User login
2023 USPSTF mammography age to start screening in average-risk patients: What’s new is old again
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

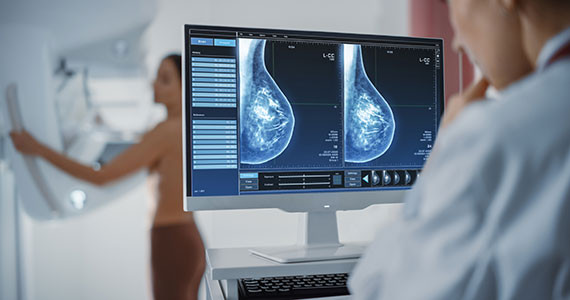
In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.
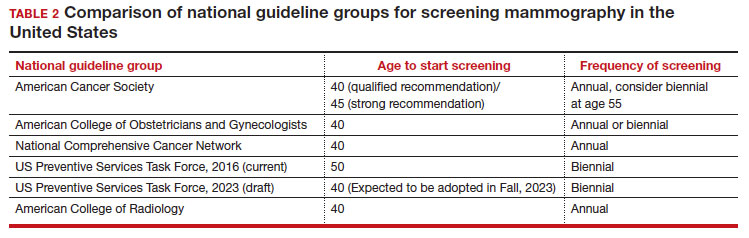
In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
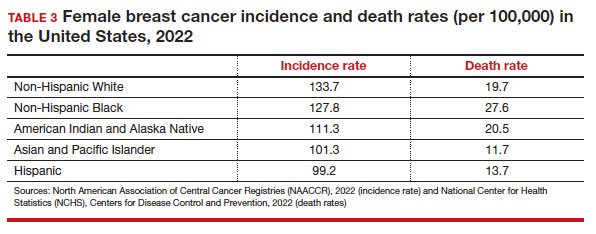
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14
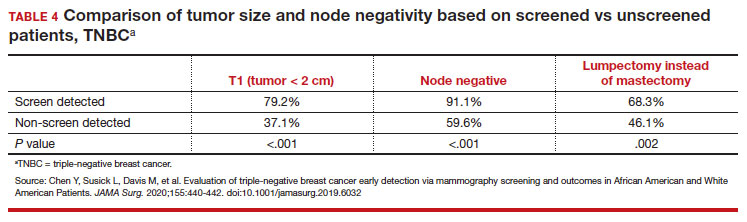
While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.
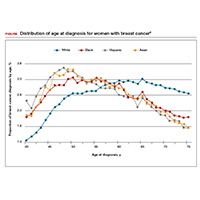
Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
The US Preventive Services Task Force (USPSTF)1 is comprised of an independent panel of preventive services clinician experts who make evidence-based recommendations, with the letter grade assigned based on the strength of the evidence, from A through D (TABLE 1), on preventive services such as health screenings, shared decision making patient counseling, and preventive medications. Both A and B recommendations are generally accepted by both government and most private health insurance companies as a covered preventive benefit with no or minimal co-pays.

In 2002, the USPSTF released a Grade B recommendation that screening mammography for average-risk patients (with patients referring to persons assigned female at birth who have not undergone bilateral mastectomy) should take place starting at age 40 and be repeated every 1 to 2 years.2 This was consistent with or endorsed by most other national breast cancer screening guidelines, including the American College of Obstetricians and Gynecologists (ACOG), National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology.
In 2009, the USPSTF changed this Grade B recommendation, instead recommending biennial screening mammography for women aged 50 to 74.3 The most significant change in the revised guideline was for patients aged 40 to 49, where the recommendation was “against routine screening mammography.” They went on to say that the decision to start “biennial screening mammography before the age of 50 years should be an individual one and take patient context into account, including the patient’s values regarding specific benefits and harms.” Other prominent national guideline groups (ACOG, NCCN, ACS) did not agree with this recommendation and maintained that patients aged 40 to 49 should continue to be offered routine screening mammography either annually (NCCN, ACS) or at 1-to-2-year intervals (ACOG).4-6 The American College of Physicians and the American Academy of Family Practice endorsed the 2016 USPSTF guidelines, creating a disparity in breast cancer mammography counseling for averagerisk patients in their 40s.7
In 2016, the USPSTF revisited their breast cancer screening recommendation and renewed their 2009 recommendation against routine screening in patients aged 40 to 49, with the American College of Physicians and the American Academy of Family Practice again endorsing these guidelines.8 ACOG, ACS, NCCN, and ACR continued to recommend age 40 as a starting age for routine mammography screening (TABLE 2). As a result, over the past 14 years, patients aged 40 to 49 were placed in an awkward position of potentially hearing different recommendations from their health care providers, those differences often depending on the specialty of the provider they were seeing.

In 2023. On May 9, the USPSTF released a draft of their latest recommendation statement stating that all patients at average risk for breast cancer should get screened every other year beginning at age 40, bringing most of the national guideline groups into alignment with regard to age to start mammographic screening.9
- With an estimated more than 300,000 new cases in 2023, breast cancer has the highest incidence rate of any cancer in the United States
- The median age of patients with breast cancer in the United States is 58.0 years
- 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49
- Despite lower incidence rates among Black vs White patients, Black patients have higher death rates from breast cancer
Why the change?
To answer this question, we need to examine the relevant epidemiology of breast cancer.
Continue to: Incidence...
Incidence
It is estimated that, in the United States in 2023, there will be 300,590 new cases of breast cancer, resulting in 43,700 deaths.10 From 2015–2019, there were 128.1 new breast cancer cases/100,000 population, which is the highest rate of cancer in the United States, regardless of sex.11 Diagnoses among patients aged 40 to 49 are rising at a faster rate than previously, about 2% per year between 2015 and 2019.
Racial and ethnic differences
In addition to the racial and ethnic epidemiologic differences in breast cancer, there are also disparities in breast cancer care and outcomes that need to be considered when making national guidelines/policy recommendations.
Black women have high mortality rates from breast cancer. While non-Hispanic White patients have the highest rates of breast cancer (TABLE 3), non-Hispanic Black patients have the highest rates of death due to breast cancer.10 There appear to be several reasons for the estimated 40%-higher rate of mortality among Black women, including:
- systemic racism in primary research, guidelines, and policy
- inequities in diagnostic follow-up and access to evidence-based cancer treatments
- biologic differences in breast cancer (ie, the incidence of triple-negative breast cancer (TNBC) is 2-fold higher in Black women compared with the other racial and ethnic groups in the United States).12-14

While prior studies have suggested that screening mammography might be less effective for patients with TNBC, a recent study demonstrated that patients who had mammography–screened-detected TNBC tumors were smaller and more likely to be node- negative compared with non-screened patients with TNBC.(14) Patients with screened-detected TNBCs were also more likely to undergo a lumpectomy instead of a mastectomy compared with non–screened detected TNBC (68.3% vs 46.1%; P = .002) (TABLE 4). These data strongly suggest that screening mammography is indeed effective in detecting TNBC at earlier stages, one of the best proxies for breast cancer mortality.

