User login
What Is Your Diagnosis? Onychomadesis Following Hand-foot-and-mouth Disease
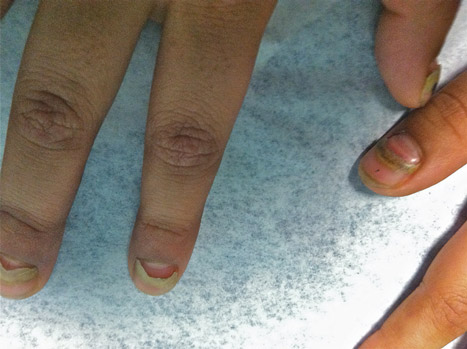
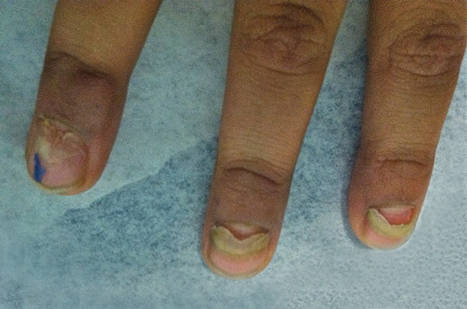
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.