User login
Worsening motor function tied to post COVID syndrome in Parkinson’s disease
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Novel drug effective for essential tremor, but with significant side effects
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.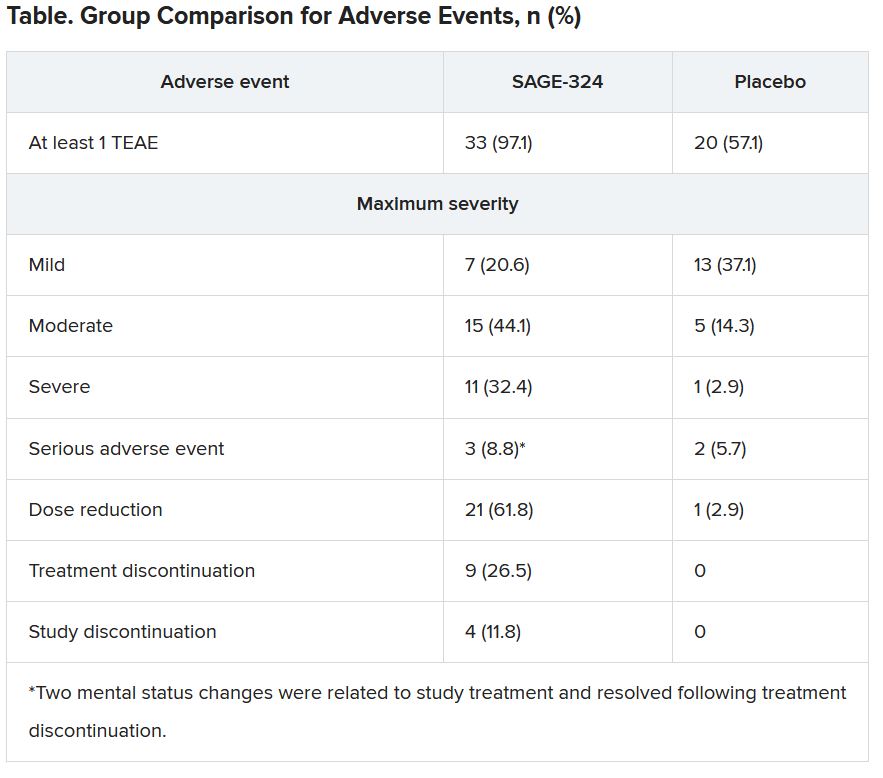
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Apple devices identify early Parkinson’s disease
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
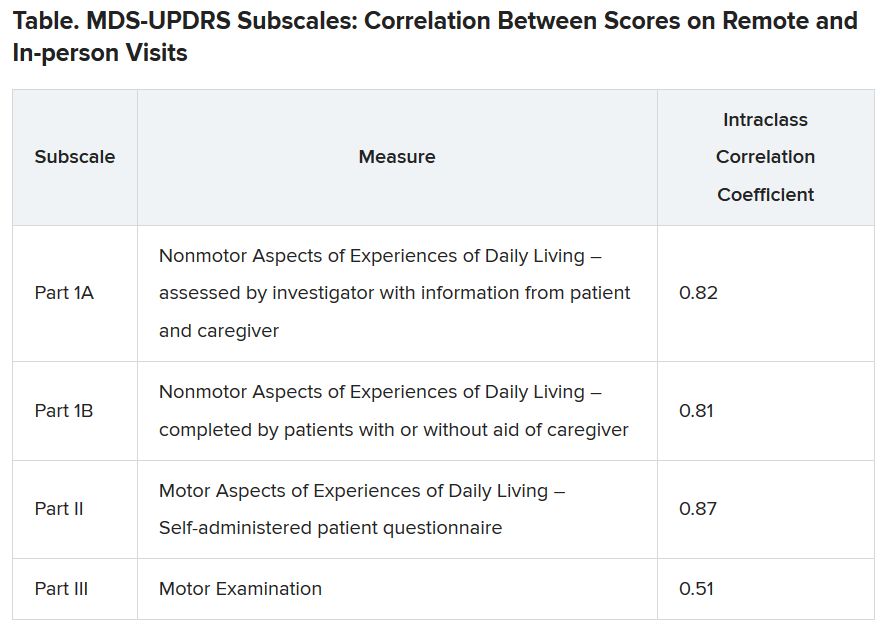
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.
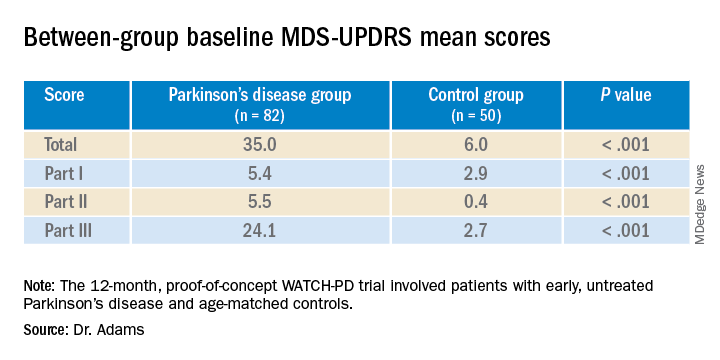
Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.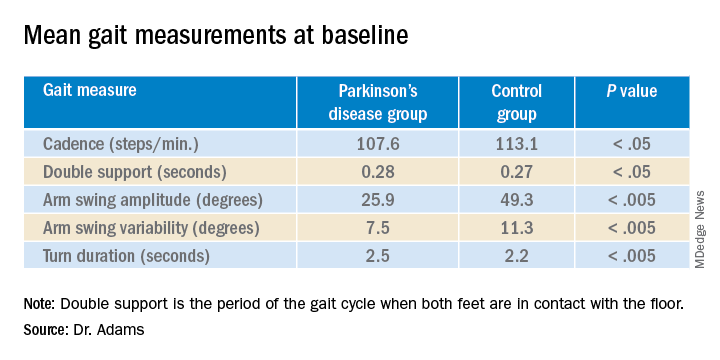
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.

Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.

Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Surge in new-onset tics in adults tied to COVID-19 stress
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a large, single-center study show several cases of tic-like movements and vocalizations with abrupt onset among older adolescents and adults during the pandemic. None had a previous diagnosis of a tic disorder. Among 10 patients, two were diagnosed with a purely functional movement disorder, four with an organic tic disorder, and four with both.
“Within our movement disorders clinic specifically ... we’ve been seeing an increased number of patients with an almost explosive onset of these tic-like movements and vocalizations later in life than what is typically seen with organic tic disorders and Tourette syndrome, which is typically in school-aged children,” said study investigator Caroline Olvera, MD, Rush University Medical Center, Chicago.
“Abrupt onset of symptoms can be seen in patients with tic disorders, although this is typically quoted as less than 10%, or even 5% is more characteristic of functional neurological disorders in general and also with psychogenic tics,” she added.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Anxiety, other psychiatric conditions
Tic disorders typically start in childhood. However, the researchers observed an increase in the number of patients with abrupt onset of tic-like movements and vocalizations later in life, which is more often characteristic of functional neurological disorders.
