User login
Transapical valve replacement relieves mitral regurgitation
, relief of mitral regurgitation, and increases in cardiac hemodynamics and quality of life sustained at 1 year.
Further, patients with severe mitral annular calcification (MAC) showed improvements in hemodynamics, functional status, and quality of life after the procedure.
With 70 centers participating in the Tendyne SUMMIT trial, the first 100 trial roll-in patients accrued from the first one or two patients from each site without previous Tendyne TMVR experience.
“For this new procedure, with new operators, there was no intraprocedural mortality, and procedural survival was 100%,” co-primary investigator Jason Rogers, MD, of the University of California Davis Medical Center, Sacramento, told attendees at a Late-Breaking Clinical Science session at the Transcatheter Cardiovascular Therapeutics annual meeting.
“The survival was 74% at 12 months. The valve was very effective at eliminating much regurgitation, and 96.5% of patients had either zero or 1+ at a year, and 97% at 30 days had no mitral regurgitation,” he reported. As follow-up was during the COVID-19 pandemic, several of the deaths were attributed to COVID.
Device and trial designs
The Tendyne TMVR is placed through the cardiac apex. It has an outer frame contoured to comport with the shape of the native mitral valve. Inside is a circular, self-expanding, tri-leaflet bioprosthetic valve.
A unique aspect of the design is a tether attached to the outflow side of the valve to allow positioning and control of the valve. At the end of the tether is an apical pad that is placed over the apical access site to control bleeding. The device is currently limited to investigational use in the United States.
The trial enrolled patients with grade III/IV MR or severe MAC if valve anatomy was deemed amenable to transcatheter repair or met MitraClip indications and if these treatments were considered more appropriate than surgery.
Dr. Rogers reported on the first 100 roll-in (early experimental) patients who received Tendyne TMVR. There was a separate severe MAC cohort receiving Tendyne implantation (N = 103). A further 1:1 randomized study of 382 patients compared Tendyne investigational treatment with a MitraClip control group.
At baseline, the 100 roll-in patients had an average age of 75 years, 54% were men, 46% had a frailty score of 2 or greater, and 41% had been hospitalized in the prior 12 months for heart failure. Left ventricular ejection fraction (LVEF) was 48.6% ± 10.3%.
Improved cardiac function
Procedural survival was 100%, technical success 94%, and valve implantation occurred in 97%. Of the first 100 patients, 26 had died by 1 year, and two withdrew consent, leaving 72 for evaluation.
Immediate post-procedure survival was 98%, 87.9% at 3 months, 83.7% at 6 months, and 74.3% at 1 year. MR severity decreased from 29% 3+ and 69% 4+ at baseline to 96.5% 0/1+ and 3.5% 2+ at 1 year.
Cumulative adverse outcomes at 1 year were 27% all-cause mortality, 21.6% cardiovascular mortality, 5.4% all-cause stroke, 2.3% myocardial infarction (MI), 2.2% post-operative mitral reintervention, no major but 2.3% minor device thrombosis, and 32.4% major bleeding.
Most adverse events occurred peri-procedurally or within the first month, representing, “I think, a new procedure with new operators and a high real risk population,” Dr. Rogers said.
Echocardiography at 1 year compared with baseline showed significant changes with decreases in left ventricular end diastolic volume (LVEDV), increases in cardiac output (CO) and forward stroke volume, and no change in mitral valve gradient or left ventricular outflow tract (LVOT) gradient. New York Heart Association (NYHA) classification decreased from 69% class III/IV at baseline to 20% at 1 year, at which point 80% of patients were in class I/II.
“There was a consistent and steady improvement in KCCQ [Kansas City Cardiomyopathy Questionnaire] score, as expected, as patients recovered from this invasive procedure,” Dr. Rogers said. The 1-year score was 68.7, representing fair to good quality of life.
Outcomes with severe MAC
After screening for MR 3+ or greater, severe mitral stenosis, or moderate MR plus mitral stenosis, 103 eligible patients were treated with the Tendyne device. The median MAC volume of the cohort was 4000 mm3, with a maximum of 38,000 mm3.
Patients averaged 78 years old, 44.7% male, 55.3% had a frailty score of 2 or greater, 73.8% were in NYHA class III or greater, and 29.1% had been hospitalized within the prior 12 months for heart failure. Grade III or IV MR severity was present in 89%, with MR being primary in 90.3% of patients, and 10.7% had severe mitral stenosis.
Tendyne procedure survival was 98.1%, technical success was 94.2%, and valves were implanted in all patients. Emergency surgery or other intervention was required in 5.8%.
As co-presenter of the SUMMIT results, Vinod Thourani, MD, of the Piedmont Heart Institute in Atlanta, said at 30 days there was 6.8% all-cause mortality, all of it cardiovascular. There was one disabling stroke, one MI, no device thrombosis, and 21.4% major bleeding.
“At 1 month, there was less than grade 1 mitral regurgitation in all patients,” he reported, vs. 89% grade 3+/4+ at baseline. “At 1 month, it was an improvement in the NYHA classification to almost 70% in class I or II, which was improved from baseline of 26% in NYHA class I or II.”
Hemodynamic parameters all showed improvement, with a reduction in LVEF, LVEDV, and mitral valve gradient and increases in CO and forward stroke volume. There was no significant increase in LVOT gradient.
There was a small improvement in the KCCQ quality of life score from a baseline score of 49.2 to 52.3 at 30 days. “We’re expecting the KCCQ overall score to improve on 1 year follow up since the patients [are] still recovering from their thoracotomy incision,” Dr. Thourani predicted.
The primary endpoint will be evaluated at 1 year post procedure, he said at the meeting, sponsored by the Cardiovascular Research Foundation.
No good option
Designated discussant Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles, first thanked the presenters for their trial, saying, “What an absolute pleasure to be a mitral surgeon at a meeting where you’re presenting a solution for something that we find incredibly challenging. There’s no good transcatheter option for MAC. There’s no great surgical option for MAC.”
She noted the small size of the MAC cohort and asked what drove failure in patient screening, starting with 474 patients, identifying 120 who would be eligible, and enrolling 103 in the MAC cohort. The presenters identified neo-LVOT, the residual LVOT created after implanting the mitral valve prosthesis. Screening also eliminated patients with a too large or too small annulus.
Dr. Thourani said in Europe, surgeons have used anterior leaflet splitting before Tendyne, which may help to expand the population of eligible patients, but no leaflet modification was allowed in the SUMMIT trial.
Dr. Chikwe then pointed to the six deaths in the MAC arm and 11 deaths in the roll-in arm and asked about the mechanism of these deaths. “Was it [that] the 22% major bleeding is transapical? Really the Achilles heel of this procedure? Is this something that could become a transcatheter device?”
“We call it a transcatheter procedure, but it’s very much a surgical procedure,” Dr. Rogers answered. “And, you know, despite having great experienced sites...many surgeons don’t deal with the apex very much.” Furthermore, catheter insertion can lead to bleeding complications.
He noted that the roll-in patients were the first one or two cases at each site, and there have been improvements with site experience. The apical pads assist in hemostasis. He said the current design of the Tendyne catheter-delivered valve does not allow it to be adapted to a transfemoral transseptal approach.
Dr. Rogers is a consultant to and co-national principal investigator of the SUMMIT Pivotal Trial for Abbott. He is a consultant to Boston Scientific and a consultant/equity holder in Laminar. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, AtriCure, Boston Scientific, Croivalve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, AtriCure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Chikwe reports no relevant financial relationships. The SUMMIT trial was sponsored by Abbott.
A version of this article first appeared on Medscape.com.
, relief of mitral regurgitation, and increases in cardiac hemodynamics and quality of life sustained at 1 year.
Further, patients with severe mitral annular calcification (MAC) showed improvements in hemodynamics, functional status, and quality of life after the procedure.
With 70 centers participating in the Tendyne SUMMIT trial, the first 100 trial roll-in patients accrued from the first one or two patients from each site without previous Tendyne TMVR experience.
“For this new procedure, with new operators, there was no intraprocedural mortality, and procedural survival was 100%,” co-primary investigator Jason Rogers, MD, of the University of California Davis Medical Center, Sacramento, told attendees at a Late-Breaking Clinical Science session at the Transcatheter Cardiovascular Therapeutics annual meeting.
“The survival was 74% at 12 months. The valve was very effective at eliminating much regurgitation, and 96.5% of patients had either zero or 1+ at a year, and 97% at 30 days had no mitral regurgitation,” he reported. As follow-up was during the COVID-19 pandemic, several of the deaths were attributed to COVID.
Device and trial designs
The Tendyne TMVR is placed through the cardiac apex. It has an outer frame contoured to comport with the shape of the native mitral valve. Inside is a circular, self-expanding, tri-leaflet bioprosthetic valve.
A unique aspect of the design is a tether attached to the outflow side of the valve to allow positioning and control of the valve. At the end of the tether is an apical pad that is placed over the apical access site to control bleeding. The device is currently limited to investigational use in the United States.
The trial enrolled patients with grade III/IV MR or severe MAC if valve anatomy was deemed amenable to transcatheter repair or met MitraClip indications and if these treatments were considered more appropriate than surgery.
Dr. Rogers reported on the first 100 roll-in (early experimental) patients who received Tendyne TMVR. There was a separate severe MAC cohort receiving Tendyne implantation (N = 103). A further 1:1 randomized study of 382 patients compared Tendyne investigational treatment with a MitraClip control group.
At baseline, the 100 roll-in patients had an average age of 75 years, 54% were men, 46% had a frailty score of 2 or greater, and 41% had been hospitalized in the prior 12 months for heart failure. Left ventricular ejection fraction (LVEF) was 48.6% ± 10.3%.
Improved cardiac function
Procedural survival was 100%, technical success 94%, and valve implantation occurred in 97%. Of the first 100 patients, 26 had died by 1 year, and two withdrew consent, leaving 72 for evaluation.
Immediate post-procedure survival was 98%, 87.9% at 3 months, 83.7% at 6 months, and 74.3% at 1 year. MR severity decreased from 29% 3+ and 69% 4+ at baseline to 96.5% 0/1+ and 3.5% 2+ at 1 year.
Cumulative adverse outcomes at 1 year were 27% all-cause mortality, 21.6% cardiovascular mortality, 5.4% all-cause stroke, 2.3% myocardial infarction (MI), 2.2% post-operative mitral reintervention, no major but 2.3% minor device thrombosis, and 32.4% major bleeding.
Most adverse events occurred peri-procedurally or within the first month, representing, “I think, a new procedure with new operators and a high real risk population,” Dr. Rogers said.
Echocardiography at 1 year compared with baseline showed significant changes with decreases in left ventricular end diastolic volume (LVEDV), increases in cardiac output (CO) and forward stroke volume, and no change in mitral valve gradient or left ventricular outflow tract (LVOT) gradient. New York Heart Association (NYHA) classification decreased from 69% class III/IV at baseline to 20% at 1 year, at which point 80% of patients were in class I/II.
“There was a consistent and steady improvement in KCCQ [Kansas City Cardiomyopathy Questionnaire] score, as expected, as patients recovered from this invasive procedure,” Dr. Rogers said. The 1-year score was 68.7, representing fair to good quality of life.
Outcomes with severe MAC
After screening for MR 3+ or greater, severe mitral stenosis, or moderate MR plus mitral stenosis, 103 eligible patients were treated with the Tendyne device. The median MAC volume of the cohort was 4000 mm3, with a maximum of 38,000 mm3.
Patients averaged 78 years old, 44.7% male, 55.3% had a frailty score of 2 or greater, 73.8% were in NYHA class III or greater, and 29.1% had been hospitalized within the prior 12 months for heart failure. Grade III or IV MR severity was present in 89%, with MR being primary in 90.3% of patients, and 10.7% had severe mitral stenosis.
Tendyne procedure survival was 98.1%, technical success was 94.2%, and valves were implanted in all patients. Emergency surgery or other intervention was required in 5.8%.
As co-presenter of the SUMMIT results, Vinod Thourani, MD, of the Piedmont Heart Institute in Atlanta, said at 30 days there was 6.8% all-cause mortality, all of it cardiovascular. There was one disabling stroke, one MI, no device thrombosis, and 21.4% major bleeding.
“At 1 month, there was less than grade 1 mitral regurgitation in all patients,” he reported, vs. 89% grade 3+/4+ at baseline. “At 1 month, it was an improvement in the NYHA classification to almost 70% in class I or II, which was improved from baseline of 26% in NYHA class I or II.”
Hemodynamic parameters all showed improvement, with a reduction in LVEF, LVEDV, and mitral valve gradient and increases in CO and forward stroke volume. There was no significant increase in LVOT gradient.
There was a small improvement in the KCCQ quality of life score from a baseline score of 49.2 to 52.3 at 30 days. “We’re expecting the KCCQ overall score to improve on 1 year follow up since the patients [are] still recovering from their thoracotomy incision,” Dr. Thourani predicted.
The primary endpoint will be evaluated at 1 year post procedure, he said at the meeting, sponsored by the Cardiovascular Research Foundation.
No good option
Designated discussant Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles, first thanked the presenters for their trial, saying, “What an absolute pleasure to be a mitral surgeon at a meeting where you’re presenting a solution for something that we find incredibly challenging. There’s no good transcatheter option for MAC. There’s no great surgical option for MAC.”
She noted the small size of the MAC cohort and asked what drove failure in patient screening, starting with 474 patients, identifying 120 who would be eligible, and enrolling 103 in the MAC cohort. The presenters identified neo-LVOT, the residual LVOT created after implanting the mitral valve prosthesis. Screening also eliminated patients with a too large or too small annulus.
Dr. Thourani said in Europe, surgeons have used anterior leaflet splitting before Tendyne, which may help to expand the population of eligible patients, but no leaflet modification was allowed in the SUMMIT trial.
Dr. Chikwe then pointed to the six deaths in the MAC arm and 11 deaths in the roll-in arm and asked about the mechanism of these deaths. “Was it [that] the 22% major bleeding is transapical? Really the Achilles heel of this procedure? Is this something that could become a transcatheter device?”
“We call it a transcatheter procedure, but it’s very much a surgical procedure,” Dr. Rogers answered. “And, you know, despite having great experienced sites...many surgeons don’t deal with the apex very much.” Furthermore, catheter insertion can lead to bleeding complications.
He noted that the roll-in patients were the first one or two cases at each site, and there have been improvements with site experience. The apical pads assist in hemostasis. He said the current design of the Tendyne catheter-delivered valve does not allow it to be adapted to a transfemoral transseptal approach.
Dr. Rogers is a consultant to and co-national principal investigator of the SUMMIT Pivotal Trial for Abbott. He is a consultant to Boston Scientific and a consultant/equity holder in Laminar. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, AtriCure, Boston Scientific, Croivalve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, AtriCure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Chikwe reports no relevant financial relationships. The SUMMIT trial was sponsored by Abbott.
A version of this article first appeared on Medscape.com.
, relief of mitral regurgitation, and increases in cardiac hemodynamics and quality of life sustained at 1 year.
Further, patients with severe mitral annular calcification (MAC) showed improvements in hemodynamics, functional status, and quality of life after the procedure.
With 70 centers participating in the Tendyne SUMMIT trial, the first 100 trial roll-in patients accrued from the first one or two patients from each site without previous Tendyne TMVR experience.
“For this new procedure, with new operators, there was no intraprocedural mortality, and procedural survival was 100%,” co-primary investigator Jason Rogers, MD, of the University of California Davis Medical Center, Sacramento, told attendees at a Late-Breaking Clinical Science session at the Transcatheter Cardiovascular Therapeutics annual meeting.
“The survival was 74% at 12 months. The valve was very effective at eliminating much regurgitation, and 96.5% of patients had either zero or 1+ at a year, and 97% at 30 days had no mitral regurgitation,” he reported. As follow-up was during the COVID-19 pandemic, several of the deaths were attributed to COVID.
Device and trial designs
The Tendyne TMVR is placed through the cardiac apex. It has an outer frame contoured to comport with the shape of the native mitral valve. Inside is a circular, self-expanding, tri-leaflet bioprosthetic valve.
A unique aspect of the design is a tether attached to the outflow side of the valve to allow positioning and control of the valve. At the end of the tether is an apical pad that is placed over the apical access site to control bleeding. The device is currently limited to investigational use in the United States.
The trial enrolled patients with grade III/IV MR or severe MAC if valve anatomy was deemed amenable to transcatheter repair or met MitraClip indications and if these treatments were considered more appropriate than surgery.
Dr. Rogers reported on the first 100 roll-in (early experimental) patients who received Tendyne TMVR. There was a separate severe MAC cohort receiving Tendyne implantation (N = 103). A further 1:1 randomized study of 382 patients compared Tendyne investigational treatment with a MitraClip control group.
At baseline, the 100 roll-in patients had an average age of 75 years, 54% were men, 46% had a frailty score of 2 or greater, and 41% had been hospitalized in the prior 12 months for heart failure. Left ventricular ejection fraction (LVEF) was 48.6% ± 10.3%.
Improved cardiac function
Procedural survival was 100%, technical success 94%, and valve implantation occurred in 97%. Of the first 100 patients, 26 had died by 1 year, and two withdrew consent, leaving 72 for evaluation.
Immediate post-procedure survival was 98%, 87.9% at 3 months, 83.7% at 6 months, and 74.3% at 1 year. MR severity decreased from 29% 3+ and 69% 4+ at baseline to 96.5% 0/1+ and 3.5% 2+ at 1 year.
Cumulative adverse outcomes at 1 year were 27% all-cause mortality, 21.6% cardiovascular mortality, 5.4% all-cause stroke, 2.3% myocardial infarction (MI), 2.2% post-operative mitral reintervention, no major but 2.3% minor device thrombosis, and 32.4% major bleeding.
Most adverse events occurred peri-procedurally or within the first month, representing, “I think, a new procedure with new operators and a high real risk population,” Dr. Rogers said.
Echocardiography at 1 year compared with baseline showed significant changes with decreases in left ventricular end diastolic volume (LVEDV), increases in cardiac output (CO) and forward stroke volume, and no change in mitral valve gradient or left ventricular outflow tract (LVOT) gradient. New York Heart Association (NYHA) classification decreased from 69% class III/IV at baseline to 20% at 1 year, at which point 80% of patients were in class I/II.
“There was a consistent and steady improvement in KCCQ [Kansas City Cardiomyopathy Questionnaire] score, as expected, as patients recovered from this invasive procedure,” Dr. Rogers said. The 1-year score was 68.7, representing fair to good quality of life.
Outcomes with severe MAC
After screening for MR 3+ or greater, severe mitral stenosis, or moderate MR plus mitral stenosis, 103 eligible patients were treated with the Tendyne device. The median MAC volume of the cohort was 4000 mm3, with a maximum of 38,000 mm3.
