User login
Right upper-abdominal pain in a 97-year-old
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
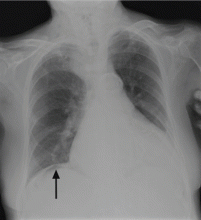
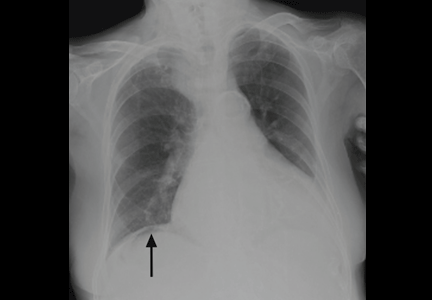
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
A 97-year-old man has had right upper-abdominal pain intermittently for 2 weeks. He has hypertension, stage IV chronic kidney disease, chronic obstructive pulmonary disease, and constipation. He has never had abdominal surgery.
He describes his pain as mild and dull. It does not radiate to the right lower quadrant or the back and is not aggravated by eating. He reports no fever or changes in appetite or bowel habits during the last 2 weeks. His body temperature is 36.8°C, blood pressure 114/68 mm Hg, heart rate 86 beats per minute, and respiratory rate 16 times per minute.
On physical examination, his abdomen is soft with no guarding and with hypoactive bowel sounds. No Murphy sign is noted. Hemography shows a normal white blood cell count of 7.8 × 109/L) (reference range 4.5–11.0). Serum biochemistry studies show an alanine transaminase level of 23 U/L (5–50) and a lipase level of 40 U/L (12–70); the C-reactive protein level is 0.5 mg/dL (0.0–1.0). A sitting chest radiograph shows a focal gas collection over the right subdiaphragmatic area (Figure 1).
Q: Based on the information above, which is most likely the cause of this man’s upper-abdominal pain?
- Perforated viscera
- Diverticulitis
- Chilaiditi syndrome
- Subdiaphragmatic abscess
- Emphysematous cholecystitis
A: The workup of this patient did not indicate active disease, so the subphrenic gas on the radiograph most likely is the Chilaiditi sign. This is a benign finding that, in a patient with gastrointestinal symptoms (nausea, vomiting, constipation, upper-abdominal pain), is labeled Chilaiditi syndrome.
CHILAIDITI SIGN AND SYNDROME
The Chilaiditi sign1 describes a benign, incidental radiologic finding of subphrenic gas caused by interposition of colonic segments (or small intestine in rare cases) between the liver and the diaphragm. The radiologic finding is called the Chilaiditi sign if the patient is asymptomatic or Chilaiditi syndrome if the patient has gastrointestinal symptoms, as our patient did. The Chilaiditi sign is reportedly found in 0.02% to 0.2% of all chest and abdominal films.
Chilaiditi syndrome has a male predominance.2 Predisposing factors include an atrophic liver, laxity of the hepatic or the transverse colon suspension ligament, abnormal fixation of the mesointestine, and diaphragmatic weakness. Other factors include advanced age; a history of abdominal surgery, adhesion, or intestinal obstruction3; chronic lung disease; and cirrhosis.4
Management is usually conservative, with a prokinetic agent or enema for constipation, and bed-rest or bowel decompression as needed, unless complications occur. Our patient’s extreme age, underlying chronic pulmonary disease, and constipation predisposed him to this rare gastrointestinal disorder.
In this patient, pain in the right upper quadrant initially suggested an inflammatory disorder involving the liver, gallbladder, and transverse or ascending colon. Right upper-quadrant pain with radiologic evidence of subphrenic air collection further raises suspicion of pneumoperitoneum from diverticulitis, bowel perforation, or gas-forming abscess. However, this patient’s normal transaminase level, low C-reactive protein value, and prolonged symptom course made hepatitis, cholecystitis, diverticulitis, and subdiaphragmatic abscess less likely. Nonetheless, severe intra-abdominal pathology can sometimes manifest with only minor symptoms in very elderly patients. Consequently, the main concern in this scenario was whether he had minor and undetected perforated viscera causing pneumoperitoneum with an indolent course, or rather a benign condition such as Chilaiditi syndrome causing pain and subphrenic air.
IS IT CHILAIDITI SYNDROME OR PNEUMOPERITONEUM?
