User login
What’s the best test for underlying osteomyelitis in patients with diabetic foot ulcers?
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)
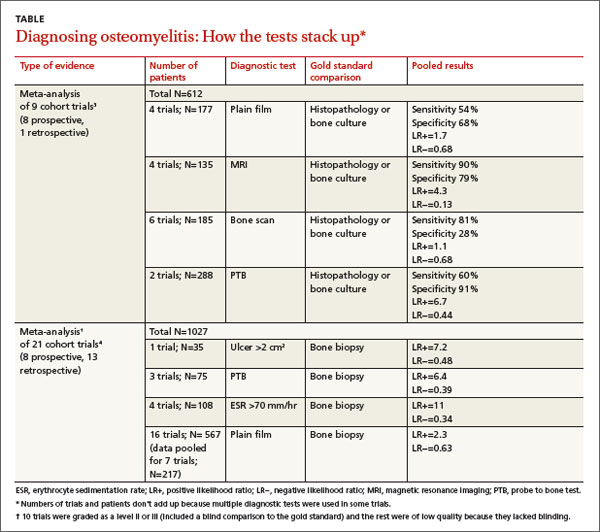
A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Evidence-based answers from the Family Physicians Inquiries Network
Are inhaled steroids effective for a postviral cough?
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
No. Inhaled corticosteroids (ICS) don’t improve postviral cough in adults with subacute (3-8 weeks) or chronic (>8 weeks) cough, adolescents with a history of asthma but without recent asthma activity, or children with a history of episodic viral wheezing without asthma (strength of recommendation [SOR]: B, preponderance of small randomized controlled trials [RCTs]).
EVIDENCE SUMMARY
A systematic review of 7 RCTs with a total of 477 adults that examined the efficacy of ICS compared with placebo for treating subacute (3-8 weeks) and chronic (>8 weeks) cough found inconsistent, but mostly negative results.1 Most trials combined patients with nonspecific subacute and chronic cough.
The evaluated steroids included beclomethasone, budesonide, fluticasone, and mometasone; daily “budesonide equivalent” doses ranged from 320 mcg to 1600 mcg. Six of the 7 trials found that ICS didn’t improve cough. The seventh didn’t treat patients with postviral cough. The authors of the review couldn’t pool data because of heterogeneity.
Steroids don’t affect methacholine challenge in teens
A double-blind, placebo-controlled RCT of 56 adolescents found that giving ICS after viral upper respiratory infection didn’t change the methacholine dosing necessary to produce a 20% reduction in the forced expiratory volume in one second (FEV1).2 Investigators included patients if they had a previous diagnosis of asthma but no use of asthma medications in 2 years, a baseline FEV1 greater than 70% of predicted, and a concentration of methacholine that produced a 20% fall in FEV1 less than 8 mg/mL.
They randomized patients to inhaled budesonide (2 200-mcg puffs bid) or placebo (2 500-mcg puffs micronized lactose bid). Patients underwent spirometry and methacholine challenge testing every 3 months over a 9-month period. The groups didn’t differ in bronchial hyperresponsiveness or FEV1.
Lower respiratory symptoms don’t respond to ICS in nonasthmatic children
A systematic review of 5 RCTs with a total of 339 patients found that in 4 of the 5, ICS didn’t improve lower respiratory symptoms in children with episodic viral wheeze and no history of asthma.3 Investigators evaluated ICS efficacy using lower respiratory symptom scores (based primarily on cough and wheeze) and decreased use of oral steroids or reduced emergency room visits.
Four trials found no benefit from ICS; one trial (52 children with viral-induced wheeze) found that nebulized budesonide (400 mg qid for 2 days, then bid for 7 days) decreased respiratory symptom scores (weighted mean difference= -0.17; 95% confidence interval, -0.34 to -0.003) compared with placebo. Investigators didn’t assess cough separately from wheezing, however.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
1. Johnstone KJ, Chang AB, Fong KM, et al. Inhaled corticosteroids for subacute and chronic cough in adults. Cochrane Database Syst Rev. 2013;3:CD009305.