To examine the profile, associated conditions, and risk factors in this population, the investigators conducted a thorough chart review of patients attending movement disorder clinics between March 2020, when the COVID pandemic was officially declared, and March 2021.
Patients with acute onset of tics were identified using the International Classification of Diseases codes for behavioral tics, tic vocalizations, and Tourette syndrome.
The charts were then narrowed down to patients with no previous diagnosis of these conditions. Most patients were videotaped for assessment by the rest of the movement disorder neurologists in the practice. Since the end of the study inclusion period in March 2021, Dr. Olvera estimates that the clinic experienced a doubling or tripling of the number of similar patients.
In the study cohort of 10 patients, the median age at presentation was 19 years (range, 15-41 years), nine were female, the gender of the other one was unknown, and the duration of tics was 8 weeks (range, 1-24 weeks) by the time they were first seen in the clinic. Four patients reported having COVID infection before tic onset.
All exhibited motor tics and nine had vocal tics. Two were diagnosed with a purely functional neurologic disorder, four with only an organic tic disorder, and four with organic tics with a functional overlay.
“All patients, including those with organic tic disorders, had a history of anxiety and also reported worsening anxiety in the setting of the COVID pandemic,” Dr. Olvera said.
The majority of patients were on a psychotropic medication prior to coming to the clinic, and these were primarily for anxiety and depression. Three patients had a history of suicidality, often very severe and leading to hospitalization, she noted.
“In terms of our conclusions from the project, we feel that this phenotype of acute explosive onset of tic-like movements and vocalizations in this older population of adults, compared with typical organic tic disorders and Tourette syndrome, appears novel to the pandemic,” she said.
She cautioned that functional and organic tics share many characteristics and therefore may be difficult to differentiate.
COVID stress
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said the research highlights how clinicians’ understanding of particular diseases can be challenged during extraordinary events such as COVID-19 and the heightened stress it causes.
“I’m not surprised that these [disorders] might have had a spike during a stressful time as COVID,” he said.
Patients are “really scared and really anxious, they’re afraid to die, and they’re afraid that their life will be over. So they might express their psychological difficulty, their discomfort, with these calls for help that look like tics. But they’re not what we consider physiological or organic things,” he added.
Dr. Tagliati added that he doesn’t believe rapid tic onset in adults is not a complication of the coronavirus infection, but rather a consequence of psychological pressure brought on by the pandemic.
Treating underlying anxiety may be a useful approach, possibly with the support of psychiatrists, which in many cases is enough to relieve the conditions and overcome the symptoms, he noted.
However, at other times, it’s not that simple, he added. Sometimes patients “fall through the cracks between neurology and psychiatry,” Dr. Tagliati said.
Dr. Olvera and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Sublingual film well tolerated for Parkinson ‘off’ episodes
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.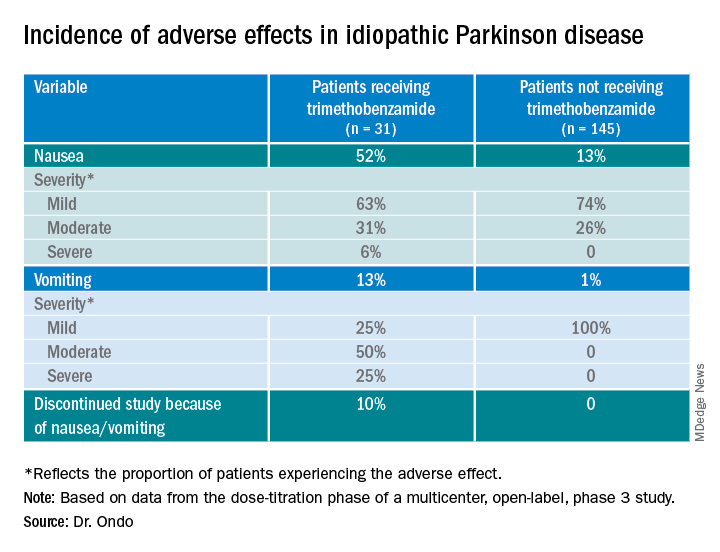
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Sublingual apomorphine alleviates off episodes in Parkinson’s disease
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS SOCIETY 2020
Prodrug infusion beats oral Parkinson’s disease therapy for motor symptoms
, according to a new study. The beneficial effects of these phosphate prodrugs of levodopa and carbidopa were most noticeable in the early morning, results of the phase 1B study showed.
As Parkinson’s disease progresses and dosing of oral levodopa/carbidopa (LD/CD) increases, its therapeutic window narrows, resulting in troublesome dyskinesia at peak drug levels and tremors and rigidity when levels fall.
“Foslevodopa/foscarbidopa shows lower ‘off’ time than oral levodopa/carbidopa, and this was statistically significant. Also, foslevodopa/foscarbidopa (fosL/fosC) showed more ‘on’ time without dyskinesia, compared with oral levodopa/carbidopa. This was also statistically significant,” lead author Sven Stodtmann, PhD, of AbbVie GmbH, Ludwigshafen, Germany, reported in his recorded presentation at the Movement Disorders Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorder (Virtual) 2020.
Continuous infusion versus oral therapy
The analysis included 20 patients, and all data from these individuals were collected between 4:30 a.m. and 9:30 p.m.
Participants were 12 men and 8 women, aged 30-80 years, with advanced, idiopathic Parkinson’s disease responsive to levodopa but inadequately controlled on their current stable therapy, having a minimum of 2.5 off hours/day. Mean age was 61.3 plus or minus 10.5 years (range 35-77 years).
In this single-arm, open-label study, they received subcutaneous infusions of personalized therapeutic doses of fosL/fosC 24 hours/day for 28 days after a 10- to 30-day screening period during which they recorded LD/CD doses in a diary and had motor symptoms monitored using a wearable device.
Following the screening period, fosL/fosC doses were titrated over up to 5 days, with subsequent weekly study visits, for a total time on fosL/fosC of 28 days. Drug titration was aimed at maximizing functional on time and minimizing the number of off episodes while minimizing troublesome dyskinesia.
Continuous infusion of fosL/fosC performed better than oral LD/CD on all counts.
“The off time is much lower in the morning for people on foslevodopa/foscarbidopa [compared with oral LD/CD] because this is a 24-hour infusion product,” Dr. Stodtmann explained.
The effect was maintained over the course of the day with little fluctuation with fosL/fosC, off periods never exceeding about 25% between 4:30 a.m. and 9 p.m. For LD/CD, off periods were highest in the early morning and peaked at about 50% on a 3- to 4-hour cycle during the course of the day.
Increased on time without dyskinesia varied between about 60% and 80% during the day with fosL/fosC, showing the greatest difference between fosL/fosC and oral LD/CD in the early morning hours.
“On time with nontroublesome dyskinesia was lower for foscarbidopa/foslevodopa, compared to oral levodopa/carbidopa, but this was not statistically significant,” Dr. Stodtmann said. On time with troublesome dyskinesia followed the same pattern, again, not statistically significant.