Chilaiditi syndrome and perforated viscera both involve subphrenic air, but they differ radiologically and clinically. Radiologically, identification of haustra or plicae circulares within the gas collection or fixed subphrenic air upon postural change indicates the Chilaiditi sign and favors Chilaiditi syndrome as the origin of the symptoms. Pneumoperitoneum from perforated viscera is more likely if the abnormal gas collection changes its position upon postural change. Abdominal ultrasonography can also assist in diagnosis by showing a fixed air collection around the hepatic surface in the Chilaiditi sign. Definite radiologic diagnosis can be reached through abdominal computed tomography. Clinically, these two disorders may manifest different severity, as perforated viscera often mandate surgical attention, whereas Chilaiditi syndrome seldom requires surgical treatment (25% of cases).2
Patients with the Chilaiditi sign also may develop abdominal pathology other than Chilaiditi syndrome per se. In our patient, the subphrenic air displayed a faint contour of bowel segments. His symptom course, benign physical examination, and the lack of laboratory evidence of other intra-abdominal pathology led us to suspect Chilaiditi syndrome as the cause of his abdominal pain. A normal leukocyte count and stable vital signs made the diagnosis of a major life-threatening condition extremely unlikely. Subsequently, abdominal sonography done at the bedside disclosed fixed colonic segments between the liver and the diaphragm. No hepatic or gallbladder lesions were detected. Chilaiditi syndrome was confirmed.
TAKE-HOME MESSAGE
As seen in this case, the accurate diagnosis rests on a careful physical examination and laboratory evaluation but, most importantly, on sound clinical judgment. Right upper-quadrant pain is often encountered in primary care practice and has many diagnostic possibilities, including benign, self-limited conditions such as Chilaiditi syndrome. It is vital to distinguish between benign conditions and severe life-threatening disorders such as hollow organ perforation so as not to operate on patients who can be managed conservatively.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
- Chilaiditi D. Zur Frage der Hepatoptose und Ptose in allegemeinen in Anschluss an drei Fälle von temporärer, partieller Lebersverlagerung. Fortschr Geb Röntgenstr Nuklearmed Erganzungsband 1910; 16:173–208.
- Saber AA, Boros MJ. Chilaiditi’s syndrome: what should every surgeon know? Am Surg 2005; 71:261–263.
- Lo BM. Radiographic look-alikes: distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med 2010; 38:36–39.
- Fisher AA, Davis MW. An elderly man with chest pain, shortness of breath, and constipation. Postgrad Med J 2003; 79:180,183–184.
Atrial fibrillation management: Issues of concern
To the Editor: I read with interest the article by Drs. Callahan and Baranowski1 in your April 2011 issue about managing newly diagnosed atrial fibrillation. I believe several issues merit further discussion.
First of all, as mentioned in the article, pulmonary vein isolation, or radiofrequency catheter ablation of the left atrium, can cure paroxysmal atrial fibrillation. Callahan and Baranowski described the optimal indication for this procedure, but they failed to mention the potential adverse effects, that is, esophageal ulcer and atrio-esophageal fistula.2 Owing to the proximity of the esophagus and the accompanying vagus nerve to the posterior wall of the left atrium, it is estimated that 47% of patients develop thermal mucosal injury and 18% develop esophageal ulcer after ablation, while 0.5% develop atrio-esophageal fistula.3 Gastric hypomotility and pyloric spasm are reported as well. It would therefore be prudent to inform patients of such risks if a persistently symptomatic young patient demands this procedure, since the damage might be long-lasting.
In addition, in deciding on long-term anticoagulation for patients with atrial fibrillation, the CHADS2 score is often utilized (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack). Although it is validated and widely applicable, the CHADS2 score carries the disadvantages of oversimplification and of overclassifying atrial fibrillation patients into the intermediate-risk category.4 Lip et al,5 in a seminal article surveying a large group of patients who had nonvalvular atrial fibrillation, proposed using a new and also simple risk stratification scheme, the 2009 Birmingham scheme. This scheme uses the acronym CHA2DS2-VASc and differs from the CHADS2 score in that patients age 75 or older get 2 points, those age 65 to 74 get 1 point, those with vascular disease get 1 point, and women get 1 point. They show that this new scheme fares marginally better than the original CHADS2 score, with fewer patients wrongly assigned to the intermediate-risk category. That means a lower percentage of patients will receive unnecessary anticoagulation and suffer from unneeded anguish. Subsequent studies also prove that this newer scoring index possesses higher sensitivity and predicts thromboembolic events more accurately than the CHADS2 score. Thus, I believe this should also be factored into the decision process when initiating warfarin in atrial fibrillation patients, especially in light of the fact that scanty evidence exists for the use of newer anticoagulants based on the CHADS2 score.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Ginzburg L. Esophageal ulceration: a complication of radiofrequency ablation treatment of atrial fibrillation. Gastrointest Endosc 2009; 70:551–552.
