User login
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
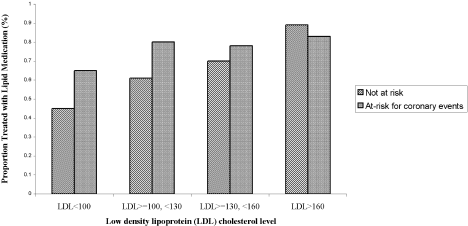
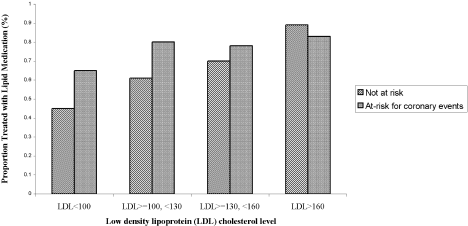
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).
DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).

DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
Aortocervicocephalic atherosclerotic disease and coronary artery disease share common risk factors, and patients with one condition are at high risk of harboring or developing the other.1, 2 Over the past decade, several randomized clinical trials of lipid‐lowering medications designed to reduce low‐density lipoprotein cholesterol (LDL‐C) have shown a significant decrease in the risk of coronary events and ischemic stroke among patients who have a history of or are at risk for coronary artery disease, regardless of whether serum cholesterol is elevated.3, 4 Results from more than 3000 stroke patients enrolled in the Heart Protection Study also provide evidence that aggressive lipid‐lowering therapy may prevent recurrent vascular events in individuals who have a total cholesterol level as low as 135 mg/dL and cerebrovascular disease, with or without known coronary artery disease.5
Guidelines from the National Cholesterol Evaluation Program Adult Treatment Panel (ATP) provide target LDL‐C levels for persons with atherosclerotic disease depending on the extent of their vascular risk.6 However, despite the broad dissemination of these guidelines, several published studies of patients with coronary artery disease or dyslipidemia have shown that a large proportion of patients with high vascular risk continue to be underscreened, underdiagnosed, and undertreated for dyslipidemia.79
Few studies have evaluated the quality of cholesterol management among hospitalized patients who have experienced an acute ischemic cerebrovascular event10, 11 So the data are scarce on the management of patients hospitalized for ischemic stroke or transient ischemic attack (TIA) who are, according to ATP criteria, at high risk for future coronary events and on the factors that may govern that management. Systematic reviews have suggested that incorporating a lipid profile during acute stroke presentation could assure baseline assessment and serve as a potential cue for physicians to change their behavior,12 and an American Stroke Association advisory recommends lipid treatment during hospitalization for most patients with ischemic stroke or TIA as it may increase the rate of long‐term use.13
The objectives of this study were to determine the rates of testing for and treatment of dyslipidemia according to national cholesterol guidelines among individuals hospitalized with acute ischemic stroke or TIA and to identify predictors of performance.
METHODS
The California Acute Stroke Prototype Registry (CASPR) is a Centers for Disease Controlsponsored cohort that captured detailed data on patients admitted to 11 hospitals over a 2‐year period. The methods of study have been described elsewhere.14 In brief, CASPR prospectively collected information on acute stroke care at 11 representative hospitals in 5 major population regions of California. Data were collected on diagnostic evaluation, appropriate use of treatment strategies, and disposition on discharge from the hospital. The main goal of CASPR was to pilot‐test a prototype prospective registry of acute stroke and transient ischemic attack to be used as a quality improvement tool. The study population was patients with an admitting or discharge diagnosis of suspected stroke or TIA from November 1, 2002, through January 31, 2003, and from November 1, 2003, through January 31, 2004. The human subjects review board at each participating center approved the study.
For the present analysis, data on all patients with a discharge diagnosis of ischemic stroke or TIA who were admitted during either period were included. We examined the possible association of several variables with 2 primary outcomes: (1) testing lipid profile during hospitalization (as indicated by a documented LDL‐C level) and (2) prescribing lipid‐lowering medication at discharge. In those analyses in which lipid profile testing was the outcome, no variables were considered acceptable reasons for not performing an LDL‐C assessment.