Looking at the data another way, the investigators calculated the odds ratios of motor symptoms using fosL/fosC, compared with oral LD/CD. Use of fosL/fosC was associated with a 59% lower risk of being in the off state during the day, compared with oral LD/CD (odds ratio, 0.4; 95% confidence interval, 0.2-0.7; P < .01). Similarly, the probability of being in the on state without dyskinesia was much greater with fosL/fosC (OR, 2.75; 95% CI, 1.08-6.99; P < .05).
Encouraging, but more data needed
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that the field has been waiting to see data on fosL/fosC.
“It seems like it’s pretty reasonable in terms of what the goals were, which is to improve stability of Parkinson’s symptoms, to improve off time and give on time without troublesome dyskinesia,” she said. “So I think those [goals] have been met.”
Dr. Subramanian, who was not involved with the research, said she would have liked to have seen results concerning safety of this drug formulation, which the presentation lacked, “because historically, there have been issues with nodule formation and skin breakdown, things like that, due to the stability of the product in the subcutaneous form. … So, always to my understanding, there has been this search for things that are tolerated in the subcutaneous delivery.”
If this formulation proves safe and tolerable, Dr. Subramanian sees a potential place for it for some patients with advanced Parkinson’s disease.
“Certainly a subcutaneous formulation will be better than something that requires … deep brain surgery or even a pump insertion like Duopa [carbidopa/levodopa enteral suspension, AbbVie] or something like that,” she said. “I think [it] would be beneficial over something with the gut because the gut historically has been a problem to rely on in advanced Parkinson’s patients due to slower transit times, and the gut itself is affected with Parkinson’s disease.”
Dr. Stodtmann and all coauthors are employees of AbbVie, which was the sponsor of the study and was responsible for all aspects of it. Dr. Subramanian has given talks for Acadia Pharmaceuticals and Acorda Therapeutics in the past.
A version of this article originally appeared on Medscape.com.
, according to a new study. The beneficial effects of these phosphate prodrugs of levodopa and carbidopa were most noticeable in the early morning, results of the phase 1B study showed.
As Parkinson’s disease progresses and dosing of oral levodopa/carbidopa (LD/CD) increases, its therapeutic window narrows, resulting in troublesome dyskinesia at peak drug levels and tremors and rigidity when levels fall.
“Foslevodopa/foscarbidopa shows lower ‘off’ time than oral levodopa/carbidopa, and this was statistically significant. Also, foslevodopa/foscarbidopa (fosL/fosC) showed more ‘on’ time without dyskinesia, compared with oral levodopa/carbidopa. This was also statistically significant,” lead author Sven Stodtmann, PhD, of AbbVie GmbH, Ludwigshafen, Germany, reported in his recorded presentation at the Movement Disorders Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorder (Virtual) 2020.
Continuous infusion versus oral therapy
The analysis included 20 patients, and all data from these individuals were collected between 4:30 a.m. and 9:30 p.m.
Participants were 12 men and 8 women, aged 30-80 years, with advanced, idiopathic Parkinson’s disease responsive to levodopa but inadequately controlled on their current stable therapy, having a minimum of 2.5 off hours/day. Mean age was 61.3 plus or minus 10.5 years (range 35-77 years).
In this single-arm, open-label study, they received subcutaneous infusions of personalized therapeutic doses of fosL/fosC 24 hours/day for 28 days after a 10- to 30-day screening period during which they recorded LD/CD doses in a diary and had motor symptoms monitored using a wearable device.
Following the screening period, fosL/fosC doses were titrated over up to 5 days, with subsequent weekly study visits, for a total time on fosL/fosC of 28 days. Drug titration was aimed at maximizing functional on time and minimizing the number of off episodes while minimizing troublesome dyskinesia.
Continuous infusion of fosL/fosC performed better than oral LD/CD on all counts.
“The off time is much lower in the morning for people on foslevodopa/foscarbidopa [compared with oral LD/CD] because this is a 24-hour infusion product,” Dr. Stodtmann explained.
The effect was maintained over the course of the day with little fluctuation with fosL/fosC, off periods never exceeding about 25% between 4:30 a.m. and 9 p.m. For LD/CD, off periods were highest in the early morning and peaked at about 50% on a 3- to 4-hour cycle during the course of the day.
Increased on time without dyskinesia varied between about 60% and 80% during the day with fosL/fosC, showing the greatest difference between fosL/fosC and oral LD/CD in the early morning hours.
“On time with nontroublesome dyskinesia was lower for foscarbidopa/foslevodopa, compared to oral levodopa/carbidopa, but this was not statistically significant,” Dr. Stodtmann said. On time with troublesome dyskinesia followed the same pattern, again, not statistically significant.
Looking at the data another way, the investigators calculated the odds ratios of motor symptoms using fosL/fosC, compared with oral LD/CD. Use of fosL/fosC was associated with a 59% lower risk of being in the off state during the day, compared with oral LD/CD (odds ratio, 0.4; 95% confidence interval, 0.2-0.7; P < .01). Similarly, the probability of being in the on state without dyskinesia was much greater with fosL/fosC (OR, 2.75; 95% CI, 1.08-6.99; P < .05).
Encouraging, but more data needed
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that the field has been waiting to see data on fosL/fosC.
“It seems like it’s pretty reasonable in terms of what the goals were, which is to improve stability of Parkinson’s symptoms, to improve off time and give on time without troublesome dyskinesia,” she said. “So I think those [goals] have been met.”
Dr. Subramanian, who was not involved with the research, said she would have liked to have seen results concerning safety of this drug formulation, which the presentation lacked, “because historically, there have been issues with nodule formation and skin breakdown, things like that, due to the stability of the product in the subcutaneous form. … So, always to my understanding, there has been this search for things that are tolerated in the subcutaneous delivery.”
If this formulation proves safe and tolerable, Dr. Subramanian sees a potential place for it for some patients with advanced Parkinson’s disease.
“Certainly a subcutaneous formulation will be better than something that requires … deep brain surgery or even a pump insertion like Duopa [carbidopa/levodopa enteral suspension, AbbVie] or something like that,” she said. “I think [it] would be beneficial over something with the gut because the gut historically has been a problem to rely on in advanced Parkinson’s patients due to slower transit times, and the gut itself is affected with Parkinson’s disease.”
Dr. Stodtmann and all coauthors are employees of AbbVie, which was the sponsor of the study and was responsible for all aspects of it. Dr. Subramanian has given talks for Acadia Pharmaceuticals and Acorda Therapeutics in the past.
A version of this article originally appeared on Medscape.com.
, according to a new study. The beneficial effects of these phosphate prodrugs of levodopa and carbidopa were most noticeable in the early morning, results of the phase 1B study showed.
As Parkinson’s disease progresses and dosing of oral levodopa/carbidopa (LD/CD) increases, its therapeutic window narrows, resulting in troublesome dyskinesia at peak drug levels and tremors and rigidity when levels fall.
“Foslevodopa/foscarbidopa shows lower ‘off’ time than oral levodopa/carbidopa, and this was statistically significant. Also, foslevodopa/foscarbidopa (fosL/fosC) showed more ‘on’ time without dyskinesia, compared with oral levodopa/carbidopa. This was also statistically significant,” lead author Sven Stodtmann, PhD, of AbbVie GmbH, Ludwigshafen, Germany, reported in his recorded presentation at the Movement Disorders Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorder (Virtual) 2020.
