User login
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
THE DIAGNOSIS: Folliculitis Decalvans
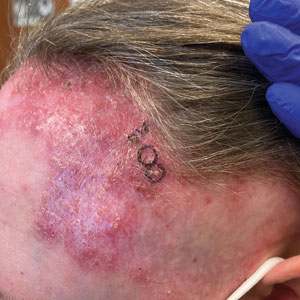
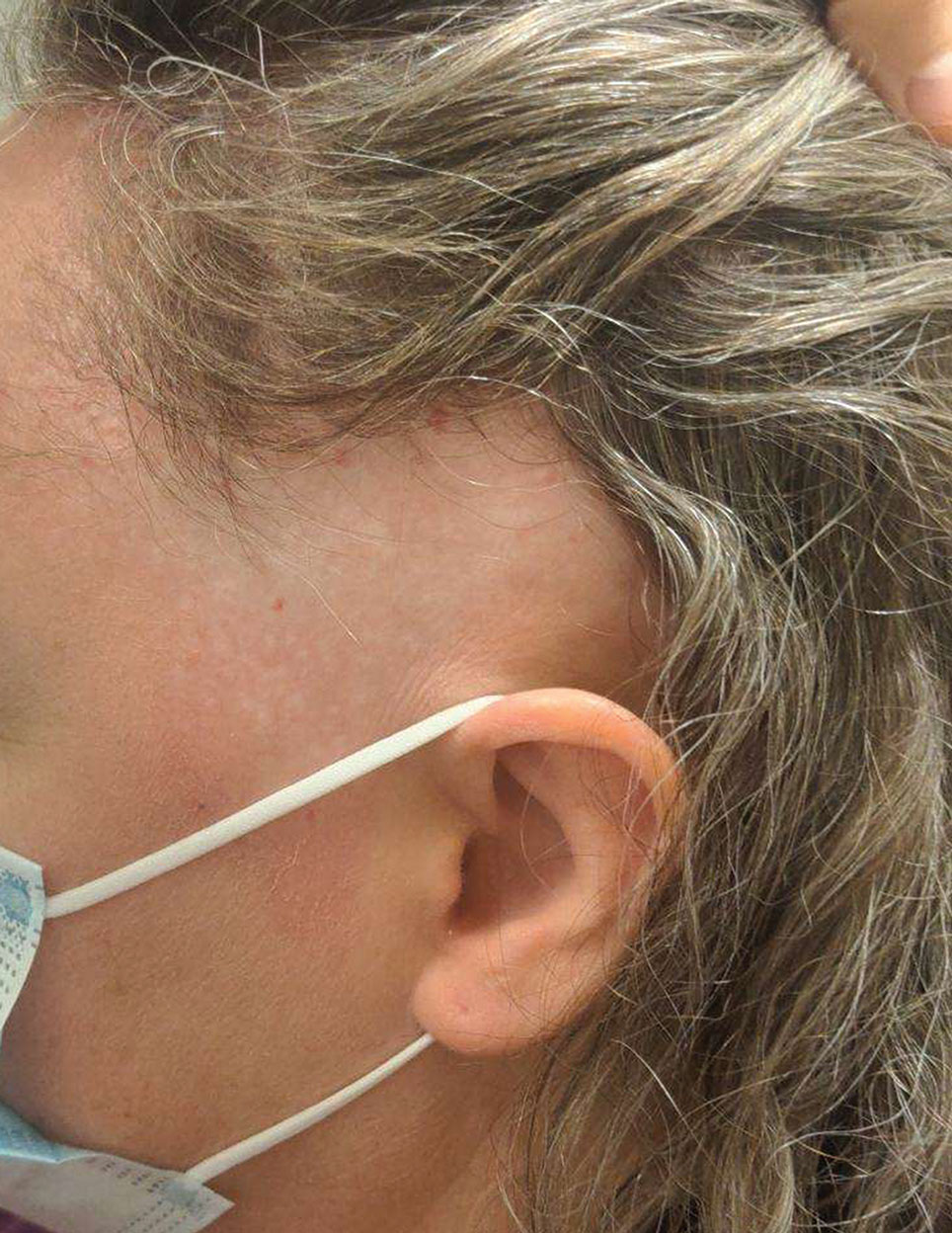
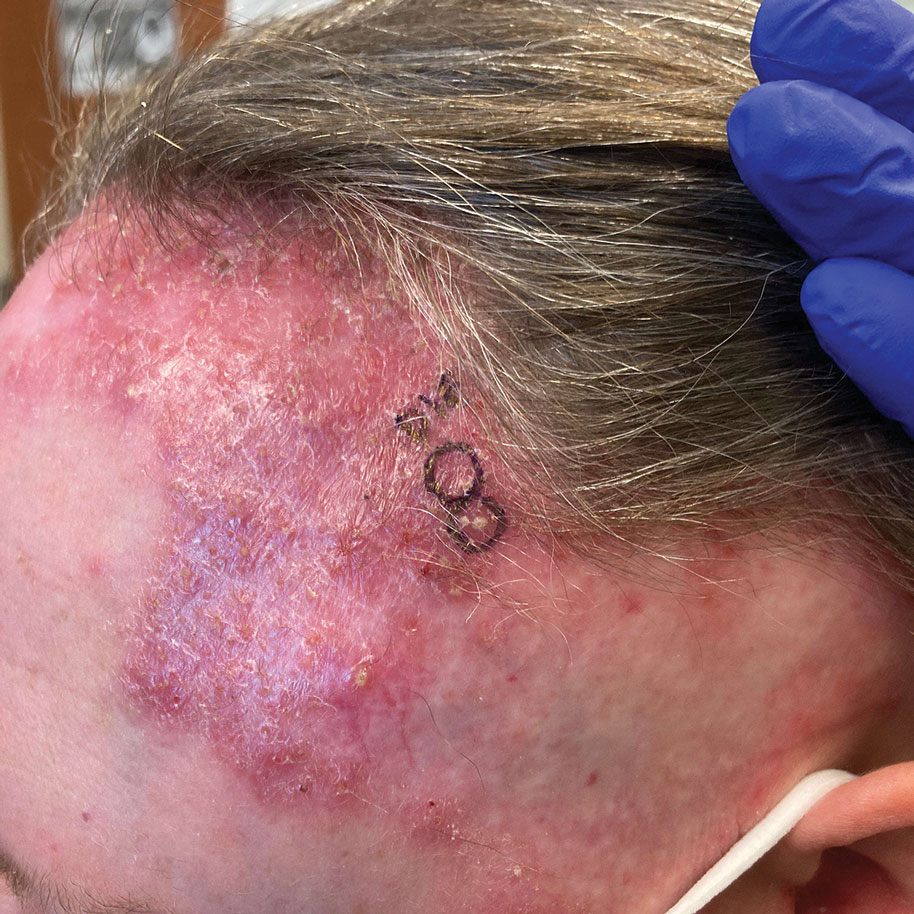
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.
Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10
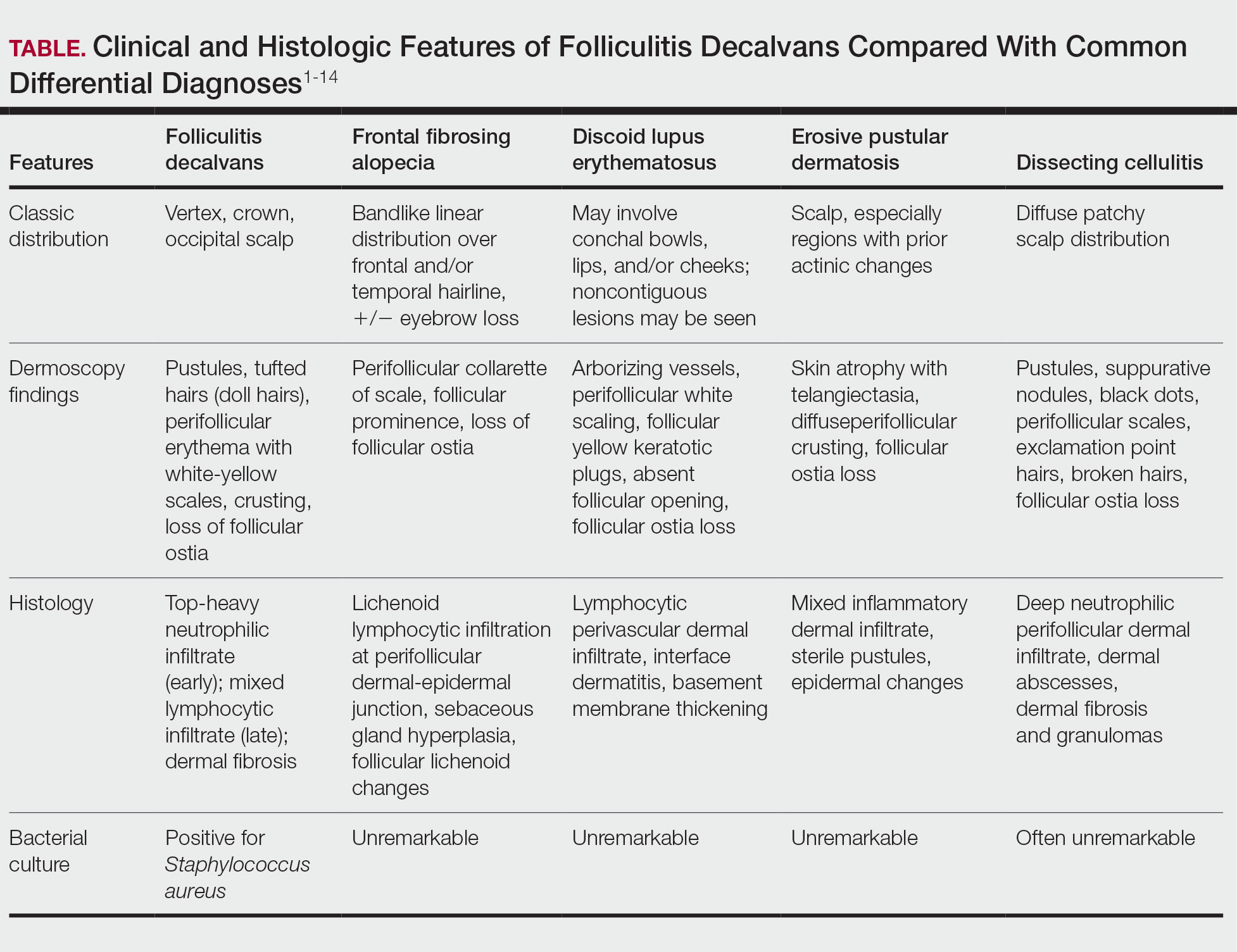
While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
THE DIAGNOSIS: Folliculitis Decalvans
Biopsy results revealed a brisk perifollicular and intrafollicular mixed inflammatory infiltrate comprising lymphocytes, neutrophils, and plasma cells filling the upper dermis and encircling dilated hair follicles. Elastic stain (Verhoeff-van Gieson) demonstrated loss of elastic fibers in areas of scarring. Periodic acid–Schiff with diastase staining was negative for fungal elements, while Gram staining revealed colonies of bacterial cocci in the stratum corneum and within the hair follicles. Immunofluorescence was unremarkable, and culture revealed methicillin-sensitive Staphylococcus aureus, leading to a diagnosis of folliculitis decalvans (FD). The patient was treated with doxycycline 100 mg twice daily and received intralesional triamcinolone 2.5 mg/mL (total volume, 2 mL) every 6 weeks with considerable improvement in pustules, erythema, and scaling (Figure). While not yet in complete remission, our patient demonstrated short regrowing hairs in areas of incomplete scarring and focal remaining perifollicular erythema and scale along the midline frontal scalp 5 months after initial presentation.

Folliculitis decalvans is an uncommon subtype of cicatricial alopecia that may mimic other forms of alopecia. Cicatricial alopecia often is difficult to diagnose due to its overlapping clinical characteristics, but early diagnosis is essential for appropriate management and prevention of further permanent hair loss. Traditionally classified as a primary neutrophilic cicatricial alopecia, lymphocyte-predominant variants of FD now are recognized.1
Patients with FD typically present with patchy scarring alopecia at the vertex scalp that gradually expands and may demonstrate secondary features of follicular tufting and pustules.1-3 While the epidemiology of FD is poorly characterized, Vañó-Galván et al4 reported that FD accounted for 2.8% of all alopecia cases and 10.5% of cicatricial alopecia cases in a multicenter study of 2835 patients. The pathophysiology of FD still is under investigation but is thought to result from a dysregulated immune response to a chronic bacterial infection (eg, S aureus), with resulting neutrophilpredominant inflammation in early stages.1-3 Vañó-Galván et al4 reported that, among 35 patients with FD cultured for bacteria, 74% (26/35) returned positive results, 96% (25/26) of which grew S aureus.5
A systematic review of 20 studies that included 263 patients found rifampin and clindamycin to be the most common treatments for FD; however, there is insufficient evidence to determine if this treatment is the most effective.6 In our patient, clindamycin was avoided due to its propensity to negatively alter the gut microbiome long term.7 Other therapies such as oral tetracyclines, high-potency topical steroids, and intralesional triamcinolone also can be used to achieve disease remission.5,6 Other treatments such as isotretinoin, red-light photodynamic therapy, tacrolimus, and external beam radiation have been reported in the literature but vary in efficacy.6 Our patient improved on a regimen of topical benzoyl peroxide wash, oral doxycycline, and intralesional triamcinolone.
Notably, FD may share clinical features with other causes of cicatricial alopecia. In our patient, FD mimicked other entities including discoid lupus erythematosus, frontal fibrosing alopecia, dissecting cellulitis, and erosive pustular dermatosis (Table).1-14 Discoid lupus erythematosus manifests as round hypopigmented and hyperpigmented plaques with associated atrophy, perifollicular erythema, and follicular plugging. Frontal fibrosing alopecia is a primary lymphocytic scarring alopecia that manifests in a bandlike linear distribution over the frontal scalp and may involve the temporal scalp, posterior hairline, and/or eyebrows. Isolated hairs (known as lonely hairs) often are seen. Dissecting cellulitis is characterized by boggy nodules associated with alopecia on the scalp without notable epidermal change, although pustules and sinus tracts may develop.9 Erosive pustular dermatosis is a diagnosis of exclusion but often is seen in older adults with chronic sun damage and clinically manifests with eroded plaques with adherent crusts.10

While our patient presented with several overlapping clinical features, including progressive hair loss along the frontal scalp in a bandlike pattern suspicious for frontal fibrosing alopecia as well as atrophic depigmented plaques with adherent peripheral scaling suspicious for discoid lupus erythematosus, the presence of pustules was an important clue. The biopsy demonstrating a mixed infiltrate inclusive of neutrophils confirmed the diagnosis of FD.
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
- Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48:103-110. doi:10.1067/mjd.2003.68
- Filbrandt R, Rufaut N, Jones L. Primary cicatricial alopecia: diagnosis and treatment. CMAJ. 2013;185:1579-1585. doi:10.1503/cmaj.111570
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Vañó-Galván S, Saceda-Corralo D, Blume-Peytavi U, et al. Frequency of the types of alopecia at twenty-two specialist hair clinics: a multicenter study. Skin Appendage Disord. 2019;5:309-315. doi:10.1159/000496708
- Vañó-Galván S, Molina-Ruiz AM, Fernández-Crehuet P, et al. Folliculitis decalvans: a multicentre review of 82 patients. J Eur Acad Dermatol Venereol. 2015;29:1750-1757. doi:10.1111/jdv.12993
- Rambhia PH, Conic RRZ, Murad A, et al. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80:794-801. doi:10.1016/j.jaad.2018.07.050
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471-489. doi:10.1016/j.jinf.2019.10.008
- Kanti V, Röwert-Huber J, Vogt A, et al. Cicatricial alopecia. J Dtsch Dermatol Ges. 2018;16:435-461. doi:10.1111/ddg.13498
- Melo DF, Slaibi EB, Siqueira TMFM, et al. Trichoscopy findings in dissecting cellulitis. An Bras Dermatol. 2019;94:608-611. doi:10.1016/j.abd.2019.09.006
- Anzai A, Pirmez R, Vincenzi C, et al. Trichoscopy findings of frontal fibrosing alopecia on the eyebrows: a study of 151 cases. J Am Acad Dermatol. 2021;85:1130-1134. doi:10.1016/j.jaad.2019.12.023
- Starace M, Loi C, Bruni F, et al. Erosive pustular dermatosis of the scalp: clinical, trichoscopic, and histopathologic features of 20 cases. J Am Acad Dermatol. 2017;76:1109-1114. doi:10.1016/j.jaad.2016.12.016
- Rongioletti F, Christana K. Cicatricial (scarring) alopecias: an overview of pathogenesis, classification, diagnosis, and treatment. Am J Clin Dermatol. 2012;13:247-260. doi:10.2165/11596960-000000000-00000
- Badaoui A, Reygagne P, Cavelier-Balloy B, et al. Dissecting cellulitis of the scalp: a retrospective study of 51 patients and review of literature. Br J Dermatol. 2016;174:421-423. doi:10.1111/bjd.13999
- Michelerio A, Vassallo C, Fiandrino G, et al. Erosive pustular dermatosis of the scalp: a clinicopathologic study of fifty cases. Dermatopathology (Basel). 2021;8:450-462. doi:10.3390/dermatopathology8040048
Alopecia and Pruritic Rash on the Forehead and Scalp
Alopecia and Pruritic Rash on the Forehead and Scalp
A 52-year-old woman presented to the dermatology department with an intermittently pruritic rash in a bandlike distribution on the left upper forehead and the frontal and temporal scalp of 4 years’ duration. The rash initially was diagnosed as psoriasis at an outside facility. Treatment over the year prior to presentation included tildrakizumab-asmn; topical crisaborole 2%; and excimer laser, which was complicated by blistering. The patient reported no history of topical or injected steroid use in the involved areas. Physical examination at the current presentation revealed arcuate erythematous plaques with follicular prominence, perifollicular scaling, pustules, and lone hairs. There also were porcelain-white atrophic plaques with loss of follicular ostia that were most prominent over the temporal scalp. A biopsy of the left lateral forehead was performed.
