User login
Left atrial appendage closure: An emerging option in atrial fibrillation when oral anticoagulants are not tolerated
Can patients with atrial fibrillation undergo a percutaneous procedure to reduce their risk of stroke, thereby eliminating the need for lifelong treatment with an oral anticoagulant drug? The data are preliminary, but this is an emerging option that physicians should be aware of.
We review here the current evidence and techniques aimed at isolating the left atrial appendage to prevent stroke, and we emphasize the need for continued systematic comparisons between oral anticoagulation and percutaneous treatment options.
NOVEL TREATMENTS ARE NEEDED
Atrial fibrillation is the most common cardiac arrhythmia,1 affecting an estimated 1% to 2% of people worldwide. In 2001, an estimated 2.3 million persons in the United States had atrial fibrillation, and that number is expected to more than double by 2050.2
Atrial fibrillation independently increases the risk of stroke by a factor of 4 to 5.3 The American Heart Association ranks stroke as the fourth most common cause of death and the leading cause of disability in the United States.4 Atrial fibrillation accounts for 15% of strokes in people of all ages and 30% in those over age 80.5 Untreated, 2% to 5% of patients with atrial fibrillation suffer a stroke in any given year.6 Most of these strokes are cardioembolic, with thrombi originating in the left atrial appendage.7 Furthermore, it has been estimated8,9 that patients with atrial fibrillation who have already had a stroke and cannot tolerate oral anticoagulants have an annual risk of stroke close to 12% and a relative risk of approximately 3.0 compared with those with atrial fibrillation and prior stroke who can tolerate anticoagulation.
Oral anticoagulation effectively prevents thromboembolic events associated with atrial fibrillation,10 but several factors limit its efficacy and applicability. The risk of bleeding complications, the need for frequent monitoring, and challenges with compliance create a large population of patients who would benefit from alternative approaches. Consequently, physicians have looked for other ways to prevent stroke—especially surgical and transcatheter procedures—that are not associated with an ongoing risk of hemorrhage and a lifelong need to take an anticoagulant.
THE LEFT ATRIAL APPENDAGE: A SITE OF CLOT FORMATION
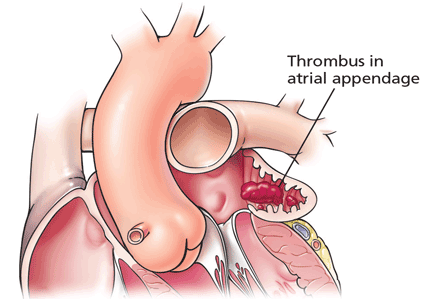
The left atrial appendage is the most common site of thrombus formation, particularly in patients with nonvalvular atrial fibrillation. Nearly 90% of thrombi discovered in the left atrium form in the appendage.7 A study of 233 patients not on long-term anticoagulation revealed that after 48 hours of atrial fibrillation, 15% had a left atrial thrombus, and all but one of the thrombi were in the appendage.11
Believed to function as a decompression chamber during left ventricular systole, the left atrial appendage is embryologically derived from the left wall of the primary atrium. It is in close proximity to the free wall of the left ventricle, and therefore its flow can vary with left ventricular function. Relative stasis due to its location and extensive trabeculations, especially in times of poor forward flow, make it a high-risk site for clot formation.12
ANTICOAGULATION: EFFECTIVE BUT IMPERFECT
In deciding whether a patient with atrial fibrillation should be prescribed anticoagulation therapy, the physician must balance the risk of stroke against the risk of bleeding. Several tools for assessing these two risks have been developed. Of note, some of the risk factors for stroke are the same as some of the risk factors for bleeding.
Calculating the risk of stroke
CHADS2 and CHA2DS2-VASc are the two most commonly used tools for assessing the risk of stroke, but only the newer CHA2DS2-VASc has received a class I recommendation (the highest) from the European Society of Cardiology (ESC).13
CHADS2 risk factors are Congestive heart failure (1 point), Hypertension (1 point), Age 75 or older (1 point), Diabetes (1 point), and Stroke or transient ischemic attack (2 points). Risk of stroke is considered low with a score of 0, intermediate with a score of 1, and high with a score of 2 or more.