The distribution of LDL‐C levels in this portion of the cohort was determined. Patients were then categorized according to their risk for future coronary events. Patients were classified as at risk for coronary events (ACE) if they either had a documented history of myocardial infarction, coronary artery disease, or diabetes or had undergone carotid endarterectomy or carotid angioplasty/stenting during hospitalization. Criteria for initiating lipid‐lowering therapy were defined according to the ATP III guidelines,6 which were in effect during both CASPR study periods. Continuing the recommendation in ATP II, the ATP III recommendations emphasized that persons with documented coronary artery disease (CAD) receive the most aggressive lipid‐lowering treatment. But this recommendation was expanded to include patients without established CAD, whose coronary risk is equivalent to that of patients with diagnosed CAD.6
As per the ATP III guidelines, CASPR‐ACE patients were considered optimally treated if they were prescribed a lipid‐lowering agent at discharge or if their documented LDL‐C was less than 130 mg/dL. A concurrent history of liver disease, abnormal prothrombin time, life expectancy of less than 1 year, and terminal illness were each considered a valid contraindication to treatment with lipid‐lowering medication. Optimal treatment for non‐ACE patients was defined as receipt of lipid‐lowering medication at discharge or a documented LDL‐C of 160 mg/dL. The rate of optimal treatment of ACE patients was compared to that of non‐ACE patients. The ACE and non‐ACE patients were then further categorized into 1 of 4 groups according to LDL‐C level<100, 100130, 130160, and >160 mg/dLand an assessment for trend of the rate of treatment in each of the 4 categories in the ACE and non‐ACE groups was performed.
Data Analysis
Univariate analyses of potential risk factors with lipid testing and treatment were performed using generalized estimating equations (GEEs) in order to account for both within‐hospital and between‐hospital variance and to acknowledge the impact of clustered observations on confidence intervals. Variables significant at the = .10 level were included in the multivariate models. In the subanalyses of patients with documented LDL‐C tests, GEE models were also used to examine factors associated with having an LDL‐C level below 100 mg/dL. A chi‐square test was used to compare the rate of optimal treatment (as defined above) in the group at risk for coronary disease with that in the group not at risk. The Mantel‐Haenszel chi‐squared test was used to compare trends in treatment rate with increasing LDL‐C level. All analyses were performed using SAS (version 8e, SAS Institute, Cary, NC).
RESULTS
Data were available from the 11 CASPR hospitals for 764 patients diagnosed with either ischemic stroke or TIA. Overall, 53.4% of subjects were women, and the average age at hospitalization of 70.4 ( 15.4) years. In the cohort, 55.3% of the patients were non‐Hispanic white, 9.7% were African American, 13.4% were Hispanic, 13% were Asian, and 8.6% were classified as other. Three hundred and nine individuals (40.5% of the cohort) were classified as at risk for coronary events. Of these, 148 (47.8%) had diabetes only, and 160 (51.8%) had a history of MI, CAD, or both. One patient (0.4%) had undergone angioplasty/stenting during hospitalization but had no history of MI, CAD, or diabetes. Only 4 patients (0.52% of the entire cohort) had undergone a carotid endarterectomy or angioplasty/stenting during hospitalization. Rates of lipid assessment and optimal treatment varied widely between hospitals, but testing and treatment were correlated for each hospital. Overall, however, testing and treatment were correlated (Pearson correlation coefficient = 0.35, P < .0001). On an individual hospital level, the correlation was positive and significant for 6 hospitals, positive but not significant for 2 hospitals, and negative but not significant for 3 hospitals.
