User login
Acute and Recurrent Bacterial Vaginosis
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of reproductive age, with a prevalence in North America of 29.2% among women ages 14 to 49.1-3 BV is a condition in which the normal vaginal flora are altered, primarily due to a reduction in hydrogen peroxide–producing strains of lactobacilli. This leads to an elevated vaginal pH and increased levels of proteolytic enzymes (eg, sialidase), organic acids, and volatile amines.4 This change in pH allows an overgrowth of multiple types of anaerobic, mycoplasmic, and gram-negative bacteria.
In most cases of BV, the predominant microbe is the facultative anaerobe Gardnerella vaginalis. However, evidence from recent studies of the pathogenesis of BV suggests that this bacterium forms a biofilm in the vaginal epithelium that serves as a “scaffolding” to which other bacterial species adhere in a symbiotic fashion, colonizing the vagina.5 Though asymptomatic in at least half of affected women,2,6,7 this polymicrobial condition can produce a thin, white, homogenous discharge with a distinct “fishy” odor.
The changes in the vaginal flora seen in BV are associated with serious sequelae, such as preterm delivery, spontaneous abortion, postpartum endometritis, and increased susceptibility to HIV and other sexually transmitted infections (STIs).4,8,9 The polymicrobial nature of BV and its propensity for recurrence make treatment a challenge.
PATIENT PRESENTATION/HISTORY
The most common symptom of BV is increased vaginal discharge, which usually is thin and white or dull gray.4 Some women report a strong fishy odor, especially after sex. Vaginal pain, itching, or burning may also be present, especially if the discharge is copious. Dyspareunia and dysuria are rare, but possible, symptoms. Fever, malaise, and other systemic symptoms are not associated with BV and should prompt the clinician to consider other causes. About half of women with bacterial vaginosis have no symptoms.2,6
The typical finding on physical examination is a homogeneous, off-white, creamy, malodorous discharge that adheres to the vaginal walls and pools in the vaginal vault. There are usually no or minimal signs of vaginal inflammation, and the vulva, labia, and cervix are typically normal. In some cases, BV can lead to cervicitis.6,9,10
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of BV can be made based on the history, physical examination, and microscopic examination of the vaginal discharge. Unlike with many other bacterial diseases, culture is not recommended for diagnosis of BV because many of the implicated organisms cannot be easily isolated in the laboratory, and because asymptomatic women also have small numbers of these flora in the vagina.
In 1991, Nugent et al11 described a Gram stain scoring system of vaginal smears to diagnose BV, which has a sensitivity and specificity of 96% and 96%, respectively; it remains the gold standard for diagnosis.7 However, because this method requires considerable time and skill, it is not routinely used in most clinic settings.
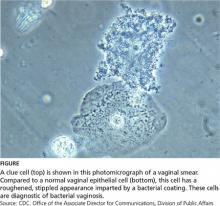
A widely used method of diagnosing BV is the Amsel criteria (see Table 1). The Amsel method has a sensitivity and specificity of 81% and 94%, respectively.1,12 The presence of clue cells is the most reliable indicator of BV (see figure). The positive predictive value of this test for the presence of BV is 95%.14 The Amsel method requires microscopy,4,12 which is not always available in clinics.
There are several commercially available point-of-care tests for BV that do not require microscopy. These include rapid antigen and nucleic acid amplification tests to detect elevated levels of G vaginalis, as well as tests that detect the presence of bacterial amines, elevated vaginal pH, and bacterial sialidase.4,7,15 Compared with the Nugent and Amsel methods, one test that detects elevated vaginal fluid sialidase activity was shown to have a sensitivity of 88% and specificity of 91% to 95%.4,7,15 These point-of-care tests are most effective for diagnosing BV when the vaginal pH exceeds 4.5 and when they are used in conjunction with other clinical criteria.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Treatment is recommended for women with symptoms. The established benefit of therapy in nonpregnant women is relief of vaginal symptoms and signs of infection. Other potential benefits to treatment include reduction in the risk for Chlamydia trachomatis or Neisseria gonorrhea infection, HIV, and other viral STIs. Table 2 includes the recommended and alternative treatment regimens for BV, according to the CDC’s 2015 treatment guidelines for sexually transmitted infections.7 These regimens are also recommended by the American Congress of Obstetricians and Gynecologists (ACOG).16
Treatment is also recommended for all symptomatic pregnant women. Older CDC guidelines noted a preference for oral therapy in pregnant women with BV, due to the possibility of subclinical upper genital tract infection.17 However, the 2015 CDC guidelinesstate that symptomatic pregnant women can be treated with either the oral or vaginal regimens recommended for nonpregnant women, as oral therapy has not been shown to outperform vaginal therapy in effecting cure or preventing adverse outcomes.7
PATIENT EDUCATION AND FOLLOW-UP
Patient preferences, possible adverse effects, drug interactions, and other coinfections should be considered when selecting a treatment regimen. Women should be advised to refrain from sexual intercourse or to use condoms consistently and correctly during treatment. Douching may increase the risk for relapse, and no data support its use for treatment or relief of symptoms. Follow-up visits are unnecessary unless symptoms do not resolve. Because recurrence of BV is common, however, women should be advised to return for evaluation if symptoms recur.1,8,18
Continue for when BV recurs >>
WHEN BV RECURS
Recurrence rates of 15% to 30% have been reported at three months,18,19 and of 28% when patients were tested cumulatively over six months,1 but few researchers have looked at long-term recurrence rates. In one observational study, recurrence rates of 51% were reported during a six-year follow-up period among women previously treated with oral metronidazole.20 Whether these high recurrence rates are due to treatment failure to eradicate the causative organism or to a reinfection from sexual partners remains unclear.21 Some studies have shown that treatment of male partners does not affect recurrence rates.21,22
Risk factors
Various research teams have identified risk factors associated with BV recurrence, but study results have been inconsistent. The strongest risk factor appears to be sexual activity, specifically with increased numbers of sexual partners and inconsistent condom use.1,23,24 Women who have sex with women also appear to be at increased risk for BV recurrence.9,10
BV tends to recur around the time of menstruation, and some suppressive therapies include administration of antibiotics during this time.1,8 Although reports conflict, other risk factors that may be implicated in recurrent BV include vaginal douching, cigarette smoking, and increased BMI.2,18 Use of an oral contraceptive may have a protective effect against BV recurrence.1
Caring for patients with multiple recurrences of BV can be challenging for many clinicians. Although a few studies have evaluated suppressive therapy for recurrent BV, there are no clear treatment guidelines for multiple recurrent infections. Sobel and colleagues evaluated twice-weekly use of metronidazole gel for 16 weeks and found a significant reduction in BV recurrence during treatment.25 However, there was only a 34% to 37% probability of patients’ remaining clinically cured at seven months posttreatment. Similarly, Reichman et al evaluated suppressive therapy with oral metronidazole, topical boric acid, and metronidazole gel. They found an 88% to 92% initial cure rate, but a 50% failure rate at 36 weeks’ follow-up.26
Management
Studies examining the use of probiotics for the prevention and treatment of BV have yielded mixed results. The theory is that probiotics containing lactobacillus organisms may protect women from infection by maintaining or restoring vaginal pH and preventing adhesion of bacteria to the epithelium of the vaginal walls.27 Despite the conflicting results, no adverse effects have been reported and, as a consequence, many experts recommend probiotics to reduce the risk for recurrent BV. When discussing suppressive therapy options with patients, clinicians should be mindful of the limited data and the clinically unfavorable long-term cure rates demonstrated.
In addition to treatment limitations for recurrent BV, clinicians often find it challenging to effectively address the psychosocial implications of distress, embarrassment, and lack of control that are commonly associated with recurrent BV.28 Beyond its impact on sexual activity, women have also reported refraining from their daily activities out of fear that others around them may detect their vaginal odor. Helping women take a proactive approach in the treatment and prevention of BV may ease some of this distress.
Women with recurrent BV are often eager to hear about measures they can take to reduce their risk for acute and recurrent infection. Patients should be counseled on the association of BV with douching, numerous sexual partners, unprotected sex, increased psychosocial stress, and cigarette smoking.7,18,29-31 Patients may inquire about the potential risk for BV when they use feminine hygiene spray, panty liners or pads, and underwear made from synthetic fabrics; however, one longitudinal study30 showed no association between any of these hygienic behaviors and BV.
Continue for the conclusion >>
CONCLUSION
Bacterial vaginosis is a common cause of vaginal discharge in women. Current recommendations for treatment are not very effective, with up to half of women experiencing recurrence. The likelihood of recurrence can result in significant frustration for both patient and clinician. Although recent studies have advanced our understanding of the pathophysiology of BV, further research is needed to develop more effective treatments that reduce recurrence. Addressing modifiable risk factors and considering the use of suppressive and/or probiotic therapy may improve quality of life for women affected by this condition.
REFERENCES
1. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
2. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Trans Dis. 2007;34(11): 864-869.
3. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Obstet Gynecol. 2007;109(1):114-120.
4. Bradshaw CS, Morton AN, Garland SM, et al. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
5. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97.e1-e6.
6. Schwebke JR. Vaginal discharge. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2007. http://accessmedicine.mhmedical.com/content.aspx?bookid=369&Sectionid=39914778. Accessed November 11, 2015.
7. Workowski KA, Bolan GA; CDC. Bacterial vaginosis. In: Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):69-72.
8. Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis. 2009;22(1): 82-86.
9. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40(2):117-122.
10. Marrazzo JM, Wiesenfeld HC, Murray PG, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193(5):617-624.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301.
12. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74(1):14-22.
13. Girerd PH. Bacterial vaginosis workup (2014). http://emedicine.medscape.com/article/254342-workup. Accessed November 11, 2015.
14. Hoffman BL, Schorge JO, Schaffer JI, et al. Gynecologic infection. In: Hoffman BL, Schorge JO, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill; 2012.
15. Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807-815.
16. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, No. 72: Vaginitis. Obstet Gynecol. 2006;107(5):1195-1206.
17. Workowski KA, Berman S; CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12): 1-110.
18. Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect. 2004;80(1):8-11.
19. Cook RL, Redondo-Lopez V, Schmitt C, et al. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30(4):870-877.
20. Boris J, Påhlson C, Larsson P-G. Six years observation after successful treatment of bacterial vaginosis. Infect Dis Obstetr Gynecol. 1997;5(4): 297-302.
21. Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacterial vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
22. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11): 1478-1486.
23. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(11):1426-1435.
24. Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44(2):213-219.
25. Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
26. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
27. Homayouni A, Bastani P, Ziyadi S, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79-86.
28. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE. 2013;8(9):e74378.
29. Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80(1):58-62.
30. Klebanoff MA, Nansel TR, Brotman RM, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis. 2010;37(2):94-99.
31. Nansel TR, Riggs MA, Yu K-F, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381-386.
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of reproductive age, with a prevalence in North America of 29.2% among women ages 14 to 49.1-3 BV is a condition in which the normal vaginal flora are altered, primarily due to a reduction in hydrogen peroxide–producing strains of lactobacilli. This leads to an elevated vaginal pH and increased levels of proteolytic enzymes (eg, sialidase), organic acids, and volatile amines.4 This change in pH allows an overgrowth of multiple types of anaerobic, mycoplasmic, and gram-negative bacteria.
In most cases of BV, the predominant microbe is the facultative anaerobe Gardnerella vaginalis. However, evidence from recent studies of the pathogenesis of BV suggests that this bacterium forms a biofilm in the vaginal epithelium that serves as a “scaffolding” to which other bacterial species adhere in a symbiotic fashion, colonizing the vagina.5 Though asymptomatic in at least half of affected women,2,6,7 this polymicrobial condition can produce a thin, white, homogenous discharge with a distinct “fishy” odor.
The changes in the vaginal flora seen in BV are associated with serious sequelae, such as preterm delivery, spontaneous abortion, postpartum endometritis, and increased susceptibility to HIV and other sexually transmitted infections (STIs).4,8,9 The polymicrobial nature of BV and its propensity for recurrence make treatment a challenge.
PATIENT PRESENTATION/HISTORY
The most common symptom of BV is increased vaginal discharge, which usually is thin and white or dull gray.4 Some women report a strong fishy odor, especially after sex. Vaginal pain, itching, or burning may also be present, especially if the discharge is copious. Dyspareunia and dysuria are rare, but possible, symptoms. Fever, malaise, and other systemic symptoms are not associated with BV and should prompt the clinician to consider other causes. About half of women with bacterial vaginosis have no symptoms.2,6
The typical finding on physical examination is a homogeneous, off-white, creamy, malodorous discharge that adheres to the vaginal walls and pools in the vaginal vault. There are usually no or minimal signs of vaginal inflammation, and the vulva, labia, and cervix are typically normal. In some cases, BV can lead to cervicitis.6,9,10
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of BV can be made based on the history, physical examination, and microscopic examination of the vaginal discharge. Unlike with many other bacterial diseases, culture is not recommended for diagnosis of BV because many of the implicated organisms cannot be easily isolated in the laboratory, and because asymptomatic women also have small numbers of these flora in the vagina.
In 1991, Nugent et al11 described a Gram stain scoring system of vaginal smears to diagnose BV, which has a sensitivity and specificity of 96% and 96%, respectively; it remains the gold standard for diagnosis.7 However, because this method requires considerable time and skill, it is not routinely used in most clinic settings.
A widely used method of diagnosing BV is the Amsel criteria (see Table 1). The Amsel method has a sensitivity and specificity of 81% and 94%, respectively.1,12 The presence of clue cells is the most reliable indicator of BV (see figure). The positive predictive value of this test for the presence of BV is 95%.14 The Amsel method requires microscopy,4,12 which is not always available in clinics.