Continuous infusion versus oral therapy
The analysis included 20 patients, and all data from these individuals were collected between 4:30 a.m. and 9:30 p.m.
Participants were 12 men and 8 women, aged 30-80 years, with advanced, idiopathic Parkinson’s disease responsive to levodopa but inadequately controlled on their current stable therapy, having a minimum of 2.5 off hours/day. Mean age was 61.3 plus or minus 10.5 years (range 35-77 years).
In this single-arm, open-label study, they received subcutaneous infusions of personalized therapeutic doses of fosL/fosC 24 hours/day for 28 days after a 10- to 30-day screening period during which they recorded LD/CD doses in a diary and had motor symptoms monitored using a wearable device.
Following the screening period, fosL/fosC doses were titrated over up to 5 days, with subsequent weekly study visits, for a total time on fosL/fosC of 28 days. Drug titration was aimed at maximizing functional on time and minimizing the number of off episodes while minimizing troublesome dyskinesia.
Continuous infusion of fosL/fosC performed better than oral LD/CD on all counts.
“The off time is much lower in the morning for people on foslevodopa/foscarbidopa [compared with oral LD/CD] because this is a 24-hour infusion product,” Dr. Stodtmann explained.
The effect was maintained over the course of the day with little fluctuation with fosL/fosC, off periods never exceeding about 25% between 4:30 a.m. and 9 p.m. For LD/CD, off periods were highest in the early morning and peaked at about 50% on a 3- to 4-hour cycle during the course of the day.
Increased on time without dyskinesia varied between about 60% and 80% during the day with fosL/fosC, showing the greatest difference between fosL/fosC and oral LD/CD in the early morning hours.
“On time with nontroublesome dyskinesia was lower for foscarbidopa/foslevodopa, compared to oral levodopa/carbidopa, but this was not statistically significant,” Dr. Stodtmann said. On time with troublesome dyskinesia followed the same pattern, again, not statistically significant.
Looking at the data another way, the investigators calculated the odds ratios of motor symptoms using fosL/fosC, compared with oral LD/CD. Use of fosL/fosC was associated with a 59% lower risk of being in the off state during the day, compared with oral LD/CD (odds ratio, 0.4; 95% confidence interval, 0.2-0.7; P < .01). Similarly, the probability of being in the on state without dyskinesia was much greater with fosL/fosC (OR, 2.75; 95% CI, 1.08-6.99; P < .05).
Encouraging, but more data needed
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that the field has been waiting to see data on fosL/fosC.
“It seems like it’s pretty reasonable in terms of what the goals were, which is to improve stability of Parkinson’s symptoms, to improve off time and give on time without troublesome dyskinesia,” she said. “So I think those [goals] have been met.”
Dr. Subramanian, who was not involved with the research, said she would have liked to have seen results concerning safety of this drug formulation, which the presentation lacked, “because historically, there have been issues with nodule formation and skin breakdown, things like that, due to the stability of the product in the subcutaneous form. … So, always to my understanding, there has been this search for things that are tolerated in the subcutaneous delivery.”
If this formulation proves safe and tolerable, Dr. Subramanian sees a potential place for it for some patients with advanced Parkinson’s disease.
“Certainly a subcutaneous formulation will be better than something that requires … deep brain surgery or even a pump insertion like Duopa [carbidopa/levodopa enteral suspension, AbbVie] or something like that,” she said. “I think [it] would be beneficial over something with the gut because the gut historically has been a problem to rely on in advanced Parkinson’s patients due to slower transit times, and the gut itself is affected with Parkinson’s disease.”
Dr. Stodtmann and all coauthors are employees of AbbVie, which was the sponsor of the study and was responsible for all aspects of it. Dr. Subramanian has given talks for Acadia Pharmaceuticals and Acorda Therapeutics in the past.
A version of this article originally appeared on Medscape.com.
Telemedicine feasible and reliable in Parkinson’s trial
, a 1-year, phase 3 clinical trial has shown. The trial was an add-on study involving a subset of subjects from the STEADY-PD III trial of isradipine in early Parkinson’s disease.
Although the trial was conducted before SARS-CoV-2 arrived on the scene, the findings have particular relevance for being able to conduct a variety of clinical trials in the face of COVID-19 and the need to limit in-person interactions.
The 40 participants used tablets to complete three remote, video-based assessments during 1 year, with each remote visit planned to be completed within 4 weeks of an in-person visit. It was easy to enroll patients, and they completed about 95% of planned visits, said neurologist Christopher Tarolli, MD, of the University of Rochester (N.Y.).
He presented the study findings at the Movement Disorder Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
“The visits were clearly feasible, and we were able to do them [84%] within that 4-week time frame around the in-person visit,” he said. “The visits were also reasonably reliable, particularly so for what we call the nonmotor outcomes and the patient-reported outcomes.”
In-person versus remote assessment
For the remote visits, participants completed primarily the same battery of tests as the in-person visits. Responses on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) subscales demonstrated “that there was excellent correlation between patient-reported and nonmotor outcome measures and moderate correlation between in-person and remote-performed motor assessments,” Dr. Tarolli said.
He explained that the study used modified motor assessments (MDS-UPDRS Part III) that excluded testing of rigidity and postural instability, which require hands-on testing by a trained examiner and thus are impossible to do remotely.
Additionally, the somewhat lower correlation on this subscale was probably the result of different investigators conducting in-person versus remote assessments, with a subset of in-person investigators who tended to rate participants more severely driving down the correlation. “I think if these methods were applied in future trials, the in-person and remote investigators would optimally be the same person,” Dr. Tarolli suggested.
Room for error?
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that “the reliability of UPDRS [part] III is where I would want to have, for sure, a little bit more of a deep dive. … possibly the same patient be rated by the same person.”
She also noted that doing remote and in-person assessments within 4 weeks of each other leaves a lot of room for variability. “You could see the same patient in the morning and then do UPDRS in the afternoon, and it can be totally different depending on when you meet the person,” she said.
Only so much testing can be done remotely. Nonetheless, she questioned whether it is really a valid UPDRS if rigidity and postural stability measures are eliminated. “[Is] this now a new modified UPDRS that we’re going to use that is as good as the old UPDRS moving forward, a home version of UPDRS or whatever we’re going to call it?”
Dr. Subramanian mentioned that patients have told her that UPDRS part III does not really measure what is most important to them, such as making pastries for their grandchildren rather than rapidly tapping their fingers.
“That speaks a little bit to the fact that we should have more patient-centered outcomes and things that patients can report. … things that are not going to require necessarily an in-person exam as maybe measures that really can be used moving forward in studies,” she suggested.
Patient satisfaction with remote visits
Greater than 90% of the patients were satisfied or very satisfied overall with the remote visits, including the convenience, comfort, and connection (using the devices and Internet connection), with “patients describing enjoying being able to do these visits from the comfort of their own home, not having to travel,” Dr. Tarolli said. Not having to drive in an ‘off’ state “was actually something that some participants identified as a safety benefit from this as well.”
