User login
Screening for Metabolic Syndrome in People with Severe Mental Illness
From the University of California San Francisco, Department of Psychiatry, Weill Institute for Neurosciences, San Francisco, CA.
Abstract
- Objective: To review screening for metabolic syndrome in people with severe mental illness (SMI).
- Methods: Review of the literature.
- Results: Despite evidence-based metabolic screening guidelines, rates of metabolic screening remain low among people with SMI. Barriers to screening exist at the individual, organizational, and systems levels. Interventions to address these barriers range from point-of-care tools to systems-level reorganization towards population-based care.
- Conclusion: Greater systems-level interventions, particularly those that improve collaboration between mental health and primary care, are needed to improve metabolic monitoring and identify cardiovascular disease risk among people with SMI.
Key words: metabolic monitoring; severe mental illness; metabolic syndrome; integrated care.
People with severe mental illness (SMI) have a life expectancy 10 to 20 years shorter than the general population, and cardiometabolic risk factors contribute significantly to the increased morbidity and mortality seen in this population. To address this health disparity, metabolic monitoring guidelines have been proposed as a mechanism to identify metabolic risk factors. This paper aims to discuss metabolic syndrome and its risk factors, describe metabolic monitoring including current rates and barriers to screening, and identify interventions that may improve rates of screening for metabolic syndrome among people with SMI.
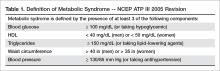
Metabolic syndrome has been conceptualized as a state of chronic low-grade inflammation and hypercoagulation associated with hypertension, dyslipidemia, glucose intolerance, insulin resistance, and visceral adiposity [1]. Per the modified National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines, metabolic syndrome is defined as the presence of 3 of the following 5 parameters: (1) blood glucose > 100 mg/dL (or a person is taking a hypoglycemic medication), (2) high density lipoprotein (HDL) < 40 mg/dL in men or < 50 mg/dL in women, (3) triglycerides > 150 mg/dL (or taking a lipid lowering agent), (4) waist circumference > 40 inches in men or > 35 inches in women, and/or (5) blood pressure > 130/85 mm Hg (or taking an antihypertensive medication) [2,3] (Table 1).
Metabolic syndrome is associated with an increased risk of diabetes mellitus, cardiovascular disease (including myocardial infarction and cerebrovascular accident), and all-cause mortality [3]. Other systemic effects related to metabolic syndrome include renal, hepatic, and skin manifestations such as chronic kidney disease, non-alcoholic steatohepatitis, and obstructive sleep apnea [1].
Epidemiology and Risk Factors
An estimated 34% of people in the United States meet criteria for metabolic syndrome, with worldwide estimates ranging widely from less than 10% to 84%. People with SMI (eg, bipolar disorder, schizoaffective disorder, schizophrenia) are at even greater risk of developing metabolic syndrome than the general population [4,5]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated metabolic syndrome rates of 40.9% and 51.6% in men and women with a diagnosis of schizophrenia, respectively [6]. In a systematic review of bipolar disorder and metabolic syndrome, people with bipolar disorder showed higher rates of hypertriglyceridemia and hyperglycemia than controls [5].
People with SMI have been found to have significantly increased morbidity and mortality as compared to people without an SMI diagnosis, much of which has been attributed to increased cardiometabolic risk related to multiple factors [7]. Among adults with schizophrenia receiving Medicaid, Olfson et al found diabetes mellitus, ischemic heart disease, nonischemic heart disease, and cerebrovascular accident to be among the top 10 causes of death [7]. The mortality rate for people with SMI is estimated to be 2 to 3 times higher than the general population, and the life expectancy for people with SMI is estimated to be 10 to 20 years shorter than the general population [8–10]. Contributors to this disparity include modifiable health-related behaviors, social determinants of health, and iatrogenic sequelae of prescribed medications. Behavioral factors include poor nutrition, food insecurity, sedentary lifestyle, and smoking; side effects of commonly prescribed psychotropic medications, most notably atypical antipsychotics and mood stabilizers, also contribute to this disparity [7,11].
Both first- and second-generation antipsychotics have been shown to be associated with metabolic sequelae, including weight gain, elevated blood glucose, and insulin resistance [12–14]. Among psychotropic medications, the atypical or second-generation antipsychotics (SGAs) are a class of medications known to have significant metabolic side effects [15,16]. Studies comparing the metabolic consequences of individual SGAs have found significant variation within the class. Clozapine, olanzapine, quetiapine, and risperidone show significant likelihood of weight gain, hyperlipidemia, and hyperglycemia as well as other metabolic consequences [17]. Aripiprazole, lurasidone, and ziprasidone have shown little to no risk of metabolic sequelae [17].
Metabolic side effects of SGAs have been demonstrated in children, adolescents, and adults. There is evidence that adolescents may be particularly sensitive to these sequelae. Galling and colleagues found that adolescents treated with antipsychotics were at greater risk of developing type 2 diabetes mellitus as compared to both healthy controls and controls with psychiatric illness [18]. Kryzhanovskaya et al, looking at metabolic parameters associated with olanzapine use in adolescents and adults, found that both adolescents and adults showed metabolic sequelae and that adolescents had larger changes in weight gain and lipids compared with adults [19].
The mechanism of SGA impact on metabolic parameters remains incompletely understood, though is thought to be multifactorial, mediated primarily through weight gain with increased adiposity. SGA histamine (H1) receptor binding affinity is implicated in weight gain [20] and 5HT2C antagonism may also lead to an increase in appetite [21]. Other proposed mechanisms include changes in appetite through leptin resistance or decreased sensitivity to leptin, the hormone that mediates satiety. Zhang and colleagues found an increase in leptin levels in patients with schizophrenia prescribed antipsychotics, suggesting leptin dysregulation [21]. Additional studies suggest metabolic disturbances independent of weight gain including direct effects of SGAs on glucose and lipid metabolism [22].
If a person experiences a weight gain of 5% after starting an SGA, it is recommended that the dose be decreased or that they be switched to another psychotropic medication with lower likelihood of metabolic consequences [23]. The effectiveness of switching antipsychotic medications to one with lower metabolic risk to improve weight and lipids has been previously demonstrated [24]. If a patient develops diabetes in the context of an antipsychotic prescription, it is also recommended that the medication be switched to an antipsychotic with less risk of hyperglycemia, and if not possible, to target additional risk factors including weight, poor nutrition, and sedentary lifestyle [25]. The decision to switch medications or decrease dosage is often weighed against the psychiatric stability of the person and their overall response to the medication in the context of their treatment course [14].
Metabolic Monitoring
Given the increased risk of metabolic syndrome among people with SMI, and the association of metabolic syndrome with increased morbidity and all-cause mortality, there has been a growing awareness of the importance of screening for metabolic syndrome among people with SMI. Metabolic monitoring involves routine screening for metabolic parameters and assessment of metabolic risk factors among people with SMI who are prescribed antipsychotic medications. Various practice guidelines have been developed in the United States and internationally to assess for metabolic risk factors in people prescribed antipsychotic medications [26]. Current metabolic monitoring guidelines in the United States stem from 2004 consensus recommendations of the American Diabetes Association and American Psychiatric Association along with the American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity for metabolic monitoring among people prescribed SGAs [23]. These recommendations include a time line for routine monitoring of weight/body mass index, waist circumference, blood pressure, fasting blood glucose or hemoglobin A1c, and fasting lipids (Table 2). Guidelines recommend screening at baseline, more frequently within the first 3 months, and then annually [23].
Though guidelines recommend measurement of waist circumference as a marker for metabolic health, body mass index is often used alone as a measure of obesity [27,28]. This may be due to the relative ease of obtaining weight over waist circumference. For example, weight is more likely to be part of clinic workflows and many providers may not be accustomed to measuring waist circumference. However, waist circumference does provide additional information regarding metabolic health [29], as central adiposity is a marker of cardiometabolic risk and related to insulin resistance [21]. Further modifications of the guidelines have included ethnicity-specific waist measurements [30].
There is evidence that non-fasting lipids may be substituted for fasting lipid panels, particularly for patients who may have difficulty adhering to fasting due to cognitive difficulties. Vanderlip and colleagues argue that fasting serum cholesterol panels are not necessary for screening for dyslipidemia given that non-HDL cholesterol is calculated based on total cholesterol and HDL, which do not substantially differ between fasting and non-fasting values [31]. Hemoglobin A1c is recommended as a screening test for blood glucose abnormalities given that it does not require a fasting state and can therefore be more easily obtained for many patients. The choice to obtain a fasting blood glucose versus hemoglobin A1c may depend on multiple factors, including that a person can adhere to fasting and the cost of the laboratory test.
Routine monitoring of metabolic parameters is an integral step in targeting interventions to treat metabolic syndrome. These interventions include lifestyle modifications and evidence-based treatment guidelines for management of associated dyslipidemia, hypertension, and type 2 diabetes mellitus.
Current Metabolic Screening Practices
Despite the presence of defined guidelines, estimates show persistently low rates of metabolic monitoring among adults prescribed SGAs [32]. One study of 3 state Medicaid programs showed little to no improvement in screening rates for glucose and lipids post dissemination of the 2004 APA/ADA guidelines [33]. They noted a nonsignificant change in rates of glucose testing from 27% to 30% and small change in lipid testing from 10% to 11% among patients prescribed SGAs between 2002–2005 [33]. Examining screening rates among Medicaid recipients in Missouri between 2010–2012, Morrato and colleagues found glucose testing rates of 80% with lipid testing remaining at 41% [34]. A retrospective study of adult Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 showed rates of screening for lipids and glucose to increase over time; glucose monitoring increased from 56.6% to 72.6% and lipids from 38.3% to 41.2% [35]. A review by Mangurian and colleagues suggested rates of glucose (fasting blood glucose or hemoglobin A1c) and lipid screening as low as 30% among people prescribed antipsychotic medications [14]. Furthermore, they underscore the impact of low screening rates, stating that if 20% of adults with SMI have diabetes and 70% remain unscreened, then approximately 2 million adults with SMI and diabetes in the United States would not be identified within our current system [14].