There are several commercially available point-of-care tests for BV that do not require microscopy. These include rapid antigen and nucleic acid amplification tests to detect elevated levels of G vaginalis, as well as tests that detect the presence of bacterial amines, elevated vaginal pH, and bacterial sialidase.4,7,15 Compared with the Nugent and Amsel methods, one test that detects elevated vaginal fluid sialidase activity was shown to have a sensitivity of 88% and specificity of 91% to 95%.4,7,15 These point-of-care tests are most effective for diagnosing BV when the vaginal pH exceeds 4.5 and when they are used in conjunction with other clinical criteria.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Treatment is recommended for women with symptoms. The established benefit of therapy in nonpregnant women is relief of vaginal symptoms and signs of infection. Other potential benefits to treatment include reduction in the risk for Chlamydia trachomatis or Neisseria gonorrhea infection, HIV, and other viral STIs. Table 2 includes the recommended and alternative treatment regimens for BV, according to the CDC’s 2015 treatment guidelines for sexually transmitted infections.7 These regimens are also recommended by the American Congress of Obstetricians and Gynecologists (ACOG).16
Treatment is also recommended for all symptomatic pregnant women. Older CDC guidelines noted a preference for oral therapy in pregnant women with BV, due to the possibility of subclinical upper genital tract infection.17 However, the 2015 CDC guidelinesstate that symptomatic pregnant women can be treated with either the oral or vaginal regimens recommended for nonpregnant women, as oral therapy has not been shown to outperform vaginal therapy in effecting cure or preventing adverse outcomes.7
PATIENT EDUCATION AND FOLLOW-UP
Patient preferences, possible adverse effects, drug interactions, and other coinfections should be considered when selecting a treatment regimen. Women should be advised to refrain from sexual intercourse or to use condoms consistently and correctly during treatment. Douching may increase the risk for relapse, and no data support its use for treatment or relief of symptoms. Follow-up visits are unnecessary unless symptoms do not resolve. Because recurrence of BV is common, however, women should be advised to return for evaluation if symptoms recur.1,8,18
Continue for when BV recurs >>
WHEN BV RECURS
Recurrence rates of 15% to 30% have been reported at three months,18,19 and of 28% when patients were tested cumulatively over six months,1 but few researchers have looked at long-term recurrence rates. In one observational study, recurrence rates of 51% were reported during a six-year follow-up period among women previously treated with oral metronidazole.20 Whether these high recurrence rates are due to treatment failure to eradicate the causative organism or to a reinfection from sexual partners remains unclear.21 Some studies have shown that treatment of male partners does not affect recurrence rates.21,22
Risk factors
Various research teams have identified risk factors associated with BV recurrence, but study results have been inconsistent. The strongest risk factor appears to be sexual activity, specifically with increased numbers of sexual partners and inconsistent condom use.1,23,24 Women who have sex with women also appear to be at increased risk for BV recurrence.9,10
BV tends to recur around the time of menstruation, and some suppressive therapies include administration of antibiotics during this time.1,8 Although reports conflict, other risk factors that may be implicated in recurrent BV include vaginal douching, cigarette smoking, and increased BMI.2,18 Use of an oral contraceptive may have a protective effect against BV recurrence.1
Caring for patients with multiple recurrences of BV can be challenging for many clinicians. Although a few studies have evaluated suppressive therapy for recurrent BV, there are no clear treatment guidelines for multiple recurrent infections. Sobel and colleagues evaluated twice-weekly use of metronidazole gel for 16 weeks and found a significant reduction in BV recurrence during treatment.25 However, there was only a 34% to 37% probability of patients’ remaining clinically cured at seven months posttreatment. Similarly, Reichman et al evaluated suppressive therapy with oral metronidazole, topical boric acid, and metronidazole gel. They found an 88% to 92% initial cure rate, but a 50% failure rate at 36 weeks’ follow-up.26
Management
Studies examining the use of probiotics for the prevention and treatment of BV have yielded mixed results. The theory is that probiotics containing lactobacillus organisms may protect women from infection by maintaining or restoring vaginal pH and preventing adhesion of bacteria to the epithelium of the vaginal walls.27 Despite the conflicting results, no adverse effects have been reported and, as a consequence, many experts recommend probiotics to reduce the risk for recurrent BV. When discussing suppressive therapy options with patients, clinicians should be mindful of the limited data and the clinically unfavorable long-term cure rates demonstrated.
In addition to treatment limitations for recurrent BV, clinicians often find it challenging to effectively address the psychosocial implications of distress, embarrassment, and lack of control that are commonly associated with recurrent BV.28 Beyond its impact on sexual activity, women have also reported refraining from their daily activities out of fear that others around them may detect their vaginal odor. Helping women take a proactive approach in the treatment and prevention of BV may ease some of this distress.
Women with recurrent BV are often eager to hear about measures they can take to reduce their risk for acute and recurrent infection. Patients should be counseled on the association of BV with douching, numerous sexual partners, unprotected sex, increased psychosocial stress, and cigarette smoking.7,18,29-31 Patients may inquire about the potential risk for BV when they use feminine hygiene spray, panty liners or pads, and underwear made from synthetic fabrics; however, one longitudinal study30 showed no association between any of these hygienic behaviors and BV.
Continue for the conclusion >>
CONCLUSION
Bacterial vaginosis is a common cause of vaginal discharge in women. Current recommendations for treatment are not very effective, with up to half of women experiencing recurrence. The likelihood of recurrence can result in significant frustration for both patient and clinician. Although recent studies have advanced our understanding of the pathophysiology of BV, further research is needed to develop more effective treatments that reduce recurrence. Addressing modifiable risk factors and considering the use of suppressive and/or probiotic therapy may improve quality of life for women affected by this condition.
REFERENCES
1. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
2. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Trans Dis. 2007;34(11): 864-869.
3. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Obstet Gynecol. 2007;109(1):114-120.
4. Bradshaw CS, Morton AN, Garland SM, et al. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
5. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97.e1-e6.
6. Schwebke JR. Vaginal discharge. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2007. http://accessmedicine.mhmedical.com/content.aspx?bookid=369&Sectionid=39914778. Accessed November 11, 2015.
7. Workowski KA, Bolan GA; CDC. Bacterial vaginosis. In: Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):69-72.
8. Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis. 2009;22(1): 82-86.
9. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40(2):117-122.
10. Marrazzo JM, Wiesenfeld HC, Murray PG, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193(5):617-624.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301.
12. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74(1):14-22.
13. Girerd PH. Bacterial vaginosis workup (2014). http://emedicine.medscape.com/article/254342-workup. Accessed November 11, 2015.
14. Hoffman BL, Schorge JO, Schaffer JI, et al. Gynecologic infection. In: Hoffman BL, Schorge JO, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill; 2012.
15. Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807-815.
16. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, No. 72: Vaginitis. Obstet Gynecol. 2006;107(5):1195-1206.
17. Workowski KA, Berman S; CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12): 1-110.
18. Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect. 2004;80(1):8-11.
19. Cook RL, Redondo-Lopez V, Schmitt C, et al. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30(4):870-877.
20. Boris J, Påhlson C, Larsson P-G. Six years observation after successful treatment of bacterial vaginosis. Infect Dis Obstetr Gynecol. 1997;5(4): 297-302.
21. Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacterial vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
22. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11): 1478-1486.
23. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(11):1426-1435.
24. Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44(2):213-219.
25. Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
26. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
27. Homayouni A, Bastani P, Ziyadi S, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79-86.
28. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE. 2013;8(9):e74378.
29. Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80(1):58-62.
30. Klebanoff MA, Nansel TR, Brotman RM, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis. 2010;37(2):94-99.
31. Nansel TR, Riggs MA, Yu K-F, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381-386.
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of reproductive age, with a prevalence in North America of 29.2% among women ages 14 to 49.1-3 BV is a condition in which the normal vaginal flora are altered, primarily due to a reduction in hydrogen peroxide–producing strains of lactobacilli. This leads to an elevated vaginal pH and increased levels of proteolytic enzymes (eg, sialidase), organic acids, and volatile amines.4 This change in pH allows an overgrowth of multiple types of anaerobic, mycoplasmic, and gram-negative bacteria.
In most cases of BV, the predominant microbe is the facultative anaerobe Gardnerella vaginalis. However, evidence from recent studies of the pathogenesis of BV suggests that this bacterium forms a biofilm in the vaginal epithelium that serves as a “scaffolding” to which other bacterial species adhere in a symbiotic fashion, colonizing the vagina.5 Though asymptomatic in at least half of affected women,2,6,7 this polymicrobial condition can produce a thin, white, homogenous discharge with a distinct “fishy” odor.
The changes in the vaginal flora seen in BV are associated with serious sequelae, such as preterm delivery, spontaneous abortion, postpartum endometritis, and increased susceptibility to HIV and other sexually transmitted infections (STIs).4,8,9 The polymicrobial nature of BV and its propensity for recurrence make treatment a challenge.
PATIENT PRESENTATION/HISTORY
The most common symptom of BV is increased vaginal discharge, which usually is thin and white or dull gray.4 Some women report a strong fishy odor, especially after sex. Vaginal pain, itching, or burning may also be present, especially if the discharge is copious. Dyspareunia and dysuria are rare, but possible, symptoms. Fever, malaise, and other systemic symptoms are not associated with BV and should prompt the clinician to consider other causes. About half of women with bacterial vaginosis have no symptoms.2,6
The typical finding on physical examination is a homogeneous, off-white, creamy, malodorous discharge that adheres to the vaginal walls and pools in the vaginal vault. There are usually no or minimal signs of vaginal inflammation, and the vulva, labia, and cervix are typically normal. In some cases, BV can lead to cervicitis.6,9,10
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of BV can be made based on the history, physical examination, and microscopic examination of the vaginal discharge. Unlike with many other bacterial diseases, culture is not recommended for diagnosis of BV because many of the implicated organisms cannot be easily isolated in the laboratory, and because asymptomatic women also have small numbers of these flora in the vagina.
In 1991, Nugent et al11 described a Gram stain scoring system of vaginal smears to diagnose BV, which has a sensitivity and specificity of 96% and 96%, respectively; it remains the gold standard for diagnosis.7 However, because this method requires considerable time and skill, it is not routinely used in most clinic settings.
A widely used method of diagnosing BV is the Amsel criteria (see Table 1). The Amsel method has a sensitivity and specificity of 81% and 94%, respectively.1,12 The presence of clue cells is the most reliable indicator of BV (see figure). The positive predictive value of this test for the presence of BV is 95%.14 The Amsel method requires microscopy,4,12 which is not always available in clinics.
There are several commercially available point-of-care tests for BV that do not require microscopy. These include rapid antigen and nucleic acid amplification tests to detect elevated levels of G vaginalis, as well as tests that detect the presence of bacterial amines, elevated vaginal pH, and bacterial sialidase.4,7,15 Compared with the Nugent and Amsel methods, one test that detects elevated vaginal fluid sialidase activity was shown to have a sensitivity of 88% and specificity of 91% to 95%.4,7,15 These point-of-care tests are most effective for diagnosing BV when the vaginal pH exceeds 4.5 and when they are used in conjunction with other clinical criteria.
Continue for treatment/management >>
TREATMENT/MANAGEMENT
Treatment is recommended for women with symptoms. The established benefit of therapy in nonpregnant women is relief of vaginal symptoms and signs of infection. Other potential benefits to treatment include reduction in the risk for Chlamydia trachomatis or Neisseria gonorrhea infection, HIV, and other viral STIs. Table 2 includes the recommended and alternative treatment regimens for BV, according to the CDC’s 2015 treatment guidelines for sexually transmitted infections.7 These regimens are also recommended by the American Congress of Obstetricians and Gynecologists (ACOG).16
Treatment is also recommended for all symptomatic pregnant women. Older CDC guidelines noted a preference for oral therapy in pregnant women with BV, due to the possibility of subclinical upper genital tract infection.17 However, the 2015 CDC guidelinesstate that symptomatic pregnant women can be treated with either the oral or vaginal regimens recommended for nonpregnant women, as oral therapy has not been shown to outperform vaginal therapy in effecting cure or preventing adverse outcomes.7
PATIENT EDUCATION AND FOLLOW-UP
Patient preferences, possible adverse effects, drug interactions, and other coinfections should be considered when selecting a treatment regimen. Women should be advised to refrain from sexual intercourse or to use condoms consistently and correctly during treatment. Douching may increase the risk for relapse, and no data support its use for treatment or relief of symptoms. Follow-up visits are unnecessary unless symptoms do not resolve. Because recurrence of BV is common, however, women should be advised to return for evaluation if symptoms recur.1,8,18
Continue for when BV recurs >>
WHEN BV RECURS
Recurrence rates of 15% to 30% have been reported at three months,18,19 and of 28% when patients were tested cumulatively over six months,1 but few researchers have looked at long-term recurrence rates. In one observational study, recurrence rates of 51% were reported during a six-year follow-up period among women previously treated with oral metronidazole.20 Whether these high recurrence rates are due to treatment failure to eradicate the causative organism or to a reinfection from sexual partners remains unclear.21 Some studies have shown that treatment of male partners does not affect recurrence rates.21,22
Risk factors
Various research teams have identified risk factors associated with BV recurrence, but study results have been inconsistent. The strongest risk factor appears to be sexual activity, specifically with increased numbers of sexual partners and inconsistent condom use.1,23,24 Women who have sex with women also appear to be at increased risk for BV recurrence.9,10
BV tends to recur around the time of menstruation, and some suppressive therapies include administration of antibiotics during this time.1,8 Although reports conflict, other risk factors that may be implicated in recurrent BV include vaginal douching, cigarette smoking, and increased BMI.2,18 Use of an oral contraceptive may have a protective effect against BV recurrence.1
Caring for patients with multiple recurrences of BV can be challenging for many clinicians. Although a few studies have evaluated suppressive therapy for recurrent BV, there are no clear treatment guidelines for multiple recurrent infections. Sobel and colleagues evaluated twice-weekly use of metronidazole gel for 16 weeks and found a significant reduction in BV recurrence during treatment.25 However, there was only a 34% to 37% probability of patients’ remaining clinically cured at seven months posttreatment. Similarly, Reichman et al evaluated suppressive therapy with oral metronidazole, topical boric acid, and metronidazole gel. They found an 88% to 92% initial cure rate, but a 50% failure rate at 36 weeks’ follow-up.26
Management
Studies examining the use of probiotics for the prevention and treatment of BV have yielded mixed results. The theory is that probiotics containing lactobacillus organisms may protect women from infection by maintaining or restoring vaginal pH and preventing adhesion of bacteria to the epithelium of the vaginal walls.27 Despite the conflicting results, no adverse effects have been reported and, as a consequence, many experts recommend probiotics to reduce the risk for recurrent BV. When discussing suppressive therapy options with patients, clinicians should be mindful of the limited data and the clinically unfavorable long-term cure rates demonstrated.
