User login
What to Do When Your Depressed Patient Develops Mania
This article has been adapted from an article originally published in Current Psychiatry (http://currentpsychiatry.com):Current Psychiatry. 2015;14(10):29-32,35-40,e6.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does Disease Exist on a Unipolar-Bipolar Continuum.
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
- Formulate a definitive, overarching diagnosis
- Provide psycho-education
- Forecast return to work or school
- Discuss prognosis and likelihood of relapse
- Address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
- Determine whether indefinite maintenance pharmacotherapy is indicated—and, if so, with which medication(s).
A Diagnostic Formulation Isn’t Always Black and White
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine,
225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for > 20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How Does One Approach A Case Such As Ms. J's?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
- A first-lifetime episode of mania or hypomania is rare after age 50
- Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
- Years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
- None of Ms. J’s 4 pregnancies led to postpartum mood episodes
- At least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
- Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial Assessment and Treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptoms:
- Distractibility
- Indiscretion/impulsivity
- Grandiosity
- Flight of ideas
- Activity increase
- Sleep deficit
- Talkativeness.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the
present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes,current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state, and have no demonstrated value in preventing post-manic depression.3 Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short halflife serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
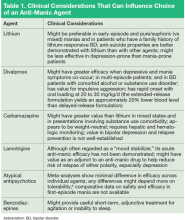
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than firstgeneration antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
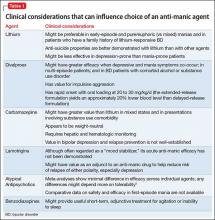
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep-wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What Medical and Neurologic Workup is Appropriate?
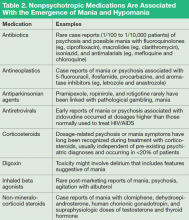
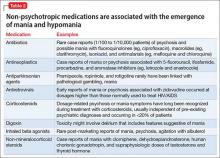
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
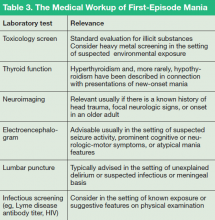
Nevertheless, evaluation of almost all first presentations of mania should include:
- Urine toxicology screen
- Complete blood count
- Comprehensive metabolic panel
- Thyroid-stimulating hormone assay
- Serum vitamin B12 level assay
- Serum folic acid level assay
- Rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
- Onset age > 40
- Absence of a family history of mood disorder
- Symptoms arising during a major medical illness
- Multiple medications
- Suspicion of a degenerative or hereditary neurologic disorder
- Altered state of consciousness
- Signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
- Abnormal vital signs.
Depending on the presentation, additional testing might include:
- Tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
- Heavy metal screening (when suggested by environmental exposure)
- Lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
- Neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
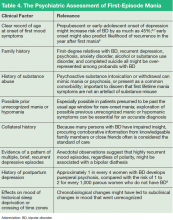
Making An Overarching Diagnosis: Is Mania Always Bipolar Disorder?
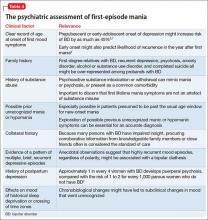
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Tables 3 and 4). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant (although antidepressants should be stopped when symptoms of mania emerge).11 Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
- Early (pre-adolescent) age at onset of first mood disorder episode6
- Family history of BD, highly recurrent depression, or psychosis12,13
- Psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15 In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressantresistant depression. Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
Indefinite Pharmacotherapy for Bipolar Disorder
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode or early age at onset.8,17
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
General practice at this time is lifetime medication following 2 manic episodes, or 1 episode if it was a severe episode and/or significant family history of bipolar or major depressive disorder is present. For a first episode of bipolar mania with no family history of bipolar or major depressive disorders, medication tapering and discontinuation may be considered after the continuation period is completed (usually 6 months in remission), depending on the severity of the first episode, surrounding factors, and prodromal history.18
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder, 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode.19 No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or Reintroduce an Antidepressant if Depression Recurs After a First Mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD).20 Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
- Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
- After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
- Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/hypomanic symptoms.22
- Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
- Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
- Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5
Psychoeducation and Forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks, it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.23
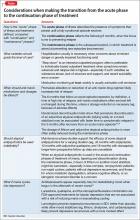
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (See this article at CurrentPsychiatry.com for a Table of considerations when making the transition from the acute phase to the continuation phase of treatment.20,24,25)
To minimize risk of relapse, psycho-education should include discussion of:
- Psychiatrically deleterious effects of alcohol and illicit drug use
- Suicide risk, including what to do in an emergency
- Protecting a regular sleep schedule and avoiding sleep deprivation
- The potential for poor medication adherence and management of side effects
- The role of adjunctive psychotherapy and effective stress management
- Familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Click here to read the digital edition.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11): 1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4): 509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective followup of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1): 125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar
disorder: a review. J Clin Psychiatry. 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002; 63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005; 7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar
depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2): 156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4): 476-488.