Higher rates of screening have been shown for Medicaid populations than commercially insured populations [36]. Haupt et al compared lipid and glucose testing pre- and post- ADA/APA guideline implementation among commercially insured patients. They found an increase from 8.4% to 10.5% post guideline implementation for baseline lipid testing and from 6.8% to 9.0% for lipid testing at 12 weeks post-antipsychotic initiation [36]. Baseline glucose testing increased from 17.3% to 21.8% and from 14.1% to 17.9 % at 12-week post antipsychotic initiation. In alignment with findings from other studies, testing rates were particularly low for children [36].
Low screening rates have been found among children and adolescents prescribed SGAs [37] despite evidence that youth may be at risk of developing more significant metabolic sequelae from SGAs [19]. Edelsohn and colleagues found an increase from 30% to 50% for glucose screening and from 19% to 28% for lipid screening among youth Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 [35]. Connolly and colleagues reported on metabolic screening rates for children and adolescents prescribed SGAs over the 8 years following announcement of the 2004 ADA/APA guidelines. Using insurance claims data, they found screening rates for fasting blood glucose and hemoglobin A1c temporarily increased following guideline dissemination, then dropped during the period 2004–2008, and again increased slightly [38].
Barriers to Screening
Barriers to screening exist at the level of the individual patient and provider as well as at the clinic and larger systems levels. Lack of provider awareness of evidence-based guidelines for metabolic monitoring despite the presence of the 2004 ADA/APA guidelines has been cited by researchers as an impediment to screening. In a survey of primary care clinicians in San Francisco, Mangurian et al found that 40% of primary care providers did not know about the ADA/APA consensus guidelines for metabolic monitoring. The same survey of primary care providers identified additional impediments to screening, including obstacles to collaboration with psychiatric providers and to scheduling patients for psychiatric follow-up [39]. Another clinician survey conducted by Parameswaran et al found that psychiatrists viewed psychiatric illness severity, lack of staff time, and lack of clinician time as significant barriers to metabolic screening. In addition, clinicians identified factors related to the complexity of coordinating care across systems as obstacles; these included barriers to coordinating follow-up with medical providers, long wait times for patients to see medical providers, and difficulty collaborating with medical providers [40].
Other systems-level barriers include lack of a population-based approach to screening (eg, registries) and lack of electronic record integration, which impedes the ability of primary care and psychiatry providers to share information related to the ordering of metabolic screening tests and prescribing of medications [41]. Mangurian calls for integration of electronic medical record systems between primary care and psychiatry, a population-based approach to metabolic monitoring utilizing registries and other elements of collaborative care models, and primary care consultation to aid in the treatment of metabolic abnormalities [41]. Amiel et al point to systems-level factors “including but not limited to … poor access to general medical services, inadequate medical record-keeping infrastructure, lack of in-system compliance incentives and lack of centralized oversight” [26].
Based on their experience implementing a computer-based intervention for metabolic monitoring, Lai et al propose that the following factors may influence providers’ engagement in metabolic monitoring: lack of apparent symptoms to suggest metabolic syndrome, patients’ lack of engagement in care, and poor access to care. They identify additional factors at the clinician level to include under-recognition of the need for metabolic monitoring, lack of familiarity with screening guidelines, lack of agreement with guidelines, and the potential for individual clinicians to forget to order tests [42]. At the systems-level, they identify the absence of ongoing training as a potential reason why sustained testing was not observed in their intervention [42].
In a 2011 survey of providers prescribing antipsychotic medication to Medicaid beneficiaries in Missouri, Morrato and colleagues found that factors limiting frequency of health care utilization were closely linked to lack of metabolic testing. They also noted disparities in screening guidelines may lead to lack of routine metabolic monitoring; providers may screen based on prescribed medication, diagnosis, or other risk factor based stratification depending on the guidelines followed [34].
Current Unmet Needs
Vulnerable Populations
Though rates of metabolic screening remain low for all groups prescribed antipsychotic medications, studies have consistently shown low rates of screening among children and adolescents [35,36]. Edelsohn and colleagues hypothesize that the cause of these low rates is multifactorial, including that guardians may be reluctant to have young people undergo blood draws [35]. Morrato and colleagues suggest that policymakers should focus initiatives on younger, healthier adults, who they found to have lower rates of screening [37].
Racial and ethnic minorities with SMI constitute another particularly vulnerable population, with some studies showing an increased risk of metabolic sequelae and lower likelihood of treatment for diabetes and other metabolic derangements among African American and Latino populations with SMI [14,43,44].
Integration of Care
Lack of widespread integration of care between mental health and primary care remains another unmet need [41]. Hasnain and colleagues recommend improved communication between mental health and primary care clinicians to coordinate care to improve rates of monitoring, facilitate early follow-up of metabolic abnormalities, and avoid duplication of monitoring efforts [45]. Morrato and colleagues recommend that efforts to increase rates of metabolic monitoring be targeted not only to providers practicing in community mental health centers, but also to other practice settings including primary care. They found that for 75% of people prescribed antipsychotic medications, the prescriptions were started by prescribing providers who practiced outside of a community mental health center [34] and recommend that educational initiatives and performance improvement interventions broaden to include primary care and other care settings [34].
Potential Interventions for Improvement
Early interventions to improve metabolic screening rates have included educational initiatives to teach providers about consensus guidelines. However, initiatives to educate clinicians on metabolic monitoring have shown to be inadequate to significantly improve rates of screening [33]. Therefore, subsequent initiatives have sought to influence screening rates by targeting behavior of individual clinicians with point-of-care tools, electronic reminders, or through systems-level reorganization towards population-based care [27,42,46].
A variety of clinical interventions focus on technologies that remind clinicians to order metabolic monitoring tests according to screening guidelines. One public mental health service in Queensland, Australia, created a standardized metabolic monitoring form to be uploaded to the electronic medical record. In their implementation study examining the efficacy of the metabolic monitoring form, they found that only 36% of the forms contained data. When data were recorded, there were significantly higher rates of documentation of measurements (weight, body mass index, blood pressure) rather than laboratory tests (including lipids and fasting blood glucose) [27].
Computerized reminder systems for metabolic monitoring have been studied in both outpatient and inpatient settings. Lai and colleagues studied the impact of a computerized reminder system on lab monitoring for metabolic parameters among outpatients with schizophrenia prescribed SGAs [42]. This intervention also included an educational component with discussion of metabolic monitoring for people prescribed SGAs at meetings with attending psychiatrists. Computer reminders were displayed when a provider failed to order fasting plasma glucose or lipids (cholesterol, triglyceride) for patients prescribed clozapine, olanzapine, quetiapine, or risperidone. The study found a statistically significant improvement in laboratory metabolic screening for patients prescribed SGAs after implementation, with the greatest impact 6-months post-intervention, though with subsequent decline in screening rates [42].
Psychiatric inpatient hospitalizations provide an opportunity to obtain testing at the time of treatment initiation and also for ongoing monitoring in a location where fasting laboratory tests may be more easily obtained given onsite phlebotomy. One intervention targeting psychiatric inpatients utilized a computerized physician order entry system with the goal to improve metabolic screening among patients prescribed SGAs. Set in a large academic medical setting, the study found inpatient metabolic monitoring rates did not change significantly after implementation of these pop-up computer alerts, comparing rates immediately and 4 years after implementation [46].
There has been increasing focus on integrating mental health and medical care in an effort to improve the health of people with mental illness [47]. Mangurian and colleagues found that the likelihood of diabetes mellitus screening doubled for people with severe mental illness who were seen for at least one primary care visit in addition to mental health treatment [48]. Haupt similarly found higher rates of metabolic screening among patients who had greater than one primary care visit [36]. Models of integration include both integration of medical services into mental health treatment as well as incorporation of mental health services into primary care. For people with SMI, integration efforts have largely focused on integrating primary care services into community mental health settings [49]. The Substance Abuse and Mental Health Service Administration’s (SAMHSA) Primary and Behavioral Health Care Integration (PBHCI) grants program and the Affordable Care Act’s Health Home Initiative are examples of federal incentive programs for improved integration between behavioral health and primary care [49]. In their evaluation of the PBHCI grant program, Scharf and colleagues presented findings that patients at 3 matched clinics with PCBHI grants showed improvement in some lipids, diastolic blood pressure, and fasting blood glucose, though not smoking or body mass index [50].
Conclusion
Several risk factors contribute to an increase in cardiometabolic risk for people with severe mental illness, including poor nutrition, sedentary lifestyle, social determinants of health, and prescribed antipsychotic medications. Metabolic monitoring aims to address these health disparities by screening for metabolic parameters and identifying abnormalities in order to target appropriate health interventions. Screening rates for metabolic parameters remain low for children, adolescents, and adults prescribed second-generation antipsychotics despite published guidelines and clinical interventions to improve screening. More system-wide interventions to improve collaboration between mental health and primary care are needed to enhance screening and prevent cardiovascular disease risk in this vulnerable population.
Corresponding author: Carrie Cunningham, MD, MPH, Zuckerberg San Francisco General Hospital, 1001 Potrero Ave, Suite 7M, San Francisco, CA 94110, [email protected].