In addition to treatment limitations for recurrent BV, clinicians often find it challenging to effectively address the psychosocial implications of distress, embarrassment, and lack of control that are commonly associated with recurrent BV.28 Beyond its impact on sexual activity, women have also reported refraining from their daily activities out of fear that others around them may detect their vaginal odor. Helping women take a proactive approach in the treatment and prevention of BV may ease some of this distress.
Women with recurrent BV are often eager to hear about measures they can take to reduce their risk for acute and recurrent infection. Patients should be counseled on the association of BV with douching, numerous sexual partners, unprotected sex, increased psychosocial stress, and cigarette smoking.7,18,29-31 Patients may inquire about the potential risk for BV when they use feminine hygiene spray, panty liners or pads, and underwear made from synthetic fabrics; however, one longitudinal study30 showed no association between any of these hygienic behaviors and BV.
Continue for the conclusion >>
CONCLUSION
Bacterial vaginosis is a common cause of vaginal discharge in women. Current recommendations for treatment are not very effective, with up to half of women experiencing recurrence. The likelihood of recurrence can result in significant frustration for both patient and clinician. Although recent studies have advanced our understanding of the pathophysiology of BV, further research is needed to develop more effective treatments that reduce recurrence. Addressing modifiable risk factors and considering the use of suppressive and/or probiotic therapy may improve quality of life for women affected by this condition.
REFERENCES
1. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis. 2013;56(6):777-786.
2. Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004: associations with symptoms, sexual behaviors, and reproductive health. Sex Trans Dis. 2007;34(11): 864-869.
3. Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey Data. Obstet Gynecol. 2007;109(1):114-120.
4. Bradshaw CS, Morton AN, Garland SM, et al. Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J Clin Microbiol. 2005;43(3):1304-1308.
5. Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97.e1-e6.
6. Schwebke JR. Vaginal discharge. In: Klausner JD, Hook EW III, eds. Current Diagnosis & Treatment of Sexually Transmitted Diseases. New York, NY: McGraw-Hill; 2007. http://accessmedicine.mhmedical.com/content.aspx?bookid=369&Sectionid=39914778. Accessed November 11, 2015.
7. Workowski KA, Bolan GA; CDC. Bacterial vaginosis. In: Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):69-72.
8. Hay P. Recurrent bacterial vaginosis. Curr Opin Infect Dis. 2009;22(1): 82-86.
9. Taylor BD, Darville T, Haggerty CL. Does bacterial vaginosis cause pelvic inflammatory disease? Sex Transm Dis. 2013;40(2):117-122.
10. Marrazzo JM, Wiesenfeld HC, Murray PG, et al. Risk factors for cervicitis among women with bacterial vaginosis. J Infect Dis. 2006;193(5):617-624.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301.
12. Amsel R, Totten PA, Spiegel CA, et al. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983; 74(1):14-22.
13. Girerd PH. Bacterial vaginosis workup (2014). http://emedicine.medscape.com/article/254342-workup. Accessed November 11, 2015.
14. Hoffman BL, Schorge JO, Schaffer JI, et al. Gynecologic infection. In: Hoffman BL, Schorge JO, Schaffer JI, et al, eds. Williams Gynecology. 2nd ed. New York, NY: McGraw-Hill; 2012.
15. Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807-815.
16. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists, No. 72: Vaginitis. Obstet Gynecol. 2006;107(5):1195-1206.
17. Workowski KA, Berman S; CDC. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12): 1-110.
18. Wilson J. Managing recurrent bacterial vaginosis. Sex Transm Infect. 2004;80(1):8-11.
19. Cook RL, Redondo-Lopez V, Schmitt C, et al. Clinical, microbiological, and biochemical factors in recurrent bacterial vaginosis. J Clin Microbiol. 1992;30(4):870-877.
20. Boris J, Påhlson C, Larsson P-G. Six years observation after successful treatment of bacterial vaginosis. Infect Dis Obstetr Gynecol. 1997;5(4): 297-302.
21. Mehta SD. Systematic review of randomized trials of treatment of male sexual partners for improved bacterial vaginosis outcomes in women. Sex Transm Dis. 2012;39(10):822-830.
22. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193(11): 1478-1486.
23. Fethers KA, Fairley CK, Hocking JS, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(11):1426-1435.
24. Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44(2):213-219.
25. Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283-1289.
26. Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734.
27. Homayouni A, Bastani P, Ziyadi S, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis. 2014;18(1):79-86.
28. Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS ONE. 2013;8(9):e74378.
29. Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect. 2004;80(1):58-62.
30. Klebanoff MA, Nansel TR, Brotman RM, et al. Personal hygienic behaviors and bacterial vaginosis. Sex Transm Dis. 2010;37(2):94-99.
31. Nansel TR, Riggs MA, Yu K-F, et al. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381-386.
Herpes Zoster Infection
CE/CME No: CR-1308
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the etiology of herpes zoster infection (HZ), typical and atypical clinical presentation, and diagnostic confirmation, when needed.
• Describe treatment interventions for acute HZ infection, including topical measures, use of antiviral agents, and pain management options.
• Discuss complications of HZ infection, including risk factors and prevention.
• Explain risks, benefits, contraindications, and other considerations for vaccination use to prevent HZ in at-risk adults.
FACULTY
Emily Jacobsen is an Assistant Professor in the Department of Family Medicine and in the Division of Physician Assistant Education at Oregon Health & Science University (OHSU) in Portland, Oregon; she is a practicing Physician Assistant at OHSU Family Medicine at Richmond in Portland. Claire E. Hull is an Assistant Professor in the Department of Family Medicine and in the Division of Physician Assistant Education at OHSU.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Herpes zoster (HZ) infection, commonly called shingles, represents a reactivation of the chickenpox virus. Persons older than 50 and those with compromised immune systems are at greatest risk. Most cases resolve spontaneously, but about one-third of patients develop postherpetic neuralgia or other complications, and 1% to 4% require hospitalization. Treatment involves antiviral medications and pain management. Vaccination against HZ, which is recommended for adults 60 and older, incurs benefits and risks that the clinician must be prepared to explain to eligible patients.
Infection with herpes zoster (HZ) affects approximately one million individuals in the United States each year.1-3 The disease is caused by a reactivation of the varicella zoster virus (VZV), which causes chickenpox. Once chickenpox has resolved, VZV remains dormant in the dorsal (spinal) root ganglia, trigeminal nerve, and autonomic ganglia of the nervous system.4 At some later time, VZV may reactivate, causing an extremely painful vesicular rash along the distribution of one or more sensory dermatomes; the rash (as well as the condition in general) is commonly referred to as shingles.
It has been estimated that 90% or more of US adults older than 40 are infected with VZV.1,3 Because the virus is so ubiquitous, virtually anyone may be at risk for the reactivation of VZV in the form of shingles. It is estimated that 10% to 20% of the US population will develop HZ in their lifetime,3 with age and immune status the most significant determinants of persons to be affected.3-6
About half of all cases of shingles in the US occur in persons age 50 or older. Incidence among those older than 75 is approximately 10 cases per 1,000 individuals, compared with about two cases per 1,000 individuals in those younger than 50.3
In addition to age, the integrity of an individual’s immune system plays a key role in the development of shingles. Reactivation of VZV is usually suppressed by the host’s cell-mediated immune response, particularly the T cells.3,5 Thus, if the cell-mediated immune system is compromised, reactivation and widespread dissemination are more likely to occur. Adults with cancer or HIV infection and those taking immunosuppressive drugs have a significantly increased risk for HZ. Psychological or physical stress and trauma have also been shown to play a role in the development of HZ.5 In contrast to chickenpox, HZ has no seasonal predilection.7
Since 1995, with the licensing of Varivax (the vaccination to prevent varicella), the incidence of wild-type varicella infection is now quite low in the US. From 2000 to 2010, varicella wild-type infection declined by 82%.8 Efforts to further quantify the incidence of varicella have been hampered by the absence of reporting requirements for this infection.
Due to the live nature of the Varivax vaccine, patients who have received it remain at risk for HZ infection by way of reactivation of vaccine-type VZV. A population-based surveillance study conducted in California from 2000 to 2006 showed that the incidence of HZ infection decreased by 55% in children 10 years or younger who were vaccinated against varicella.9 This finding, along with similar results in other, older research in immunocompromised hosts, supports the notion that the risk for HZ is substantially reduced among children who have been vaccinated against varicella.10
Incidence of HZ infection seems to be on the rise, both in the US and worldwide1; however, the causes for this are a point of controversy. Fears have been expressed that incidence of HZ infection in adults would increase once varicella vaccination in children became commonplace, based on reasoning that exposure to the virus (which is thought to boost cell-mediated immunity and keep the virus from reactivating) would decline. This concern has put a halt to vaccination against varicella in some European countries.11 At least one US researcher considers the evidence strong for a causal link between the increase in incidence of HZ and the widespread implementation of varicella vaccination.12
Other research has led to different conclusions. Authors of a nationwide, retrospective review of claims data noted an increase in HZ prior to Varivax licensure but did not find any association between vaccination rates and HZ rates geographically.13 Similarly, researchers conducting a case-control study in a Wisconsin clinic found no relationship between HZ and exposure to VZV in the previous 10 years.14
On the next page: Clinical presentation and laboratory diagnosis >>
CLINICAL PRESENTATION
Identifying HZ infection is primarily a clinical diagnosis and not particularly difficult. Approximately 20% of patients will present with prodromal symptoms of fatigue, headache, malaise, and fever. Paresthesias in the involved dermatome often precede the rash by several days and may be manifested as itching, tingling, burning, or severe pain. Physical examination at this stage may reveal tenderness and hyperesthesia of the skin in the involved dermatome.3,5,15,16
Pain and abnormal skin sensations are the most common symptoms of HZ. They often precede and usually accompany the rash. The prodromal pain of HZ can mimic a variety of other conditions, including pleurisy, myocardial infarction, peptic ulcer, appendicitis, or biliary or renal colic, prompting some clinicians to undertake an extensive workup and treatment plan.15,17
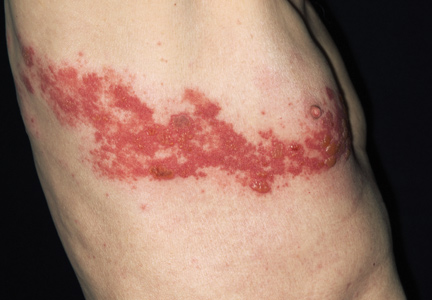
Consistent with other herpes infections, the HZ rash initially starts in the form of erythematous papules, which quickly evolve into grouped vesicles or bullae. Within three to four days, these vesicular lesions can become more pustular. In contrast to chickenpox, the rash of shingles is manifested in a dermatomal distribution. The two most commonly affected dermatomes are the first (ophthalmic) division of the trigeminal nerve and the spinal sensory ganglia from T1 to L2.3,5,15,16 The infection is generally limited to one dermatome in previously healthy hosts but can occasionally affect two or three neighboring dermatomes. Some patients have a few scattered vesicles located some distance away from the involved dermatome.15
In immunocompetent hosts, the lesions crust over within seven to 10 days and are no longer considered infectious. The development of new lesions more than a week after presentation should raise concerns regarding possible underlying immunodeficiency.3,5,15,16
LABORATORY DIAGNOSIS
While HZ is generally a clinical diagnosis based on the history and physical exam findings, laboratory testing may be appropriate to confirm the diagnosis when the presentation is atypical, the host is immunocompromised, lesions recur, or serious complications are suspected.17,18
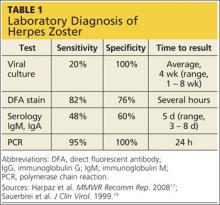
Several laboratory tests are currently available (see Table 117,19). Detection of VZV DNA following amplification of appropriate specimens (most reliably, clear fluid from recently erupted vesicles) by polymerase chain reaction (PCR) is generally recommended if testing is required, because of its high sensitivity and specificity and quick turnaround. However, this test is not available at every laboratory.17-19
When PCR is not available, a suitable alternative is direct fluorescent antibody (DFA) staining of cellular material from fresh vesicles or prevesicular lesions.1 This test uses a modified Tzanck technique to view fluorescein-conjugated monoclonal antibodies. DFA staining can differentiate between herpes simplex and herpes zoster.16,17,20
The original Tzanck smear is inexpensive and may reveal multinucleated giant cells and epithelial cells containing acidophilic intranuclear inclusion bodies.17 However, this test is not often used to confirm a diagnosis of HZ; rather, it is most helpful for distinguishing herpesvirus infections from vesicular lesions of other etiologies (eg, coxsackievirus, echovirus).15,17
Serologic tests measuring immunoglobulin M and A titers (IgM, IgA) may be helpful in cases of zoster without rash (zoster sine herpete), but their sensitivity and specificity are low.15 Positive results may be indicative of primary infection, reinfection, or reactivation.1
On the next page: Treatment >>
TREATMENT
Treatment of HZ infection is focused on limiting the extent, duration, and severity of pain and rash in the primary dermatome, as well as decreasing the risk for complications.