2. Koh YY, Sun YH, Lim HS, et al. Effect of inhaled budesonide on bronchial hyperresponsiveness in adolescents with clinical remission of asthma. Chest. 2001;120:1140-1146.
3. McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database Syst Rev. 2000;(2):CD001107.
Evidence-based answers from the Family Physicians Inquiries Network
What is the best treatment for nocturnal enuresis in children?
For children with primary nocturnal enuresis, treatment with enuresis alarms reduced the number of wet nights by almost 4 per week, with almost half of patients remaining dry for 3 months after treatment (strength of recommendation [SOR]: A, based on a systematic review of homogeneous randomized control trials [RCTs]). Desmopressin (DDAVP) and tricyclic drugs reduce the number of wet nights by 1 to 2 per week during treatment, although the effect is not sustained after treatment is finished (SOR: A, based on a SR of homogeneous RCTs). Dry bed training with an alarm results in an additional reduction of wet nights over alarms alone (SOR: A, based on a systematic review of homogeneous RCTs].
Alarms have a high success rate with commitment; desmopressin good for temporary reduction
Nocturnal enuresis is embarrassing to children and frustrating to parents. Even though it has a usually benign, self-limited course, many families want to hear about treatment options. Enuresis alarms have a high success rate in achieving dry nights during treatment and maintaining dry nights once treatment stops. The success of alarms requires a motivated child and family plus a significant time and effort commitment for 3 to 6 months.
Since desmopressin rapidly reduces bedwetting, it is a good choice for situational use such as sleepovers, camping, and holidays. Desmopressin has minimal adverse reactions such as nasal irritation, nausea, and headaches, but parents should minimize evening water intake to prevent rare water intoxication side effects. The lack of benefit of desmopressin and alarm combination therapy may be partly explained by the loss of learning, if desmopressin negates the alarm needing to trigger. Since tricyclic medications do not show a benefit over desmopressin, they should be considered second-line agents due to cardiotoxic side effects and life-threatening overdose outcomes.
Evidence summary
Nocturnal enuresis is an involuntary loss of urine at night in the absence of congenital or acquired central nervous system defect among children over 5 years of age.1-4 Approximately 15% of children aged >5years wet their bed at night.5 The spontaneous resolution rate is about 15% per year.5 Before primary care treatment, indications for urological referral should be excluded, including daytime wetting, abnormal voiding (unusual posturing, discomfort, straining, or poor urine stream), recurrent urinary tract infections, neurological and anatomical anomalies, and urgency symptoms.4
The Cochrane Incontinence Group Trials demonstrated that enuresis alarms led to nearly 4 fewer wet nights per week compared with no treatment or placebo (weighted mean difference [WMD]=–3.65; 95% confidence interval [CI], –4.52 to –2.78).1 The relative risk of failure was 0.36 compared with placebo (95% CI, 0.26 to 0.40). The number needed to treat (NNT) to achieve 14 consecutive dry nights is 2. About half the children relapse after stopping treatment, compared with nearly all children after control interventions (55% vs 99%). Evidence is insufficient to say whether the addition of dry bed training (scheduled awakenings, cleanliness training, social reinforcement, positive practice) improves the outcomes. Alarms that wake the child immediately (vs a time delay) and alarms that wake the child (instead of the parents) were slightly more effective.
A meta-analysis also showed that desmopressin (10-60 μg) at bedtime reduced bedwetting by 1 to 2 nights per week compared with placebo (WMD=1.34; 95% CI, -1.57 to –1.11 with a dose of 20 μg).6 The NNT to achieve 14 consecutive dry nights is 7. However, the data suggest once treatment stops, there is little difference between desmopressin and placebo. Some evidence suggested that a higher dose was more likely to decrease the number of wet nights; however, there was no difference in cure rates. Evidence comparing intranasal with oral administration is insufficient.2
In the Cochrane review, children treated with desmopressin had 1.7 fewer wet nights (WMD=1.7; 95% CI, –2.95 to –0.45) in the first week compared with children treated with alarms.6 However, at the end of 3 months, alarms were associated with 1.4 fewer wet nights per week than children treated with desmopressin (WMD=1.4; 95% CI, 0.14 to 2.66).