Culprits of Medication-Induced Telogen Effluvium, Part 2
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30 β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30 β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30 β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Practice Points
- Medications are a common culprit of telogen effluvium (TE), and medication-induced TE should be suspected in patients presenting with diffuse nonscarring alopecia who are taking systemic medication(s) such as heparin and its derivatives.
- Infection, illness, or hospitalization around the time of initiation of the suspected culprit medication may complicate identification of the inciting cause and may contribute to TE.
- Angiotensin-converting enzyme inhibitors and β-blockers are unlikely culprits of medication-induced TE, and the benefits of discontinuing a suspected culprit medication should be weighed carefully against the risks of medication cessation.
Culprits of Medication-Induced Telogen Effluvium, Part 1
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
Alopecia is a commonly reported side effect of various medications. Anagen effluvium and telogen effluvium (TE) are considered the most common mechanisms underlying medication-related hair loss. Anagen effluvium is associated with chemotherapeutic agents and radiation therapy, with anagen shedding typically occurring within 2 weeks of medication administration.1,2 Medication-induced TE is a diffuse nonscarring alopecia that is a reversible reactive process.3-5 Telogen effluvium is clinically apparent as a generalized shedding of scalp hair 1 to 6 months after an inciting cause.6 The underlying cause of TE may be multifactorial and difficult to identify given the delay between the trigger and the onset of clinically apparent hair loss. Other known triggers of TE include acute illness,7,8 nutritional deficiencies,4,9 and/or major surgery.10
Each hair follicle independently and sequentially progresses through anagen growth, catagen transition, and telogen resting phases. In the human scalp, the telogen phase typically lasts 3 months, at the end of which the telogen hair is extruded from the scalp. Anagen and telogen follicles typically account for an average of 90% and 10% of follicles on the human scalp, respectively.11 Immediate anagen release is hypothesized to be the mechanism underlying medication-induced TE.12 This theory suggests that an increased percentage of anagen follicles prematurely enter the telogen phase, with a notable increase in hair shedding at the conclusion of the telogen phase approximately 1 to 6 months later.12 First-line management of medication-induced TE is identification and cessation of the causative agent, if possible. Notable regrowth of hair is expected several months after removal of the inciting medication. In part 1 of this 2-part series, we review the existing literature to identify common culprits of medication-induced TE, including retinoids, antifungals, and psychotropic medications.
Retinoids
Retinoids are vitamin A derivatives used in the treatment of a myriad of dermatologic and nondermatologic conditions.13,14 Retinoids modulate sebum production,15 keratinocyte proliferation,16 and epithelial differentiation through signal transduction downstream of the ligand-activated nuclear retinoic acid receptors and retinoid X receptors.13,14,17 The recommended daily dosage of retinol is 900 µg retinol activity equivalent (3000 IU) for men and 700 µg retinol activity equivalent (2333 IU) for women. Retinoids are used in the treatment of acne vulgaris,18 psoriasis,19 and ichthyosis.20 The most commonly reported adverse effects of systemic retinoid therapy include cheilitis, alopecia, and xerosis.21 Retinoid-associated alopecia is dose and duration dependent.19,21-24 A prospective study of acitretin therapy in plaque psoriasis reported that more than 63% (42/66) of patients on 50 mg or more of acitretin daily for 6 months or longer experienced alopecia that reversed with discontinuation.23 A systematic review of isotretinoin use in acne showed alopecia was seen in 3.2% (18/565) of patients on less than 0.5 mg/kg/d of isotretinoin and in 5.7% (192/3375) of patients on 0.5 mg/kg/d or less of isotretinoin.24 In a phase 2 clinical trial of orally administered 9-cis-retinoic acid (alitretinoin) in the treatment of Kaposi sarcoma related to AIDS, 42% (24/57) of adult male patients receiving 60, 100, or 140 mg/m2 alitretinoin daily (median treatment duration, 15.1 weeks) reported alopecia as an adverse effect of treatment.25 In one case report, a patient who ingested 500,000 IU of vitamin A daily for 4 months and then 100,000 IU monthly for 6 months experienced diffusely increased shedding of scalp hair along with muscle soreness, nail dystrophy, diffuse skin rash, and refractory ascites; he was found to have severe liver damage secondary to hypervitaminosis A that required liver transplantation.26 Regarding the pathomechanism of retinoid-induced alopecia, animal and in vitro studies similarly have demonstrated that all-trans-retinoic acid appears to exert its inhibitory effects on hair follicle growth via the influence of the transforming growth factor β2 and SMAD2/3 pathway influence on dermal papillae cells.14,27 Development of hair loss secondary to systemic retinoid therapy may be managed with dose reduction or cessation.
Antifungals
Azole medications have broad-spectrum fungistatic activity against a wide range of yeast and filamentous fungi. Azoles inhibit sterol 14α-demethylase activity, impairing ergosterol synthesis and thereby disrupting plasma membrane synthesis and activity of membrane-bound enzymes.28 Fluconazole is a systemic oral agent in this class that was first approved by the US Food and Drug Administration (FDA) for use in the 1990s.29 A retrospective study by the National Institute of Allergy and Infectious Disease Mycoses Study Group followed the clinical course of 33 patients who developed alopecia while receiving fluconazole therapy for various mycoses.30 The majority (88% [29/33]) of patients received 400 mg or more of fluconazole daily. The median time to hair loss after starting fluconazole was 3 months, and the scalp was involved in all cases. In 97% (32/33) of patients, resolution of alopecia was noted following discontinuation of fluconazole or a dose reduction of 50% or more. In 85% (28/33) of patients, complete resolution of alopecia occurred within 6 months of fluconazole cessation or dose reduction.30 Fluconazole-induced TE was reproducible in an animal model using Wistar rats31; however, further studies are required to clarify the molecular pathways of its effect on hair growth.
Voriconazole is an azole approved for the treatment of invasive aspergillosis, candidemia, and fungal infections caused by Scedosporium apiospermum and Fusarium species. A retrospective survey study of patients who received voriconazole for 1 month or longer found a considerable proportion of patients developed diffuse reversible hair loss.32 Scalp alopecia was noted in 79% (120/152) of patients who completed the survey, with a mean (SD) time to alopecia of 75 (54) days after initiation of voriconazole. Notable regrowth was reported in 69% (79/114) of patients who discontinued voriconazole for at least 3 months. A subgroup of 32 patients were changed to itraconazole or posaconazole, and hair loss stopped in 84% (27/32) with regrowth noted in 69% (22/32) of patients.32 Voriconazole and fluconazole share structural similarity not present with other triazoles.33,34 Because voriconazole-associated alopecia was reversed in the majority of patients who switched to itraconazole or posaconazole, the authors hypothesized that structural similarity of fluconazole and voriconazole may underly the greater risk for TE that is not a class effect of azole medications.31
Psychotropic Medications
Various psychotropic medications have been associated with hair loss. Valproic acid (or sodium valproate) is an anticonvulsant and mood-stabilizing agent used for the treatment of seizures, bipolar disorder (BD), migraines, and neuropathic pain.35,36 Divalproex sodium (or divalproex) is an enteric-coated formulation of sodium valproate and valproic acid with similar indications. Valproate is a notorious culprit of medication-induced hair loss, with alopecia listed among the most common adverse reactions (reported >5%) on its structure product labeling document.37 A systemic review and meta-analysis by Wang et al38 estimated the overall incidence of valproate-related alopecia to be 11% (95% CI, 0.08-0.13). Although this meta-analysis did not find an association between incidence of alopecia and dose or duration of valproate therapy,38 a separate review suggested that valproate-induced alopecia is dose dependent and can be managed with dose reduction.39 A 12-month, randomized, double-blind study of treatment of BD with divalproex (valproate derivative), lithium, or placebo (2:1:1 ratio) showed a significantly higher frequency of alopecia in the divalproex group compared with placebo (16% [30/187] vs 6% [6/94]; P=.03).40 Valproate-related hair loss is characteristically diffuse and nonscarring, often noted 3 to 6 months following initiation of valproate.41,42 The proposed mechanism of valproate-induced alopecia includes chelation of zinc and selenium,43 and a reduction in serum biotinidase activity, thereby decreasing the availability of these essential micronutrients required for hair growth.41 Studies examining the effects of valproate administration and serum biotinidase activity in patients have yielded conflicting results.44-46 In a study of children with seizures including 57 patients treated with valproic acid, 17 treated with carbamazepine, and 75 age- and sex-matched healthy controls, the authors found no significant differences in serum biotinidase enzyme activity across the 3 groups.44 In contrast, a study of 75 children with seizures on valproic acid therapy stratified by dose (mean [SD])—group A: 28.7 [8.5] mg/kg/d; group B: 41.6 [4.9] mg/kg/d; group C: 64.5 [5.8] mg/kg/d—found that patients receiving higher doses (groups B and C) had significantly reduced serum biotinidase activity (1.22