Topical Therapy
Patients should keep the cutaneous lesions clean and dry to reduce the risk for bacterial superinfection. A sterile, nonocclusive, nonadherent dressing placed over the involved dermatome will protect the lesions from contact with clothing. To hasten the drying of vesicular lesions and alleviate pruritus associated with rash, the application of cool compresses, calamine lotion, cornstarch, or baking soda may be helpful.16,21
While antipruritic agents may help prevent infections that can develop when the affected area is scratched, there is no evidence that any of these agents have any real therapeutic effect on the HZ rash or lesions. Topical antiviral agents are not effective.15,18
Antiviral Therapy
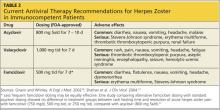
Treatment for acute HZ with oral antiviral medication should be considered for any patient who presents within 72 hours of rash onset; antiviral agents initiated within this time frame have been shown to reduce the duration and severity of pain associated with acute HZ.21 Antivirals are recommended and should be given routinely to patients older than 50 and those who have moderate to severe symptoms.15,21
Among patients who present longer than 72 hours after rash onset, antiviral therapy should be considered only for those with new vesicular formation, ophthalmic involvement, or motor or neurologic complications, although evidence is lacking for this recommendation.15,21 A modest reduction in the duration of rash (by 1 to 3 days) has also been reported in patients treated with antivirals,21 most likely because viral replication is slowed within the dorsal root ganglion.16,22
Acyclovir, valacyclovir, and famciclovir—nucleoside analogs that block viral replication—are the only FDA-approved medications for treatment of HZ.18,22 When choosing among these agents, the prescribing clinician should be aware of their differences in bioavailability and pharmacokinetics. Acyclovir, for example, is a second-generation antiviral drug with poor pharmacokinetics, which explains the frequent dosing its use generally requires.22,23 However, the inhibitory dose of acyclovir required for patients with HZ is much lower than that required to treat primary VZV infection.
Valacyclovir and famciclovir, which are third-generation antivirals, feature enhanced absorption from the gastrointestinal tract (77% vs 30% for acyclovir),24 thus improving their bioavailability by three to five times, compared with acyclovir. The superior pharmacokinetics of valacyclovir and famciclovir has been confirmed clinically by researchers who demonstrated median pain duration of 38 days in patients taking valacyclovir, compared with 51 days in those treated with acyclovir.25 In a direct comparison of valacyclovir and famciclovir, resolution of rash and pain times were found comparable.26 Table 218,27 summarizes the current recommendations for antiviral therapy in patients with HZ.
Pain Management
Pain is almost universal once the HZ rash appears. Pain associated with the prodromal period is variable but may be present in 70% to 80% of patients.28,29 The severity of acute pain in HZ is highly variable, ranging from mild to quite severe. Pain can begin weeks or a single day before the rash emerges and persist for several weeks after the rash disappears.
Aggressive pain management is appropriate. A variety of opiate analgesics (eg, hydrocodone, oxycodone, hydromorphone, morphine) and nonopiate analgesics (acetaminophen, NSAIDs) may be effective.17,28 Drug choices, dosage, and scheduling should be tailored to the patient’s level of pain and disability, with any potential contraindications also taken into account. Mild pain can be treated with as-needed dosing, whereas scheduled dosing is preferred for moderate to severe pain.16
If acute pain persists, addition of gabapentin, pregabalin, or nortriptyline is reasonable.17,22,28,30 Although these medications have been studied in the treatment of postherpetic neuralgia (PHN), there is little evidence to support their use for acute zoster pain.22,30
Additional interventions that have been studied for relief of acute HZ pain include topical lidocaine, acupuncture, and interventional pain injections. However, the evidence is either scant or of poor quality. More research is needed before these modalities can be routinely recommended in the clinical setting.17,22
Corticosteroids have been used for acute HZ, but conflicting study results make their routine use controversial.17,31,32 In some studies, corticosteroids reduced acute pain and speeded lesion healing and return to daily activities; others have yielded little evidence to support these findings.22,33 Corticosteroids may offer the greatest benefit when used in combination with effective antiviral therapy.18,22,31,32 In one randomized clinical trial comparing acyclovir with acyclovir plus prednisolone (40 mg/d for three weeks, tapered down) combination therapy was associated with a significant decrease in pain during the initial two weeks.32
Historically, corticosteroids have also been prescribed with the hope that their anti-inflammatory properties might help reduce the risk for PHN. However, a recent Cochrane Review found that these agents do not reliably prevent PHN six months after HZ rash onset.34 Glucocorticoids may improve motor outcomes and acute pain in VZV-induced facial paralysis and cranial polyneuritis, in which compression of affected nerves may contribute to disability.
Before prescribing steroids, clinicians must consider contraindications to their use, including diabetes, osteoporosis, hypertension, glaucoma, and gastritis.16
On the next page: Patient education and complications >>
PATIENT EDUCATION
Patients must be instructed in how to avoid transmitting the HZ virus. The mechanism of transmission was long thought to be restricted to direct contact with lesions; however, molecular studies have shown that the HZ virus can be transmitted via the respiratory route, either through aerosolized virus from skin lesions or from respiratory droplets, as early as 24 to 48 hours before the rash appears.6,17 The risk of transmission by airborne virus is increased in patients with HZ rash that is disseminated beyond the primary and secondary dermatomes. The rash, patients should be informed, generally persists for two to four weeks.1,16,20
HZ continues to be contagious until the lesions crust over.17 Covering the rash greatly reduces patients’ risk for transmitting the virus via airborne or direct contact routes.15,17 A patient with HZ rash can infect a nonimmune person with primary varicella, causing chickenpox.28 The patient with HZ should be advised to avoid exposure to infants younger than 1 year, unvaccinated older children, anyone who is not immune to varicella (either by vaccination or primary infection), susceptible pregnant women, and potentially susceptible immunocompromised persons.16
COMPLICATIONS AND SEQUELAE
The majority of cases of shingles resolve without any complications or long-term sequelae. Complications of HZ that do occur may include superimposed skin infections, such as Streptococcus or Staphylococcus. The virus may be reactivated in the nasociliary branch of the trigeminal nerve and, in 10% to 25% of cases, herpes zoster ophthalmicus (HZO) may develop.17,35 Associated morbidity includes keratitis, corneal ulceration, conjunctivitis, uveitis, episcleritis and scleritis, retinitis, choroiditis, optic neuritis, lid retraction, ptosis, and glaucoma. Patients with HZO should be referred to an ophthalmologist promptly, as this condition can result in permanent loss of vision.35
Other less common complications of HZ include Ramsay Hunt syndrome (facial nerve palsy associated with reactivation in the geniculate ganglion) and zoster paresis (motor weakness in noncranial nerve distributions).17,36,37 Autonomic dysfunction has also been reported in patients with HZ, leading to colonic pseudo-obstruction and urinary retention. Rare but serious neurologic complications include Guillain-Barré syndrome, myelitis, aseptic meningitis, and meningoencephalitis.15,17
Postherpetic Neuralgia
By far, the most common complication of shingles is postherpetic neuralgia, a painfully debilitating and difficult-to-treat condition. Of the one million persons affected by HZ each year, between 9% and 34% will develop PHN.3,7,18 The criteria for diagnosing PHN is variable: While all definitions include the presence of persistent pain after rash resolution, they differ in how long this pain must persist. Some define PHN as pain persisting from 30 days to six months after rash resolution, while others define it as pain continuing three months or longer.3,17,18 While most patients with PHN experience complete resolution, the pain can endure from weeks to months to years.3,18,38
The pathophysiology of PHN is thought to involve replication of the VZV in the basal ganglia, damaging the nerves and thereby causing pain in the affected dermatome.22 Other possible factors include axonal and cell body degeneration, atrophy of the dorsal horn of the spinal cord, scarring of the dorsal root ganglion, and loss of epidermal innervations of the dermatome.17
The risk for PHN increases significantly in patients of advancing age. While PHN is rare in those younger than 50, it complicates HZ in 20% of patients between ages 60 and 65 and in 30% of those 80 and older. Additional risk factors for PHN include female gender, prodromal pain preceding the HZ rash, rash that is moderate to severe, moderate to severe acute pain associated with the rash, and ophthalmic involvement.39
Evidence conflicts regarding the impact of antivirals in patients with HZ on the subsequent development of PHN. Researchers performing a meta-analysis of five randomized clinical trials found no significant difference in the incidence of PHN among patients treated for HZ with oral acyclovir, famciclovir, or placebo.34 In an older, placebo-controlled randomized clinical trial, however, famciclovir-treated patients experienced PHN of reduced duration, compared with controls (63 days vs 119 days, respectively). Six months after development of the HZ rash, 15% of treated patients continued to experience PHN symptoms, compared with 23% of controls.23 More evidence is needed.
Additional Concerns
Recurrence of HZ is uncommon in immunocompetent persons. Despite its ordinarily benign course, 1% to 4% of people with shingles require hospitalization each year, mostly elderly patients.1 In a recent study, it was estimated that 96 US deaths are attributable to HZ each year.40 Almost all HZ-associated deaths occur in elderly patients with compromised or suppressed immune systems.1
On the next page: Prevention >>
PREVENTION
The varicella vaccine was licensed for use in children by the FDA in 1995. In June 2006, the Advisory Committee on Immunization Practices (ACIP) recommended a second dose to boost waning immunity.41
In 2006, the HZ vaccine (Zostavax), a more concentrated formulation of the varicella vaccine, was approved by the FDA for use in adults age 60 or older; in 2011, approval of the vaccine was extended to adults 50 or older.42 As of November 2011, ACIP has continued to recommend routine administration of the HZ vaccine for immunocompetent adults 60 and older, citing lack of evidence for long-term protection in patients vaccinated before age 60, as well as concerns about maintaining sufficient vaccine supplies.
Although ACIP does not recommend routine vaccination against HZ in patients age 50 to 59, health care providers may wish to consider it for patients in this age-group, based on the potential for poor tolerance of HZ or PHN symptoms, anticipated difficulty tolerating the required medications used to treat them, and employment-related considerations.43
Use of the HZ Vaccine
Zostavax is a live attenuated vaccine that increases varicella-specific, cell-mediated immunity in immunocompetent persons.17,42 It should be administered as a single 0.65-mL dose subcutaneously in the deltoid region of the upper arm42; a booster dose is not licensed for the vaccine.17 Adverse effects of the HZ vaccine generally include mild injection-site reactions: pain (54%), erythema (48%), swelling (40%), and pruritus (11%).42 According to researchers for the Shingles Prevention Study38,44 (SPS), these reactions were more common in treated patients than in controls and increasingly common in study participants of advancing age. Less than 2% of patients receiving either the HZ vaccine or placebo experienced serious adverse effects.44
The evidence to support vaccination against HZ comes mainly from the original SPS,38 a randomized, double-blind, placebo-controlled trial in which more than 38,500 adults 60 and older were enrolled. The SPS researchers showed that the vaccine reduced the incidence of HZ by 51.3%, reduced the incidence of PHN by 67%, and reduced the HZ-associated burden of illness (ie, its incidence, severity, and duration of associated pain and discomfort) by 61%2,38,45; they also found vaccination against HZ effective for at least three years.
An ongoing substudy involving 14,270 of the original SPS participants produced data showing that from year 4 to year 5 postvaccination, vaccine efficacy in terms of HZ incidence declined from 51% to 40%, respectively, and its efficacy regarding incidence of PHN, from 67% to 60%.46 Since there is no strong evidence that any treatment intervention started after shingles presents can reduce the risk for PHN, perhaps the vaccine’s most valuable attribute is its potential for preventing this debilitating and common complication of shingles.
Who Should or Should Not Be Vaccinated?
According to the ACIP, there is no upper age limit on vaccination against shingles. This judgment is supported by the fact that the incidence of zoster and PHN both continue to increase among patients of advancing age.17
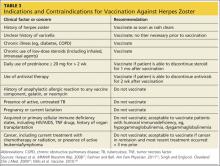
While vaccination is appropriate for most individuals 60 or older, some contraindications exist (see Table 317,22,47,48). In cases of anticipated immunosuppression (as in patients scheduled to undergo chemotherapy), vaccination is recommended one month before the start of therapy. Additionally, the safety and efficacy of vaccination is unknown in patients receiving immune modulators and recombinant human immune mediators (eg, adalimumab, etanercept, infliximab); thus, these patients too should be vaccinated one month before starting these treatments or one month after their completion.47
On the next page: Conclusion >>
CONCLUSION
Herpes zoster remains a common disease in the US, despite the availability of an effective vaccine. While most cases of shingles resolve spontaneously, life-threatening and permanent complications can occur. Treatment may shorten the length of illness and prevent these complications. Primary care providers should recommend routine vaccination against HZ for their immunocompetent patients 60 or older.
1. CDC. Shingles (herpes zoster). www.cdc.gov/shingles/hcp/clinical-overview.html. Accessed June 26, 2013.
2. Tseng HF, Smith N, Harpaz R, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160-166.
3. Weinberg JM. Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol. 2007;57:S130-S135.
4. Kennedy PG, Cohrs RJ. Varicella-zoster virus human ganglionic latency: a current summary. J Neurovirol. 2010;16:411-418.
5. Wilson DD. Herpes zoster: prevention, diagnosis and treatment. Nurse Pract. 2007;32:19-24.
6. Chen TM, George S, Woodruff CA, Hsu S. Clinical manifestations of varicella-zoster virus infection. Dermatol Clin. 2002;20:267-282.
7. Gilden D, Mahalingam R, Nagel MA, et al. Review: the neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441-463.
8. CDC. Chickenpox (varicella): monitoring the impact of varicella vaccination. www.cdc.gov/chickenpox/hcp/monitoring-varicella.html. Accessed June 26, 2013.