Evidence is conflicting for increased efficacy for combining desmopressin with enuresis alarms. There is some limited evidence that children receiving combination treatment with desmopressin and alarms had fewer wet nights than children treated with alarms and placebo.6 This combination treatment did not show a benefit with failure rates (not attaining 14 consecutive dry nights) or a statistically significant difference in failure and relapse rates once treatment stopped. In addition, one RCT in which desmopressin nonresponders were supplemented with alarms showed no added benefit in remission rates compared with conditioning alarms plus placebo (51% vs 48% in achieving 28 dry nights).7 Neither was there added benefit in relapse rates once treatment stopped.
Children treated with tricyclic drugs compared with those treated with placebo had approximately 1 less night of enuresis per week (WMD=1.19; 95% CI, –1.56 to-0.82).8 More children achieved 14 dry nights while on imipramine compared with placebo (21% vs 5%; NNT=6); however, this advantage was not sustained once treatment finished (96% vs 97% relapsed). Little evidence exists to compare desmopressin with tricyclic drugs.2,8
Simple behavior methods may be more effective than no treatment, but there is little evidence for how these methods compare with one another, or with more successful means of treatment.9 Behavior techniques include lifting (taking a sleeping child to urinate in the bath-room), waking, rewards, and evening fluid restriction.
Dry bed training refers to comprehensive regimes, including enuresis alarms, waking routines, positive practice, cleanliness training, and bladder training in various combinations. A meta-analysis examining dry bed training including an enuresis alarm showed children had fewer wet nights compared with children receiving no treatment (relative risk [RR] of failure=0.17; 95% CI, 0.11-0.28).2,10 Additionally, more children remained dry after treatment stopped (RR of relapse=0.25; 95% CI, 0.16-0.39). However, evidence was not sufficient to show a remission benefit for dry bed training without an alarm (RR of failure=0.82; 95% CI, 0.6-1.02), highlighting the key role for alarm therapy. On the other hand, dry bed training including bed alarms may reduce the relapse rate compared with alarm monotherapy (RR for failure or relapse=0.5; 95% CI, 0.31-0.8)
TABLE
Nocturnal enuresis treatments and efficacy
| TREATMENT | EFFICACY |
|---|---|
| Enuresis alarms |
|
| Desmopressin |
|
| Tricyclic drugs |
|
| Dry bed training with an alarm |
|
Recommendations from others
A recent evidence-based practice parameter from the American Academy of Child and Adolescent Psychiatry states once the history and physical suggest primary nocturnal enuresis, treatment should include education demystification and withholding punishment.4 Although insufficient evidence exists to recommend behavioral interventions such as journal keeping, fluid restrictions, and night awakenings, these supportive approaches are acceptable, benign starting points. Conditioning with an enuresis alarm and overlearning, which involves giving extra fluids at bedtime after successfully becoming dry and intermittent reinforcement before ending treatment, is a highly effective first-line management approach. Medication choices include desmopressin and imipramine, although relapse rates are high. Short-term use of desmopressin may be used for sleepovers or camping trips.
1. Glazener CM, Evans H, Peto RE. Alarm interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2003;(2):CD002911.-
2. Lyth N, Bosson S. Nocturnal enuresis. Clin Evid 2004;(11):468-477.
3. Butler RJ. Childhood nocturnal enuresis: developing a conceptual framework. Clin Psychol Rev 2004;24:909-931
4. Fritz G, Rockney R. American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry 2004;43:123-125.
5. Mammen AA, Ferrer FA. Nocturnal enuresis: medical management. Urol Clin North Am 2004;31:491-498.
6. Glazener CM, Evans JH. Desmopressin for nocturnal enuresis in children. Cochrane Database Syst Rev 2002;(3):CD002112.-
7. Gibb S, Nolan T, South M, Noad L, Bates G, Vidmar S. Evidence against a synergistic effect of desmopressin with conditioning in the treatment of nocturnal enuresis. J Pediatr 2004;144:351-357.