Patients averaged 78 years old, 44.7% male, 55.3% had a frailty score of 2 or greater, 73.8% were in NYHA class III or greater, and 29.1% had been hospitalized within the prior 12 months for heart failure. Grade III or IV MR severity was present in 89%, with MR being primary in 90.3% of patients, and 10.7% had severe mitral stenosis.
Tendyne procedure survival was 98.1%, technical success was 94.2%, and valves were implanted in all patients. Emergency surgery or other intervention was required in 5.8%.
As co-presenter of the SUMMIT results, Vinod Thourani, MD, of the Piedmont Heart Institute in Atlanta, said at 30 days there was 6.8% all-cause mortality, all of it cardiovascular. There was one disabling stroke, one MI, no device thrombosis, and 21.4% major bleeding.
“At 1 month, there was less than grade 1 mitral regurgitation in all patients,” he reported, vs. 89% grade 3+/4+ at baseline. “At 1 month, it was an improvement in the NYHA classification to almost 70% in class I or II, which was improved from baseline of 26% in NYHA class I or II.”
Hemodynamic parameters all showed improvement, with a reduction in LVEF, LVEDV, and mitral valve gradient and increases in CO and forward stroke volume. There was no significant increase in LVOT gradient.
There was a small improvement in the KCCQ quality of life score from a baseline score of 49.2 to 52.3 at 30 days. “We’re expecting the KCCQ overall score to improve on 1 year follow up since the patients [are] still recovering from their thoracotomy incision,” Dr. Thourani predicted.
The primary endpoint will be evaluated at 1 year post procedure, he said at the meeting, sponsored by the Cardiovascular Research Foundation.
No good option
Designated discussant Joanna Chikwe, MD, chair of cardiac surgery at Cedars-Sinai Medical Center in Los Angeles, first thanked the presenters for their trial, saying, “What an absolute pleasure to be a mitral surgeon at a meeting where you’re presenting a solution for something that we find incredibly challenging. There’s no good transcatheter option for MAC. There’s no great surgical option for MAC.”
She noted the small size of the MAC cohort and asked what drove failure in patient screening, starting with 474 patients, identifying 120 who would be eligible, and enrolling 103 in the MAC cohort. The presenters identified neo-LVOT, the residual LVOT created after implanting the mitral valve prosthesis. Screening also eliminated patients with a too large or too small annulus.
Dr. Thourani said in Europe, surgeons have used anterior leaflet splitting before Tendyne, which may help to expand the population of eligible patients, but no leaflet modification was allowed in the SUMMIT trial.
Dr. Chikwe then pointed to the six deaths in the MAC arm and 11 deaths in the roll-in arm and asked about the mechanism of these deaths. “Was it [that] the 22% major bleeding is transapical? Really the Achilles heel of this procedure? Is this something that could become a transcatheter device?”
“We call it a transcatheter procedure, but it’s very much a surgical procedure,” Dr. Rogers answered. “And, you know, despite having great experienced sites...many surgeons don’t deal with the apex very much.” Furthermore, catheter insertion can lead to bleeding complications.
He noted that the roll-in patients were the first one or two cases at each site, and there have been improvements with site experience. The apical pads assist in hemostasis. He said the current design of the Tendyne catheter-delivered valve does not allow it to be adapted to a transfemoral transseptal approach.
Dr. Rogers is a consultant to and co-national principal investigator of the SUMMIT Pivotal Trial for Abbott. He is a consultant to Boston Scientific and a consultant/equity holder in Laminar. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, AtriCure, Boston Scientific, Croivalve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, AtriCure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Chikwe reports no relevant financial relationships. The SUMMIT trial was sponsored by Abbott.
A version of this article first appeared on Medscape.com.
FROM TCT 2023
Short aspirin therapy noninferior to DAPT for 1 year after PCI for ACS
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.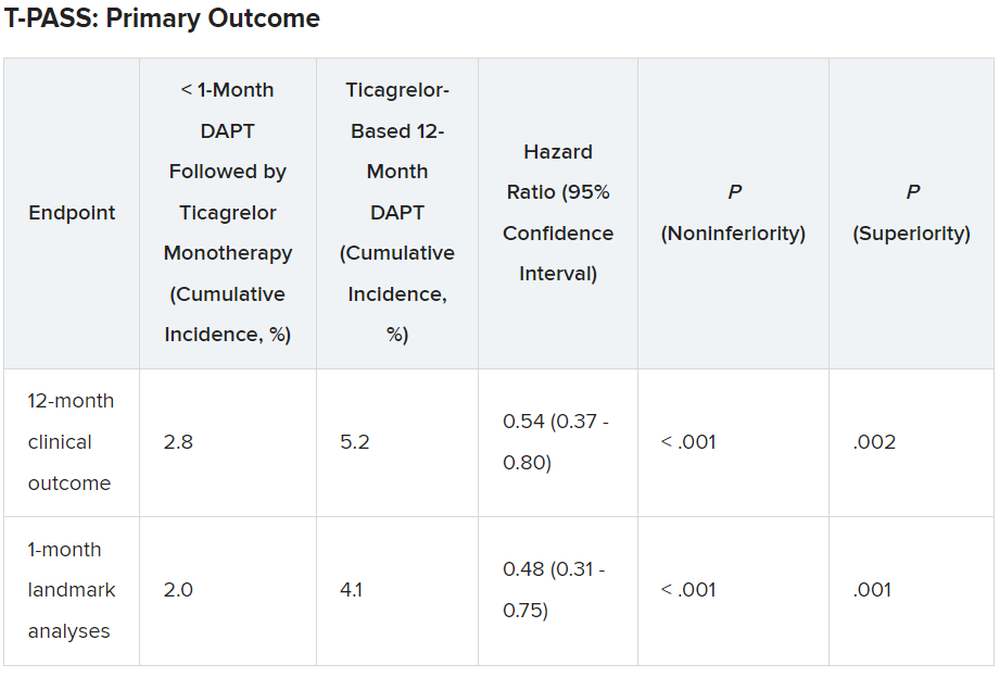
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Stopping aspirin within 1 month of implanting a drug-eluting stent (DES) for acute coronary syndrome (ACS) followed by ticagrelor monotherapy was shown to be noninferior to 12 months of dual antiplatelet therapy (DAPT) in net adverse cardiovascular and bleeding events in the T-PASS trial.
of death, myocardial infarction, stent thrombosis, stroke, and major bleeding, primarily due to a significant reduction in bleeding events,” senior author Myeong-Ki Hong, MD, PhD, Yonsei University, Seoul, Korea, told attendees at the Transcatheter Cardiovascular Therapeutics annual meeting, sponsored by the Cardiovascular Research Foundation.
“This study provides evidence that stopping aspirin within 1 month after implantation of drug-eluting stents for ticagrelor monotherapy is a reasonable alternative to 12-month DAPT as for adverse cardiovascular and bleeding events,” Dr. Hong concluded.
The study was published in Circulation ahead of print to coincide with the presentation.
Three months to 1 month
Previous trials (TICO and TWILIGHT) have shown that ticagrelor monotherapy after 3 months of DAPT can be safe and effectively prevent ischemic events after percutaneous coronary intervention (PCI) in ACS or high-risk PCI patients.
The current study aimed to investigate whether ticagrelor monotherapy after less than 1 month of DAPT was noninferior to 12 months of ticagrelor-based DAPT for preventing adverse cardiovascular and bleeding events in patients with ACS undergoing PCI with a DES implant.
T-PASS, carried out at 24 centers in Korea, enrolled ACS patients aged 19 years or older who received an ultrathin, bioresorbable polymer sirolimus-eluting stent (Orsiro, Biotronik). They were randomized 1:1 to ticagrelor monotherapy after less than 1 month of DAPT (n = 1,426) or to ticagrelor-based DAPT for 12 months (n = 1,424).
The primary outcome measure was net adverse clinical events (NACE) at 12 months, consisting of major bleeding plus major adverse cardiovascular events. All patients were included in the intention-to-treat analysis.
The study could enroll patients aged 19-80 years. It excluded anyone with active bleeding, at increased risk for bleeding, with anemia (hemoglobin ≤ 8 g/dL), platelets less than 100,000/mcL, need for oral anticoagulation therapy, current or potential pregnancy, or a life expectancy less than 1 year.
Baseline characteristics of the two groups were well balanced. The extended monotherapy and DAPT arms had an average age of 61 ± 10 years, were 84% and 83% male and had diabetes mellitus in 30% and 29%, respectively, with 74% of each group admitted via the emergency room. ST-elevation myocardial infarction occurred in 40% and 41% of patients in each group, respectively.
Results showed that stopping aspirin early was noninferior and possibly superior to 12 months of DAPT.
For the 12-month clinical outcome, fewer patients in the less than 1 month DAPT followed by ticagrelor monotherapy arm reached the primary clinical endpoint of NACE versus the ticagrelor-based 12-month DAPT arm, both in terms of noninferiority (P < .001) and superiority (P = .002). Similar results were found for the 1-month landmark analyses.
For both the 12-month clinical outcome and the 1-month landmark analyses, the curves for the two arms began to diverge at about 150 days, with the one for ticagrelor monotherapy essentially flattening out just after that and the one for the 12-month DAPT therapy continuing to rise out to the 1-year point.
In the less than 1 month DAPT arm, aspirin was stopped at a median of 16 days. Panelist Adnan Kastrati, MD, Deutsches Herzzentrum München, Technische Universität, Munich, Germany, asked Dr. Hong about the criteria for the point at which aspirin was stopped in the less than 1 month arm.
Dr. Hong replied: “Actually, we recommend less than 1 month, so therefore in some patients, it was the operator’s decision,” depending on risk factors for stopping or continuing aspirin. He said that in some patients it may be reasonable to stop aspirin even in 7-10 days. Fewer than 10% of patients in the less than 1 month arm continued on aspirin past 30 days, but a few continued on it to the 1-year point.
There was no difference between the less than 1 month DAPT followed by ticagrelor monotherapy arm and the 12-month DAPT arm in terms of major adverse cardiac and cerebrovascular events at 1 year (1.8% vs. 2.2%, respectively; hazard ratio, 0.84; 95% confidence interval, 0.50-1.41; log-rank, P = .51).
However, the 12-month DAPT arm showed a significantly greater incidence of major bleeding at 1 year: 3.4% versus 1.2% for less than 1 month aspirin arm (HR, 0.35; 95% CI, 0.20-0.61; log-rank, P < .001).
Dr. Hong said that a limitation of the study was that it was open label and not placebo controlled. However, an independent clinical event adjudication committee assessed all clinical outcomes.
Lead discussant Marco Valgimigli, MD, PhD, Cardiocentro Ticino Foundation, Lugano, Switzerland, noted that T-PASS is the fifth study to investigate ticagrelor monotherapy versus a DAPT, giving randomized data on almost 22,000 patients.
“T-PASS showed very consistently with the prior four studies that by dropping aspirin and continuation with ticagrelor therapy, compared with the standard DAPT regimen, is associated with no penalty ... and in fact leading to a very significant and clinically very convincing risk reduction, and I would like to underline major bleeding risk reduction,” he said, pointing out that this study comes from the same research group that carried out the TICO trial.
Dr. Hong has received institutional research grants from Samjin Pharmaceutical and Chong Kun Dang Pharmaceutical, and speaker’s fees from Medtronic and Edwards Lifesciences. Dr. Kastrati has disclosed no relevant financial relationships. Dr. Valgimigli has received grant support/research contracts from Terumo Medical and AstraZeneca; consultant fees/honoraria/speaker’s bureau for Terumo Medical Corporation, Bayer, Daiichi Sankyo/Eli Lilly, Amgen, Alvimedica, AstraZenca, Idorsia, Coreflow, Vifor, Bristol-Myers Squibb, and iVascular. The study was funded by Biotronik.
A version of this article first appeared on Medscape.com.
AT TCT 2023
Drug-coated balloon beats conventional angioplasty for high-risk patients with in-stent restenosis
SAN FRANCISCO – For the treatment of coronary artery in-stent restenosis, angioplasty with a drug-coated balloon (AGENT DCB; Boston Scientific) was superior to conventional balloon angioplasty in preventing target lesion failure at 1 year in a high-risk patient population.
Approximate 50% reductions in the rates of target lesion restenosis and target vessel myocardial infarction (MI) accounted for the superior findings with the AGENT DCB over conventional balloon angioplasty.
Robert Yeh, MD, of Beth Israel Deaconess Medical Center in Boston reported at the annual Transcatheter Cardiovascular Therapeutics congress. “This represented a 38% relative risk reduction as well as a 10% absolute risk reduction in the endpoint. The P value for superiority was 0.0063, highly statistically significant.”
In-stent restenosis is clinically challenging and accounts for about 10% of all percutaneous coronary interventions. “Sometimes these patients have multiple layers, and that could be a third or fourth layer of stent, something that we try to avoid,” he said.
Drug-coated balloons, which are not currently approved in the United States, can deliver drugs that inhibit blockages from reforming, “without leaving additional layers of metal behind,” he added. Such devices are already available in Europe and Japan.
AGENT IDE was a prospective, multicenter, superiority trial that randomly assigned 480 patients 2:1 to the AGENT DCB (n = 321) or to conventional balloon angioplasty (n = 159). Randomization occurred after successful pre-dilation of the target vessel.
The trial included patients with in-stent restenosis previously treated with a bare metal or a drug-eluting stent with lesion lengths < 26 mm (reference vessel diameter: > 2 mm to ≤ 4), and percent diameter stenosis of more than 70% if they were asymptomatic or of more than 50% if they were symptomatic. Patients were excluded if they had a recent ST-elevation MI, bifurcation, saphenous vein or arterial graft, or thrombus in the target vessel.
All received dual antiplatelet therapy for at least 1 month and then antiplatelet monotherapy for the duration of the trial. The primary endpoint was target lesion failure at 1 year, a composite of target lesion restenosis, target vessel-related MI, or cardiac death. More than 93% of patients in each arm were available for evaluation of the primary endpoint.
The two groups were well balanced at baseline: Approximate age was 68 years, 27% were women, and three quarters were White. Approximately 28%-32% had had a prior coronary artery bypass graft, 20%-22% had previous heart failure, and about 22% had a history of left main coronary artery disease. Half had diabetes, and about half had stable angina.
Multiple stent layers were common in 43% of each group. Stenosis diameter was about 65% at baseline for the two groups and was reduced to 22% post procedure.
Outcomes all favored AGENT DCB
In the AGENT DCB group, the technical success rate was 92.9% vs 89.3% for balloon angioplasty. Intravascular imaging was used during the procedure in 72.3% of DCB cases and in 76.7% of balloon cases.
Besides demonstrating a nearly 38% reduction in the primary endpoint of target lesion failure at 1 year for the DCB over conventional balloon angioplasty, DCB nearly halved the rate of target lesion revascularization and target vessel MI and was superior on other measures of clinical outcome.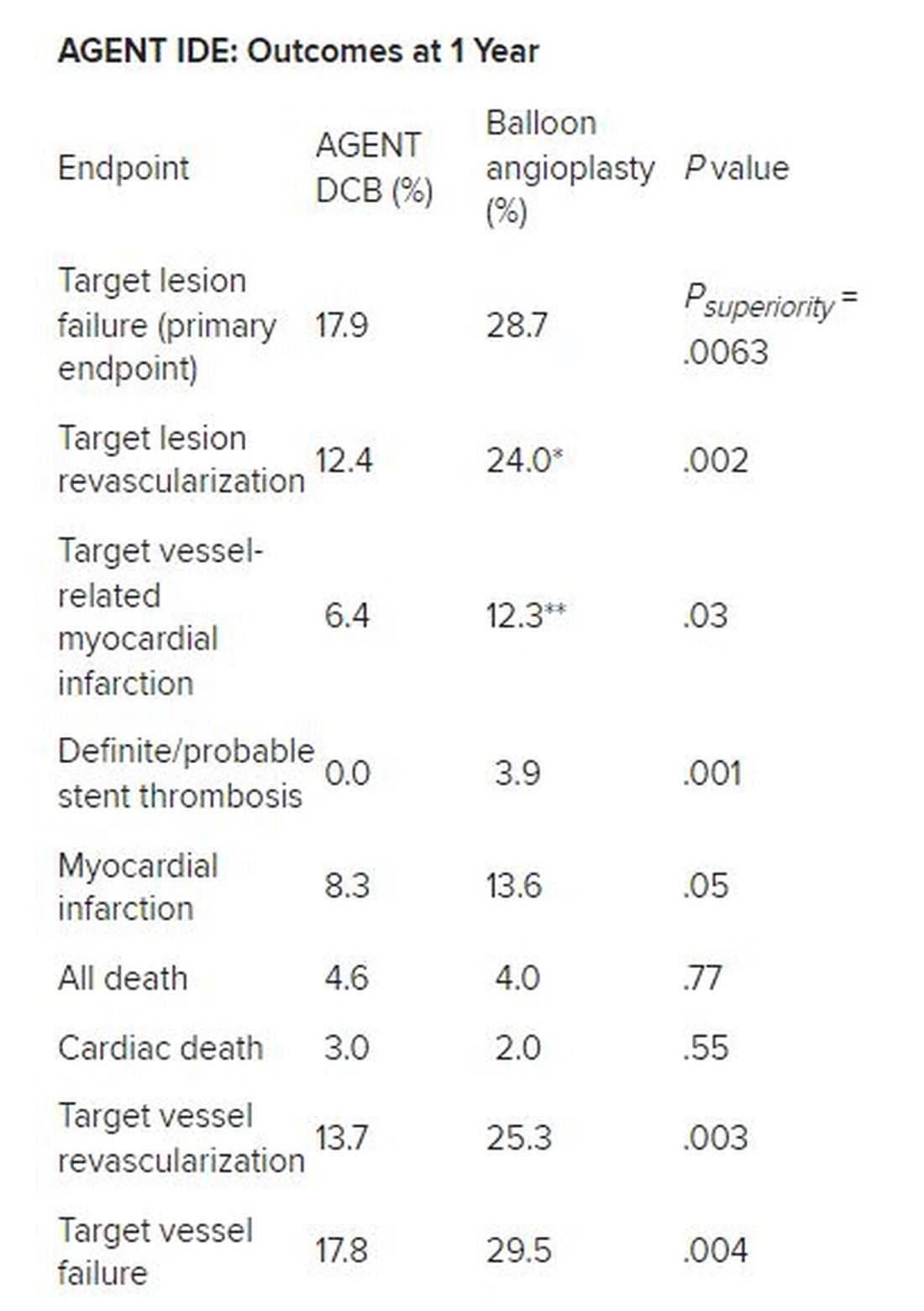
*Hazard ratio, 0.49; 95% CI, 0.31-0.79; ** HR, 0.51; 95% CI, 0.27-0.95
There was no stent rethrombosis with the DCB vs 3.9% with the conventional balloon angioplasty. Of note, there were no differences between the groups in terms of cardiac or noncardiac death.