9. Civen R, Chaves SS, Jumaan A, et all. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
10. Hardy I, Gershon AA, Steinberg SP, LaRussa P; Varicella Vaccine Collaborative Study Group. The incidence of zoster after immunization with live attenuated varicella vaccine: a study in children with leukemia. N Engl J Med. 1991;325:1545-1550.
11. Poletti P, Melegaro A, Ajelli M, et al. Perspectives on the impact of varicella immunization on herpes zoster: a model-based evaluation from three European countries. PLoS One. 2013;8:e60732.
12. Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
13. Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332-340.
14. Donahue JG, Kieke BA, Gargiullo PM, et al. Herpes zoster and exposure to the varicella zoster virus in an era of varicella vaccination. Am J Public Health. 2010;100:1116-1122.
15. Schmader KE, Oxman MN. Varicella and herpes zoster. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
16. Wilson JF. Herpes zoster. Ann Intern Med. 2011;154:ITC31-ITC15.
17. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1-30.
18. Gnann JW Jr, Whitley RJ. Clinical practice: herpes zoster. N Engl J Med. 2002; 347:340-346.
19. Sauerbrei A, Eichhorn U, Schacke M, Wutzler P. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
20. Whitley RJ. A 70-year-old woman with shingles. JAMA. 2009;302:73-80.
21. Galluzzi KE. Managing herpes zoster and postherpetic neuralgia. J Am Osteopath Assoc. 2009;109(6 suppl 2):S7-S12.
22. Fashner J, Bell AL. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2011;83:1432-1437.
23. Tyring S, Barbarash RA, Nahlik JE, et al; Collaborative Famciclovir Herpes Zoster Study Group. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:89-96.
24. Pavan-Langston D. Herpes zoster: antivirals and pain management. Ophthalmology. 2008;115(2 suppl):S13-S20.
25. Opstelten W, Eekhof J, Neven AK, Verheij T. Treatment of herpes zoster. Can Fam Physician. 2008;54:373-377.
26. Tyring SK, Beutner KR, Tucker BA, et al. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9: 863-869.
27. Shafran SD, Tyring SK, Ashton R, et al. Once, twice, or three times daily famciclovir compared with aciclovir for the oral treatment of herpes zoster in immunocompetent adults: a randomized, multicenter, double-blind clinical trial. J Clin Virol. 2004;29:248-253.
28. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
29. Benbernou A, Drolet M, Levin MJ, et al. Association between prodromal pain and the severity of acute herpes zoster and utilization of health care resources. Eur J Pain. 2011;15:1100-1106.
30. Gan EY, Tian EA, Tey HL. Management of herpes zoster and post-herpetic neuralgia. Am J Clin Dermatol. 2013;14:77-85.
31. Whitley RJ, Weiss H, Gnann JW Jr, et al; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Acyclovir with and without prednisone for the treatment of herpes zoster: a randomized, placebo-controlled trial. Ann Intern Med. 1996;125:376-383.
32. Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896-900.
33. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211–1215.
34. Chen N, Yang M, He L, et al. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2010;(12):CD005582.
35. Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002;66:1723-1730.
36. Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149-154.
37. Tilki HE, Mutluer N, Selçuki D, Stålberg E. Zoster paresis. Electromyogr Clin Neurophysiol. 2003;43:231-234.
38. Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
39. Opstelten W, Zuithoff NP, van Essen GA, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain. 2007;132(suppl 1):S52-S59.
40. Mahamud A, Marin M, Nickell SP, et al. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979-2007. Clin Infect Dis. 2012;55:960-6.
41. Marin M, Güris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1-40.
42. Zostavax® (zoster vaccine live). Highlights of prescribing information (2013). www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf. Accessed June 26, 2013.
43. CDC. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528.
44. Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152:545-554.
45. Levin MJ, Oxman MN, Zhang JH, et al; Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825-835.
46. Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin Infect Dis. 2012;55:1320-1328.
47. Singh A, Englund K. Q: Who should receive the shingles vaccine? Cleveland Clin J Med. 2009;76:45-48.
48. Mills R, Tyring SK, Levin MJ, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine. 2010;28:4204-4209.
CE/CME No: CR-1308
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the etiology of herpes zoster infection (HZ), typical and atypical clinical presentation, and diagnostic confirmation, when needed.
• Describe treatment interventions for acute HZ infection, including topical measures, use of antiviral agents, and pain management options.
• Discuss complications of HZ infection, including risk factors and prevention.
• Explain risks, benefits, contraindications, and other considerations for vaccination use to prevent HZ in at-risk adults.
FACULTY
Emily Jacobsen is an Assistant Professor in the Department of Family Medicine and in the Division of Physician Assistant Education at Oregon Health & Science University (OHSU) in Portland, Oregon; she is a practicing Physician Assistant at OHSU Family Medicine at Richmond in Portland. Claire E. Hull is an Assistant Professor in the Department of Family Medicine and in the Division of Physician Assistant Education at OHSU.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Herpes zoster (HZ) infection, commonly called shingles, represents a reactivation of the chickenpox virus. Persons older than 50 and those with compromised immune systems are at greatest risk. Most cases resolve spontaneously, but about one-third of patients develop postherpetic neuralgia or other complications, and 1% to 4% require hospitalization. Treatment involves antiviral medications and pain management. Vaccination against HZ, which is recommended for adults 60 and older, incurs benefits and risks that the clinician must be prepared to explain to eligible patients.
Infection with herpes zoster (HZ) affects approximately one million individuals in the United States each year.1-3 The disease is caused by a reactivation of the varicella zoster virus (VZV), which causes chickenpox. Once chickenpox has resolved, VZV remains dormant in the dorsal (spinal) root ganglia, trigeminal nerve, and autonomic ganglia of the nervous system.4 At some later time, VZV may reactivate, causing an extremely painful vesicular rash along the distribution of one or more sensory dermatomes; the rash (as well as the condition in general) is commonly referred to as shingles.
It has been estimated that 90% or more of US adults older than 40 are infected with VZV.1,3 Because the virus is so ubiquitous, virtually anyone may be at risk for the reactivation of VZV in the form of shingles. It is estimated that 10% to 20% of the US population will develop HZ in their lifetime,3 with age and immune status the most significant determinants of persons to be affected.3-6
About half of all cases of shingles in the US occur in persons age 50 or older. Incidence among those older than 75 is approximately 10 cases per 1,000 individuals, compared with about two cases per 1,000 individuals in those younger than 50.3
In addition to age, the integrity of an individual’s immune system plays a key role in the development of shingles. Reactivation of VZV is usually suppressed by the host’s cell-mediated immune response, particularly the T cells.3,5 Thus, if the cell-mediated immune system is compromised, reactivation and widespread dissemination are more likely to occur. Adults with cancer or HIV infection and those taking immunosuppressive drugs have a significantly increased risk for HZ. Psychological or physical stress and trauma have also been shown to play a role in the development of HZ.5 In contrast to chickenpox, HZ has no seasonal predilection.7
Since 1995, with the licensing of Varivax (the vaccination to prevent varicella), the incidence of wild-type varicella infection is now quite low in the US. From 2000 to 2010, varicella wild-type infection declined by 82%.8 Efforts to further quantify the incidence of varicella have been hampered by the absence of reporting requirements for this infection.
Due to the live nature of the Varivax vaccine, patients who have received it remain at risk for HZ infection by way of reactivation of vaccine-type VZV. A population-based surveillance study conducted in California from 2000 to 2006 showed that the incidence of HZ infection decreased by 55% in children 10 years or younger who were vaccinated against varicella.9 This finding, along with similar results in other, older research in immunocompromised hosts, supports the notion that the risk for HZ is substantially reduced among children who have been vaccinated against varicella.10
Incidence of HZ infection seems to be on the rise, both in the US and worldwide1; however, the causes for this are a point of controversy. Fears have been expressed that incidence of HZ infection in adults would increase once varicella vaccination in children became commonplace, based on reasoning that exposure to the virus (which is thought to boost cell-mediated immunity and keep the virus from reactivating) would decline. This concern has put a halt to vaccination against varicella in some European countries.11 At least one US researcher considers the evidence strong for a causal link between the increase in incidence of HZ and the widespread implementation of varicella vaccination.12
Other research has led to different conclusions. Authors of a nationwide, retrospective review of claims data noted an increase in HZ prior to Varivax licensure but did not find any association between vaccination rates and HZ rates geographically.13 Similarly, researchers conducting a case-control study in a Wisconsin clinic found no relationship between HZ and exposure to VZV in the previous 10 years.14
On the next page: Clinical presentation and laboratory diagnosis >>
CLINICAL PRESENTATION
Identifying HZ infection is primarily a clinical diagnosis and not particularly difficult. Approximately 20% of patients will present with prodromal symptoms of fatigue, headache, malaise, and fever. Paresthesias in the involved dermatome often precede the rash by several days and may be manifested as itching, tingling, burning, or severe pain. Physical examination at this stage may reveal tenderness and hyperesthesia of the skin in the involved dermatome.3,5,15,16
Pain and abnormal skin sensations are the most common symptoms of HZ. They often precede and usually accompany the rash. The prodromal pain of HZ can mimic a variety of other conditions, including pleurisy, myocardial infarction, peptic ulcer, appendicitis, or biliary or renal colic, prompting some clinicians to undertake an extensive workup and treatment plan.15,17
Consistent with other herpes infections, the HZ rash initially starts in the form of erythematous papules, which quickly evolve into grouped vesicles or bullae. Within three to four days, these vesicular lesions can become more pustular. In contrast to chickenpox, the rash of shingles is manifested in a dermatomal distribution. The two most commonly affected dermatomes are the first (ophthalmic) division of the trigeminal nerve and the spinal sensory ganglia from T1 to L2.3,5,15,16 The infection is generally limited to one dermatome in previously healthy hosts but can occasionally affect two or three neighboring dermatomes. Some patients have a few scattered vesicles located some distance away from the involved dermatome.15
In immunocompetent hosts, the lesions crust over within seven to 10 days and are no longer considered infectious. The development of new lesions more than a week after presentation should raise concerns regarding possible underlying immunodeficiency.3,5,15,16
LABORATORY DIAGNOSIS
While HZ is generally a clinical diagnosis based on the history and physical exam findings, laboratory testing may be appropriate to confirm the diagnosis when the presentation is atypical, the host is immunocompromised, lesions recur, or serious complications are suspected.17,18
Several laboratory tests are currently available (see Table 117,19). Detection of VZV DNA following amplification of appropriate specimens (most reliably, clear fluid from recently erupted vesicles) by polymerase chain reaction (PCR) is generally recommended if testing is required, because of its high sensitivity and specificity and quick turnaround. However, this test is not available at every laboratory.17-19
When PCR is not available, a suitable alternative is direct fluorescent antibody (DFA) staining of cellular material from fresh vesicles or prevesicular lesions.1 This test uses a modified Tzanck technique to view fluorescein-conjugated monoclonal antibodies. DFA staining can differentiate between herpes simplex and herpes zoster.16,17,20
The original Tzanck smear is inexpensive and may reveal multinucleated giant cells and epithelial cells containing acidophilic intranuclear inclusion bodies.17 However, this test is not often used to confirm a diagnosis of HZ; rather, it is most helpful for distinguishing herpesvirus infections from vesicular lesions of other etiologies (eg, coxsackievirus, echovirus).15,17
Serologic tests measuring immunoglobulin M and A titers (IgM, IgA) may be helpful in cases of zoster without rash (zoster sine herpete), but their sensitivity and specificity are low.15 Positive results may be indicative of primary infection, reinfection, or reactivation.1
On the next page: Treatment >>
TREATMENT
Treatment of HZ infection is focused on limiting the extent, duration, and severity of pain and rash in the primary dermatome, as well as decreasing the risk for complications.
Topical Therapy
Patients should keep the cutaneous lesions clean and dry to reduce the risk for bacterial superinfection. A sterile, nonocclusive, nonadherent dressing placed over the involved dermatome will protect the lesions from contact with clothing. To hasten the drying of vesicular lesions and alleviate pruritus associated with rash, the application of cool compresses, calamine lotion, cornstarch, or baking soda may be helpful.16,21
While antipruritic agents may help prevent infections that can develop when the affected area is scratched, there is no evidence that any of these agents have any real therapeutic effect on the HZ rash or lesions. Topical antiviral agents are not effective.15,18
Antiviral Therapy
Treatment for acute HZ with oral antiviral medication should be considered for any patient who presents within 72 hours of rash onset; antiviral agents initiated within this time frame have been shown to reduce the duration and severity of pain associated with acute HZ.21 Antivirals are recommended and should be given routinely to patients older than 50 and those who have moderate to severe symptoms.15,21
Among patients who present longer than 72 hours after rash onset, antiviral therapy should be considered only for those with new vesicular formation, ophthalmic involvement, or motor or neurologic complications, although evidence is lacking for this recommendation.15,21 A modest reduction in the duration of rash (by 1 to 3 days) has also been reported in patients treated with antivirals,21 most likely because viral replication is slowed within the dorsal root ganglion.16,22
Acyclovir, valacyclovir, and famciclovir—nucleoside analogs that block viral replication—are the only FDA-approved medications for treatment of HZ.18,22 When choosing among these agents, the prescribing clinician should be aware of their differences in bioavailability and pharmacokinetics. Acyclovir, for example, is a second-generation antiviral drug with poor pharmacokinetics, which explains the frequent dosing its use generally requires.22,23 However, the inhibitory dose of acyclovir required for patients with HZ is much lower than that required to treat primary VZV infection.