Lithium carbonate (lithium) is used in the treatment of BD. Despite its efficacy and low cost, its potential for adverse effects, narrow therapeutic index, and subsequent need for routine monitoring are factors that limit its use.48 Some reported dermatologic adverse reactions on its structure product labeling include xerosis, thinning of hair, alopecia, xerosis cutis, psoriasis onset/exacerbation, and generalized pruritus.49 A systematic review and meta-analysis of 385 studies identified 24 publications reporting adverse effects of lithium on hair with no significantly increased risk of alopecia overall.50 The analysis included 2 randomized controlled trials comparing the effects of lithium and placebo on hair loss in patients with BD. Hair loss was reported in 7% (7/94) of patients taking lithium and 6% (6/94) of the placebo group in the 12-month study40 and in 3% (1/32) of the lithium group and 0% (0/28) of the divalproex group in the 20-month study.51 Despite anecdotal reports of alopecia associated with lithium, there is a lack of high-quality evidence to support this claim. Of note, hypothyroidism is a known complication of lithium use, and serum testing of thyroid function at 6-month intervals is recommended for patients on lithium treatment.52 Because thyroid abnormalities can cause alopecia distinct from TE, new-onset alopecia during lithium use should prompt serum testing of thyroid function. The development of hypothyroidism secondary to lithium is not a direct contraindication to its use53; rather, treatment should be focused on correction with thyroid replacement therapy (eg, supplementation with thyroxine).54
Commonly prescribed antidepressant medications include selective serotonin reuptake inhibitors (SSRIs) and bupropion. Selective serotonin reuptake inhibitors affect the neuronal serotonin transporter, increasing the concentration of serotonin in the synaptic cleft available for stimulation of postsynaptic serotonin receptors55,56; bupropion is an antidepressant medication that inhibits norepinephrine and dopamine reuptake at the synaptic cleft.57 Alopecia is an infrequent (1 in 100 to 1 in 1000 patients) adverse effect for several SSRIs.58-62 A recent systematic review identified a total of 71 cases of alopecia associated with SSRI use including citalopram (n=11), escitalopram (n=7), fluoxetine (n=27), fluoxvamine (n=5), paroxetine (n=4), and sertraline (n=20), with a median time to onset of hair shedding of 8.6 weeks (range, 3 days to 5 years). Discontinuation of the suspected culprit SSRI led to improvement and/or resolution in 63% (51/81) episodes of alopecia, with a median time to improvement and/or resolution of 4 weeks.63 A comparative retrospective cohort study using a large US health claims database from 2006 to 2014 included more than 1 million new and mutually exclusive patients taking fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram, paroxetine, duloxetine, venlafaxine, desvenlafaxine, and bupropion.64 Overall, 1% (1569/150,404) of patients treated with bupropion received 1 or more physician visits for alopecia. Patients on SSRIs generally had a lower risk for hair loss compared with patients using bupropion (citalopram: hazard ratio [HR], 0.80 [95% CI, 0.74-0.86]; escitalopram: HR, 0.79 [95% CI, 0.74-0.86]; fluoxetine: HR, 0.68 [95% CI, 0.63-0.74]; paroxetine: HR, 0.68 [95% CI, 0.62-0.74]; sertraline: HR, 0.74 [95% CI, 0.69-0.79]), with the exception of fluvoxamine (HR, 0.93 [95% CI, 0.64-1.37]). However, the type of alopecia, time to onset, and time to resolution were not reported, making it difficult to assess whether the reported hair loss was consistent with medication-induced TE. Additionally, the authors acknowledged that bupropion may have been prescribed for smoking cessation, which may carry a different risk profile for the development of alopecia.64 Several other case reports have described alopecia following treatment with SSRIs, including sertraline,65 fluvoxamine,66 paroxetine,67 fluoxetine,68 and escitalopram.69
Overall, it appears that the use of SSRIs portends relatively low risk for alopecia and medication-induced TE. Little is known regarding the molecular effects of SSRIs on hair growth and the pathomechanism of SSRI-induced TE. The potential benefits of discontinuing a suspected culprit medication should be carefully weighed against the risks of medication cessation, and consideration should be given to alternative medications in the same class that also may be associated with TE. In patients requiring antidepressant therapy with suspected medication-induced TE, consider transitioning to a different class of medication with lower risk of medication-induced alopecia; for example, discontinuing bupropion in favor of an SSRI.
Final Thoughts
Medication-induced alopecia is an undesired side effect of many commonly used drugs and drug classes, including retinoids, azole antifungals, and mood stabilizers. Although the precise pathomechanisms of medication-induced TE remain unclear, the recommended management often requires identification of the likely causative agent and its discontinuation, if possible. Suspicion for medication-induced TE should prompt a thorough history of recent changes to medications, risk factors for nutritional deficiencies, underlying illnesses, and recent surgical procedures. Underlying nutritional, electrolyte, and/or metabolic disturbances should be corrected. In part 2 of this series, we will discuss medication-induced alopecia associated with anticoagulant and antihypertensive medications.
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
- Saleh D, Nassereddin A, Cook C. Anagen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482293/
- Guerrero-Putz MD, Flores-Dominguez AC, Castillo-de la Garza RJ, et al. Anagen effluvium after neurointerventional radiation: trichoscopy as a diagnostic ally. Skin Appendage Disord. 2021;8:102-107. doi:10.1159/000518743
- Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67-73. doi:https://doi.org/10.1016/j.det.2012.08.002
- Chen V, Strazzulla L, Asbeck SM, et al. Etiology, management, and outcomes of pediatric telogen effluvium: a single-center study in the United States. Pediatr Dermatol. 2023;40:120-124. doi:10.1111/pde.15154
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Hughes EC, Saleh D. Telogen effluvium. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK430848/
- Nguyen B, Tosti A. Alopecia in patients with COVID-19: a systematic review and meta-analysis. JAAD Int. 2022;7:67-77. doi:10.1016/j.jdin.2022.02.006
- Starace M, Piraccini BM, Evangelista V, et al. Acute telogen effluvium due to dengue fever mimicking androgenetic alopecia. Ital J Dermatol Venerol. 2023;158:66-67. doi:10.23736/s2784-8671.22.07369-8
- Patel KV, Farrant P, Sanderson JD, et al. Hair loss in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1753-1763. doi:10.1097/MIB.0b013e31828132de
- Cohen-Kurzrock RA, Cohen PR. Bariatric surgery–induced telogen effluvium (bar site): case report and a review of hair loss following weight loss surgery. Cureus. 2021;13:E14617. doi:10.7759/cureus.14617
- Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964-973. doi:10.1056/nejm199909233411307
- Headington JT. Telogen effluvium: new concepts and review. Arch Dermatol. 1993;129:356-363. doi:10.1001/arcderm.1993.01680240096017
- Lee DD, Stojadinovic O, Krzyzanowska A, et al. Retinoid-responsive transcriptional changes in epidermal keratinocytes. J Cell Physiol. 2009;220:427-439. doi:10.1002/jcp.21784
- Foitzik K, Spexard T, Nakamura M, et al. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla. J Invest Dermatol. 2005;124:1119-1126. doi:10.1111/j.0022-202X.2005.23686.x
- Karlsson T, Vahlquist A, Kedishvili N, et al. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273-278. doi:10.1016/s0006-291x(03)00332-2
- Chapellier B, Mark M, Messaddeq N, et al. Physiological and retinoid-induced proliferations of epidermis basal keratinocytes are differently controlled. EMBO J. 2002;21:3402-3413. doi:10.1093/emboj/cdf331
- Geiger JM. Retinoids and sebaceous gland activity. Dermatology. 1995;191:305-310. doi:10.1159/000246581
- Oge LK, Broussard A, Marshall MD. Acne vulgaris: diagnosis and treatment. Am Fam Physician. 2019;100:475-484.