There was also a time benefit to the patients and investigators. The average length of the remote visits was 54.3 minutes each versus 74 minutes of interaction for in-person visits, mainly a result of more efficient hand-offs between the neurologist and the study coordinator during the remote visits, plus being able to pause the remote visit to give a medication dose time to take effect.
For the patient, there was a large amount of time saved when travel time was considered – a total of 190.2 minutes on average for travel and testing for the in-person visits.
About three-quarters (76%) of the study patients said that remote visits would increase their likelihood of participating in future trials. However, that result may be skewed by the fact that these were already people willing to participate in a remote trial, so the generalizability of the result may be affected. Nonetheless, Dr. Tarolli said he thinks that, as technology gets better and older people become more comfortable with it, remote visits within Parkinson’s research studies may become more common.
One caveat he mentioned is that, with remote visits, the neurologist misses a chance to observe a patient’s whole body and construct a global impression of how he or she is moving. On the other hand, remote video gives the investigator the chance to see the living environment of the patient and suggest changes for safety, such as to reduce the risk of falling for a person with unsteadiness of gait living in a crowded house.
“It really allows us to make a more holistic assessment of how our patient is functioning outside the clinic, which I think we’ve traditionally had really no way of doing,” Dr. Tarolli said.
His final suggestion for anyone contemplating conducting studies with remote visits is to develop a team that is comfortable troubleshooting the technological aspects of those visits.
UCLA’s Dr. Subramanian lauded the University of Rochester team for their efforts in moving remote visits forward. “They’re at the cutting edge of these sorts of things,” she said. “So I’m assuming that they’ll come out with more things [for visits] to become better that are going to move this forward, which is exciting.”
Dr. Tarolli has disclosed no relevant financial relationships. Dr. Subramanian has given talks for Acorda Pharmaceuticals and Acadia Pharmaceuticals in the past. The study had only university, government, foundation, and other nonprofit support.
A version of this article originally appeared on Medscape.com.
, a 1-year, phase 3 clinical trial has shown. The trial was an add-on study involving a subset of subjects from the STEADY-PD III trial of isradipine in early Parkinson’s disease.
Although the trial was conducted before SARS-CoV-2 arrived on the scene, the findings have particular relevance for being able to conduct a variety of clinical trials in the face of COVID-19 and the need to limit in-person interactions.
The 40 participants used tablets to complete three remote, video-based assessments during 1 year, with each remote visit planned to be completed within 4 weeks of an in-person visit. It was easy to enroll patients, and they completed about 95% of planned visits, said neurologist Christopher Tarolli, MD, of the University of Rochester (N.Y.).
He presented the study findings at the Movement Disorder Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
“The visits were clearly feasible, and we were able to do them [84%] within that 4-week time frame around the in-person visit,” he said. “The visits were also reasonably reliable, particularly so for what we call the nonmotor outcomes and the patient-reported outcomes.”
In-person versus remote assessment
For the remote visits, participants completed primarily the same battery of tests as the in-person visits. Responses on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) subscales demonstrated “that there was excellent correlation between patient-reported and nonmotor outcome measures and moderate correlation between in-person and remote-performed motor assessments,” Dr. Tarolli said.
He explained that the study used modified motor assessments (MDS-UPDRS Part III) that excluded testing of rigidity and postural instability, which require hands-on testing by a trained examiner and thus are impossible to do remotely.
Additionally, the somewhat lower correlation on this subscale was probably the result of different investigators conducting in-person versus remote assessments, with a subset of in-person investigators who tended to rate participants more severely driving down the correlation. “I think if these methods were applied in future trials, the in-person and remote investigators would optimally be the same person,” Dr. Tarolli suggested.
Room for error?
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that “the reliability of UPDRS [part] III is where I would want to have, for sure, a little bit more of a deep dive. … possibly the same patient be rated by the same person.”
She also noted that doing remote and in-person assessments within 4 weeks of each other leaves a lot of room for variability. “You could see the same patient in the morning and then do UPDRS in the afternoon, and it can be totally different depending on when you meet the person,” she said.
Only so much testing can be done remotely. Nonetheless, she questioned whether it is really a valid UPDRS if rigidity and postural stability measures are eliminated. “[Is] this now a new modified UPDRS that we’re going to use that is as good as the old UPDRS moving forward, a home version of UPDRS or whatever we’re going to call it?”
Dr. Subramanian mentioned that patients have told her that UPDRS part III does not really measure what is most important to them, such as making pastries for their grandchildren rather than rapidly tapping their fingers.
“That speaks a little bit to the fact that we should have more patient-centered outcomes and things that patients can report. … things that are not going to require necessarily an in-person exam as maybe measures that really can be used moving forward in studies,” she suggested.
Patient satisfaction with remote visits
Greater than 90% of the patients were satisfied or very satisfied overall with the remote visits, including the convenience, comfort, and connection (using the devices and Internet connection), with “patients describing enjoying being able to do these visits from the comfort of their own home, not having to travel,” Dr. Tarolli said. Not having to drive in an ‘off’ state “was actually something that some participants identified as a safety benefit from this as well.”
There was also a time benefit to the patients and investigators. The average length of the remote visits was 54.3 minutes each versus 74 minutes of interaction for in-person visits, mainly a result of more efficient hand-offs between the neurologist and the study coordinator during the remote visits, plus being able to pause the remote visit to give a medication dose time to take effect.
For the patient, there was a large amount of time saved when travel time was considered – a total of 190.2 minutes on average for travel and testing for the in-person visits.
About three-quarters (76%) of the study patients said that remote visits would increase their likelihood of participating in future trials. However, that result may be skewed by the fact that these were already people willing to participate in a remote trial, so the generalizability of the result may be affected. Nonetheless, Dr. Tarolli said he thinks that, as technology gets better and older people become more comfortable with it, remote visits within Parkinson’s research studies may become more common.
One caveat he mentioned is that, with remote visits, the neurologist misses a chance to observe a patient’s whole body and construct a global impression of how he or she is moving. On the other hand, remote video gives the investigator the chance to see the living environment of the patient and suggest changes for safety, such as to reduce the risk of falling for a person with unsteadiness of gait living in a crowded house.
“It really allows us to make a more holistic assessment of how our patient is functioning outside the clinic, which I think we’ve traditionally had really no way of doing,” Dr. Tarolli said.
His final suggestion for anyone contemplating conducting studies with remote visits is to develop a team that is comfortable troubleshooting the technological aspects of those visits.
UCLA’s Dr. Subramanian lauded the University of Rochester team for their efforts in moving remote visits forward. “They’re at the cutting edge of these sorts of things,” she said. “So I’m assuming that they’ll come out with more things [for visits] to become better that are going to move this forward, which is exciting.”
Dr. Tarolli has disclosed no relevant financial relationships. Dr. Subramanian has given talks for Acorda Pharmaceuticals and Acadia Pharmaceuticals in the past. The study had only university, government, foundation, and other nonprofit support.
A version of this article originally appeared on Medscape.com.
, a 1-year, phase 3 clinical trial has shown. The trial was an add-on study involving a subset of subjects from the STEADY-PD III trial of isradipine in early Parkinson’s disease.
