User login
What’s the best test for underlying osteomyelitis in patients with diabetic foot ulcers?
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)
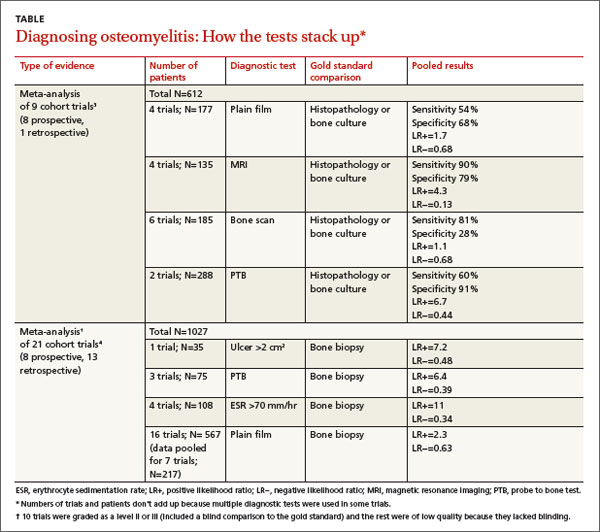
A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Evidence-based answers from the Family Physicians Inquiries Network
Bilateral hand cramping and weakness • broad fingers • coarse facial features • Dx?
THE CASE
A 37-year-old right-hand dominant woman came to our clinic seeking treatment for bilateral generalized hand cramping and weakness that she had been experiencing for approximately 2 to 3 years. She was dropping objects and had finger locking, yet had no numbness, tingling, or morning stiffness.
Ten months earlier, she had given birth to a healthy 3715 g girl. Our patient’s prenatal glucose tolerance test had been normal. Her pregnancy and delivery had been significant for oligohydramnios, failed post-term (41 weeks 4 days) induction, and emergent low transverse cesarean section due to fetal bradycardia. Since giving birth, our patient had 3 menstrual periods while breastfeeding. She had a copper intrauterine device inserted at her 6-week postpartum visit. She also had 2 truncal acrochordons removed 3 months postpartum. She had no history of neck trauma, overuse injury, or occupational exposures.
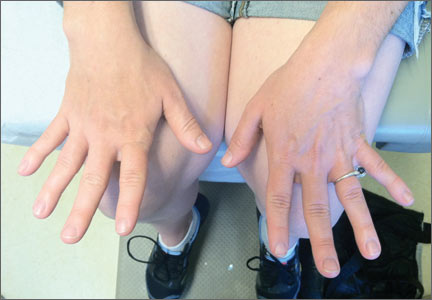
Her blood pressure and vital signs were within normal limits. Physical exam was notable for subtly coarse facial features and broad fingers (FIGURE 1).
She had normal wrist and hand joint range of motion; her wrist and hand strengths, including grip strength, were 5 out of 5. Tinel’s sign, Phalen’s maneuver, and Finkelstein’s test were negative.
Her upper extremity neurovascular exams were completely normal. Initial laboratory studies—including a comprehensive metabolic panel—were normal. The only exception was her creatine kinase, which was 265 U/L (normal, 24-195 U/L).
At a follow-up appointment 7 weeks later, we gathered a more detailed history and learned that over the past 2 to 3 years, the patient had noticed that her shoe and ring sizes had been increasing. She also mentioned some mild weight gain following her pregnancy.
Occasionally, she had generalized hand swelling, headaches, and saw floaters, but she denied losing peripheral vision. Additional lab work at this time revealed a fasting growth hormone (GH) level of 27.3 ng/mL (normal, 0.05-8 ng/mL) and an insulin-like growth factor 1 (IGF-1) level of 848 ng/mL (normal, 106-368 ng/mL). An anterior pituitary hormone panel and cortisol level were normal. A urine pregnancy test was negative.
THE DIAGNOSIS
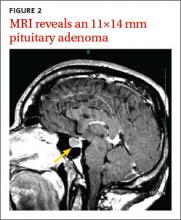
Magnetic resonance imaging (MRI) of our patient’s brain revealed a pituitary adenoma (FIGURE 2). Based on that and the patient’s elevated GH and IGF-1 levels, we diagnosed acromegaly due to a pituitary adenoma.