- Pilkington T, Brogden RN. Acitretin. Drugs. 1992;43:597-627. doi:10.2165/00003495-199243040-00010
- Zaenglein AL, Levy ML, Stefanko NS, et al. Consensus recommendations for the use of retinoids in ichthyosis and other disorders of cornification in children and adolescents. Pediatr Dermatol. 2021;38:164-180. doi:10.1111/pde.14408
- Katz HI, Waalen J, Leach EE. Acitretin in psoriasis: an overview of adverse effects. J Am Acad Dermatol. 1999;41(3 suppl):S7-S12. doi:10.1016/s0190-9622(99)70359-2
- Tran PT, Evron E, Goh C. Characteristics of patients with hair loss after isotretinoin treatment: a retrospective review study. Int J Trichology. 2022;14:125-127. doi:10.4103/ijt.ijt_80_20
- Gupta AK, Goldfarb MT, Ellis CN, et al. Side-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088-1093. doi:10.1016/s0190-9622(89)70138-9
- Lytvyn Y, McDonald K, Mufti A, et al. Comparing the frequency of isotretinoin-induced hair loss at <0.5-mg/kg/d versus ≥0.5-mg/kg/d dosing in acne patients: a systematic review. JAAD Int. 2022;6:125-142. doi:10.1016/j.jdin.2022.01.002
- Aboulafia DM, Norris D, Henry D, et al. 9-cis-Retinoic acid capsules in the treatment of AIDS-related Kaposi sarcoma: results of a phase 2 multicenter clinical trial. Arch Dermatol. 2003;139:178-186. doi:10.1001/archderm.139.2.178
- Cheruvattath R, Orrego M, Gautam M, et al. Vitamin A toxicity: when one a day doesn’t keep the doctor away. Liver Transpl. 2006;12:1888-1891. doi:10.1002/lt.21007
- Nan W, Li G, Si H, et al. All-trans-retinoic acid inhibits mink hair follicle growth via inhibiting proliferation and inducing apoptosis of dermal papilla cells through TGF-β2/Smad2/3 pathway. Acta Histochem. 2020;122:151603. doi:10.1016/j.acthis.2020.151603
- Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40:279-291. doi:10.1128/aac.40.2.279
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40-79. doi:10.1128/cmr.12.1.40
- Pappas PG, Kauffman CA, Perfect J, et al. Alopecia associated with fluconazole therapy. Ann Intern Med. 1995;123:354-357. doi:10.7326/0003-4819-123-5-199509010-00006
- Thompson GR 3rd, Krois CR, Affolter VK, et al. Examination of fluconazole-induced alopecia in an animal model and human cohort. Antimicrob Agents Chemother. 2019;63:e01384-18. doi:10.1128/aac.01384-18
- Malani AN, Kerr L, Obear J, et al. Alopecia and nail changes associated with voriconazole therapy. Clin Infect Dis. 2014;59:E61-E65. doi:10.1093/cid/ciu275
- Greer ND. Voriconazole: the newest triazole antifungal agent. Proc (Bayl Univ Med Cent). 2003;16:241-248. doi:10.1080/08998280.2003.11927910
- Drabin´ska B, Dettlaff K, Kossakowski K, et al. Structural and spectroscopic properties of voriconazole and fluconazole—experimental and theoretical studies. Open Chemistry. 2022;20:1575-1590. doi:10.1515/chem-2022-0253
- Löscher W. Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol. 1999;58:31-59. doi:10.1016/s0301-0082(98)00075-6
- Gill D, Derry S, Wiffen PJ, et al. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011:CD009183. doi:10.1002/14651858.CD009183.pub2
- Depakote, Prescribing information. Abbott Laboratories; 2011. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018723s037lbl.pdf
- Wang X, Wang H, Xu D, et al. Risk of valproic acid-related alopecia: a systematic review and meta-analysis. Seizure. 2019;69:61-69. doi:10.1016/j.seizure.2019.04.003
- Mercke Y, Sheng H, Khan T, et al. Hair loss in psychopharmacology. Ann Clin Psychiatry. 2000;12:35-42. doi:10.1023/a:1009074926921
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481-489. doi:10.1001/archpsyc.57.5.481
- Praharaj SK, Munoli RN, Udupa ST, et al. Valproate-associated hair abnormalities: pathophysiology and management strategies. Hum Psychopharmacol. 2022;37:E2814. doi:10.1002/hup.2814
- Wilting I, van Laarhoven JH, de Koning-Verest IF, et al. Valproic acid-induced hair-texture changes in a white woman. Epilepsia. 2007;48:400-401. doi:10.1111/j.1528-1167.2006.00933.x
- Potter WZ, Ketter TA. Pharmacological issues in the treatment of bipolar disorder: focus on mood-stabilizing compounds. Can J Psychiatry. 1993;38(3 suppl 2):S51-S56.
- Castro-Gago M, Gómez-Lado C, Eirís-Pun´al J, et al. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J Child Neurol. 2009;25:32-35. doi:10.1177/0883073809336118
- Schulpis KH, Karikas GA, Tjamouranis J, et al. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia. 2001;42:1359-1362. doi:10.1046/j.1528-1157.2001.47000.x
- Yilmaz Y, Tasdemir HA, Paksu MS. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur J Paediatr Neurol. 2009;13:439-443. doi:10.1016/j.ejpn.2008.08.007
- Henriksen O, Johannessen SI. Clinical and pharmacokinetic observations on sodium valproate—a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand. 1982;65:504-523. doi:10.1111/j.1600-0404.1982.tb03106.x
- Fountoulakis KN, Tohen M, Zarate CA Jr. Lithium treatment of bipolar disorder in adults: a systematic review of randomized trials and meta-analyses. Eur Neuropsychopharmacol. 2022;54:100-115. doi:10.1016/j.euroneuro.2021.10.003
- Lithium carbonate. Prescribing information. West-Ward Pharmaceuticals; 2018. Accessed November 20, 2023. https://ww.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf
- McKnight RF, Adida M, Budge K, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721-728. doi:10.1016/s0140-6736(11)61516-x
- Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162:2152-2161. doi:10.1176/appi.ajp.162.11.2152.
- Duce HL, Duff CJ, Zaidi S, et al. Evaluation of thyroid function monitoring in people treated with lithium: advice based on real-world data. Bipolar Disord. 2023;25:402-409. doi:10.1111/bdi.13298
- Bocchetta A, Loviselli A. Lithium treatment and thyroid abnormalities. Clin Pract Epidemiol Ment Health. 2006;2:23. doi:10.1186/1745-0179-2-23.
- Joffe RT. How should lithium-induced thyroid dysfunction be managed in patients with bipolar disorder? J Psychiatry Neurosci. 2002;27:392.
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors. an overview with emphasis on pharmacokinetics and effects on oxidative drug metabolism. Clin Pharmacokinet. 1997;32(suppl 1):1-21. doi:10.2165/00003088-199700321-00003
- Chu A, Wadhwa R. Selective serotonin reuptake inhibitors. StatPearls. StatPearls Publishing; 2023.
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159-166. doi:10.4088/pcc.v06n0403
- Escitalopram. Prescribing information. Solco Healthcare US, LLC; 2022. Accessed November 20, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/2ffc6ec3-830f-46bc-9b3f-7c42cefa39b2/spl-doc
- Fluoxetine. Eli Lilly & Company; 2017. Prescribing information. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018936s108lbl.pdf
- Paxil. Prescribing information. GlaxoSmithKline; 2012. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020031s067,020710s031.pdf
- Zoloft. Prescribing information. Pfizer; 2016. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/019839s74s86s87_20990s35s44s45lbl.pdf
- Celexa. Prescribing information. Allergan; 2022. Accessed November 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- Pejcic AV, Paudel V. Alopecia associated with the use of selective serotonin reuptake inhibitors: systematic review. Psychiatry Res. 2022;313:114620. 10.1016/j.psychres.2022.114620
- Etminan M, Sodhi M, Procyshyn RM, et al. Risk of hair loss with different antidepressants: a comparative retrospective cohort study. Int Clin Psychopharmacol. 2018;33:44-48.
- Ghanizadeh A. Sertraline-associated hair loss. J Drugs Dermatol. 2008;7:693-694.
- Parameshwar E. Hair loss associated with fluvoxamine use. Am J Psychiatry. 1996;153:581-582. doi:10.1176/ajp.153.4.581
- Zalsman G, Sever J, Munitz H. Hair loss associated with paroxetine treatment: a case report. Clin Neuropharmacol. 1999;22:246-247.
- Ananth J, Elmishaugh A. Hair loss associated with fluoxetinetreatment. Can J Psychiatry. 1991;36:621. doi:10.1177/070674379103600824
- Tirmazi SI, Imran H, Rasheed A, et al. Escitalopram-induced hair loss. Prim Care Companion CNS Disord. 2020;22:19l02496. doi:10.4088/PCC.19l02496
Practice Points
- Medications are a common culprit of telogen effluvium (TE), and medication-induced TE should be suspected in patients presenting with diffuse nonscarring alopecia who are taking systemic medication(s).
- A careful history of new medications and dose adjustments 1 to 6 months prior to notable hair loss may identify the most likely inciting cause.
- Medication-induced TE often improves with cessation or dose reduction of the culprit medication.
Serum Ferritin Levels: A Clinical Guide in Patients With Hair Loss
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.
Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
Ferritin is an iron storage protein crucial to human iron homeostasis. Because serum ferritin levels are in dynamic equilibrium with the body’s iron stores, ferritin often is measured as a reflection of iron status; however, ferritin also is an acute-phase reactant whose levels may be nonspecifically elevated in a wide range of inflammatory conditions. The various processes that alter serum ferritin levels complicate the clinical interpretation of this laboratory value. In this article, we review the structure and function of ferritin and provide a guide for clinical use.