8. Glazener CM, Evans JH. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev 2003;(3):CD002117.-
9. Glazner CM, Evans JH. Simple behavioural and physical interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2004;(2):CD003637.-
10. Glazener CM, Evans JH, Peto RE. Complex behavioural and educational interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2004;(1):CD004668.-
For children with primary nocturnal enuresis, treatment with enuresis alarms reduced the number of wet nights by almost 4 per week, with almost half of patients remaining dry for 3 months after treatment (strength of recommendation [SOR]: A, based on a systematic review of homogeneous randomized control trials [RCTs]). Desmopressin (DDAVP) and tricyclic drugs reduce the number of wet nights by 1 to 2 per week during treatment, although the effect is not sustained after treatment is finished (SOR: A, based on a SR of homogeneous RCTs). Dry bed training with an alarm results in an additional reduction of wet nights over alarms alone (SOR: A, based on a systematic review of homogeneous RCTs].
Alarms have a high success rate with commitment; desmopressin good for temporary reduction
Nocturnal enuresis is embarrassing to children and frustrating to parents. Even though it has a usually benign, self-limited course, many families want to hear about treatment options. Enuresis alarms have a high success rate in achieving dry nights during treatment and maintaining dry nights once treatment stops. The success of alarms requires a motivated child and family plus a significant time and effort commitment for 3 to 6 months.
Since desmopressin rapidly reduces bedwetting, it is a good choice for situational use such as sleepovers, camping, and holidays. Desmopressin has minimal adverse reactions such as nasal irritation, nausea, and headaches, but parents should minimize evening water intake to prevent rare water intoxication side effects. The lack of benefit of desmopressin and alarm combination therapy may be partly explained by the loss of learning, if desmopressin negates the alarm needing to trigger. Since tricyclic medications do not show a benefit over desmopressin, they should be considered second-line agents due to cardiotoxic side effects and life-threatening overdose outcomes.
Evidence summary
Nocturnal enuresis is an involuntary loss of urine at night in the absence of congenital or acquired central nervous system defect among children over 5 years of age.1-4 Approximately 15% of children aged >5years wet their bed at night.5 The spontaneous resolution rate is about 15% per year.5 Before primary care treatment, indications for urological referral should be excluded, including daytime wetting, abnormal voiding (unusual posturing, discomfort, straining, or poor urine stream), recurrent urinary tract infections, neurological and anatomical anomalies, and urgency symptoms.4
The Cochrane Incontinence Group Trials demonstrated that enuresis alarms led to nearly 4 fewer wet nights per week compared with no treatment or placebo (weighted mean difference [WMD]=–3.65; 95% confidence interval [CI], –4.52 to –2.78).1 The relative risk of failure was 0.36 compared with placebo (95% CI, 0.26 to 0.40). The number needed to treat (NNT) to achieve 14 consecutive dry nights is 2. About half the children relapse after stopping treatment, compared with nearly all children after control interventions (55% vs 99%). Evidence is insufficient to say whether the addition of dry bed training (scheduled awakenings, cleanliness training, social reinforcement, positive practice) improves the outcomes. Alarms that wake the child immediately (vs a time delay) and alarms that wake the child (instead of the parents) were slightly more effective.
A meta-analysis also showed that desmopressin (10-60 μg) at bedtime reduced bedwetting by 1 to 2 nights per week compared with placebo (WMD=1.34; 95% CI, -1.57 to –1.11 with a dose of 20 μg).6 The NNT to achieve 14 consecutive dry nights is 7. However, the data suggest once treatment stops, there is little difference between desmopressin and placebo. Some evidence suggested that a higher dose was more likely to decrease the number of wet nights; however, there was no difference in cure rates. Evidence comparing intranasal with oral administration is insufficient.2
In the Cochrane review, children treated with desmopressin had 1.7 fewer wet nights (WMD=1.7; 95% CI, –2.95 to –0.45) in the first week compared with children treated with alarms.6 However, at the end of 3 months, alarms were associated with 1.4 fewer wet nights per week than children treated with desmopressin (WMD=1.4; 95% CI, 0.14 to 2.66).
