User login
A Veteran With Acute Progressive Encephalopathy of Unknown Etiology
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
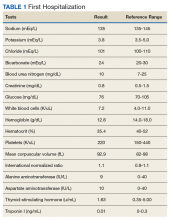
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
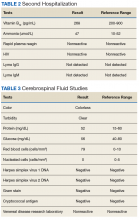
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
Case Presentation. A 70-year-old US Marine Corps veteran of the Vietnam War with no significant past medical history was brought by ambulance to VA Boston Healthcare System (VABHS) after being found on the floor at home by his wife, awake, but with minimally coherent speech. He was moving all extremities, and there was no loss of bowel or bladder continence. He had last been seen well by his wife 30 minutes prior. When emergency medical services arrived, his finger stick blood glucose and vital signs were within normal range. In the emergency department, he was able to state his first name but then continuously repeated “7/11” to other questions. A neurologic examination revealed intact cranial nerves, full strength in all extremities, and normal reflexes. A National Institute of Health Stroke Scale (NIHSS) was 3, and a code stroke was activated. At the time of presentation, the patient was an active smoker of 15 cigarettes per day for 50 years and did not use alcohol or recreational drugs.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. Fehnel, the patient’s medical team was most worried about a transient ischemic attack (TIA) or cerebrovascular accident (CVA). Is his presentation consistent with these diagnoses, and what else is on your differential diagnosis?
►Corey R. Fehnel, MD, Neuro-Intensivist, BIDMC, and Assistant Professor of Neurology, Harvard Medical School. This patient is presenting with what appears to be an acute encephalopathy—a sudden onset of global alteration in mental status. The most worrisome underlying etiology for this presentation would be acute stroke, but this is an uncommon cause of acute encephalopathy. The differential diagnosis at this stage remains broad, but a careful neurologic examination can help narrow the possibilities. In particular, I would aim to differentiate an apparent language deficit (ie, aphasia) from a deficit of attention. A key finding that may help is the ability to name high- or low-frequency objects. If the patient can successfully name objects, aphasia is less likely. Based on the limited examination at present, the patient produces some normal speech, but perseverates; therefore, the finding remains nonspecific. My leading diagnoses are complex partial seizure and toxic/metabolic encephalopathy.
►Dr. Li. This patient’s NIHSS score is 3. How do you use this score in your management decisions for the patient?
►Dr. Fehnel. The NIHSS is a useful tool for gauging severity of ischemic and hemorrhagic stroke. However the score is not specific for establishing the diagnosis of stroke. Many common and chronic neurologic problems will score on the NIHSS, so it can never be interpreted in isolation. If the clinical history and complete neurologic examination support the diagnosis of stroke, then the NIHSS can be used with the understanding that it is biased toward anterior circulation strokes, and posterior circulation strokes will score lower even though they are potentially more life threatening.1 In this case, even though a complex partial seizure appears more likely, it is difficult to rule out the possibility of an acute stroke affecting the thalamus or, less likely, a distal middle cerebral artery occlusion. I would consider IV thrombolysis pending further history and neuroimaging results.
►Dr. Li. Initial laboratory data include a hemoglobin of 12.8 mg/dL. The white cell count, platelet count, chemistry panel, liver function tests, thyroid-stimulating hormone, and troponin were within normal range (Table 1).
Do you agree with inpatient workup for this patient whose mental status has now returned to baseline? If so, what workup would you pursue next?
►Dr. Fehnel. This patient requires inpatient admission to further evaluate the underlying etiology for his acute change in mental status. The improvement of his presenting deficit and largely normal neurovascular imaging make a neurovascular etiology less likely, but a careful risk factor evaluation for CVA/TIA should be performed, including continuous cardiac telemetry to detect atrial fibrillation. Magnetic resonance imaging (MRI) of the brain should be performed to rule out occult stroke and evaluate for a structural etiology given the more likely diagnosis of complex partial seizure. An electroencephalogram (EEG), preferably 24-hour continuous recording, should be performed. Without a clear toxic or metabolic etiology thus far to explain his acute global waxing-waning alteration in mental status and likely new-onset complex partial seizures, I would also pursue lumbar puncture for cerebrospinal fluid examination.
►Dr. Li. The hospital course was notable for episodes of acute combativeness and confusion. An MRI of the brain was deferred due to reports from the patient’s family of retained shrapnel in the lumbar spine. Routine EEG showed no seizure activity. This was followed by continuous video EEG monitoring, which showed subclinical seizure activity with a right temporal focus. He was started on valproic acid with improvement in his agitation, though confusion continued. He was discharged to an inpatient geriatric psychiatry nursing home with diagnosis of seizures and acute delirium.
Dr. Fehnel, seizures are often part of the workup for unexplained encephalopathy. In this case, the routine EEG was unrevealing, while the continuous video EEG proved valuable. In what situations would you pursue a continuous video EEG in addition to a routine EEG?
►Dr. Fehnel. EEG monitoring is only as good as the window of time during which the study is performed. If the suspicious clinical event is captured during a routine recording or an area of focal slowing is detected, a shorter study may be entirely sufficient. However, in cases where there is no clear alternative explanation, a patient’s mental status does not return to normal, or in the setting of mental status fluctuations without explanation, continuous video-EEG monitoring for at least 24 hours is indicated. While the prolonged study raises sensitivity, the exact duration of EEG recording required outside of the intensive care unit setting remains debated.2
►Dr. Li. If his encephalopathy were due to seizures alone, I would expect improvement in his mental status during interictal periods, which does not appear to be the case here. Do you feel the seizures alone can explain his encephalopathy?
