User login
Should breast cancer screening guidelines be tailored to a patient’s race and ethnicity?
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
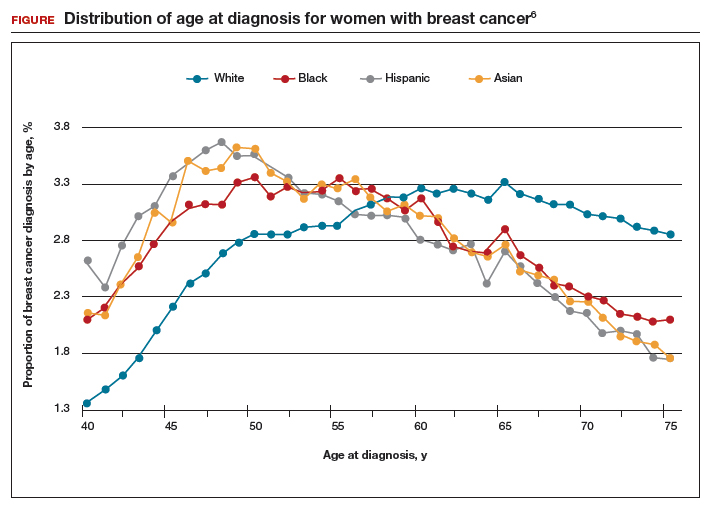
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.
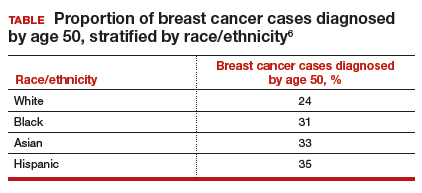
Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).
These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
How does oral contraceptive use affect one’s risk of ovarian, endometrial, breast, and colorectal cancers?
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.
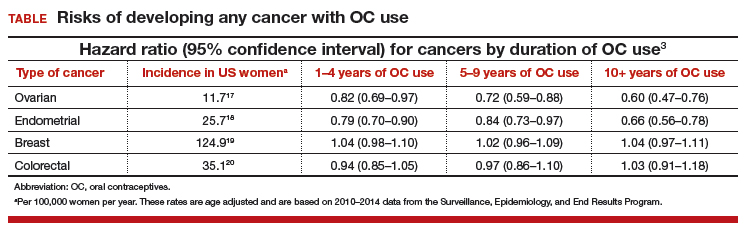
There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.

There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.

There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.