Evidence is conflicting for increased efficacy for combining desmopressin with enuresis alarms. There is some limited evidence that children receiving combination treatment with desmopressin and alarms had fewer wet nights than children treated with alarms and placebo.6 This combination treatment did not show a benefit with failure rates (not attaining 14 consecutive dry nights) or a statistically significant difference in failure and relapse rates once treatment stopped. In addition, one RCT in which desmopressin nonresponders were supplemented with alarms showed no added benefit in remission rates compared with conditioning alarms plus placebo (51% vs 48% in achieving 28 dry nights).7 Neither was there added benefit in relapse rates once treatment stopped.
Children treated with tricyclic drugs compared with those treated with placebo had approximately 1 less night of enuresis per week (WMD=1.19; 95% CI, –1.56 to-0.82).8 More children achieved 14 dry nights while on imipramine compared with placebo (21% vs 5%; NNT=6); however, this advantage was not sustained once treatment finished (96% vs 97% relapsed). Little evidence exists to compare desmopressin with tricyclic drugs.2,8
Simple behavior methods may be more effective than no treatment, but there is little evidence for how these methods compare with one another, or with more successful means of treatment.9 Behavior techniques include lifting (taking a sleeping child to urinate in the bath-room), waking, rewards, and evening fluid restriction.
Dry bed training refers to comprehensive regimes, including enuresis alarms, waking routines, positive practice, cleanliness training, and bladder training in various combinations. A meta-analysis examining dry bed training including an enuresis alarm showed children had fewer wet nights compared with children receiving no treatment (relative risk [RR] of failure=0.17; 95% CI, 0.11-0.28).2,10 Additionally, more children remained dry after treatment stopped (RR of relapse=0.25; 95% CI, 0.16-0.39). However, evidence was not sufficient to show a remission benefit for dry bed training without an alarm (RR of failure=0.82; 95% CI, 0.6-1.02), highlighting the key role for alarm therapy. On the other hand, dry bed training including bed alarms may reduce the relapse rate compared with alarm monotherapy (RR for failure or relapse=0.5; 95% CI, 0.31-0.8)
TABLE
Nocturnal enuresis treatments and efficacy
| TREATMENT | EFFICACY |
|---|---|
| Enuresis alarms |
|
| Desmopressin |
|
| Tricyclic drugs |
|
| Dry bed training with an alarm |
|
Recommendations from others
A recent evidence-based practice parameter from the American Academy of Child and Adolescent Psychiatry states once the history and physical suggest primary nocturnal enuresis, treatment should include education demystification and withholding punishment.4 Although insufficient evidence exists to recommend behavioral interventions such as journal keeping, fluid restrictions, and night awakenings, these supportive approaches are acceptable, benign starting points. Conditioning with an enuresis alarm and overlearning, which involves giving extra fluids at bedtime after successfully becoming dry and intermittent reinforcement before ending treatment, is a highly effective first-line management approach. Medication choices include desmopressin and imipramine, although relapse rates are high. Short-term use of desmopressin may be used for sleepovers or camping trips.
For children with primary nocturnal enuresis, treatment with enuresis alarms reduced the number of wet nights by almost 4 per week, with almost half of patients remaining dry for 3 months after treatment (strength of recommendation [SOR]: A, based on a systematic review of homogeneous randomized control trials [RCTs]). Desmopressin (DDAVP) and tricyclic drugs reduce the number of wet nights by 1 to 2 per week during treatment, although the effect is not sustained after treatment is finished (SOR: A, based on a SR of homogeneous RCTs). Dry bed training with an alarm results in an additional reduction of wet nights over alarms alone (SOR: A, based on a systematic review of homogeneous RCTs].
Alarms have a high success rate with commitment; desmopressin good for temporary reduction
Nocturnal enuresis is embarrassing to children and frustrating to parents. Even though it has a usually benign, self-limited course, many families want to hear about treatment options. Enuresis alarms have a high success rate in achieving dry nights during treatment and maintaining dry nights once treatment stops. The success of alarms requires a motivated child and family plus a significant time and effort commitment for 3 to 6 months.