Non-White patients have higher incidence rates of breast cancer in their 40s. A second factor to consider in racial differences is the relatively higher incidence of breast cancer in Hispanic, Black, and Asian patients in their 40s compared with non-Hispanic White patients. In a recent analysis of data from 1973 to 2010 from the Surveillance, Epidemiology, and End Results (SEER) Program, the median age of patients with breast cancer in the United States was 58.0 years (interquartile range [IQR], 50.0–67.0 years).16 Across all US demographic populations by age at diagnosis, more than 20% of patients will have their initial diagnosis of breast cancer under the age of 50, and 1.55% (1 in 65) patients between ages 40 and 49 years will be diagnosed with breast cancer.4 However, among patients aged 50 and younger diagnosed with breast cancer, a significantly higher proportion are Black (31%), Hispanic (34.9%), or Asian (32.8%) versus White (23.1%) (P < .001 for all).16 So, for there to be similar racial and ethnic mammography capture rates with White patients, starting mammography screening ages would need to be lower for Black (age 47 years), Hispanic (and 46 years), and Asian (age 47 years) patients. Data from this study of the SEER database16 also demonstrated that more Black and Hispanic patients at age of diagnosis were diagnosed with advanced (regional or distant) breast cancer (46.6% and 42.9%, respectively) versus White or Asian patients (37.1% and 35.6%, respectively; P < .001 for all).
These findings led the authors of the study to conclude that the “Current [2016] USPSTF breast cancer screening recommendations do not reflect age-specific patterns based on race.” The USPSTF stated that this is one of the reasons why they reconsidered their stance on screening , and now recommend screening for all patients starting at age 40.
My current counseling approach
I encourage all racial and ethnic patients between the ages of 40 and 49 to undergo screening mammography because of the associated relative risk mortality reduction rates, which range from 15% to 50%. I also share that with my patients that, because of the younger average age of onset of breast cancer in Black, Hispanic, and Asian patients, they may derive additional benefit from screening starting at age 40.4
Impact of draft guidelines on breast cancer screening and mortality in younger patients
There is clear, unequivocal, and repeatable Level 1 evidence that screening mammography in the general population of patients aged 40 to 49 reduces breast cancer mortality. Breast cancer is the leading cause of cancer in the United States, the second leading cause of cancer mortality in patients, and 1 in 5 new breast cancer diagnoses occur in patients between the ages of 40 and 49. While recent efforts have been made to come to consensus on a screening starting age of 40 for patients at average risk for breast cancer, the USPSTF appeared to be an outlier with their 2016 recommendation to routinely start mammography screening at age 50 instead of 40.17
The USPSTF is a very important national voice in cancer prevention, and their 2023 (draft) revised guidelines to age 40 as the recommended starting screening age now agrees with the leading US guideline groups listed in Table 2. These guideline groups have gone through varying processes, and now have finally arrived at the same conclusion for age to start screening mammography in women of average risk. This agreement should come as a significant comfort to health care providers and patients alike. Changing the starting age to 40 years will result in thousands of lives and hundreds of thousands of life-years saved for patients aged 40 to 49. ●
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
- US Preventive Services Task Force website. Task Force at a glance. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce.org /uspstf/about-uspstf/task-force-at-a-glance
- Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137(5_Part_1):347-360.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
- Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- American College of Obstetricans and Gynecologists. ACOG Practice Bulletin number 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1e16. doi: 10.1097/AOG. 0000000000002158.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Qaseem A, Lin JS, Mustafa RA, et al. Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170: 547-560.
- Siu AL, US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- US Preventive Services Task Force. Draft Recommendation Statement Breast Cancer: Screening. May 9, 2023. Accessed October 25, 2023. https://www.uspreventiveservicestaskforce .org/uspstf/draft-recommendation/breast -cancer-screening-adults#bcei-recommendation -title-area
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: Cancer J Clin. 2023;73:17-48.
- American Cancer Society. Cancer Statistics Center: Breast. 2023. Accessed October 25, 2023. https ://cancerstatisticscenter.cancer.org/#!/cancer-site /Breast
- Bailey ZD, Krieger N, Agénor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Collin LJ, Gaglioti AH, Beyer KM, et al. Neighborhood-level redlining and lending bias are associated with breast cancer mortality in a large and diverse metropolitan area. Cancer Epidemiol, Biomarkers Prev. 2021;30:53-60.
- Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg. 2022;275:776-783.
- Chen Y, Susick L, Davis M, et al. Evaluation of triple-negative breast cancer early detection via mammography screening and outcomes in African American and White American patients. JAMA Surg. 2020;155:440-442.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Chelmow D, Pearlman MD, Young A, et al. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet Gynecol. 2020;135:1457-1478.
Does tamoxifen use increase the risk of endometrial cancer in premenopausal patients?
Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951.
EXPERT COMMENTARY
Tamoxifen is a selective estrogen receptor modulator (SERM) approved by the US Food and Drug Administration (FDA) for both adjuvant treatment of invasive or metastatic breast cancer with hormone receptor (HR)–positive tumors (duration, 5 to 10 years) and for reduction of future breast cancers in certain high-risk individuals (duration, 5 years). It is also occasionally used for non-FDA approved indications, such as cyclic mastodynia.
Because breast cancer is among the most frequently diagnosed cancers in the United States (297,790 new cases expected in 2023) and approximately 80% are HR-positive tumors that will require hormonal adjuvant therapy,1 physicians and other gynecologic clinicians should have a working understanding of tamoxifen, including the risks and benefits associated with its use. Among the recognized serious adverse effects of tamoxifen is the increased risk of endometrial cancer in menopausal patients. This adverse effect creates a potential conundrum for clinicians who may be managing patients with tamoxifen to treat or prevent breast cancer, while also increasing the risk of another cancer. Prior prospective studies of tamoxifen have demonstrated a statistically and clinically significant increased risk of endometrial cancer in menopausal patients but not in premenopausal patients.
A recent study challenged those previous findings, suggesting that the risk of endometrial cancer is similar in both premenopausal and postmenopausal patients taking tamoxifen for treatment of breast cancer.2
Details of the study
The study by Ryu and colleagues used data from the Korean National Health Insurance Service, which covers 97% of the Korean population.2 The authors selected patients being treated for invasive breast cancer from January 1, 2003, through December 31, 2018, who were between the ages of 20 and 50 years when the breast cancer diagnosis was first made. Patients with a diagnostic code entered into their electronic health record that was consistent with menopausal status were excluded, along with any patients with a current or prior history of aromatase inhibitor use (for which one must be naturally, medically, or surgically menopausal to use). Based on these exclusions, the study cohort was then assumed to be premenopausal.
The study group included patients diagnosed with invasive breast cancer who were treated with adjuvant hormonal therapy with tamoxifen (n = 34,637), and the control group included patients with invasive breast cancer who were not treated with adjuvant hormonal therapy (n = 43,683). The primary study end point was the finding of endometrial or uterine pathology, including endometrial polyps, endometrial hyperplasia, endometrial cancer, and other uterine malignant neoplasms not originating in the endometrium (for example, uterine sarcomas).
Because this was a retrospective cohort study that included all eligible patients, the 2 groups were not matched. The treatment group was statistically older, had a higher body mass index (BMI) and a larger waist circumference, were more likely to be hypertensive, and included more patients with diabetes than the control group—all known risk factors for endometrial cancer. However, after adjusting for these 4 factors, an increased risk of endometrial cancer remained in the tamoxifen group compared with the control group (hazard ratio [HR], 3.77; 95% confidence interval [CI], 3.04–4.66). In addition, tamoxifen use was independently associated with an increased risk of endometrial polyps (HR, 3.90; 95% CI, 3.65–4.16), endometrial hyperplasia (HR, 5.56; 95% CI, 5.06–6.12), and other uterine cancers (HR, 2.27; 95% CI, 1.54–3.33). In a subgroup analysis, the risk for endometrial cancer was not higher in patients treated for more than 5 years of tamoxifen compared with those treated for 5 years or less.
Study strengths and limitations
A major strength of this study was the large number of study participants (n = 34,637 tamoxifen; n = 43,683 control), the long duration of follow-up (up to 15 years), and use of a single source of data with coverage of nearly the entire population of Korea. While the 2 study populations (tamoxifen vs no tamoxifen) were initially unbalanced in terms of endometrial cancer risk (age, BMI, concurrent diagnoses of hypertension and diabetes), the authors corrected for this with a multivariate analysis.
Furthermore, while the likely homogeneity of the study population may not make the results generalizable, the authors noted that Korean patients have a higher tendency toward early-onset breast cancer. This observation could make this cohort better suited for a study on premenopausal effects of tamoxifen.
Limitations. These data are provocative as they conflict with level 1 evidence based on multiple well-designed, double-blind, placebo-controlled randomized trials in which tamoxifen use for 5 years did not demonstrate a statistically increased risk of endometrial cancer in patients younger than age 50.3-5 Because of the importance of the question and the implications for many premenopausal women being treated with tamoxifen, we carefully evaluated the study methodology to better understand this discrepancy.
Continue to: Methodological concerns...
Methodological concerns
In the study by Ryu and colleagues, we found the definition of premenopausal to be problematic. Ultimately, if patients did not have a diagnosis of menopause in the problem summary list, they were assumed to be premenopausal if they were between the ages of 20 and 50 and not taking an aromatase inhibitor. However, important considerations in this population include the cancer stage and treatment regimens that can and do directly impact menopausal status.
Data demonstrate that early-onset breast cancer tends to be associated with more biologically aggressive characteristics that frequently require adjuvant or neoadjuvant chemotherapy.6,7 This chemotherapy regimen is comprised most commonly of Adriamycin (doxorubicin), paclitaxel, and cyclophosphamide. Cyclophosphamide is an alkylating agent that is a known gonadotoxin, and it often renders patients either temporarily or permanently menopausal due to chemotherapy-induced ovarian failure. Prior studies have demonstrated that for patients in their 40s, approximately 90% of those treated with cyclophosphamide-containing chemo-therapy for breast cancer will experience chemotherapy-induced amenorrhea (CIA).8 Although some patients in their 40s with CIA will resume ovarian function, the majority will not.8,9
Due to the lack of reliability in diagnosing CIA, blood levels of estradiol and follicle stimulating hormone are often necessary for confirmation and, even so, may be only temporary. One prospective analysis of 4 randomized neoadjuvant/adjuvant breast cancer trials used this approach and demonstrated that 85.1% of the study cohort experienced chemotherapy-induced ovarian failure at the end of their treatment, with some fluctuating back to premenopausal hormonal levels at 6 and 12 months.10
Furthermore, in the study by Ryu and colleagues, there is no description or confirmation of menstrual patterns in the study group to support the diagnosis of ongoing premenopausal status. Data on CIA and loss of ovarian function, therefore, are critical to the accurate categorization of patients as premenopausal or menopausal in this study. The study also relied on consistent and accurate recording of appropriate medical codes to capture a patient’s menopausal status, which is unclear for this particular population and health system.
In evaluating prior research, multiple studies demonstrated no increased risk of endometrial cancer in premenopausal women taking tamoxifen for breast cancer prevention (TABLE).3,5 These breast cancer prevention trials have several major advantages in assessing tamoxifen-associated endometrial cancer risk for premenopausal patients compared with the current study:
- Both studies were prospective double-blind, placebo-controlled randomized clinical breast cancer prevention trials with carefully designed and measured outcomes.
- Since these were breast cancer prevention trials, administration of gonadotoxic chemotherapy was not a concern. As a result, miscategorizing patients with chemotherapy-induced menopause as premenopausal would not be expected, and premature menopause would not be expected at a higher rate than the general population.
- Careful histories were required prior to study entry and throughout the study, including data on menopausal status and menstrual and uterine bleeding histories.11
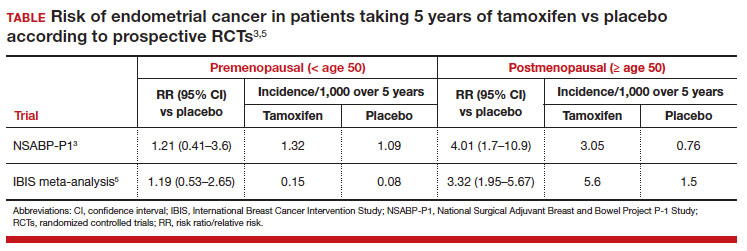
In these prevention trials, the effect of tamoxifen on uterine pathology demonstratedrepeatable evidence that there was a statistically significant increased risk of endometrial cancer in postmenopausal women, but there was no similar increased risk of endometrial cancer in premenopausal women (TABLE).3,5 Interestingly, the magnitude of the endometrial cancer risk found in the premenopausal patients in the study by Ryu and colleagues (RR, 3.77) is comparable to that of the menopausal group in the prevention trials, raising concern that many or most of the patients in the treatment group assumed to be premenopausal may have indeed been “menopausal” for some or all the time they were taking tamoxifen due to the possible aforementioned reasons. ●
While the data from the study by Ryu and colleagues are provocative, the findings that premenopausal women are at an increased risk of endometrial cancer do not agree with those of well-designed previous trials. Our concerns about categorization bias (that is, women in the treatment group may have been menopausal for some or all the time they were taking tamoxifen but were not formally diagnosed) make the conclusion that endometrial cancer risk is increased in truly premenopausal women somewhat specious. In a Committee Opinion (last endorsed in 2020), the American College of Obstetricians and Gynecologists (ACOG) stated the following: “Postmenopausal women taking tamoxifen should be closely monitored for symptoms of endometrial hyperplasia or cancer. Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer and as such require no additional monitoring beyond routine gynecologic care.”12 Based on multiple previously published studies with solid level 1 evidence and the challenges with the current study design, we continue to agree with this ACOG statement.
VERSHA PLEASANT, MD, MPH; MARK D. PEARLMAN, MD
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48.
- Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951-e.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
- Iqbal J, Ginsburg OM, Wijeratne TD, et al. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38:318-328.
- Kumar R, Abreu C, Toi M, et al. Oncobiology and treatment of breast cancer in young women. Cancer Metastasis Rev. 2022;41:749-770.
- Tesch ME, Partidge AH. Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book. 2022;42:1-12.
- Han HS, Ro J, Lee KS, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115:335-342.
- Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011-2016.
- Furlanetto J, Marme F, Seiler S, et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: prospective analysis of four randomised neoadjuvant/ adjuvant breast cancer trials. Eur J Cancer. 2021;152: 193-203.
- Runowicz CD, Costantino JP, Wickerham DL, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205:535.e1-535.e5.
- American College of Obstetricians and Gynecologists. Committee opinion no. 601: tamoxifen and uterine cancer. Obstet Gynecol. 2014;123:1394-1397.
Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951.
EXPERT COMMENTARY
Tamoxifen is a selective estrogen receptor modulator (SERM) approved by the US Food and Drug Administration (FDA) for both adjuvant treatment of invasive or metastatic breast cancer with hormone receptor (HR)–positive tumors (duration, 5 to 10 years) and for reduction of future breast cancers in certain high-risk individuals (duration, 5 years). It is also occasionally used for non-FDA approved indications, such as cyclic mastodynia.
Because breast cancer is among the most frequently diagnosed cancers in the United States (297,790 new cases expected in 2023) and approximately 80% are HR-positive tumors that will require hormonal adjuvant therapy,1 physicians and other gynecologic clinicians should have a working understanding of tamoxifen, including the risks and benefits associated with its use. Among the recognized serious adverse effects of tamoxifen is the increased risk of endometrial cancer in menopausal patients. This adverse effect creates a potential conundrum for clinicians who may be managing patients with tamoxifen to treat or prevent breast cancer, while also increasing the risk of another cancer. Prior prospective studies of tamoxifen have demonstrated a statistically and clinically significant increased risk of endometrial cancer in menopausal patients but not in premenopausal patients.
A recent study challenged those previous findings, suggesting that the risk of endometrial cancer is similar in both premenopausal and postmenopausal patients taking tamoxifen for treatment of breast cancer.2
Details of the study
The study by Ryu and colleagues used data from the Korean National Health Insurance Service, which covers 97% of the Korean population.2 The authors selected patients being treated for invasive breast cancer from January 1, 2003, through December 31, 2018, who were between the ages of 20 and 50 years when the breast cancer diagnosis was first made. Patients with a diagnostic code entered into their electronic health record that was consistent with menopausal status were excluded, along with any patients with a current or prior history of aromatase inhibitor use (for which one must be naturally, medically, or surgically menopausal to use). Based on these exclusions, the study cohort was then assumed to be premenopausal.
The study group included patients diagnosed with invasive breast cancer who were treated with adjuvant hormonal therapy with tamoxifen (n = 34,637), and the control group included patients with invasive breast cancer who were not treated with adjuvant hormonal therapy (n = 43,683). The primary study end point was the finding of endometrial or uterine pathology, including endometrial polyps, endometrial hyperplasia, endometrial cancer, and other uterine malignant neoplasms not originating in the endometrium (for example, uterine sarcomas).
Because this was a retrospective cohort study that included all eligible patients, the 2 groups were not matched. The treatment group was statistically older, had a higher body mass index (BMI) and a larger waist circumference, were more likely to be hypertensive, and included more patients with diabetes than the control group—all known risk factors for endometrial cancer. However, after adjusting for these 4 factors, an increased risk of endometrial cancer remained in the tamoxifen group compared with the control group (hazard ratio [HR], 3.77; 95% confidence interval [CI], 3.04–4.66). In addition, tamoxifen use was independently associated with an increased risk of endometrial polyps (HR, 3.90; 95% CI, 3.65–4.16), endometrial hyperplasia (HR, 5.56; 95% CI, 5.06–6.12), and other uterine cancers (HR, 2.27; 95% CI, 1.54–3.33). In a subgroup analysis, the risk for endometrial cancer was not higher in patients treated for more than 5 years of tamoxifen compared with those treated for 5 years or less.
Study strengths and limitations
A major strength of this study was the large number of study participants (n = 34,637 tamoxifen; n = 43,683 control), the long duration of follow-up (up to 15 years), and use of a single source of data with coverage of nearly the entire population of Korea. While the 2 study populations (tamoxifen vs no tamoxifen) were initially unbalanced in terms of endometrial cancer risk (age, BMI, concurrent diagnoses of hypertension and diabetes), the authors corrected for this with a multivariate analysis.
Furthermore, while the likely homogeneity of the study population may not make the results generalizable, the authors noted that Korean patients have a higher tendency toward early-onset breast cancer. This observation could make this cohort better suited for a study on premenopausal effects of tamoxifen.
Limitations. These data are provocative as they conflict with level 1 evidence based on multiple well-designed, double-blind, placebo-controlled randomized trials in which tamoxifen use for 5 years did not demonstrate a statistically increased risk of endometrial cancer in patients younger than age 50.3-5 Because of the importance of the question and the implications for many premenopausal women being treated with tamoxifen, we carefully evaluated the study methodology to better understand this discrepancy.
Continue to: Methodological concerns...
Methodological concerns
In the study by Ryu and colleagues, we found the definition of premenopausal to be problematic. Ultimately, if patients did not have a diagnosis of menopause in the problem summary list, they were assumed to be premenopausal if they were between the ages of 20 and 50 and not taking an aromatase inhibitor. However, important considerations in this population include the cancer stage and treatment regimens that can and do directly impact menopausal status.
Data demonstrate that early-onset breast cancer tends to be associated with more biologically aggressive characteristics that frequently require adjuvant or neoadjuvant chemotherapy.6,7 This chemotherapy regimen is comprised most commonly of Adriamycin (doxorubicin), paclitaxel, and cyclophosphamide. Cyclophosphamide is an alkylating agent that is a known gonadotoxin, and it often renders patients either temporarily or permanently menopausal due to chemotherapy-induced ovarian failure. Prior studies have demonstrated that for patients in their 40s, approximately 90% of those treated with cyclophosphamide-containing chemo-therapy for breast cancer will experience chemotherapy-induced amenorrhea (CIA).8 Although some patients in their 40s with CIA will resume ovarian function, the majority will not.8,9
Due to the lack of reliability in diagnosing CIA, blood levels of estradiol and follicle stimulating hormone are often necessary for confirmation and, even so, may be only temporary. One prospective analysis of 4 randomized neoadjuvant/adjuvant breast cancer trials used this approach and demonstrated that 85.1% of the study cohort experienced chemotherapy-induced ovarian failure at the end of their treatment, with some fluctuating back to premenopausal hormonal levels at 6 and 12 months.10
Furthermore, in the study by Ryu and colleagues, there is no description or confirmation of menstrual patterns in the study group to support the diagnosis of ongoing premenopausal status. Data on CIA and loss of ovarian function, therefore, are critical to the accurate categorization of patients as premenopausal or menopausal in this study. The study also relied on consistent and accurate recording of appropriate medical codes to capture a patient’s menopausal status, which is unclear for this particular population and health system.
In evaluating prior research, multiple studies demonstrated no increased risk of endometrial cancer in premenopausal women taking tamoxifen for breast cancer prevention (TABLE).3,5 These breast cancer prevention trials have several major advantages in assessing tamoxifen-associated endometrial cancer risk for premenopausal patients compared with the current study:
- Both studies were prospective double-blind, placebo-controlled randomized clinical breast cancer prevention trials with carefully designed and measured outcomes.
- Since these were breast cancer prevention trials, administration of gonadotoxic chemotherapy was not a concern. As a result, miscategorizing patients with chemotherapy-induced menopause as premenopausal would not be expected, and premature menopause would not be expected at a higher rate than the general population.
- Careful histories were required prior to study entry and throughout the study, including data on menopausal status and menstrual and uterine bleeding histories.11

In these prevention trials, the effect of tamoxifen on uterine pathology demonstratedrepeatable evidence that there was a statistically significant increased risk of endometrial cancer in postmenopausal women, but there was no similar increased risk of endometrial cancer in premenopausal women (TABLE).3,5 Interestingly, the magnitude of the endometrial cancer risk found in the premenopausal patients in the study by Ryu and colleagues (RR, 3.77) is comparable to that of the menopausal group in the prevention trials, raising concern that many or most of the patients in the treatment group assumed to be premenopausal may have indeed been “menopausal” for some or all the time they were taking tamoxifen due to the possible aforementioned reasons. ●
While the data from the study by Ryu and colleagues are provocative, the findings that premenopausal women are at an increased risk of endometrial cancer do not agree with those of well-designed previous trials. Our concerns about categorization bias (that is, women in the treatment group may have been menopausal for some or all the time they were taking tamoxifen but were not formally diagnosed) make the conclusion that endometrial cancer risk is increased in truly premenopausal women somewhat specious. In a Committee Opinion (last endorsed in 2020), the American College of Obstetricians and Gynecologists (ACOG) stated the following: “Postmenopausal women taking tamoxifen should be closely monitored for symptoms of endometrial hyperplasia or cancer. Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer and as such require no additional monitoring beyond routine gynecologic care.”12 Based on multiple previously published studies with solid level 1 evidence and the challenges with the current study design, we continue to agree with this ACOG statement.
VERSHA PLEASANT, MD, MPH; MARK D. PEARLMAN, MD
Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951.
EXPERT COMMENTARY
Tamoxifen is a selective estrogen receptor modulator (SERM) approved by the US Food and Drug Administration (FDA) for both adjuvant treatment of invasive or metastatic breast cancer with hormone receptor (HR)–positive tumors (duration, 5 to 10 years) and for reduction of future breast cancers in certain high-risk individuals (duration, 5 years). It is also occasionally used for non-FDA approved indications, such as cyclic mastodynia.
Because breast cancer is among the most frequently diagnosed cancers in the United States (297,790 new cases expected in 2023) and approximately 80% are HR-positive tumors that will require hormonal adjuvant therapy,1 physicians and other gynecologic clinicians should have a working understanding of tamoxifen, including the risks and benefits associated with its use. Among the recognized serious adverse effects of tamoxifen is the increased risk of endometrial cancer in menopausal patients. This adverse effect creates a potential conundrum for clinicians who may be managing patients with tamoxifen to treat or prevent breast cancer, while also increasing the risk of another cancer. Prior prospective studies of tamoxifen have demonstrated a statistically and clinically significant increased risk of endometrial cancer in menopausal patients but not in premenopausal patients.
A recent study challenged those previous findings, suggesting that the risk of endometrial cancer is similar in both premenopausal and postmenopausal patients taking tamoxifen for treatment of breast cancer.2
Details of the study
The study by Ryu and colleagues used data from the Korean National Health Insurance Service, which covers 97% of the Korean population.2 The authors selected patients being treated for invasive breast cancer from January 1, 2003, through December 31, 2018, who were between the ages of 20 and 50 years when the breast cancer diagnosis was first made. Patients with a diagnostic code entered into their electronic health record that was consistent with menopausal status were excluded, along with any patients with a current or prior history of aromatase inhibitor use (for which one must be naturally, medically, or surgically menopausal to use). Based on these exclusions, the study cohort was then assumed to be premenopausal.
The study group included patients diagnosed with invasive breast cancer who were treated with adjuvant hormonal therapy with tamoxifen (n = 34,637), and the control group included patients with invasive breast cancer who were not treated with adjuvant hormonal therapy (n = 43,683). The primary study end point was the finding of endometrial or uterine pathology, including endometrial polyps, endometrial hyperplasia, endometrial cancer, and other uterine malignant neoplasms not originating in the endometrium (for example, uterine sarcomas).
Because this was a retrospective cohort study that included all eligible patients, the 2 groups were not matched. The treatment group was statistically older, had a higher body mass index (BMI) and a larger waist circumference, were more likely to be hypertensive, and included more patients with diabetes than the control group—all known risk factors for endometrial cancer. However, after adjusting for these 4 factors, an increased risk of endometrial cancer remained in the tamoxifen group compared with the control group (hazard ratio [HR], 3.77; 95% confidence interval [CI], 3.04–4.66). In addition, tamoxifen use was independently associated with an increased risk of endometrial polyps (HR, 3.90; 95% CI, 3.65–4.16), endometrial hyperplasia (HR, 5.56; 95% CI, 5.06–6.12), and other uterine cancers (HR, 2.27; 95% CI, 1.54–3.33). In a subgroup analysis, the risk for endometrial cancer was not higher in patients treated for more than 5 years of tamoxifen compared with those treated for 5 years or less.
Study strengths and limitations
A major strength of this study was the large number of study participants (n = 34,637 tamoxifen; n = 43,683 control), the long duration of follow-up (up to 15 years), and use of a single source of data with coverage of nearly the entire population of Korea. While the 2 study populations (tamoxifen vs no tamoxifen) were initially unbalanced in terms of endometrial cancer risk (age, BMI, concurrent diagnoses of hypertension and diabetes), the authors corrected for this with a multivariate analysis.
Furthermore, while the likely homogeneity of the study population may not make the results generalizable, the authors noted that Korean patients have a higher tendency toward early-onset breast cancer. This observation could make this cohort better suited for a study on premenopausal effects of tamoxifen.
Limitations. These data are provocative as they conflict with level 1 evidence based on multiple well-designed, double-blind, placebo-controlled randomized trials in which tamoxifen use for 5 years did not demonstrate a statistically increased risk of endometrial cancer in patients younger than age 50.3-5 Because of the importance of the question and the implications for many premenopausal women being treated with tamoxifen, we carefully evaluated the study methodology to better understand this discrepancy.
Continue to: Methodological concerns...
Methodological concerns
In the study by Ryu and colleagues, we found the definition of premenopausal to be problematic. Ultimately, if patients did not have a diagnosis of menopause in the problem summary list, they were assumed to be premenopausal if they were between the ages of 20 and 50 and not taking an aromatase inhibitor. However, important considerations in this population include the cancer stage and treatment regimens that can and do directly impact menopausal status.
Data demonstrate that early-onset breast cancer tends to be associated with more biologically aggressive characteristics that frequently require adjuvant or neoadjuvant chemotherapy.6,7 This chemotherapy regimen is comprised most commonly of Adriamycin (doxorubicin), paclitaxel, and cyclophosphamide. Cyclophosphamide is an alkylating agent that is a known gonadotoxin, and it often renders patients either temporarily or permanently menopausal due to chemotherapy-induced ovarian failure. Prior studies have demonstrated that for patients in their 40s, approximately 90% of those treated with cyclophosphamide-containing chemo-therapy for breast cancer will experience chemotherapy-induced amenorrhea (CIA).8 Although some patients in their 40s with CIA will resume ovarian function, the majority will not.8,9
Due to the lack of reliability in diagnosing CIA, blood levels of estradiol and follicle stimulating hormone are often necessary for confirmation and, even so, may be only temporary. One prospective analysis of 4 randomized neoadjuvant/adjuvant breast cancer trials used this approach and demonstrated that 85.1% of the study cohort experienced chemotherapy-induced ovarian failure at the end of their treatment, with some fluctuating back to premenopausal hormonal levels at 6 and 12 months.10
Furthermore, in the study by Ryu and colleagues, there is no description or confirmation of menstrual patterns in the study group to support the diagnosis of ongoing premenopausal status. Data on CIA and loss of ovarian function, therefore, are critical to the accurate categorization of patients as premenopausal or menopausal in this study. The study also relied on consistent and accurate recording of appropriate medical codes to capture a patient’s menopausal status, which is unclear for this particular population and health system.
In evaluating prior research, multiple studies demonstrated no increased risk of endometrial cancer in premenopausal women taking tamoxifen for breast cancer prevention (TABLE).3,5 These breast cancer prevention trials have several major advantages in assessing tamoxifen-associated endometrial cancer risk for premenopausal patients compared with the current study:
- Both studies were prospective double-blind, placebo-controlled randomized clinical breast cancer prevention trials with carefully designed and measured outcomes.
- Since these were breast cancer prevention trials, administration of gonadotoxic chemotherapy was not a concern. As a result, miscategorizing patients with chemotherapy-induced menopause as premenopausal would not be expected, and premature menopause would not be expected at a higher rate than the general population.
- Careful histories were required prior to study entry and throughout the study, including data on menopausal status and menstrual and uterine bleeding histories.11