Valacyclovir and famciclovir, which are third-generation antivirals, feature enhanced absorption from the gastrointestinal tract (77% vs 30% for acyclovir),24 thus improving their bioavailability by three to five times, compared with acyclovir. The superior pharmacokinetics of valacyclovir and famciclovir has been confirmed clinically by researchers who demonstrated median pain duration of 38 days in patients taking valacyclovir, compared with 51 days in those treated with acyclovir.25 In a direct comparison of valacyclovir and famciclovir, resolution of rash and pain times were found comparable.26 Table 218,27 summarizes the current recommendations for antiviral therapy in patients with HZ.
Pain Management
Pain is almost universal once the HZ rash appears. Pain associated with the prodromal period is variable but may be present in 70% to 80% of patients.28,29 The severity of acute pain in HZ is highly variable, ranging from mild to quite severe. Pain can begin weeks or a single day before the rash emerges and persist for several weeks after the rash disappears.
Aggressive pain management is appropriate. A variety of opiate analgesics (eg, hydrocodone, oxycodone, hydromorphone, morphine) and nonopiate analgesics (acetaminophen, NSAIDs) may be effective.17,28 Drug choices, dosage, and scheduling should be tailored to the patient’s level of pain and disability, with any potential contraindications also taken into account. Mild pain can be treated with as-needed dosing, whereas scheduled dosing is preferred for moderate to severe pain.16
If acute pain persists, addition of gabapentin, pregabalin, or nortriptyline is reasonable.17,22,28,30 Although these medications have been studied in the treatment of postherpetic neuralgia (PHN), there is little evidence to support their use for acute zoster pain.22,30
Additional interventions that have been studied for relief of acute HZ pain include topical lidocaine, acupuncture, and interventional pain injections. However, the evidence is either scant or of poor quality. More research is needed before these modalities can be routinely recommended in the clinical setting.17,22
Corticosteroids have been used for acute HZ, but conflicting study results make their routine use controversial.17,31,32 In some studies, corticosteroids reduced acute pain and speeded lesion healing and return to daily activities; others have yielded little evidence to support these findings.22,33 Corticosteroids may offer the greatest benefit when used in combination with effective antiviral therapy.18,22,31,32 In one randomized clinical trial comparing acyclovir with acyclovir plus prednisolone (40 mg/d for three weeks, tapered down) combination therapy was associated with a significant decrease in pain during the initial two weeks.32
Historically, corticosteroids have also been prescribed with the hope that their anti-inflammatory properties might help reduce the risk for PHN. However, a recent Cochrane Review found that these agents do not reliably prevent PHN six months after HZ rash onset.34 Glucocorticoids may improve motor outcomes and acute pain in VZV-induced facial paralysis and cranial polyneuritis, in which compression of affected nerves may contribute to disability.
Before prescribing steroids, clinicians must consider contraindications to their use, including diabetes, osteoporosis, hypertension, glaucoma, and gastritis.16
On the next page: Patient education and complications >>
PATIENT EDUCATION
Patients must be instructed in how to avoid transmitting the HZ virus. The mechanism of transmission was long thought to be restricted to direct contact with lesions; however, molecular studies have shown that the HZ virus can be transmitted via the respiratory route, either through aerosolized virus from skin lesions or from respiratory droplets, as early as 24 to 48 hours before the rash appears.6,17 The risk of transmission by airborne virus is increased in patients with HZ rash that is disseminated beyond the primary and secondary dermatomes. The rash, patients should be informed, generally persists for two to four weeks.1,16,20
HZ continues to be contagious until the lesions crust over.17 Covering the rash greatly reduces patients’ risk for transmitting the virus via airborne or direct contact routes.15,17 A patient with HZ rash can infect a nonimmune person with primary varicella, causing chickenpox.28 The patient with HZ should be advised to avoid exposure to infants younger than 1 year, unvaccinated older children, anyone who is not immune to varicella (either by vaccination or primary infection), susceptible pregnant women, and potentially susceptible immunocompromised persons.16
COMPLICATIONS AND SEQUELAE
The majority of cases of shingles resolve without any complications or long-term sequelae. Complications of HZ that do occur may include superimposed skin infections, such as Streptococcus or Staphylococcus. The virus may be reactivated in the nasociliary branch of the trigeminal nerve and, in 10% to 25% of cases, herpes zoster ophthalmicus (HZO) may develop.17,35 Associated morbidity includes keratitis, corneal ulceration, conjunctivitis, uveitis, episcleritis and scleritis, retinitis, choroiditis, optic neuritis, lid retraction, ptosis, and glaucoma. Patients with HZO should be referred to an ophthalmologist promptly, as this condition can result in permanent loss of vision.35
Other less common complications of HZ include Ramsay Hunt syndrome (facial nerve palsy associated with reactivation in the geniculate ganglion) and zoster paresis (motor weakness in noncranial nerve distributions).17,36,37 Autonomic dysfunction has also been reported in patients with HZ, leading to colonic pseudo-obstruction and urinary retention. Rare but serious neurologic complications include Guillain-Barré syndrome, myelitis, aseptic meningitis, and meningoencephalitis.15,17
Postherpetic Neuralgia
By far, the most common complication of shingles is postherpetic neuralgia, a painfully debilitating and difficult-to-treat condition. Of the one million persons affected by HZ each year, between 9% and 34% will develop PHN.3,7,18 The criteria for diagnosing PHN is variable: While all definitions include the presence of persistent pain after rash resolution, they differ in how long this pain must persist. Some define PHN as pain persisting from 30 days to six months after rash resolution, while others define it as pain continuing three months or longer.3,17,18 While most patients with PHN experience complete resolution, the pain can endure from weeks to months to years.3,18,38
The pathophysiology of PHN is thought to involve replication of the VZV in the basal ganglia, damaging the nerves and thereby causing pain in the affected dermatome.22 Other possible factors include axonal and cell body degeneration, atrophy of the dorsal horn of the spinal cord, scarring of the dorsal root ganglion, and loss of epidermal innervations of the dermatome.17
The risk for PHN increases significantly in patients of advancing age. While PHN is rare in those younger than 50, it complicates HZ in 20% of patients between ages 60 and 65 and in 30% of those 80 and older. Additional risk factors for PHN include female gender, prodromal pain preceding the HZ rash, rash that is moderate to severe, moderate to severe acute pain associated with the rash, and ophthalmic involvement.39
Evidence conflicts regarding the impact of antivirals in patients with HZ on the subsequent development of PHN. Researchers performing a meta-analysis of five randomized clinical trials found no significant difference in the incidence of PHN among patients treated for HZ with oral acyclovir, famciclovir, or placebo.34 In an older, placebo-controlled randomized clinical trial, however, famciclovir-treated patients experienced PHN of reduced duration, compared with controls (63 days vs 119 days, respectively). Six months after development of the HZ rash, 15% of treated patients continued to experience PHN symptoms, compared with 23% of controls.23 More evidence is needed.
Additional Concerns
Recurrence of HZ is uncommon in immunocompetent persons. Despite its ordinarily benign course, 1% to 4% of people with shingles require hospitalization each year, mostly elderly patients.1 In a recent study, it was estimated that 96 US deaths are attributable to HZ each year.40 Almost all HZ-associated deaths occur in elderly patients with compromised or suppressed immune systems.1
On the next page: Prevention >>
PREVENTION
The varicella vaccine was licensed for use in children by the FDA in 1995. In June 2006, the Advisory Committee on Immunization Practices (ACIP) recommended a second dose to boost waning immunity.41
In 2006, the HZ vaccine (Zostavax), a more concentrated formulation of the varicella vaccine, was approved by the FDA for use in adults age 60 or older; in 2011, approval of the vaccine was extended to adults 50 or older.42 As of November 2011, ACIP has continued to recommend routine administration of the HZ vaccine for immunocompetent adults 60 and older, citing lack of evidence for long-term protection in patients vaccinated before age 60, as well as concerns about maintaining sufficient vaccine supplies.
Although ACIP does not recommend routine vaccination against HZ in patients age 50 to 59, health care providers may wish to consider it for patients in this age-group, based on the potential for poor tolerance of HZ or PHN symptoms, anticipated difficulty tolerating the required medications used to treat them, and employment-related considerations.43
Use of the HZ Vaccine
Zostavax is a live attenuated vaccine that increases varicella-specific, cell-mediated immunity in immunocompetent persons.17,42 It should be administered as a single 0.65-mL dose subcutaneously in the deltoid region of the upper arm42; a booster dose is not licensed for the vaccine.17 Adverse effects of the HZ vaccine generally include mild injection-site reactions: pain (54%), erythema (48%), swelling (40%), and pruritus (11%).42 According to researchers for the Shingles Prevention Study38,44 (SPS), these reactions were more common in treated patients than in controls and increasingly common in study participants of advancing age. Less than 2% of patients receiving either the HZ vaccine or placebo experienced serious adverse effects.44
The evidence to support vaccination against HZ comes mainly from the original SPS,38 a randomized, double-blind, placebo-controlled trial in which more than 38,500 adults 60 and older were enrolled. The SPS researchers showed that the vaccine reduced the incidence of HZ by 51.3%, reduced the incidence of PHN by 67%, and reduced the HZ-associated burden of illness (ie, its incidence, severity, and duration of associated pain and discomfort) by 61%2,38,45; they also found vaccination against HZ effective for at least three years.
An ongoing substudy involving 14,270 of the original SPS participants produced data showing that from year 4 to year 5 postvaccination, vaccine efficacy in terms of HZ incidence declined from 51% to 40%, respectively, and its efficacy regarding incidence of PHN, from 67% to 60%.46 Since there is no strong evidence that any treatment intervention started after shingles presents can reduce the risk for PHN, perhaps the vaccine’s most valuable attribute is its potential for preventing this debilitating and common complication of shingles.
Who Should or Should Not Be Vaccinated?
According to the ACIP, there is no upper age limit on vaccination against shingles. This judgment is supported by the fact that the incidence of zoster and PHN both continue to increase among patients of advancing age.17
While vaccination is appropriate for most individuals 60 or older, some contraindications exist (see Table 317,22,47,48). In cases of anticipated immunosuppression (as in patients scheduled to undergo chemotherapy), vaccination is recommended one month before the start of therapy. Additionally, the safety and efficacy of vaccination is unknown in patients receiving immune modulators and recombinant human immune mediators (eg, adalimumab, etanercept, infliximab); thus, these patients too should be vaccinated one month before starting these treatments or one month after their completion.47
On the next page: Conclusion >>
CONCLUSION
Herpes zoster remains a common disease in the US, despite the availability of an effective vaccine. While most cases of shingles resolve spontaneously, life-threatening and permanent complications can occur. Treatment may shorten the length of illness and prevent these complications. Primary care providers should recommend routine vaccination against HZ for their immunocompetent patients 60 or older.
CE/CME No: CR-1308
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the etiology of herpes zoster infection (HZ), typical and atypical clinical presentation, and diagnostic confirmation, when needed.
• Describe treatment interventions for acute HZ infection, including topical measures, use of antiviral agents, and pain management options.
• Discuss complications of HZ infection, including risk factors and prevention.
• Explain risks, benefits, contraindications, and other considerations for vaccination use to prevent HZ in at-risk adults.
FACULTY
Emily Jacobsen is an Assistant Professor in the Department of Family Medicine and in the Division of Physician Assistant Education at Oregon Health & Science University (OHSU) in Portland, Oregon; she is a practicing Physician Assistant at OHSU Family Medicine at Richmond in Portland. Claire E. Hull is an Assistant Professor in the Department of Family Medicine and in the Division of Physician Assistant Education at OHSU.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Herpes zoster (HZ) infection, commonly called shingles, represents a reactivation of the chickenpox virus. Persons older than 50 and those with compromised immune systems are at greatest risk. Most cases resolve spontaneously, but about one-third of patients develop postherpetic neuralgia or other complications, and 1% to 4% require hospitalization. Treatment involves antiviral medications and pain management. Vaccination against HZ, which is recommended for adults 60 and older, incurs benefits and risks that the clinician must be prepared to explain to eligible patients.
Infection with herpes zoster (HZ) affects approximately one million individuals in the United States each year.1-3 The disease is caused by a reactivation of the varicella zoster virus (VZV), which causes chickenpox. Once chickenpox has resolved, VZV remains dormant in the dorsal (spinal) root ganglia, trigeminal nerve, and autonomic ganglia of the nervous system.4 At some later time, VZV may reactivate, causing an extremely painful vesicular rash along the distribution of one or more sensory dermatomes; the rash (as well as the condition in general) is commonly referred to as shingles.
It has been estimated that 90% or more of US adults older than 40 are infected with VZV.1,3 Because the virus is so ubiquitous, virtually anyone may be at risk for the reactivation of VZV in the form of shingles. It is estimated that 10% to 20% of the US population will develop HZ in their lifetime,3 with age and immune status the most significant determinants of persons to be affected.3-6
About half of all cases of shingles in the US occur in persons age 50 or older. Incidence among those older than 75 is approximately 10 cases per 1,000 individuals, compared with about two cases per 1,000 individuals in those younger than 50.3
In addition to age, the integrity of an individual’s immune system plays a key role in the development of shingles. Reactivation of VZV is usually suppressed by the host’s cell-mediated immune response, particularly the T cells.3,5 Thus, if the cell-mediated immune system is compromised, reactivation and widespread dissemination are more likely to occur. Adults with cancer or HIV infection and those taking immunosuppressive drugs have a significantly increased risk for HZ. Psychological or physical stress and trauma have also been shown to play a role in the development of HZ.5 In contrast to chickenpox, HZ has no seasonal predilection.7
Since 1995, with the licensing of Varivax (the vaccination to prevent varicella), the incidence of wild-type varicella infection is now quite low in the US. From 2000 to 2010, varicella wild-type infection declined by 82%.8 Efforts to further quantify the incidence of varicella have been hampered by the absence of reporting requirements for this infection.
