotid‐artery stenting versus endarterectomy in high‐risk patients.N Engl J Med.2004;351:1493–1501.
- ,,.New evidence for stroke prevention: scientific review.JAMA.2002;288:1388–1395.
- ,,,,.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363:915–924.
- .Carotid artery surgery vs. stent: a cardiovascular perspective.Catheter Cardiovasc Interv.2004;63:377–384.
- ,,, et al.Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis.Chest.2004;126:1938–1945.
- Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143(1‐2):1–13.
- A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).CAPRIE Steering Committee.Lancet.1996;348:1329–1339.
- ,,,,.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med. Apr 202006;354(16):1706–1717.
- ,,, et al.PROTECT: A Coordinated Stroke Treatment Program to Prevent Recurrent Thromboembolic Events.Neurology. Vol63;2004:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow up.Stroke.2004;35:2879–2883.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- ,,, et al.Early effects of statins in patients with coronary artery disease and high C‐reactive protein.Am J Cardiol.2004;94:1107–1112.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol. Apr 12001;87(7):819–822.
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review.Am J Cardiol.2004;94:1155–1160.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:1024.
- ,,, et al.Intensive versus moderate lipid lowering with statins after acute coronary syndromes.N Engl J Med.2004;350:1495–1504.
- ,,.Niacin therapy in atherosclerosis.Curr Opin Lipidol.2004;15:659–665.
- ,,, et al.Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA‐HIT).Circulation.2001;103:2828–2833.
- ,,.The effect of long‐term intensified insulin treatment on the development of microvascular complications of diabetes mellitus.N Engl J Med.1993;329:304–309.
- .Stroke prevention: windows of opportunity and failed expectations? A discussion of modifiable cardiovascular risk factors and a prevention proposal.Neuroepidemiology.1997;16(4):163–173.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Stroke.2006;37:577–617.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.,.Lipid Assessment and treatment patterns in hospitalized TIA and ischemic stroke patients.J Hosp Med.2006;1:214–220.
- ,,, et al.Heart disease and stroke statistics—2006 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2006;113(6):e85–e151.
- .Long‐term outcome after ischaemic stroke/transient ischaemic attack.Cerebrovasc Dis.2003;16(Suppl 1):14–19.
- ,,,,.Ten‐year survival after first‐ever stroke in the perth community stroke study.Stroke.2003;34:1842–1846.
- NINDS rt‐PA Stroke Group.Tissue plasminogen activator for acute ischemic stroke.N Engl J Med.1995;333:1581–1587.
- ,,,,,.Assessing patterns of t‐PA use in acute stroke.Stroke.2002;33:354.
- Growth of hospital medicine nationwide. Available at: http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNationwide/Growth_of_ Hospital_M.htm. Accessed April 12,2006.
- American Academy of Neurology. Available at: http://www.aan.com/students/medical/faq.cfm. Accessed June 25,2006.
- .The neurologist's role in stroke management.Stroke.1996;27:1935–1936.
- .Prevention and management of medical complications of the hospitalized elderly stroke patient.Clin Geriatr Med.1991;7:475–482.
- ,,, et al.The Stroke Prevention Policy Model: linking evidence and clinical decisions.Ann Intern Med.1997;127:704–711.
- ,,.Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services.BMJ.2004;328:326.
- ,,, et al.Incidence and short‐term prognosis of transient ischemic attack in a population‐based study.Stroke.2005;36:720–723.
- ,,.Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services.BMJ.2004;328:326.
- ,,,.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,,,.Ten‐year risk of first recurrent stroke and disability after first‐ever stroke in the Perth Community Stroke Study.Stroke.2004;35:731–735.
- .New strategies for prevention of ischemic stroke: the LIFE study.Curr Neurol Neurosci Rep.2003;3(1):46–51.
- ,,, et al.[Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis].Med Klin.2002;97(4):221–228.
- ,,,,,.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134(3):224–238.
- .Prevention of strokes and recurrent strokes.J Neurol Neurosurg Psychiatry.1998;64:716.
- ,.Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital‐based geriatrics practice.J Am Geriatr Soc.1999;47:1255–1256.
- ,,.Risk factors and their management for stroke prevention: outlook for 1999 and beyond.Neurology.1999;53(7 Suppl 4):S15–S24.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest. Sep2004;126(3 Suppl):483S–512S.
- Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.Lancet.2001;358:1033–1041.
- ,,.Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review.Stroke.2003;34:2741–2748.
- Post‐stroke antihypertensive treatment study. A preliminary result.PATS Collaborating Group.Chin Med J (Engl).1995;108:710–717.
- ,,.Should beta blockers remain first choice in the treatment of primary hypertension? A meta‐analysis.Lancet.2005;366:1545–1553.
- ,,, et al.Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines.Circulation.2004;110(2):227–239.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with Simvastatin on stroke and other major vascular events in 20, 536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- , et al.The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study. Presented at the 15th European Stroke Conference, Brussels, Belgium, May 16‐19,2006.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38: UK Prospective Diabetes Study Group.BMJ.1998;317:703–713.
- ,,,,, for theHeart Protection Study Collaborative Group.MRC/BHF Heart Protection Study of cholesterol‐lowering with simvastatin in 5963 people with diabetes: a randomised placebo‐controlled trial.Lancet.2003;361:2005–2016.
- ,,,.The effect of angiotensin‐converting‐enzyme inhibition on diabetic nephropathy: the Collaborative Study Group.N Engl J Med.1993;329:1456–1462.
- ,,, for theCollaborative Study Group.Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy.N Engl J Med.2001;345:861–869.
- ,,., et al.Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study.Diabetes Res Clin Pract.1995;28:103–117.
- Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis.North American Symptomatic Carotid Endarterectomy Trial Collaborators.N Engl J Med.1991;325:445–453.
- ,,, et al.Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis.North American Symptomatic Carotid Endarterectomy Trial Collaborators.N Engl J Med.1998;339:1415–1425.
- Endarterectomy for asymptomatic carotid artery stenosis.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study.JAMA.1995;273:1421–1428.
- ,,, et al.Protected carotid‐artery stenting versus endarterectomy in high‐risk patients.N Engl J Med.2004;351:1493–1501.
- ,,.New evidence for stroke prevention: scientific review.JAMA.2002;288:1388–1395.
- ,,,,.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363:915–924.
- .Carotid artery surgery vs. stent: a cardiovascular perspective.Catheter Cardiovasc Interv.2004;63:377–384.
- ,,, et al.Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis.Chest.2004;126:1938–1945.
- Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324:71–86.
- ,,,,,.European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke.J Neurol Sci.1996;143(1‐2):1–13.
- A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).CAPRIE Steering Committee.Lancet.1996;348:1329–1339.
- ,,,,.Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial.Lancet.2006;367:1665–1673.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364:331–337.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med. Apr 202006;354(16):1706–1717.
- ,,, et al.PROTECT: A Coordinated Stroke Treatment Program to Prevent Recurrent Thromboembolic Events.Neurology. Vol63;2004:1217–1222.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow up.Stroke.2004;35:2879–2883.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- ,,, et al.Early effects of statins in patients with coronary artery disease and high C‐reactive protein.Am J Cardiol.2004;94:1107–1112.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol. Apr 12001;87(7):819–822.
- ,,.Importance of in‐hospital initiation of evidence‐based medical therapies for heart failure‐a review.Am J Cardiol.2004;94:1155–1160.
- ,,,.Blood pressure and stroke: an overview of published reviews.Stroke.2004;35:1024.
- ,,, et al.Intensive versus moderate lipid lowering with statins after acute coronary syndromes.N Engl J Med.2004;350:1495–1504.
- ,,.Niacin therapy in atherosclerosis.Curr Opin Lipidol.2004;15:659–665.
- ,,, et al.Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA‐HIT).Circulation.2001;103:2828–2833.
- ,,.The effect of long‐term intensified insulin treatment on the development of microvascular complications of diabetes mellitus.N Engl J Med.1993;329:304–309.
Lipid Management during Stroke Hospitalization
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).
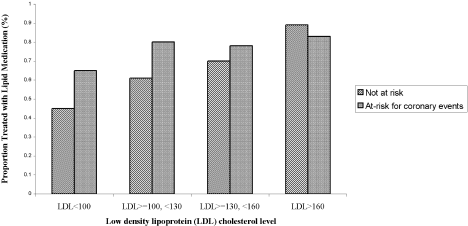
DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).

DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).

DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
Copyright © 2006 Society of Hospital Medicine