Subgroup analyses of the primary outcome in terms of sex, age, diabetes, vessel size, or single or multiple stent layers all trended in favor of AGENT DCB but were not statistically significant for interaction.
The study is being expanded to include 600 patients. This device is a US Food and Drug Administration–designated breakthrough device, “and this pivotal trial will be the primary evidence used to support FDA approval,” Dr. Yeh said. “And given the marked superiority over conventional balloon angioplasty, I believe that the AGENT DCB is likely to become an important new treatment option for patients with coronary stenosis in the United States.”
Long overdue
Róisín Colleran, MBBCh, of the Cardiovascular Research Institute Dublin at Mater Private Hospital in Ireland, the designated discussant, first congratulated Dr. Yeh and his coinvestigators on the study’s conduct and findings.
“This study is long overdue,” she said. As Dr. Yeh noted, about 10% of PCI procedures are done for in-stent restenosis, Dr. Colleran said, but in 2023, there is still no coronary drug eluting balloon approved for this indication in the US, despite the class 1 recommendation in the 2014 European guidelines.
She pointed to the trial results, saying they are “clear...a significant reduction in target lesion failure driven by halving in rates of both target lesion revascularization and target vessel MI.”
Strengths of the study are it is the largest of its kind to date, with 480 patients, conducted at 40 US centers, using device-specific endpoints. There was a “very high” intravascular imaging rate of 75% in a cohort with a high risk for in-stent restenosis, consisting of 50% of patients with diabetes and more than 40% with multiple stents.
“The main limitation is the choice of comparator,” Dr. Colleran said. Balloon angioplasty is inferior to both stenting and drug coated balloon therapy for treatment of in-stent restenosis but is the standard of care in the United States, she noted. “I think...for regulatory reasons this was the comparator chosen,” she said.
“I think the implications are clear,” Dr. Colleran added. “This trial should provide a basis for regulatory approval of the drug coated balloon treatment of in-stent restenosis in the U.S. and finally provide this as an available treatment option for such patients.”
Dr. Yeh reported receiving grant/research support from Abbott Vascular, BD Bard, Boston Scientific, Cook Medical, Philips Medical, and Medtronic, and consulting for Abbott Vascular, Boston Scientific, CathWorks, Elixir Medical, Infraredx, Medtronic, Shockwave Medical, and Zol. Dr. Colleran had no disclosures. The trial was supported by Boston Scientific.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – For the treatment of coronary artery in-stent restenosis, angioplasty with a drug-coated balloon (AGENT DCB; Boston Scientific) was superior to conventional balloon angioplasty in preventing target lesion failure at 1 year in a high-risk patient population.
Approximate 50% reductions in the rates of target lesion restenosis and target vessel myocardial infarction (MI) accounted for the superior findings with the AGENT DCB over conventional balloon angioplasty.
Robert Yeh, MD, of Beth Israel Deaconess Medical Center in Boston reported at the annual Transcatheter Cardiovascular Therapeutics congress. “This represented a 38% relative risk reduction as well as a 10% absolute risk reduction in the endpoint. The P value for superiority was 0.0063, highly statistically significant.”
In-stent restenosis is clinically challenging and accounts for about 10% of all percutaneous coronary interventions. “Sometimes these patients have multiple layers, and that could be a third or fourth layer of stent, something that we try to avoid,” he said.
Drug-coated balloons, which are not currently approved in the United States, can deliver drugs that inhibit blockages from reforming, “without leaving additional layers of metal behind,” he added. Such devices are already available in Europe and Japan.
AGENT IDE was a prospective, multicenter, superiority trial that randomly assigned 480 patients 2:1 to the AGENT DCB (n = 321) or to conventional balloon angioplasty (n = 159). Randomization occurred after successful pre-dilation of the target vessel.
The trial included patients with in-stent restenosis previously treated with a bare metal or a drug-eluting stent with lesion lengths < 26 mm (reference vessel diameter: > 2 mm to ≤ 4), and percent diameter stenosis of more than 70% if they were asymptomatic or of more than 50% if they were symptomatic. Patients were excluded if they had a recent ST-elevation MI, bifurcation, saphenous vein or arterial graft, or thrombus in the target vessel.
All received dual antiplatelet therapy for at least 1 month and then antiplatelet monotherapy for the duration of the trial. The primary endpoint was target lesion failure at 1 year, a composite of target lesion restenosis, target vessel-related MI, or cardiac death. More than 93% of patients in each arm were available for evaluation of the primary endpoint.
The two groups were well balanced at baseline: Approximate age was 68 years, 27% were women, and three quarters were White. Approximately 28%-32% had had a prior coronary artery bypass graft, 20%-22% had previous heart failure, and about 22% had a history of left main coronary artery disease. Half had diabetes, and about half had stable angina.
Multiple stent layers were common in 43% of each group. Stenosis diameter was about 65% at baseline for the two groups and was reduced to 22% post procedure.
Outcomes all favored AGENT DCB
In the AGENT DCB group, the technical success rate was 92.9% vs 89.3% for balloon angioplasty. Intravascular imaging was used during the procedure in 72.3% of DCB cases and in 76.7% of balloon cases.
Besides demonstrating a nearly 38% reduction in the primary endpoint of target lesion failure at 1 year for the DCB over conventional balloon angioplasty, DCB nearly halved the rate of target lesion revascularization and target vessel MI and was superior on other measures of clinical outcome.
*Hazard ratio, 0.49; 95% CI, 0.31-0.79; ** HR, 0.51; 95% CI, 0.27-0.95
There was no stent rethrombosis with the DCB vs 3.9% with the conventional balloon angioplasty. Of note, there were no differences between the groups in terms of cardiac or noncardiac death.
Subgroup analyses of the primary outcome in terms of sex, age, diabetes, vessel size, or single or multiple stent layers all trended in favor of AGENT DCB but were not statistically significant for interaction.
The study is being expanded to include 600 patients. This device is a US Food and Drug Administration–designated breakthrough device, “and this pivotal trial will be the primary evidence used to support FDA approval,” Dr. Yeh said. “And given the marked superiority over conventional balloon angioplasty, I believe that the AGENT DCB is likely to become an important new treatment option for patients with coronary stenosis in the United States.”
Long overdue
Róisín Colleran, MBBCh, of the Cardiovascular Research Institute Dublin at Mater Private Hospital in Ireland, the designated discussant, first congratulated Dr. Yeh and his coinvestigators on the study’s conduct and findings.
“This study is long overdue,” she said. As Dr. Yeh noted, about 10% of PCI procedures are done for in-stent restenosis, Dr. Colleran said, but in 2023, there is still no coronary drug eluting balloon approved for this indication in the US, despite the class 1 recommendation in the 2014 European guidelines.
She pointed to the trial results, saying they are “clear...a significant reduction in target lesion failure driven by halving in rates of both target lesion revascularization and target vessel MI.”
Strengths of the study are it is the largest of its kind to date, with 480 patients, conducted at 40 US centers, using device-specific endpoints. There was a “very high” intravascular imaging rate of 75% in a cohort with a high risk for in-stent restenosis, consisting of 50% of patients with diabetes and more than 40% with multiple stents.
“The main limitation is the choice of comparator,” Dr. Colleran said. Balloon angioplasty is inferior to both stenting and drug coated balloon therapy for treatment of in-stent restenosis but is the standard of care in the United States, she noted. “I think...for regulatory reasons this was the comparator chosen,” she said.
“I think the implications are clear,” Dr. Colleran added. “This trial should provide a basis for regulatory approval of the drug coated balloon treatment of in-stent restenosis in the U.S. and finally provide this as an available treatment option for such patients.”
Dr. Yeh reported receiving grant/research support from Abbott Vascular, BD Bard, Boston Scientific, Cook Medical, Philips Medical, and Medtronic, and consulting for Abbott Vascular, Boston Scientific, CathWorks, Elixir Medical, Infraredx, Medtronic, Shockwave Medical, and Zol. Dr. Colleran had no disclosures. The trial was supported by Boston Scientific.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – For the treatment of coronary artery in-stent restenosis, angioplasty with a drug-coated balloon (AGENT DCB; Boston Scientific) was superior to conventional balloon angioplasty in preventing target lesion failure at 1 year in a high-risk patient population.
Approximate 50% reductions in the rates of target lesion restenosis and target vessel myocardial infarction (MI) accounted for the superior findings with the AGENT DCB over conventional balloon angioplasty.
Robert Yeh, MD, of Beth Israel Deaconess Medical Center in Boston reported at the annual Transcatheter Cardiovascular Therapeutics congress. “This represented a 38% relative risk reduction as well as a 10% absolute risk reduction in the endpoint. The P value for superiority was 0.0063, highly statistically significant.”
In-stent restenosis is clinically challenging and accounts for about 10% of all percutaneous coronary interventions. “Sometimes these patients have multiple layers, and that could be a third or fourth layer of stent, something that we try to avoid,” he said.
Drug-coated balloons, which are not currently approved in the United States, can deliver drugs that inhibit blockages from reforming, “without leaving additional layers of metal behind,” he added. Such devices are already available in Europe and Japan.
AGENT IDE was a prospective, multicenter, superiority trial that randomly assigned 480 patients 2:1 to the AGENT DCB (n = 321) or to conventional balloon angioplasty (n = 159). Randomization occurred after successful pre-dilation of the target vessel.
The trial included patients with in-stent restenosis previously treated with a bare metal or a drug-eluting stent with lesion lengths < 26 mm (reference vessel diameter: > 2 mm to ≤ 4), and percent diameter stenosis of more than 70% if they were asymptomatic or of more than 50% if they were symptomatic. Patients were excluded if they had a recent ST-elevation MI, bifurcation, saphenous vein or arterial graft, or thrombus in the target vessel.
All received dual antiplatelet therapy for at least 1 month and then antiplatelet monotherapy for the duration of the trial. The primary endpoint was target lesion failure at 1 year, a composite of target lesion restenosis, target vessel-related MI, or cardiac death. More than 93% of patients in each arm were available for evaluation of the primary endpoint.
The two groups were well balanced at baseline: Approximate age was 68 years, 27% were women, and three quarters were White. Approximately 28%-32% had had a prior coronary artery bypass graft, 20%-22% had previous heart failure, and about 22% had a history of left main coronary artery disease. Half had diabetes, and about half had stable angina.
Multiple stent layers were common in 43% of each group. Stenosis diameter was about 65% at baseline for the two groups and was reduced to 22% post procedure.
Outcomes all favored AGENT DCB
In the AGENT DCB group, the technical success rate was 92.9% vs 89.3% for balloon angioplasty. Intravascular imaging was used during the procedure in 72.3% of DCB cases and in 76.7% of balloon cases.
Besides demonstrating a nearly 38% reduction in the primary endpoint of target lesion failure at 1 year for the DCB over conventional balloon angioplasty, DCB nearly halved the rate of target lesion revascularization and target vessel MI and was superior on other measures of clinical outcome.
*Hazard ratio, 0.49; 95% CI, 0.31-0.79; ** HR, 0.51; 95% CI, 0.27-0.95
There was no stent rethrombosis with the DCB vs 3.9% with the conventional balloon angioplasty. Of note, there were no differences between the groups in terms of cardiac or noncardiac death.
Subgroup analyses of the primary outcome in terms of sex, age, diabetes, vessel size, or single or multiple stent layers all trended in favor of AGENT DCB but were not statistically significant for interaction.
The study is being expanded to include 600 patients. This device is a US Food and Drug Administration–designated breakthrough device, “and this pivotal trial will be the primary evidence used to support FDA approval,” Dr. Yeh said. “And given the marked superiority over conventional balloon angioplasty, I believe that the AGENT DCB is likely to become an important new treatment option for patients with coronary stenosis in the United States.”
Long overdue
Róisín Colleran, MBBCh, of the Cardiovascular Research Institute Dublin at Mater Private Hospital in Ireland, the designated discussant, first congratulated Dr. Yeh and his coinvestigators on the study’s conduct and findings.
“This study is long overdue,” she said. As Dr. Yeh noted, about 10% of PCI procedures are done for in-stent restenosis, Dr. Colleran said, but in 2023, there is still no coronary drug eluting balloon approved for this indication in the US, despite the class 1 recommendation in the 2014 European guidelines.
She pointed to the trial results, saying they are “clear...a significant reduction in target lesion failure driven by halving in rates of both target lesion revascularization and target vessel MI.”
Strengths of the study are it is the largest of its kind to date, with 480 patients, conducted at 40 US centers, using device-specific endpoints. There was a “very high” intravascular imaging rate of 75% in a cohort with a high risk for in-stent restenosis, consisting of 50% of patients with diabetes and more than 40% with multiple stents.
“The main limitation is the choice of comparator,” Dr. Colleran said. Balloon angioplasty is inferior to both stenting and drug coated balloon therapy for treatment of in-stent restenosis but is the standard of care in the United States, she noted. “I think...for regulatory reasons this was the comparator chosen,” she said.
“I think the implications are clear,” Dr. Colleran added. “This trial should provide a basis for regulatory approval of the drug coated balloon treatment of in-stent restenosis in the U.S. and finally provide this as an available treatment option for such patients.”
Dr. Yeh reported receiving grant/research support from Abbott Vascular, BD Bard, Boston Scientific, Cook Medical, Philips Medical, and Medtronic, and consulting for Abbott Vascular, Boston Scientific, CathWorks, Elixir Medical, Infraredx, Medtronic, Shockwave Medical, and Zol. Dr. Colleran had no disclosures. The trial was supported by Boston Scientific.
A version of this article first appeared on Medscape.com.
AT TCT 2023
Drug-eluting resorbable scaffold beats angioplasty for infrapopliteal artery disease
SAN FRANCISCO – An everolimus-eluting stent with a resorbable scaffold showed superior efficacy in a randomized multicenter trial when compared with angioplasty for the treatment of patients with chronic limb threatening ischemia (CLTI) resulting from infrapopliteal artery disease.
The stent (Esprit BTK, Abbott Vascular) was also noninferior to angioplasty in terms of safety.
Presenting results of the LIFE-BTK trial at the Transcatheter Cardiovascular Therapeutics annual meeting, Ramon Varcoe, MBBS, MS, PhD, MMed, of Prince of Wales Hospital, Sydney, said that peripheral artery disease is a global epidemic, the most serious manifestation of which is CTLI.
“If not treated expeditiously, this could lead to high rates of amputation, which, as we all know, has a severe impact on patients’ quality of life and even worse impact on their life prognosis, with prognosis rates worse than most cancers.”
He said that for infrapopliteal or below-the-knee (BTK) arterial disease, treatment with angioplasty has proven superior to bypass graft surgery, but some limitations of angioplasty are elastic recoil, dissection, and restenosis, thus limiting its durability. Coronary drug-eluting stents showed promise in BTK procedures but can interfere with reintervention. Thus, LIFE-BTK compared a drug-eluting stent with resorbable scaffolding to surgery.
Esprit BTK is a drug-eluting resorbable scaffold consisting of a temporary scaffold backbone of poly(L-lactide) and a strut thickness of 99 μm. It is coated with everolimus and bioresorbable poly(D,L-lactide). Two platinum markers at each end provide radiopacity.
LIFE-BTK enrolled patients aged 18 years or older with CLTI associated with ischemic rest pain or minor tissue loss and who had infrapopliteal artery stenosis or occlusion. The trial was prospective, international multicenter, and single-blind and randomly assigned 261 patients aged 18 years or older in the ratio of 2:1 to Esprit BTK (n = 173) or to angioplasty (n = 88). Treatment of up to two target lesions was allowed with a total scaffold length less than 170 mm.
The primary efficacy endpoint was superiority of Esprit BTK over angioplasty in terms of freedom of above-ankle amputation in the index limb, binary restenosis of the target lesion, and clinically driven target lesion revascularization evaluated at 1 year.
The primary safety endpoint, evaluated at 6 months, consisted of freedom from above-ankle amputation, major reintervention at 6 months, and perioperative mortality at 30 days.
An independent committee adjudicated clinical events, and core laboratories with assessors blinded to trial group assignment adjudicated imaging results and wound assessments.
Superior efficacy, noninferior safety
Participants were about two-thirds men, largely White, with about 15% of participants being Black/African American, and more than 90% of patients in each arm had hypertension. Lesion lengths were approximately 44 mm in each group with reference vessel diameters averaging 2.82-2.94 mm before intervention. Less than 4% in each group had severe lesion calcification.
Clinical follow-up rate at 1 year in the Esprit BTK arm was 90.2% and in the angioplasty arm 90.9%. Six patients in the former arm died versus five in the latter.
At the meeting, sponsored by the Cardiovascular Research Foundation, Dr. Varcoe showed a graph of the primary efficacy results at 453 days, the extra time being allowed to achieve a diagnostic ultrasound. (P < .0001).
“As you can see, those bars start to diverge in about 5 months and continue to separate over time, showing clear superiority of the scaffold over angioplasty, absolute risk difference of 30.8% and a highly statistically significant P value,” he said. Very similar primary efficacy outcomes were seen at 393 days.
At 1 year, the secondary efficacy endpoint of binary restenosis of the target lesion occurred in 24.2% of scaffold patients versus 46.5% of the angioplasty group (P < .0001). Another secondary endpoint, freedom from above-ankle amputation, 100% total occlusion of the target vessel, or clinically driven target lesion revascularization, occurred in 82.5% of the scaffold group versus 70.4% in the angioplasty group (P = .0081).
The primary safety endpoint of freedom from a major adverse limb event plus perioperative death was 100% for angioplasty and 96.9% for Esprit BTK (P = .0019)
All subgroup analyses assessing interaction by sex, race, geographic region, or age showed Esprit BTK was superior to angioplasty, with relative risks ranging from 0.27 to 0.61.
“If this technology is approved by the FDA, it will provide a new option for our patients with very difficult-to-treat disease, which will provide them additional durability and fewer reinterventions,” Dr. Varcoe concluded. “And I think we all know deep down that’s going to translate to improved clinical outcomes and few amputations.”
David Kandzari, MD, of Peidmont Heart Institute, Atlanta, was asked to comment on the study. He said that as with other vascular interventions, the ideal technology “would be first to provide a safe and effective antiproliferative therapy that would mitigate against restenosis and for scaffolding to prevent elastic recoil and reocclusion ... and ultimately fulfill these two promises without the requisite of a permanent implant.
“Despite their common use in femoral popliteal disease, drug-coated balloons had at best demonstrated inconsistent results below the knee, and drug-coated balloons, therefore, are not approved for such indications.”
He said that drug-eluting stents have demonstrated efficacy in this indication but that these studies have been limited to fairly discrete proximal disease.