Due to the live nature of the Varivax vaccine, patients who have received it remain at risk for HZ infection by way of reactivation of vaccine-type VZV. A population-based surveillance study conducted in California from 2000 to 2006 showed that the incidence of HZ infection decreased by 55% in children 10 years or younger who were vaccinated against varicella.9 This finding, along with similar results in other, older research in immunocompromised hosts, supports the notion that the risk for HZ is substantially reduced among children who have been vaccinated against varicella.10
Incidence of HZ infection seems to be on the rise, both in the US and worldwide1; however, the causes for this are a point of controversy. Fears have been expressed that incidence of HZ infection in adults would increase once varicella vaccination in children became commonplace, based on reasoning that exposure to the virus (which is thought to boost cell-mediated immunity and keep the virus from reactivating) would decline. This concern has put a halt to vaccination against varicella in some European countries.11 At least one US researcher considers the evidence strong for a causal link between the increase in incidence of HZ and the widespread implementation of varicella vaccination.12
Other research has led to different conclusions. Authors of a nationwide, retrospective review of claims data noted an increase in HZ prior to Varivax licensure but did not find any association between vaccination rates and HZ rates geographically.13 Similarly, researchers conducting a case-control study in a Wisconsin clinic found no relationship between HZ and exposure to VZV in the previous 10 years.14
On the next page: Clinical presentation and laboratory diagnosis >>
CLINICAL PRESENTATION
Identifying HZ infection is primarily a clinical diagnosis and not particularly difficult. Approximately 20% of patients will present with prodromal symptoms of fatigue, headache, malaise, and fever. Paresthesias in the involved dermatome often precede the rash by several days and may be manifested as itching, tingling, burning, or severe pain. Physical examination at this stage may reveal tenderness and hyperesthesia of the skin in the involved dermatome.3,5,15,16
Pain and abnormal skin sensations are the most common symptoms of HZ. They often precede and usually accompany the rash. The prodromal pain of HZ can mimic a variety of other conditions, including pleurisy, myocardial infarction, peptic ulcer, appendicitis, or biliary or renal colic, prompting some clinicians to undertake an extensive workup and treatment plan.15,17
Consistent with other herpes infections, the HZ rash initially starts in the form of erythematous papules, which quickly evolve into grouped vesicles or bullae. Within three to four days, these vesicular lesions can become more pustular. In contrast to chickenpox, the rash of shingles is manifested in a dermatomal distribution. The two most commonly affected dermatomes are the first (ophthalmic) division of the trigeminal nerve and the spinal sensory ganglia from T1 to L2.3,5,15,16 The infection is generally limited to one dermatome in previously healthy hosts but can occasionally affect two or three neighboring dermatomes. Some patients have a few scattered vesicles located some distance away from the involved dermatome.15
In immunocompetent hosts, the lesions crust over within seven to 10 days and are no longer considered infectious. The development of new lesions more than a week after presentation should raise concerns regarding possible underlying immunodeficiency.3,5,15,16
LABORATORY DIAGNOSIS
While HZ is generally a clinical diagnosis based on the history and physical exam findings, laboratory testing may be appropriate to confirm the diagnosis when the presentation is atypical, the host is immunocompromised, lesions recur, or serious complications are suspected.17,18
Several laboratory tests are currently available (see Table 117,19). Detection of VZV DNA following amplification of appropriate specimens (most reliably, clear fluid from recently erupted vesicles) by polymerase chain reaction (PCR) is generally recommended if testing is required, because of its high sensitivity and specificity and quick turnaround. However, this test is not available at every laboratory.17-19
When PCR is not available, a suitable alternative is direct fluorescent antibody (DFA) staining of cellular material from fresh vesicles or prevesicular lesions.1 This test uses a modified Tzanck technique to view fluorescein-conjugated monoclonal antibodies. DFA staining can differentiate between herpes simplex and herpes zoster.16,17,20
The original Tzanck smear is inexpensive and may reveal multinucleated giant cells and epithelial cells containing acidophilic intranuclear inclusion bodies.17 However, this test is not often used to confirm a diagnosis of HZ; rather, it is most helpful for distinguishing herpesvirus infections from vesicular lesions of other etiologies (eg, coxsackievirus, echovirus).15,17
Serologic tests measuring immunoglobulin M and A titers (IgM, IgA) may be helpful in cases of zoster without rash (zoster sine herpete), but their sensitivity and specificity are low.15 Positive results may be indicative of primary infection, reinfection, or reactivation.1
On the next page: Treatment >>
TREATMENT
Treatment of HZ infection is focused on limiting the extent, duration, and severity of pain and rash in the primary dermatome, as well as decreasing the risk for complications.
Topical Therapy
Patients should keep the cutaneous lesions clean and dry to reduce the risk for bacterial superinfection. A sterile, nonocclusive, nonadherent dressing placed over the involved dermatome will protect the lesions from contact with clothing. To hasten the drying of vesicular lesions and alleviate pruritus associated with rash, the application of cool compresses, calamine lotion, cornstarch, or baking soda may be helpful.16,21
While antipruritic agents may help prevent infections that can develop when the affected area is scratched, there is no evidence that any of these agents have any real therapeutic effect on the HZ rash or lesions. Topical antiviral agents are not effective.15,18
Antiviral Therapy
Treatment for acute HZ with oral antiviral medication should be considered for any patient who presents within 72 hours of rash onset; antiviral agents initiated within this time frame have been shown to reduce the duration and severity of pain associated with acute HZ.21 Antivirals are recommended and should be given routinely to patients older than 50 and those who have moderate to severe symptoms.15,21
Among patients who present longer than 72 hours after rash onset, antiviral therapy should be considered only for those with new vesicular formation, ophthalmic involvement, or motor or neurologic complications, although evidence is lacking for this recommendation.15,21 A modest reduction in the duration of rash (by 1 to 3 days) has also been reported in patients treated with antivirals,21 most likely because viral replication is slowed within the dorsal root ganglion.16,22
Acyclovir, valacyclovir, and famciclovir—nucleoside analogs that block viral replication—are the only FDA-approved medications for treatment of HZ.18,22 When choosing among these agents, the prescribing clinician should be aware of their differences in bioavailability and pharmacokinetics. Acyclovir, for example, is a second-generation antiviral drug with poor pharmacokinetics, which explains the frequent dosing its use generally requires.22,23 However, the inhibitory dose of acyclovir required for patients with HZ is much lower than that required to treat primary VZV infection.
Valacyclovir and famciclovir, which are third-generation antivirals, feature enhanced absorption from the gastrointestinal tract (77% vs 30% for acyclovir),24 thus improving their bioavailability by three to five times, compared with acyclovir. The superior pharmacokinetics of valacyclovir and famciclovir has been confirmed clinically by researchers who demonstrated median pain duration of 38 days in patients taking valacyclovir, compared with 51 days in those treated with acyclovir.25 In a direct comparison of valacyclovir and famciclovir, resolution of rash and pain times were found comparable.26 Table 218,27 summarizes the current recommendations for antiviral therapy in patients with HZ.
Pain Management
Pain is almost universal once the HZ rash appears. Pain associated with the prodromal period is variable but may be present in 70% to 80% of patients.28,29 The severity of acute pain in HZ is highly variable, ranging from mild to quite severe. Pain can begin weeks or a single day before the rash emerges and persist for several weeks after the rash disappears.
Aggressive pain management is appropriate. A variety of opiate analgesics (eg, hydrocodone, oxycodone, hydromorphone, morphine) and nonopiate analgesics (acetaminophen, NSAIDs) may be effective.17,28 Drug choices, dosage, and scheduling should be tailored to the patient’s level of pain and disability, with any potential contraindications also taken into account. Mild pain can be treated with as-needed dosing, whereas scheduled dosing is preferred for moderate to severe pain.16
If acute pain persists, addition of gabapentin, pregabalin, or nortriptyline is reasonable.17,22,28,30 Although these medications have been studied in the treatment of postherpetic neuralgia (PHN), there is little evidence to support their use for acute zoster pain.22,30
Additional interventions that have been studied for relief of acute HZ pain include topical lidocaine, acupuncture, and interventional pain injections. However, the evidence is either scant or of poor quality. More research is needed before these modalities can be routinely recommended in the clinical setting.17,22
Corticosteroids have been used for acute HZ, but conflicting study results make their routine use controversial.17,31,32 In some studies, corticosteroids reduced acute pain and speeded lesion healing and return to daily activities; others have yielded little evidence to support these findings.22,33 Corticosteroids may offer the greatest benefit when used in combination with effective antiviral therapy.18,22,31,32 In one randomized clinical trial comparing acyclovir with acyclovir plus prednisolone (40 mg/d for three weeks, tapered down) combination therapy was associated with a significant decrease in pain during the initial two weeks.32
Historically, corticosteroids have also been prescribed with the hope that their anti-inflammatory properties might help reduce the risk for PHN. However, a recent Cochrane Review found that these agents do not reliably prevent PHN six months after HZ rash onset.34 Glucocorticoids may improve motor outcomes and acute pain in VZV-induced facial paralysis and cranial polyneuritis, in which compression of affected nerves may contribute to disability.
Before prescribing steroids, clinicians must consider contraindications to their use, including diabetes, osteoporosis, hypertension, glaucoma, and gastritis.16
On the next page: Patient education and complications >>
PATIENT EDUCATION
Patients must be instructed in how to avoid transmitting the HZ virus. The mechanism of transmission was long thought to be restricted to direct contact with lesions; however, molecular studies have shown that the HZ virus can be transmitted via the respiratory route, either through aerosolized virus from skin lesions or from respiratory droplets, as early as 24 to 48 hours before the rash appears.6,17 The risk of transmission by airborne virus is increased in patients with HZ rash that is disseminated beyond the primary and secondary dermatomes. The rash, patients should be informed, generally persists for two to four weeks.1,16,20
HZ continues to be contagious until the lesions crust over.17 Covering the rash greatly reduces patients’ risk for transmitting the virus via airborne or direct contact routes.15,17 A patient with HZ rash can infect a nonimmune person with primary varicella, causing chickenpox.28 The patient with HZ should be advised to avoid exposure to infants younger than 1 year, unvaccinated older children, anyone who is not immune to varicella (either by vaccination or primary infection), susceptible pregnant women, and potentially susceptible immunocompromised persons.16
COMPLICATIONS AND SEQUELAE
The majority of cases of shingles resolve without any complications or long-term sequelae. Complications of HZ that do occur may include superimposed skin infections, such as Streptococcus or Staphylococcus. The virus may be reactivated in the nasociliary branch of the trigeminal nerve and, in 10% to 25% of cases, herpes zoster ophthalmicus (HZO) may develop.17,35 Associated morbidity includes keratitis, corneal ulceration, conjunctivitis, uveitis, episcleritis and scleritis, retinitis, choroiditis, optic neuritis, lid retraction, ptosis, and glaucoma. Patients with HZO should be referred to an ophthalmologist promptly, as this condition can result in permanent loss of vision.35
Other less common complications of HZ include Ramsay Hunt syndrome (facial nerve palsy associated with reactivation in the geniculate ganglion) and zoster paresis (motor weakness in noncranial nerve distributions).17,36,37 Autonomic dysfunction has also been reported in patients with HZ, leading to colonic pseudo-obstruction and urinary retention. Rare but serious neurologic complications include Guillain-Barré syndrome, myelitis, aseptic meningitis, and meningoencephalitis.15,17
Postherpetic Neuralgia
By far, the most common complication of shingles is postherpetic neuralgia, a painfully debilitating and difficult-to-treat condition. Of the one million persons affected by HZ each year, between 9% and 34% will develop PHN.3,7,18 The criteria for diagnosing PHN is variable: While all definitions include the presence of persistent pain after rash resolution, they differ in how long this pain must persist. Some define PHN as pain persisting from 30 days to six months after rash resolution, while others define it as pain continuing three months or longer.3,17,18 While most patients with PHN experience complete resolution, the pain can endure from weeks to months to years.3,18,38
The pathophysiology of PHN is thought to involve replication of the VZV in the basal ganglia, damaging the nerves and thereby causing pain in the affected dermatome.22 Other possible factors include axonal and cell body degeneration, atrophy of the dorsal horn of the spinal cord, scarring of the dorsal root ganglion, and loss of epidermal innervations of the dermatome.17
The risk for PHN increases significantly in patients of advancing age. While PHN is rare in those younger than 50, it complicates HZ in 20% of patients between ages 60 and 65 and in 30% of those 80 and older. Additional risk factors for PHN include female gender, prodromal pain preceding the HZ rash, rash that is moderate to severe, moderate to severe acute pain associated with the rash, and ophthalmic involvement.39
Evidence conflicts regarding the impact of antivirals in patients with HZ on the subsequent development of PHN. Researchers performing a meta-analysis of five randomized clinical trials found no significant difference in the incidence of PHN among patients treated for HZ with oral acyclovir, famciclovir, or placebo.34 In an older, placebo-controlled randomized clinical trial, however, famciclovir-treated patients experienced PHN of reduced duration, compared with controls (63 days vs 119 days, respectively). Six months after development of the HZ rash, 15% of treated patients continued to experience PHN symptoms, compared with 23% of controls.23 More evidence is needed.
Additional Concerns
Recurrence of HZ is uncommon in immunocompetent persons. Despite its ordinarily benign course, 1% to 4% of people with shingles require hospitalization each year, mostly elderly patients.1 In a recent study, it was estimated that 96 US deaths are attributable to HZ each year.40 Almost all HZ-associated deaths occur in elderly patients with compromised or suppressed immune systems.1
On the next page: Prevention >>
PREVENTION
The varicella vaccine was licensed for use in children by the FDA in 1995. In June 2006, the Advisory Committee on Immunization Practices (ACIP) recommended a second dose to boost waning immunity.41
In 2006, the HZ vaccine (Zostavax), a more concentrated formulation of the varicella vaccine, was approved by the FDA for use in adults age 60 or older; in 2011, approval of the vaccine was extended to adults 50 or older.42 As of November 2011, ACIP has continued to recommend routine administration of the HZ vaccine for immunocompetent adults 60 and older, citing lack of evidence for long-term protection in patients vaccinated before age 60, as well as concerns about maintaining sufficient vaccine supplies.