CHA2DS2-VASc risk factors are Congestive heart failure or left ventricular ejection fraction ≤ 40% (1 point), Hypertension (1 point), Age ≥ 75 (2 points), Age 65–74 (1 point), Diabetes mellitus (1 point), Stroke, transient ischemic attack, or thromboembolism (2 points), Vascular disease (1 point), and female Sex (1 point). Low risk is defined as a score of 0 for a man or, for a woman with no other risk factors, a score of 1. A score of 1 for a man indicates moderate risk, and a score of 2 or more is high risk.
Calculating the risk of bleeding
Tools for assessing bleeding risk include ATRIA2 and HAS-BLED,14 the latter carrying a class I recommendation from the ESC.13
HAS-BLED risk factors are Hypertension (1 point), Abnormal renal or liver function (1 point each), Stroke (1 point), Bleeding (1 point), Labile international normalized ratio (INR) (1 point), Elderly (age > 65) (1 point), and Drug or alcohol use (1 point each). The risk of bleeding is considered high with a score of 3 or higher.
Disadvantages of oral anticoagulation
Oral anticoagulation is the standard treatment for preventing stroke in patients with atrial fibrillation, and the vitamin K antagonist warfarin remains the foundation.
Though highly effective, warfarin requires close monitoring and frequent dose adjustments because of its numerous food and drug interactions. Bleeding risk and the challenge of frequent monitoring rule out treatment with warfarin in 14% to 44% of patients with atrial fibrillation.15 Even in “ideal” candidates, warfarin is underused, with one study reporting that only 38% of those with clinical indications for it had been prescribed warfarin, and of those for whom it had not been prescribed, 63% were also not taking aspirin.16 Moreover, a meta-analysis suggested that the average patient treated with warfarin has his or her INR in the therapeutic range only about 55% of the time.17
Newer, target-specific oral anticoagulants such as dabigatran (a direct thrombin inhibitor) and rivaroxaban and apixaban (both factor Xa inhibitors) do not require monitoring and have fewer drug interactions. But like warfarin, they also confer a risk of serious bleeding.18–20 Most of the studies of these newer drugs have compared them with warfarin, with the preponderance of evidence showing them to be either noninferior or superior to warfarin for stroke reduction. But bleeding complication rates remain significant, apixaban having lower rates of major bleeding than dabigatran and rivaroxaban.
In a meta-analysis, Ruff et al21 concluded that the target-specific oral anticoagulants provide a favorable balance of risk and benefit. Compared with warfarin, these new drugs reduced the rate of stroke or systemic embolic events by 19%. There was also a significant reduction in rates of intracranial hemorrhage and all-cause mortality. The risk of major bleeding was similar to that with warfarin, though there was a higher risk of gastrointestinal bleeding with target-specific agents. These effects were consistent across a wide range of patients.
Given the difficulties, risks, and serious side effects of anticoagulant therapy, many patients stop taking these drugs soon after starting them, either on their own or on their physician’s recommendation. In the RE-LY trial (Dabigatran vs Warfarin in Patients With Atrial Fibrillation), 10% of patients receiving dabigatran and 17% of those receiving warfarin stopped the treatment within 1 to 2 years.22 In a similar trial of rivaroxaban vs warfarin in nonvalvular atrial fibrillation (the ROCKET-AF trial), 24% of those treated with rivaroxaban and 22% of those treated with warfarin stopped treatment during the study.19 In the ARISTOTLE trial (Apixaban vs Warfarin in Patients With Atrial Fibrillation), 25% of patients discontinued apixaban and 28% discontinued warfarin.20
The results of these trials show a clear need for treatments without high attrition rates, since patients with atrial fibrillation need protection from stroke for the rest of their life.