Funding/support: Dr. Cunningham was supported by the UCSF-Zuckerberg San Francisco General Public Psychiatry Fellowship. Mr. Riano was supported by the NIH Center Grant from the National Institute of Diabetes and Digestive and Kidney Diseases for The Health Delivery Systems-Center for Diabetes Translational Research (CDTR) (P30DK092924) and by the UCSF-San Francisco General Hospital Public Psychiatry Fellowship. Dr. Mangurian received support from a grant from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R03 DK101857), as well as NIH Career Development Award (K23MH093689).
1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014.
2. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
3. American Heart Association. What is metabolic syndrome? 2015.
4. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339–47.
5. Czepielewski L, Daruy Filho L, Brietzke E, Grassi-Oliveira R. Bipolar disorder and metabolic syndrome: a systematic review. Rev Bras Psiquiatria 2013;35:88–93.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19–32.
7. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015:1–10.
8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123–31.
9. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41.
10. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chron Dis 2006;3:A42.
11. Williams J, Stubbs B, Gaughran F, Craig T. ‘Walk This Way’–a pilot of a health coaching intervention to reduce sedentary behaviour and increase low intensity exercise in people with serious mental illness: study protocol for a randomised controlled trial. Trials 2016;17:594.
12. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–96.
13. Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: differences between antipsychotic-naïve and treated patients. J Pharmacol Pharmacother 2013;4:176–86.
14. Mangurian C, Newcomer JW, Modlin C, Schillinger D. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med 2016:1–9.
15. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005;19(Suppl 1):1–93.
16. Baptista T, De Mendoza S, Beaulieu S, et al. The metabolic syndrome during atypical antipsychotic drug treatment: mechanisms and management. Metab Syndr Relat Disord 2004;2:290–307.
17. Hert MDE, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52–77.
18. Galling B, Roldan A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:247–59.
19. Kryzhanovskaya LA, Xu W, Millen BA, et al. Comparison of long-term (at least 24 weeks) weight gain and metabolic changes between adolescents and adults treated with olanzapine. J Child Adol Psychopharmacol 2012;22:157–65.
20. Nasrallah H. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008;13:27–35.
21. Zhang Z-J, YAO Z-J, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Br J Psychiatry 2004;184:58–62.
22. Kang SH, Lee JI. Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatr Invest 2015;12:242–8.
23. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 596–601.
24. Weiden PJ, Newcomer JW, Loebel AD, et al. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33:985–94.
25. Henderson DC. Atypical antipsychotic-induced diabetes mellitus. CNS Drugs 2002;16:77–89.
26. Amiel JM, Mangurian CV, Ganguli R, Newcomer JW. Addressing cardiometabolic risk during treatment with antipsychotic medications. Curr Opin Psychiatry 2008;21:613–8.
27. Happell B, Platania-Phung C, Gaskin CJ, Stanton R. Use of an electronic metabolic monitoring form in a mental health service–a retrospective file audit. BMC Psychiatry 2016;16:109.
28. Rosenbaum S, Nijjar S, Watkins A, et al. Nurse‐assessed metabolic monitoring: A file audit of risk factor prevalence and impact of an intervention to enhance measurement of waist circumference. Int J Ment Health Nurs 2014;23:252–6.
29. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 2007;15:1061–7.
30. Tan C-E, Ma S, Wai D, et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004;27:1182–6.
31. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv 2014;65:573–6.
32. Mitchell A, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012;42:125–47.
33. Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry 2010;67:17–24.
34. Morrato EH, Campagna EJ, Brewer SE, et al. Metabolic testing for adults in a state Medicaid program receiving antipsychotics: remaining barriers to achieving population health prevention goals. JAMA Psychiatry 2016;73:721–30.
35. Edelsohn GA, Parthasarathy M, Terhorst L, et al. Measurement of metabolic monitoring in youth and adult Medicaid recipients prescribed antipsychotics. J Manage Care Specialty Pharm 2015;21:769–77.
36. Haupt DW, Rosenblatt LC, Kim E, et al. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 2009;166:345–53.
37. Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010;164:344–51.
38. Connolly JG, Toomey TJ, Schneeweiss MC. Metabolic monitoring for youths initiating use of second-generation antipsychotics, 2003–2011. Psychiatr Serv 2015;66:604–9.
39. Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv 2013;64:597–9.
40. Parameswaran SG, Chang C, Swenson AK, et al. Roles in and barriers to metabolic screening for people taking antipsychotic medications: a survey of psychiatrists. Schizophren Res 2013;143:395–6.
41. Mangurian C. Patient-centered medical care in community mental health settings. Psychiatr Serv 2017;68:213-.
42. Lai C-L, Chan H-Y, Pan Y-J, Chen C-H. The effectiveness of a computer reminder system for laboratory monitoring of metabolic syndrome in schizophrenic outpatients using second-generation antipsychotics. Pharmacopsychiatry 2015;48:25–9.
43. Lambert BL, Chou C-H, Chang K-Y, et al. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf 2005;14:417–25.
44. Ramaswamy K, Kozma CM, Nasrallah H. Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine. Drug Saf 2007;30:589–99.
45. Hasnain M, Vieweg WVR, Fredrickson SK, et al. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim Care Diab 2009;3:5–15.
46. Lee J, Dalack G, Casher M, et al. Persistence of metabolic monitoring for psychiatry inpatients treated with second‐generation antipsychotics utilizing a computer‐based intervention. J Clin Pharm Therap 2016;41:209–13.
47. Katz MH. Improving the health of persons with serious mental illness. JAMA Intern Med 2015;175:1979–80.
48. Mangurian C, Newcomer JW, Vittinghoff E, et al. Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med 2015;175:1977–9.
49. Gerrity M. Integrating primary care into behavioral health settings: What works. New York: Milbank Memorial Fund; 2014.
50. Scharf DM EN, Hackbarth NS, Horvitz-Lennon M, et al. Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: Final Report (Task 13). 2014.
From the University of California San Francisco, Department of Psychiatry, Weill Institute for Neurosciences, San Francisco, CA.
Abstract
- Objective: To review screening for metabolic syndrome in people with severe mental illness (SMI).
- Methods: Review of the literature.
- Results: Despite evidence-based metabolic screening guidelines, rates of metabolic screening remain low among people with SMI. Barriers to screening exist at the individual, organizational, and systems levels. Interventions to address these barriers range from point-of-care tools to systems-level reorganization towards population-based care.
- Conclusion: Greater systems-level interventions, particularly those that improve collaboration between mental health and primary care, are needed to improve metabolic monitoring and identify cardiovascular disease risk among people with SMI.
Key words: metabolic monitoring; severe mental illness; metabolic syndrome; integrated care.
People with severe mental illness (SMI) have a life expectancy 10 to 20 years shorter than the general population, and cardiometabolic risk factors contribute significantly to the increased morbidity and mortality seen in this population. To address this health disparity, metabolic monitoring guidelines have been proposed as a mechanism to identify metabolic risk factors. This paper aims to discuss metabolic syndrome and its risk factors, describe metabolic monitoring including current rates and barriers to screening, and identify interventions that may improve rates of screening for metabolic syndrome among people with SMI.
Metabolic syndrome has been conceptualized as a state of chronic low-grade inflammation and hypercoagulation associated with hypertension, dyslipidemia, glucose intolerance, insulin resistance, and visceral adiposity [1]. Per the modified National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines, metabolic syndrome is defined as the presence of 3 of the following 5 parameters: (1) blood glucose > 100 mg/dL (or a person is taking a hypoglycemic medication), (2) high density lipoprotein (HDL) < 40 mg/dL in men or < 50 mg/dL in women, (3) triglycerides > 150 mg/dL (or taking a lipid lowering agent), (4) waist circumference > 40 inches in men or > 35 inches in women, and/or (5) blood pressure > 130/85 mm Hg (or taking an antihypertensive medication) [2,3] (Table 1).
Metabolic syndrome is associated with an increased risk of diabetes mellitus, cardiovascular disease (including myocardial infarction and cerebrovascular accident), and all-cause mortality [3]. Other systemic effects related to metabolic syndrome include renal, hepatic, and skin manifestations such as chronic kidney disease, non-alcoholic steatohepatitis, and obstructive sleep apnea [1].
Epidemiology and Risk Factors
An estimated 34% of people in the United States meet criteria for metabolic syndrome, with worldwide estimates ranging widely from less than 10% to 84%. People with SMI (eg, bipolar disorder, schizoaffective disorder, schizophrenia) are at even greater risk of developing metabolic syndrome than the general population [4,5]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated metabolic syndrome rates of 40.9% and 51.6% in men and women with a diagnosis of schizophrenia, respectively [6]. In a systematic review of bipolar disorder and metabolic syndrome, people with bipolar disorder showed higher rates of hypertriglyceridemia and hyperglycemia than controls [5].
People with SMI have been found to have significantly increased morbidity and mortality as compared to people without an SMI diagnosis, much of which has been attributed to increased cardiometabolic risk related to multiple factors [7]. Among adults with schizophrenia receiving Medicaid, Olfson et al found diabetes mellitus, ischemic heart disease, nonischemic heart disease, and cerebrovascular accident to be among the top 10 causes of death [7]. The mortality rate for people with SMI is estimated to be 2 to 3 times higher than the general population, and the life expectancy for people with SMI is estimated to be 10 to 20 years shorter than the general population [8–10]. Contributors to this disparity include modifiable health-related behaviors, social determinants of health, and iatrogenic sequelae of prescribed medications. Behavioral factors include poor nutrition, food insecurity, sedentary lifestyle, and smoking; side effects of commonly prescribed psychotropic medications, most notably atypical antipsychotics and mood stabilizers, also contribute to this disparity [7,11].