Note: Page numbers differ between the print issue and digital edition.
This article has been adapted from an article originally published in Current Psychiatry (http://currentpsychiatry.com):Current Psychiatry. 2015;14(10):29-32,35-40,e6.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does Disease Exist on a Unipolar-Bipolar Continuum.
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
- Formulate a definitive, overarching diagnosis
- Provide psycho-education
- Forecast return to work or school
- Discuss prognosis and likelihood of relapse
- Address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
- Determine whether indefinite maintenance pharmacotherapy is indicated—and, if so, with which medication(s).
A Diagnostic Formulation Isn’t Always Black and White
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine,
225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for > 20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How Does One Approach A Case Such As Ms. J's?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
- A first-lifetime episode of mania or hypomania is rare after age 50
- Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
- Years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
- None of Ms. J’s 4 pregnancies led to postpartum mood episodes
- At least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
- Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial Assessment and Treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptoms:
- Distractibility
- Indiscretion/impulsivity
- Grandiosity
- Flight of ideas
- Activity increase
- Sleep deficit
- Talkativeness.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the
present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes,current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state, and have no demonstrated value in preventing post-manic depression.3 Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short halflife serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than firstgeneration antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep-wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What Medical and Neurologic Workup is Appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
- Urine toxicology screen
- Complete blood count
- Comprehensive metabolic panel
- Thyroid-stimulating hormone assay
- Serum vitamin B12 level assay
- Serum folic acid level assay
- Rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
- Onset age > 40
- Absence of a family history of mood disorder
- Symptoms arising during a major medical illness
- Multiple medications
- Suspicion of a degenerative or hereditary neurologic disorder
- Altered state of consciousness
- Signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
- Abnormal vital signs.
Depending on the presentation, additional testing might include:
- Tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
- Heavy metal screening (when suggested by environmental exposure)
- Lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
- Neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making An Overarching Diagnosis: Is Mania Always Bipolar Disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Tables 3 and 4). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant (although antidepressants should be stopped when symptoms of mania emerge).11 Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
- Early (pre-adolescent) age at onset of first mood disorder episode6
- Family history of BD, highly recurrent depression, or psychosis12,13
- Psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15 In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressantresistant depression. Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
Indefinite Pharmacotherapy for Bipolar Disorder
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode or early age at onset.8,17
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
General practice at this time is lifetime medication following 2 manic episodes, or 1 episode if it was a severe episode and/or significant family history of bipolar or major depressive disorder is present. For a first episode of bipolar mania with no family history of bipolar or major depressive disorders, medication tapering and discontinuation may be considered after the continuation period is completed (usually 6 months in remission), depending on the severity of the first episode, surrounding factors, and prodromal history.18
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder, 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode.19 No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or Reintroduce an Antidepressant if Depression Recurs After a First Mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD).20 Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
- Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
- After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
- Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/hypomanic symptoms.22
- Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
- Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
- Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5
Psychoeducation and Forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks, it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.23
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (See this article at CurrentPsychiatry.com for a Table of considerations when making the transition from the acute phase to the continuation phase of treatment.20,24,25)
To minimize risk of relapse, psycho-education should include discussion of:
- Psychiatrically deleterious effects of alcohol and illicit drug use
- Suicide risk, including what to do in an emergency
- Protecting a regular sleep schedule and avoiding sleep deprivation
- The potential for poor medication adherence and management of side effects
- The role of adjunctive psychotherapy and effective stress management
- Familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Click here to read the digital edition.