DISCUSSION
Acromegaly is a rare, progressively disfiguring disease with a prevalence of 40 cases per million people.1 It affects middle-aged adults, with no gender difference.2 In most cases, the cause is a benign pituitary adenoma.1-4
Physical changes include coarse facial features, generalized expansion of the skull, brow protrusion, ocular distension, prognathism, macroglossia, acral overgrowth, and dental malocclusion; these changes typically occur slowly over a long time period.1-5 For example, when we looked at the 3-year-old photo on our patient’s driver’s license, we noticed only subtle changes from her current appearance. Common clinical manifestations include headache, hyperpigmentation, hypertrichosis, hyperhidrosis, goiter, arthropathy, carpal tunnel syndrome, visual disturbances, and acrochordons.1,5
Acromegaly is associated with an increased risk of cardiovascular disease, metabolic disorders, infertility, sleep apnea, arthritis, thyroid tumors, colon adenomas, and carcinoma.1,2,4,5 Due to the insidious progression of acromegaly’s clinical manifestations, diagnosis is delayed for 4 to 10 years, on average.1 The diagnosis of acromegaly is typically based on an elevation of GH and IGF-1 levels.1,5 A brain MRI is essential in the diagnosis of a pituitary adenoma.1
Pregnancy among patients with acromegaly is uncommon. In fact, fewer than 150 cases have been reported in the literature.2,6 In most cases, it appears that pregnancy among patients with acromegaly is safe for mothers and newborns.6,7
The goals of treatment for acromegaly caused by a pituitary adenoma are to remove/ reduce the tumor and its mechanical effects, relieve symptoms, reduce serum GH and IGF-1, and restore pituitary function. Transsphenoidal surgical resection is the preferred treatment for pituitary adenomas.1,2,4 Radiation therapy and pharmacologic treatment may be necessary as adjuncts to surgery or for patients for whom surgery is contraindicated.1,4,5
Pharmacologic management of acromegaly includes dopamine agonists (cabergoline), somatostatin analogues (octreotide, lanreotide), and GH receptor antagonists (pegvisomant).1,3 Patients who receive effective early treatment of acromegaly have a life expectancy similar to that of the general population.1,5
Our patient
Our patient was referred to Neurosurgery and underwent transnasal transsphenoidal resection of the pituitary adenoma. Two weeks postop, her GH level had decreased to 0.66 ng/mL and her IGF-1 level was down to 386 ng/mL. Four months later, her GH (2.32 ng/mL) and IGF-1 levels (277 ng/mL) were within normal range and our patient reported improvement in all of her symptoms.
THE TAKEAWAY
Because it may take years for the classical clinical features of acromegaly such as coarse facial features, protruding jaw, and broad fingers to become apparent, diligent history taking is essential to diagnose the condition early. Patients may present with nonspecific and confusing symptoms such as muscle weakness.8 Early nonspecific symptoms and signs in the presence of normal basic laboratory tests should warrant an evaluation of fasting GH and IGF-1. Early treatment with surgery, radiation therapy, or pharmacotherapy may prevent or decrease the intensity of rheumatologic, cardiovascular, respiratory, and metabolic complications of acromegaly.1
1. Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9: 297-303.
2. Hossain B, Drake WM. Acromegaly. Medicine. 2009;37: 407-410.
3. Chan MR, Ziebert M, Maas DL, et al. “My rings won’t fit anymore”. Ectopic growth hormone-secreting tumor. Am Fam Physician. 2005;71:1766-1767.
4. Lake MG, Krook LS, Cruz SV. Pituitary adenomas: an overview. Am Fam Physician. 2013;88:319-327.
5. Vilar L, Valenzuela A, Ribeiro-Oliveira A Jr, et al. Multiple facets in the control of acromegaly. Pituitary. 2014;17 suppl 1:S11-S17.
6. Cheng V, Faiman C, Kennedy L, et al. Pregnancy and acromegaly: a review. Pituitary. 2012;15:59-63.
7. Caron P, Broussaud S, Bertherat J, et al. Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95:4680-4687.
8. Saguil A. Evaluation of the patient with muscle weakness. Am Fam Physician. 2005;71:1327-1336.
THE CASE
A 37-year-old right-hand dominant woman came to our clinic seeking treatment for bilateral generalized hand cramping and weakness that she had been experiencing for approximately 2 to 3 years. She was dropping objects and had finger locking, yet had no numbness, tingling, or morning stiffness.