Both first- and second-generation antipsychotics have been shown to be associated with metabolic sequelae, including weight gain, elevated blood glucose, and insulin resistance [12–14]. Among psychotropic medications, the atypical or second-generation antipsychotics (SGAs) are a class of medications known to have significant metabolic side effects [15,16]. Studies comparing the metabolic consequences of individual SGAs have found significant variation within the class. Clozapine, olanzapine, quetiapine, and risperidone show significant likelihood of weight gain, hyperlipidemia, and hyperglycemia as well as other metabolic consequences [17]. Aripiprazole, lurasidone, and ziprasidone have shown little to no risk of metabolic sequelae [17].
Metabolic side effects of SGAs have been demonstrated in children, adolescents, and adults. There is evidence that adolescents may be particularly sensitive to these sequelae. Galling and colleagues found that adolescents treated with antipsychotics were at greater risk of developing type 2 diabetes mellitus as compared to both healthy controls and controls with psychiatric illness [18]. Kryzhanovskaya et al, looking at metabolic parameters associated with olanzapine use in adolescents and adults, found that both adolescents and adults showed metabolic sequelae and that adolescents had larger changes in weight gain and lipids compared with adults [19].
The mechanism of SGA impact on metabolic parameters remains incompletely understood, though is thought to be multifactorial, mediated primarily through weight gain with increased adiposity. SGA histamine (H1) receptor binding affinity is implicated in weight gain [20] and 5HT2C antagonism may also lead to an increase in appetite [21]. Other proposed mechanisms include changes in appetite through leptin resistance or decreased sensitivity to leptin, the hormone that mediates satiety. Zhang and colleagues found an increase in leptin levels in patients with schizophrenia prescribed antipsychotics, suggesting leptin dysregulation [21]. Additional studies suggest metabolic disturbances independent of weight gain including direct effects of SGAs on glucose and lipid metabolism [22].
If a person experiences a weight gain of 5% after starting an SGA, it is recommended that the dose be decreased or that they be switched to another psychotropic medication with lower likelihood of metabolic consequences [23]. The effectiveness of switching antipsychotic medications to one with lower metabolic risk to improve weight and lipids has been previously demonstrated [24]. If a patient develops diabetes in the context of an antipsychotic prescription, it is also recommended that the medication be switched to an antipsychotic with less risk of hyperglycemia, and if not possible, to target additional risk factors including weight, poor nutrition, and sedentary lifestyle [25]. The decision to switch medications or decrease dosage is often weighed against the psychiatric stability of the person and their overall response to the medication in the context of their treatment course [14].
Metabolic Monitoring
Given the increased risk of metabolic syndrome among people with SMI, and the association of metabolic syndrome with increased morbidity and all-cause mortality, there has been a growing awareness of the importance of screening for metabolic syndrome among people with SMI. Metabolic monitoring involves routine screening for metabolic parameters and assessment of metabolic risk factors among people with SMI who are prescribed antipsychotic medications. Various practice guidelines have been developed in the United States and internationally to assess for metabolic risk factors in people prescribed antipsychotic medications [26]. Current metabolic monitoring guidelines in the United States stem from 2004 consensus recommendations of the American Diabetes Association and American Psychiatric Association along with the American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity for metabolic monitoring among people prescribed SGAs [23]. These recommendations include a time line for routine monitoring of weight/body mass index, waist circumference, blood pressure, fasting blood glucose or hemoglobin A1c, and fasting lipids (Table 2). Guidelines recommend screening at baseline, more frequently within the first 3 months, and then annually [23].
Though guidelines recommend measurement of waist circumference as a marker for metabolic health, body mass index is often used alone as a measure of obesity [27,28]. This may be due to the relative ease of obtaining weight over waist circumference. For example, weight is more likely to be part of clinic workflows and many providers may not be accustomed to measuring waist circumference. However, waist circumference does provide additional information regarding metabolic health [29], as central adiposity is a marker of cardiometabolic risk and related to insulin resistance [21]. Further modifications of the guidelines have included ethnicity-specific waist measurements [30].
There is evidence that non-fasting lipids may be substituted for fasting lipid panels, particularly for patients who may have difficulty adhering to fasting due to cognitive difficulties. Vanderlip and colleagues argue that fasting serum cholesterol panels are not necessary for screening for dyslipidemia given that non-HDL cholesterol is calculated based on total cholesterol and HDL, which do not substantially differ between fasting and non-fasting values [31]. Hemoglobin A1c is recommended as a screening test for blood glucose abnormalities given that it does not require a fasting state and can therefore be more easily obtained for many patients. The choice to obtain a fasting blood glucose versus hemoglobin A1c may depend on multiple factors, including that a person can adhere to fasting and the cost of the laboratory test.
Routine monitoring of metabolic parameters is an integral step in targeting interventions to treat metabolic syndrome. These interventions include lifestyle modifications and evidence-based treatment guidelines for management of associated dyslipidemia, hypertension, and type 2 diabetes mellitus.
Current Metabolic Screening Practices
Despite the presence of defined guidelines, estimates show persistently low rates of metabolic monitoring among adults prescribed SGAs [32]. One study of 3 state Medicaid programs showed little to no improvement in screening rates for glucose and lipids post dissemination of the 2004 APA/ADA guidelines [33]. They noted a nonsignificant change in rates of glucose testing from 27% to 30% and small change in lipid testing from 10% to 11% among patients prescribed SGAs between 2002–2005 [33]. Examining screening rates among Medicaid recipients in Missouri between 2010–2012, Morrato and colleagues found glucose testing rates of 80% with lipid testing remaining at 41% [34]. A retrospective study of adult Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 showed rates of screening for lipids and glucose to increase over time; glucose monitoring increased from 56.6% to 72.6% and lipids from 38.3% to 41.2% [35]. A review by Mangurian and colleagues suggested rates of glucose (fasting blood glucose or hemoglobin A1c) and lipid screening as low as 30% among people prescribed antipsychotic medications [14]. Furthermore, they underscore the impact of low screening rates, stating that if 20% of adults with SMI have diabetes and 70% remain unscreened, then approximately 2 million adults with SMI and diabetes in the United States would not be identified within our current system [14].
Higher rates of screening have been shown for Medicaid populations than commercially insured populations [36]. Haupt et al compared lipid and glucose testing pre- and post- ADA/APA guideline implementation among commercially insured patients. They found an increase from 8.4% to 10.5% post guideline implementation for baseline lipid testing and from 6.8% to 9.0% for lipid testing at 12 weeks post-antipsychotic initiation [36]. Baseline glucose testing increased from 17.3% to 21.8% and from 14.1% to 17.9 % at 12-week post antipsychotic initiation. In alignment with findings from other studies, testing rates were particularly low for children [36].
Low screening rates have been found among children and adolescents prescribed SGAs [37] despite evidence that youth may be at risk of developing more significant metabolic sequelae from SGAs [19]. Edelsohn and colleagues found an increase from 30% to 50% for glucose screening and from 19% to 28% for lipid screening among youth Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 [35]. Connolly and colleagues reported on metabolic screening rates for children and adolescents prescribed SGAs over the 8 years following announcement of the 2004 ADA/APA guidelines. Using insurance claims data, they found screening rates for fasting blood glucose and hemoglobin A1c temporarily increased following guideline dissemination, then dropped during the period 2004–2008, and again increased slightly [38].
Barriers to Screening
Barriers to screening exist at the level of the individual patient and provider as well as at the clinic and larger systems levels. Lack of provider awareness of evidence-based guidelines for metabolic monitoring despite the presence of the 2004 ADA/APA guidelines has been cited by researchers as an impediment to screening. In a survey of primary care clinicians in San Francisco, Mangurian et al found that 40% of primary care providers did not know about the ADA/APA consensus guidelines for metabolic monitoring. The same survey of primary care providers identified additional impediments to screening, including obstacles to collaboration with psychiatric providers and to scheduling patients for psychiatric follow-up [39]. Another clinician survey conducted by Parameswaran et al found that psychiatrists viewed psychiatric illness severity, lack of staff time, and lack of clinician time as significant barriers to metabolic screening. In addition, clinicians identified factors related to the complexity of coordinating care across systems as obstacles; these included barriers to coordinating follow-up with medical providers, long wait times for patients to see medical providers, and difficulty collaborating with medical providers [40].
Other systems-level barriers include lack of a population-based approach to screening (eg, registries) and lack of electronic record integration, which impedes the ability of primary care and psychiatry providers to share information related to the ordering of metabolic screening tests and prescribing of medications [41]. Mangurian calls for integration of electronic medical record systems between primary care and psychiatry, a population-based approach to metabolic monitoring utilizing registries and other elements of collaborative care models, and primary care consultation to aid in the treatment of metabolic abnormalities [41]. Amiel et al point to systems-level factors “including but not limited to … poor access to general medical services, inadequate medical record-keeping infrastructure, lack of in-system compliance incentives and lack of centralized oversight” [26].
Based on their experience implementing a computer-based intervention for metabolic monitoring, Lai et al propose that the following factors may influence providers’ engagement in metabolic monitoring: lack of apparent symptoms to suggest metabolic syndrome, patients’ lack of engagement in care, and poor access to care. They identify additional factors at the clinician level to include under-recognition of the need for metabolic monitoring, lack of familiarity with screening guidelines, lack of agreement with guidelines, and the potential for individual clinicians to forget to order tests [42]. At the systems-level, they identify the absence of ongoing training as a potential reason why sustained testing was not observed in their intervention [42].
