User login
Retrospective Analysis of Liraglutide as Add-On Therapy in Type 2 Diabetes Mellitus: Quantifying the Changes in Insulin Requirements
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
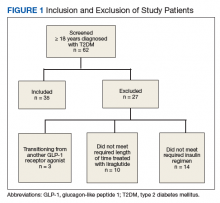
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
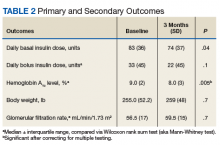
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
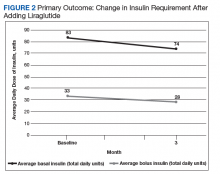
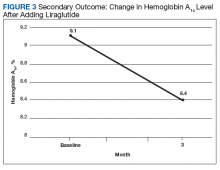
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.