SURGICAL CLOSURE AS AN ADD-ON TO OTHER PROCEDURES
If the patient is undergoing cardiac surgery for another reason, the surgeon can excise, suture, staple, or clip the left atrial appendage shut at the same time. Closure is recommended as part of valve replacement.8 In a 2008 retrospective study, left atrial appendage closure was successfully performed in 40% of those undergoing the procedure during cardiac surgery, and complete closure occurred more often with excision than with suture exclusion and stapler exclusion.23 A study of patients who underwent ligation of the left atrial appendage during mitral value replacement found that 35% demonstrated incomplete closure as determined by transesophageal echocardiography.24
Newer devices have shown more success. The AtriClip (AtriCure Inc., West Chester, OH) is a self-closing, implantable clip applied epicardially by either an open surgical or a minimally invasive technique.25 Successful closure was confirmed in 60 of 61 patients at 90 days as determined by computed tomography or transesophageal echocardiography, and there were no adverse events related to implantation of the device.25 The TigerPaw system (Terumo Cardiovascular Systems, Ann Arbor, MI)26 is a fastener delivered surgically around the base of the ostium of the left atrial appendage. In an initial trial, 90 days after the procedure, transesophageal echocardiography showed no leaks in any of those who were examined (54 of 60 patients).
Amputation of the left atrial appendage is also considered part of the maze procedure for atrial fibrillation, in which the operator creates multiple small scars in the atria to prevent irregular impulses from being conducted.27
Results of these surgical approaches have been mixed, as incomplete closure or clipping actually increases the risk of left atrial thrombus formation and embolization.28 Moreover, these invasive surgical techniques are associated with significant periprocedural morbidity.29 Because of the high risk of surgical complications, cardiac specialists have sought less invasive percutaneous procedures to manage stroke risk in patients with atrial fibrillation.
PERCUTANEOUS OCCLUSION
One option for closing the left atrial appendage is a percutaneous transseptal approach in which a plug is placed in the opening connecting the appendage to the rest of the atrium.
The PLAATO device
The Percutaneous LAA Transcatheter Occlusion (PLAATO) device (Appriva Medical Inc., Sunnyvale, CA) contains an expandable nitinol-covered cage designed to be placed in the orifice of the left atrial appendage. Over time, tissue grows into the device, entirely isolating the appendage from the rest of the atrium.
In 2002, Sievert et al30 reported using this device in 15 patients. Subsequently, in a nonrandomized trial in patients with contraindications to lifelong anticoagulation, total occlusion was achieved in 108 of 111 patients, with no thrombosis or migration of the device at 6 months. The annual risk of stroke was 2.2%, a reduction in relative risk of 65% based on the CHADS2 score.31
But despite this apparent success, the PLAATO device was discontinued for unspecified commercial reasons.
Amplatzer cardiac plug
Modeled after an atrial septal occluder, the Amplatzer cardiac plug (St. Jude Medical, St. Paul, MN) consists of a lobe and a disk connected by a central waist.
In 2011, Park et al32 published their initial experience implanting this device in patients who either could not tolerate or did not desire long-term anticoagulation. They reported a 96% closure rate (137 of 143 patients), but there were serious complications in 10 patients: 3 with ischemic stroke, 2 with device embolism, and 5 with pericardial effusions.
Urena et al33 reported similar results in 52 patients with absolute contraindications to warfarin, with a 98.1% implantation rate. Patients were then maintained on either single or dual antiplatelet therapy at the discretion of the operator. At 20-month follow-up, there had been one stroke, one transient ischemic attack, and one major bleeding event. The leakage rate was 16.2% as determined by transesophageal echocardiography.
While initial results were promising, a clinical trial comparing this device and optimal medical treatment is currently on hold. Thus, there are no clear data comparing the Amplatzer device with oral anticoagulation.34
The Watchman device
The Watchman device (Boston Scientific, Marlborough, MA), an evolution of the PLAATO device, is a self-expanding nitinol structure with fixation barbs and a polyethylene membrane to protect the atrium-facing side of the device (Figure 1).