In a 2011 survey of providers prescribing antipsychotic medication to Medicaid beneficiaries in Missouri, Morrato and colleagues found that factors limiting frequency of health care utilization were closely linked to lack of metabolic testing. They also noted disparities in screening guidelines may lead to lack of routine metabolic monitoring; providers may screen based on prescribed medication, diagnosis, or other risk factor based stratification depending on the guidelines followed [34].
Current Unmet Needs
Vulnerable Populations
Though rates of metabolic screening remain low for all groups prescribed antipsychotic medications, studies have consistently shown low rates of screening among children and adolescents [35,36]. Edelsohn and colleagues hypothesize that the cause of these low rates is multifactorial, including that guardians may be reluctant to have young people undergo blood draws [35]. Morrato and colleagues suggest that policymakers should focus initiatives on younger, healthier adults, who they found to have lower rates of screening [37].
Racial and ethnic minorities with SMI constitute another particularly vulnerable population, with some studies showing an increased risk of metabolic sequelae and lower likelihood of treatment for diabetes and other metabolic derangements among African American and Latino populations with SMI [14,43,44].
Integration of Care
Lack of widespread integration of care between mental health and primary care remains another unmet need [41]. Hasnain and colleagues recommend improved communication between mental health and primary care clinicians to coordinate care to improve rates of monitoring, facilitate early follow-up of metabolic abnormalities, and avoid duplication of monitoring efforts [45]. Morrato and colleagues recommend that efforts to increase rates of metabolic monitoring be targeted not only to providers practicing in community mental health centers, but also to other practice settings including primary care. They found that for 75% of people prescribed antipsychotic medications, the prescriptions were started by prescribing providers who practiced outside of a community mental health center [34] and recommend that educational initiatives and performance improvement interventions broaden to include primary care and other care settings [34].
Potential Interventions for Improvement
Early interventions to improve metabolic screening rates have included educational initiatives to teach providers about consensus guidelines. However, initiatives to educate clinicians on metabolic monitoring have shown to be inadequate to significantly improve rates of screening [33]. Therefore, subsequent initiatives have sought to influence screening rates by targeting behavior of individual clinicians with point-of-care tools, electronic reminders, or through systems-level reorganization towards population-based care [27,42,46].
A variety of clinical interventions focus on technologies that remind clinicians to order metabolic monitoring tests according to screening guidelines. One public mental health service in Queensland, Australia, created a standardized metabolic monitoring form to be uploaded to the electronic medical record. In their implementation study examining the efficacy of the metabolic monitoring form, they found that only 36% of the forms contained data. When data were recorded, there were significantly higher rates of documentation of measurements (weight, body mass index, blood pressure) rather than laboratory tests (including lipids and fasting blood glucose) [27].
Computerized reminder systems for metabolic monitoring have been studied in both outpatient and inpatient settings. Lai and colleagues studied the impact of a computerized reminder system on lab monitoring for metabolic parameters among outpatients with schizophrenia prescribed SGAs [42]. This intervention also included an educational component with discussion of metabolic monitoring for people prescribed SGAs at meetings with attending psychiatrists. Computer reminders were displayed when a provider failed to order fasting plasma glucose or lipids (cholesterol, triglyceride) for patients prescribed clozapine, olanzapine, quetiapine, or risperidone. The study found a statistically significant improvement in laboratory metabolic screening for patients prescribed SGAs after implementation, with the greatest impact 6-months post-intervention, though with subsequent decline in screening rates [42].
Psychiatric inpatient hospitalizations provide an opportunity to obtain testing at the time of treatment initiation and also for ongoing monitoring in a location where fasting laboratory tests may be more easily obtained given onsite phlebotomy. One intervention targeting psychiatric inpatients utilized a computerized physician order entry system with the goal to improve metabolic screening among patients prescribed SGAs. Set in a large academic medical setting, the study found inpatient metabolic monitoring rates did not change significantly after implementation of these pop-up computer alerts, comparing rates immediately and 4 years after implementation [46].
There has been increasing focus on integrating mental health and medical care in an effort to improve the health of people with mental illness [47]. Mangurian and colleagues found that the likelihood of diabetes mellitus screening doubled for people with severe mental illness who were seen for at least one primary care visit in addition to mental health treatment [48]. Haupt similarly found higher rates of metabolic screening among patients who had greater than one primary care visit [36]. Models of integration include both integration of medical services into mental health treatment as well as incorporation of mental health services into primary care. For people with SMI, integration efforts have largely focused on integrating primary care services into community mental health settings [49]. The Substance Abuse and Mental Health Service Administration’s (SAMHSA) Primary and Behavioral Health Care Integration (PBHCI) grants program and the Affordable Care Act’s Health Home Initiative are examples of federal incentive programs for improved integration between behavioral health and primary care [49]. In their evaluation of the PBHCI grant program, Scharf and colleagues presented findings that patients at 3 matched clinics with PCBHI grants showed improvement in some lipids, diastolic blood pressure, and fasting blood glucose, though not smoking or body mass index [50].
Conclusion
Several risk factors contribute to an increase in cardiometabolic risk for people with severe mental illness, including poor nutrition, sedentary lifestyle, social determinants of health, and prescribed antipsychotic medications. Metabolic monitoring aims to address these health disparities by screening for metabolic parameters and identifying abnormalities in order to target appropriate health interventions. Screening rates for metabolic parameters remain low for children, adolescents, and adults prescribed second-generation antipsychotics despite published guidelines and clinical interventions to improve screening. More system-wide interventions to improve collaboration between mental health and primary care are needed to enhance screening and prevent cardiovascular disease risk in this vulnerable population.
Corresponding author: Carrie Cunningham, MD, MPH, Zuckerberg San Francisco General Hospital, 1001 Potrero Ave, Suite 7M, San Francisco, CA 94110, [email protected].
Funding/support: Dr. Cunningham was supported by the UCSF-Zuckerberg San Francisco General Public Psychiatry Fellowship. Mr. Riano was supported by the NIH Center Grant from the National Institute of Diabetes and Digestive and Kidney Diseases for The Health Delivery Systems-Center for Diabetes Translational Research (CDTR) (P30DK092924) and by the UCSF-San Francisco General Hospital Public Psychiatry Fellowship. Dr. Mangurian received support from a grant from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R03 DK101857), as well as NIH Career Development Award (K23MH093689).
From the University of California San Francisco, Department of Psychiatry, Weill Institute for Neurosciences, San Francisco, CA.
Abstract
- Objective: To review screening for metabolic syndrome in people with severe mental illness (SMI).
- Methods: Review of the literature.
- Results: Despite evidence-based metabolic screening guidelines, rates of metabolic screening remain low among people with SMI. Barriers to screening exist at the individual, organizational, and systems levels. Interventions to address these barriers range from point-of-care tools to systems-level reorganization towards population-based care.
- Conclusion: Greater systems-level interventions, particularly those that improve collaboration between mental health and primary care, are needed to improve metabolic monitoring and identify cardiovascular disease risk among people with SMI.
Key words: metabolic monitoring; severe mental illness; metabolic syndrome; integrated care.
People with severe mental illness (SMI) have a life expectancy 10 to 20 years shorter than the general population, and cardiometabolic risk factors contribute significantly to the increased morbidity and mortality seen in this population. To address this health disparity, metabolic monitoring guidelines have been proposed as a mechanism to identify metabolic risk factors. This paper aims to discuss metabolic syndrome and its risk factors, describe metabolic monitoring including current rates and barriers to screening, and identify interventions that may improve rates of screening for metabolic syndrome among people with SMI.
Metabolic syndrome has been conceptualized as a state of chronic low-grade inflammation and hypercoagulation associated with hypertension, dyslipidemia, glucose intolerance, insulin resistance, and visceral adiposity [1]. Per the modified National Cholesterol Education Program Adult Treatment Plan III (NCEP ATP III) guidelines, metabolic syndrome is defined as the presence of 3 of the following 5 parameters: (1) blood glucose > 100 mg/dL (or a person is taking a hypoglycemic medication), (2) high density lipoprotein (HDL) < 40 mg/dL in men or < 50 mg/dL in women, (3) triglycerides > 150 mg/dL (or taking a lipid lowering agent), (4) waist circumference > 40 inches in men or > 35 inches in women, and/or (5) blood pressure > 130/85 mm Hg (or taking an antihypertensive medication) [2,3] (Table 1).
Metabolic syndrome is associated with an increased risk of diabetes mellitus, cardiovascular disease (including myocardial infarction and cerebrovascular accident), and all-cause mortality [3]. Other systemic effects related to metabolic syndrome include renal, hepatic, and skin manifestations such as chronic kidney disease, non-alcoholic steatohepatitis, and obstructive sleep apnea [1].
Epidemiology and Risk Factors
An estimated 34% of people in the United States meet criteria for metabolic syndrome, with worldwide estimates ranging widely from less than 10% to 84%. People with SMI (eg, bipolar disorder, schizoaffective disorder, schizophrenia) are at even greater risk of developing metabolic syndrome than the general population [4,5]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study demonstrated metabolic syndrome rates of 40.9% and 51.6% in men and women with a diagnosis of schizophrenia, respectively [6]. In a systematic review of bipolar disorder and metabolic syndrome, people with bipolar disorder showed higher rates of hypertriglyceridemia and hyperglycemia than controls [5].