Overview of Iron
Iron is an essential element of key biologic functions including DNA synthesis and repair, oxygen transport, and oxidative phosphorylation. The body’s iron stores are mainly derived from internal iron recycling following red blood cell breakdown, while 5% to 10% is supplied by dietary intake.1-3 Iron metabolism is of particular importance in cells of the reticuloendothelial system (eg, spleen, liver, bone marrow), where excess iron must be appropriately sequestered and from which iron can be mobilized.4 Sufficient iron stores are necessary for proper cellular function and survival, as iron is a necessary component of hemoglobin for oxygen delivery, iron-sulfur clusters in electron transport, and enzyme cofactors in other cellular processes.
Although labile pools of biologically active free iron exist in limited amounts within cells, excess free iron can generate free radicals that damage cellular proteins, lipids, and nucleic acids.5-7 As such, most intracellular iron is captured within ferritin molecules. The excretion of iron is unregulated and occurs through loss in sweat, menstruation, hair shedding, skin desquamation, and enterocyte turnover.8 The lack of regulated excretion highlights the need for a tightly regulated system of uptake, transportation, storage, and sequestration to maintain iron homeostasis.
Overview of Ferritin Structure and Function
Ferritin is a key regulator of iron homeostasis that also serves as an important clinical indicator of body iron status. Ferritin mainly is found as an intracellular cytosolic iron storage and detoxification protein structured as a hollow 24-subunit polymer shell that can sequester up to 4500 atoms of iron within its core.9,10 The 24-mer is composed of both ferritin L (FTL) and ferritin H (FTH) subunits, with dynamic regulation of the H:L ratio dependent on the context and tissue in which ferritin is found.6
Ferritin H possesses ferroxidase, which facilitates oxidation of ferrous (Fe2+) iron into ferric (Fe3+) iron, which can then be incorporated into the mineral core of the ferritin heteropolymer.11 Ferritin L is more abundant in the spleen and liver, while FTH is found predominantly in the heart and kidneys where the increased ferroxidase activity may confer an increased ability to oxidize Fe2+ and limit oxidative stress.6
Regulation of Ferritin Synthesis and Secretion
Ferritin synthesis is regulated by intracellular nonheme iron levels, governed mainly by an iron response element (IRE) and iron response protein (IRP) translational repression system. Both FTH and FTL messenger RNA (mRNA) contain an IRE that is a regulatory stem-loop structure in the 5´ untranslated region. When the IRE is bound by IRP1 or IRP2, mRNA translation of ferritin subunits is suppressed.6 In low iron conditions, IRPs have greater affinity for IRE, and binding suppresses ferritin translation.12 In high iron conditions, IRPs have a decreased affinity for IRE, and ferritin mRNA synthesis is increased.13 Additionally, inflammatory cytokines such as tumor necrosis factor α and IL-1α transcriptionally induce FTH synthesis, resulting in an increased population of H-rich ferritins.11,14-16 A study using cultured human primary skin fibroblasts demonstrated UV radiation–induced increases in free intracellular iron content.17,18 Pourzand et al18 suggested that UV-mediated damage of lysosomal membranes results in leakage of lysosomal proteases into the cytosol, contributing to degradation of intracellular ferritin and subsequent release of iron within skin fibroblasts. The increased intracellular iron downregulates IRPs and increases ferritin mRNA synthesis,18 consistent with prior findings of increased ferritin synthesis in skin that is induced by UV radiation.19
Molecular analysis of serum ferritin in iron-overloaded mice revealed that extracellular ferritin found in the serum is composed of a greater fraction of FTL and has lower iron content than intracellular ferritin. The low iron content of serum ferritin compared with intracellular ferritin and transferrin suggests that serum ferritin is not a major pathway of systemic iron transport.10 However, locally secreted ferritins may play a greater role in iron transport and release in selected tissues. Additionally, in vitro studies of cell cultures from humans and mice have demonstrated the ability of macrophages to secrete ferritin, suggesting that macrophages are an important cellular source of serum ferritin.10,20 As such, serum ferritin generally may reflect body iron status but more specifically reflects macrophage iron status.10 Although the exact pathways of ferritin release are unknown, it is hypothesized that ferritin secretion occurs through cytosolic autophagy followed by secretion of proteins from the lysosomal compartment.10,18,21
Clinical Utility of Serum Ferritin
Low Ferritin and Iron Deficiency—Although bone marrow biopsy with iron staining remains the gold standard for diagnosis of iron deficiency, serum ferritin is a much more accessible and less invasive tool for evaluation of iron status. A serum ferritin level below 12 μg/L is highly specific for iron depletion,22 with a higher cutoff recommended in clinical practice to improve diagnostic sensitivity.23,24 Conditions independent of iron deficiency that may reduce serum ferritin include hypothyroidism and ascorbate deficiency, though neither condition has been reported to interfere with appropriate diagnosis of iron deficiency.25 Guyatt et al26 conducted a systematic review of laboratory tests used in the diagnosis of iron deficiency anemia and identified 55 studies suitable for inclusion. Based on an area under the receiver operating characteristic curve (AUROC) of 0.95, serum ferritin values were superior to transferrin saturation (AUROC, 0.74), red cell protoporphyrin (AUROC, 0.77), red cell volume distribution width (AUROC, 0.62), and mean cell volume (AUROC, 0.76) for diagnosis of IDA, verified by histologic examination of aspirated bone marrow.26 The likelihood ratio of iron deficiency begins to decrease for serum ferritin values of 40 μg/L or greater. For patients with inflammatory conditions—patients with concomitant chronic renal failure, inflammatory disease, infection, rheumatoid arthritis, liver disease, inflammatory bowel disease, and malignancy—the likelihood of iron deficiency begins to decrease at serum ferritin levels of 70 μg/L or greater.26 Similarly, the World Health Organization recommends that in adults with infection or inflammation, serum ferritin levels lower than 70 μg/L may be used to indicate iron deficiency.24 A serum ferritin level of 41 μg/L or lower was found to have a sensitivity and specificity of 98% for discriminating between iron-deficiency anemia and anemia of chronic disease (diagnosed based on bone marrow biopsy with iron staining), with an AUROC of 0.98.27 As such, we recommend using a serum ferritin level of 40 μg/L or lower in patients who are otherwise healthy as an indicator of iron deficiency.
The threshold for iron supplementation may vary based on age, sex, and race. In women, ferritin levels increase during menopause and peak after menopause; ferritin levels are higher in men than in women.28-30 A multisite longitudinal cohort study of 70 women in the United States found that the mean (SD) ferritin valuewas 69.5 (81.7) μg/L premenopause and 128.8 (125.7) μg/L postmenopause (P<.01).31 A separate longitudinal survey study of 8564 patients in China found that the mean (SE) ferritin value was 201.55 (3.60) μg/L for men and 80.46 (1.64) μg/L for women (P<.0001).32 Analysis of serum ferritin levels of 3554 male patients from the third National Health and Nutrition Examination Survey demonstrated that patients who self-reported as non-Hispanic Black (n=1616) had significantly higher serum ferritin levels than non-Hispanic White patients (n=1938)(serum ferritin difference of 37.1 μg/L)(P<.0001).33 The British Society for Haematology guidelines recommend that the threshold of serum ferritin for diagnosing iron deficiency should take into account age-, sex-, and race-based differences.34 Ferritin and Hair—Cutaneous manifestations of iron deficiency include koilonychia, glossitis, pruritus, angular cheilitis, and telogen effluvium (TE).1 A case-control study of 30 females aged 15 to 45 years demonstrated that the mean (SD) ferritin level was significantly lower in patients with TE than those with no hair loss (16.3 [12.6] ng/mL vs 60.3 [50.1] ng/mL; P<.0001). Using a threshold of 30 μg/L or lower, the investigators found that the odds ratio for TE was 21.0 (95% CI, 4.2-105.0) in patients with low serum ferritin.35
Another retrospective review of 54 patients with diffuse hair loss and 55 controls compared serum vitamin B12, folate, thyroid-stimulating hormone, zinc, ferritin, and 25-hydroxy vitamin D levels between the 2 groups.36 Exclusion criteria were clinical diagnoses of female pattern hair loss (androgenetic alopecia), pregnancy, menopause, metabolic and endocrine disorders, hormone replacement therapy, chemotherapy, immunosuppressive therapy, vitamin and mineral supplementation, scarring alopecia, eating disorders, and restrictive diets. Compared with controls, patients with diffuse nonscarring hair loss were found to have significantly lower ferritin (mean [SD], 14.72 [10.70] ng/mL vs 25.30 [14.41] ng/mL; P<.001) and 25-hydroxy vitamin D levels (mean [SD], 14.03 [8.09] ng/mL vs 17.01 [8.59] ng/mL; P=.01).36
In contrast, a separate case-control study of 381 cases and 76 controls found no increase in the rate of iron deficiency—defined as ferritin ≤15 μg/L or ≤40 μg/L—among women with female pattern hair loss or chronic TE vs controls.37 Taken together, these studies suggest that iron status may play a role in TE, a process that may result from nutritional deficiency, trauma, or physical or psychological stress38; however, there is insufficient evidence to suggest that low iron status impacts androgenetic alopecia, in which its multifactorial pathogenesis implicates genetic and hormonal factors.39 More research is needed to clarify the potential associations between iron deficiency and types of hair loss. Additionally, it is unclear whether iron supplementation improves hair growth parameters such as density and caliber.40