In these prevention trials, the effect of tamoxifen on uterine pathology demonstratedrepeatable evidence that there was a statistically significant increased risk of endometrial cancer in postmenopausal women, but there was no similar increased risk of endometrial cancer in premenopausal women (TABLE).3,5 Interestingly, the magnitude of the endometrial cancer risk found in the premenopausal patients in the study by Ryu and colleagues (RR, 3.77) is comparable to that of the menopausal group in the prevention trials, raising concern that many or most of the patients in the treatment group assumed to be premenopausal may have indeed been “menopausal” for some or all the time they were taking tamoxifen due to the possible aforementioned reasons. ●
While the data from the study by Ryu and colleagues are provocative, the findings that premenopausal women are at an increased risk of endometrial cancer do not agree with those of well-designed previous trials. Our concerns about categorization bias (that is, women in the treatment group may have been menopausal for some or all the time they were taking tamoxifen but were not formally diagnosed) make the conclusion that endometrial cancer risk is increased in truly premenopausal women somewhat specious. In a Committee Opinion (last endorsed in 2020), the American College of Obstetricians and Gynecologists (ACOG) stated the following: “Postmenopausal women taking tamoxifen should be closely monitored for symptoms of endometrial hyperplasia or cancer. Premenopausal women treated with tamoxifen have no known increased risk of uterine cancer and as such require no additional monitoring beyond routine gynecologic care.”12 Based on multiple previously published studies with solid level 1 evidence and the challenges with the current study design, we continue to agree with this ACOG statement.
VERSHA PLEASANT, MD, MPH; MARK D. PEARLMAN, MD
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48.
- Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951-e.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
- Iqbal J, Ginsburg OM, Wijeratne TD, et al. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38:318-328.
- Kumar R, Abreu C, Toi M, et al. Oncobiology and treatment of breast cancer in young women. Cancer Metastasis Rev. 2022;41:749-770.
- Tesch ME, Partidge AH. Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book. 2022;42:1-12.
- Han HS, Ro J, Lee KS, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115:335-342.
- Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011-2016.
- Furlanetto J, Marme F, Seiler S, et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: prospective analysis of four randomised neoadjuvant/ adjuvant breast cancer trials. Eur J Cancer. 2021;152: 193-203.
- Runowicz CD, Costantino JP, Wickerham DL, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205:535.e1-535.e5.
- American College of Obstetricians and Gynecologists. Committee opinion no. 601: tamoxifen and uterine cancer. Obstet Gynecol. 2014;123:1394-1397.
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48.
- Ryu KJ, Kim MS, Lee JY, et al. Risk of endometrial polyps, hyperplasia, carcinoma, and uterine cancer after tamoxifen treatment in premenopausal women with breast cancer. JAMA Netw Open. 2022;5:e2243951-e.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
- Iqbal J, Ginsburg OM, Wijeratne TD, et al. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38:318-328.
- Kumar R, Abreu C, Toi M, et al. Oncobiology and treatment of breast cancer in young women. Cancer Metastasis Rev. 2022;41:749-770.
- Tesch ME, Partidge AH. Treatment of breast cancer in young adults. Am Soc Clin Oncol Educ Book. 2022;42:1-12.
- Han HS, Ro J, Lee KS, et al. Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat. 2009;115:335-342.
- Henry NL, Xia R, Banerjee M, et al. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24:2011-2016.
- Furlanetto J, Marme F, Seiler S, et al. Chemotherapy-induced ovarian failure in young women with early breast cancer: prospective analysis of four randomised neoadjuvant/ adjuvant breast cancer trials. Eur J Cancer. 2021;152: 193-203.
- Runowicz CD, Costantino JP, Wickerham DL, et al. Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR). Am J Obstet Gynecol. 2011;205:535.e1-535.e5.
- American College of Obstetricians and Gynecologists. Committee opinion no. 601: tamoxifen and uterine cancer. Obstet Gynecol. 2014;123:1394-1397.
Progress in breast cancer screening over the past 50 years: A remarkable story, but still work to do
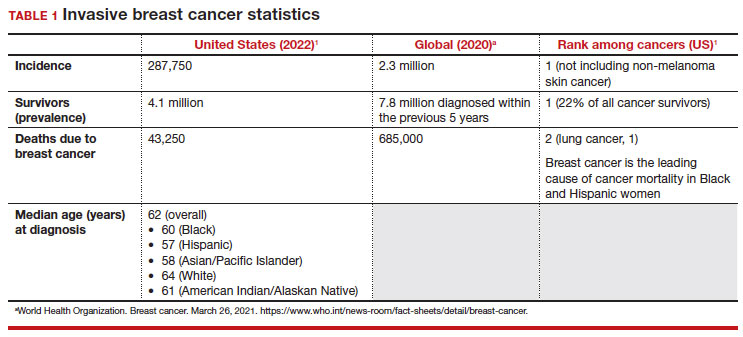
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3
While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.
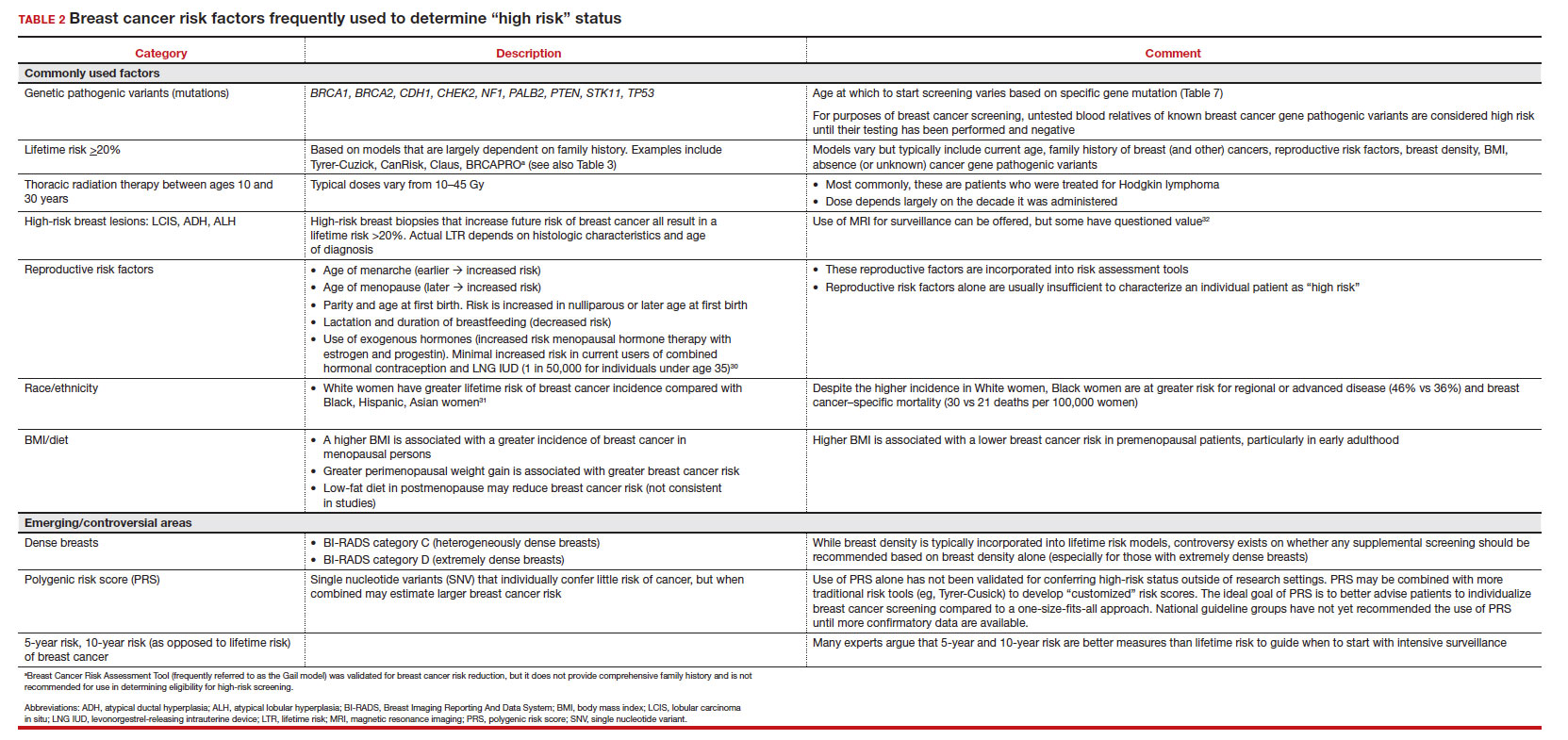
Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7
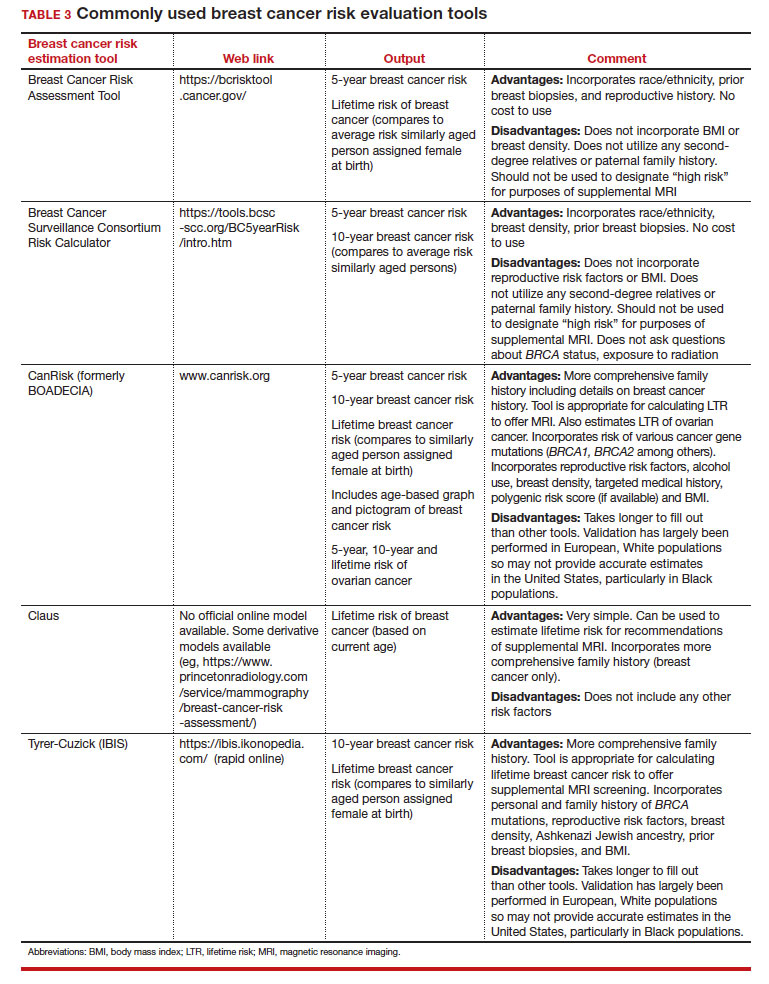
Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10
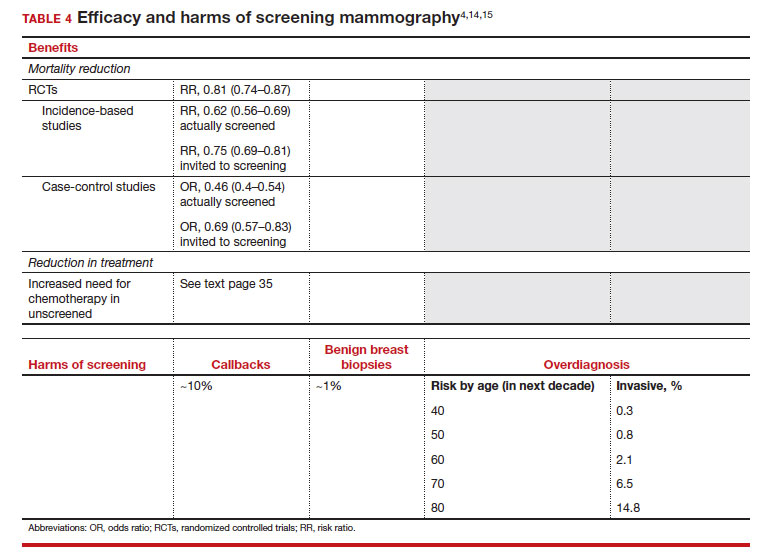
With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).
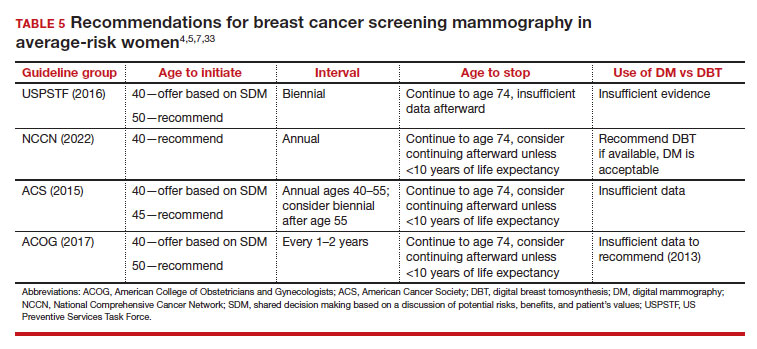
Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.
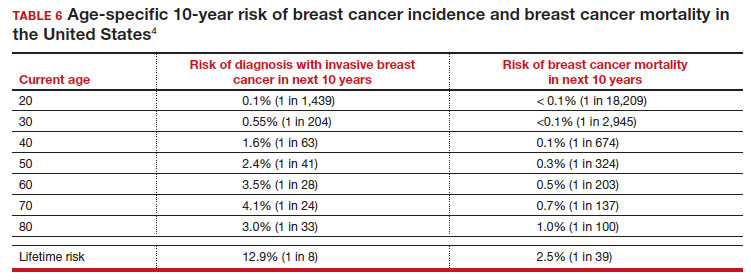
For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.
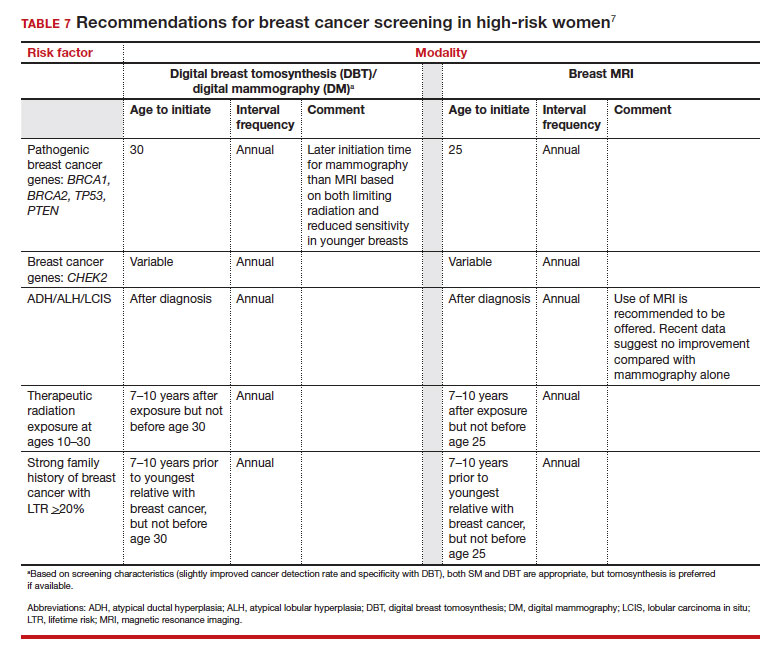
Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).
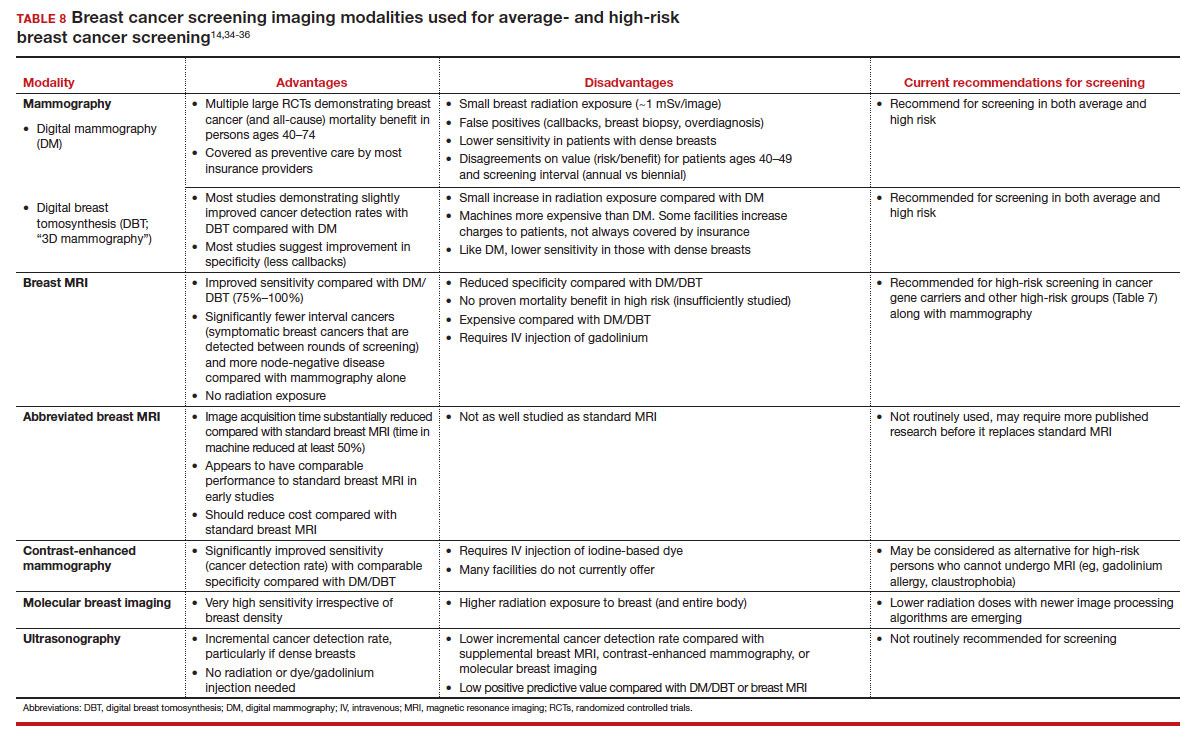
Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
Meaningful progress has been made in reducing deaths due to breast cancer over the last half century, with a 43% decrease in mortality rate (breast cancer deaths per 100,000 population).1 Screening mammography (SM) has contributed greatly to that success, accounting for 30% to 70% of the reduced mortality rate, with the remainder due to advancements in breast cancer treatment.2 Despite these improvements, invasive breast cancer remains the highest incident cancer in the United States and in the world, is the second leading cause of cancer death in the United States, and results in more years of life lost than any other cancer (TABLE 1).1,3