Since desmopressin rapidly reduces bedwetting, it is a good choice for situational use such as sleepovers, camping, and holidays. Desmopressin has minimal adverse reactions such as nasal irritation, nausea, and headaches, but parents should minimize evening water intake to prevent rare water intoxication side effects. The lack of benefit of desmopressin and alarm combination therapy may be partly explained by the loss of learning, if desmopressin negates the alarm needing to trigger. Since tricyclic medications do not show a benefit over desmopressin, they should be considered second-line agents due to cardiotoxic side effects and life-threatening overdose outcomes.
Evidence summary
Nocturnal enuresis is an involuntary loss of urine at night in the absence of congenital or acquired central nervous system defect among children over 5 years of age.1-4 Approximately 15% of children aged >5years wet their bed at night.5 The spontaneous resolution rate is about 15% per year.5 Before primary care treatment, indications for urological referral should be excluded, including daytime wetting, abnormal voiding (unusual posturing, discomfort, straining, or poor urine stream), recurrent urinary tract infections, neurological and anatomical anomalies, and urgency symptoms.4
The Cochrane Incontinence Group Trials demonstrated that enuresis alarms led to nearly 4 fewer wet nights per week compared with no treatment or placebo (weighted mean difference [WMD]=–3.65; 95% confidence interval [CI], –4.52 to –2.78).1 The relative risk of failure was 0.36 compared with placebo (95% CI, 0.26 to 0.40). The number needed to treat (NNT) to achieve 14 consecutive dry nights is 2. About half the children relapse after stopping treatment, compared with nearly all children after control interventions (55% vs 99%). Evidence is insufficient to say whether the addition of dry bed training (scheduled awakenings, cleanliness training, social reinforcement, positive practice) improves the outcomes. Alarms that wake the child immediately (vs a time delay) and alarms that wake the child (instead of the parents) were slightly more effective.
A meta-analysis also showed that desmopressin (10-60 μg) at bedtime reduced bedwetting by 1 to 2 nights per week compared with placebo (WMD=1.34; 95% CI, -1.57 to –1.11 with a dose of 20 μg).6 The NNT to achieve 14 consecutive dry nights is 7. However, the data suggest once treatment stops, there is little difference between desmopressin and placebo. Some evidence suggested that a higher dose was more likely to decrease the number of wet nights; however, there was no difference in cure rates. Evidence comparing intranasal with oral administration is insufficient.2
In the Cochrane review, children treated with desmopressin had 1.7 fewer wet nights (WMD=1.7; 95% CI, –2.95 to –0.45) in the first week compared with children treated with alarms.6 However, at the end of 3 months, alarms were associated with 1.4 fewer wet nights per week than children treated with desmopressin (WMD=1.4; 95% CI, 0.14 to 2.66).
Evidence is conflicting for increased efficacy for combining desmopressin with enuresis alarms. There is some limited evidence that children receiving combination treatment with desmopressin and alarms had fewer wet nights than children treated with alarms and placebo.6 This combination treatment did not show a benefit with failure rates (not attaining 14 consecutive dry nights) or a statistically significant difference in failure and relapse rates once treatment stopped. In addition, one RCT in which desmopressin nonresponders were supplemented with alarms showed no added benefit in remission rates compared with conditioning alarms plus placebo (51% vs 48% in achieving 28 dry nights).7 Neither was there added benefit in relapse rates once treatment stopped.
Children treated with tricyclic drugs compared with those treated with placebo had approximately 1 less night of enuresis per week (WMD=1.19; 95% CI, –1.56 to-0.82).8 More children achieved 14 dry nights while on imipramine compared with placebo (21% vs 5%; NNT=6); however, this advantage was not sustained once treatment finished (96% vs 97% relapsed). Little evidence exists to compare desmopressin with tricyclic drugs.2,8
Simple behavior methods may be more effective than no treatment, but there is little evidence for how these methods compare with one another, or with more successful means of treatment.9 Behavior techniques include lifting (taking a sleeping child to urinate in the bath-room), waking, rewards, and evening fluid restriction.