A pilot trial reported successful implantation in 66 of 75 patients, though the device was found to migrate after placement in 5 of the first 16 patients using the original device and delivery system. The device was modified, and no further embolization of the device occurred.35
The PROTECT-AF trial (Protection in Patients With Atrial Fibrillation)36 was the first completed and published randomized controlled trial evaluating left atrial appendage closure using a device vs long-term warfarin therapy. This study randomized 707 people with nonvalvular atrial fibrillation from 59 centers worldwide to receive the Watchman device or a control treatment. The study included patients age 18 or older with nonvalvular atrial fibrillation who were able to tolerate warfarin therapy. Patients in the control group received warfarin for the duration of the study and were monitored every 2 weeks for a goal INR of 2 to 3, achieving a therapeutic INR 66% of the time. The device group was also treated with warfarin for 45 days to allow device endothelialization. Warfarin was discontinued if transesophageal echocardiography showed complete closure or significantly decreased flow around the device. Patients in the device group were then treated with aspirin and clopidogrel for 6 months, and then with aspirin indefinitely.
At 1,065 patient-years of follow-up, PROTECT-AF showed that in patients with atrial fibrillation who were candidates for warfarin therapy, percutaneous left atrial appendage closure using the Watchman device reduced the rate of hemorrhagic stroke compared with warfarin and was noninferior to warfarin in terms of all-cause mortality and stroke. A 4-year follow-up to the PROTECT-AF trial found that receiving the Watchman was better than taking warfarin in terms of risk of cardiovascular death, stroke and other systemic embolization, and all-cause mortality. The adverse event rates were 2.3% in the device group and 3.8% in the control group, a 40% relative risk reduction in the Watchman group.37
The PREVAIL trial (Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation vs Long-Term Warfarin Therapy) aimed to confirm the safety and efficacy of the Watchman device compared with long-term warfarin therapy.38 The event rate (defined as 7-day occurrence of death, ischemic stroke, systemic embolism, and procedure- or device-related complications requiring major cardiovascular or endovascular intervention) was 2.2%. But the PREVAIL trial was unable to show that the device was noninferior to warfarin in terms of its second primary end point of stroke, systemic embolism, and cardiovascular or unexplained death at 18 months. When performed by physicians who were new to the procedure, the procedure was successful (ie, the device was successfully implanted) in 93.2%; the rate was slightly higher (96.3%) when performed by experienced implanters.
Safety data gathered in PREVAIL in conjunction with demonstrated efficacy from PROTECT-AF suggest that the Watchman device may be a safe and effective alternative to long-term oral anticoagulation in patients with nonvalvular atrial fibrillation.
In patients with contraindications to warfarin
Most of the published data have been about the efficacy of occlusion devices compared with long-term warfarin therapy. Unfortunately, the population that has not been studied extensively is patients who have contraindications to long-term oral anticoagulation, who would benefit the most from an occlusive device.
The ASA Plavix Feasibility Study (ASAP) focused on this population, specifically those who had a CHADS2 score of 1 or higher and who were considered ineligible for warfarin, to determine whether closure using the Watchman device could be safely performed without a transition period with warfarin.39 After device implantation, trial participants were given clopidogrel for 6 months and aspirin indefinitely. The trial enrolled 150 patients and followed them for a mean of 14.4 (± 8.6) months. In that time, there were four strokes, five pericardial effusions, and six instances of device-related thrombus by transesophageal echocardiography. Three of the strokes were ischemic (1.7% per year), which is a 77% reduction from the expected rate of 7.3% based on the CHADS2 scores of the patient cohort.
These data suggest that implantation of the Watchman device may be appropriate for those who cannot tolerate warfarin even in the short term.
The Lariat system
The Lariat suture delivery device (SentreHeart, Inc., Redwood City, CA) is approved by the US Food and Drug Administration (FDA) for soft-tissue closure and has been used for percutaneous left atrial appendage closure. It uses a magnet-tipped wire that is passed to the epicardial side of the left atrial appendage via pericardial access to meet a second magnet-tipped wire introduced into the appendage via transseptal access. A “lasso” is then advanced over the epicardial guide wire and tightened down around the ostium of the left atrial appendage. This tool facilitates deployment of a nonabsorbable polyester suture, which effectively ligates off the appendage from the rest of the left atrium (Figure 2).40 In theory, the Lariat’s epicardial approach could eliminate the need for short- and long-term anticoagulation, as there would be no foreign body left within the heart.