Although the trial was conducted before SARS-CoV-2 arrived on the scene, the findings have particular relevance for being able to conduct a variety of clinical trials in the face of COVID-19 and the need to limit in-person interactions.
The 40 participants used tablets to complete three remote, video-based assessments during 1 year, with each remote visit planned to be completed within 4 weeks of an in-person visit. It was easy to enroll patients, and they completed about 95% of planned visits, said neurologist Christopher Tarolli, MD, of the University of Rochester (N.Y.).
He presented the study findings at the Movement Disorder Society’s 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
“The visits were clearly feasible, and we were able to do them [84%] within that 4-week time frame around the in-person visit,” he said. “The visits were also reasonably reliable, particularly so for what we call the nonmotor outcomes and the patient-reported outcomes.”
In-person versus remote assessment
For the remote visits, participants completed primarily the same battery of tests as the in-person visits. Responses on the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) subscales demonstrated “that there was excellent correlation between patient-reported and nonmotor outcome measures and moderate correlation between in-person and remote-performed motor assessments,” Dr. Tarolli said.
He explained that the study used modified motor assessments (MDS-UPDRS Part III) that excluded testing of rigidity and postural instability, which require hands-on testing by a trained examiner and thus are impossible to do remotely.
Additionally, the somewhat lower correlation on this subscale was probably the result of different investigators conducting in-person versus remote assessments, with a subset of in-person investigators who tended to rate participants more severely driving down the correlation. “I think if these methods were applied in future trials, the in-person and remote investigators would optimally be the same person,” Dr. Tarolli suggested.
Room for error?
Indu Subramanian, MD, of the department of neurology at the University of California, Los Angeles, and director of the Parkinson’s Disease Research, Education, and Clinical Center at the West Los Angeles Veterans Affairs Hospital, commented that “the reliability of UPDRS [part] III is where I would want to have, for sure, a little bit more of a deep dive. … possibly the same patient be rated by the same person.”
She also noted that doing remote and in-person assessments within 4 weeks of each other leaves a lot of room for variability. “You could see the same patient in the morning and then do UPDRS in the afternoon, and it can be totally different depending on when you meet the person,” she said.
Only so much testing can be done remotely. Nonetheless, she questioned whether it is really a valid UPDRS if rigidity and postural stability measures are eliminated. “[Is] this now a new modified UPDRS that we’re going to use that is as good as the old UPDRS moving forward, a home version of UPDRS or whatever we’re going to call it?”
Dr. Subramanian mentioned that patients have told her that UPDRS part III does not really measure what is most important to them, such as making pastries for their grandchildren rather than rapidly tapping their fingers.
“That speaks a little bit to the fact that we should have more patient-centered outcomes and things that patients can report. … things that are not going to require necessarily an in-person exam as maybe measures that really can be used moving forward in studies,” she suggested.
Patient satisfaction with remote visits
Greater than 90% of the patients were satisfied or very satisfied overall with the remote visits, including the convenience, comfort, and connection (using the devices and Internet connection), with “patients describing enjoying being able to do these visits from the comfort of their own home, not having to travel,” Dr. Tarolli said. Not having to drive in an ‘off’ state “was actually something that some participants identified as a safety benefit from this as well.”
There was also a time benefit to the patients and investigators. The average length of the remote visits was 54.3 minutes each versus 74 minutes of interaction for in-person visits, mainly a result of more efficient hand-offs between the neurologist and the study coordinator during the remote visits, plus being able to pause the remote visit to give a medication dose time to take effect.
For the patient, there was a large amount of time saved when travel time was considered – a total of 190.2 minutes on average for travel and testing for the in-person visits.
About three-quarters (76%) of the study patients said that remote visits would increase their likelihood of participating in future trials. However, that result may be skewed by the fact that these were already people willing to participate in a remote trial, so the generalizability of the result may be affected. Nonetheless, Dr. Tarolli said he thinks that, as technology gets better and older people become more comfortable with it, remote visits within Parkinson’s research studies may become more common.
One caveat he mentioned is that, with remote visits, the neurologist misses a chance to observe a patient’s whole body and construct a global impression of how he or she is moving. On the other hand, remote video gives the investigator the chance to see the living environment of the patient and suggest changes for safety, such as to reduce the risk of falling for a person with unsteadiness of gait living in a crowded house.
“It really allows us to make a more holistic assessment of how our patient is functioning outside the clinic, which I think we’ve traditionally had really no way of doing,” Dr. Tarolli said.
His final suggestion for anyone contemplating conducting studies with remote visits is to develop a team that is comfortable troubleshooting the technological aspects of those visits.
UCLA’s Dr. Subramanian lauded the University of Rochester team for their efforts in moving remote visits forward. “They’re at the cutting edge of these sorts of things,” she said. “So I’m assuming that they’ll come out with more things [for visits] to become better that are going to move this forward, which is exciting.”
Dr. Tarolli has disclosed no relevant financial relationships. Dr. Subramanian has given talks for Acorda Pharmaceuticals and Acadia Pharmaceuticals in the past. The study had only university, government, foundation, and other nonprofit support.
A version of this article originally appeared on Medscape.com.
Hypnosis may relieve pain, cut reliance on morphine at atrial flutter ablation
used as a control, in a small randomized trial.
Ablation is typically performed using conscious sedation and “requires sometimes very high dosages of morphine, and there are sometimes some complications, blood pressure drop, or oxygen desaturation,” Rodrigue Garcia, MD, Poitiers (France) University Hospital, said in an interview.
But patients in the study assigned to undergo hypnosis during the AFlut ablation, performed by practitioners hailing from the French Hypnosis Association, consistently perceived significantly less pain throughout the procedure than those in the active-control group.
They also used almost two-thirds less morphine, which was available to both groups on demand, reported Dr. Garcia, who presented the results of the PAINLESS study at the European Heart Rhythm Association 2020 Virtual Congress. The annual meeting was conducted online this year because of the COVID-19 pandemic.
Hypnotism for pain control may not be widely available in hospitals, “but it’s becoming more and more frequent in the different centers, especially in France,” he said.
The technique is probably also suitable for catheter ablation of ventricular tachycardia, Dr. Garcia said, and “we already use it for atrial fibrillation ablation, because it’s a very common procedure and because, in France, for example, there is a lack of anesthesiologists.” One limitation of hypnosis for such procedures, he said, is that it requires a practitioner with a lot of training and experience.
The current study, “I think, is one of the few, if not the first, randomized trial on this topic, at least for flutter,” Elena Arbelo, MD, PhD, MSc, Hospital Clinic of Barcelona and the University of Barcelona, said in an interview.
“I thought it was very interesting. Many centers have the issue of not having anesthesiology support for their procedures. We have the option of having anesthesiology with us only a few days a week,” said Dr. Arbelo, who was not an investigator with the study.
“If it’s validated in larger cohorts and in different cultures, it may be an interesting way of reducing the need for anesthesiology support, which is a main issue. I know for sure in Europe,” she said, that “some centers do struggle to have anesthesiology support for their EP procedures.”
