User login
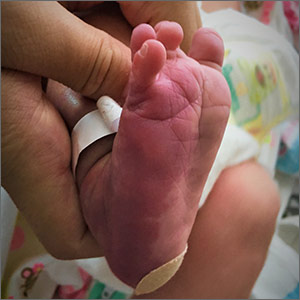
Violaceous patches on baby’s foot/leg

The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.

The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.

The presence of the large red to purple, well-demarcated patches with a lateral predilection led the FP to diagnose a port-wine-stain.1,2
Port-wine-stains are a type of capillary malformation that fall under the over-arching category of “simple vascular malformations.”3 Occurring in approximately 3/1000 live births, port-wine-stains have no gender predilection and can occur anywhere on the body, however, 80% of cases are associated with the head and neck.1,4 Lesions tend to be present at birth and grow in proportion with the child.1-4 While port-wine-stains may lighten during the infant’s first year of life, they tend to darken and become more nodular with time.1,3-5 Darkening of lesions is thought to be due to a lack of neural input to the capillaries, leading to poor vascular tone and dilation.5
Port-wine-stains are often isolated and benign, but their presence may indicate an underlying syndrome. Two of the more common syndromes associated with port-wine-stains include Sturge-Webber syndrome and Klippel-Trenaunay syndrome.1,4
Sturge-Webber syndrome is characterized by a port-wine-stain in the distribution of the first trigeminal division (V1), with possible involvement of the second or third trigeminal divisions (V2 and V3).1,4 Central nervous system abnormalities are also characteristic of Sturge-Webber Syndrome and can include cerebral atrophy, leptomeningeal angiomatosis, and cortical calcifications that can cause seizures, mental retardation, and hemiparesis.1,2,4
Ophthalmologic complications of Sturge-Webber syndrome can include glaucoma, and are seen in 10% to 30% of patients with a port-wine-stain in the periocular region and in 30% to 70% of patients with leptomeningeal involvement.2 A larger facial distribution of a port-wine-stain correlates to a stronger association with Sturge-Webber syndrome.2
Klippel-Trenaunay syndrome is characterized by port-wine-stains on the lower extremities with limb hypertrophy and length discrepancy, varicose veins, lymphedema, and phleboliths.1,4 Diagnosis is typically clinical and based on physical exam findings. However, an elevated d-dimer, magnetic resonance imaging (MRI), or ultrasound may aid in confirmation. The MRI or ultrasound may reveal tissue hypertrophy and the associated vascular malformations.6
The differential diagnosis for a port-wine stain includes nevus simplex, another type of capillary malformation. Nevus simplex is the most common capillary malformation, occurring in up to 82% of newborns.2 Depending on the location, nevus simplex is also referred to as a “stork bite” (lesion on nape of neck) or “angel’s kiss” (lesion on forehead).2 Nevus simplex is distinguished from a port-wine-stain by a more central location, indistinct borders, and a pale pink to red coloring.2,3 Nevus simplex lesions tend to fade as the child grows, while port-wine-stains tend to darken.2,3
Port-wine-stains also can be confused with infantile or congenital hemangiomas, which were considered in this case. Congenital hemangiomas are present at birth, while infantile hemangiomas appear within the first few weeks of life.1,2 Superficial hemangiomas can be red and macular, and often have well-defined borders, which makes distinction from port-wine-stains difficult at times.1 Hemangiomas will typically go through proliferations and involution stages making them dynamic lesions, whereas port-wine-stains grow in proportion to the child.1,2
Pulsed-dye laser (PDL) treatments are the gold standard for treatment of port-wine-stains.1,4 PDL selectively targets the vascular chromophore, which minimizes the appearance of the vascular stain but can’t completely eradicate it.1,4 Treatment is generally initiated after 6 months of life.1 In this case, the patient was referred to Dermatology for a discussion of the benefits of PDL therapy.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.
1. Slaughter KA, Chen T, Williams E. Vascular lesions. Facial Plast Surg Clin North Am. 2016;24:559-571.
2. Rozas-Muñoz E, Frieden IJ, Roé E1, et al. Vascular stains: proposal for a clinical classification to improve diagnosis and management. Pediatr Dermatol. 2016;33:570-584.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136:e203-e214.
4. Lam SM, Williams EF III. Practical considerations in the treatment of capillary vascular malformations, or port wine stains. Facial Plast Surg. 2004;20:71-76.
5. Cordoro KM, Speetzen LS, Doerper MA, et al. Physiologic changes in vascular birthmarks during early infancy: mechanisms and clinical implications. J Am Acad Dermatol. 2009;60:669-675.
6. Wang, SK, Drucker NA, Gupta AK, et al. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5:587-595.
History of posttraumatic stress disorder • priapism • Dx?
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.