People with SMI have been found to have significantly increased morbidity and mortality as compared to people without an SMI diagnosis, much of which has been attributed to increased cardiometabolic risk related to multiple factors [7]. Among adults with schizophrenia receiving Medicaid, Olfson et al found diabetes mellitus, ischemic heart disease, nonischemic heart disease, and cerebrovascular accident to be among the top 10 causes of death [7]. The mortality rate for people with SMI is estimated to be 2 to 3 times higher than the general population, and the life expectancy for people with SMI is estimated to be 10 to 20 years shorter than the general population [8–10]. Contributors to this disparity include modifiable health-related behaviors, social determinants of health, and iatrogenic sequelae of prescribed medications. Behavioral factors include poor nutrition, food insecurity, sedentary lifestyle, and smoking; side effects of commonly prescribed psychotropic medications, most notably atypical antipsychotics and mood stabilizers, also contribute to this disparity [7,11].
Both first- and second-generation antipsychotics have been shown to be associated with metabolic sequelae, including weight gain, elevated blood glucose, and insulin resistance [12–14]. Among psychotropic medications, the atypical or second-generation antipsychotics (SGAs) are a class of medications known to have significant metabolic side effects [15,16]. Studies comparing the metabolic consequences of individual SGAs have found significant variation within the class. Clozapine, olanzapine, quetiapine, and risperidone show significant likelihood of weight gain, hyperlipidemia, and hyperglycemia as well as other metabolic consequences [17]. Aripiprazole, lurasidone, and ziprasidone have shown little to no risk of metabolic sequelae [17].
Metabolic side effects of SGAs have been demonstrated in children, adolescents, and adults. There is evidence that adolescents may be particularly sensitive to these sequelae. Galling and colleagues found that adolescents treated with antipsychotics were at greater risk of developing type 2 diabetes mellitus as compared to both healthy controls and controls with psychiatric illness [18]. Kryzhanovskaya et al, looking at metabolic parameters associated with olanzapine use in adolescents and adults, found that both adolescents and adults showed metabolic sequelae and that adolescents had larger changes in weight gain and lipids compared with adults [19].
The mechanism of SGA impact on metabolic parameters remains incompletely understood, though is thought to be multifactorial, mediated primarily through weight gain with increased adiposity. SGA histamine (H1) receptor binding affinity is implicated in weight gain [20] and 5HT2C antagonism may also lead to an increase in appetite [21]. Other proposed mechanisms include changes in appetite through leptin resistance or decreased sensitivity to leptin, the hormone that mediates satiety. Zhang and colleagues found an increase in leptin levels in patients with schizophrenia prescribed antipsychotics, suggesting leptin dysregulation [21]. Additional studies suggest metabolic disturbances independent of weight gain including direct effects of SGAs on glucose and lipid metabolism [22].
If a person experiences a weight gain of 5% after starting an SGA, it is recommended that the dose be decreased or that they be switched to another psychotropic medication with lower likelihood of metabolic consequences [23]. The effectiveness of switching antipsychotic medications to one with lower metabolic risk to improve weight and lipids has been previously demonstrated [24]. If a patient develops diabetes in the context of an antipsychotic prescription, it is also recommended that the medication be switched to an antipsychotic with less risk of hyperglycemia, and if not possible, to target additional risk factors including weight, poor nutrition, and sedentary lifestyle [25]. The decision to switch medications or decrease dosage is often weighed against the psychiatric stability of the person and their overall response to the medication in the context of their treatment course [14].
Metabolic Monitoring
Given the increased risk of metabolic syndrome among people with SMI, and the association of metabolic syndrome with increased morbidity and all-cause mortality, there has been a growing awareness of the importance of screening for metabolic syndrome among people with SMI. Metabolic monitoring involves routine screening for metabolic parameters and assessment of metabolic risk factors among people with SMI who are prescribed antipsychotic medications. Various practice guidelines have been developed in the United States and internationally to assess for metabolic risk factors in people prescribed antipsychotic medications [26]. Current metabolic monitoring guidelines in the United States stem from 2004 consensus recommendations of the American Diabetes Association and American Psychiatric Association along with the American Association of Clinical Endocrinologists and the North American Association for the Study of Obesity for metabolic monitoring among people prescribed SGAs [23]. These recommendations include a time line for routine monitoring of weight/body mass index, waist circumference, blood pressure, fasting blood glucose or hemoglobin A1c, and fasting lipids (Table 2). Guidelines recommend screening at baseline, more frequently within the first 3 months, and then annually [23].
Though guidelines recommend measurement of waist circumference as a marker for metabolic health, body mass index is often used alone as a measure of obesity [27,28]. This may be due to the relative ease of obtaining weight over waist circumference. For example, weight is more likely to be part of clinic workflows and many providers may not be accustomed to measuring waist circumference. However, waist circumference does provide additional information regarding metabolic health [29], as central adiposity is a marker of cardiometabolic risk and related to insulin resistance [21]. Further modifications of the guidelines have included ethnicity-specific waist measurements [30].
There is evidence that non-fasting lipids may be substituted for fasting lipid panels, particularly for patients who may have difficulty adhering to fasting due to cognitive difficulties. Vanderlip and colleagues argue that fasting serum cholesterol panels are not necessary for screening for dyslipidemia given that non-HDL cholesterol is calculated based on total cholesterol and HDL, which do not substantially differ between fasting and non-fasting values [31]. Hemoglobin A1c is recommended as a screening test for blood glucose abnormalities given that it does not require a fasting state and can therefore be more easily obtained for many patients. The choice to obtain a fasting blood glucose versus hemoglobin A1c may depend on multiple factors, including that a person can adhere to fasting and the cost of the laboratory test.
Routine monitoring of metabolic parameters is an integral step in targeting interventions to treat metabolic syndrome. These interventions include lifestyle modifications and evidence-based treatment guidelines for management of associated dyslipidemia, hypertension, and type 2 diabetes mellitus.
Current Metabolic Screening Practices
Despite the presence of defined guidelines, estimates show persistently low rates of metabolic monitoring among adults prescribed SGAs [32]. One study of 3 state Medicaid programs showed little to no improvement in screening rates for glucose and lipids post dissemination of the 2004 APA/ADA guidelines [33]. They noted a nonsignificant change in rates of glucose testing from 27% to 30% and small change in lipid testing from 10% to 11% among patients prescribed SGAs between 2002–2005 [33]. Examining screening rates among Medicaid recipients in Missouri between 2010–2012, Morrato and colleagues found glucose testing rates of 80% with lipid testing remaining at 41% [34]. A retrospective study of adult Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 showed rates of screening for lipids and glucose to increase over time; glucose monitoring increased from 56.6% to 72.6% and lipids from 38.3% to 41.2% [35]. A review by Mangurian and colleagues suggested rates of glucose (fasting blood glucose or hemoglobin A1c) and lipid screening as low as 30% among people prescribed antipsychotic medications [14]. Furthermore, they underscore the impact of low screening rates, stating that if 20% of adults with SMI have diabetes and 70% remain unscreened, then approximately 2 million adults with SMI and diabetes in the United States would not be identified within our current system [14].
Higher rates of screening have been shown for Medicaid populations than commercially insured populations [36]. Haupt et al compared lipid and glucose testing pre- and post- ADA/APA guideline implementation among commercially insured patients. They found an increase from 8.4% to 10.5% post guideline implementation for baseline lipid testing and from 6.8% to 9.0% for lipid testing at 12 weeks post-antipsychotic initiation [36]. Baseline glucose testing increased from 17.3% to 21.8% and from 14.1% to 17.9 % at 12-week post antipsychotic initiation. In alignment with findings from other studies, testing rates were particularly low for children [36].
Low screening rates have been found among children and adolescents prescribed SGAs [37] despite evidence that youth may be at risk of developing more significant metabolic sequelae from SGAs [19]. Edelsohn and colleagues found an increase from 30% to 50% for glucose screening and from 19% to 28% for lipid screening among youth Medicaid recipients prescribed first- and second-generation antipsychotics between 2008 and 2012 [35]. Connolly and colleagues reported on metabolic screening rates for children and adolescents prescribed SGAs over the 8 years following announcement of the 2004 ADA/APA guidelines. Using insurance claims data, they found screening rates for fasting blood glucose and hemoglobin A1c temporarily increased following guideline dissemination, then dropped during the period 2004–2008, and again increased slightly [38].
Barriers to Screening
Barriers to screening exist at the level of the individual patient and provider as well as at the clinic and larger systems levels. Lack of provider awareness of evidence-based guidelines for metabolic monitoring despite the presence of the 2004 ADA/APA guidelines has been cited by researchers as an impediment to screening. In a survey of primary care clinicians in San Francisco, Mangurian et al found that 40% of primary care providers did not know about the ADA/APA consensus guidelines for metabolic monitoring. The same survey of primary care providers identified additional impediments to screening, including obstacles to collaboration with psychiatric providers and to scheduling patients for psychiatric follow-up [39]. Another clinician survey conducted by Parameswaran et al found that psychiatrists viewed psychiatric illness severity, lack of staff time, and lack of clinician time as significant barriers to metabolic screening. In addition, clinicians identified factors related to the complexity of coordinating care across systems as obstacles; these included barriers to coordinating follow-up with medical providers, long wait times for patients to see medical providers, and difficulty collaborating with medical providers [40].
Other systems-level barriers include lack of a population-based approach to screening (eg, registries) and lack of electronic record integration, which impedes the ability of primary care and psychiatry providers to share information related to the ordering of metabolic screening tests and prescribing of medications [41]. Mangurian calls for integration of electronic medical record systems between primary care and psychiatry, a population-based approach to metabolic monitoring utilizing registries and other elements of collaborative care models, and primary care consultation to aid in the treatment of metabolic abnormalities [41]. Amiel et al point to systems-level factors “including but not limited to … poor access to general medical services, inadequate medical record-keeping infrastructure, lack of in-system compliance incentives and lack of centralized oversight” [26].