This article has been adapted from an article originally published in Current Psychiatry (http://currentpsychiatry.com):Current Psychiatry. 2015;14(10):29-32,35-40,e6.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does Disease Exist on a Unipolar-Bipolar Continuum.
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
- Formulate a definitive, overarching diagnosis
- Provide psycho-education
- Forecast return to work or school
- Discuss prognosis and likelihood of relapse
- Address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
- Determine whether indefinite maintenance pharmacotherapy is indicated—and, if so, with which medication(s).
A Diagnostic Formulation Isn’t Always Black and White
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine,
225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for > 20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How Does One Approach A Case Such As Ms. J's?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
- A first-lifetime episode of mania or hypomania is rare after age 50
- Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
- Years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
- None of Ms. J’s 4 pregnancies led to postpartum mood episodes
- At least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
- Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial Assessment and Treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptoms:
- Distractibility
- Indiscretion/impulsivity
- Grandiosity
- Flight of ideas
- Activity increase
- Sleep deficit
- Talkativeness.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the
present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes,current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state, and have no demonstrated value in preventing post-manic depression.3 Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short halflife serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than firstgeneration antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep-wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What Medical and Neurologic Workup is Appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
- Urine toxicology screen
- Complete blood count
- Comprehensive metabolic panel
- Thyroid-stimulating hormone assay
- Serum vitamin B12 level assay
- Serum folic acid level assay
- Rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
- Onset age > 40
- Absence of a family history of mood disorder
- Symptoms arising during a major medical illness
- Multiple medications
- Suspicion of a degenerative or hereditary neurologic disorder
- Altered state of consciousness
- Signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
- Abnormal vital signs.
Depending on the presentation, additional testing might include:
- Tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
- Heavy metal screening (when suggested by environmental exposure)
- Lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
- Neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making An Overarching Diagnosis: Is Mania Always Bipolar Disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Tables 3 and 4). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant (although antidepressants should be stopped when symptoms of mania emerge).11 Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
- Early (pre-adolescent) age at onset of first mood disorder episode6
- Family history of BD, highly recurrent depression, or psychosis12,13
- Psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15 In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressantresistant depression. Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
Indefinite Pharmacotherapy for Bipolar Disorder
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode or early age at onset.8,17
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
General practice at this time is lifetime medication following 2 manic episodes, or 1 episode if it was a severe episode and/or significant family history of bipolar or major depressive disorder is present. For a first episode of bipolar mania with no family history of bipolar or major depressive disorders, medication tapering and discontinuation may be considered after the continuation period is completed (usually 6 months in remission), depending on the severity of the first episode, surrounding factors, and prodromal history.18
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder, 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode.19 No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or Reintroduce an Antidepressant if Depression Recurs After a First Mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD).20 Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
- Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
- After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
- Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/hypomanic symptoms.22
- Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
- Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
- Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5
Psychoeducation and Forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks, it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.23
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (See this article at CurrentPsychiatry.com for a Table of considerations when making the transition from the acute phase to the continuation phase of treatment.20,24,25)
To minimize risk of relapse, psycho-education should include discussion of:
- Psychiatrically deleterious effects of alcohol and illicit drug use
- Suicide risk, including what to do in an emergency
- Protecting a regular sleep schedule and avoiding sleep deprivation
- The potential for poor medication adherence and management of side effects
- The role of adjunctive psychotherapy and effective stress management
- Familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Click here to read the digital edition.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11): 1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4): 509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective followup of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1): 125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar
disorder: a review. J Clin Psychiatry. 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002; 63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005; 7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar
depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2): 156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4): 476-488.
Note: Page numbers differ between the print issue and digital edition.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11): 1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4): 509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective followup of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1): 125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar
disorder: a review. J Clin Psychiatry. 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002; 63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005; 7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar
depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2): 156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4): 476-488.
Note: Page numbers differ between the print issue and digital edition.
What to do when your depressed patient develops mania
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.