- Bahnson TD. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2009; 32:248–260.
- Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation—friend or foe? Thromb Haemost 2010; 104:45–48.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–172.
To the Editor: I read with interest the article by Drs. Callahan and Baranowski1 in your April 2011 issue about managing newly diagnosed atrial fibrillation. I believe several issues merit further discussion.
First of all, as mentioned in the article, pulmonary vein isolation, or radiofrequency catheter ablation of the left atrium, can cure paroxysmal atrial fibrillation. Callahan and Baranowski described the optimal indication for this procedure, but they failed to mention the potential adverse effects, that is, esophageal ulcer and atrio-esophageal fistula.2 Owing to the proximity of the esophagus and the accompanying vagus nerve to the posterior wall of the left atrium, it is estimated that 47% of patients develop thermal mucosal injury and 18% develop esophageal ulcer after ablation, while 0.5% develop atrio-esophageal fistula.3 Gastric hypomotility and pyloric spasm are reported as well. It would therefore be prudent to inform patients of such risks if a persistently symptomatic young patient demands this procedure, since the damage might be long-lasting.
In addition, in deciding on long-term anticoagulation for patients with atrial fibrillation, the CHADS2 score is often utilized (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack). Although it is validated and widely applicable, the CHADS2 score carries the disadvantages of oversimplification and of overclassifying atrial fibrillation patients into the intermediate-risk category.4 Lip et al,5 in a seminal article surveying a large group of patients who had nonvalvular atrial fibrillation, proposed using a new and also simple risk stratification scheme, the 2009 Birmingham scheme. This scheme uses the acronym CHA2DS2-VASc and differs from the CHADS2 score in that patients age 75 or older get 2 points, those age 65 to 74 get 1 point, those with vascular disease get 1 point, and women get 1 point. They show that this new scheme fares marginally better than the original CHADS2 score, with fewer patients wrongly assigned to the intermediate-risk category. That means a lower percentage of patients will receive unnecessary anticoagulation and suffer from unneeded anguish. Subsequent studies also prove that this newer scoring index possesses higher sensitivity and predicts thromboembolic events more accurately than the CHADS2 score. Thus, I believe this should also be factored into the decision process when initiating warfarin in atrial fibrillation patients, especially in light of the fact that scanty evidence exists for the use of newer anticoagulants based on the CHADS2 score.
To the Editor: I read with interest the article by Drs. Callahan and Baranowski1 in your April 2011 issue about managing newly diagnosed atrial fibrillation. I believe several issues merit further discussion.
First of all, as mentioned in the article, pulmonary vein isolation, or radiofrequency catheter ablation of the left atrium, can cure paroxysmal atrial fibrillation. Callahan and Baranowski described the optimal indication for this procedure, but they failed to mention the potential adverse effects, that is, esophageal ulcer and atrio-esophageal fistula.2 Owing to the proximity of the esophagus and the accompanying vagus nerve to the posterior wall of the left atrium, it is estimated that 47% of patients develop thermal mucosal injury and 18% develop esophageal ulcer after ablation, while 0.5% develop atrio-esophageal fistula.3 Gastric hypomotility and pyloric spasm are reported as well. It would therefore be prudent to inform patients of such risks if a persistently symptomatic young patient demands this procedure, since the damage might be long-lasting.