Based on their experience implementing a computer-based intervention for metabolic monitoring, Lai et al propose that the following factors may influence providers’ engagement in metabolic monitoring: lack of apparent symptoms to suggest metabolic syndrome, patients’ lack of engagement in care, and poor access to care. They identify additional factors at the clinician level to include under-recognition of the need for metabolic monitoring, lack of familiarity with screening guidelines, lack of agreement with guidelines, and the potential for individual clinicians to forget to order tests [42]. At the systems-level, they identify the absence of ongoing training as a potential reason why sustained testing was not observed in their intervention [42].
In a 2011 survey of providers prescribing antipsychotic medication to Medicaid beneficiaries in Missouri, Morrato and colleagues found that factors limiting frequency of health care utilization were closely linked to lack of metabolic testing. They also noted disparities in screening guidelines may lead to lack of routine metabolic monitoring; providers may screen based on prescribed medication, diagnosis, or other risk factor based stratification depending on the guidelines followed [34].
Current Unmet Needs
Vulnerable Populations
Though rates of metabolic screening remain low for all groups prescribed antipsychotic medications, studies have consistently shown low rates of screening among children and adolescents [35,36]. Edelsohn and colleagues hypothesize that the cause of these low rates is multifactorial, including that guardians may be reluctant to have young people undergo blood draws [35]. Morrato and colleagues suggest that policymakers should focus initiatives on younger, healthier adults, who they found to have lower rates of screening [37].
Racial and ethnic minorities with SMI constitute another particularly vulnerable population, with some studies showing an increased risk of metabolic sequelae and lower likelihood of treatment for diabetes and other metabolic derangements among African American and Latino populations with SMI [14,43,44].
Integration of Care
Lack of widespread integration of care between mental health and primary care remains another unmet need [41]. Hasnain and colleagues recommend improved communication between mental health and primary care clinicians to coordinate care to improve rates of monitoring, facilitate early follow-up of metabolic abnormalities, and avoid duplication of monitoring efforts [45]. Morrato and colleagues recommend that efforts to increase rates of metabolic monitoring be targeted not only to providers practicing in community mental health centers, but also to other practice settings including primary care. They found that for 75% of people prescribed antipsychotic medications, the prescriptions were started by prescribing providers who practiced outside of a community mental health center [34] and recommend that educational initiatives and performance improvement interventions broaden to include primary care and other care settings [34].
Potential Interventions for Improvement
Early interventions to improve metabolic screening rates have included educational initiatives to teach providers about consensus guidelines. However, initiatives to educate clinicians on metabolic monitoring have shown to be inadequate to significantly improve rates of screening [33]. Therefore, subsequent initiatives have sought to influence screening rates by targeting behavior of individual clinicians with point-of-care tools, electronic reminders, or through systems-level reorganization towards population-based care [27,42,46].
A variety of clinical interventions focus on technologies that remind clinicians to order metabolic monitoring tests according to screening guidelines. One public mental health service in Queensland, Australia, created a standardized metabolic monitoring form to be uploaded to the electronic medical record. In their implementation study examining the efficacy of the metabolic monitoring form, they found that only 36% of the forms contained data. When data were recorded, there were significantly higher rates of documentation of measurements (weight, body mass index, blood pressure) rather than laboratory tests (including lipids and fasting blood glucose) [27].
Computerized reminder systems for metabolic monitoring have been studied in both outpatient and inpatient settings. Lai and colleagues studied the impact of a computerized reminder system on lab monitoring for metabolic parameters among outpatients with schizophrenia prescribed SGAs [42]. This intervention also included an educational component with discussion of metabolic monitoring for people prescribed SGAs at meetings with attending psychiatrists. Computer reminders were displayed when a provider failed to order fasting plasma glucose or lipids (cholesterol, triglyceride) for patients prescribed clozapine, olanzapine, quetiapine, or risperidone. The study found a statistically significant improvement in laboratory metabolic screening for patients prescribed SGAs after implementation, with the greatest impact 6-months post-intervention, though with subsequent decline in screening rates [42].
Psychiatric inpatient hospitalizations provide an opportunity to obtain testing at the time of treatment initiation and also for ongoing monitoring in a location where fasting laboratory tests may be more easily obtained given onsite phlebotomy. One intervention targeting psychiatric inpatients utilized a computerized physician order entry system with the goal to improve metabolic screening among patients prescribed SGAs. Set in a large academic medical setting, the study found inpatient metabolic monitoring rates did not change significantly after implementation of these pop-up computer alerts, comparing rates immediately and 4 years after implementation [46].
There has been increasing focus on integrating mental health and medical care in an effort to improve the health of people with mental illness [47]. Mangurian and colleagues found that the likelihood of diabetes mellitus screening doubled for people with severe mental illness who were seen for at least one primary care visit in addition to mental health treatment [48]. Haupt similarly found higher rates of metabolic screening among patients who had greater than one primary care visit [36]. Models of integration include both integration of medical services into mental health treatment as well as incorporation of mental health services into primary care. For people with SMI, integration efforts have largely focused on integrating primary care services into community mental health settings [49]. The Substance Abuse and Mental Health Service Administration’s (SAMHSA) Primary and Behavioral Health Care Integration (PBHCI) grants program and the Affordable Care Act’s Health Home Initiative are examples of federal incentive programs for improved integration between behavioral health and primary care [49]. In their evaluation of the PBHCI grant program, Scharf and colleagues presented findings that patients at 3 matched clinics with PCBHI grants showed improvement in some lipids, diastolic blood pressure, and fasting blood glucose, though not smoking or body mass index [50].
Conclusion
Several risk factors contribute to an increase in cardiometabolic risk for people with severe mental illness, including poor nutrition, sedentary lifestyle, social determinants of health, and prescribed antipsychotic medications. Metabolic monitoring aims to address these health disparities by screening for metabolic parameters and identifying abnormalities in order to target appropriate health interventions. Screening rates for metabolic parameters remain low for children, adolescents, and adults prescribed second-generation antipsychotics despite published guidelines and clinical interventions to improve screening. More system-wide interventions to improve collaboration between mental health and primary care are needed to enhance screening and prevent cardiovascular disease risk in this vulnerable population.
Corresponding author: Carrie Cunningham, MD, MPH, Zuckerberg San Francisco General Hospital, 1001 Potrero Ave, Suite 7M, San Francisco, CA 94110, [email protected].
Funding/support: Dr. Cunningham was supported by the UCSF-Zuckerberg San Francisco General Public Psychiatry Fellowship. Mr. Riano was supported by the NIH Center Grant from the National Institute of Diabetes and Digestive and Kidney Diseases for The Health Delivery Systems-Center for Diabetes Translational Research (CDTR) (P30DK092924) and by the UCSF-San Francisco General Hospital Public Psychiatry Fellowship. Dr. Mangurian received support from a grant from the NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R03 DK101857), as well as NIH Career Development Award (K23MH093689).
1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014.
2. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
3. American Heart Association. What is metabolic syndrome? 2015.
4. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339–47.
5. Czepielewski L, Daruy Filho L, Brietzke E, Grassi-Oliveira R. Bipolar disorder and metabolic syndrome: a systematic review. Rev Bras Psiquiatria 2013;35:88–93.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19–32.
7. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015:1–10.
8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123–31.
9. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41.
10. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chron Dis 2006;3:A42.
11. Williams J, Stubbs B, Gaughran F, Craig T. ‘Walk This Way’–a pilot of a health coaching intervention to reduce sedentary behaviour and increase low intensity exercise in people with serious mental illness: study protocol for a randomised controlled trial. Trials 2016;17:594.
12. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–96.
13. Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: differences between antipsychotic-naïve and treated patients. J Pharmacol Pharmacother 2013;4:176–86.
14. Mangurian C, Newcomer JW, Modlin C, Schillinger D. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med 2016:1–9.
15. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005;19(Suppl 1):1–93.
16. Baptista T, De Mendoza S, Beaulieu S, et al. The metabolic syndrome during atypical antipsychotic drug treatment: mechanisms and management. Metab Syndr Relat Disord 2004;2:290–307.
17. Hert MDE, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52–77.
18. Galling B, Roldan A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:247–59.
19. Kryzhanovskaya LA, Xu W, Millen BA, et al. Comparison of long-term (at least 24 weeks) weight gain and metabolic changes between adolescents and adults treated with olanzapine. J Child Adol Psychopharmacol 2012;22:157–65.
20. Nasrallah H. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008;13:27–35.
21. Zhang Z-J, YAO Z-J, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Br J Psychiatry 2004;184:58–62.
22. Kang SH, Lee JI. Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatr Invest 2015;12:242–8.
23. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 596–601.
24. Weiden PJ, Newcomer JW, Loebel AD, et al. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33:985–94.
25. Henderson DC. Atypical antipsychotic-induced diabetes mellitus. CNS Drugs 2002;16:77–89.
26. Amiel JM, Mangurian CV, Ganguli R, Newcomer JW. Addressing cardiometabolic risk during treatment with antipsychotic medications. Curr Opin Psychiatry 2008;21:613–8.
27. Happell B, Platania-Phung C, Gaskin CJ, Stanton R. Use of an electronic metabolic monitoring form in a mental health service–a retrospective file audit. BMC Psychiatry 2016;16:109.