Ten months earlier, she had given birth to a healthy 3715 g girl. Our patient’s prenatal glucose tolerance test had been normal. Her pregnancy and delivery had been significant for oligohydramnios, failed post-term (41 weeks 4 days) induction, and emergent low transverse cesarean section due to fetal bradycardia. Since giving birth, our patient had 3 menstrual periods while breastfeeding. She had a copper intrauterine device inserted at her 6-week postpartum visit. She also had 2 truncal acrochordons removed 3 months postpartum. She had no history of neck trauma, overuse injury, or occupational exposures.
Her blood pressure and vital signs were within normal limits. Physical exam was notable for subtly coarse facial features and broad fingers (FIGURE 1).
She had normal wrist and hand joint range of motion; her wrist and hand strengths, including grip strength, were 5 out of 5. Tinel’s sign, Phalen’s maneuver, and Finkelstein’s test were negative.
Her upper extremity neurovascular exams were completely normal. Initial laboratory studies—including a comprehensive metabolic panel—were normal. The only exception was her creatine kinase, which was 265 U/L (normal, 24-195 U/L).
At a follow-up appointment 7 weeks later, we gathered a more detailed history and learned that over the past 2 to 3 years, the patient had noticed that her shoe and ring sizes had been increasing. She also mentioned some mild weight gain following her pregnancy.
Occasionally, she had generalized hand swelling, headaches, and saw floaters, but she denied losing peripheral vision. Additional lab work at this time revealed a fasting growth hormone (GH) level of 27.3 ng/mL (normal, 0.05-8 ng/mL) and an insulin-like growth factor 1 (IGF-1) level of 848 ng/mL (normal, 106-368 ng/mL). An anterior pituitary hormone panel and cortisol level were normal. A urine pregnancy test was negative.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of our patient’s brain revealed a pituitary adenoma (FIGURE 2). Based on that and the patient’s elevated GH and IGF-1 levels, we diagnosed acromegaly due to a pituitary adenoma.
DISCUSSION
Acromegaly is a rare, progressively disfiguring disease with a prevalence of 40 cases per million people.1 It affects middle-aged adults, with no gender difference.2 In most cases, the cause is a benign pituitary adenoma.1-4
Physical changes include coarse facial features, generalized expansion of the skull, brow protrusion, ocular distension, prognathism, macroglossia, acral overgrowth, and dental malocclusion; these changes typically occur slowly over a long time period.1-5 For example, when we looked at the 3-year-old photo on our patient’s driver’s license, we noticed only subtle changes from her current appearance. Common clinical manifestations include headache, hyperpigmentation, hypertrichosis, hyperhidrosis, goiter, arthropathy, carpal tunnel syndrome, visual disturbances, and acrochordons.1,5
Acromegaly is associated with an increased risk of cardiovascular disease, metabolic disorders, infertility, sleep apnea, arthritis, thyroid tumors, colon adenomas, and carcinoma.1,2,4,5 Due to the insidious progression of acromegaly’s clinical manifestations, diagnosis is delayed for 4 to 10 years, on average.1 The diagnosis of acromegaly is typically based on an elevation of GH and IGF-1 levels.1,5 A brain MRI is essential in the diagnosis of a pituitary adenoma.1
Pregnancy among patients with acromegaly is uncommon. In fact, fewer than 150 cases have been reported in the literature.2,6 In most cases, it appears that pregnancy among patients with acromegaly is safe for mothers and newborns.6,7
The goals of treatment for acromegaly caused by a pituitary adenoma are to remove/ reduce the tumor and its mechanical effects, relieve symptoms, reduce serum GH and IGF-1, and restore pituitary function. Transsphenoidal surgical resection is the preferred treatment for pituitary adenomas.1,2,4 Radiation therapy and pharmacologic treatment may be necessary as adjuncts to surgery or for patients for whom surgery is contraindicated.1,4,5
Pharmacologic management of acromegaly includes dopamine agonists (cabergoline), somatostatin analogues (octreotide, lanreotide), and GH receptor antagonists (pegvisomant).1,3 Patients who receive effective early treatment of acromegaly have a life expectancy similar to that of the general population.1,5
Our patient
Our patient was referred to Neurosurgery and underwent transnasal transsphenoidal resection of the pituitary adenoma. Two weeks postop, her GH level had decreased to 0.66 ng/mL and her IGF-1 level was down to 386 ng/mL. Four months later, her GH (2.32 ng/mL) and IGF-1 levels (277 ng/mL) were within normal range and our patient reported improvement in all of her symptoms.