Dry bed training refers to comprehensive regimes, including enuresis alarms, waking routines, positive practice, cleanliness training, and bladder training in various combinations. A meta-analysis examining dry bed training including an enuresis alarm showed children had fewer wet nights compared with children receiving no treatment (relative risk [RR] of failure=0.17; 95% CI, 0.11-0.28).2,10 Additionally, more children remained dry after treatment stopped (RR of relapse=0.25; 95% CI, 0.16-0.39). However, evidence was not sufficient to show a remission benefit for dry bed training without an alarm (RR of failure=0.82; 95% CI, 0.6-1.02), highlighting the key role for alarm therapy. On the other hand, dry bed training including bed alarms may reduce the relapse rate compared with alarm monotherapy (RR for failure or relapse=0.5; 95% CI, 0.31-0.8)
TABLE
Nocturnal enuresis treatments and efficacy
| TREATMENT | EFFICACY |
|---|---|
| Enuresis alarms |
|
| Desmopressin |
|
| Tricyclic drugs |
|
| Dry bed training with an alarm |
|
Recommendations from others
A recent evidence-based practice parameter from the American Academy of Child and Adolescent Psychiatry states once the history and physical suggest primary nocturnal enuresis, treatment should include education demystification and withholding punishment.4 Although insufficient evidence exists to recommend behavioral interventions such as journal keeping, fluid restrictions, and night awakenings, these supportive approaches are acceptable, benign starting points. Conditioning with an enuresis alarm and overlearning, which involves giving extra fluids at bedtime after successfully becoming dry and intermittent reinforcement before ending treatment, is a highly effective first-line management approach. Medication choices include desmopressin and imipramine, although relapse rates are high. Short-term use of desmopressin may be used for sleepovers or camping trips.
1. Glazener CM, Evans H, Peto RE. Alarm interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2003;(2):CD002911.-
2. Lyth N, Bosson S. Nocturnal enuresis. Clin Evid 2004;(11):468-477.
3. Butler RJ. Childhood nocturnal enuresis: developing a conceptual framework. Clin Psychol Rev 2004;24:909-931
4. Fritz G, Rockney R. American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry 2004;43:123-125.
5. Mammen AA, Ferrer FA. Nocturnal enuresis: medical management. Urol Clin North Am 2004;31:491-498.
6. Glazener CM, Evans JH. Desmopressin for nocturnal enuresis in children. Cochrane Database Syst Rev 2002;(3):CD002112.-
7. Gibb S, Nolan T, South M, Noad L, Bates G, Vidmar S. Evidence against a synergistic effect of desmopressin with conditioning in the treatment of nocturnal enuresis. J Pediatr 2004;144:351-357.
8. Glazener CM, Evans JH. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev 2003;(3):CD002117.-
9. Glazner CM, Evans JH. Simple behavioural and physical interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2004;(2):CD003637.-
10. Glazener CM, Evans JH, Peto RE. Complex behavioural and educational interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2004;(1):CD004668.-
1. Glazener CM, Evans H, Peto RE. Alarm interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2003;(2):CD002911.-
2. Lyth N, Bosson S. Nocturnal enuresis. Clin Evid 2004;(11):468-477.
3. Butler RJ. Childhood nocturnal enuresis: developing a conceptual framework. Clin Psychol Rev 2004;24:909-931
4. Fritz G, Rockney R. American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry 2004;43:123-125.
5. Mammen AA, Ferrer FA. Nocturnal enuresis: medical management. Urol Clin North Am 2004;31:491-498.
6. Glazener CM, Evans JH. Desmopressin for nocturnal enuresis in children. Cochrane Database Syst Rev 2002;(3):CD002112.-
7. Gibb S, Nolan T, South M, Noad L, Bates G, Vidmar S. Evidence against a synergistic effect of desmopressin with conditioning in the treatment of nocturnal enuresis. J Pediatr 2004;144:351-357.
8. Glazener CM, Evans JH. Tricyclic and related drugs for nocturnal enuresis in children. Cochrane Database Syst Rev 2003;(3):CD002117.-
9. Glazner CM, Evans JH. Simple behavioural and physical interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2004;(2):CD003637.-
10. Glazener CM, Evans JH, Peto RE. Complex behavioural and educational interventions for nocturnal enuresis in children. Cochrane Database Syst Rev 2004;(1):CD004668.-
Evidence-based answers from the Family Physicians Inquiries Network