Overall, LDL‐C levels were determined in 383 patients (50.1%). The likelihood that a patient would have an LDL‐C test performed during hospitalization varied widely by hospital, ranging from 12% to 88% (P < .0001). Univariate variables significantly associated with documented LDL‐C measurement in the overall cohort at the = .10 level were diagnosis of ischemic stroke (as compared to TIA) and history of dyslipidemia (Table 1). In the CASPR cohort, 53% of the ACE subjects received a lipid profile assessment compared to 48% in the rest of the cohort (P = .14). In multivariate analysis, diagnosis of ischemic stroke and history of dyslipidemia remained significantly associated with documented LDL‐C measurement (Table 1).
| Characteristic | n | With LDL‐C | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 210 | (54.5) | Ref | |||||
| > 73 years | 379 | 173 | (45.6) | 0.95 | (0.68, 1.34) | .78 | |||
| Sex | |||||||||
| Female | 408 | 189 | (46.3) | Ref | |||||
| Male | 356 | 194 | (54.5) | 1.05 | (0.84, 1.39) | .53 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (56.3) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.60, 1.30) | .53 | |||
| Event type | |||||||||
| TIA | 172 | 62 | (36) | Ref | Ref | ||||
| Ischemic stroke | 592 | 321 | (54) | 1.70 | (1.14, 2.54) | .01 | 1.52 | (1.06, 2.19) | .02 |
| Risk of coronary events | 309 | 165 | (53.4) | 1.14 | (0.78, 1.68) | .50 | |||
| History of:b | |||||||||
| Stroke/TIA | 277 | 122 | (44.0) | 0.85 | (0.58, 1.24) | .39 | |||
| Dyslipidemia | 67 | 32 | (47.8) | 0.94 | (0.47, 1.90) | .86 | |||
| MI | 132 | 63 | (47.7) | 0.84 | (0.65, 1.08) | .17 | |||
| CAD | 158 | 96 | (60.8) | 0.95 | (0.67, 1.34) | .76 | |||
| Smoking | 83 | 31 | (37.3) | 0.67 | (0.40, 1.10) | .12 | |||
| Heart failure | 199 | 109 | (54.8) | 1.13 | (0.74, 1.73) | .58 | |||
| Diabetes | 516 | 259 | (50.2) | 1.09 | (0.83, 1.44) | .54 | |||
| Hypertension | 243 | 140 | (57.6) | 1.45 | (0.98, 2.14) | .07 | 1.41 | (1.01, 1.97) | .05 |
| Atrial fibrillation | 125 | 56 | (44.8) | 0.95 | (0.69, 1.32) | .76 | |||
| Received tPA | |||||||||
| No | 748 | 371 | (49.6) | Ref | |||||
| Yes | 16 | 12 | (75.0) | 2.01 | (0.79, 5.11) | .14 | |||
Lipid‐lowering drugs were prescribed at discharge to 370 patients (48.4%); however, treatment rate varied among hospitals, from a low of 13% of patients to a high of 84% of patients (P < .0001). Univariate factors associated with a higher treatment rate at the = .10 level were diagnosis of ischemic stroke, history of stroke/TIA, history of diabetes, hypertension, history of dyslipidemia, independent ambulation at discharge, and ACE status (Table 2). Patients were less likely to receive lipid‐lowering medication if they had a history of heart failure. Fifty‐nine percent of the CASPR ACE subjects were discharged on lipid‐modifying agents compared to 42% in the rest of the cohort (P = .0006). Multivariate analyses revealed several independent predictors of treatment with lipid‐lowering medication. Diagnosis of ischemic stroke, ACE status, and history of heart failure were negative predictors (less likely to be treated), and history of dyslipidemia was a positive predictor (Table 2). Status as an academic hospital was a hospital characteristic for which a significant association was found. Academic hospitals were significantly more likely to both perform LDL profiles and administer lipid‐lowering medications at discharge than were nonacademic hospitals. This association was found in a logistic regression analysis that did not account for between‐hospital variance. However, when we used GEE analysis, which adjusted for the variance, the difference between academic and nonacademic hospitals was no longer significant.