In an initial cohort of 89 patients in Poland,41 the investigators reported a 96% closure rate as determined by transesophageal echocardiography immediately after the procedure. At 1-year follow-up, there was 98% complete closure, including cases of incomplete closure detected earlier.41 Adverse events were limited, with only two cases of severe pericarditis, two strokes, and one pericardial effusion. These results were replicated in the United States in a cohort of 25 patients, with a 100% closure rate and no stroke events.42
There have been three published case reports of left atrial clot formation after successful left atrial appendage ligation using the Lariat device.43–45 These experiences further emphasize that closure does not necessarily confer instant stroke prevention, and there remains a need to investigate the need for routine imaging and possibly periprocedural anticoagulation after ligation.
More recently, Pillai et al46 published their initial experience following 71 patients with echocardiograms 3 months after left atrial appendage closure using the Lariat device. They reported leaks in 6 of the 71 patients; five of the leaks were successfully closed using the Amplatzer Septal Occluder, and one was closed with a repeat Lariat procedure.
Although the Lariat system has been used in more than 2,000 patients worldwide (SentreHeart, personal communication), there has been no published systematic comparison between it and oral anticoagulation to date.
AN EMERGING OPTION
Established guidelines help determine which patients with atrial fibrillation should receive oral anticoagulant therapy. For patients who have absolute contraindications to oral anticoagulants or who are undergoing cardiac surgery, surgical ligation of the left atrial appendage is an option. But for those with contraindications to oral anticoagulation in both the short term and the long term, there is a growing body of evidence suggesting that a percutaneous intervention is at least noninferior to—and in some cases is superior to—warfarin. Figure 3 shows our recommendations for the steps to determine which patients would be most appropriate to consider for left atrial appendage closure.
Holmes et al47 propose that we may now have enough evidence to support an expedited regulatory approval process of these occlusion devices. But there are still a number of areas in which further investigation is clearly needed before left atrial appendage occlusion devices can be widely adopted.
The trials discussed above had specific inclusion and exclusion criteria, and therefore, although they support percutaneous intervention, the generalizability of their results remains in question. Indeed, the patients in PROTECT-AF36 had an average CHADS2 score of only 2.2. This study also included only patients who were able to tolerate both aspirin and clopidogrel simultaneously for a significant amount of time. Hence, one cannot assume the results would be the same in patients who have strict contraindications to warfarin or any target-specific oral anticoagulant. Concern regarding the generalizability of the conclusions from PROTECT-AF and PREVAIL has led to mixed votes (three assessments to date) from the FDA Circulatory Device Panel.48
In an encouraging review of cases, Gafoor et al49 reported safe and efficacious occlusion in octogenarians using the devices mentioned above. These patients often pose the greatest challenge in initiating long-term anticoagulation because of the many drug-drug interactions and the risk of intracranial hemorrhage secondary to falls.
Further, while occlusion devices would clearly be useful for patients in whom traditional oral anticoagulation is not an option, the newer oral anticoagulants might complicate the picture somewhat. As shown by Ruff et al,21 the risk-benefit ratio of these target-specific oral anticoagulants is quite favorable and by some measurements is superior to that of warfarin. Could there be a group of patients who cannot take warfarin but could instead do well on one of the newer anticoagulants, thus alleviating the need for percutaneous intervention? As the newer oral anticoagulants become more commonly used, the cost-benefit analysis of implanting an occlusion device could shift.
Lastly, in this era of high-value medical care, one must consider the cost-effectiveness of these novel interventions. As with any new technology, the up-front cost of implantation is certainly greater than that of warfarin therapy. If device implantation can prevent a hospitalization from a major bleed secondary to warfarin use or prevent a catastrophic stroke due to untreated atrial fibrillation, then the cost-benefit analysis may be tipped in the other direction. As these devices become more widely available and physicians have more experience implanting them, the costs will likely decrease.