“LIFE-BTK therefore represents one of the most rigorous trials in the space of endovascular interventions as a single line and randomized trial,” Dr. Kandzari said, “showing a primary composite endpoint of both safety and effectiveness relative to conventional angioplasty.”
Dr. Kandzari pointed to strengths of the study in that it used standardization of technique with independent adjudication of both imaging and wound healing assessments. “And the study population, too, was relevant to clinical practice, representing oftentimes underrepresented groups, including those with extensive disease burden [and] clinical severity,” he said. “Importantly, in this study, nearly one-third of the population will be women.”
Panelist Jennifer Rymer, MD, of Duke University Medical Center, Durham, N.C., commented that she treats a lot of African American patients with CTLI and applauded the researchers for including those patients. “I think that this will be a groundbreaking new change in our practice,” she said.
The trial results were published simultaneously with the presentation at TCT 2023 in the New England Journal of Medicine.
Dr. Varcoe reported receiving consulting fees/honoraria from Boston Scientific Corporation, Medtronic, Abbott, BD, Intervene, Surmodics, Philips, Nectero, Alucent, W.L. Gore, Vesteck, Bard Medical, Cook Medical, and R3 Vascular. He has equity, stock, or stock options in EBR Systems and has an executive role or ownership interest in Provisio Medical and Vesteck. Dr. Kandzari received grant support/research contract from Medtronic, Teleflex, Biotronik, and CSI; consultant fees/honoraria from and is on the speaker’s bureau of CSI and Medtronic; and has equity, stock, or options in Biostar Ventures. Dr. Rymer receives grant support/research contract from Chiesi, Abbott Vascular, Abiomed, and Pfizer. The study was funded by Abbott.
A version of this article appeared on Medscape.com.
SAN FRANCISCO – An everolimus-eluting stent with a resorbable scaffold showed superior efficacy in a randomized multicenter trial when compared with angioplasty for the treatment of patients with chronic limb threatening ischemia (CLTI) resulting from infrapopliteal artery disease.
The stent (Esprit BTK, Abbott Vascular) was also noninferior to angioplasty in terms of safety.
Presenting results of the LIFE-BTK trial at the Transcatheter Cardiovascular Therapeutics annual meeting, Ramon Varcoe, MBBS, MS, PhD, MMed, of Prince of Wales Hospital, Sydney, said that peripheral artery disease is a global epidemic, the most serious manifestation of which is CTLI.
“If not treated expeditiously, this could lead to high rates of amputation, which, as we all know, has a severe impact on patients’ quality of life and even worse impact on their life prognosis, with prognosis rates worse than most cancers.”
He said that for infrapopliteal or below-the-knee (BTK) arterial disease, treatment with angioplasty has proven superior to bypass graft surgery, but some limitations of angioplasty are elastic recoil, dissection, and restenosis, thus limiting its durability. Coronary drug-eluting stents showed promise in BTK procedures but can interfere with reintervention. Thus, LIFE-BTK compared a drug-eluting stent with resorbable scaffolding to surgery.
Esprit BTK is a drug-eluting resorbable scaffold consisting of a temporary scaffold backbone of poly(L-lactide) and a strut thickness of 99 μm. It is coated with everolimus and bioresorbable poly(D,L-lactide). Two platinum markers at each end provide radiopacity.
LIFE-BTK enrolled patients aged 18 years or older with CLTI associated with ischemic rest pain or minor tissue loss and who had infrapopliteal artery stenosis or occlusion. The trial was prospective, international multicenter, and single-blind and randomly assigned 261 patients aged 18 years or older in the ratio of 2:1 to Esprit BTK (n = 173) or to angioplasty (n = 88). Treatment of up to two target lesions was allowed with a total scaffold length less than 170 mm.
The primary efficacy endpoint was superiority of Esprit BTK over angioplasty in terms of freedom of above-ankle amputation in the index limb, binary restenosis of the target lesion, and clinically driven target lesion revascularization evaluated at 1 year.
The primary safety endpoint, evaluated at 6 months, consisted of freedom from above-ankle amputation, major reintervention at 6 months, and perioperative mortality at 30 days.
An independent committee adjudicated clinical events, and core laboratories with assessors blinded to trial group assignment adjudicated imaging results and wound assessments.
Superior efficacy, noninferior safety
Participants were about two-thirds men, largely White, with about 15% of participants being Black/African American, and more than 90% of patients in each arm had hypertension. Lesion lengths were approximately 44 mm in each group with reference vessel diameters averaging 2.82-2.94 mm before intervention. Less than 4% in each group had severe lesion calcification.
Clinical follow-up rate at 1 year in the Esprit BTK arm was 90.2% and in the angioplasty arm 90.9%. Six patients in the former arm died versus five in the latter.
At the meeting, sponsored by the Cardiovascular Research Foundation, Dr. Varcoe showed a graph of the primary efficacy results at 453 days, the extra time being allowed to achieve a diagnostic ultrasound. (P < .0001).
“As you can see, those bars start to diverge in about 5 months and continue to separate over time, showing clear superiority of the scaffold over angioplasty, absolute risk difference of 30.8% and a highly statistically significant P value,” he said. Very similar primary efficacy outcomes were seen at 393 days.
At 1 year, the secondary efficacy endpoint of binary restenosis of the target lesion occurred in 24.2% of scaffold patients versus 46.5% of the angioplasty group (P < .0001). Another secondary endpoint, freedom from above-ankle amputation, 100% total occlusion of the target vessel, or clinically driven target lesion revascularization, occurred in 82.5% of the scaffold group versus 70.4% in the angioplasty group (P = .0081).
The primary safety endpoint of freedom from a major adverse limb event plus perioperative death was 100% for angioplasty and 96.9% for Esprit BTK (P = .0019)
All subgroup analyses assessing interaction by sex, race, geographic region, or age showed Esprit BTK was superior to angioplasty, with relative risks ranging from 0.27 to 0.61.
“If this technology is approved by the FDA, it will provide a new option for our patients with very difficult-to-treat disease, which will provide them additional durability and fewer reinterventions,” Dr. Varcoe concluded. “And I think we all know deep down that’s going to translate to improved clinical outcomes and few amputations.”
David Kandzari, MD, of Peidmont Heart Institute, Atlanta, was asked to comment on the study. He said that as with other vascular interventions, the ideal technology “would be first to provide a safe and effective antiproliferative therapy that would mitigate against restenosis and for scaffolding to prevent elastic recoil and reocclusion ... and ultimately fulfill these two promises without the requisite of a permanent implant.
“Despite their common use in femoral popliteal disease, drug-coated balloons had at best demonstrated inconsistent results below the knee, and drug-coated balloons, therefore, are not approved for such indications.”
He said that drug-eluting stents have demonstrated efficacy in this indication but that these studies have been limited to fairly discrete proximal disease.
“LIFE-BTK therefore represents one of the most rigorous trials in the space of endovascular interventions as a single line and randomized trial,” Dr. Kandzari said, “showing a primary composite endpoint of both safety and effectiveness relative to conventional angioplasty.”
Dr. Kandzari pointed to strengths of the study in that it used standardization of technique with independent adjudication of both imaging and wound healing assessments. “And the study population, too, was relevant to clinical practice, representing oftentimes underrepresented groups, including those with extensive disease burden [and] clinical severity,” he said. “Importantly, in this study, nearly one-third of the population will be women.”
Panelist Jennifer Rymer, MD, of Duke University Medical Center, Durham, N.C., commented that she treats a lot of African American patients with CTLI and applauded the researchers for including those patients. “I think that this will be a groundbreaking new change in our practice,” she said.
The trial results were published simultaneously with the presentation at TCT 2023 in the New England Journal of Medicine.
Dr. Varcoe reported receiving consulting fees/honoraria from Boston Scientific Corporation, Medtronic, Abbott, BD, Intervene, Surmodics, Philips, Nectero, Alucent, W.L. Gore, Vesteck, Bard Medical, Cook Medical, and R3 Vascular. He has equity, stock, or stock options in EBR Systems and has an executive role or ownership interest in Provisio Medical and Vesteck. Dr. Kandzari received grant support/research contract from Medtronic, Teleflex, Biotronik, and CSI; consultant fees/honoraria from and is on the speaker’s bureau of CSI and Medtronic; and has equity, stock, or options in Biostar Ventures. Dr. Rymer receives grant support/research contract from Chiesi, Abbott Vascular, Abiomed, and Pfizer. The study was funded by Abbott.
A version of this article appeared on Medscape.com.
SAN FRANCISCO – An everolimus-eluting stent with a resorbable scaffold showed superior efficacy in a randomized multicenter trial when compared with angioplasty for the treatment of patients with chronic limb threatening ischemia (CLTI) resulting from infrapopliteal artery disease.
The stent (Esprit BTK, Abbott Vascular) was also noninferior to angioplasty in terms of safety.
Presenting results of the LIFE-BTK trial at the Transcatheter Cardiovascular Therapeutics annual meeting, Ramon Varcoe, MBBS, MS, PhD, MMed, of Prince of Wales Hospital, Sydney, said that peripheral artery disease is a global epidemic, the most serious manifestation of which is CTLI.
“If not treated expeditiously, this could lead to high rates of amputation, which, as we all know, has a severe impact on patients’ quality of life and even worse impact on their life prognosis, with prognosis rates worse than most cancers.”
He said that for infrapopliteal or below-the-knee (BTK) arterial disease, treatment with angioplasty has proven superior to bypass graft surgery, but some limitations of angioplasty are elastic recoil, dissection, and restenosis, thus limiting its durability. Coronary drug-eluting stents showed promise in BTK procedures but can interfere with reintervention. Thus, LIFE-BTK compared a drug-eluting stent with resorbable scaffolding to surgery.
Esprit BTK is a drug-eluting resorbable scaffold consisting of a temporary scaffold backbone of poly(L-lactide) and a strut thickness of 99 μm. It is coated with everolimus and bioresorbable poly(D,L-lactide). Two platinum markers at each end provide radiopacity.
LIFE-BTK enrolled patients aged 18 years or older with CLTI associated with ischemic rest pain or minor tissue loss and who had infrapopliteal artery stenosis or occlusion. The trial was prospective, international multicenter, and single-blind and randomly assigned 261 patients aged 18 years or older in the ratio of 2:1 to Esprit BTK (n = 173) or to angioplasty (n = 88). Treatment of up to two target lesions was allowed with a total scaffold length less than 170 mm.
The primary efficacy endpoint was superiority of Esprit BTK over angioplasty in terms of freedom of above-ankle amputation in the index limb, binary restenosis of the target lesion, and clinically driven target lesion revascularization evaluated at 1 year.
The primary safety endpoint, evaluated at 6 months, consisted of freedom from above-ankle amputation, major reintervention at 6 months, and perioperative mortality at 30 days.
An independent committee adjudicated clinical events, and core laboratories with assessors blinded to trial group assignment adjudicated imaging results and wound assessments.
Superior efficacy, noninferior safety
Participants were about two-thirds men, largely White, with about 15% of participants being Black/African American, and more than 90% of patients in each arm had hypertension. Lesion lengths were approximately 44 mm in each group with reference vessel diameters averaging 2.82-2.94 mm before intervention. Less than 4% in each group had severe lesion calcification.
Clinical follow-up rate at 1 year in the Esprit BTK arm was 90.2% and in the angioplasty arm 90.9%. Six patients in the former arm died versus five in the latter.
At the meeting, sponsored by the Cardiovascular Research Foundation, Dr. Varcoe showed a graph of the primary efficacy results at 453 days, the extra time being allowed to achieve a diagnostic ultrasound. (P < .0001).
“As you can see, those bars start to diverge in about 5 months and continue to separate over time, showing clear superiority of the scaffold over angioplasty, absolute risk difference of 30.8% and a highly statistically significant P value,” he said. Very similar primary efficacy outcomes were seen at 393 days.
At 1 year, the secondary efficacy endpoint of binary restenosis of the target lesion occurred in 24.2% of scaffold patients versus 46.5% of the angioplasty group (P < .0001). Another secondary endpoint, freedom from above-ankle amputation, 100% total occlusion of the target vessel, or clinically driven target lesion revascularization, occurred in 82.5% of the scaffold group versus 70.4% in the angioplasty group (P = .0081).
The primary safety endpoint of freedom from a major adverse limb event plus perioperative death was 100% for angioplasty and 96.9% for Esprit BTK (P = .0019)
All subgroup analyses assessing interaction by sex, race, geographic region, or age showed Esprit BTK was superior to angioplasty, with relative risks ranging from 0.27 to 0.61.
“If this technology is approved by the FDA, it will provide a new option for our patients with very difficult-to-treat disease, which will provide them additional durability and fewer reinterventions,” Dr. Varcoe concluded. “And I think we all know deep down that’s going to translate to improved clinical outcomes and few amputations.”
David Kandzari, MD, of Peidmont Heart Institute, Atlanta, was asked to comment on the study. He said that as with other vascular interventions, the ideal technology “would be first to provide a safe and effective antiproliferative therapy that would mitigate against restenosis and for scaffolding to prevent elastic recoil and reocclusion ... and ultimately fulfill these two promises without the requisite of a permanent implant.
“Despite their common use in femoral popliteal disease, drug-coated balloons had at best demonstrated inconsistent results below the knee, and drug-coated balloons, therefore, are not approved for such indications.”
He said that drug-eluting stents have demonstrated efficacy in this indication but that these studies have been limited to fairly discrete proximal disease.
“LIFE-BTK therefore represents one of the most rigorous trials in the space of endovascular interventions as a single line and randomized trial,” Dr. Kandzari said, “showing a primary composite endpoint of both safety and effectiveness relative to conventional angioplasty.”
Dr. Kandzari pointed to strengths of the study in that it used standardization of technique with independent adjudication of both imaging and wound healing assessments. “And the study population, too, was relevant to clinical practice, representing oftentimes underrepresented groups, including those with extensive disease burden [and] clinical severity,” he said. “Importantly, in this study, nearly one-third of the population will be women.”
Panelist Jennifer Rymer, MD, of Duke University Medical Center, Durham, N.C., commented that she treats a lot of African American patients with CTLI and applauded the researchers for including those patients. “I think that this will be a groundbreaking new change in our practice,” she said.
The trial results were published simultaneously with the presentation at TCT 2023 in the New England Journal of Medicine.
Dr. Varcoe reported receiving consulting fees/honoraria from Boston Scientific Corporation, Medtronic, Abbott, BD, Intervene, Surmodics, Philips, Nectero, Alucent, W.L. Gore, Vesteck, Bard Medical, Cook Medical, and R3 Vascular. He has equity, stock, or stock options in EBR Systems and has an executive role or ownership interest in Provisio Medical and Vesteck. Dr. Kandzari received grant support/research contract from Medtronic, Teleflex, Biotronik, and CSI; consultant fees/honoraria from and is on the speaker’s bureau of CSI and Medtronic; and has equity, stock, or options in Biostar Ventures. Dr. Rymer receives grant support/research contract from Chiesi, Abbott Vascular, Abiomed, and Pfizer. The study was funded by Abbott.
A version of this article appeared on Medscape.com.
AT TCT 2023
Trilogy TAVR safe, effective in aortic regurgitation
SAN FRANCISCO – , achieving a 1-year all-cause mortality rate of 7.8%.
New pacemaker implantation was 24%, similar to previously reported outcomes.
Vinod Thourani, MD, Piedmont Heart Institute, Atlanta, presented initial outcome results of the ALIGN-AR trial at the Transcatheter Cardiovascular Therapeutics annual meeting.
Dr. Thourani concluded that the Trilogy system provides the first dedicated transcatheter aortic valve replacement options “for symptomatic patients with moderate to severe or severe aortic regurgitation or at high risk for surgery and is well positioned to become the preferred therapy upon approval for this population.”
Currently, Trilogy is not approved by the U.S. Food and Drug Administration in the United States and is for investigational use only.
Untreated, severe symptomatic aortic regurgitation (AR) is associated with high mortality, especially for those with NYHA class 3 or 4 symptoms, Dr. Thourani explained. “While surgery remains the only recommended intervention for patients with native severe AR, there are a multitude of high-risk patients who are not offered therapy.”
Off-label use of transcatheter valves for AR has been associated with “higher rates of complications, including paravalvular regurgitation and embolization,” he noted.
Dr. Thourani described the unique features of the JenaValve Trilogy valve. The system has a set of three “locators” in its own sheath that allows it to be rotated to align with the three cusps of the native aortic valve, falling into the sinuses and securely anchored to the native valve leaflets – then the valve is deployed. Inside a self-expanding nitinol frame is porcine pericardial tissue. A sealing ring provides sufficient anchoring while conforming to the annulus.
ALIGN-AR was a multicenter, single arm, non-blinded trial with follow-up out to 5 years involving patients with 3-plus or greater AR at high risk for surgical aortic valve replacement. Exclusion criteria included an aortic root diameter greater than 5 cm, a previous prosthetic aortic valve, mitral regurgitation greater than moderate, or coronary artery disease requiring revascularization.
After Trilogy valve implantation, patients were followed for 1, 6, and 12 months, as well as annually out to 5 years. Safety and efficacy endpoints were compared with prespecified performance goals. Of 180 patients enrolled, 177 were successfully implanted with the Trilogy device.
Patients had an average age of 75.5 years, 47.2% were women, 67.2% were in NYHA class III/IV, 82.8% were hypertensive, and one-third were frail. Severe AR was present in 62.4%, and 31.7% had moderate to severe AR.
The primary composite safety endpoint included all-cause mortality, any stroke, major vascular complication, major bleeding, a new pacemaker, acute kidney injury, valve dysfunction, or any intervention related to the device. The primary efficacy endpoint was all-cause mortality at 12 months.
The performance goal for primary efficacy was a weighted average of 25%, derived mainly from 1-year mortality figures for NYHA class I/II and class II/IV with conservative management.
Non-inferiority margin met
With a 25% prespecified non-inferiority margin for the primary efficacy endpoint, “We have observed a rate of 7.8%,” Dr. Thourani reported during a late-breaking clinical trials session. “The non-inferiority margin was met for the primary efficacy endpoint with a P value of less than .0001.”
“With a 40.5% prespecified non-inferiority margin of our primary safety endpoint, with a Trilogy [heart valve] we have observed a rate of 26.7%,” he said. “At 30 days there was a 2.2% mortality and a 2.2% stroke rate. There was a 26.7% primary safety endpoint, mainly driven by the 24% new pacemaker implantation rate. Without pacemaker implantation, the rate of safety events was less than 8%,” (P noninferiority < .0001).
Procedure technical success was 95%, device success 96.7%, and procedure success 92.8%. There was one ascending aortic dissection (0.6%). Moderate or greater paravalvular regurgitation also occurred in one patient. There were four cases of valve embolization.