| Characteristic | n | Use of lipid‐lowering medication | Univariatea | P value | Adjusteda | P value | |||
|---|---|---|---|---|---|---|---|---|---|
| no. | % | OR | (95% CI) | OR | (95% CI) | ||||
| |||||||||
| Median age | |||||||||
| 73 years | 385 | 208 | (54.0) | Ref | |||||
| > 73 years | 379 | 162 | (42.7) | 0.79 | (0.59, 1.06) | .11 | |||
| Sex | |||||||||
| Female | 408 | 184 | (45.1) | Ref | |||||
| Male | 356 | 186 | (52.2) | 1.05 | (0.89, 1.25) | .55 | |||
| Ethnicity | |||||||||
| Other | 341 | 190 | (55.7) | Ref | |||||
| White | 423 | 193 | (45.6) | 0.88 | (0.61, 1.27) | .55 | |||
| Event type | |||||||||
| TIA | 172 | 58 | (34) | Ref | Ref | ||||
| Ischemic stroke | 592 | 312 | (53) | 1.92 | (1.39, 2.65) | < .0001 | 1.95 | (1.33, 2.85) | .0009 |
| At risk, coronary events | 309 | 181 | (58.6) | 1.83 | (1.30, 2.59) | .0006 | 1.49 | (1.06, 2.10) | .02 |
| History of:b | |||||||||
| Stroke/TIA | 277 | 141 | (50.9) | 1.43 | (0.97, 2.12) | .07 | 1.30 | 4(0.87, 2.08) | .18 |
| Dyslipidemia | 243 | 192 | (79.0) | 6.62 | (3.28, 13.36) | < .00 01 | 5.77 | 2.65, 12.54) | < .0001 |
| MI | 67 | 42 | (62.7) | 1.77 | (0.90, 3.45) | .10 | a | ||
| CAD | 132 | 28 | (21.2) | 1.49 | (0.87, 2.54) | .14 | |||
| Smoking | 158 | 89 | (56.3) | 1.00 | (0.74, 1.28) | .86 | |||
| Heart failure | 83 | 28 | (33.7) | 0.60 | (0.41, 0.87) | .007 | 0.40 | 0.26, 0.61) | < .0001 |
| Diabetes | 199 | 119 | (59.8) | 1.67 | (1.26, 2.20) | .007 | a | ||
| Hypertension | 516 | 271 | (52.5)1.82 | (1.45, 2.27) | < .0001 | 1.36 | 7(0.88, 2.212) | .16 | |
| Atrial fibrillation | 125 | 51 | (40.8) | 0.79 | (0.55, 1.12) | 18 | |||
| Received lipid profile | 383 | 253 | (66.1) | 2.77 | (1.75, 4.38) | < .0001 | 2.46 | (1.53, 3.97) | .0002 |
| Received tPA | |||||||||
| No | 748 | 360 | (48.1) | Ref | |||||
| Yes | 16 | 9 | (56.3) | 1.26 | (0.58, 2.71) | .56 | |||
| Ambulatory at discharge | 400 | 206 | (51.5) | 1.36 | (1.05, 1.78) | .02 | 1.33 | (0.96, 1.80) | 0.09 |
Three of the patients with documented LDL‐C levels (0.8%) had documented contraindications to therapy. Among all those who had documented LDL‐C levels, the rate of appropriate treatment with lipid‐lowering medications was high in both the ACE and non‐ACE groups (94.6% and 98.6%, respectively; P = .02). However, because only a small number of patients did not receive optimal treatment, the odds ratio of 0.24 had a fairly wide confidence interval (95% CI = 0.06, 0.91). Although a trend toward a higher rate of treatment with increasing LDL‐C level was seen in both the ACE and non‐ACE groups, this trend was only significant for the group with non‐ACE patients (Figure 1).

DISCUSSION
We found that only half the patients hospitalized for ischemic stroke or TIA had LDL‐C levels tested while in the hospital, even among those identified by the ATP guidelines as at high risk for future coronary events. Our findings are in accord with those of the Coverdell Project, which evaluated key features of acute stroke care from 4 prototype registries, those in Georgia, Massachusetts, Michigan, and Ohio, finding that fewer than 40% of acute stroke patients had had lipid profiles checked during hospitalization.11 Our study also evaluated predictors for in‐hospital lipid testing and lipid‐lowering treatment during hospitalization for an acute ischemic cerebrovascular event. We found that lipid testing was correlated with treatment during stroke or TIA hospitalization, suggesting that in‐hospital lipid management is related to an overall appreciation of the importance of lipids.