As with oral anticoagulation therapy, all interventions, whether surgical or percutaneous, carry a risk of bleeding and stroke. There remains no substitute for frank and clear discussions between the physician and patient regarding the risks and benefits of each approach.
While a growing body of evidence surrounds left atrial appendage occlusion devices, many questions remain. Notably, could these devices be used in patients who can tolerate oral anticoagulants? And if so, which subgroups would benefit most? Does occlusion or ligation of the left atrial appendage affect electrical connections between it and the left atrium, thereby lowering the burden of atrial fibrillation?
We expect that continued investigation of and experience with left atrial appendage closure devices will position them one day as a viable and equal option for preventing stroke in patients with atrial fibrillation.
- Rosamond W, Flegal K, Furie K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25–146.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987; 147:1561–1564.
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154:1449–1457.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996; 61:755–759.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Odell JA, Blackshear JL, Davies E, et al. Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann Thorac Surg 1996; 61:565–569.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999; 131:492–501.
- Manning WJ, Silverman DI, Keighley CS, Oettgen P, Douglas PS. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol 1995; 25:1354–1361.
- Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart 1999; 82:547–554.
- Lip GY. Recommendations for thromboprophylaxis in the 2012 focused update of the ESC guidelines on atrial fibrillation: a commentary. J Thromb Haemost 2013; 11:615–626.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Onalan O, Lashevsky I, Hamad A, Crystal E. Nonpharmacologic stroke prevention in atrial fibrillation. Expert Rev Cardiovasc Ther 2005; 3:619–633.
- Brass LM, Krumholz HM, Scinto JM, Radford M. Warfarin use among patients with atrial fibrillation. Stroke 1997; 28:2382–2389.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm 2009; 15:244–252.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost 2014; 111:933–942.
- Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008; 52:924–929.
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000; 36:468–471.
- Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011; 142:1002–1009.e1.
- Slater AD, Tatooles AJ, Coffey A, et al. Prospective clinical study of a novel left atrial appendage occlusion device. Ann Thorac Surg 2012; 93:2035-2040.
- Pinho-Gomes AC, Amorim MJ, Oliveira SM, Leite-Moreira AF. Surgical treatment of atrial fibrillation: an updated review. Eur J Cardiothorac Surg 2014; 46:167–178.
- Aryana A, Cavaco D, Arthur A, O’Neill PG, Adragão P, D’Avila A. Percutaneous endocardial occlusion of incompletely surgically ligated left atrial appendage. J Cardiovasc Electrophysiol 2013; 24:968–974.
- García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 2002; 105:1887–1889.
- Ostermayer SH, Reisman M, Kramer PH, et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol 2005; 46:9–14.
- Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011; 77:700–706.
- Urena M, Rodés-Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol 2013; 62:96–102.
- ClinicalTrials.gov. http://clinicaltrials.gov/show/NCT01118299. Accessed January 30, 2015.
- Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007; 49:1490–1495.
- Holmes DR, Reddy VY, Turi ZG, et al; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
- Boston Scientific. WATCHMAN™ Left Atrial Appendage Closure Device. http://www.bostonscientific.com/watchman-eu/assets/pdf/SH-158101-AA-PROTECT-AF-Reddy-HRS-2013.pdf. Accessed January 30, 2015.
- David Holmes M. Boston Scientific. March 9, 2013. Available at: http://www.bostonscientific.com/watchman-eu/assets/downloads/PREVAIL-Clinical-Results.ppt.pdf. Accessed January 30, 2015.
- Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Koneru JN, Badhwar N, Ellenbogen KA, Lee RJ. LAA ligation using the LARIAT suture delivery device: tips and tricks for a successful procedure. Heart Rhythm 2014; 11:911–921.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Massumi A, Chelu MG, Nazeri A, et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol 2013; 111:869–873.
- Giedrimas E, Lin AC, Knight BP. Left atrial thrombus after appendage closure using LARIAT. Circ Arrhythm Electrophysiol 2013; 6:e52–e53.