Although ACIP does not recommend routine vaccination against HZ in patients age 50 to 59, health care providers may wish to consider it for patients in this age-group, based on the potential for poor tolerance of HZ or PHN symptoms, anticipated difficulty tolerating the required medications used to treat them, and employment-related considerations.43
Use of the HZ Vaccine
Zostavax is a live attenuated vaccine that increases varicella-specific, cell-mediated immunity in immunocompetent persons.17,42 It should be administered as a single 0.65-mL dose subcutaneously in the deltoid region of the upper arm42; a booster dose is not licensed for the vaccine.17 Adverse effects of the HZ vaccine generally include mild injection-site reactions: pain (54%), erythema (48%), swelling (40%), and pruritus (11%).42 According to researchers for the Shingles Prevention Study38,44 (SPS), these reactions were more common in treated patients than in controls and increasingly common in study participants of advancing age. Less than 2% of patients receiving either the HZ vaccine or placebo experienced serious adverse effects.44
The evidence to support vaccination against HZ comes mainly from the original SPS,38 a randomized, double-blind, placebo-controlled trial in which more than 38,500 adults 60 and older were enrolled. The SPS researchers showed that the vaccine reduced the incidence of HZ by 51.3%, reduced the incidence of PHN by 67%, and reduced the HZ-associated burden of illness (ie, its incidence, severity, and duration of associated pain and discomfort) by 61%2,38,45; they also found vaccination against HZ effective for at least three years.
An ongoing substudy involving 14,270 of the original SPS participants produced data showing that from year 4 to year 5 postvaccination, vaccine efficacy in terms of HZ incidence declined from 51% to 40%, respectively, and its efficacy regarding incidence of PHN, from 67% to 60%.46 Since there is no strong evidence that any treatment intervention started after shingles presents can reduce the risk for PHN, perhaps the vaccine’s most valuable attribute is its potential for preventing this debilitating and common complication of shingles.
Who Should or Should Not Be Vaccinated?
According to the ACIP, there is no upper age limit on vaccination against shingles. This judgment is supported by the fact that the incidence of zoster and PHN both continue to increase among patients of advancing age.17
While vaccination is appropriate for most individuals 60 or older, some contraindications exist (see Table 317,22,47,48). In cases of anticipated immunosuppression (as in patients scheduled to undergo chemotherapy), vaccination is recommended one month before the start of therapy. Additionally, the safety and efficacy of vaccination is unknown in patients receiving immune modulators and recombinant human immune mediators (eg, adalimumab, etanercept, infliximab); thus, these patients too should be vaccinated one month before starting these treatments or one month after their completion.47
On the next page: Conclusion >>
CONCLUSION
Herpes zoster remains a common disease in the US, despite the availability of an effective vaccine. While most cases of shingles resolve spontaneously, life-threatening and permanent complications can occur. Treatment may shorten the length of illness and prevent these complications. Primary care providers should recommend routine vaccination against HZ for their immunocompetent patients 60 or older.
1. CDC. Shingles (herpes zoster). www.cdc.gov/shingles/hcp/clinical-overview.html. Accessed June 26, 2013.
2. Tseng HF, Smith N, Harpaz R, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160-166.
3. Weinberg JM. Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol. 2007;57:S130-S135.
4. Kennedy PG, Cohrs RJ. Varicella-zoster virus human ganglionic latency: a current summary. J Neurovirol. 2010;16:411-418.
5. Wilson DD. Herpes zoster: prevention, diagnosis and treatment. Nurse Pract. 2007;32:19-24.
6. Chen TM, George S, Woodruff CA, Hsu S. Clinical manifestations of varicella-zoster virus infection. Dermatol Clin. 2002;20:267-282.
7. Gilden D, Mahalingam R, Nagel MA, et al. Review: the neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441-463.
8. CDC. Chickenpox (varicella): monitoring the impact of varicella vaccination. www.cdc.gov/chickenpox/hcp/monitoring-varicella.html. Accessed June 26, 2013.
9. Civen R, Chaves SS, Jumaan A, et all. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
10. Hardy I, Gershon AA, Steinberg SP, LaRussa P; Varicella Vaccine Collaborative Study Group. The incidence of zoster after immunization with live attenuated varicella vaccine: a study in children with leukemia. N Engl J Med. 1991;325:1545-1550.
11. Poletti P, Melegaro A, Ajelli M, et al. Perspectives on the impact of varicella immunization on herpes zoster: a model-based evaluation from three European countries. PLoS One. 2013;8:e60732.
12. Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
13. Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332-340.
14. Donahue JG, Kieke BA, Gargiullo PM, et al. Herpes zoster and exposure to the varicella zoster virus in an era of varicella vaccination. Am J Public Health. 2010;100:1116-1122.
15. Schmader KE, Oxman MN. Varicella and herpes zoster. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
16. Wilson JF. Herpes zoster. Ann Intern Med. 2011;154:ITC31-ITC15.
17. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1-30.
18. Gnann JW Jr, Whitley RJ. Clinical practice: herpes zoster. N Engl J Med. 2002; 347:340-346.
19. Sauerbrei A, Eichhorn U, Schacke M, Wutzler P. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
20. Whitley RJ. A 70-year-old woman with shingles. JAMA. 2009;302:73-80.
21. Galluzzi KE. Managing herpes zoster and postherpetic neuralgia. J Am Osteopath Assoc. 2009;109(6 suppl 2):S7-S12.
22. Fashner J, Bell AL. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2011;83:1432-1437.
23. Tyring S, Barbarash RA, Nahlik JE, et al; Collaborative Famciclovir Herpes Zoster Study Group. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:89-96.
24. Pavan-Langston D. Herpes zoster: antivirals and pain management. Ophthalmology. 2008;115(2 suppl):S13-S20.
25. Opstelten W, Eekhof J, Neven AK, Verheij T. Treatment of herpes zoster. Can Fam Physician. 2008;54:373-377.
26. Tyring SK, Beutner KR, Tucker BA, et al. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9: 863-869.
27. Shafran SD, Tyring SK, Ashton R, et al. Once, twice, or three times daily famciclovir compared with aciclovir for the oral treatment of herpes zoster in immunocompetent adults: a randomized, multicenter, double-blind clinical trial. J Clin Virol. 2004;29:248-253.
28. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
29. Benbernou A, Drolet M, Levin MJ, et al. Association between prodromal pain and the severity of acute herpes zoster and utilization of health care resources. Eur J Pain. 2011;15:1100-1106.
30. Gan EY, Tian EA, Tey HL. Management of herpes zoster and post-herpetic neuralgia. Am J Clin Dermatol. 2013;14:77-85.
31. Whitley RJ, Weiss H, Gnann JW Jr, et al; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Acyclovir with and without prednisone for the treatment of herpes zoster: a randomized, placebo-controlled trial. Ann Intern Med. 1996;125:376-383.
32. Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896-900.
33. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211–1215.
34. Chen N, Yang M, He L, et al. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2010;(12):CD005582.
35. Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002;66:1723-1730.
36. Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149-154.
37. Tilki HE, Mutluer N, Selçuki D, Stålberg E. Zoster paresis. Electromyogr Clin Neurophysiol. 2003;43:231-234.
38. Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
39. Opstelten W, Zuithoff NP, van Essen GA, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain. 2007;132(suppl 1):S52-S59.
40. Mahamud A, Marin M, Nickell SP, et al. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979-2007. Clin Infect Dis. 2012;55:960-6.
41. Marin M, Güris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1-40.
42. Zostavax® (zoster vaccine live). Highlights of prescribing information (2013). www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf. Accessed June 26, 2013.
43. CDC. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528.
44. Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152:545-554.
45. Levin MJ, Oxman MN, Zhang JH, et al; Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825-835.
46. Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin Infect Dis. 2012;55:1320-1328.
47. Singh A, Englund K. Q: Who should receive the shingles vaccine? Cleveland Clin J Med. 2009;76:45-48.
48. Mills R, Tyring SK, Levin MJ, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine. 2010;28:4204-4209.
1. CDC. Shingles (herpes zoster). www.cdc.gov/shingles/hcp/clinical-overview.html. Accessed June 26, 2013.
2. Tseng HF, Smith N, Harpaz R, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160-166.
3. Weinberg JM. Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol. 2007;57:S130-S135.
4. Kennedy PG, Cohrs RJ. Varicella-zoster virus human ganglionic latency: a current summary. J Neurovirol. 2010;16:411-418.
5. Wilson DD. Herpes zoster: prevention, diagnosis and treatment. Nurse Pract. 2007;32:19-24.
6. Chen TM, George S, Woodruff CA, Hsu S. Clinical manifestations of varicella-zoster virus infection. Dermatol Clin. 2002;20:267-282.
7. Gilden D, Mahalingam R, Nagel MA, et al. Review: the neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441-463.
8. CDC. Chickenpox (varicella): monitoring the impact of varicella vaccination. www.cdc.gov/chickenpox/hcp/monitoring-varicella.html. Accessed June 26, 2013.
9. Civen R, Chaves SS, Jumaan A, et all. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954-959.
10. Hardy I, Gershon AA, Steinberg SP, LaRussa P; Varicella Vaccine Collaborative Study Group. The incidence of zoster after immunization with live attenuated varicella vaccine: a study in children with leukemia. N Engl J Med. 1991;325:1545-1550.
11. Poletti P, Melegaro A, Ajelli M, et al. Perspectives on the impact of varicella immunization on herpes zoster: a model-based evaluation from three European countries. PLoS One. 2013;8:e60732.
12. Goldman GS, King PG. Review of the United States universal varicella vaccination program: herpes zoster incidence rates, cost-effectiveness, and vaccine efficacy based primarily on the Antelope Valley Varicella Active Surveillance Project data. Vaccine. 2013;31:1680-1694.
13. Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332-340.
14. Donahue JG, Kieke BA, Gargiullo PM, et al. Herpes zoster and exposure to the varicella zoster virus in an era of varicella vaccination. Am J Public Health. 2010;100:1116-1122.
15. Schmader KE, Oxman MN. Varicella and herpes zoster. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
16. Wilson JF. Herpes zoster. Ann Intern Med. 2011;154:ITC31-ITC15.
17. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1-30.
18. Gnann JW Jr, Whitley RJ. Clinical practice: herpes zoster. N Engl J Med. 2002; 347:340-346.
19. Sauerbrei A, Eichhorn U, Schacke M, Wutzler P. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
20. Whitley RJ. A 70-year-old woman with shingles. JAMA. 2009;302:73-80.
21. Galluzzi KE. Managing herpes zoster and postherpetic neuralgia. J Am Osteopath Assoc. 2009;109(6 suppl 2):S7-S12.
22. Fashner J, Bell AL. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2011;83:1432-1437.
23. Tyring S, Barbarash RA, Nahlik JE, et al; Collaborative Famciclovir Herpes Zoster Study Group. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:89-96.
24. Pavan-Langston D. Herpes zoster: antivirals and pain management. Ophthalmology. 2008;115(2 suppl):S13-S20.
25. Opstelten W, Eekhof J, Neven AK, Verheij T. Treatment of herpes zoster. Can Fam Physician. 2008;54:373-377.
26. Tyring SK, Beutner KR, Tucker BA, et al. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9: 863-869.
27. Shafran SD, Tyring SK, Ashton R, et al. Once, twice, or three times daily famciclovir compared with aciclovir for the oral treatment of herpes zoster in immunocompetent adults: a randomized, multicenter, double-blind clinical trial. J Clin Virol. 2004;29:248-253.
28. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
29. Benbernou A, Drolet M, Levin MJ, et al. Association between prodromal pain and the severity of acute herpes zoster and utilization of health care resources. Eur J Pain. 2011;15:1100-1106.
30. Gan EY, Tian EA, Tey HL. Management of herpes zoster and post-herpetic neuralgia. Am J Clin Dermatol. 2013;14:77-85.
31. Whitley RJ, Weiss H, Gnann JW Jr, et al; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Acyclovir with and without prednisone for the treatment of herpes zoster: a randomized, placebo-controlled trial. Ann Intern Med. 1996;125:376-383.
32. Wood MJ, Johnson RW, McKendrick MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. 1994;330:896-900.
33. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211–1215.
34. Chen N, Yang M, He L, et al. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2010;(12):CD005582.
35. Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002;66:1723-1730.
36. Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71:149-154.
37. Tilki HE, Mutluer N, Selçuki D, Stålberg E. Zoster paresis. Electromyogr Clin Neurophysiol. 2003;43:231-234.
38. Oxman MN, Levin MJ, Johnson GR, et al; Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
39. Opstelten W, Zuithoff NP, van Essen GA, et al. Predicting postherpetic neuralgia in elderly primary care patients with herpes zoster: prospective prognostic study. Pain. 2007;132(suppl 1):S52-S59.
40. Mahamud A, Marin M, Nickell SP, et al. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979-2007. Clin Infect Dis. 2012;55:960-6.
41. Marin M, Güris D, Chaves SS, et al. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1-40.
42. Zostavax® (zoster vaccine live). Highlights of prescribing information (2013). www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf. Accessed June 26, 2013.
43. CDC. Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011;60:1528.
44. Simberkoff MS, Arbeit RD, Johnson GR, et al; Shingles Prevention Study Group. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152:545-554.
45. Levin MJ, Oxman MN, Zhang JH, et al; Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825-835.
46. Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin Infect Dis. 2012;55:1320-1328.
47. Singh A, Englund K. Q: Who should receive the shingles vaccine? Cleveland Clin J Med. 2009;76:45-48.
48. Mills R, Tyring SK, Levin MJ, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine. 2010;28:4204-4209.