28. Rosenbaum S, Nijjar S, Watkins A, et al. Nurse‐assessed metabolic monitoring: A file audit of risk factor prevalence and impact of an intervention to enhance measurement of waist circumference. Int J Ment Health Nurs 2014;23:252–6.
29. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 2007;15:1061–7.
30. Tan C-E, Ma S, Wai D, et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004;27:1182–6.
31. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv 2014;65:573–6.
32. Mitchell A, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012;42:125–47.
33. Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry 2010;67:17–24.
34. Morrato EH, Campagna EJ, Brewer SE, et al. Metabolic testing for adults in a state Medicaid program receiving antipsychotics: remaining barriers to achieving population health prevention goals. JAMA Psychiatry 2016;73:721–30.
35. Edelsohn GA, Parthasarathy M, Terhorst L, et al. Measurement of metabolic monitoring in youth and adult Medicaid recipients prescribed antipsychotics. J Manage Care Specialty Pharm 2015;21:769–77.
36. Haupt DW, Rosenblatt LC, Kim E, et al. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 2009;166:345–53.
37. Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010;164:344–51.
38. Connolly JG, Toomey TJ, Schneeweiss MC. Metabolic monitoring for youths initiating use of second-generation antipsychotics, 2003–2011. Psychiatr Serv 2015;66:604–9.
39. Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv 2013;64:597–9.
40. Parameswaran SG, Chang C, Swenson AK, et al. Roles in and barriers to metabolic screening for people taking antipsychotic medications: a survey of psychiatrists. Schizophren Res 2013;143:395–6.
41. Mangurian C. Patient-centered medical care in community mental health settings. Psychiatr Serv 2017;68:213-.
42. Lai C-L, Chan H-Y, Pan Y-J, Chen C-H. The effectiveness of a computer reminder system for laboratory monitoring of metabolic syndrome in schizophrenic outpatients using second-generation antipsychotics. Pharmacopsychiatry 2015;48:25–9.
43. Lambert BL, Chou C-H, Chang K-Y, et al. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf 2005;14:417–25.
44. Ramaswamy K, Kozma CM, Nasrallah H. Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine. Drug Saf 2007;30:589–99.
45. Hasnain M, Vieweg WVR, Fredrickson SK, et al. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim Care Diab 2009;3:5–15.
46. Lee J, Dalack G, Casher M, et al. Persistence of metabolic monitoring for psychiatry inpatients treated with second‐generation antipsychotics utilizing a computer‐based intervention. J Clin Pharm Therap 2016;41:209–13.
47. Katz MH. Improving the health of persons with serious mental illness. JAMA Intern Med 2015;175:1979–80.
48. Mangurian C, Newcomer JW, Vittinghoff E, et al. Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med 2015;175:1977–9.
49. Gerrity M. Integrating primary care into behavioral health settings: What works. New York: Milbank Memorial Fund; 2014.
50. Scharf DM EN, Hackbarth NS, Horvitz-Lennon M, et al. Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: Final Report (Task 13). 2014.
1. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014.
2. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97.
3. American Heart Association. What is metabolic syndrome? 2015.
4. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta‐analysis. World Psychiatry 2015;14:339–47.
5. Czepielewski L, Daruy Filho L, Brietzke E, Grassi-Oliveira R. Bipolar disorder and metabolic syndrome: a systematic review. Rev Bras Psiquiatria 2013;35:88–93.
6. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19–32.
7. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry 2015:1–10.
8. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123–31.
9. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334–41.
10. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chron Dis 2006;3:A42.
11. Williams J, Stubbs B, Gaughran F, Craig T. ‘Walk This Way’–a pilot of a health coaching intervention to reduce sedentary behaviour and increase low intensity exercise in people with serious mental illness: study protocol for a randomised controlled trial. Trials 2016;17:594.
12. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686–96.
13. Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: differences between antipsychotic-naïve and treated patients. J Pharmacol Pharmacother 2013;4:176–86.
14. Mangurian C, Newcomer JW, Modlin C, Schillinger D. Diabetes and cardiovascular care among people with severe mental illness: a literature review. J Gen Intern Med 2016:1–9.
15. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005;19(Suppl 1):1–93.
16. Baptista T, De Mendoza S, Beaulieu S, et al. The metabolic syndrome during atypical antipsychotic drug treatment: mechanisms and management. Metab Syndr Relat Disord 2004;2:290–307.
17. Hert MDE, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52–77.
18. Galling B, Roldan A, Nielsen RE, et al. Type 2 diabetes mellitus in youth exposed to antipsychotics: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:247–59.
19. Kryzhanovskaya LA, Xu W, Millen BA, et al. Comparison of long-term (at least 24 weeks) weight gain and metabolic changes between adolescents and adults treated with olanzapine. J Child Adol Psychopharmacol 2012;22:157–65.
20. Nasrallah H. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008;13:27–35.
21. Zhang Z-J, YAO Z-J, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels. Br J Psychiatry 2004;184:58–62.
22. Kang SH, Lee JI. Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatr Invest 2015;12:242–8.
23. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 596–601.
24. Weiden PJ, Newcomer JW, Loebel AD, et al. Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 2008;33:985–94.
25. Henderson DC. Atypical antipsychotic-induced diabetes mellitus. CNS Drugs 2002;16:77–89.
26. Amiel JM, Mangurian CV, Ganguli R, Newcomer JW. Addressing cardiometabolic risk during treatment with antipsychotic medications. Curr Opin Psychiatry 2008;21:613–8.
27. Happell B, Platania-Phung C, Gaskin CJ, Stanton R. Use of an electronic metabolic monitoring form in a mental health service–a retrospective file audit. BMC Psychiatry 2016;16:109.
28. Rosenbaum S, Nijjar S, Watkins A, et al. Nurse‐assessed metabolic monitoring: A file audit of risk factor prevalence and impact of an intervention to enhance measurement of waist circumference. Int J Ment Health Nurs 2014;23:252–6.
29. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Obesity 2007;15:1061–7.
30. Tan C-E, Ma S, Wai D, et al. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care 2004;27:1182–6.
31. Vanderlip ER, Chwastiak LA, McCarron RM. Integrated care: nonfasting screening for cardiovascular risk among individuals taking second-generation antipsychotics. Psychiatr Serv 2014;65:573–6.
32. Mitchell A, Delaffon V, Vancampfort D, et al. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 2012;42:125–47.
33. Morrato EH, Druss B, Hartung DM, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry 2010;67:17–24.
34. Morrato EH, Campagna EJ, Brewer SE, et al. Metabolic testing for adults in a state Medicaid program receiving antipsychotics: remaining barriers to achieving population health prevention goals. JAMA Psychiatry 2016;73:721–30.
35. Edelsohn GA, Parthasarathy M, Terhorst L, et al. Measurement of metabolic monitoring in youth and adult Medicaid recipients prescribed antipsychotics. J Manage Care Specialty Pharm 2015;21:769–77.
36. Haupt DW, Rosenblatt LC, Kim E, et al. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry 2009;166:345–53.
37. Morrato EH, Nicol GE, Maahs D, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med 2010;164:344–51.
38. Connolly JG, Toomey TJ, Schneeweiss MC. Metabolic monitoring for youths initiating use of second-generation antipsychotics, 2003–2011. Psychiatr Serv 2015;66:604–9.
39. Mangurian C, Giwa F, Shumway M, et al. Primary care providers’ views on metabolic monitoring of outpatients taking antipsychotic medication. Psychiatr Serv 2013;64:597–9.
40. Parameswaran SG, Chang C, Swenson AK, et al. Roles in and barriers to metabolic screening for people taking antipsychotic medications: a survey of psychiatrists. Schizophren Res 2013;143:395–6.
41. Mangurian C. Patient-centered medical care in community mental health settings. Psychiatr Serv 2017;68:213-.
42. Lai C-L, Chan H-Y, Pan Y-J, Chen C-H. The effectiveness of a computer reminder system for laboratory monitoring of metabolic syndrome in schizophrenic outpatients using second-generation antipsychotics. Pharmacopsychiatry 2015;48:25–9.
43. Lambert BL, Chou C-H, Chang K-Y, et al. Antipsychotic exposure and type 2 diabetes among patients with schizophrenia: a matched case-control study of California Medicaid claims. Pharmacoepidemiol Drug Saf 2005;14:417–25.
44. Ramaswamy K, Kozma CM, Nasrallah H. Risk of diabetic ketoacidosis after exposure to risperidone or olanzapine. Drug Saf 2007;30:589–99.
45. Hasnain M, Vieweg WVR, Fredrickson SK, et al. Clinical monitoring and management of the metabolic syndrome in patients receiving atypical antipsychotic medications. Prim Care Diab 2009;3:5–15.
46. Lee J, Dalack G, Casher M, et al. Persistence of metabolic monitoring for psychiatry inpatients treated with second‐generation antipsychotics utilizing a computer‐based intervention. J Clin Pharm Therap 2016;41:209–13.
47. Katz MH. Improving the health of persons with serious mental illness. JAMA Intern Med 2015;175:1979–80.
48. Mangurian C, Newcomer JW, Vittinghoff E, et al. Diabetes screening among underserved adults with severe mental illness who take antipsychotic medications. JAMA Intern Med 2015;175:1977–9.
49. Gerrity M. Integrating primary care into behavioral health settings: What works. New York: Milbank Memorial Fund; 2014.
50. Scharf DM EN, Hackbarth NS, Horvitz-Lennon M, et al. Evaluation of the SAMHSA Primary and Behavioral Health Care Integration (PBHCI) Grant Program: Final Report (Task 13). 2014.