- Briceno DF, Fernando RR, Laing ST. Left atrial appendage thrombus post LARIAT closure device. Heart Rhythm 2014; 11:1600–1601.
- Baker MS, Paul Mounsey J, Gehi AK, Chung EH. Left atrial thrombus after appendage ligation with LARIAT. Heart Rhythm 2014; 11:1489.
- Pillai AM, Kanmanthareddy A, Earnest M, et al. Initial experience with post Lariat left atrial appendage leak closure with Amplatzer septal occluder device and repeat Lariat application. Heart Rhythm 2014; 11:1877–1883.
- Holmes DR Jr, Lakkireddy DR, Whitlock RP, Waksman R, Mack MJ. Left atrial appendage occlusion: opportunities and challenges. J Am Coll Cardiol 2014; 63:291–298.
- Wood S. FDA Advisors cool on Watchman approval amid ischemic-stroke data. Medscape Multispecialty October 8, 2014. www.medscape.com/viewarticle/832993.
- Gafoor S, Franke J, Bertog S, et al. Left atrial appendage occlusion in octogenarians: short-term and 1-year follow-up. Catheter Cardiovasc Interv 2014; 83:805–810.
Can patients with atrial fibrillation undergo a percutaneous procedure to reduce their risk of stroke, thereby eliminating the need for lifelong treatment with an oral anticoagulant drug? The data are preliminary, but this is an emerging option that physicians should be aware of.
We review here the current evidence and techniques aimed at isolating the left atrial appendage to prevent stroke, and we emphasize the need for continued systematic comparisons between oral anticoagulation and percutaneous treatment options.
NOVEL TREATMENTS ARE NEEDED
Atrial fibrillation is the most common cardiac arrhythmia,1 affecting an estimated 1% to 2% of people worldwide. In 2001, an estimated 2.3 million persons in the United States had atrial fibrillation, and that number is expected to more than double by 2050.2
Atrial fibrillation independently increases the risk of stroke by a factor of 4 to 5.3 The American Heart Association ranks stroke as the fourth most common cause of death and the leading cause of disability in the United States.4 Atrial fibrillation accounts for 15% of strokes in people of all ages and 30% in those over age 80.5 Untreated, 2% to 5% of patients with atrial fibrillation suffer a stroke in any given year.6 Most of these strokes are cardioembolic, with thrombi originating in the left atrial appendage.7 Furthermore, it has been estimated8,9 that patients with atrial fibrillation who have already had a stroke and cannot tolerate oral anticoagulants have an annual risk of stroke close to 12% and a relative risk of approximately 3.0 compared with those with atrial fibrillation and prior stroke who can tolerate anticoagulation.
Oral anticoagulation effectively prevents thromboembolic events associated with atrial fibrillation,10 but several factors limit its efficacy and applicability. The risk of bleeding complications, the need for frequent monitoring, and challenges with compliance create a large population of patients who would benefit from alternative approaches. Consequently, physicians have looked for other ways to prevent stroke—especially surgical and transcatheter procedures—that are not associated with an ongoing risk of hemorrhage and a lifelong need to take an anticoagulant.
THE LEFT ATRIAL APPENDAGE: A SITE OF CLOT FORMATION
The left atrial appendage is the most common site of thrombus formation, particularly in patients with nonvalvular atrial fibrillation. Nearly 90% of thrombi discovered in the left atrium form in the appendage.7 A study of 233 patients not on long-term anticoagulation revealed that after 48 hours of atrial fibrillation, 15% had a left atrial thrombus, and all but one of the thrombi were in the appendage.11
Believed to function as a decompression chamber during left ventricular systole, the left atrial appendage is embryologically derived from the left wall of the primary atrium. It is in close proximity to the free wall of the left ventricle, and therefore its flow can vary with left ventricular function. Relative stasis due to its location and extensive trabeculations, especially in times of poor forward flow, make it a high-risk site for clot formation.12
ANTICOAGULATION: EFFECTIVE BUT IMPERFECT
In deciding whether a patient with atrial fibrillation should be prescribed anticoagulation therapy, the physician must balance the risk of stroke against the risk of bleeding. Several tools for assessing these two risks have been developed. Of note, some of the risk factors for stroke are the same as some of the risk factors for bleeding.