THE TAKEAWAY
Because it may take years for the classical clinical features of acromegaly such as coarse facial features, protruding jaw, and broad fingers to become apparent, diligent history taking is essential to diagnose the condition early. Patients may present with nonspecific and confusing symptoms such as muscle weakness.8 Early nonspecific symptoms and signs in the presence of normal basic laboratory tests should warrant an evaluation of fasting GH and IGF-1. Early treatment with surgery, radiation therapy, or pharmacotherapy may prevent or decrease the intensity of rheumatologic, cardiovascular, respiratory, and metabolic complications of acromegaly.1
THE CASE
A 37-year-old right-hand dominant woman came to our clinic seeking treatment for bilateral generalized hand cramping and weakness that she had been experiencing for approximately 2 to 3 years. She was dropping objects and had finger locking, yet had no numbness, tingling, or morning stiffness.
Ten months earlier, she had given birth to a healthy 3715 g girl. Our patient’s prenatal glucose tolerance test had been normal. Her pregnancy and delivery had been significant for oligohydramnios, failed post-term (41 weeks 4 days) induction, and emergent low transverse cesarean section due to fetal bradycardia. Since giving birth, our patient had 3 menstrual periods while breastfeeding. She had a copper intrauterine device inserted at her 6-week postpartum visit. She also had 2 truncal acrochordons removed 3 months postpartum. She had no history of neck trauma, overuse injury, or occupational exposures.
Her blood pressure and vital signs were within normal limits. Physical exam was notable for subtly coarse facial features and broad fingers (FIGURE 1).
She had normal wrist and hand joint range of motion; her wrist and hand strengths, including grip strength, were 5 out of 5. Tinel’s sign, Phalen’s maneuver, and Finkelstein’s test were negative.
Her upper extremity neurovascular exams were completely normal. Initial laboratory studies—including a comprehensive metabolic panel—were normal. The only exception was her creatine kinase, which was 265 U/L (normal, 24-195 U/L).
At a follow-up appointment 7 weeks later, we gathered a more detailed history and learned that over the past 2 to 3 years, the patient had noticed that her shoe and ring sizes had been increasing. She also mentioned some mild weight gain following her pregnancy.
Occasionally, she had generalized hand swelling, headaches, and saw floaters, but she denied losing peripheral vision. Additional lab work at this time revealed a fasting growth hormone (GH) level of 27.3 ng/mL (normal, 0.05-8 ng/mL) and an insulin-like growth factor 1 (IGF-1) level of 848 ng/mL (normal, 106-368 ng/mL). An anterior pituitary hormone panel and cortisol level were normal. A urine pregnancy test was negative.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of our patient’s brain revealed a pituitary adenoma (FIGURE 2). Based on that and the patient’s elevated GH and IGF-1 levels, we diagnosed acromegaly due to a pituitary adenoma.