►Dr. Fehnel. Complex partial seizures and the medications used to treat them can confound the examination of patients during the interictal period. We commonly debate postictal encephalopathy vs residual effect of benzodiazepines and rapid dose escalation of antiepileptic drugs as culprit in a patient’s prolonged alteration in mental status. Serial clinical examinations, continuous EEG monitoring to rule out ongoing subclinical seizures when appropriate, and judicious use of potentially sedating medications is the most helpful approach. The key issue here is the bimodal distribution of new-onset seizures. Among children there is a higher incidence of genetically related seizure disorders; whereas among adults, “acquired” and structural etiologies are more common. For this case, a more careful evaluation of acquired/structural etiologies for new-onset seizures is indicated.
►Dr. Li. At the geriatric psychiatry nursing home, the patient continued to be combative and refused medications. He was readmitted to the VABHS with encephalopathy of unclear etiology. An expanded encephalopathy workup was unrevealing (Table 2).
Dr. Fehnel, this patient’s initial cerebrospinal fluid (CSF) cell count and chemistries were completely normal. Is this sufficient to rule out encephalitis? If not, what other diagnostic tests would you send?
►Dr. Fehnel. A fully normal CSF profile reduces the likelihood of a broad range of neuro-infectious etiologies but does not completely rule those out. For example, there are reports of herpes simplex virus (HSV) encephalitis producing relatively normal profiles and even negative polymerase chain reaction assays for antibodies to HSV if the specimen is obtained very early in the course of the disease.3,4 That was not the case here as the CSF was obtained several days after his initial presentation. Given this patient’s clinical syndrome, normal CSF findings, and long smoking history without regular screening examinations, I would send a CSF specimen screening for paraneoplastic and autoimmune encephalitis. Most autoimmune encephalitis syndromes are associated with CSF lymphocytic pleocytosis or slight elevation in CSF protein levels. This patient’s diagnosis is most likely an anti-Hu paraneoplastic syndrome, which can be distinguished from other autoimmune and paraneoplastic processes by the characteristically normal CSF profile. Anti-Hu antibodies are strongly associated with non-small cell lung cancer (NSCLC). I would, therefore, also obtain more advanced chest imaging.
►Dr. Li. An autoimmune and paraneoplastic encephalitis panel was sent. While this send-out panel was pending, a CT torso was obtained to evaluate for occult malignancy in light of his significant smoking history. This showed a 3-cm spiculated mass originating from the left hilum. Bronchoalviolar lavage washings returned positive for small cell lung cancer.
Dr. Fehnel, can you explain the mechanism by which certain neoplasms can cause encephalitis?
►Dr. Fehnel. Onconeuronal antibodies Hu (NSCLC) and Ma2 (testicular seminoma), when identified, are strongly associated with the presence of an underlying malignancy. The work of Dr. Josep Dalmau and others in this area has dramatically improved our understanding of these syndromes over the past 25 years.5 The exact mechanism is not fully understood but is thought to be mediated by cytotoxic T-cell response directed at the malignancy itself with homology to intraneuronal structures, which are readily absorbed and result in neuronal cell death.6
►Dr. Li. Is there a specific treatment for paraneoplastic encephalitis, other than treating the underlying malignancy?
►Dr. Fehnel. Early treatment is associated with improved outcome and should not be delayed while waiting for laboratory confirmation in cases of high clinical suspicion. Treatment directed at the underlying tumor is the mainstay along with less specific immunosuppressive agents. Unfortunately Anti-Hu (as well as Ma2) antibodies are intraneuronal and less responsive to standard treatments relative to other paraneoplastic auto-antibodies identified on the cell surface. Immunosuppressive agents typically used in this setting include high-dose IV methylprednisolone, IV immune globulin (IVIG), rituximab, and cyclophosphamide.7
►Dr. Li. The patient was started on IVIG, methylprednisolone, cisplatin, and etoposide. His course was complicated by aspiration pneumonia, autonomic dysfunction causing tachy- and brady-arrhythmias, urosepsis, worsening somnolence, chemotherapy-induced neutropenic fevers, and ultimately septic shock. The palliative care team was closely involved throughout the final stages of his hospital course. After multiple family meetings, the patient was transitioned to comfort-focused care per family discussion and died 6 weeks after his initial presentation.
This patient had a very atypical initial presentation of small cell lung cancer. Despite the fact that a diagnosis eluded his doctors, they persisted in a thoughtful and exhaustive workup and through this perseverance were able to make the final diagnosis, which serves as an important learning case for us all.
Acknowledgments
We thank the family of this veteran for sharing his story and allowing us to learn from this case for the benefit of our future patients. We also thank Dr. Michelle Hankins, who provided oncologic expertise.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.
1. Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. 2013;44(4):1153-1157.
2. Herman ST, Abend NS, Bleck TP, et al; Critical Care Continuous EEG Task Force of the American Clinical Neurophysiology Society. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87-95.
3. DeBiasi RL, Kleinschmidt-DeMasters BK, Weinberg A, Tyler KL. Use of PCR for the diagnosis of herpesvirus infections of the central nervous system. J Clin Virol. 2002;25(suppl 1):S5-S11.
4. Buerger KJ, Zerr K, Salazar R. An unusual presentation of herpes simplex encephalitis with negative PCR. BMJ Case Rep. 2015;2015:pii:bcr201521052.
5. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
6. Greenlee JE, Clawson SA, Hill KE, et al. Neuronal uptake of anti-Hu antibody, but not anti-Ri antibody, leads to cell death in brain slice cultures. J Neuroinflammation. 2014;11:160.
7. Bradshaw MJ, Linnoila JJ. An overview of autoimmune and paraneoplastic encephalitides. Semin Neurol. 2018;38(3):330-343.