While the benefits and harms of SM are reasonably well understood, different guidelines groups have approached the relative value of the risks and benefits differently, which has led to challenges in implementation of shared decision making, particularly around the age to initiate routine screening.4-6 In this article, we will focus on the data behind the controversy, current gaps in knowledge, challenges related to breast density and screening in diverse groups, and emerging technologies to address these gaps and provide a construct for appropriate counseling of the patient across the risk spectrum.
In recognition of 35 years of publication of OBG Management, this article on breast cancer screening by Mark D. Pearlman, MD, kicks off a series that focuses on various cancer screening modalities and expert recommendations.
Stay tuned for articles on the future of cervical cancer screening and genetic testing for cancer risk beyond BRCA testing.
We look forward to continuing OBG Management’s mission of enhancing the quality of reproductive health care and the professional development of ObGyns and all women’s health care clinicians.
Breast cancer risk
Variables that affect risk
While female sex and older age are the 2 greatest risks for the development of breast cancer, many other factors can either increase or decrease breast cancer risk in a person’s lifetime. The importance of identifying risk factors is 3-fold:
- to perform risk assessment to determine if individuals would benefit from average-risk versus high-risk breast cancer surveillance
- to identify persons who might benefit from BRCA genetic counseling and screening, risk reduction medications or procedures, and
- to allow patients to determine whether any modification in their lifestyle or reproductive choices would make sense to them to reduce their future breast cancer risk.
Most of these risk variables are largely inalterable (for example, family history of breast cancer, carriage of genetic pathogenic variants such as BRCA1 and BRCA2, age of menarche and menopause), but some are potentially modifiable, such as parity, age at first birth, lactation and duration, and dietary factors, among others. TABLE 2 lists common breast cancer risk factors.

Breast cancer risk assessment
Several validated tools have been developed to estimate a person’s breast cancer risk (TABLE 3). These tools combine known risk factors and, depending on the specific tool, can provide estimates of 5-year, 10-year, or lifetime risk of breast cancer. Patients at highest risk can benefit from earlier screening, supplemental screening with breast magnetic resonance imaging (MRI), or risk reduction (see the section, “High-risk screening”). Ideally, a risk assessment should be done by age 30 so that patients at high risk can be identified for earlier or more intensive screening and for possible genetic testing in those at risk for carriage of the BRCA or other breast cancer gene pathogenic variants.5,7

Continue to: Breast cancer screening: Efficacy and harms...
Breast cancer screening: Efficacy and harms
The earliest studies of breast cancer screening with mammography were randomized controlled trials (RCTs) that compared screened and unscreened patients aged 40 to 74. Nearly all the RCTs and numerous well-designed incidence-based and case-control studies have demonstrated that SM results in a clinically and statistically significant reduction in breast cancer mortality (TABLE 4).4,6,8 Since the mid-1980s and continuing to the current day, SM programs are routinely recommended in the United States. In addition to the mortality benefit outlined in TABLE 4, SM also is associated with a need for less invasive treatments if breast cancer is diagnosed.9,10

With several decades of experience, SM programs have demonstrated that multiple harms are associated with SM, including callbacks, false-positive mammograms that result in a benign biopsy, and overdiagnosis of breast cancer (TABLE 4). Overdiagnosis is a mammographic detection of a breast cancer that would not have harmed that woman in her lifetime. Overdiagnosis leads to overtreatment of breast cancers with its attendant side effects, the emotional harms of a breast cancer diagnosis, and the substantial financial cost of cancer treatment. Estimates of overdiagnosis range from 0% to 50%, with the most likely estimate of invasive breast cancer overdiagnosis from SM between 5% and 15%.11-13 Some of these overdiagnosed cancers are due to very slow growing cancers or breast cancers that may even regress. However, the higher rates of overdiagnosis occur in older persons who are screened and in whom competing causes of mortality become more prevalent. It is estimated that overdiagnosis of invasive breast cancer in patients younger than age 60 is less than 1%, but it exceeds 14% in those older than age 80 (TABLE 4).14
A structured approach is needed to counsel patients about SM so that they understand both the substantial benefit (earlier-stage diagnosis, reduced need for treatment, reduced breast cancer and all-cause mortality) and the potential harms (callback, false-positive results, and overdiagnosis). Moreover, the relative balance of the benefits and harms are influenced throughout their lifetime by both aging and changes in their personal and family medical history.
Counseling should consider factors beyond just the performance of mammography (sensitivity and specificity), such as the patient’s current health and age (competing causes of mortality), likelihood of developing breast cancer based on risk assessment (more benefit in higher-risk persons), and the individual patient’s values on the importance of the benefits and harms. The differing emphases on mammography performance and the relative value of the benefits and harms have led experts to produce disparate national guideline recommendations (TABLE 5).

Should SM start at age 40, 45, or 50 in average-risk persons?
There is not clear consensus about the age at which to begin to recommend routine SM in patients at average risk. The National Comprehensive Cancer Network (NCCN),7 American Cancer Society (ACS),4 and the US Preventive Services Task Force (USPSTF)5 recommend that those at average risk start SM at age 40, 45, and 50, respectively (TABLE 5). While the guideline groups listed in TABLE 5 agree that there is level 1 evidence that SM reduces breast cancer mortality in the general population for persons starting at age 40, because the incidence of breast cancer is lower in younger persons (TABLE 6),4 the net population-based screening benefit is lower in this group, and the number needed to invite to screening to save a single life due to breast cancer varies.

For patients in their 40s, it is estimated that 1,904 individuals need to be invited to SM to save 1 life, whereas for patients in their 50s, it is 1,339.15 However, for patients in their 40s, the number needed to screen to save 1 life due to breast cancer decreases from 1 in 1,904 if invited to be screened to 1 in 588 if they are actually screened.16 Furthermore, if a patient is diagnosed with breast cancer at age 40–50, the likelihood of dying is reduced at least 22% and perhaps as high as 48% if her cancer was diagnosed on SM compared with an unscreened individual with a symptomatic presentation (for example, palpable mass).4,15,17,18 Another benefit of SM in the fifth decade of life (40s) is the decreased need for more extensive treatment, including a higher risk of need for chemotherapy (odds ratio [OR], 2.81; 95% confidence interval [CI], 1.16–6.84); need for mastectomy (OR, 3.41; 95% CI, 1.36–8.52); and need for axillary lymph node dissection (OR, 5.76; 95% CI, 2.40–13.82) in unscreened (compared with screened) patients diagnosed with breast cancer.10
The harms associated with SM are not inconsequential and include callbacks (approximately 1 in 10), false-positive biopsy (approximately 1 in 100), and overdiagnosis (likely <1% of all breast cancers in persons younger than age 50). Because most patients in their 40s will not develop breast cancer (TABLE 6), the benefit of reduced breast cancer mortality will not be experienced by most in this decade of life, but they are still just as likely to experience a callback, false-positive biopsy, or the possibility of overdiagnosis. Interpretation of this balance on a population level is the crux of the various guideline groups’ development of differing recommendations as to when screening should start. Despite this seeming disagreement, all the guideline groups listed in TABLE 5 concur that persons at average risk for breast cancer should be offered SM if they desire starting at age 40 after a shared decision-making conversation that incorporates the patient’s view on the relative value of the benefits and risks.
Continue to: High-risk screening...
High-risk screening
Unlike in screening average-risk patients, there is less disagreement about screening in high-risk groups. TABLE 7 outlines the various categories and recommended strategies that qualify for screening at younger ages or more intensive screening. Adding breast MRI to SM in high-risk individuals results in both higher cancer detection rates and less interval breast cancers (cancers diagnosed between screening rounds) diagnosed compared with SM alone.19,20 Interval breast cancer tends to be more aggressive and is used as a surrogate marker for more recognized factors, such as breast cancer mortality. In addition to less interval breast cancers, high-risk patients are more likely to be diagnosed with node-negative disease if screening breast MRI is added to SM.
Long-term mortality benefit studies using MRI have not been conducted due to the prolonged follow-up times needed. Expense, lower specificity compared with mammography (that is, more false-positive results), and need for the use of gadolinium limit more widespread use of breast MRI screening in average-risk persons.

Screening in patients with dense breasts
Half of patients undergoing SM in the United States have dense breasts (heterogeneously dense breasts, 40%; extremely dense breasts, 10%). Importantly, increasing breast density is associated with a lower cancer detection rate with SM and is an independent risk factor for developing breast cancer. While most states already require patients to be notified if they have dense breasts identified on SM, the US Food and Drug Administration will soon make breast density patient notification a national standard (see: https://delauro.house.gov/media-center/press-releases/delauro-secures-timeline-fda-rollout-breast-density-notification-rule).
Most of the risk assessment tools listed in TABLE 3 incorporate breast density into their calculation of breast cancer risk. If that calculation places a patient into one of the highest-risk groups (based on additional factors like strong family history of breast cancer, reproductive risk factors, BRCA carriage, and so on), more intensive surveillance should be recommended (TABLE 7).7 However, once these risk calculations are done, most persons with dense breasts will remain in an average-risk category.
Because of the frequency and risks associated with dense breasts, different and alternative strategies have been recommended for screening persons who are at average risk with dense breasts. Supplemental screening with MRI, ultrasonography, contrast-enhanced mammography, and molecular breast imaging are all being considered but have not been studied sufficiently to demonstrate mortality benefit or cost-effectiveness.
Of all the supplemental modalities used to screen patients with dense breasts, MRI has been the best studied. A large RCT in the Netherlands evaluated supplemental MRI screening in persons with extremely dense breasts after a negative mammogram.21 Compared with no supplemental screening, the MRI group had 17 additional cancers detected per 1,000 screened and a 50% reduction in interval breast cancers; in addition, MRI was associated with a positive predictive value of 26% for biopsies. At present, high cost and limited access to standard breast MRI has not allowed its routine use for persons with dense breasts in the United States, but this may change with more experience and more widespread introduction and experience with abbreviated (or rapid) breast MRI in the future (TABLE 8).