Pacemaker implants occurred in 30% of patients in the first tercile enrolled and decreased to 14% for the third tercile enrolled. “Lower rates are most likely due to the change in the insertion technique, placing locators above the nadir of the native cusps, reduction in oversizing, and also evolution in the management of periprocedural conduction abnormalities,” Dr. Thourani proposed.
The hemodynamics of the valve improved from a gradient of 8.7 mm Hg at baseline to 3.9 mm Hg at 30 days and remained fairly stable out to 1 year. Paravalvular regurgitation was absent in 80.8% of patients at 30 days and mild in 18%. It improved over time, being absent in 93.5% at 6 months and in 92.2% at 1 year.
Left ventricular (LV) remodeling occurred over one year, with LV end systolic diameter, LV end systolic volume, LV mass, and LV mass index all decreasing significantly from baseline to one year (all P < .0001). Importantly to patients, NYHA class improved, from 32% class II, 63% class III, and 5% class IV at baseline to 54% class I, 37% class II, and 10% class III at 30 days and improving slightly out to 1 year.
These improvements resulted in better quality of life, as reflected in a 21.8-point improvement in the self-reported Kansas City Cardiomyopathy Questionnaire Overall Summary Score, with a score of 77.6 at 1 year, indicating self-perceived good health.
Encouraging data
During the session, Robert Bonow, MD, of Northwestern University Feinberg School of Medicine, Chicago, commented that Dr. Thourani presented very encouraging data from the ALIGN-AR trial of high-risk surgical patients with significant aortic regurgitation. However, he had a couple of questions for Dr. Thourani.
One related to the efficacy data being compared with historical survival data. “So, are you planning to do a randomized study of these patients? You could argue, unlike aortic stenosis, where there’s no medical therapy, there could be medical therapies for the patients.” He noted that one-third of the patients are only in NYHA functional class II, so those patients “might do well over the long haul, with medical therapy as an agent.”
Dr. Thourani said it was an excellent question. “Doing a randomized trial with a high-risk patient [is] probably less likely,” he said. “I think there is a lot of interest among physicians on the cardiology side and on the surgery side of looking at lower-risk patients, and this could include those that are intermediate and or low risk.”
He said he believes investigators and leadership of the ALIGN-AR trial have conceived of such a trial involving all comers. “I think that’s warranted if we go into younger patients,” he said.
Dr. Bonow then asked if there was significant aortic valve calcification in the study population, because it is common with regurgitation, “which is why standard approaches are not effective ... And how does this device behave in calcified valves?” But Dr. Thourani said calcification was an exclusion criterion for this trial.
He said, “deep dives are going to come,” looking at the ventricular outcomes and also looking at a lot of the echocardiographic parameters.
Dr. Bonow related this study’s findings on ventricular remodeling to what is seen with surgical aortic valve replacement, where the ventricle decompresses within days. “And that’s predictive of good outcome if you have early data and these patients show how the ventricle remodels quickly,” he said.
The trial was supported by JenaValve. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, Atricure, Boston Scientific, CroiValve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, Atricure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Bonow had no disclosures.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , achieving a 1-year all-cause mortality rate of 7.8%.
New pacemaker implantation was 24%, similar to previously reported outcomes.
Vinod Thourani, MD, Piedmont Heart Institute, Atlanta, presented initial outcome results of the ALIGN-AR trial at the Transcatheter Cardiovascular Therapeutics annual meeting.
Dr. Thourani concluded that the Trilogy system provides the first dedicated transcatheter aortic valve replacement options “for symptomatic patients with moderate to severe or severe aortic regurgitation or at high risk for surgery and is well positioned to become the preferred therapy upon approval for this population.”
Currently, Trilogy is not approved by the U.S. Food and Drug Administration in the United States and is for investigational use only.
Untreated, severe symptomatic aortic regurgitation (AR) is associated with high mortality, especially for those with NYHA class 3 or 4 symptoms, Dr. Thourani explained. “While surgery remains the only recommended intervention for patients with native severe AR, there are a multitude of high-risk patients who are not offered therapy.”
Off-label use of transcatheter valves for AR has been associated with “higher rates of complications, including paravalvular regurgitation and embolization,” he noted.
Dr. Thourani described the unique features of the JenaValve Trilogy valve. The system has a set of three “locators” in its own sheath that allows it to be rotated to align with the three cusps of the native aortic valve, falling into the sinuses and securely anchored to the native valve leaflets – then the valve is deployed. Inside a self-expanding nitinol frame is porcine pericardial tissue. A sealing ring provides sufficient anchoring while conforming to the annulus.
ALIGN-AR was a multicenter, single arm, non-blinded trial with follow-up out to 5 years involving patients with 3-plus or greater AR at high risk for surgical aortic valve replacement. Exclusion criteria included an aortic root diameter greater than 5 cm, a previous prosthetic aortic valve, mitral regurgitation greater than moderate, or coronary artery disease requiring revascularization.
After Trilogy valve implantation, patients were followed for 1, 6, and 12 months, as well as annually out to 5 years. Safety and efficacy endpoints were compared with prespecified performance goals. Of 180 patients enrolled, 177 were successfully implanted with the Trilogy device.
Patients had an average age of 75.5 years, 47.2% were women, 67.2% were in NYHA class III/IV, 82.8% were hypertensive, and one-third were frail. Severe AR was present in 62.4%, and 31.7% had moderate to severe AR.
The primary composite safety endpoint included all-cause mortality, any stroke, major vascular complication, major bleeding, a new pacemaker, acute kidney injury, valve dysfunction, or any intervention related to the device. The primary efficacy endpoint was all-cause mortality at 12 months.
The performance goal for primary efficacy was a weighted average of 25%, derived mainly from 1-year mortality figures for NYHA class I/II and class II/IV with conservative management.
Non-inferiority margin met
With a 25% prespecified non-inferiority margin for the primary efficacy endpoint, “We have observed a rate of 7.8%,” Dr. Thourani reported during a late-breaking clinical trials session. “The non-inferiority margin was met for the primary efficacy endpoint with a P value of less than .0001.”
“With a 40.5% prespecified non-inferiority margin of our primary safety endpoint, with a Trilogy [heart valve] we have observed a rate of 26.7%,” he said. “At 30 days there was a 2.2% mortality and a 2.2% stroke rate. There was a 26.7% primary safety endpoint, mainly driven by the 24% new pacemaker implantation rate. Without pacemaker implantation, the rate of safety events was less than 8%,” (P noninferiority < .0001).
Procedure technical success was 95%, device success 96.7%, and procedure success 92.8%. There was one ascending aortic dissection (0.6%). Moderate or greater paravalvular regurgitation also occurred in one patient. There were four cases of valve embolization.
Pacemaker implants occurred in 30% of patients in the first tercile enrolled and decreased to 14% for the third tercile enrolled. “Lower rates are most likely due to the change in the insertion technique, placing locators above the nadir of the native cusps, reduction in oversizing, and also evolution in the management of periprocedural conduction abnormalities,” Dr. Thourani proposed.
The hemodynamics of the valve improved from a gradient of 8.7 mm Hg at baseline to 3.9 mm Hg at 30 days and remained fairly stable out to 1 year. Paravalvular regurgitation was absent in 80.8% of patients at 30 days and mild in 18%. It improved over time, being absent in 93.5% at 6 months and in 92.2% at 1 year.
Left ventricular (LV) remodeling occurred over one year, with LV end systolic diameter, LV end systolic volume, LV mass, and LV mass index all decreasing significantly from baseline to one year (all P < .0001). Importantly to patients, NYHA class improved, from 32% class II, 63% class III, and 5% class IV at baseline to 54% class I, 37% class II, and 10% class III at 30 days and improving slightly out to 1 year.
These improvements resulted in better quality of life, as reflected in a 21.8-point improvement in the self-reported Kansas City Cardiomyopathy Questionnaire Overall Summary Score, with a score of 77.6 at 1 year, indicating self-perceived good health.
Encouraging data
During the session, Robert Bonow, MD, of Northwestern University Feinberg School of Medicine, Chicago, commented that Dr. Thourani presented very encouraging data from the ALIGN-AR trial of high-risk surgical patients with significant aortic regurgitation. However, he had a couple of questions for Dr. Thourani.
One related to the efficacy data being compared with historical survival data. “So, are you planning to do a randomized study of these patients? You could argue, unlike aortic stenosis, where there’s no medical therapy, there could be medical therapies for the patients.” He noted that one-third of the patients are only in NYHA functional class II, so those patients “might do well over the long haul, with medical therapy as an agent.”
Dr. Thourani said it was an excellent question. “Doing a randomized trial with a high-risk patient [is] probably less likely,” he said. “I think there is a lot of interest among physicians on the cardiology side and on the surgery side of looking at lower-risk patients, and this could include those that are intermediate and or low risk.”
He said he believes investigators and leadership of the ALIGN-AR trial have conceived of such a trial involving all comers. “I think that’s warranted if we go into younger patients,” he said.
Dr. Bonow then asked if there was significant aortic valve calcification in the study population, because it is common with regurgitation, “which is why standard approaches are not effective ... And how does this device behave in calcified valves?” But Dr. Thourani said calcification was an exclusion criterion for this trial.
He said, “deep dives are going to come,” looking at the ventricular outcomes and also looking at a lot of the echocardiographic parameters.
Dr. Bonow related this study’s findings on ventricular remodeling to what is seen with surgical aortic valve replacement, where the ventricle decompresses within days. “And that’s predictive of good outcome if you have early data and these patients show how the ventricle remodels quickly,” he said.
The trial was supported by JenaValve. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, Atricure, Boston Scientific, CroiValve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, Atricure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Bonow had no disclosures.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – , achieving a 1-year all-cause mortality rate of 7.8%.
New pacemaker implantation was 24%, similar to previously reported outcomes.
Vinod Thourani, MD, Piedmont Heart Institute, Atlanta, presented initial outcome results of the ALIGN-AR trial at the Transcatheter Cardiovascular Therapeutics annual meeting.
Dr. Thourani concluded that the Trilogy system provides the first dedicated transcatheter aortic valve replacement options “for symptomatic patients with moderate to severe or severe aortic regurgitation or at high risk for surgery and is well positioned to become the preferred therapy upon approval for this population.”
Currently, Trilogy is not approved by the U.S. Food and Drug Administration in the United States and is for investigational use only.
Untreated, severe symptomatic aortic regurgitation (AR) is associated with high mortality, especially for those with NYHA class 3 or 4 symptoms, Dr. Thourani explained. “While surgery remains the only recommended intervention for patients with native severe AR, there are a multitude of high-risk patients who are not offered therapy.”
Off-label use of transcatheter valves for AR has been associated with “higher rates of complications, including paravalvular regurgitation and embolization,” he noted.
Dr. Thourani described the unique features of the JenaValve Trilogy valve. The system has a set of three “locators” in its own sheath that allows it to be rotated to align with the three cusps of the native aortic valve, falling into the sinuses and securely anchored to the native valve leaflets – then the valve is deployed. Inside a self-expanding nitinol frame is porcine pericardial tissue. A sealing ring provides sufficient anchoring while conforming to the annulus.
ALIGN-AR was a multicenter, single arm, non-blinded trial with follow-up out to 5 years involving patients with 3-plus or greater AR at high risk for surgical aortic valve replacement. Exclusion criteria included an aortic root diameter greater than 5 cm, a previous prosthetic aortic valve, mitral regurgitation greater than moderate, or coronary artery disease requiring revascularization.
After Trilogy valve implantation, patients were followed for 1, 6, and 12 months, as well as annually out to 5 years. Safety and efficacy endpoints were compared with prespecified performance goals. Of 180 patients enrolled, 177 were successfully implanted with the Trilogy device.
Patients had an average age of 75.5 years, 47.2% were women, 67.2% were in NYHA class III/IV, 82.8% were hypertensive, and one-third were frail. Severe AR was present in 62.4%, and 31.7% had moderate to severe AR.
The primary composite safety endpoint included all-cause mortality, any stroke, major vascular complication, major bleeding, a new pacemaker, acute kidney injury, valve dysfunction, or any intervention related to the device. The primary efficacy endpoint was all-cause mortality at 12 months.
The performance goal for primary efficacy was a weighted average of 25%, derived mainly from 1-year mortality figures for NYHA class I/II and class II/IV with conservative management.
Non-inferiority margin met
With a 25% prespecified non-inferiority margin for the primary efficacy endpoint, “We have observed a rate of 7.8%,” Dr. Thourani reported during a late-breaking clinical trials session. “The non-inferiority margin was met for the primary efficacy endpoint with a P value of less than .0001.”
“With a 40.5% prespecified non-inferiority margin of our primary safety endpoint, with a Trilogy [heart valve] we have observed a rate of 26.7%,” he said. “At 30 days there was a 2.2% mortality and a 2.2% stroke rate. There was a 26.7% primary safety endpoint, mainly driven by the 24% new pacemaker implantation rate. Without pacemaker implantation, the rate of safety events was less than 8%,” (P noninferiority < .0001).
Procedure technical success was 95%, device success 96.7%, and procedure success 92.8%. There was one ascending aortic dissection (0.6%). Moderate or greater paravalvular regurgitation also occurred in one patient. There were four cases of valve embolization.
Pacemaker implants occurred in 30% of patients in the first tercile enrolled and decreased to 14% for the third tercile enrolled. “Lower rates are most likely due to the change in the insertion technique, placing locators above the nadir of the native cusps, reduction in oversizing, and also evolution in the management of periprocedural conduction abnormalities,” Dr. Thourani proposed.
The hemodynamics of the valve improved from a gradient of 8.7 mm Hg at baseline to 3.9 mm Hg at 30 days and remained fairly stable out to 1 year. Paravalvular regurgitation was absent in 80.8% of patients at 30 days and mild in 18%. It improved over time, being absent in 93.5% at 6 months and in 92.2% at 1 year.
Left ventricular (LV) remodeling occurred over one year, with LV end systolic diameter, LV end systolic volume, LV mass, and LV mass index all decreasing significantly from baseline to one year (all P < .0001). Importantly to patients, NYHA class improved, from 32% class II, 63% class III, and 5% class IV at baseline to 54% class I, 37% class II, and 10% class III at 30 days and improving slightly out to 1 year.
These improvements resulted in better quality of life, as reflected in a 21.8-point improvement in the self-reported Kansas City Cardiomyopathy Questionnaire Overall Summary Score, with a score of 77.6 at 1 year, indicating self-perceived good health.
Encouraging data
During the session, Robert Bonow, MD, of Northwestern University Feinberg School of Medicine, Chicago, commented that Dr. Thourani presented very encouraging data from the ALIGN-AR trial of high-risk surgical patients with significant aortic regurgitation. However, he had a couple of questions for Dr. Thourani.
One related to the efficacy data being compared with historical survival data. “So, are you planning to do a randomized study of these patients? You could argue, unlike aortic stenosis, where there’s no medical therapy, there could be medical therapies for the patients.” He noted that one-third of the patients are only in NYHA functional class II, so those patients “might do well over the long haul, with medical therapy as an agent.”
Dr. Thourani said it was an excellent question. “Doing a randomized trial with a high-risk patient [is] probably less likely,” he said. “I think there is a lot of interest among physicians on the cardiology side and on the surgery side of looking at lower-risk patients, and this could include those that are intermediate and or low risk.”
He said he believes investigators and leadership of the ALIGN-AR trial have conceived of such a trial involving all comers. “I think that’s warranted if we go into younger patients,” he said.
Dr. Bonow then asked if there was significant aortic valve calcification in the study population, because it is common with regurgitation, “which is why standard approaches are not effective ... And how does this device behave in calcified valves?” But Dr. Thourani said calcification was an exclusion criterion for this trial.
He said, “deep dives are going to come,” looking at the ventricular outcomes and also looking at a lot of the echocardiographic parameters.
Dr. Bonow related this study’s findings on ventricular remodeling to what is seen with surgical aortic valve replacement, where the ventricle decompresses within days. “And that’s predictive of good outcome if you have early data and these patients show how the ventricle remodels quickly,” he said.
The trial was supported by JenaValve. Dr. Thourani has received grant/research support from Abbott Vascular, Artivion, Atricure, Boston Scientific, CroiValve, Edwards Lifesciences, JenaValve, Medtronic, and Trisol; consultant fees/honoraria from Abbott Vascular, Artivion, Atricure, Boston Scientific, Croivalve, and Edwards Lifesciences; and has an executive role/ownership interest in DASI Simulations. Dr. Bonow had no disclosures.
A version of this article first appeared on Medscape.com.
AT TCT 2023
Surprisingly more nonsustained VT shown in HCM using extended ECG monitoring
BARCELONA – , suggests a study that questions current risk stratification practices in HCM.
In the registry study, such arrythmias were observed in about six times as many HCM patients during 30 days of ambulatory electrocardiographic monitoring as would have been identified based on the first 24 hours of the monitoring period: 65% vs. 11% of the cohort.
Also, about 62% of the patients showed NSVT at “extended” 30-day monitoring, compared with an 8% prevalence of the arrhythmia based on the more conventional ECG monitoring period of 24 hours.
Nonsustained ventricular tachycardia, an important arrhythmia used every day in clinical practice to make decisions, is “much, much more prevalent than we thought” in patients with HCM, Juan Caro Codón, MD, the study’s principal investigator, said in an interview. “We should invest in further research regarding extended ECG monitoring in these patients.”
Dr. Caro Codón, of La Paz University Hospital, Madrid, presented the findings from the TEMPO-HCM study at the European Heart Rhythm Association 2023 Congress, held in Barcelona and virtually.
Its results, he said, have implications for stratifying HCM patients according to their risk for sudden cardiac death in deciding who should be offered an implantable cardioverter-defibrillator (ICD).
The life-incidence of atrial fibrillation (AF) in patients like those in the current analysis has previously been found to be about 20%, and the life-prevalence of NSVT about 20%-30%, using traditional 24- or 48-hour Holter monitoring, Dr. Caro Codón said.
“These arrhythmias are clinically relevant events because they are linked to very meaningful clinical endpoints,” including stroke and thromboembolism, he said, “but also for sudden cardiac death.”
Extended ECG monitoring has been shown useful in the setting of cryptogenic stroke and after AF ablation, but similar findings have been scarce in HCM. Patients using personal wearable monitors such as smart watches, Dr. Caro Codón said, have come to his clinic with concerns that the devices may have signaled a problem. But the lack of relevant data leaves them without a sufficient answer.
In other findings, invited discussant Isabelle van Gelder, MD, PhD, observed after Dr. Caro Codón’s presentation that the number of patients with AF almost doubled based on extended monitoring, compared with the first 24 hours of monitoring.