In addition, in deciding on long-term anticoagulation for patients with atrial fibrillation, the CHADS2 score is often utilized (1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack). Although it is validated and widely applicable, the CHADS2 score carries the disadvantages of oversimplification and of overclassifying atrial fibrillation patients into the intermediate-risk category.4 Lip et al,5 in a seminal article surveying a large group of patients who had nonvalvular atrial fibrillation, proposed using a new and also simple risk stratification scheme, the 2009 Birmingham scheme. This scheme uses the acronym CHA2DS2-VASc and differs from the CHADS2 score in that patients age 75 or older get 2 points, those age 65 to 74 get 1 point, those with vascular disease get 1 point, and women get 1 point. They show that this new scheme fares marginally better than the original CHADS2 score, with fewer patients wrongly assigned to the intermediate-risk category. That means a lower percentage of patients will receive unnecessary anticoagulation and suffer from unneeded anguish. Subsequent studies also prove that this newer scoring index possesses higher sensitivity and predicts thromboembolic events more accurately than the CHADS2 score. Thus, I believe this should also be factored into the decision process when initiating warfarin in atrial fibrillation patients, especially in light of the fact that scanty evidence exists for the use of newer anticoagulants based on the CHADS2 score.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Ginzburg L. Esophageal ulceration: a complication of radiofrequency ablation treatment of atrial fibrillation. Gastrointest Endosc 2009; 70:551–552.
- Bahnson TD. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2009; 32:248–260.
- Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation—friend or foe? Thromb Haemost 2010; 104:45–48.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–172.
- Callahan T, Baranowski B. Managing newly diagnosed atrial fibrillation: rate, rhythm, and risk. Cleve Clin J Med 2011; 78:258–264.
- Ginzburg L. Esophageal ulceration: a complication of radiofrequency ablation treatment of atrial fibrillation. Gastrointest Endosc 2009; 70:551–552.
- Bahnson TD. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2009; 32:248–260.
- Karthikeyan G, Eikelboom JW. The CHADS2 score for stroke risk stratification in atrial fibrillation—friend or foe? Thromb Haemost 2010; 104:45–48.
- Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–172.
Iron therapy and infection
To the Editor: In their article, “Is iron therapy for anemia harmful in the setting of infection?” in the March 2011 issue, Daoud et al1 illustrated an interesting aspect we often encounter, especially in nephrology practice. However, I believe several points should be clarified in this context.
First, “iron therapy” and “iron stores” are quite different things when we talk about infection. As Daoud et al state, human studies involving iron therapy and infection are conflicting in their results. The explanation is likely that iron therapy per se does not always translate to increased iron stores, while iron stores do correlate with increased risk of infection and death, whether in hemodialysis patients2 or in the general population.3 Intravenous iron therapy mostly gains the association with risk of infection when dosed greater than a certain amount or for an extended duration. In addition, Pieracci et al4 showed that oral iron therapy for anemia does not boost the infection rate during critical illness when equivalent iron markers are achieved.
This mounting evidence solidifies the view that iron stores underlie the infection susceptibility. But to prove this concept, a randomized controlled study consisting of achieving similar iron stores by component therapy or intravenous iron supplementation would be the best option.
Second, I wish to add a category of infection omitted in their article, ie, fungal infection (mucormycosis). Mucormycosis, a rare but life-threatening disease, is caused by fungi of the class Zygomycetes that spread systemically in immunocompromised hosts, with a high death rate. Iron overload, whether or not accompanied by the use of deferoxamine (Desferal), is an established risk factor for mucormycosis. These fungi possess a high-affinity iron permease and produce siderophores, both of which facilitate the uptake of iron.5 An abundant host iron pool further enhances their scavenging process, resulting in devastating proliferation and tissue damage. This disease category should be borne in mind when dealing with immunocompromised patients undergoing iron therapy.
- Daoud E, Nakhla E, Sharma R. Is iron therapy for anemia harmful in the setting of infection? Cleve Clin J Med 2011; 78:168–170.
- Pieracci FM, Barie PS. Iron and the risk of infection. Surg Infect 2005; 6(suppl 1):S41–S46.
- Ellervik C, Tybjærg-Hansen A, Nordestgaard BG. Total mortality by transferrin saturation levels: two general population studies and a metaanalysis. Clin Chem 2011; 57:459–466.
- Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect 2009; 10:9–19.
- Ibrahim A, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
To the Editor: In their article, “Is iron therapy for anemia harmful in the setting of infection?” in the March 2011 issue, Daoud et al1 illustrated an interesting aspect we often encounter, especially in nephrology practice. However, I believe several points should be clarified in this context.