DISCUSSION
Acromegaly is a rare, progressively disfiguring disease with a prevalence of 40 cases per million people.1 It affects middle-aged adults, with no gender difference.2 In most cases, the cause is a benign pituitary adenoma.1-4
Physical changes include coarse facial features, generalized expansion of the skull, brow protrusion, ocular distension, prognathism, macroglossia, acral overgrowth, and dental malocclusion; these changes typically occur slowly over a long time period.1-5 For example, when we looked at the 3-year-old photo on our patient’s driver’s license, we noticed only subtle changes from her current appearance. Common clinical manifestations include headache, hyperpigmentation, hypertrichosis, hyperhidrosis, goiter, arthropathy, carpal tunnel syndrome, visual disturbances, and acrochordons.1,5
Acromegaly is associated with an increased risk of cardiovascular disease, metabolic disorders, infertility, sleep apnea, arthritis, thyroid tumors, colon adenomas, and carcinoma.1,2,4,5 Due to the insidious progression of acromegaly’s clinical manifestations, diagnosis is delayed for 4 to 10 years, on average.1 The diagnosis of acromegaly is typically based on an elevation of GH and IGF-1 levels.1,5 A brain MRI is essential in the diagnosis of a pituitary adenoma.1
Pregnancy among patients with acromegaly is uncommon. In fact, fewer than 150 cases have been reported in the literature.2,6 In most cases, it appears that pregnancy among patients with acromegaly is safe for mothers and newborns.6,7
The goals of treatment for acromegaly caused by a pituitary adenoma are to remove/ reduce the tumor and its mechanical effects, relieve symptoms, reduce serum GH and IGF-1, and restore pituitary function. Transsphenoidal surgical resection is the preferred treatment for pituitary adenomas.1,2,4 Radiation therapy and pharmacologic treatment may be necessary as adjuncts to surgery or for patients for whom surgery is contraindicated.1,4,5
Pharmacologic management of acromegaly includes dopamine agonists (cabergoline), somatostatin analogues (octreotide, lanreotide), and GH receptor antagonists (pegvisomant).1,3 Patients who receive effective early treatment of acromegaly have a life expectancy similar to that of the general population.1,5
Our patient
Our patient was referred to Neurosurgery and underwent transnasal transsphenoidal resection of the pituitary adenoma. Two weeks postop, her GH level had decreased to 0.66 ng/mL and her IGF-1 level was down to 386 ng/mL. Four months later, her GH (2.32 ng/mL) and IGF-1 levels (277 ng/mL) were within normal range and our patient reported improvement in all of her symptoms.
THE TAKEAWAY
Because it may take years for the classical clinical features of acromegaly such as coarse facial features, protruding jaw, and broad fingers to become apparent, diligent history taking is essential to diagnose the condition early. Patients may present with nonspecific and confusing symptoms such as muscle weakness.8 Early nonspecific symptoms and signs in the presence of normal basic laboratory tests should warrant an evaluation of fasting GH and IGF-1. Early treatment with surgery, radiation therapy, or pharmacotherapy may prevent or decrease the intensity of rheumatologic, cardiovascular, respiratory, and metabolic complications of acromegaly.1
1. Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9: 297-303.
2. Hossain B, Drake WM. Acromegaly. Medicine. 2009;37: 407-410.
3. Chan MR, Ziebert M, Maas DL, et al. “My rings won’t fit anymore”. Ectopic growth hormone-secreting tumor. Am Fam Physician. 2005;71:1766-1767.
4. Lake MG, Krook LS, Cruz SV. Pituitary adenomas: an overview. Am Fam Physician. 2013;88:319-327.
5. Vilar L, Valenzuela A, Ribeiro-Oliveira A Jr, et al. Multiple facets in the control of acromegaly. Pituitary. 2014;17 suppl 1:S11-S17.
6. Cheng V, Faiman C, Kennedy L, et al. Pregnancy and acromegaly: a review. Pituitary. 2012;15:59-63.
7. Caron P, Broussaud S, Bertherat J, et al. Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95:4680-4687.
8. Saguil A. Evaluation of the patient with muscle weakness. Am Fam Physician. 2005;71:1327-1336.
1. Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9: 297-303.
2. Hossain B, Drake WM. Acromegaly. Medicine. 2009;37: 407-410.
3. Chan MR, Ziebert M, Maas DL, et al. “My rings won’t fit anymore”. Ectopic growth hormone-secreting tumor. Am Fam Physician. 2005;71:1766-1767.
4. Lake MG, Krook LS, Cruz SV. Pituitary adenomas: an overview. Am Fam Physician. 2013;88:319-327.
5. Vilar L, Valenzuela A, Ribeiro-Oliveira A Jr, et al. Multiple facets in the control of acromegaly. Pituitary. 2014;17 suppl 1:S11-S17.
6. Cheng V, Faiman C, Kennedy L, et al. Pregnancy and acromegaly: a review. Pituitary. 2012;15:59-63.
7. Caron P, Broussaud S, Bertherat J, et al. Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95:4680-4687.
8. Saguil A. Evaluation of the patient with muscle weakness. Am Fam Physician. 2005;71:1327-1336.