Based on European Society of Cardiology guidelines from 2020, “Once clinical AF has been documented, there is a class IIA recommendation to start anticoagulation,” said Dr. van Gelder, University of Groningen, the Netherlands. “Therefore, your data really are a call for more data on screening for AF in hypertrophic cardiomyopathy patients.”
Prospective multicenter registry
The TEMPO-HCM registry includes patients with HCM and a clinical indication for standard Holter monitoring at five hospitals in Spain. It excludes patients with an HCM-like phenotype but who lack the telltale genotype, as well as those already implanted with an ICD.
Those in the current analysis underwent 30-day ECG monitoring with a small, wearable device that Dr. Caro Codón described as about 7 cm long, worn in what is essentially a T-shirt with a pocket. Patients could remove the shirt and device to bathe or go swimming, for example, and still be monitored for most of the day.
The analysis included the registry’s first 100 patients (mean age, 57 years; 78% male). Hypertension was present in 47%, 58% were on beta-blockers, 16% had prior AF or atrial flutter, and 19% were taking anticoagulants. Only 8% were on antiarrhythmic drugs, Dr. Caro Codón reported.
The patients had good functional status (68% and 29% were in NYHA class 1 and 2, respectively) and their left ventricular ejection fraction averaged 66%. Of the 71 patients who underwent MRI, 28.2% showed late gadolinium enhancement suggesting myocardial scarring.
More arrhythmias on 30-day monitoring
The primary endpoint of clinically relevant arrhythmia (AF, atrial flutter, or NSVT) was identified during the first 24 hours of monitoring in 11% of patients. The prevalence rose to 65% (P < .001) based on 30-day monitoring.
Similarly, prevalences of the composite primary endpoint components grew on extended monitoring, but the increases reached statistical significance only for NSVT; its prevalence went from 8% to 62% (P < .001). Prevalences rose nonsignificantly from 6% to 10% for AF and 0% to 1% for sustained ventricular tachycardia.
The incidence of NSVT during monitoring climbed fastest from day 0 through about day 19 and then rose more slowly through day 30, Dr. Caro Codón said. “It actually didn’t reach a plateau during this time period, so there is the possibility that if we had continued monitoring patients, the difference between both periods may have been even higher.”
Three variables predicted the incidence of nonsustained VT during monitoring, he said: age, atrial wall thickness, and whether there was late gadolinium enhancement at MRI.
An exploratory analysis looked at the 5-year risk of sudden cardiac death using the European Society of Cardiology HCM-SCD risk calculator recommended in guidelines. Risk assessment based on the 30-day extended monitoring period, compared with the first 24 hours of monitoring alone, predicted a significantly higher 5-year risk of sudden death, Dr. Caro Codón said.
“Even more importantly,” he added, “over 20%” of patients would have been reclassified into a higher-risk group and possibly considered for an ICD based on extended monitoring, compared to 24-hour monitoring.
However, given that more than 50% of patients were found to have NSVT during extended monitoring, Dr. Caro Codón proposed that decisions on whether to implant an ICD should not be so “binary” based on the presence or absence of symptoms, and proposed further investigations be conducted into the complete phenotype of these arrhythmias.
The study has limitations, he observed, including a relatively small size; but it was able to detect important differences between 24-hour and 30-day monitoring outcomes even with only 100 patients. It was also limited by a lack of clinical follow-up for information on endpoints like stroke, thromboembolism, and sudden cardiac death.
Extended monitoring detected more cases of NSVT in the study’s relatively low-risk HCM patients who would not generally have an indication for ICD implantation, observed Dr. van Gelder. Also, at present the prognostic value of NSVT for SCD “seems to be more important at younger age” – that is, younger than 30 years – in patients with HCM.
Dr. van Gelder echoed Dr. Caro Codón’s call for more data from prolonged monitoring to help stratify patients according to risk; she proposed NSVT frequency, duration, and rate as possible targets.
The study was supported by an unrestricted grant from Nuubo, which provided the ECG monitoring systems. Dr. Caro Codón and Dr. van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – , suggests a study that questions current risk stratification practices in HCM.
In the registry study, such arrythmias were observed in about six times as many HCM patients during 30 days of ambulatory electrocardiographic monitoring as would have been identified based on the first 24 hours of the monitoring period: 65% vs. 11% of the cohort.
Also, about 62% of the patients showed NSVT at “extended” 30-day monitoring, compared with an 8% prevalence of the arrhythmia based on the more conventional ECG monitoring period of 24 hours.
Nonsustained ventricular tachycardia, an important arrhythmia used every day in clinical practice to make decisions, is “much, much more prevalent than we thought” in patients with HCM, Juan Caro Codón, MD, the study’s principal investigator, said in an interview. “We should invest in further research regarding extended ECG monitoring in these patients.”
Dr. Caro Codón, of La Paz University Hospital, Madrid, presented the findings from the TEMPO-HCM study at the European Heart Rhythm Association 2023 Congress, held in Barcelona and virtually.
Its results, he said, have implications for stratifying HCM patients according to their risk for sudden cardiac death in deciding who should be offered an implantable cardioverter-defibrillator (ICD).
The life-incidence of atrial fibrillation (AF) in patients like those in the current analysis has previously been found to be about 20%, and the life-prevalence of NSVT about 20%-30%, using traditional 24- or 48-hour Holter monitoring, Dr. Caro Codón said.
“These arrhythmias are clinically relevant events because they are linked to very meaningful clinical endpoints,” including stroke and thromboembolism, he said, “but also for sudden cardiac death.”
Extended ECG monitoring has been shown useful in the setting of cryptogenic stroke and after AF ablation, but similar findings have been scarce in HCM. Patients using personal wearable monitors such as smart watches, Dr. Caro Codón said, have come to his clinic with concerns that the devices may have signaled a problem. But the lack of relevant data leaves them without a sufficient answer.
In other findings, invited discussant Isabelle van Gelder, MD, PhD, observed after Dr. Caro Codón’s presentation that the number of patients with AF almost doubled based on extended monitoring, compared with the first 24 hours of monitoring.
Based on European Society of Cardiology guidelines from 2020, “Once clinical AF has been documented, there is a class IIA recommendation to start anticoagulation,” said Dr. van Gelder, University of Groningen, the Netherlands. “Therefore, your data really are a call for more data on screening for AF in hypertrophic cardiomyopathy patients.”
Prospective multicenter registry
The TEMPO-HCM registry includes patients with HCM and a clinical indication for standard Holter monitoring at five hospitals in Spain. It excludes patients with an HCM-like phenotype but who lack the telltale genotype, as well as those already implanted with an ICD.
Those in the current analysis underwent 30-day ECG monitoring with a small, wearable device that Dr. Caro Codón described as about 7 cm long, worn in what is essentially a T-shirt with a pocket. Patients could remove the shirt and device to bathe or go swimming, for example, and still be monitored for most of the day.
The analysis included the registry’s first 100 patients (mean age, 57 years; 78% male). Hypertension was present in 47%, 58% were on beta-blockers, 16% had prior AF or atrial flutter, and 19% were taking anticoagulants. Only 8% were on antiarrhythmic drugs, Dr. Caro Codón reported.
The patients had good functional status (68% and 29% were in NYHA class 1 and 2, respectively) and their left ventricular ejection fraction averaged 66%. Of the 71 patients who underwent MRI, 28.2% showed late gadolinium enhancement suggesting myocardial scarring.
More arrhythmias on 30-day monitoring
The primary endpoint of clinically relevant arrhythmia (AF, atrial flutter, or NSVT) was identified during the first 24 hours of monitoring in 11% of patients. The prevalence rose to 65% (P < .001) based on 30-day monitoring.
Similarly, prevalences of the composite primary endpoint components grew on extended monitoring, but the increases reached statistical significance only for NSVT; its prevalence went from 8% to 62% (P < .001). Prevalences rose nonsignificantly from 6% to 10% for AF and 0% to 1% for sustained ventricular tachycardia.
The incidence of NSVT during monitoring climbed fastest from day 0 through about day 19 and then rose more slowly through day 30, Dr. Caro Codón said. “It actually didn’t reach a plateau during this time period, so there is the possibility that if we had continued monitoring patients, the difference between both periods may have been even higher.”
Three variables predicted the incidence of nonsustained VT during monitoring, he said: age, atrial wall thickness, and whether there was late gadolinium enhancement at MRI.
An exploratory analysis looked at the 5-year risk of sudden cardiac death using the European Society of Cardiology HCM-SCD risk calculator recommended in guidelines. Risk assessment based on the 30-day extended monitoring period, compared with the first 24 hours of monitoring alone, predicted a significantly higher 5-year risk of sudden death, Dr. Caro Codón said.
“Even more importantly,” he added, “over 20%” of patients would have been reclassified into a higher-risk group and possibly considered for an ICD based on extended monitoring, compared to 24-hour monitoring.
However, given that more than 50% of patients were found to have NSVT during extended monitoring, Dr. Caro Codón proposed that decisions on whether to implant an ICD should not be so “binary” based on the presence or absence of symptoms, and proposed further investigations be conducted into the complete phenotype of these arrhythmias.
The study has limitations, he observed, including a relatively small size; but it was able to detect important differences between 24-hour and 30-day monitoring outcomes even with only 100 patients. It was also limited by a lack of clinical follow-up for information on endpoints like stroke, thromboembolism, and sudden cardiac death.
Extended monitoring detected more cases of NSVT in the study’s relatively low-risk HCM patients who would not generally have an indication for ICD implantation, observed Dr. van Gelder. Also, at present the prognostic value of NSVT for SCD “seems to be more important at younger age” – that is, younger than 30 years – in patients with HCM.
Dr. van Gelder echoed Dr. Caro Codón’s call for more data from prolonged monitoring to help stratify patients according to risk; she proposed NSVT frequency, duration, and rate as possible targets.
The study was supported by an unrestricted grant from Nuubo, which provided the ECG monitoring systems. Dr. Caro Codón and Dr. van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – , suggests a study that questions current risk stratification practices in HCM.
In the registry study, such arrythmias were observed in about six times as many HCM patients during 30 days of ambulatory electrocardiographic monitoring as would have been identified based on the first 24 hours of the monitoring period: 65% vs. 11% of the cohort.
Also, about 62% of the patients showed NSVT at “extended” 30-day monitoring, compared with an 8% prevalence of the arrhythmia based on the more conventional ECG monitoring period of 24 hours.
Nonsustained ventricular tachycardia, an important arrhythmia used every day in clinical practice to make decisions, is “much, much more prevalent than we thought” in patients with HCM, Juan Caro Codón, MD, the study’s principal investigator, said in an interview. “We should invest in further research regarding extended ECG monitoring in these patients.”
Dr. Caro Codón, of La Paz University Hospital, Madrid, presented the findings from the TEMPO-HCM study at the European Heart Rhythm Association 2023 Congress, held in Barcelona and virtually.
Its results, he said, have implications for stratifying HCM patients according to their risk for sudden cardiac death in deciding who should be offered an implantable cardioverter-defibrillator (ICD).
The life-incidence of atrial fibrillation (AF) in patients like those in the current analysis has previously been found to be about 20%, and the life-prevalence of NSVT about 20%-30%, using traditional 24- or 48-hour Holter monitoring, Dr. Caro Codón said.
“These arrhythmias are clinically relevant events because they are linked to very meaningful clinical endpoints,” including stroke and thromboembolism, he said, “but also for sudden cardiac death.”
Extended ECG monitoring has been shown useful in the setting of cryptogenic stroke and after AF ablation, but similar findings have been scarce in HCM. Patients using personal wearable monitors such as smart watches, Dr. Caro Codón said, have come to his clinic with concerns that the devices may have signaled a problem. But the lack of relevant data leaves them without a sufficient answer.
In other findings, invited discussant Isabelle van Gelder, MD, PhD, observed after Dr. Caro Codón’s presentation that the number of patients with AF almost doubled based on extended monitoring, compared with the first 24 hours of monitoring.
Based on European Society of Cardiology guidelines from 2020, “Once clinical AF has been documented, there is a class IIA recommendation to start anticoagulation,” said Dr. van Gelder, University of Groningen, the Netherlands. “Therefore, your data really are a call for more data on screening for AF in hypertrophic cardiomyopathy patients.”
Prospective multicenter registry
The TEMPO-HCM registry includes patients with HCM and a clinical indication for standard Holter monitoring at five hospitals in Spain. It excludes patients with an HCM-like phenotype but who lack the telltale genotype, as well as those already implanted with an ICD.
Those in the current analysis underwent 30-day ECG monitoring with a small, wearable device that Dr. Caro Codón described as about 7 cm long, worn in what is essentially a T-shirt with a pocket. Patients could remove the shirt and device to bathe or go swimming, for example, and still be monitored for most of the day.
The analysis included the registry’s first 100 patients (mean age, 57 years; 78% male). Hypertension was present in 47%, 58% were on beta-blockers, 16% had prior AF or atrial flutter, and 19% were taking anticoagulants. Only 8% were on antiarrhythmic drugs, Dr. Caro Codón reported.
The patients had good functional status (68% and 29% were in NYHA class 1 and 2, respectively) and their left ventricular ejection fraction averaged 66%. Of the 71 patients who underwent MRI, 28.2% showed late gadolinium enhancement suggesting myocardial scarring.
More arrhythmias on 30-day monitoring
The primary endpoint of clinically relevant arrhythmia (AF, atrial flutter, or NSVT) was identified during the first 24 hours of monitoring in 11% of patients. The prevalence rose to 65% (P < .001) based on 30-day monitoring.
Similarly, prevalences of the composite primary endpoint components grew on extended monitoring, but the increases reached statistical significance only for NSVT; its prevalence went from 8% to 62% (P < .001). Prevalences rose nonsignificantly from 6% to 10% for AF and 0% to 1% for sustained ventricular tachycardia.
The incidence of NSVT during monitoring climbed fastest from day 0 through about day 19 and then rose more slowly through day 30, Dr. Caro Codón said. “It actually didn’t reach a plateau during this time period, so there is the possibility that if we had continued monitoring patients, the difference between both periods may have been even higher.”
Three variables predicted the incidence of nonsustained VT during monitoring, he said: age, atrial wall thickness, and whether there was late gadolinium enhancement at MRI.
An exploratory analysis looked at the 5-year risk of sudden cardiac death using the European Society of Cardiology HCM-SCD risk calculator recommended in guidelines. Risk assessment based on the 30-day extended monitoring period, compared with the first 24 hours of monitoring alone, predicted a significantly higher 5-year risk of sudden death, Dr. Caro Codón said.
“Even more importantly,” he added, “over 20%” of patients would have been reclassified into a higher-risk group and possibly considered for an ICD based on extended monitoring, compared to 24-hour monitoring.
However, given that more than 50% of patients were found to have NSVT during extended monitoring, Dr. Caro Codón proposed that decisions on whether to implant an ICD should not be so “binary” based on the presence or absence of symptoms, and proposed further investigations be conducted into the complete phenotype of these arrhythmias.
The study has limitations, he observed, including a relatively small size; but it was able to detect important differences between 24-hour and 30-day monitoring outcomes even with only 100 patients. It was also limited by a lack of clinical follow-up for information on endpoints like stroke, thromboembolism, and sudden cardiac death.
Extended monitoring detected more cases of NSVT in the study’s relatively low-risk HCM patients who would not generally have an indication for ICD implantation, observed Dr. van Gelder. Also, at present the prognostic value of NSVT for SCD “seems to be more important at younger age” – that is, younger than 30 years – in patients with HCM.
Dr. van Gelder echoed Dr. Caro Codón’s call for more data from prolonged monitoring to help stratify patients according to risk; she proposed NSVT frequency, duration, and rate as possible targets.
The study was supported by an unrestricted grant from Nuubo, which provided the ECG monitoring systems. Dr. Caro Codón and Dr. van Gelder reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EHRA
Statins tied to lower stroke risk in atrial fibrillation
Among patients with atrial fibrillation (AFib), initiation of statins soon after diagnosis was protective against stroke and related vascular events, and longer duration of use was associated with greater protection, a new cohort study shows.
Statin use was associated with lower risks of ischemic stroke or systemic embolism, hemorrhagic stroke, and transient ischemic attack (TIA), regardless of whether patients were also taking anticoagulant medications.
Lead author Jiayi Huang, a PhD student at Hong Kong University at Shenzhen (China) Hospital, concluded that the study’s findings support the use of statins to prevent stroke for patients with new-onset AFib.
“The findings have important clinical implications, particularly given that in atrial fibrillation, patients’ ischemic strokes are often fatal or disabling and have a high risk of recurrence,” she said.
The results were presented in a moderated poster session at the European Heart Rhythm Association 2023 Congress.
Widely prescribed
Anticoagulant drugs are prescribed to lower the fivefold increased risk of stroke among individuals with AFib, compared with those without AFib, but the therapy does not eliminate the higher risk, Ms. Huang explained. And although statins are widely prescribed to reduce the likelihood of myocardial infarction and stroke, “the benefit of statins for stroke prevention in patients with atrial fibrillation has been unclear.”
Ms. Huang and colleagues analyzed data from 51,472 patients newly diagnosed with AFib between 2010 and 2018. The population was divided into statin users (n = 11,866), defined as patients who had taken statins for at least 19 consecutive days in the first year after AFib diagnosis, and statin nonusers (n = 39,606), based on whether they were prescribed statin therapy after their first diagnosis of AFib.
The median age of the cohort was 74.9 years, and 47.7% were women. The investigators used statistical methods to balance baseline covariates between the two groups.
The primary outcomes were ischemic stroke or systemic embolism, hemorrhagic stroke, and TIA. Median follow-up was 5.1 years.
Statin use was associated with a significantly lower risk of all outcomes, compared with nonuse. Statin users had a 17% reduced risk of ischemic stroke or systemic embolism, a 7% reduced risk of hemorrhagic stroke, and a 15% rate of reduced risk of TIA, Ms. Huang reported.
“We also found long-term statin use was associated with greater protection than short-term use,” she said. For statin use of 6 years or longer, in comparison with use of 3 months to 2 years, the risk of ischemic stroke or systemic embolism was lowered by 43%; for hemorrhagic stroke, it was lowered by 44%, and for TIA, it was lowered by 42%.
These associations were consistent regardless of whether patients used anticoagulant medications or the type of anticoagulant.
Oussama Wazni, MD, MBA, section head of cardiac electrophysiology and pacing at the Cleveland Clinic, was a moderator of the poster session at which Ms. Huang presented her study. In an interview, he called the study “very important.”
“The message should be that all patients who have atrial fibrillation should be checked for cholesterol levels, and we should consider placing them on statins,” he said. “Is there an opportunity? Probably there is, and that’s why we’re seeing this effect in this group of patients.”