First, “iron therapy” and “iron stores” are quite different things when we talk about infection. As Daoud et al state, human studies involving iron therapy and infection are conflicting in their results. The explanation is likely that iron therapy per se does not always translate to increased iron stores, while iron stores do correlate with increased risk of infection and death, whether in hemodialysis patients2 or in the general population.3 Intravenous iron therapy mostly gains the association with risk of infection when dosed greater than a certain amount or for an extended duration. In addition, Pieracci et al4 showed that oral iron therapy for anemia does not boost the infection rate during critical illness when equivalent iron markers are achieved.
This mounting evidence solidifies the view that iron stores underlie the infection susceptibility. But to prove this concept, a randomized controlled study consisting of achieving similar iron stores by component therapy or intravenous iron supplementation would be the best option.
Second, I wish to add a category of infection omitted in their article, ie, fungal infection (mucormycosis). Mucormycosis, a rare but life-threatening disease, is caused by fungi of the class Zygomycetes that spread systemically in immunocompromised hosts, with a high death rate. Iron overload, whether or not accompanied by the use of deferoxamine (Desferal), is an established risk factor for mucormycosis. These fungi possess a high-affinity iron permease and produce siderophores, both of which facilitate the uptake of iron.5 An abundant host iron pool further enhances their scavenging process, resulting in devastating proliferation and tissue damage. This disease category should be borne in mind when dealing with immunocompromised patients undergoing iron therapy.
To the Editor: In their article, “Is iron therapy for anemia harmful in the setting of infection?” in the March 2011 issue, Daoud et al1 illustrated an interesting aspect we often encounter, especially in nephrology practice. However, I believe several points should be clarified in this context.
First, “iron therapy” and “iron stores” are quite different things when we talk about infection. As Daoud et al state, human studies involving iron therapy and infection are conflicting in their results. The explanation is likely that iron therapy per se does not always translate to increased iron stores, while iron stores do correlate with increased risk of infection and death, whether in hemodialysis patients2 or in the general population.3 Intravenous iron therapy mostly gains the association with risk of infection when dosed greater than a certain amount or for an extended duration. In addition, Pieracci et al4 showed that oral iron therapy for anemia does not boost the infection rate during critical illness when equivalent iron markers are achieved.
This mounting evidence solidifies the view that iron stores underlie the infection susceptibility. But to prove this concept, a randomized controlled study consisting of achieving similar iron stores by component therapy or intravenous iron supplementation would be the best option.
Second, I wish to add a category of infection omitted in their article, ie, fungal infection (mucormycosis). Mucormycosis, a rare but life-threatening disease, is caused by fungi of the class Zygomycetes that spread systemically in immunocompromised hosts, with a high death rate. Iron overload, whether or not accompanied by the use of deferoxamine (Desferal), is an established risk factor for mucormycosis. These fungi possess a high-affinity iron permease and produce siderophores, both of which facilitate the uptake of iron.5 An abundant host iron pool further enhances their scavenging process, resulting in devastating proliferation and tissue damage. This disease category should be borne in mind when dealing with immunocompromised patients undergoing iron therapy.
- Daoud E, Nakhla E, Sharma R. Is iron therapy for anemia harmful in the setting of infection? Cleve Clin J Med 2011; 78:168–170.
- Pieracci FM, Barie PS. Iron and the risk of infection. Surg Infect 2005; 6(suppl 1):S41–S46.
- Ellervik C, Tybjærg-Hansen A, Nordestgaard BG. Total mortality by transferrin saturation levels: two general population studies and a metaanalysis. Clin Chem 2011; 57:459–466.
- Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect 2009; 10:9–19.
- Ibrahim A, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.
- Daoud E, Nakhla E, Sharma R. Is iron therapy for anemia harmful in the setting of infection? Cleve Clin J Med 2011; 78:168–170.
- Pieracci FM, Barie PS. Iron and the risk of infection. Surg Infect 2005; 6(suppl 1):S41–S46.
- Ellervik C, Tybjærg-Hansen A, Nordestgaard BG. Total mortality by transferrin saturation levels: two general population studies and a metaanalysis. Clin Chem 2011; 57:459–466.
- Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect 2009; 10:9–19.
- Ibrahim A, Spellberg B, Edwards J. Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis 2008; 21:620–625.