Understanding the factors resulting in such underperformance is critical for improving patient care and outcomes. Lipid assessment and treatment rates varied widely between CASPR hospitals, reflecting dramatic differences in hospital practice. This finding is similar to that noted in a recent study performed in Europe10 and underscores the need to promote a more uniform approach to in‐hospital care of patients with ischemic stroke or TIA. Our study also found that ischemic stroke patients were much more likely to have their lipid level measured and to be discharged on a lipid‐lowering agent than were TIA patients. This may be so because many treating health care professionals perceive TIAs as benign events that carry a more favorable prognosis than do strokes, or it could be that the length of stay for a TIA, often shorter than that for a stroke, limited in‐hospital testing or planning for patient follow‐up.
A high proportion of non‐ACE, lipid‐tested stroke/TIA patients received lipid‐lowering drug treatment, even when their lipid levels were within the treatment range categorized as nonpharmacologic by the national guidelines. This finding could be a result of one of the goals of the primary study.15 In the primary study, the effect of standardized orders implemented during the second observational period were analyzed by comparing them to those in place during the first observational period to see if they had improved the in‐hospital stroke care process. One of the study goals was optimal discharge utilization of a lipid‐lowering agent, defined as prescription of a lipid modifier or an LDL < 100 mg/dL. There was a significant increase in the number of prescriptions for lipid modifiers at discharge after implementing the standardized orders.15 However, as this study has shown, when existing national cholesterol guidelines were strictly applied to all the patients,6 overall there was a suboptimal rate of utilization of lipid modifiers at discharge.
Lipid profile assessment during stroke admission is one of the 10 performance measures in the performance measure set of the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) Stroke Disease‐Specific Care.16 Initiating therapy with lipid‐lowering agents before discharge may help to maintain continuity of care and clarify therapeutic intent, especially when a different physician is responsible for care after discharge from the hospital. Recent studies indicated that in‐hospital initiation of medication following admission for a vascular event tends to improve longer‐term patient adherence to treatment,17, 18 as well as vascular outcomes,19, 20 and is a strategy favored by the American Stroke Association.13, 21
This study had several limitations. Our definitions of dyslipidemia and of adherence to ATP III goals were based on single measurements of LDL‐C, rather than multiple determinations of lipoprotein subfractions. However, we believe that this approach parallels actual clinical practice more closely. Although LDL‐C is the most important of all the components of the lipid profile,6 because lipid subfractions other than LDL‐C were not collected in the CASPR registry, we may have misclassified a few patients. For instance, extremely high trigylceride levels can render LDL‐C levels inaccurate, and as such, not having a documented LDL‐C may not have always indicated that a lipid panel was not performed. It is also conceivable that physicians might actually have been more thorough in measuring LDL‐C, identifying contraindications to lipid‐lowering therapy, or instituting lipid‐lowering therapy than were noted in the hospital charts. However, for quality assurance purposes, what is documented is the only traceable record of what was actually asked for or done. As such, health care professionals are frequently encouraged to keep updated chart notes. This study was an assessment of in‐hospital behavior; the low utilization of lipid‐lowering agents observed may underestimate the final treatment rate, as we did not evaluate the postdischarge rate of therapy. However, recent data suggest in‐hospital prescription patterns are a major predictor of longer‐term care in the community.17, 22 Last, the CASPR investigators did not collect data on the rate of utilization of lipid agents prior to hospitalization or on the mechanisms by which the strokes and TIAs had occurred. Prehospital utilization of lipid agents has previously been revealed to influence the prescribing of lipid‐lowering agents at discharge.10 Knowledge of the mechanisms of the stroke and TIA events would have increased the number of those eligible for lipid treatment, particularly those whose events were to the result of an atherosclerotic mechanism per ATP III's more expansive definition of CHD risk equivalents, which includes carotid and other forms of clinical atherosclerotic disease.6 However, because the results of other studies that evaluated lipid management in all hospitalized stroke patients (regardless of mechanism)11, 23 or in all patients with any form of clinical atherosclerotic disease24 were in accord with those of our study, it would appear unlikely that such information would have made an overwhelming difference to our results.