Equitable screening
Black persons who are diagnosed with breast cancer have a 40% higher risk of dying than White patients due to multiple factors, including systemic racial factors (implicit and unconscious bias), reduced access to care, and a lower likelihood of receiving standard of care once diagnosed.22-24 In addition, Black patients have twice the likelihood of being diagnosed with triple-negative breast cancers, a biologically more aggressive tumor.22-24 Among Black, Asian, and Hispanic persons diagnosed with breast cancer, one-third are diagnosed younger than age 50, which is higher than for non-Hispanic White persons. Prior to the age of 50, Black, Asian, and Hispanic patients also have a 72% more likelihood of being diagnosed with invasive breast cancer, have a 58% greater risk of advanced-stage disease, and have a 127% higher risk of dying from breast cancer compared with White patients.25,26 Based on all of these factors, delaying SM until age 50 may adversely affect the Black, Asian, and Hispanic populations.
Persons in the LGBTQ+ community do not present for SM as frequently as the general population, often because they feel threatened or unwelcome.27 Clinicians and breast imaging units should review their inclusivity policies and training to provide a welcoming and respectful environment to all persons in an effort to reduce these barriers. While data are limited and largely depend on expert opinion, current recommendations for screening in the transgender patient depend on sex assigned at birth, the type and duration of hormone use, and surgical history. In patients assigned female sex at birth, average-risk and high-risk screening recommendations are similar to those for the general population unless bilateral mastectomy has been performed.28 In transfeminine patients who have used hormones for longer than 5 years, some groups recommend annual screening starting at age 40, although well-designed studies are lacking.29
Continue to: We have done well, can we do better?...
We have done well, can we do better?
Screening mammography clearly has been an important and effective tool in the effort to reduce breast cancer mortality, but there are clear limitations. These include moderate sensitivity of mammography, particularly in patients with dense breasts, and a specificity that results in either callbacks (10%), breast biopsies for benign disease (1%), or the reality of overdiagnosis, which becomes increasingly important in older patients.
With the introduction of mammography in the mid-1980s, a one-size-fits-all approach has proved challenging more recently due to an increased recognition of the harms of screening. As a result of this evolving understanding, different recommendations for average-risk screening have emerged. With the advent of breast MRI, risk-based screening is an important but underutilized tool to identify highest-risk individuals, which is associated with improved cancer detection rates, reduced node-positive disease, and fewer diagnosed interval breast cancers. Assuring that nearly all of this highest-risk group is identified through routine breast cancer risk assessment remains a challenge for clinicians.
But what SM recommendations should be offered to persons who fall into an intermediate-risk group (15%–20%), very low-risk groups (<5%), or patients with dense breasts? These are challenges that could be met through novel and individualized approaches (for example, polygenic risk scoring, further research on newer modalities of screening [TABLE 8]), improved screening algorithms for persons with dense breasts, and enhanced clinician engagement to achieve universal breast cancer and BRCA risk assessment of patients by age 25 to 30.
In 2023, best practice and consensus guidelines for intermediate- and low-risk breast cancer groups remain unclear, and one of the many ongoing challenges is to further reduce the impact of breast cancer on the lives of persons affected and the recognized harms of SM.
In the meantime, there is consensus in average-risk patients to provide counseling about SM by age 40. My approach has been to counsel all average-risk patients on the risks and benefits of mammography using the acronym TIP-V:
- Use a Tool to calculate breast cancer risk (TABLE 3). If they are at high risk, provide recommendations for high-risk management (TABLE 7).7
- For average-risk patients, counsel that their Incidence of developing breast cancer in the next decade is approximately 1 in 70 (TABLE 6).4
- Provide data and guidance on the benefits of SM for patients in their 40s (mortality improvement, decreased treatment) and the likelihood of harm from breast cancer screening (10% callback, 1% benign biopsy, and <1% likelihood of overdiagnosis [TABLE 4]).4,14,15
- Engage the patient to better understand their relative Values of the benefits and harms and make a shared decision on screening starting at age 40, 45, or 50.
Looking forward
In summary, SM remains an important tool in the effort to decrease the risk of mortality due to breast cancer. Given the limitations of SM, however, newer tools and methods—abbreviated MRI, contrast-enhanced mammography, molecular breast imaging, customized screening intervals depending on individual risk/polygenic risk score, and customized counseling and screening based on risk factors (TABLES 2 and 7)—will play an increased role in recommendations for breast cancer screening in the future. ●
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
- Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72:524-541.
- Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784-1792.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Drist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force Recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Cantor A, Humphrey L, et al. Screening for breast cancer: a systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence synthesis no 124. AHRQ publication no 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Karzai S, Port E, Siderides C, et al. Impact of screening mammography on treatment in young women diagnosed with breast cancer. Ann Surg Oncol. 2022. doi:10.1245/ s10434-022-11581-6.
- Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol. 2018;25:2979-2986.
- Coldman A, Phillips N. Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ. 2013;185:E492-E498.
- Etzioni R, Gulati R, Mallinger L, et al. Influence of study features and methods on overdiagnosis estimates in breast and prostate cancer screening. Ann Internal Med. 2013;158:831-838.
- Ryser MD, Lange J, Inoue LY, et al. Estimation of breast cancer overdiagnosis in a US breast screening cohort. Ann Intern Med. 2022;175:471-478.
- Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol. 2021;18:1280-1288.
- Nelson HD, Fu R, Cantor A, Pappas M, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Internal Med. 2016;164:244-255.
- Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. Am J Roentgenol. 2014;203:1379-1381.
- Broeders M, Moss S, Nyström L, et al; EUROSCREEN Working Group. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen. 2012;19(suppl 1):14-25.
- Tabár L, Yen AMF, Wu WYY, et al. Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21:13-20.
- Kriege M, Brekelmans CTM, Boetes C, et al; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
- Vreemann S, Gubern-Merida A, Lardenoije S, et al. The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat. 2018;169:323-331.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Amirikia KC, Mills P, Bush J, et al. Higher population‐based incidence rates of triple‐negative breast cancer among young African‐American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747-2753.
- Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048.
- Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152:485-493.
- Stapleton SM, Oseni TO, Bababekov YJ, et al. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. 2018;153:594-595.
- Hendrick RE, Monticciolo DL, Biggs KW, et al. Age distributions of breast cancer diagnosis and mortality by race and ethnicity in US women. Cancer. 2021;127:4384-4392.
- Perry H, Fang AJ, Tsai EM, et al. Imaging health and radiology care of transgender patients: a call to build evidence-based best practices. J Am Coll Radiol. 2021;18(3 pt B):475-480.
- Lockhart R, Kamaya A. Patient-friendly summary of the ACR Appropriateness Criteria: transgender breast cancer screening. J Am Coll Radiol. 2022;19:e19.
- Expert Panel on Breast Imaging; Brown A, Lourenco AP, Niell BL, et al. ACR Appropriateness Criteria transgender breast cancer screening. J Am Coll Radiol. 2021;18:S502-S515.
- Mørch LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377:2228-2239.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
- Laws A, Katlin F, Hans M, et al. Screening MRI does not increase cancer detection or result in an earlier stage at diagnosis for patients with high-risk breast lesions: a propensity score analysis. Ann Surg Oncol. 2023;30;68-77.
- American College of Obstetricians and Gynecologists. Practice bulletin no 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Grimm LJ, Mango VL, Harvey JA, et al. Implementation of abbreviated breast MRI for screening: AJR expert panel narrative review. AJR Am J Roentgenol. 2022;218:202-212.
- Potsch N, Vatteroini G, Clauser P, et al. Contrast-enhanced mammography versus contrast-enhanced breast MRI: a systematic review and meta-analysis. Radiology. 2022;305:94-103.
- Covington MF, Parent EE, Dibble EH, et al. Advances and future directions in molecular breast imaging. J Nucl Med. 2022;63:17-21.
Late-onset recurrence in breast cancer: Implications for women’s health clinicians in survivorship care
Improved treatments for breast cancer (BC) and effective screening programs have resulted in a BC mortality rate reduction of 41% since 1989.1 Because BC is the leading cause of cancer in women, these mortality improvements have resulted in more than 3 million BC survivors in the United States.2,3 With longer-term survival, there is increasing interest in late-onset recurrences.4,5 A recent study has provided an improved understanding of the risk of lateonset recurrence in women with 10 years of disease-free survival, an important finding for women’s health providers because oncologists do not typically follow survivors after 10 years of disease-free survival.4
Recent study looks at incidence of late-onset recurrence
Pederson and colleagues evaluated all patients diagnosed with BC in Denmark from 1987 through 2004.4 Those patients without evidence of recurrence at 10 years were then followed utilizing population-based linked registries to identify patients who subsequently developed a local, regional, or distant late-onset recurrence. The authors evaluated the frequency of late recurrence and identified associations with demographic and tumor characteristics.
What they found
A total of 36,920 patients were diagnosed with BC in Denmark between 1987-2004, of whom 20,315 (55%) were identified as disease free for at least 10 years. Late-onset recurrence occurred in 2,595 (12.8%) with the strongest associations of recurrence seen in patients who had a tumor size >2 cm and lymph node‒positive (involving 4 or more nodes) disease (24.6%), compared with 12.7% in patients with tumors <2 cm and node-negative disease. Several other factors were associated with a higher risk of late-onset recurrence and are included in the TABLE. Half of the recurrences occurred between 10 and 15 years after the primary diagnosis.
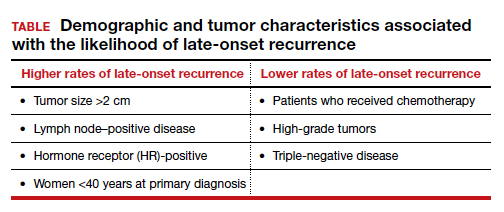
Prior research
These findings are consistent with another recent study showing that BC patients have a 1% to 2%/year risk of recurrence after 10 disease-free years.5 Strengths of this study include:
- population-based, including all women with BC
- long-term follow-up for up to 32 years
- universal health care in Denmark, which results in robust and linked databases and very few missing data points.
There were two notable weaknesses to consider:
- Treatment regimens changed considerably during the time frame of the study (1997-2018), particularly the duration of tamoxifen use in patients with HR-positive disease. In this study nearly all patients received 5 years or less of tamoxifen. Since the mid-2010s, 10 years of hormonal adjuvant therapy has become routine in HR-positive BC, which reduces recurrences, including late-onset recurrence.6 The effect of 10 years of tamoxifen would very likely have resulted in less late-onset recurrence in the HR-positive population in this study.
- There is a lack of racial diversity in the Danish population, and the study findings may not translate to Black patients who have a higher frequency of triple-negative BC with a different risk of late-onset recurrence.7
Practice takeaways
Cancer surveillance. There are 3+ million BC survivors in the United States, and a 55%+ likelihood that they will be disease free for 10 years. This is clearly an important population to the women’s health care provider. This study, and previous research, suggests that among 10-year-disease-free survivors, 1% to 2% will recur annually, with higher rates amongst HR-positive, lymph-node positive women under age 40, and in the first 5 years following the 10-year post–initial diagnosis mark, so ongoing surveillance is imperative. Annual clinical breast examinations along with annual (not biennial) mammography should be performed.8 Digital breast tomosynthesis has improved specificity and sensitivity for BC detection and is the preferred modality when it is available.
Management of menopausal symptoms. These findings also have implications for menopausal hormone therapy for patients with symptoms. Because HR-positive patients have an increased risk of late-onset recurrence, nonhormonal therapies should be considered as first-line therapy for patients with menopausal symptoms. If hormone therapy is being considered, providers and patients should use shared decision making to balance the potential benefits (reduction in symptoms, possible cardiovascular benefits, and reduction in bone loss) with the risks (increased risk of recurrence and venous thromboembolism), even if patients are remote from the original diagnosis (ie, 10-year disease-free survival).
Topical estrogen therapies would be preferred for patients with significant urogenital atrophic symptoms who fail nonhormonal therapies due to substantially less systemic absorption and the lack of need to add a progestin.9,10 If oral therapy is being considered, I carefully counsel these women about the likely increased risk of recurrence and, if possible, include their breast oncologist in the discussion. ●
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654.
- de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561- 570. doi: 10.1158/1055-9965.EPI-12-1356.
- Carreira H, Williams R, Funston G, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a matched population-based cohort study in the United Kingdom. PLOS Med. 2021;18:e1003504. doi: 10.1371/journal.pmed.1003504.
- Pedersen RN, Esen BÖ, Mellemkjær L, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. November 8, 2021. doi: 10.1093/jnci/djab202.
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836- 1846. doi: 10.1056/NEJMoa1701830.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. doi: 10.1016/S0140-6736(12)61963-1.
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple‐negative breast cancer disparities for the United States: a population‐based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412-3417. doi: 10.1002/cncr.32207.
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2021; Version 2.2022.
- Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause. 2020;27:339-360. doi: 10.1097 /GME.0000000000001468.
- Treatment of urogenital symptoms in individuals with a history of estrogen-dependent breast cancer: clinical consensus. Obstet Gynecol. 2021;138:950-960. doi: 10.1097/AOG .0000000000004601.
Improved treatments for breast cancer (BC) and effective screening programs have resulted in a BC mortality rate reduction of 41% since 1989.1 Because BC is the leading cause of cancer in women, these mortality improvements have resulted in more than 3 million BC survivors in the United States.2,3 With longer-term survival, there is increasing interest in late-onset recurrences.4,5 A recent study has provided an improved understanding of the risk of lateonset recurrence in women with 10 years of disease-free survival, an important finding for women’s health providers because oncologists do not typically follow survivors after 10 years of disease-free survival.4
Recent study looks at incidence of late-onset recurrence
Pederson and colleagues evaluated all patients diagnosed with BC in Denmark from 1987 through 2004.4 Those patients without evidence of recurrence at 10 years were then followed utilizing population-based linked registries to identify patients who subsequently developed a local, regional, or distant late-onset recurrence. The authors evaluated the frequency of late recurrence and identified associations with demographic and tumor characteristics.
What they found
A total of 36,920 patients were diagnosed with BC in Denmark between 1987-2004, of whom 20,315 (55%) were identified as disease free for at least 10 years. Late-onset recurrence occurred in 2,595 (12.8%) with the strongest associations of recurrence seen in patients who had a tumor size >2 cm and lymph node‒positive (involving 4 or more nodes) disease (24.6%), compared with 12.7% in patients with tumors <2 cm and node-negative disease. Several other factors were associated with a higher risk of late-onset recurrence and are included in the TABLE. Half of the recurrences occurred between 10 and 15 years after the primary diagnosis.

Prior research
These findings are consistent with another recent study showing that BC patients have a 1% to 2%/year risk of recurrence after 10 disease-free years.5 Strengths of this study include:
- population-based, including all women with BC
- long-term follow-up for up to 32 years
- universal health care in Denmark, which results in robust and linked databases and very few missing data points.
There were two notable weaknesses to consider:
- Treatment regimens changed considerably during the time frame of the study (1997-2018), particularly the duration of tamoxifen use in patients with HR-positive disease. In this study nearly all patients received 5 years or less of tamoxifen. Since the mid-2010s, 10 years of hormonal adjuvant therapy has become routine in HR-positive BC, which reduces recurrences, including late-onset recurrence.6 The effect of 10 years of tamoxifen would very likely have resulted in less late-onset recurrence in the HR-positive population in this study.
- There is a lack of racial diversity in the Danish population, and the study findings may not translate to Black patients who have a higher frequency of triple-negative BC with a different risk of late-onset recurrence.7
Practice takeaways
Cancer surveillance. There are 3+ million BC survivors in the United States, and a 55%+ likelihood that they will be disease free for 10 years. This is clearly an important population to the women’s health care provider. This study, and previous research, suggests that among 10-year-disease-free survivors, 1% to 2% will recur annually, with higher rates amongst HR-positive, lymph-node positive women under age 40, and in the first 5 years following the 10-year post–initial diagnosis mark, so ongoing surveillance is imperative. Annual clinical breast examinations along with annual (not biennial) mammography should be performed.8 Digital breast tomosynthesis has improved specificity and sensitivity for BC detection and is the preferred modality when it is available.
Management of menopausal symptoms. These findings also have implications for menopausal hormone therapy for patients with symptoms. Because HR-positive patients have an increased risk of late-onset recurrence, nonhormonal therapies should be considered as first-line therapy for patients with menopausal symptoms. If hormone therapy is being considered, providers and patients should use shared decision making to balance the potential benefits (reduction in symptoms, possible cardiovascular benefits, and reduction in bone loss) with the risks (increased risk of recurrence and venous thromboembolism), even if patients are remote from the original diagnosis (ie, 10-year disease-free survival).
Topical estrogen therapies would be preferred for patients with significant urogenital atrophic symptoms who fail nonhormonal therapies due to substantially less systemic absorption and the lack of need to add a progestin.9,10 If oral therapy is being considered, I carefully counsel these women about the likely increased risk of recurrence and, if possible, include their breast oncologist in the discussion. ●
Improved treatments for breast cancer (BC) and effective screening programs have resulted in a BC mortality rate reduction of 41% since 1989.1 Because BC is the leading cause of cancer in women, these mortality improvements have resulted in more than 3 million BC survivors in the United States.2,3 With longer-term survival, there is increasing interest in late-onset recurrences.4,5 A recent study has provided an improved understanding of the risk of lateonset recurrence in women with 10 years of disease-free survival, an important finding for women’s health providers because oncologists do not typically follow survivors after 10 years of disease-free survival.4
Recent study looks at incidence of late-onset recurrence
Pederson and colleagues evaluated all patients diagnosed with BC in Denmark from 1987 through 2004.4 Those patients without evidence of recurrence at 10 years were then followed utilizing population-based linked registries to identify patients who subsequently developed a local, regional, or distant late-onset recurrence. The authors evaluated the frequency of late recurrence and identified associations with demographic and tumor characteristics.
What they found
A total of 36,920 patients were diagnosed with BC in Denmark between 1987-2004, of whom 20,315 (55%) were identified as disease free for at least 10 years. Late-onset recurrence occurred in 2,595 (12.8%) with the strongest associations of recurrence seen in patients who had a tumor size >2 cm and lymph node‒positive (involving 4 or more nodes) disease (24.6%), compared with 12.7% in patients with tumors <2 cm and node-negative disease. Several other factors were associated with a higher risk of late-onset recurrence and are included in the TABLE. Half of the recurrences occurred between 10 and 15 years after the primary diagnosis.