The single-center trial randomized adults slated to undergo cavotricuspid isthmus ablation (n = 116) for AFlut to receive hypnosis or a control procedure, consisting of nonhypnotic relaxation suggestions and white noise delivered through earphones – 56 and 57 patients, respectively, after exclusion of several who ultimately did not undergo ablation. Any patient could receive 1 mg of morphine if self-reported pain was 5 or greater on a 10-point visual analog scale, or simply on demand.
The hypnosis and control groups were predominantly male and well matched for age (mean, about 69 years in both groups), prevalence of atrial fibrillation, and left ventricular ejection fraction (about 55% for both). Also, in both groups, the procedure duration was approximately 36 minutes.
Asked if all patients in the hypnosis group were actually hypnotized, Dr. Garcia said: “That’s a tricky question” because there was no prespecified definition for successful hypnosis. Between 70% and 80% achieved a hypnotized state, he estimated.
Hypnosis was superior to the control intervention for the primary outcome of pain self-assessment during the ablation procedure, as recorded 45 minutes after ablation. Also, using a 10-point visual analog scale, the hypnosis group rated the average pain intensity as 4.0, whereas the control group rated it as 5.5 (P < .001).
Similarly, instantaneous pain intensity, rated on a 10-point scale every 5 minutes, was lower throughout the procedure for the hypnosis patients than for the control patients (P < .05 at all assessments). Maximum pain intensities, which occurred at the 15- to 25-minute points, were no greater than 3 for hypnosis patients and peaked at approximately 5 for the control patients.
Two of three secondary end points favored the hypnosis group. Morphine consumption averaged 1.3 mg, compared with 3.6 mg for the control group (P < .001). Observer-assessed degrees of sedation were 8.3 and 5.4, respectively, on a 10-point scale (P < .001). And patient self-assessment of anxiety during the procedure was 1.5 in the hypnosis group and 2.5 in the control group on a similar scale.
Regarding morphine use in the two groups, Dr. Garcia said, “It was more than 2 mg of difference, and this can be very important, especially in certain types of patients,” such as those with compromised lung function.
All six complications (11%) observed during the study occurred in the control group. There were four severe hypotensive episodes, one case of oxygen desaturation, and one case of pericardial effusion (P = .03 vs the hypnosis group).
After pointing out the substantial risk for adverse events associated with deep analgesia, particularly from the use of opiates, Paulus Kirchhof, MD, PhD, said, “I think it’s a clinically relevant topic, in the context of reducing the risk of ablation procedures, to try to minimize the use of opiates or other strong anesthetics.”
A multicenter trial could be the next step, said Dr. Kirchhof, from the University Heart and Vascular Center UKE Hamburg (Germany). That would potentially provide “the first evidence for me that this is not sort of something that works in one specific setting, but that it is transferable to other centers, other countries, where practices and complication rates of analgosedation may be different.”
Dr. Kirchhof praised the study design for comparing hypnosis with an active standard-of-care control group. “That is one of the strengths of the study; they tried to design it in a way that didn’t disadvantage the control group.”
The study was funded by the University Hospital of Poitiers. Dr. Garcia and Dr. Arbelo reported no conflicts of interest. Dr. Kirchhof reported support for basic, translational, and clinical research projects from the European Union, the British Heart Foundation, the Leducq Foundation, the Medical Research Council, and the German Centre for Cardiovascular Research, and from several drug and device companies active in atrial fibrillation, from which he received honoraria more than 3 years ago; he is listed as inventor on two patents held by the University of Birmingham (England).
A version of this article originally appeared on Medscape.com.
used as a control, in a small randomized trial.
Ablation is typically performed using conscious sedation and “requires sometimes very high dosages of morphine, and there are sometimes some complications, blood pressure drop, or oxygen desaturation,” Rodrigue Garcia, MD, Poitiers (France) University Hospital, said in an interview.
But patients in the study assigned to undergo hypnosis during the AFlut ablation, performed by practitioners hailing from the French Hypnosis Association, consistently perceived significantly less pain throughout the procedure than those in the active-control group.
They also used almost two-thirds less morphine, which was available to both groups on demand, reported Dr. Garcia, who presented the results of the PAINLESS study at the European Heart Rhythm Association 2020 Virtual Congress. The annual meeting was conducted online this year because of the COVID-19 pandemic.
Hypnotism for pain control may not be widely available in hospitals, “but it’s becoming more and more frequent in the different centers, especially in France,” he said.
The technique is probably also suitable for catheter ablation of ventricular tachycardia, Dr. Garcia said, and “we already use it for atrial fibrillation ablation, because it’s a very common procedure and because, in France, for example, there is a lack of anesthesiologists.” One limitation of hypnosis for such procedures, he said, is that it requires a practitioner with a lot of training and experience.
The current study, “I think, is one of the few, if not the first, randomized trial on this topic, at least for flutter,” Elena Arbelo, MD, PhD, MSc, Hospital Clinic of Barcelona and the University of Barcelona, said in an interview.
“I thought it was very interesting. Many centers have the issue of not having anesthesiology support for their procedures. We have the option of having anesthesiology with us only a few days a week,” said Dr. Arbelo, who was not an investigator with the study.
“If it’s validated in larger cohorts and in different cultures, it may be an interesting way of reducing the need for anesthesiology support, which is a main issue. I know for sure in Europe,” she said, that “some centers do struggle to have anesthesiology support for their EP procedures.”
The single-center trial randomized adults slated to undergo cavotricuspid isthmus ablation (n = 116) for AFlut to receive hypnosis or a control procedure, consisting of nonhypnotic relaxation suggestions and white noise delivered through earphones – 56 and 57 patients, respectively, after exclusion of several who ultimately did not undergo ablation. Any patient could receive 1 mg of morphine if self-reported pain was 5 or greater on a 10-point visual analog scale, or simply on demand.
The hypnosis and control groups were predominantly male and well matched for age (mean, about 69 years in both groups), prevalence of atrial fibrillation, and left ventricular ejection fraction (about 55% for both). Also, in both groups, the procedure duration was approximately 36 minutes.
Asked if all patients in the hypnosis group were actually hypnotized, Dr. Garcia said: “That’s a tricky question” because there was no prespecified definition for successful hypnosis. Between 70% and 80% achieved a hypnotized state, he estimated.
Hypnosis was superior to the control intervention for the primary outcome of pain self-assessment during the ablation procedure, as recorded 45 minutes after ablation. Also, using a 10-point visual analog scale, the hypnosis group rated the average pain intensity as 4.0, whereas the control group rated it as 5.5 (P < .001).
Similarly, instantaneous pain intensity, rated on a 10-point scale every 5 minutes, was lower throughout the procedure for the hypnosis patients than for the control patients (P < .05 at all assessments). Maximum pain intensities, which occurred at the 15- to 25-minute points, were no greater than 3 for hypnosis patients and peaked at approximately 5 for the control patients.
Two of three secondary end points favored the hypnosis group. Morphine consumption averaged 1.3 mg, compared with 3.6 mg for the control group (P < .001). Observer-assessed degrees of sedation were 8.3 and 5.4, respectively, on a 10-point scale (P < .001). And patient self-assessment of anxiety during the procedure was 1.5 in the hypnosis group and 2.5 in the control group on a similar scale.