In conclusion, the results of the present study suggest that considerable improvement is needed in identifying appropriate candidates among those who have had stroke or TIA and treating them with lipid‐lowering agents. Performing lipid testing in individuals hospitalized with ischemic stroke or TIA is important because it may inform the identification of persons for whom treatment should be initiated or modified. Lipid assessment during hospitalization for stroke/TIA and initiation of lipid‐lowering therapy when indicated are major management steps that all patients with ischemic cerebrovascular events should receive.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
- ,,, et al.Thrombus formation on atherosclerotic plaques: pathogenesis and clinical consequences.Ann Intern Med.2001;134:224–238.
- ,,, et al.Manifestations of atherosclerosis in various vascular regions. Similarities and differences regarding epidemiology, etiology and prognosis [in German].Med Klin.2002;97:221–228.
- ,,,.Hypolipemic agents for stroke prevention.Clin Exp Hypertens.2002;24:573–594.
- ,,,,,.Differential effects of lipid‐lowering therapies on stroke prevention: a meta‐analysis of randomized trials.Arch Intern Med.2003;163:669–676.
- Heart Protection Study Collaborative Group.Effects of cholesterol‐lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high‐risk conditions.Lancet.2004;363:757–767.
- Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,,,.The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals.Arch Intern Med.2000;160:459–467.
- ,,, et al.Analysis of the degree of undertreatment of hyperlipidemia and congestive heart failure secondary to coronary artery disease.Am J Cardiol.1999;83:1303–1307.
- .Statin therapy after acute myocardial infarction: are we adequately treating high‐risk patients?Curr Atheroscler Rep.2002;4:99–106.
- ,,,.Determination of lipid profiles and use of statins in patients with ischemic stroke or transient ischemic attack.Stroke.2003;34:105–110.
- ,,, et al.Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry.Stroke.2005;36:1232–1240.
- ,,.Stroke prevention: narrowing the evidence‐practice gap.Neurology.2000;54:1899–1906.
- Statins after ischemic stroke and transient ischemic attack: an advisory statement from the Stroke Council, American Heart Association and American Stroke Association.Stroke.2004;35:1023.
- California Acute Stroke Pilot Registry (CASPR) Investigators.Prioritizing interventions to improve rates of thrombolysis for ischemic stroke.Neurology.2005;64:654–659.
- California Acute Stroke Pilot Registry (CASPR) Investigators.The impact of standardized stroke orders on adherence to best practices.Neurology.2005;65:360–365.
- JCAHO Stroke Disease‐Specific Care performance measure set. Available at: www.jcaho.org/dscc/dsc/performance+measures/stroke+measure+set.htm. Accessed November 20,2005.
- .The role of in‐hospital initiation of cardiovascular protective therapies to improve treatment rates and clinical outcomes.Rev Cardiovasc Med.2003;4(Suppl 3):S37–S46.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35:2879–2883.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87:819–822.
- ,,, et al.Impact of combination evidence‐based medical therapy on mortality in patients with acute coronary syndromes.Circulation.2004;109:745–749.
- American Heart Association Get with the Guidelines Program—Coronary Artery Disease Pilot Test Results. Available at: http://www.americanheart.org/presenter.jhtml?identifier=699. Accessed November 30,2003.
- ,,, et al.In‐hospital initiation of lipid‐lowering therapy after coronary intervention as a predictor of long‐term utilization: a propensity analysis.Arch Intern Med.2003;163:2576–2582.
- University HealthSystem Consortium Ischemic Stroke Clinical Benchmarking Project Clinical Database Analysis—2001. University HealthSystem Consortium Ischemic Stroke Database Report #3.
- ,,.Expanding indications for statins in cerebral ischemia: a quantitative study.Arch Neurol.2005;62:67–72.
Copyright © 2006 Society of Hospital Medicine