Prior research
These findings are consistent with another recent study showing that BC patients have a 1% to 2%/year risk of recurrence after 10 disease-free years.5 Strengths of this study include:
- population-based, including all women with BC
- long-term follow-up for up to 32 years
- universal health care in Denmark, which results in robust and linked databases and very few missing data points.
There were two notable weaknesses to consider:
- Treatment regimens changed considerably during the time frame of the study (1997-2018), particularly the duration of tamoxifen use in patients with HR-positive disease. In this study nearly all patients received 5 years or less of tamoxifen. Since the mid-2010s, 10 years of hormonal adjuvant therapy has become routine in HR-positive BC, which reduces recurrences, including late-onset recurrence.6 The effect of 10 years of tamoxifen would very likely have resulted in less late-onset recurrence in the HR-positive population in this study.
- There is a lack of racial diversity in the Danish population, and the study findings may not translate to Black patients who have a higher frequency of triple-negative BC with a different risk of late-onset recurrence.7
Practice takeaways
Cancer surveillance. There are 3+ million BC survivors in the United States, and a 55%+ likelihood that they will be disease free for 10 years. This is clearly an important population to the women’s health care provider. This study, and previous research, suggests that among 10-year-disease-free survivors, 1% to 2% will recur annually, with higher rates amongst HR-positive, lymph-node positive women under age 40, and in the first 5 years following the 10-year post–initial diagnosis mark, so ongoing surveillance is imperative. Annual clinical breast examinations along with annual (not biennial) mammography should be performed.8 Digital breast tomosynthesis has improved specificity and sensitivity for BC detection and is the preferred modality when it is available.
Management of menopausal symptoms. These findings also have implications for menopausal hormone therapy for patients with symptoms. Because HR-positive patients have an increased risk of late-onset recurrence, nonhormonal therapies should be considered as first-line therapy for patients with menopausal symptoms. If hormone therapy is being considered, providers and patients should use shared decision making to balance the potential benefits (reduction in symptoms, possible cardiovascular benefits, and reduction in bone loss) with the risks (increased risk of recurrence and venous thromboembolism), even if patients are remote from the original diagnosis (ie, 10-year disease-free survival).
Topical estrogen therapies would be preferred for patients with significant urogenital atrophic symptoms who fail nonhormonal therapies due to substantially less systemic absorption and the lack of need to add a progestin.9,10 If oral therapy is being considered, I carefully counsel these women about the likely increased risk of recurrence and, if possible, include their breast oncologist in the discussion. ●
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654.
- de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561- 570. doi: 10.1158/1055-9965.EPI-12-1356.
- Carreira H, Williams R, Funston G, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a matched population-based cohort study in the United Kingdom. PLOS Med. 2021;18:e1003504. doi: 10.1371/journal.pmed.1003504.
- Pedersen RN, Esen BÖ, Mellemkjær L, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. November 8, 2021. doi: 10.1093/jnci/djab202.
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836- 1846. doi: 10.1056/NEJMoa1701830.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. doi: 10.1016/S0140-6736(12)61963-1.
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple‐negative breast cancer disparities for the United States: a population‐based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412-3417. doi: 10.1002/cncr.32207.
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2021; Version 2.2022.
- Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause. 2020;27:339-360. doi: 10.1097 /GME.0000000000001468.
- Treatment of urogenital symptoms in individuals with a history of estrogen-dependent breast cancer: clinical consensus. Obstet Gynecol. 2021;138:950-960. doi: 10.1097/AOG .0000000000004601.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654.
- de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561- 570. doi: 10.1158/1055-9965.EPI-12-1356.
- Carreira H, Williams R, Funston G, et al. Associations between breast cancer survivorship and adverse mental health outcomes: a matched population-based cohort study in the United Kingdom. PLOS Med. 2021;18:e1003504. doi: 10.1371/journal.pmed.1003504.
- Pedersen RN, Esen BÖ, Mellemkjær L, et al. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. J Natl Cancer Inst. November 8, 2021. doi: 10.1093/jnci/djab202.
- Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836- 1846. doi: 10.1056/NEJMoa1701830.
- Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805-816. doi: 10.1016/S0140-6736(12)61963-1.
- Scott LC, Mobley LR, Kuo TM, et al. Update on triple‐negative breast cancer disparities for the United States: a population‐based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412-3417. doi: 10.1002/cncr.32207.
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2021; Version 2.2022.
- Crandall CJ, Diamant A, Santoro N. Safety of vaginal estrogens: a systematic review. Menopause. 2020;27:339-360. doi: 10.1097 /GME.0000000000001468.
- Treatment of urogenital symptoms in individuals with a history of estrogen-dependent breast cancer: clinical consensus. Obstet Gynecol. 2021;138:950-960. doi: 10.1097/AOG .0000000000004601.
It’s not time to abandon routine screening mammography in average-risk women in their 40s
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.
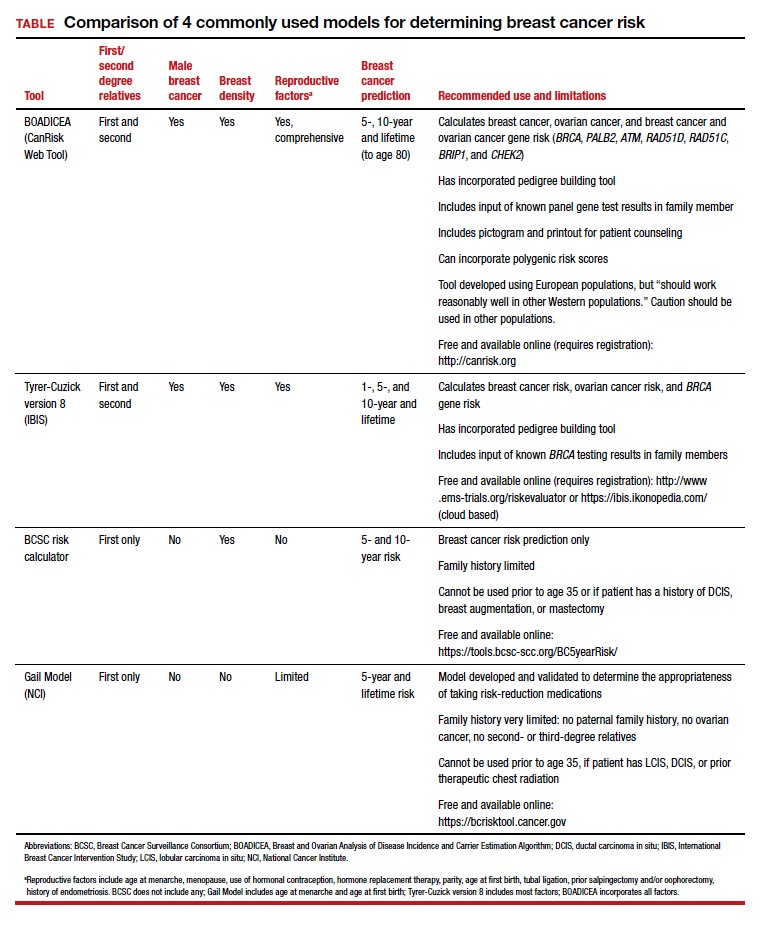
Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.

Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
In the 1970s and early 1980s, population-based screening mammography was studied in numerous randomized control trials (RCTs), with the primary outcome of reduced breast cancer mortality. Although technology and the sensitivity of mammography in the 1980s was somewhat rudimentary compared with current screening, a meta-analysis of these RCTs demonstrated a clear mortality benefit for screening mammography.1 As a result, widespread population-based mammography was introduced in the mid-1980s in the United States and has become a standard for breast cancer screening.
Since that time, few RCTs of screening mammography versus observation have been conducted because of the ethical challenges of entering women into such studies as well as the difficulty and expense of long-term follow-up to measure the effect of screening on breast cancer mortality. Without ongoing RCTs of mammography, retrospective, observational, and computer simulation trials of the efficacy and harms of screening mammography have been conducted using proxy measures of mortality (such as stage at diagnosis), and some have questioned the overall benefit of screening mammography.2,3
To further complicate this controversy, some national guidelines have recommended against routinely recommending screening mammography for women aged 40 to 49 based on concerns that the harms (callbacks, benign breast biopsies, overdiagnosis) exceed the potential benefits (earlier diagnosis, possible decrease in needed treatments, reduced breast cancer mortality).4 This has resulted in a confusing morass of national recommendations with uncertainty regarding the question of whether to routinely offer screening mammography for women in their 40s at average risk for breast cancer.4-6
Recently, to address this question Duffy and colleagues conducted a large RCT of women in their 40s to evaluate the long-term effect of mammography on breast cancer mortality.7 Here, I review the study in depth and offer some guidance to clinicians and women struggling with screening decisions.
Breast cancer mortality significantly lower in the screening group
The RCT, known as the UK Age trial, was conducted in England, Wales, and Scotland and enrolled 160,921 women from 1990 through 1997.7 Women were randomly assigned in a 2:1 ratio to observation or annual screening mammogram beginning at age 39–41 until age 48. (In the United Kingdom, all women are screened starting at age 50.) Study enrollees were followed for a median of 22.8 years, and the primary outcome was breast cancer mortality.
The study results showed a 25% relative risk (RR) reduction in breast cancer mortality at 10 years of follow-up in the mammography group compared with the unscreened women (83 breast cancer deaths in the mammography group vs 219 in the observation group [RR, 0.75; 95% confidence interval (CI), 0.58–0.97; P = .029]). Based on the prevalence of breast cancer in women in their 40s, this 25% relative risk reduction translates into approximately 1 less death per 1,000 women who undergo routine screening in their 40s.
While there was no additional significant mortality reduction beyond 10 years of follow-up, as noted mammography is offered routinely starting at age 50 to all women in the United Kingdom. The authors concluded that “reducing the lower age limit for screening from 50 to 40 years [of age] could potentially reduce breast cancer mortality.”
Was overdiagnosis a concern? Another finding in this trial was related to overdiagnosis of breast cancer in the screened group. Overdiagnosis refers to mammographic-only diagnosis (that is, no clinical findings) of nonaggressive breast cancer, which would remain indolent and not harm the patient. The study results demonstrated essentially no overdiagnosis in women screened at age 40 compared with the unscreened group.
Continue to: Large trial, long follow-up are key strengths...
Large trial, long follow-up are key strengths
The UK Age trial’s primary strength is its study design: a large population-based RCT that included diverse participants with the critical study outcome for cancer screening (mortality). The study’s long-term follow-up is another key strength, since breast cancer mortality typically occurs 7 to 10 years after diagnosis. In addition, results were available for 99.9% of the women enrolled in the trial (that is, only 0.1% of women were lost to follow-up). Interestingly, the demonstrated mortality reduction with screening mammography for women in their 40s validates the mortality benefit demonstrated in other large RCTs of women in their 40s.1
Another strong point is that the study addresses the issue of whether screening women in their 40s results in overdiagnosis compared with women who start screening in their 50s. Further, this study validates a prior observational study that mammographic findings of nonprogressive cancers do not disappear, so nonaggressive cancers that present on mammography in women in their 40s still would be detected when women start screening in their 50s.8
Study limitations should be noted
The study has several limitations. For example, significant improvements have been made in breast cancer treatments that may mitigate against the positive impact of screening mammography. The impact of changed breast cancer management over the past 20 years could not be addressed with this study’s design since women would have been treated in the 1990s. In addition, substantial improvements have occurred in breast cancer screening standards (2 views vs the single view used in the study) and technology since the 1990s. Current mammography includes nearly uniform use of either digital mammography (DM) or digital breast tomosynthesis (DBT), both of which improve breast cancer detection for women in their 40s compared with the older film-screen technology. In addition, DBT reduces false-positive results by approximately 40%, resulting in fewer callbacks and biopsies. While improved cancer detection and reduced false-positive results are seen with DM and DBT, whether these technology improvements result in improved breast cancer mortality has not yet been sufficiently studied.
Perhaps the most important limitation in this study is that the women did not undergo routine risk assessment before trial entry to assure that they all were at “average risk.” As a result, both high- and average-risk women would have been included in this population-based trial. Without risk stratification, it remains uncertain whether the reduction in breast cancer mortality disproportionately exists within a high-risk subgroup (such as breast cancer gene mutation carriers).
Finally, the cost efficacy of routine screening mammography for women in their 40s was not evaluated in this study.
The UK Age trial in perspective
The good news is that there is the clear evidence that breast cancer mortality rates (deaths per 100,000) have decreased by about 40% over the past 50 years, likely due to improvements in breast cancer treatment and routine screening mammography.9 Breast cancer mortality reduction is particularly important because breast cancer remains the most common cancer and is the second leading cause of cancer death in women in the United States. In the past decade, considerable debate has arisen arguing whether this reduction in breast cancer mortality is due to improved treatments, routine screening mammography, or both. Authors of a retrospective trial in Australia, recently reviewed in OBG Management, suggested that the majority of improvement is due to improvements in treatment.3,10 However, as the authors pointed out, due to the trial’s retrospective design, causality only can be inferred. The current UK Age trial does add to the numerous prospective trials demonstrating mortality benefit for mammography in women in their 40s.11
What remains a challenge for clinicians, and for women struggling with the mammography question, is the absence of risk assessment in these long-term RCT trials as well as in the large retrospective database studies. Without risk stratification, these studies treated all the study population as “average risk.” Because breast cancer risk assessment is sporadically performed in clinical practice and there are no published RCTs of screening mammography in risk-assessed “average risk” women in their 40s, it remains uncertain whether the women benefiting from screening in their 40s are in a high-risk group or whether women of average risk in this age group also are benefiting from routine screening mammography.
Continue to: What’s next: Incorporate routine risk assessment into clinical practice...
What’s next: Incorporate routine risk assessment into clinical practice
It is not time to abandon screening mammography for all women in their 40s. Rather, routine risk assessment should be performed using one of many available validated or widely tested tools, a recommendation supported by the American College of Obstetricians and Gynecologists, the National Comprehensive Cancer Network, and the US Preventive Services Task Force.5,6,12
Ideally, these tools can be incorporated into an electronic health record and prepopulated using already available patient data (such as age, reproductive risk factors, current medications, breast density if available, and family history). Prepopulating available data into breast cancer risk calculators would allow clinicians to spend time on counseling women regarding breast cancer risk and appropriate screening methods. The TABLE provides a summary of useful breast cancer risk calculators and includes comments about their utility and significant limitations and benefits. In addition to breast cancer risk, the more comprehensive risk calculators (Tyrer-Cuzick and BOADICEA) allow calculation of ovarian cancer risk and gene mutation risk.