When asked about a possible mechanism by which statins produced the effects seen in the study, he pointed to LDL cholesterol lowering and possibly an effect on inflammation. “If a patient had a carotid atheroma, for example, maybe it helped with that,” he said. Previous work has shown that inflammation is related to or is associated with higher risk of thrombogenic effects, including MI or stroke.
It may be a bit less clear how statins reduced the incidence of hemorrhagic strokes, but Dr. Wazni proposed that some strokes could have started as an ischemic stroke “and then had hemorrhagic conversion, so we don’t have the granularity in here to know whether that was the case or not.”
Given the fact that the effect was stronger the longer a patient had been taking a statin, Dr. Wazni said that if a patient is tolerating the drug well, there should be no reason to discontinue it, regardless of age.
He said the study provides “welcome data and evidence because it’s pointing in the right direction,” but prospective studies would be useful “so that we can see what is driving what. Otherwise, this is just an association.”
The study was supported by Sanming Project Shenzhen. Ms. Huang and Dr. Wazni disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Among patients with atrial fibrillation (AFib), initiation of statins soon after diagnosis was protective against stroke and related vascular events, and longer duration of use was associated with greater protection, a new cohort study shows.
Statin use was associated with lower risks of ischemic stroke or systemic embolism, hemorrhagic stroke, and transient ischemic attack (TIA), regardless of whether patients were also taking anticoagulant medications.
Lead author Jiayi Huang, a PhD student at Hong Kong University at Shenzhen (China) Hospital, concluded that the study’s findings support the use of statins to prevent stroke for patients with new-onset AFib.
“The findings have important clinical implications, particularly given that in atrial fibrillation, patients’ ischemic strokes are often fatal or disabling and have a high risk of recurrence,” she said.
The results were presented in a moderated poster session at the European Heart Rhythm Association 2023 Congress.
Widely prescribed
Anticoagulant drugs are prescribed to lower the fivefold increased risk of stroke among individuals with AFib, compared with those without AFib, but the therapy does not eliminate the higher risk, Ms. Huang explained. And although statins are widely prescribed to reduce the likelihood of myocardial infarction and stroke, “the benefit of statins for stroke prevention in patients with atrial fibrillation has been unclear.”
Ms. Huang and colleagues analyzed data from 51,472 patients newly diagnosed with AFib between 2010 and 2018. The population was divided into statin users (n = 11,866), defined as patients who had taken statins for at least 19 consecutive days in the first year after AFib diagnosis, and statin nonusers (n = 39,606), based on whether they were prescribed statin therapy after their first diagnosis of AFib.
The median age of the cohort was 74.9 years, and 47.7% were women. The investigators used statistical methods to balance baseline covariates between the two groups.
The primary outcomes were ischemic stroke or systemic embolism, hemorrhagic stroke, and TIA. Median follow-up was 5.1 years.
Statin use was associated with a significantly lower risk of all outcomes, compared with nonuse. Statin users had a 17% reduced risk of ischemic stroke or systemic embolism, a 7% reduced risk of hemorrhagic stroke, and a 15% rate of reduced risk of TIA, Ms. Huang reported.
“We also found long-term statin use was associated with greater protection than short-term use,” she said. For statin use of 6 years or longer, in comparison with use of 3 months to 2 years, the risk of ischemic stroke or systemic embolism was lowered by 43%; for hemorrhagic stroke, it was lowered by 44%, and for TIA, it was lowered by 42%.
These associations were consistent regardless of whether patients used anticoagulant medications or the type of anticoagulant.
Oussama Wazni, MD, MBA, section head of cardiac electrophysiology and pacing at the Cleveland Clinic, was a moderator of the poster session at which Ms. Huang presented her study. In an interview, he called the study “very important.”
“The message should be that all patients who have atrial fibrillation should be checked for cholesterol levels, and we should consider placing them on statins,” he said. “Is there an opportunity? Probably there is, and that’s why we’re seeing this effect in this group of patients.”
When asked about a possible mechanism by which statins produced the effects seen in the study, he pointed to LDL cholesterol lowering and possibly an effect on inflammation. “If a patient had a carotid atheroma, for example, maybe it helped with that,” he said. Previous work has shown that inflammation is related to or is associated with higher risk of thrombogenic effects, including MI or stroke.
It may be a bit less clear how statins reduced the incidence of hemorrhagic strokes, but Dr. Wazni proposed that some strokes could have started as an ischemic stroke “and then had hemorrhagic conversion, so we don’t have the granularity in here to know whether that was the case or not.”
Given the fact that the effect was stronger the longer a patient had been taking a statin, Dr. Wazni said that if a patient is tolerating the drug well, there should be no reason to discontinue it, regardless of age.
He said the study provides “welcome data and evidence because it’s pointing in the right direction,” but prospective studies would be useful “so that we can see what is driving what. Otherwise, this is just an association.”
The study was supported by Sanming Project Shenzhen. Ms. Huang and Dr. Wazni disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Among patients with atrial fibrillation (AFib), initiation of statins soon after diagnosis was protective against stroke and related vascular events, and longer duration of use was associated with greater protection, a new cohort study shows.
Statin use was associated with lower risks of ischemic stroke or systemic embolism, hemorrhagic stroke, and transient ischemic attack (TIA), regardless of whether patients were also taking anticoagulant medications.
Lead author Jiayi Huang, a PhD student at Hong Kong University at Shenzhen (China) Hospital, concluded that the study’s findings support the use of statins to prevent stroke for patients with new-onset AFib.
“The findings have important clinical implications, particularly given that in atrial fibrillation, patients’ ischemic strokes are often fatal or disabling and have a high risk of recurrence,” she said.
The results were presented in a moderated poster session at the European Heart Rhythm Association 2023 Congress.
Widely prescribed
Anticoagulant drugs are prescribed to lower the fivefold increased risk of stroke among individuals with AFib, compared with those without AFib, but the therapy does not eliminate the higher risk, Ms. Huang explained. And although statins are widely prescribed to reduce the likelihood of myocardial infarction and stroke, “the benefit of statins for stroke prevention in patients with atrial fibrillation has been unclear.”
Ms. Huang and colleagues analyzed data from 51,472 patients newly diagnosed with AFib between 2010 and 2018. The population was divided into statin users (n = 11,866), defined as patients who had taken statins for at least 19 consecutive days in the first year after AFib diagnosis, and statin nonusers (n = 39,606), based on whether they were prescribed statin therapy after their first diagnosis of AFib.
The median age of the cohort was 74.9 years, and 47.7% were women. The investigators used statistical methods to balance baseline covariates between the two groups.
The primary outcomes were ischemic stroke or systemic embolism, hemorrhagic stroke, and TIA. Median follow-up was 5.1 years.
Statin use was associated with a significantly lower risk of all outcomes, compared with nonuse. Statin users had a 17% reduced risk of ischemic stroke or systemic embolism, a 7% reduced risk of hemorrhagic stroke, and a 15% rate of reduced risk of TIA, Ms. Huang reported.
“We also found long-term statin use was associated with greater protection than short-term use,” she said. For statin use of 6 years or longer, in comparison with use of 3 months to 2 years, the risk of ischemic stroke or systemic embolism was lowered by 43%; for hemorrhagic stroke, it was lowered by 44%, and for TIA, it was lowered by 42%.
These associations were consistent regardless of whether patients used anticoagulant medications or the type of anticoagulant.
Oussama Wazni, MD, MBA, section head of cardiac electrophysiology and pacing at the Cleveland Clinic, was a moderator of the poster session at which Ms. Huang presented her study. In an interview, he called the study “very important.”
“The message should be that all patients who have atrial fibrillation should be checked for cholesterol levels, and we should consider placing them on statins,” he said. “Is there an opportunity? Probably there is, and that’s why we’re seeing this effect in this group of patients.”
When asked about a possible mechanism by which statins produced the effects seen in the study, he pointed to LDL cholesterol lowering and possibly an effect on inflammation. “If a patient had a carotid atheroma, for example, maybe it helped with that,” he said. Previous work has shown that inflammation is related to or is associated with higher risk of thrombogenic effects, including MI or stroke.
It may be a bit less clear how statins reduced the incidence of hemorrhagic strokes, but Dr. Wazni proposed that some strokes could have started as an ischemic stroke “and then had hemorrhagic conversion, so we don’t have the granularity in here to know whether that was the case or not.”
Given the fact that the effect was stronger the longer a patient had been taking a statin, Dr. Wazni said that if a patient is tolerating the drug well, there should be no reason to discontinue it, regardless of age.
He said the study provides “welcome data and evidence because it’s pointing in the right direction,” but prospective studies would be useful “so that we can see what is driving what. Otherwise, this is just an association.”
The study was supported by Sanming Project Shenzhen. Ms. Huang and Dr. Wazni disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM EHRA 2023
Double antiglutamatergic therapy is ‘promising’ for super-refractory status epilepticus
(SRSE), new research suggests.
In a retrospective cohort study of survivors of cardiac arrest with postanoxic sustained SRSE, resolution of the condition was achieved by 81% of those who received intensive treatment of ketamine plus perampanel, versus 41% of those who received standard care.
The novelty of the new treatment approach is the duration of therapy as well as the dual antiglutamate drugs, researchers note.
“So the logic is to continue treatment until resolution of refractory status epilepticus under continuous EEG [electroencephalographic] monitoring,” reported lead investigator Simone Beretta, MD, San Gerardo University Hospital, Monza, Italy.
Therapy was guided by data on brainstem reflexes, N20 cortical responses, neuronal serum enolase levels, and neuroimaging.
If all or most of these indicators are favorable, “we continue to treat without any time limit,” Dr. Beretta said. However, if the indicators become unfavorable, clinicians should consider lowering the intensity of care, he added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
SUPER-CAT trial
In SRSE, epileptic seizures occur one after another without patients recovering consciousness in between. Standard aggressive therapy for the condition does not include antiglutamatergic drugs, the researchers noted.
In the Super-Refractory Status Epilepticus After Cardiac Arrest: Aggressive Treatment Guided by Multimodal Prognostic Indicators (SUPER-CAT) study, researchers assessed the combination of two such medications.
The first was the anti-NMDA receptor drug ketamine, which was given by intravenous bolus and then continuous infusion for 3 days guided by continuous EEG to reach a ketamine EEG pattern, as evidenced by alpha and beta waves. It was combined with the anti-AMPA receptor antiepileptic perampanel via nasogastric tube for 5 days, followed by slow tapering.
Dr. Beretta noted that in the ongoing TELSTAR trial, which involved a similar patient population, a different drug combination is being used. A major difference between the two trials is that in the TELSTAR trial, aggressive therapy continues for only 2 days if there is no response.
“In the SUPER-CAT study, we continue far beyond 2 days in the majority of patients,” he said. In addition, ketamine and perampanel were not assessed in TELSTAR.
In SUPER-CAT, 489 survivors of cardiac arrest were recruited over 10 years. Of these, 101 had refractory status epilepticus. After excluding those with more than two indicators of poor prognosis (n = 31) or whose status epilepticus resolved (n = 14), 56 patients were determined to have SRSE. All had experienced relapse after undergoing one cycle of anesthetic.
The 56 participants received one of three treatment regimens: double antiglutamate (DAG) therapy of ketamine and perampanel (n = 26), single antiglutamate therapy with either agent (n = 8), or aggressive nonantiglutamate (NAG) therapy with antiepilepsy drugs and anesthetics other than ketamine or perampanel (n = 22).
The single-antiglutamate group was not included in the analysis of patient outcomes.
The DAG and NAG groups were well balanced at baseline. There were no significant differences in median age (60 years vs. 66 years), gender, low cerebral blood flow, presence of bilateral pupillary or corneal reflexes, neuron-specific enolase levels, cortical N20 somatosenory evoked potentials, moderate to severe postanoxic brain injury, and hypothermia/targeted temperature management.
Primary outcome met
More patients in the DAG group (42%) had moderate to severe postanoxic brain injury than in the NAG group (28%). However, the difference was not statistically significant (P = .08), possibly because of the small sample sizes. The number of antiepileptic drugs and the number of cycles of anesthetics did not differ between the groups.
Results showed that efficacy and safety outcomes favored DAG therapy.
The primary efficacy outcome was resolution of status epilepticus within 3 days after initiation of treatments. Status epilepticus resolved for 21 of 26 patients in the DAG group (81%), versus 9 of 22 patients in the NAG group (41%; odds ratio, 6.06; 95% confidence interval, 1.66-22.12; P = .005).
For secondary efficacy outcomes, there was a trend in favor of DAG, but differences from the NAG group were not statistically significant. In the groups, 46% versus 32% awakened and responded to commands before discharge from the intensive care unit, and 32% versus 23% showed good neurologic outcome at 6 months.
The primary safety outcome of all-cause mortality risk in the ICU was 90% lower for patients treated with DAG than for those treated with NAG (15% vs. 64%; OR, 0.1; 95% CI, 0.02-0.41; P < .01). Dr. Beretta explained that the high mortality rate in the NAG group was presumably a result of unresolved status epilepticus.
The secondary safety outcome of a transitory rise of gamma-glutamyl transferase greater than three times the upper limit of normal in the DAG group was expected with high-dose perampanel, the investigators noted. This outcome occurred in 77% of the DAG group versus 27% of the NAG group (OR, 9.88; 95% CI, 2.4-32.9; P < .001).
There was no statistically significant difference in incidence of recurrent cardiac arrest during therapy. This occurred in one member of the DAG group and in none in the NAG group.
Dr. Beretta reported that their investigations are still in a retrospective phase, but the researchers plan to move the work into a prospective phase and possibly a randomized trial soon.
Fascinating, promising
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center, Columbus, said the study provides “fascinating, very helpful data” about a condition that has responded well to current treatment options.
He added that his center has used “the innovative treatments” discussed in the study for a few patients.
“More concrete evidence will push us to use it more uniformly across all our patient population [that] has refractory status. So I’m very optimistic, and the data were very promising,” said Dr. Singh, who was not involved with the research.
He cautioned that the study was retrospective, not randomized or controlled, and that it involved a small number of patients but said that the data were “heading in the right direction.”
Although resolution of status epilepticus was better among patients in the DAG group than in the NAG group, the awakenings and neurologic outcomes were “pretty much same as standard medical therapy, which we commonly give to our patients,” said Dr. Singh. “We see this phenomenon all the time in our patients.”
He noted that other factors can determine how patients respond, such as conditions of the heart or kidneys, the presence of sepsis, and multiorgan dysfunction. These factors were not controlled for in the study.
Nonetheless, he said the study achieved its primary endpoint of better resolution of status epilepticus “because that’s the first thing you want to see: whether the treatment is taking care of that.”
The study received no outside funding. Dr. Beretta and Dr. Singh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SRSE), new research suggests.
In a retrospective cohort study of survivors of cardiac arrest with postanoxic sustained SRSE, resolution of the condition was achieved by 81% of those who received intensive treatment of ketamine plus perampanel, versus 41% of those who received standard care.
The novelty of the new treatment approach is the duration of therapy as well as the dual antiglutamate drugs, researchers note.
“So the logic is to continue treatment until resolution of refractory status epilepticus under continuous EEG [electroencephalographic] monitoring,” reported lead investigator Simone Beretta, MD, San Gerardo University Hospital, Monza, Italy.
Therapy was guided by data on brainstem reflexes, N20 cortical responses, neuronal serum enolase levels, and neuroimaging.
If all or most of these indicators are favorable, “we continue to treat without any time limit,” Dr. Beretta said. However, if the indicators become unfavorable, clinicians should consider lowering the intensity of care, he added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
SUPER-CAT trial
In SRSE, epileptic seizures occur one after another without patients recovering consciousness in between. Standard aggressive therapy for the condition does not include antiglutamatergic drugs, the researchers noted.
In the Super-Refractory Status Epilepticus After Cardiac Arrest: Aggressive Treatment Guided by Multimodal Prognostic Indicators (SUPER-CAT) study, researchers assessed the combination of two such medications.
The first was the anti-NMDA receptor drug ketamine, which was given by intravenous bolus and then continuous infusion for 3 days guided by continuous EEG to reach a ketamine EEG pattern, as evidenced by alpha and beta waves. It was combined with the anti-AMPA receptor antiepileptic perampanel via nasogastric tube for 5 days, followed by slow tapering.
Dr. Beretta noted that in the ongoing TELSTAR trial, which involved a similar patient population, a different drug combination is being used. A major difference between the two trials is that in the TELSTAR trial, aggressive therapy continues for only 2 days if there is no response.
“In the SUPER-CAT study, we continue far beyond 2 days in the majority of patients,” he said. In addition, ketamine and perampanel were not assessed in TELSTAR.
In SUPER-CAT, 489 survivors of cardiac arrest were recruited over 10 years. Of these, 101 had refractory status epilepticus. After excluding those with more than two indicators of poor prognosis (n = 31) or whose status epilepticus resolved (n = 14), 56 patients were determined to have SRSE. All had experienced relapse after undergoing one cycle of anesthetic.
The 56 participants received one of three treatment regimens: double antiglutamate (DAG) therapy of ketamine and perampanel (n = 26), single antiglutamate therapy with either agent (n = 8), or aggressive nonantiglutamate (NAG) therapy with antiepilepsy drugs and anesthetics other than ketamine or perampanel (n = 22).
The single-antiglutamate group was not included in the analysis of patient outcomes.
The DAG and NAG groups were well balanced at baseline. There were no significant differences in median age (60 years vs. 66 years), gender, low cerebral blood flow, presence of bilateral pupillary or corneal reflexes, neuron-specific enolase levels, cortical N20 somatosenory evoked potentials, moderate to severe postanoxic brain injury, and hypothermia/targeted temperature management.
Primary outcome met
More patients in the DAG group (42%) had moderate to severe postanoxic brain injury than in the NAG group (28%). However, the difference was not statistically significant (P = .08), possibly because of the small sample sizes. The number of antiepileptic drugs and the number of cycles of anesthetics did not differ between the groups.
Results showed that efficacy and safety outcomes favored DAG therapy.
The primary efficacy outcome was resolution of status epilepticus within 3 days after initiation of treatments. Status epilepticus resolved for 21 of 26 patients in the DAG group (81%), versus 9 of 22 patients in the NAG group (41%; odds ratio, 6.06; 95% confidence interval, 1.66-22.12; P = .005).
For secondary efficacy outcomes, there was a trend in favor of DAG, but differences from the NAG group were not statistically significant. In the groups, 46% versus 32% awakened and responded to commands before discharge from the intensive care unit, and 32% versus 23% showed good neurologic outcome at 6 months.
The primary safety outcome of all-cause mortality risk in the ICU was 90% lower for patients treated with DAG than for those treated with NAG (15% vs. 64%; OR, 0.1; 95% CI, 0.02-0.41; P < .01). Dr. Beretta explained that the high mortality rate in the NAG group was presumably a result of unresolved status epilepticus.