Low serum ferritin (<40 μg/L) with concurrent symptoms of iron deficiency, including fatigue, pallor, dyspnea on exertion, or hair loss, should prompt treatment with supplemental iron.41-43 Generally, ferrous (Fe2+) salts are preferred to ferric (Fe3+) salts, as the former is more readily absorbed through the duodenal mucosa44 and is the more common formulation in commercially available supplements in the United States.45 Oral supplementation with ferrous sulfate 325 mg (65 mg elemental iron) tablets is the first-line therapy for iron deficiency anemia.1 Alternatively, ferrous gluconate 324 mg (38 mg elemental iron) over-the-counter and its liquid form has demonstrated superior absorption compared to ferrous sulfate tablets in a clinical study with peritoneal dialysis patients.1,46 One study suggested that oral iron 40 to 80 mg should be taken every other day to increase absorption.47 Due to improved bioavailability, intravenous iron may be utilized in patients with malabsorption, renal failure, or intolerance to oral iron (including those with gastric ulcers or active inflammatory bowel disease), with the formulation chosen based on underlying comorbidities and potential risks.1,48 The theoretical risk for potentiating bacterial growth by increasing the amount of unbound iron in the blood raises concerns of iron supplementation in patients with infection or sepsis. Although far from definitive, existing data suggest that risk for infection is greater with intravenous iron supplementation and should be carefully considered prior to use.49,50Elevated Ferritin—Elevated ferritin may be difficult to interpret given the multitude of conditions that can cause it.23,51,52 Elevated serum ferritin can be broadly characterized by increased synthesis due to iron overload, increased synthesis due to inflammation, or increased ferritin release from cellular damage.34 Further complicating interpretation is the potential diurnal fluctuations in serum iron levels dependent on dietary intake and timing of laboratory evaluation, choice of assay, differences in reference standards, and variations in calibration procedures that can lead to analytic variability in the measurement of ferritin.23,53,54
Among healthy patients, serum ferritin is directly proportional to iron status.9,51 A study utilizing weekly phlebotomy of 22 healthy participants to measure serum ferritin and calculate mobilizable storage iron found a strong positive correlation between the 2 variables (r=0.83, P<.001), with each 1-μg/L increase of serum ferritin corresponding to approximately an 8-mg increase of storage iron; the initial serum ferritin values ranged from 2 to 83 μg/L in females and 36 to 224 μg/L in males.55 The correlation of ferritin with iron status also was supported by the significant correlation between the number of transfusions received in patients with transfusion-related iron overload and serum ferritin levels (r=0.89, P<.001), with an average increase of 60 μg/L per transfusion.51
Clinical guidelines on the interpretation of serum ferritin levels by Cullis et al34 recommend a normal upper limit of 200 μg/L for healthy females and 300 μg/L for healthy males. Outside of clinical syndromes associated with iron overload, Lee and Means56 found that serum ferritin of 1000 μg/L or higher was a nonspecific marker of disease, including infection and/or neoplastic disorders. We have adapted these guidelines to propose a workflow for evaluation of serum ferritin levels (Figure). In patients with inflammatory conditions or those affected by metabolic syndrome, elevated serum ferritin does not correlate with body iron status.57,58 It is believed that inflammatory cytokines, including tumor necrosis factor α and IL-1α, can upregulate ferritin synthesis independent of cellular iron stores.15,16 Several studies have examined the relationship between insulin resistance and/or metabolic syndrome with serum ferritin levels.31,32 Han et al32 found that elevated serum ferritin was significantly associated with higher risk for metabolic syndrome in men (P<.0001) but not in women.

Although cutaneous manifestations of iron overload can be seen as skin hyperpigmentation due to increased iron deposits and increased melanin production,22 the effects of elevated ferritin on the skin and hair are not well known. Iron overload is a known trigger of porphyria cutanea tarda (PCT),59 a condition in which reduced or absent enzymatic activity of uroporphyrinogen decarboxylase (UROD) leads to build up of toxic porphyrins in various organs.60 In the skin, PCT manifests as a blistering photosensitive eruption that may resolve as dyspigmentation, scarring, and milia.61 Phlebotomy is first-line therapy in PCT to reduce serum iron and subsequent formation of UROD inhibitors, with guidelines suggesting discontinuation of phlebotomy when serum ferritin levels reach 20 ng/mL or lower.60 Hyperferritinemia (serum ferritin >500 μg/L) is a common finding in several inflammatory disorders often accompanied by clinically apparent cutaneous symptoms such as adult-onset Still disease,62 hemophagocytic lymphohistiocytosis,63,64 and anti-melanoma differentiation-associated gene 5 dermatomyositis.65 Among these conditions, serum ferritin levels have been reported to correlate with disease activity, raising the question of whether ferritin is a bystander or a driver of the underlying pathology.62,66,67 However, rapid decline of serum ferritin levels with treatment and control of inflammatory cytokines suggest that ferritin is unlikely to contribute to pathology.62,67
Final Thoughts
Many clinical studies have examined the association between hair health and body iron status, the collective findings of which suggest that iron deficiency may be associated with TE. Among commonly measured serum iron parameters, low ferritin is a highly specific and sensitive marker for diagnosing iron deficiency. Serum ferritin may be a clinically useful tool for ruling out underlying iron deficiency in patients presenting with hair loss. Despite advances in our understanding of the molecular mechanisms of ferritin synthesis and regulation, whether ferritin itself contributes to cutaneous pathology is poorly understood.35,36,68-74 For patients who are otherwise healthy with low suspicion for inflammatory disorders, chronic systemic illnesses, or malignancy, serum ferritin can be used as an indicator of body iron status. The workup for slightly elevated serum ferritin should be interpreted in the context of other laboratory findings and should be reassessed over time. Serum ferritin levels above 1000 μg/L warrant further investigation into causes such as iron overload conditions and underlying inflammatory conditions or malignancy.
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
- Hoffman M, Micheletti RG, Shields BE. Nutritional dermatoses in the hospitalized patient. Cutis. 2020;105:296, 302-308, E1-E5.
- Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4:446-453. doi:10.1159/000336423
- Slusarczyk P, Mandal PK, Zurawska G, et al. Impaired iron recycling from erythrocytes is an early hallmark of aging. eLife. 2023;12:E79196. doi:10.7554/eLife.79196
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Sandnes M, Ulvik RJ, Vorland M, et al. Hyperferritinemia—a clinical overview. J Clin Med. 2021;10:2008. doi:10.3390/jcm10092008
- Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401-409. doi:10.1093/intimm/dxx031
- Wright JA, Richards T, Srai SKS. The role of iron in the skin and cutaneous wound healing. review. Front Pharmacol. 2014;5:156. doi:10.3389/fphar.2014.00156
- Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls Publishing; 2022.
- Crichton RR. Ferritin: structure, synthesis and function. N Engl J Med. 1971;284:1413-1422. doi:10.1056/nejm197106242842506
- Cohen LA, Gutierrez L, Weiss A, et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574-1584. doi:10.1182/blood-2009-11-253815
- Briat JF, Ravet K, Arnaud N, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot. 2010;105:811-822. doi:10.1093/aob/mcp128
- Kato J, Kobune M, Ohkubo S, et al. Iron/IRP-1-dependent regulation of mRNA expression for transferrin receptor, DMT1 and ferritin during human erythroid differentiation. Exp Hematol. 2007;35:879-887. doi:10.1016/j.exphem.2007.03.005
- Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal. 2014;20:1754-1769. doi:10.1089/ars.2013.5666
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505-3516. doi:10.1182/blood.V99.10.3505
- Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638-12644.
- Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289-296. doi:10.1016/0006-291x(90)91466-6
- Bissett DL, Chatterjee R, Hannon DP. Chronic ultraviolet radiation–induced increase in skin iron and the photoprotective effect of topically applied iron chelators. Photochem Photobiol. 1991;54:215-223. https://doi.org/10.1111/j.1751-1097.1991.tb02009.x
- Pourzand C, Watkin RD, Brown JE, et al. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751-6756. doi:10.1073/pnas.96.12.6751
- Applegate LA, Scaletta C, Panizzon R, et al. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol. 1998;111:159-163. https://doi.org/10.1046/j.1523-1747.1998.00254.x
- Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. Am J Respir Crit Care Med. 1994;150:690-695. doi:10.1164/ajrccm.150.3.8087339
- De Domenico I, Ward DM, Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546-4551. doi:10.1182/blood-2009-05-224188
- Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev. 2009;23:95-104. doi:10.1016/j.blre.2008.08.001
- Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chronic Dis. 2018;2018:9394060. doi:10.1155/2018/9394060
- World Health Organization. WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. Published April 21, 2020. Accessed July 23, 2023. https://www.who.int/publications/i/item/9789240000124
- Finch CA, Bellotti V, Stray S, et al. Plasma ferritin determination as a diagnostic tool. West J Med. 1986;145:657-663.