Routinely performing breast cancer risk assessment can guide discussions of screening mammography and can provide data for conducting a more individualized discussion on cancer genetic counseling and testing, risk reduction methods in high-risk women, and possible use of intensive breast cancer screening tools in identified high-risk women.
Ultimately, debating the question of whether all women should have routine breast cancer screening in their 40s should be passé. Ideally, all women should undergo breast cancer risk assessment in their 20s. Risk assessment results can then be used to guide the discussion of multiple potential interventions for women in their 40s (or earlier if appropriate), including routine screening mammography, cancer genetic counseling and testing in appropriate individuals, and intervention for women who are identified at high risk.
Absent breast cancer risk assessment, screening mammography still should be offered to women in their 40s, and the decision to proceed should be based on a discussion of risks, benefits, and the value the patient places on these factors.●
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
- Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:244-255.
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249-e.
- Nelson HD, Cantor A, Humphrey L, et al. A systematic review to update the 2009 US Preventive Services Task Force recommendation. Evidence syntheses No. 124. AHRQ Publication No. 14-05201-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2016.
- Bevers TB, Helvie M, Bonaccio E, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:1362-1389.
- ACOG Committee on Practice Bulletins–Gynecology. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165-1172.
- Arleo EK, Monticciolo DL, Monsees B, et al. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14:863-867.
- DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438-451.
- Kaunitz AM. How effective is screening mammography for preventing breast cancer mortality? OBG Manag. 2020;32(8):17,49.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:652-665.
Should supplemental MRI be used in otherwise average-risk women with extremely dense breasts?
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3
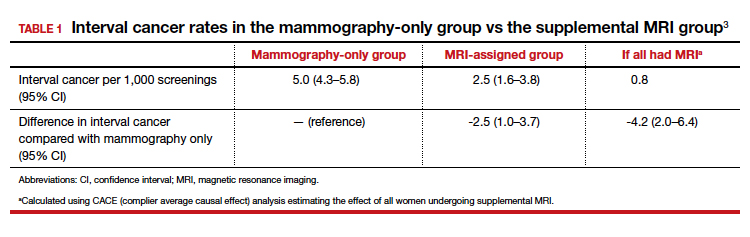
Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.
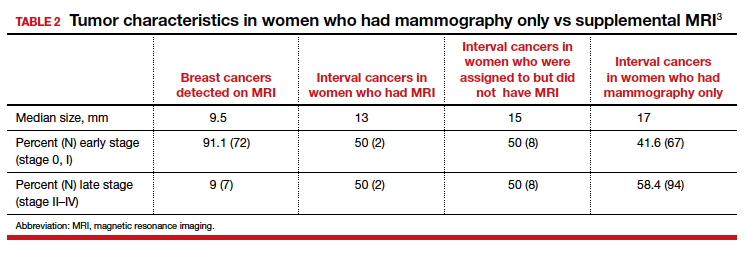
Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
While the frequency of dense breasts decreases with age, approximately 10% of women in the United States have extremely dense breasts (Breast Imaging, Reporting, and Data System [BI-RADS] category D), and another 40% have heterogeneously dense breasts (BI-RADS category C).1 Women with dense breasts have both an increased risk for developing breast cancer and reduced mammographic sensitivity for breast cancer detection compared with women who have nondense breasts.2
These 2 observations have led the majority of states to pass legislation requiring that women with dense breasts be informed of their breast density, and most require that providers discuss these results with their patients. Thoughtful clinicians who review the available literature, however, will find sparse evidence on which to counsel patients as to next steps.
Now, a recent trial adds to our knowledge about supplemental magnetic resonance imaging (MRI) breast screening in women with extremely dense breasts.
DENSE trial offers high-quality data
Bakker and colleagues studied women aged 50 to 74 who were participating in a Netherlands population-based biennial mammography screening program.3 They enrolled average-risk women with extremely dense breasts who had a negative screening digital mammogram into the Dense Tissue and Early Breast Neoplasm Screening (DENSE) multicenter trial. The women were randomly assigned to receive either continued biennial digital mammography or supplemental breast MRI.
The primary outcome was the between-group difference in the development of interval breast cancers—that is, breast cancers detected by women or their providers between rounds of screening mammography. Interval breast cancers were chosen as the primary outcome for 2 reasons:
- interval cancers appear to be more aggressive tumors than those cancers detected by screening mammography
- interval cancers can be identified over a shorter time interval, making them easier to study than outcomes such as breast cancer mortality, which typically require more than a decade to identify.
The DENSE trial’s secondary outcomes included recall rates from MRI, cancer detection rates on MRI, positive predictive value of MRIs requiring biopsy, and breast cancer characteristics (size, stage) diagnosed in the different groups.
Between-group difference in incidence of interval cancers
A total of 40,373 women with extremely dense breasts were screened; 8,061 of these were randomly assigned to receive breast MRI and 32,312 to continued mammography only (1:4 cluster randomization) across 12 mammography centers in the Netherlands. Among the women assigned to the MRI group, 59% actually underwent MRI (4,783 of the 8,061).
The interval cancer rate in the mammography-only group was 5.0 per 1,000 screenings (95% confidence interval [CI], 4.3–5.8), while the interval cancer rate in the MRI-assigned group was 2.5 per 1,000 screenings (95% CI, 1.6–3.8) (TABLE 1).3

Key secondary outcomes
Of the women who underwent supplemental MRI, 9.49% were recalled for additional imaging, follow-up, or biopsy. Of the 4,783 women who had an MRI, 300 (6.3%) underwent a breast biopsy, and 79 breast cancers (1.65%) were detected. Sixty-four of these cancers were invasive, and 15 were ductal carcinoma in situ (DCIS). Among women who underwent a biopsy for an MRI-detected abnormality, the positive predictive value was 26.3%.
Tumor characteristics. For women who developed breast cancer during the study, both tumor size at diagnosis and tumor stage (early vs late) were described. TABLE 2 shows these results in the women who had their breast cancer detected on MRI, those in the MRI-assigned group who developed interval cancer, and those in the mammography-only group who had interval cancers.3 Overall, tumor size was smaller in the interval group who underwent MRI compared with those who underwent mammography only.

Continue to: Study contributes valuable data, but we need more on long-term outcomes...
Study contributes valuable data, but we need more on long-term outcomes
The trial by Bakker and colleagues employed a solid study design as women were randomly assigned to supplemental MRI screening or ongoing biennial mammography, and nearly all cancers were identified in the short-term of follow-up. In addition, very few women were lost to follow-up, and secondary outcomes, including false-positive rates, were collected to help providers and patients better understand some of the potential downsides of supplemental screening.
The substantial reduction in interval cancers (50% in the intent-to-screen analysis and 84% in the women who actually underwent supplemental MRI) was highly statistically significant (P<.001). While there were substantially fewer interval cancers in the MRI-assigned group, the interval cancers that did occur were of similar stage as those in the women assigned to the mammography-only group (TABLE 2).
Data demonstrate that interval cancers appear to be more aggressive than screen-detected cancers.4 While reducing interval cancers should be a good thing overall, it remains unproven that using supplemental MRI in all women with dense breasts would reduce breast cancer specific mortality, all-cause mortality, or the risk of more invasive treatments (for example, the need for chemotherapy or requirement for mastectomy).
On the other hand, using routine supplemental breast MRI in women with extremely dense breasts would result in very substantial use of resources, including cost, radiologist time, provider time, and machine time. In the United States, approximately 49 million women are aged 50 to 74.5 Breast MRI charges commonly range from $1,000 to $4,000. If the 4.9 million women with extremely dense breasts underwent supplemental MRI this year, the approximate cost would be somewhere between $4.9 and $19.5 billion for imaging alone. This does not include callbacks, biopsies, or provider time for ordering, interpreting, and arranging for follow-up.
While the reduction in interval cancers seen in this study is promising, more assurance of improvement in important outcomes—such as reduced mortality or reduced need for more invasive breast cancer treatments—should precede any routine change in practice.
Unanswered questions
This study did not address a number of other important questions, including:
Should MRI be done with every round of breast cancer screening given the possibility of prevalence bias? Prevalence bias can be defined as more cancers detected in the first round of MRI screening with possible reduced benefit in future rounds of screening. The study authors indicated that they will continue to analyze the study results to see what occurs in the next round of screening.
Is there a similar impact on decreased interval cancers in women undergoing annual mammography or in women screened between ages 40 and 49? This study was conducted in women aged 50 to 74 undergoing mammography every 2 years. In the United States, annual mammography in women aged 40 to 49 is frequently recommended.
What effect does supplemental MRI screening have in women with heterogeneously dense breasts, which represents 40% of the population? The US Food and Drug Administration recommends that all women with dense breasts be counseled regarding options for management.6
Do these results translate to the more racially and ethnically diverse populations of the United States? In the Netherlands, where this study was conducted, 85% to 90% of women are either Dutch or of western European origin. Women of different racial and ancestral backgrounds have biologically different breast cancers and cancer risk (for example, higher rates of triple-negative breast cancers in African American women; 10-fold higher rates of BRCA pathogenic variants in Ashkenazi Jewish women).
Continue to: Use validated tools to assess risk comprehensively...
Use validated tools to assess risk comprehensively
Women aged 50 to 74 with extremely dense breasts have reduced interval cancers following a normal biennial mammogram if supplemental MRI is offered, but the long-term benefit of identifying these cancers earlier is unclear. Until more data are available on important long-term outcomes (such as breast cancer mortality and need for more invasive treatments), providers should consider breast density in the context of a more comprehensive assessment of breast cancer risk using a validated breast cancer risk assessment tool.
I prefer the modified version of the International Breast Cancer Intervention Study (IBIS) tool, which is readily available online (https://ibis.ikonopedia.com/).7 This tool incorporates several breast cancer risk factors, including reproductive risk factors, body mass index, BRCA gene status, breast density, and family history. The tool takes 1 to 2 minutes to complete and provides an estimate of a woman’s 10-year risk and lifetime risk of breast cancer.
If the lifetime risk exceeds 20%, I offer the patient supplemental MRI screening, consistent with current recommendations of the National Comprehensive Cancer Network and the American Cancer Society.8,9 I generally recommend starting breast imaging screening 7 to 10 years prior to the youngest breast cancer occurrence in the family, with mammography starting no earlier than age 30 and MRI no earlier than age 25. Other validated tools also can be used.10-13
Incorporating breast density and other important risk factors allows a more comprehensive analysis upon which to counsel women about the value (benefits and harms) of breast imaging.8
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
- Sprague BL, Gagnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106:dju255. doi: 10.1093/jcni/dju255.
- Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
- Bakker MF, de Lange SV, Pijnappel RM, et al; for the DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102.
- Drukker CA, Schmidt MK, Rutgers EJT, et al. Mammographic screening detects low-risk tumor biology breast cancers. Breast Cancer Res Treat. 2014;144:103-111.
- Statista website. Resident population of the United States by sex and age as of July 1, 2018. https://www.statista.com/statistics/241488/population-of-the-us-by-sex-and-age. Accessed January 6, 2020.
- US Food and Drug Administration website. Mammography: what you need to know. https://www.fda.gov/consumers/consumer-updates/mammography-what-you-need-know. Accessed January 13, 2020.
- IBIS (International Breast Cancer Intervention Study) website. Online Tyrer-Cuzick Model Breast Cancer Risk Evaluation Tool. ibis.ikonopedia.com. Accessed January 13, 2020.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. Breast cancer screening and diagnosis: NCCN practice guidelines in oncology. JNCCN. 2009;7:1060-1096.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
- Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457-1466.
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer: implications for risk prediction. Cancer. 1994;73:643-651.
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145-158.
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
Should breast cancer screening guidelines be tailored to a patient’s race and ethnicity?
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.
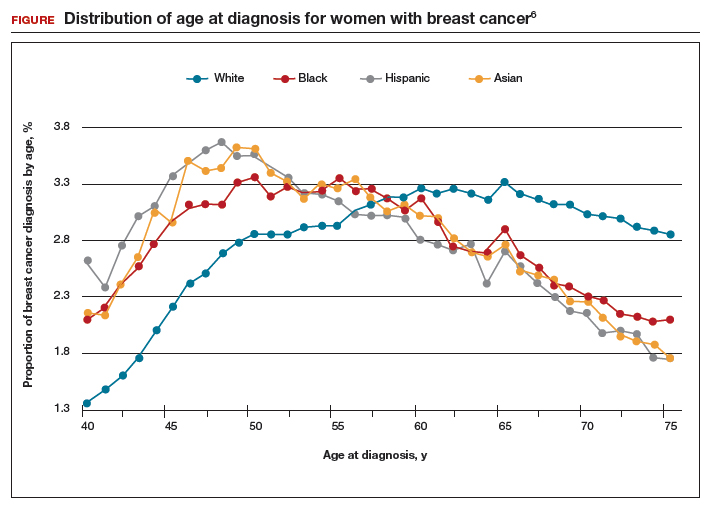
Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).
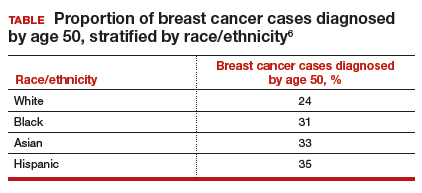
These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Breast cancer screening is an important aspect of women’s preventative health care, with proven mortality benefits.1,2 Different recommendations have been made for the age at initiation and the frequency of breast cancer screening in an effort to maximize benefit while minimizing unnecessary health care costs and harms of screening.
The American College of Obstetricians and Gynecologists (ACOG) and the National Comprehensive Cancer Network (NCCN) recommend initiating mammography screening at age 40, with annual screening (although ACOG offers deferral of screening to age 50 and biennial screening through shared decision making).3,4 The American Cancer Society (ACS) recommends offering annual mammography at ages 40 to 44 and recommends routinely starting annual mammography from 45 to 54, followed by either annual or biennial screening for women 55 and older.1 Finally, the US Preventive Services Task Force (USPSTF) recommends biennial mammography screening starting at age 50.5 No organization alters screening recommendations based on a woman’s race/ethnicity.
Details of the study
Stapleton and colleagues recently performed a retrospective population-based cohort study using the Surveillance, Epidemiology, and End Results (SEER) Program database to evaluate the age and stage at breast cancer diagnosis across different racial groups in the United States.6 The study (timeframe, January 1, 1973 to December 31, 2010) included 747,763 women, with a racial/ethnic distribution of 77.0% white, 9.3% black, 7.0% Hispanic, and 6.2% Asian.
The investigators found 2 distinct age distributions of breast cancer based on race. Among nonwhite women, the highest peak of breast cancer diagnoses occurred between 45 and 50 years (FIGURE). By contrast, breast cancer diagnoses peaked at 60 to 65 years in white women.

Similarly, a higher proportion of nonwhite women were diagnosed with their breast cancer prior to age 50 compared with white women. While one-quarter of white women with breast cancer develop disease prior to age 50, approximately one-third of black, Asian, and Hispanic women with breast cancer will be diagnosed before age 50 (TABLE).

These data suggest that the peak proportion of breast cancer diagnoses in nonwhite women occurs prior to the age of initiation of screening recommended by the USPSTF. Based on these results, Stapleton and colleagues recommend reconsideration of the current USPSTF guidelines to incorporate race/ethnicity–based differences. To diagnose the same proportion of breast cancer cases among nonwhite women as is currently possible among white women at age 50, initiation of breast cancer screening would need to be adjusted to age 47 for black women, age 46 for Hispanic women, and age 47 for Asian women.
Study strengths and weaknesses
This is a unique study that uses the SEER database to capture a large cross section of the American population. The SEER database is a valuable tool because it gathers data from numerous major US metropolitan areas, creating a diverse representative population that minimizes confounding from geographical trends. Nevertheless, any study utilizing a large database is limited by the accuracy and completeness of the data collected at the level of the individual cancer registry. Furthermore, information regarding medical comorbidities and access and adherence to breast cancer screening is lacking in the SEER database; this provides an opportunity for confounding.
Approximately one-third of breast cancer cases in nonwhite women, and one-quarter of cases in white women, occur prior to the age of initiation of screening (50 years) recommended by the USPSTF.
While some screening organizations do recommend that breast cancer screening be initiated prior to age 50, no organizations alter the recommendations for screening based on a woman's race/ethnicity.
Health care providers should be aware that initiation of breast cancer screening at age 50 in nonwhite women misses a disproportionate number of breast cancer cases compared with white women.
Providers should counsel nonwhite women about these differences in age of diagnosis and include that in their consideration of initiating breast cancer screening prior to the age of 50, more in accordance with recommendations of ACOG, NCCN, and ACS.
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
- Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice Bulletin No. 179: Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1–e16.
- Bevers TB, Anderson BO, Bonaccio E, et al; National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096.
- US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726.
- Stapleton SM, Oseni TO, Bababekov YJ, Hung Y-C, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg. Published online March 7, 2018. doi:10.1001/jamasurg.2018.0035.
How does oral contraceptive use affect one’s risk of ovarian, endometrial, breast, and colorectal cancers?
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.
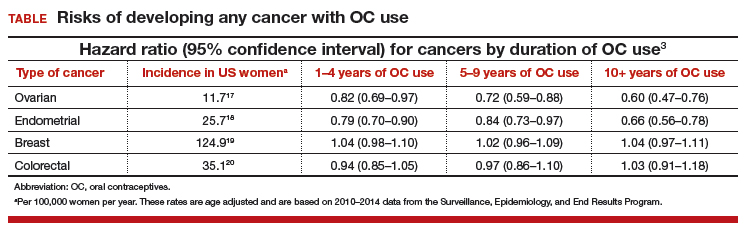
There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.

There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.