Regarding morphine use in the two groups, Dr. Garcia said, “It was more than 2 mg of difference, and this can be very important, especially in certain types of patients,” such as those with compromised lung function.
All six complications (11%) observed during the study occurred in the control group. There were four severe hypotensive episodes, one case of oxygen desaturation, and one case of pericardial effusion (P = .03 vs the hypnosis group).
After pointing out the substantial risk for adverse events associated with deep analgesia, particularly from the use of opiates, Paulus Kirchhof, MD, PhD, said, “I think it’s a clinically relevant topic, in the context of reducing the risk of ablation procedures, to try to minimize the use of opiates or other strong anesthetics.”
A multicenter trial could be the next step, said Dr. Kirchhof, from the University Heart and Vascular Center UKE Hamburg (Germany). That would potentially provide “the first evidence for me that this is not sort of something that works in one specific setting, but that it is transferable to other centers, other countries, where practices and complication rates of analgosedation may be different.”
Dr. Kirchhof praised the study design for comparing hypnosis with an active standard-of-care control group. “That is one of the strengths of the study; they tried to design it in a way that didn’t disadvantage the control group.”
The study was funded by the University Hospital of Poitiers. Dr. Garcia and Dr. Arbelo reported no conflicts of interest. Dr. Kirchhof reported support for basic, translational, and clinical research projects from the European Union, the British Heart Foundation, the Leducq Foundation, the Medical Research Council, and the German Centre for Cardiovascular Research, and from several drug and device companies active in atrial fibrillation, from which he received honoraria more than 3 years ago; he is listed as inventor on two patents held by the University of Birmingham (England).
A version of this article originally appeared on Medscape.com.
used as a control, in a small randomized trial.
Ablation is typically performed using conscious sedation and “requires sometimes very high dosages of morphine, and there are sometimes some complications, blood pressure drop, or oxygen desaturation,” Rodrigue Garcia, MD, Poitiers (France) University Hospital, said in an interview.
But patients in the study assigned to undergo hypnosis during the AFlut ablation, performed by practitioners hailing from the French Hypnosis Association, consistently perceived significantly less pain throughout the procedure than those in the active-control group.
They also used almost two-thirds less morphine, which was available to both groups on demand, reported Dr. Garcia, who presented the results of the PAINLESS study at the European Heart Rhythm Association 2020 Virtual Congress. The annual meeting was conducted online this year because of the COVID-19 pandemic.
Hypnotism for pain control may not be widely available in hospitals, “but it’s becoming more and more frequent in the different centers, especially in France,” he said.
The technique is probably also suitable for catheter ablation of ventricular tachycardia, Dr. Garcia said, and “we already use it for atrial fibrillation ablation, because it’s a very common procedure and because, in France, for example, there is a lack of anesthesiologists.” One limitation of hypnosis for such procedures, he said, is that it requires a practitioner with a lot of training and experience.
The current study, “I think, is one of the few, if not the first, randomized trial on this topic, at least for flutter,” Elena Arbelo, MD, PhD, MSc, Hospital Clinic of Barcelona and the University of Barcelona, said in an interview.
“I thought it was very interesting. Many centers have the issue of not having anesthesiology support for their procedures. We have the option of having anesthesiology with us only a few days a week,” said Dr. Arbelo, who was not an investigator with the study.
“If it’s validated in larger cohorts and in different cultures, it may be an interesting way of reducing the need for anesthesiology support, which is a main issue. I know for sure in Europe,” she said, that “some centers do struggle to have anesthesiology support for their EP procedures.”
The single-center trial randomized adults slated to undergo cavotricuspid isthmus ablation (n = 116) for AFlut to receive hypnosis or a control procedure, consisting of nonhypnotic relaxation suggestions and white noise delivered through earphones – 56 and 57 patients, respectively, after exclusion of several who ultimately did not undergo ablation. Any patient could receive 1 mg of morphine if self-reported pain was 5 or greater on a 10-point visual analog scale, or simply on demand.
The hypnosis and control groups were predominantly male and well matched for age (mean, about 69 years in both groups), prevalence of atrial fibrillation, and left ventricular ejection fraction (about 55% for both). Also, in both groups, the procedure duration was approximately 36 minutes.
Asked if all patients in the hypnosis group were actually hypnotized, Dr. Garcia said: “That’s a tricky question” because there was no prespecified definition for successful hypnosis. Between 70% and 80% achieved a hypnotized state, he estimated.
Hypnosis was superior to the control intervention for the primary outcome of pain self-assessment during the ablation procedure, as recorded 45 minutes after ablation. Also, using a 10-point visual analog scale, the hypnosis group rated the average pain intensity as 4.0, whereas the control group rated it as 5.5 (P < .001).
Similarly, instantaneous pain intensity, rated on a 10-point scale every 5 minutes, was lower throughout the procedure for the hypnosis patients than for the control patients (P < .05 at all assessments). Maximum pain intensities, which occurred at the 15- to 25-minute points, were no greater than 3 for hypnosis patients and peaked at approximately 5 for the control patients.
Two of three secondary end points favored the hypnosis group. Morphine consumption averaged 1.3 mg, compared with 3.6 mg for the control group (P < .001). Observer-assessed degrees of sedation were 8.3 and 5.4, respectively, on a 10-point scale (P < .001). And patient self-assessment of anxiety during the procedure was 1.5 in the hypnosis group and 2.5 in the control group on a similar scale.
Regarding morphine use in the two groups, Dr. Garcia said, “It was more than 2 mg of difference, and this can be very important, especially in certain types of patients,” such as those with compromised lung function.
All six complications (11%) observed during the study occurred in the control group. There were four severe hypotensive episodes, one case of oxygen desaturation, and one case of pericardial effusion (P = .03 vs the hypnosis group).
After pointing out the substantial risk for adverse events associated with deep analgesia, particularly from the use of opiates, Paulus Kirchhof, MD, PhD, said, “I think it’s a clinically relevant topic, in the context of reducing the risk of ablation procedures, to try to minimize the use of opiates or other strong anesthetics.”
A multicenter trial could be the next step, said Dr. Kirchhof, from the University Heart and Vascular Center UKE Hamburg (Germany). That would potentially provide “the first evidence for me that this is not sort of something that works in one specific setting, but that it is transferable to other centers, other countries, where practices and complication rates of analgosedation may be different.”
Dr. Kirchhof praised the study design for comparing hypnosis with an active standard-of-care control group. “That is one of the strengths of the study; they tried to design it in a way that didn’t disadvantage the control group.”
The study was funded by the University Hospital of Poitiers. Dr. Garcia and Dr. Arbelo reported no conflicts of interest. Dr. Kirchhof reported support for basic, translational, and clinical research projects from the European Union, the British Heart Foundation, the Leducq Foundation, the Medical Research Council, and the German Centre for Cardiovascular Research, and from several drug and device companies active in atrial fibrillation, from which he received honoraria more than 3 years ago; he is listed as inventor on two patents held by the University of Birmingham (England).
A version of this article originally appeared on Medscape.com.