- Guyatt GH, Oxman AD, Ali M, et al. Laboratory diagnosis of iron-deficiency anemia. J Gen Intern Med. 1992;7:145-153. doi:10.1007/BF02598003
- Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. https://doi.org/10.1182/blood.V89.3.1052
- Zacharski LR, Ornstein DL, Woloshin S, et al. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. American Heart Journal. 2000;140:98-104. https://doi.org/10.1067/mhj.2000.106646
- Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22-27. doi:10.1007/bf01811467
- Liu J-M, Hankinson SE, Stampfer MJ, et al. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160-1167. doi:10.1093/ajcn/78.6.1160
- Kim C, Nan B, Kong S, et al. Changes in iron measures over menopause and associations with insulin resistance. J Womens Health (Larchmt). 2012;21:872-877. doi:10.1089/jwh.2012.3549
- Han LL, Wang YX, Li J, et al. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58:2189-2195. doi:10.1002/mnfr.201400088
- Pan Y, Jackson RT. Insights into the ethnic differences in serum ferritin between black and white US adult men. Am J Hum Biol. 2008;20:406-416. https://doi.org/10.1002/ajhb.20745
- Cullis JO, Fitzsimons EJ, Griffiths WJ, et al. Investigation and management of a raised serum ferritin. Br J Haematol. 2018;181:331-340. doi:10.1111/bjh.15166
- Moeinvaziri M, Mansoori P, Holakooee K, et al. Iron status in diffuse telogen hair loss among women. Acta Dermatovenerol Croat. 2009;17:279-284.
- Tamer F, Yuksel ME, Karabag Y. Serum ferritin and vitamin D levels should be evaluated in patients with diffuse hair loss prior to treatment. Postepy Dermatol Alergol. 2020;37:407-411. doi:10.5114/ada.2020.96251
- Olsen EA, Reed KB, Cacchio PB, et al. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am Acad Dermatol. 2010;63:991-999. doi:10.1016/j.jaad.2009.12.006
- Asghar F, Shamim N, Farooque U, et al. Telogen effluvium: a review of the literature. Cureus. 2020;12:E8320. doi:10.7759/cureus.8320
- Brough KR, Torgerson RR. Hormonal therapy in female pattern hair loss. Int J Womens Dermatol. 2017;3:53-57. doi:10.1016/j.ijwd.2017.01.001
- Klein EJ, Karim M, Li X, et al. Supplementation and hair growth: a retrospective chart review of patients with alopecia and laboratory abnormalities. JAAD Int. 2022;9:69-71. doi:10.1016/j.jdin.2022.08.013
- Goksin S. Retrospective evaluation of clinical profile and comorbidities in patients with alopecia areata. North Clin Istanb. 2022;9:451-458. doi:10.14744/nci.2022.78790
- Beatrix J, Piales C, Berland P, et al. Non-anemic iron deficiency: correlations between symptoms and iron status parameters. Eur J Clin Nutr. 2022;76:835-840. doi:10.1038/s41430-021-01047-5
- Treister-Goltzman Y, Yarza S, Peleg R. Iron deficiency and nonscarring alopecia in women: systematic review and meta-analysis. Skin Appendage Disord. 2022;8:83-92. doi:10.1159/000519952
- Santiago P. Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. ScientificWorldJournal. 2012;2012:846824. doi:10.1100/2012/846824
- Lo JO, Benson AE, Martens KL, et al. The role of oral iron in the treatment of adults with iron deficiency. Eur J Haematol. 2023;110:123-130. doi:10.1111/ejh.13892
- Lausevic´ M, Jovanovic´ N, Ignjatovic´ S, et al. Resorption and tolerance of the high doses of ferrous sulfate and ferrous gluconate in the patients on peritoneal dialysis. Vojnosanit Pregl. 2006;63:143-147. doi:10.2298/vsp0602143l
- Stoffel NU, Zeder C, Brittenham GM, et al. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica. 2020;105:1232-1239. doi:10.3324/haematol.2019.220830
- Jimenez KM, Gasche C. Management of iron deficiency anaemia in inflammatory bowel disease. Acta Haematologica. 2019;142:30-36. doi:10.1159/000496728
- Shah AA, Donovan K, Seeley C, et al. Risk of infection associated with administration of intravenous iron: a systematic review and meta-analysis. JAMA Netw Open. 2021;4:E2133935-E2133935. doi:10.1001/jamanetworkopen.2021.33935
- Ganz T, Aronoff GR, Gaillard CAJM, et al. Iron administration, infection, and anemia management in ckd: untangling the effects of intravenous iron therapy on immunity and infection risk. Kidney Med. 2020/05/01/ 2020;2:341-353. doi: 10.1016/j.xkme.2020.01.006
- Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213-1216. doi:10.1056/nejm197405302902201
- Loveikyte R, Bourgonje AR, van der Reijden JJ, et al. Hepcidin and iron status in patients with inflammatory bowel disease undergoing induction therapy with vedolizumab or infliximab [published online February 7, 2023]. Inflamm Bowel Dis. doi:10.1093/ibd/izad010
- Borel MJ, Smith SM, Derr J, et al. Day-to-day variation in iron-status indices in healthy men and women. Am J Clin Nutr. 1991;54:729-735. doi:10.1093/ajcn/54.4.729
- Ford BA, Coyne DW, Eby CS, et al. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney International. 2009;75:104-110. doi:10.1038/ki.2008.526
- Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26:770-772. doi:10.1136/jcp.26.10.770
- Lee MH, Means RT Jr. Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med. Jun 1995;98:566-571. doi:10.1016/s0002-9343(99)80015-1
- Theil EC. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289-315. doi:10.1146/annurev.bi.56.070187.001445
- Chen LY, Chang SD, Sreenivasan GM, et al. Dysmetabolic hyperferritinemia is associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin. Ann Hematol. 2011;90:139-143. doi:10.1007/s00277-010-1050-x
- Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248-253.
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24:735-745. doi:10.1016/j.bpg.2010.07.002
- Mehta B, Efthimiou P. Ferritin in adult-onset Still’s disease: just a useful innocent bystander? Int J Inflam. 2012;2012:298405. doi:10.1155/2012/298405
- Ma AD, Fedoriw YD, Roehrs P. Hyperferritinemia and hemophagocytic lymphohistiocytosis. single institution experience in adult and pediatric patients. Blood. 2012;120:2135-2135. doi:10.1182/blood.V120.21.2135.2135
- Basu S, Maji B, Barman S, et al. Hyperferritinemia in hemophagocytic lymphohistiocytosis: a single institution experience in pediatric patients. Indian J Clin Biochem. 2018;33:108-112. doi:10.1007/s12291-017-0655-4
- Yamada K, Asai K, Okamoto A, et al. Correlation between disease activity and serum ferritin in clinically amyopathic dermatomyositis with rapidly-progressive interstitial lung disease: a case report. BMC Res Notes. 2018;11:34. doi:10.1186/s13104-018-3146-7
- Zohar DN, Seluk L, Yonath H, et al. Anti-MDA5 positive dermatomyositis associated with rapidly progressive interstitial lung disease and correlation between serum ferritin level and treatment response. Mediterr J Rheumatol. 2020;31:75-77. doi:10.31138/mjr.31.1.75
- Lin TF, Ferlic-Stark LL, Allen CE, et al. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56:154-155. doi:10.1002/pbc.22774
- Bregy A, Trueb RM. No association between serum ferritin levels >10 microg/l and hair loss activity in women. Dermatology. 2008;217:1-6. doi:10.1159/000118505
- de Queiroz M, Vaske TM, Boza JC. Serum ferritin and vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. 2022;21:2688-2690. doi:10.1111/jocd.14472
- El-Husseiny R, Alrgig NT, Abdel Fattah NSA. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J Cosmet Dermatol. 2021;20:1860-1866. doi:10.1111/jocd.13747
- Enitan AO, Olasode OA, Onayemi EO, et al. Serum ferritin levels amongst individuals with androgenetic alopecia in Ile-Ife, Nigeria. West Afr J Med. 2022;39:1026-1031.
- I˙bis¸ S, Aksoy Sarac¸ G, Akdag˘ T. Evaluation of MCV/RDW ratio and correlations with ferritin in telogen effluvium patients. Dermatol Pract Concept. 2022;12:E2022151. doi:10.5826/dpc.1203a151
- Kakpovbia E, Ogbechie-Godec OA, Shapiro J, et al. Laboratory testing in telogen effluvium. J Drugs Dermatol. 2021;20:110-111. doi:10.36849/jdd.5771
- Rasheed H, Mahgoub D, Hegazy R, et al. Serum ferritin and vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. 2013;26:101-107. doi:10.1159/000346698
Practice Points
- In patients who are otherwise healthy without chronic systemic disease, hepatic disease, or inflammatory disorders, serum ferritin levels directly correlate with body iron status.
- Elevated serum ferritin should be interpreted in the context of other indicators of iron status, including transferrin saturation, complete blood cell count, and/or liver function panel.
- Low serum ferritin is a specific marker for iron deficiency, and iron supplementation should be initiated based on age-, sex-, and condition-specific thresholds.