There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
Does hormonal contraception increase the risk of breast cancer?
Hormonal contraception (HC) has long been utilized safely in this country for a variety of indications, including pregnancy prevention, timing pregnancy appropriately, management of symptoms (dysmenorrhea, irregular menstrual cycles, heavy menstrual bleeding), and to prevent serious diseases (such as ovarian cancer, uterine cancer, osteoporosis in women with premature menopause). Like most prescription medications, there are potential adverse effects. With HC, side effects such as venous thromboembolism, a slight increase in liver cancer, and a possible increase in breast cancer risk have long been recognized.
Danish study compared HC use with breast cancer risk
In the December 7, 2017, issue of New England Journal of Medicine,1 investigators in Denmark published a study of women using HC (oral, transdermal, intravaginal routes, and levonorgestrel intrauterine device [LNG-IUD]) and breast cancer risk compared with women who did not use HC. This retrospective observational country-wide study was very large (1.8 million women followed over an average of 10.9 years), which allowed for the detection of even small changes in breast cancer risk.
Putting results in perspective
It is important to point out that this is an observational study, and small effect sizes (1 in 7,600) should be interpreted with caution. Observational studies can introduce many different types of bias (prescribing bias, confounding bias, etc). Of note, while the LNG-IUD was associated with a small increased risk of breast cancer (relative risk [RR], 1.21; 95% confidence interval [CI], 1.11-1.33]), the higher dose continuous progestin administration (medroxyprogesterone) was not (RR, 0.95; 95% CI, 0.40-2.29).1
Nonetheless, providing patients with a balanced summary of this new study along with other published and reliable information about HC that conveys both benefits and risks is important to assure that each woman makes a decision regarding HC that achieves her health and life goals. See "Counseling talking points" below.
Bottom line
This recent study demonstrated that in Denmark, a woman's risk of developing breast cancer is very slightly elevated on HC1:
- 1 in 7,690 users overall
- 1 in 50,000 women older than age 35 years.
By comparison, the risk of maternal mortality in the United States is 1 in 3,788.2 A substantial reduction in HC use would likely increase unintended and mistimed pregnancies with a potential substantial negative impact on quality of life and personal/societal cost.
The best available data indicate that a woman's risk of developing any cancer is slightly less on HC than not on HC, even with this incremental breast cancer increase.3,4
Breast cancer risk relative to benefits of pregnancy prevention
There was a very slight increase in breast cancer in women using HC in the Danish study.1
Risk of breast cancer
- Overall, the number needed to harm (NNH) was approximately 1 in 7,690, which equates to 13 incremental breast cancers for every 100,000 women using HC (0.013%).
- Breast cancer risk was not evenly distributed across the different age groups. In women younger than 35 years, the risk was 1 extra case for every 50,000 women using HC (0.002%).
Risk of pregnancy prevention failure: Maternal mortality
- By comparison, the rate of maternal mortality is considerably higher than either of these risks in the United States. Specifically, the most recently available rate of maternal mortality (2015) in the United States was 26.4 for every 100,000 women, essentially double that of developing breast cancer on HC.2
-- Most women who develop breast cancer while on HC will survive their cancer long-term.5 And most would agree that while neither is desirable, death is a worse outcome than the development of breast cancer.
Risk of pregnancy prevention failure other than maternal mortality
- Other than the copper IUD and sterilization methods, all other nonhormonal contraceptive methods are by far inferior in terms of the ability to prevent unintended pregnancy.
- Unintended pregnancy has substantial health, social, and economic consequences to women and infants, and contraception use is a well-accepted proximate determinant of unintended pregnancy.6
- Unintended pregnancy is a serious maternal-child health problem with potentially long-term burdens not only for women and families7-10 but also for society.11-13
- Unintended pregnancies generate an estimated $21 billion direct and indirect costs for the US health care system per year,14 and approximately 42% of these pregnancies end in abortion.15
HC cancer risk and HC cancer prevention
- HC use increases risk of breast and liver cancer but reduces risk of ovarian, endometrial, and colorectal cancer; the net effect is a modest reduction in total cancer.3,4
- In addition, there appears to be additional cervical cancer prevention benefit from IUD use.16
- In a recent meta-analysis, IUDs (including LNG-IUD) have been associated with a 33% reduction in cervical cancer.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch, LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775-1812.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193-200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276-2277.
- American Cancer Society. Breast Cancer Facts & Figures 2015-2016. Atlanta, Georgia: American Cancer Society, Inc; 2015.
- Sonfield A. What the Agency for Healthcare Research and Quality forgets to tell Americans about how to protect their sexual and reproductive health. Womens Health Issues. 2015;25(1):1-2.
- Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the wellbeing of children and families. Washington, DC: National Academy Press; 1995:50-90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281-286.
- Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing. The National Campaign to Prevent Teen Pregnancy and Child Trends. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf. Published May 2007. Accessed January 11, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126-127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
- Sonfield A, Kost K. Public costs from unintended pregnancy and the role of public insurance program in paying for pregnancy and infant care: Estimates for 2008. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP.pdf. Published October 2013. Accessed January 15, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6-15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: National and state estimates for 2010. Guttmacher Institute; 2015. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Accessed January 29, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Cortessis VK, Barrett M, Brown Wade N, et al. Intrauterine device use and cervical cancer risk: A systematic review and meta-analysis. Obstet Gynecol. 2017;130(6):1226-1236.
Hormonal contraception (HC) has long been utilized safely in this country for a variety of indications, including pregnancy prevention, timing pregnancy appropriately, management of symptoms (dysmenorrhea, irregular menstrual cycles, heavy menstrual bleeding), and to prevent serious diseases (such as ovarian cancer, uterine cancer, osteoporosis in women with premature menopause). Like most prescription medications, there are potential adverse effects. With HC, side effects such as venous thromboembolism, a slight increase in liver cancer, and a possible increase in breast cancer risk have long been recognized.
Danish study compared HC use with breast cancer risk
In the December 7, 2017, issue of New England Journal of Medicine,1 investigators in Denmark published a study of women using HC (oral, transdermal, intravaginal routes, and levonorgestrel intrauterine device [LNG-IUD]) and breast cancer risk compared with women who did not use HC. This retrospective observational country-wide study was very large (1.8 million women followed over an average of 10.9 years), which allowed for the detection of even small changes in breast cancer risk.
Putting results in perspective
It is important to point out that this is an observational study, and small effect sizes (1 in 7,600) should be interpreted with caution. Observational studies can introduce many different types of bias (prescribing bias, confounding bias, etc). Of note, while the LNG-IUD was associated with a small increased risk of breast cancer (relative risk [RR], 1.21; 95% confidence interval [CI], 1.11-1.33]), the higher dose continuous progestin administration (medroxyprogesterone) was not (RR, 0.95; 95% CI, 0.40-2.29).1
Nonetheless, providing patients with a balanced summary of this new study along with other published and reliable information about HC that conveys both benefits and risks is important to assure that each woman makes a decision regarding HC that achieves her health and life goals. See "Counseling talking points" below.
Bottom line
This recent study demonstrated that in Denmark, a woman's risk of developing breast cancer is very slightly elevated on HC1:
- 1 in 7,690 users overall
- 1 in 50,000 women older than age 35 years.
By comparison, the risk of maternal mortality in the United States is 1 in 3,788.2 A substantial reduction in HC use would likely increase unintended and mistimed pregnancies with a potential substantial negative impact on quality of life and personal/societal cost.
The best available data indicate that a woman's risk of developing any cancer is slightly less on HC than not on HC, even with this incremental breast cancer increase.3,4
Breast cancer risk relative to benefits of pregnancy prevention
There was a very slight increase in breast cancer in women using HC in the Danish study.1
Risk of breast cancer
- Overall, the number needed to harm (NNH) was approximately 1 in 7,690, which equates to 13 incremental breast cancers for every 100,000 women using HC (0.013%).
- Breast cancer risk was not evenly distributed across the different age groups. In women younger than 35 years, the risk was 1 extra case for every 50,000 women using HC (0.002%).
Risk of pregnancy prevention failure: Maternal mortality
- By comparison, the rate of maternal mortality is considerably higher than either of these risks in the United States. Specifically, the most recently available rate of maternal mortality (2015) in the United States was 26.4 for every 100,000 women, essentially double that of developing breast cancer on HC.2
-- Most women who develop breast cancer while on HC will survive their cancer long-term.5 And most would agree that while neither is desirable, death is a worse outcome than the development of breast cancer.
Risk of pregnancy prevention failure other than maternal mortality
- Other than the copper IUD and sterilization methods, all other nonhormonal contraceptive methods are by far inferior in terms of the ability to prevent unintended pregnancy.
- Unintended pregnancy has substantial health, social, and economic consequences to women and infants, and contraception use is a well-accepted proximate determinant of unintended pregnancy.6
- Unintended pregnancy is a serious maternal-child health problem with potentially long-term burdens not only for women and families7-10 but also for society.11-13
- Unintended pregnancies generate an estimated $21 billion direct and indirect costs for the US health care system per year,14 and approximately 42% of these pregnancies end in abortion.15
HC cancer risk and HC cancer prevention
- HC use increases risk of breast and liver cancer but reduces risk of ovarian, endometrial, and colorectal cancer; the net effect is a modest reduction in total cancer.3,4
- In addition, there appears to be additional cervical cancer prevention benefit from IUD use.16
- In a recent meta-analysis, IUDs (including LNG-IUD) have been associated with a 33% reduction in cervical cancer.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Hormonal contraception (HC) has long been utilized safely in this country for a variety of indications, including pregnancy prevention, timing pregnancy appropriately, management of symptoms (dysmenorrhea, irregular menstrual cycles, heavy menstrual bleeding), and to prevent serious diseases (such as ovarian cancer, uterine cancer, osteoporosis in women with premature menopause). Like most prescription medications, there are potential adverse effects. With HC, side effects such as venous thromboembolism, a slight increase in liver cancer, and a possible increase in breast cancer risk have long been recognized.
Danish study compared HC use with breast cancer risk
In the December 7, 2017, issue of New England Journal of Medicine,1 investigators in Denmark published a study of women using HC (oral, transdermal, intravaginal routes, and levonorgestrel intrauterine device [LNG-IUD]) and breast cancer risk compared with women who did not use HC. This retrospective observational country-wide study was very large (1.8 million women followed over an average of 10.9 years), which allowed for the detection of even small changes in breast cancer risk.
Putting results in perspective
It is important to point out that this is an observational study, and small effect sizes (1 in 7,600) should be interpreted with caution. Observational studies can introduce many different types of bias (prescribing bias, confounding bias, etc). Of note, while the LNG-IUD was associated with a small increased risk of breast cancer (relative risk [RR], 1.21; 95% confidence interval [CI], 1.11-1.33]), the higher dose continuous progestin administration (medroxyprogesterone) was not (RR, 0.95; 95% CI, 0.40-2.29).1
Nonetheless, providing patients with a balanced summary of this new study along with other published and reliable information about HC that conveys both benefits and risks is important to assure that each woman makes a decision regarding HC that achieves her health and life goals. See "Counseling talking points" below.
Bottom line
This recent study demonstrated that in Denmark, a woman's risk of developing breast cancer is very slightly elevated on HC1:
- 1 in 7,690 users overall
- 1 in 50,000 women older than age 35 years.
By comparison, the risk of maternal mortality in the United States is 1 in 3,788.2 A substantial reduction in HC use would likely increase unintended and mistimed pregnancies with a potential substantial negative impact on quality of life and personal/societal cost.
The best available data indicate that a woman's risk of developing any cancer is slightly less on HC than not on HC, even with this incremental breast cancer increase.3,4
Breast cancer risk relative to benefits of pregnancy prevention
There was a very slight increase in breast cancer in women using HC in the Danish study.1
Risk of breast cancer
- Overall, the number needed to harm (NNH) was approximately 1 in 7,690, which equates to 13 incremental breast cancers for every 100,000 women using HC (0.013%).
- Breast cancer risk was not evenly distributed across the different age groups. In women younger than 35 years, the risk was 1 extra case for every 50,000 women using HC (0.002%).
Risk of pregnancy prevention failure: Maternal mortality
- By comparison, the rate of maternal mortality is considerably higher than either of these risks in the United States. Specifically, the most recently available rate of maternal mortality (2015) in the United States was 26.4 for every 100,000 women, essentially double that of developing breast cancer on HC.2
-- Most women who develop breast cancer while on HC will survive their cancer long-term.5 And most would agree that while neither is desirable, death is a worse outcome than the development of breast cancer.
Risk of pregnancy prevention failure other than maternal mortality
- Other than the copper IUD and sterilization methods, all other nonhormonal contraceptive methods are by far inferior in terms of the ability to prevent unintended pregnancy.
- Unintended pregnancy has substantial health, social, and economic consequences to women and infants, and contraception use is a well-accepted proximate determinant of unintended pregnancy.6
- Unintended pregnancy is a serious maternal-child health problem with potentially long-term burdens not only for women and families7-10 but also for society.11-13
- Unintended pregnancies generate an estimated $21 billion direct and indirect costs for the US health care system per year,14 and approximately 42% of these pregnancies end in abortion.15
HC cancer risk and HC cancer prevention
- HC use increases risk of breast and liver cancer but reduces risk of ovarian, endometrial, and colorectal cancer; the net effect is a modest reduction in total cancer.3,4
- In addition, there appears to be additional cervical cancer prevention benefit from IUD use.16
- In a recent meta-analysis, IUDs (including LNG-IUD) have been associated with a 33% reduction in cervical cancer.16
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch, LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775-1812.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193-200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276-2277.
- American Cancer Society. Breast Cancer Facts & Figures 2015-2016. Atlanta, Georgia: American Cancer Society, Inc; 2015.
- Sonfield A. What the Agency for Healthcare Research and Quality forgets to tell Americans about how to protect their sexual and reproductive health. Womens Health Issues. 2015;25(1):1-2.
- Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the wellbeing of children and families. Washington, DC: National Academy Press; 1995:50-90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281-286.
- Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing. The National Campaign to Prevent Teen Pregnancy and Child Trends. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf. Published May 2007. Accessed January 11, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126-127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
- Sonfield A, Kost K. Public costs from unintended pregnancy and the role of public insurance program in paying for pregnancy and infant care: Estimates for 2008. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP.pdf. Published October 2013. Accessed January 15, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6-15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: National and state estimates for 2010. Guttmacher Institute; 2015. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Accessed January 29, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Cortessis VK, Barrett M, Brown Wade N, et al. Intrauterine device use and cervical cancer risk: A systematic review and meta-analysis. Obstet Gynecol. 2017;130(6):1226-1236.
- Mørch, LS, Skovlund CW, Hannaford PC, et al. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228-2239.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775-1812.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193-200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276-2277.
- American Cancer Society. Breast Cancer Facts & Figures 2015-2016. Atlanta, Georgia: American Cancer Society, Inc; 2015.
- Sonfield A. What the Agency for Healthcare Research and Quality forgets to tell Americans about how to protect their sexual and reproductive health. Womens Health Issues. 2015;25(1):1-2.
- Brown SS, Eisenberg L. The best intentions: Unintended pregnancy and the wellbeing of children and families. Washington, DC: National Academy Press; 1995:50-90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281-286.
- Logan C, Holcombe E, Manlove J, Ryan S. The consequences of unintended childbearing. The National Campaign to Prevent Teen Pregnancy and Child Trends. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf. Published May 2007. Accessed January 11, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126-127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
- Sonfield A, Kost K. Public costs from unintended pregnancy and the role of public insurance program in paying for pregnancy and infant care: Estimates for 2008. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP.pdf. Published October 2013. Accessed January 15, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6-15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: National and state estimates for 2010. Guttmacher Institute; 2015. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Accessed January 29, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Cortessis VK, Barrett M, Brown Wade N, et al. Intrauterine device use and cervical cancer risk: A systematic review and meta-analysis. Obstet Gynecol. 2017;130(6):1226-1236.
Breast cancer screening: Is the controversy of benefits versus harms resolved?
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4
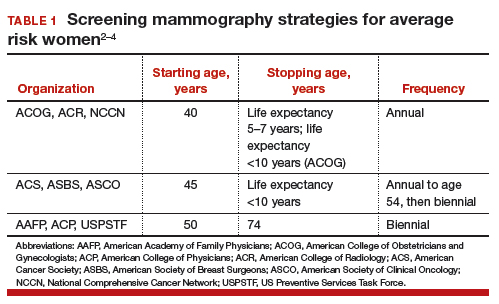
There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.
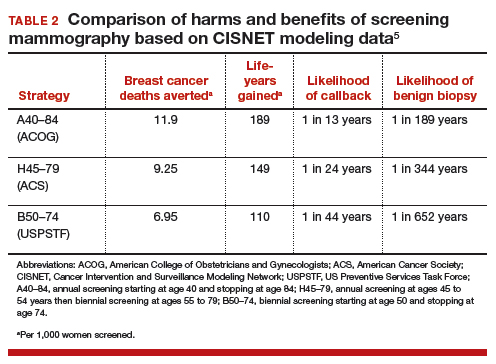
The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4

There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.

The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4

There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.

The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.