The secondary safety outcome of a transitory rise of gamma-glutamyl transferase greater than three times the upper limit of normal in the DAG group was expected with high-dose perampanel, the investigators noted. This outcome occurred in 77% of the DAG group versus 27% of the NAG group (OR, 9.88; 95% CI, 2.4-32.9; P < .001).
There was no statistically significant difference in incidence of recurrent cardiac arrest during therapy. This occurred in one member of the DAG group and in none in the NAG group.
Dr. Beretta reported that their investigations are still in a retrospective phase, but the researchers plan to move the work into a prospective phase and possibly a randomized trial soon.
Fascinating, promising
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center, Columbus, said the study provides “fascinating, very helpful data” about a condition that has responded well to current treatment options.
He added that his center has used “the innovative treatments” discussed in the study for a few patients.
“More concrete evidence will push us to use it more uniformly across all our patient population [that] has refractory status. So I’m very optimistic, and the data were very promising,” said Dr. Singh, who was not involved with the research.
He cautioned that the study was retrospective, not randomized or controlled, and that it involved a small number of patients but said that the data were “heading in the right direction.”
Although resolution of status epilepticus was better among patients in the DAG group than in the NAG group, the awakenings and neurologic outcomes were “pretty much same as standard medical therapy, which we commonly give to our patients,” said Dr. Singh. “We see this phenomenon all the time in our patients.”
He noted that other factors can determine how patients respond, such as conditions of the heart or kidneys, the presence of sepsis, and multiorgan dysfunction. These factors were not controlled for in the study.
Nonetheless, he said the study achieved its primary endpoint of better resolution of status epilepticus “because that’s the first thing you want to see: whether the treatment is taking care of that.”
The study received no outside funding. Dr. Beretta and Dr. Singh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(SRSE), new research suggests.
In a retrospective cohort study of survivors of cardiac arrest with postanoxic sustained SRSE, resolution of the condition was achieved by 81% of those who received intensive treatment of ketamine plus perampanel, versus 41% of those who received standard care.
The novelty of the new treatment approach is the duration of therapy as well as the dual antiglutamate drugs, researchers note.
“So the logic is to continue treatment until resolution of refractory status epilepticus under continuous EEG [electroencephalographic] monitoring,” reported lead investigator Simone Beretta, MD, San Gerardo University Hospital, Monza, Italy.
Therapy was guided by data on brainstem reflexes, N20 cortical responses, neuronal serum enolase levels, and neuroimaging.
If all or most of these indicators are favorable, “we continue to treat without any time limit,” Dr. Beretta said. However, if the indicators become unfavorable, clinicians should consider lowering the intensity of care, he added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
SUPER-CAT trial
In SRSE, epileptic seizures occur one after another without patients recovering consciousness in between. Standard aggressive therapy for the condition does not include antiglutamatergic drugs, the researchers noted.
In the Super-Refractory Status Epilepticus After Cardiac Arrest: Aggressive Treatment Guided by Multimodal Prognostic Indicators (SUPER-CAT) study, researchers assessed the combination of two such medications.
The first was the anti-NMDA receptor drug ketamine, which was given by intravenous bolus and then continuous infusion for 3 days guided by continuous EEG to reach a ketamine EEG pattern, as evidenced by alpha and beta waves. It was combined with the anti-AMPA receptor antiepileptic perampanel via nasogastric tube for 5 days, followed by slow tapering.
Dr. Beretta noted that in the ongoing TELSTAR trial, which involved a similar patient population, a different drug combination is being used. A major difference between the two trials is that in the TELSTAR trial, aggressive therapy continues for only 2 days if there is no response.
“In the SUPER-CAT study, we continue far beyond 2 days in the majority of patients,” he said. In addition, ketamine and perampanel were not assessed in TELSTAR.
In SUPER-CAT, 489 survivors of cardiac arrest were recruited over 10 years. Of these, 101 had refractory status epilepticus. After excluding those with more than two indicators of poor prognosis (n = 31) or whose status epilepticus resolved (n = 14), 56 patients were determined to have SRSE. All had experienced relapse after undergoing one cycle of anesthetic.
The 56 participants received one of three treatment regimens: double antiglutamate (DAG) therapy of ketamine and perampanel (n = 26), single antiglutamate therapy with either agent (n = 8), or aggressive nonantiglutamate (NAG) therapy with antiepilepsy drugs and anesthetics other than ketamine or perampanel (n = 22).
The single-antiglutamate group was not included in the analysis of patient outcomes.
The DAG and NAG groups were well balanced at baseline. There were no significant differences in median age (60 years vs. 66 years), gender, low cerebral blood flow, presence of bilateral pupillary or corneal reflexes, neuron-specific enolase levels, cortical N20 somatosenory evoked potentials, moderate to severe postanoxic brain injury, and hypothermia/targeted temperature management.
Primary outcome met
More patients in the DAG group (42%) had moderate to severe postanoxic brain injury than in the NAG group (28%). However, the difference was not statistically significant (P = .08), possibly because of the small sample sizes. The number of antiepileptic drugs and the number of cycles of anesthetics did not differ between the groups.
Results showed that efficacy and safety outcomes favored DAG therapy.
The primary efficacy outcome was resolution of status epilepticus within 3 days after initiation of treatments. Status epilepticus resolved for 21 of 26 patients in the DAG group (81%), versus 9 of 22 patients in the NAG group (41%; odds ratio, 6.06; 95% confidence interval, 1.66-22.12; P = .005).
For secondary efficacy outcomes, there was a trend in favor of DAG, but differences from the NAG group were not statistically significant. In the groups, 46% versus 32% awakened and responded to commands before discharge from the intensive care unit, and 32% versus 23% showed good neurologic outcome at 6 months.
The primary safety outcome of all-cause mortality risk in the ICU was 90% lower for patients treated with DAG than for those treated with NAG (15% vs. 64%; OR, 0.1; 95% CI, 0.02-0.41; P < .01). Dr. Beretta explained that the high mortality rate in the NAG group was presumably a result of unresolved status epilepticus.
The secondary safety outcome of a transitory rise of gamma-glutamyl transferase greater than three times the upper limit of normal in the DAG group was expected with high-dose perampanel, the investigators noted. This outcome occurred in 77% of the DAG group versus 27% of the NAG group (OR, 9.88; 95% CI, 2.4-32.9; P < .001).
There was no statistically significant difference in incidence of recurrent cardiac arrest during therapy. This occurred in one member of the DAG group and in none in the NAG group.
Dr. Beretta reported that their investigations are still in a retrospective phase, but the researchers plan to move the work into a prospective phase and possibly a randomized trial soon.
Fascinating, promising
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center, Columbus, said the study provides “fascinating, very helpful data” about a condition that has responded well to current treatment options.
He added that his center has used “the innovative treatments” discussed in the study for a few patients.
“More concrete evidence will push us to use it more uniformly across all our patient population [that] has refractory status. So I’m very optimistic, and the data were very promising,” said Dr. Singh, who was not involved with the research.
He cautioned that the study was retrospective, not randomized or controlled, and that it involved a small number of patients but said that the data were “heading in the right direction.”
Although resolution of status epilepticus was better among patients in the DAG group than in the NAG group, the awakenings and neurologic outcomes were “pretty much same as standard medical therapy, which we commonly give to our patients,” said Dr. Singh. “We see this phenomenon all the time in our patients.”
He noted that other factors can determine how patients respond, such as conditions of the heart or kidneys, the presence of sepsis, and multiorgan dysfunction. These factors were not controlled for in the study.
Nonetheless, he said the study achieved its primary endpoint of better resolution of status epilepticus “because that’s the first thing you want to see: whether the treatment is taking care of that.”
The study received no outside funding. Dr. Beretta and Dr. Singh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From WCN 2021
Is genetic testing valuable in the clinical management of epilepsy?
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From WCN 2021
A safer way to use Botox to treat challenging dystonia type?
, new research suggests.
Oromandibular dystonia causes an involuntary opening of the mouth, which can be disabling and disfiguring. Although injection of the lateral pterygoid muscle with botulinum toxin is the preferred treatment for oromandibular dystonia, a potential complication concerns the maxillary artery, which can run either lateral or medial to the lateral pterygoid muscle.
In a study of 200 Turkish patients, researchers documented significant variations between men and women in the anatomical location of the maxillary artery – and even found lateral versus medial differences on the left and right side in the same individual.
“The results showed that the maxillary artery runs lateral to the muscle in 67% of the Turkish patients,” Rezzak Yilmaz, MD, department of neurology, University of Ankara Medical School, Turkey, reported at the International Congress of Parkinson’s Disease and Movement Disorders.
Given this high rate, there is a high risk for injury “that may result in pain and hematoma” when using preauricular extraoral injections, Dr. Yilmaz and colleagues noted. Instead, they recommend an intraoral injection approach to the lateral pterygoid muscle. “However, this critical anatomical variation is still unrecognized by most clinicians performing [botulinum toxin] injections,” they wrote.
Significant gender differences
The maxillary artery is the largest branch of the external carotid artery.
In the current study, the researchers used magnetic resonance angiography to assess the relevant anatomy in a cohort of 200 individuals (mean age, 56.4 years; 64% women) without a history of facial trauma or movement disorders.
Results showed that the maxillary artery ran lateral to the lateral pterygoid muscle in 67% of the study population.
“This result was also more frequent in females compared with males. Also, there was a considerable variability between the left and the right side in 20% of the participants,” Dr. Yilmaz reported.
Statistically significant gender differences were found for the artery running lateral to the lateral pterygoid muscle on both sides (71.1% in women vs. 58.5% in men; P = .007) and for the artery running lateral to the lateral pterygoid muscle on just the left side (69.8% in women vs. 53.5% in men; P = .02).
In an email exchange, Dr. Yilmaz said if medical personnel are not trained to perform an intraoral approach, “imaging to visualize the path of the maxillary artery before an extraoral/transcutaneous injection can be recommended.”
“If the imaging reveals that the maxillary artery passes lateral to the muscle, then the patient needs to be referred to another center for an intraoral injection,” unless the clinician is trained for an intraoral approach, he added.
Useful education
Commenting on the study, Michele Tagliati, MD, director of the Movement Disorders Program at Cedars-Sinai Medical Center, Los Angeles, said the results were educational. “I didn’t know about all this variability. I was working under the assumption that the artery was medial,” said Dr. Tagliati, who was not involved with the research.
Among his large practice of about 2,000 patients, Dr. Tagliati estimated having five patients for whom he provides this type of injection – and has never encountered a problem with them.
“Maybe all my patients are medial, but now that I’m aware I’ll probably pay more attention,” Dr. Tagliati said. He does not currently perform magnetic resonance angiography before injecting them, “although maybe I should,” he said.
When asked if it is worth the time and expense to perform magnetic resonance angiography on every patient who comes in for lateral pterygoid muscle injections, Dr. Tagliati said that although he has done the injections without problems in his current patients, he may “start obtaining imaging studies to make sure that we’re not taking unnecessary risk” if the maxillary artery is lateral to the lateral pterygoid muscle in new patients.
If there is a risk, he’ll then consider talking with colleagues in oral or facial surgery. Dr. Tagliati added that the number of patients he sees with oromandibular dystonia is rather small, so this extra step would not add a lot of additional imaging.
Overall, Dr. Tagliati noted that the study outcome was significant enough to want to use it for professional education. “I can definitely tell you that I’m going to bring it to the attention of my Fellows. [Every year] I teach one or two Fellows to inject Botox,” he said.
There was no funding for the study. Dr. Yilmaz and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Oromandibular dystonia causes an involuntary opening of the mouth, which can be disabling and disfiguring. Although injection of the lateral pterygoid muscle with botulinum toxin is the preferred treatment for oromandibular dystonia, a potential complication concerns the maxillary artery, which can run either lateral or medial to the lateral pterygoid muscle.
In a study of 200 Turkish patients, researchers documented significant variations between men and women in the anatomical location of the maxillary artery – and even found lateral versus medial differences on the left and right side in the same individual.
“The results showed that the maxillary artery runs lateral to the muscle in 67% of the Turkish patients,” Rezzak Yilmaz, MD, department of neurology, University of Ankara Medical School, Turkey, reported at the International Congress of Parkinson’s Disease and Movement Disorders.
Given this high rate, there is a high risk for injury “that may result in pain and hematoma” when using preauricular extraoral injections, Dr. Yilmaz and colleagues noted. Instead, they recommend an intraoral injection approach to the lateral pterygoid muscle. “However, this critical anatomical variation is still unrecognized by most clinicians performing [botulinum toxin] injections,” they wrote.
Significant gender differences
The maxillary artery is the largest branch of the external carotid artery.
In the current study, the researchers used magnetic resonance angiography to assess the relevant anatomy in a cohort of 200 individuals (mean age, 56.4 years; 64% women) without a history of facial trauma or movement disorders.
Results showed that the maxillary artery ran lateral to the lateral pterygoid muscle in 67% of the study population.
“This result was also more frequent in females compared with males. Also, there was a considerable variability between the left and the right side in 20% of the participants,” Dr. Yilmaz reported.
Statistically significant gender differences were found for the artery running lateral to the lateral pterygoid muscle on both sides (71.1% in women vs. 58.5% in men; P = .007) and for the artery running lateral to the lateral pterygoid muscle on just the left side (69.8% in women vs. 53.5% in men; P = .02).
In an email exchange, Dr. Yilmaz said if medical personnel are not trained to perform an intraoral approach, “imaging to visualize the path of the maxillary artery before an extraoral/transcutaneous injection can be recommended.”
“If the imaging reveals that the maxillary artery passes lateral to the muscle, then the patient needs to be referred to another center for an intraoral injection,” unless the clinician is trained for an intraoral approach, he added.
Useful education
Commenting on the study, Michele Tagliati, MD, director of the Movement Disorders Program at Cedars-Sinai Medical Center, Los Angeles, said the results were educational. “I didn’t know about all this variability. I was working under the assumption that the artery was medial,” said Dr. Tagliati, who was not involved with the research.
Among his large practice of about 2,000 patients, Dr. Tagliati estimated having five patients for whom he provides this type of injection – and has never encountered a problem with them.
“Maybe all my patients are medial, but now that I’m aware I’ll probably pay more attention,” Dr. Tagliati said. He does not currently perform magnetic resonance angiography before injecting them, “although maybe I should,” he said.
When asked if it is worth the time and expense to perform magnetic resonance angiography on every patient who comes in for lateral pterygoid muscle injections, Dr. Tagliati said that although he has done the injections without problems in his current patients, he may “start obtaining imaging studies to make sure that we’re not taking unnecessary risk” if the maxillary artery is lateral to the lateral pterygoid muscle in new patients.
If there is a risk, he’ll then consider talking with colleagues in oral or facial surgery. Dr. Tagliati added that the number of patients he sees with oromandibular dystonia is rather small, so this extra step would not add a lot of additional imaging.
Overall, Dr. Tagliati noted that the study outcome was significant enough to want to use it for professional education. “I can definitely tell you that I’m going to bring it to the attention of my Fellows. [Every year] I teach one or two Fellows to inject Botox,” he said.
There was no funding for the study. Dr. Yilmaz and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Oromandibular dystonia causes an involuntary opening of the mouth, which can be disabling and disfiguring. Although injection of the lateral pterygoid muscle with botulinum toxin is the preferred treatment for oromandibular dystonia, a potential complication concerns the maxillary artery, which can run either lateral or medial to the lateral pterygoid muscle.
In a study of 200 Turkish patients, researchers documented significant variations between men and women in the anatomical location of the maxillary artery – and even found lateral versus medial differences on the left and right side in the same individual.
“The results showed that the maxillary artery runs lateral to the muscle in 67% of the Turkish patients,” Rezzak Yilmaz, MD, department of neurology, University of Ankara Medical School, Turkey, reported at the International Congress of Parkinson’s Disease and Movement Disorders.
Given this high rate, there is a high risk for injury “that may result in pain and hematoma” when using preauricular extraoral injections, Dr. Yilmaz and colleagues noted. Instead, they recommend an intraoral injection approach to the lateral pterygoid muscle. “However, this critical anatomical variation is still unrecognized by most clinicians performing [botulinum toxin] injections,” they wrote.
Significant gender differences
The maxillary artery is the largest branch of the external carotid artery.
In the current study, the researchers used magnetic resonance angiography to assess the relevant anatomy in a cohort of 200 individuals (mean age, 56.4 years; 64% women) without a history of facial trauma or movement disorders.
Results showed that the maxillary artery ran lateral to the lateral pterygoid muscle in 67% of the study population.
“This result was also more frequent in females compared with males. Also, there was a considerable variability between the left and the right side in 20% of the participants,” Dr. Yilmaz reported.
Statistically significant gender differences were found for the artery running lateral to the lateral pterygoid muscle on both sides (71.1% in women vs. 58.5% in men; P = .007) and for the artery running lateral to the lateral pterygoid muscle on just the left side (69.8% in women vs. 53.5% in men; P = .02).
In an email exchange, Dr. Yilmaz said if medical personnel are not trained to perform an intraoral approach, “imaging to visualize the path of the maxillary artery before an extraoral/transcutaneous injection can be recommended.”
“If the imaging reveals that the maxillary artery passes lateral to the muscle, then the patient needs to be referred to another center for an intraoral injection,” unless the clinician is trained for an intraoral approach, he added.
Useful education
Commenting on the study, Michele Tagliati, MD, director of the Movement Disorders Program at Cedars-Sinai Medical Center, Los Angeles, said the results were educational. “I didn’t know about all this variability. I was working under the assumption that the artery was medial,” said Dr. Tagliati, who was not involved with the research.
Among his large practice of about 2,000 patients, Dr. Tagliati estimated having five patients for whom he provides this type of injection – and has never encountered a problem with them.
“Maybe all my patients are medial, but now that I’m aware I’ll probably pay more attention,” Dr. Tagliati said. He does not currently perform magnetic resonance angiography before injecting them, “although maybe I should,” he said.
When asked if it is worth the time and expense to perform magnetic resonance angiography on every patient who comes in for lateral pterygoid muscle injections, Dr. Tagliati said that although he has done the injections without problems in his current patients, he may “start obtaining imaging studies to make sure that we’re not taking unnecessary risk” if the maxillary artery is lateral to the lateral pterygoid muscle in new patients.
If there is a risk, he’ll then consider talking with colleagues in oral or facial surgery. Dr. Tagliati added that the number of patients he sees with oromandibular dystonia is rather small, so this extra step would not add a lot of additional imaging.
Overall, Dr. Tagliati noted that the study outcome was significant enough to want to use it for professional education. “I can definitely tell you that I’m going to bring it to the attention of my Fellows. [Every year] I teach one or two Fellows to inject Botox,” he said.
There was no funding for the study. Dr. Yilmaz and Dr. Tagliati have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021