Calculating the risk of stroke
CHADS2 and CHA2DS2-VASc are the two most commonly used tools for assessing the risk of stroke, but only the newer CHA2DS2-VASc has received a class I recommendation (the highest) from the European Society of Cardiology (ESC).13
CHADS2 risk factors are Congestive heart failure (1 point), Hypertension (1 point), Age 75 or older (1 point), Diabetes (1 point), and Stroke or transient ischemic attack (2 points). Risk of stroke is considered low with a score of 0, intermediate with a score of 1, and high with a score of 2 or more.
CHA2DS2-VASc risk factors are Congestive heart failure or left ventricular ejection fraction ≤ 40% (1 point), Hypertension (1 point), Age ≥ 75 (2 points), Age 65–74 (1 point), Diabetes mellitus (1 point), Stroke, transient ischemic attack, or thromboembolism (2 points), Vascular disease (1 point), and female Sex (1 point). Low risk is defined as a score of 0 for a man or, for a woman with no other risk factors, a score of 1. A score of 1 for a man indicates moderate risk, and a score of 2 or more is high risk.
Calculating the risk of bleeding
Tools for assessing bleeding risk include ATRIA2 and HAS-BLED,14 the latter carrying a class I recommendation from the ESC.13
HAS-BLED risk factors are Hypertension (1 point), Abnormal renal or liver function (1 point each), Stroke (1 point), Bleeding (1 point), Labile international normalized ratio (INR) (1 point), Elderly (age > 65) (1 point), and Drug or alcohol use (1 point each). The risk of bleeding is considered high with a score of 3 or higher.
Disadvantages of oral anticoagulation
Oral anticoagulation is the standard treatment for preventing stroke in patients with atrial fibrillation, and the vitamin K antagonist warfarin remains the foundation.
Though highly effective, warfarin requires close monitoring and frequent dose adjustments because of its numerous food and drug interactions. Bleeding risk and the challenge of frequent monitoring rule out treatment with warfarin in 14% to 44% of patients with atrial fibrillation.15 Even in “ideal” candidates, warfarin is underused, with one study reporting that only 38% of those with clinical indications for it had been prescribed warfarin, and of those for whom it had not been prescribed, 63% were also not taking aspirin.16 Moreover, a meta-analysis suggested that the average patient treated with warfarin has his or her INR in the therapeutic range only about 55% of the time.17
Newer, target-specific oral anticoagulants such as dabigatran (a direct thrombin inhibitor) and rivaroxaban and apixaban (both factor Xa inhibitors) do not require monitoring and have fewer drug interactions. But like warfarin, they also confer a risk of serious bleeding.18–20 Most of the studies of these newer drugs have compared them with warfarin, with the preponderance of evidence showing them to be either noninferior or superior to warfarin for stroke reduction. But bleeding complication rates remain significant, apixaban having lower rates of major bleeding than dabigatran and rivaroxaban.
In a meta-analysis, Ruff et al21 concluded that the target-specific oral anticoagulants provide a favorable balance of risk and benefit. Compared with warfarin, these new drugs reduced the rate of stroke or systemic embolic events by 19%. There was also a significant reduction in rates of intracranial hemorrhage and all-cause mortality. The risk of major bleeding was similar to that with warfarin, though there was a higher risk of gastrointestinal bleeding with target-specific agents. These effects were consistent across a wide range of patients.
Given the difficulties, risks, and serious side effects of anticoagulant therapy, many patients stop taking these drugs soon after starting them, either on their own or on their physician’s recommendation. In the RE-LY trial (Dabigatran vs Warfarin in Patients With Atrial Fibrillation), 10% of patients receiving dabigatran and 17% of those receiving warfarin stopped the treatment within 1 to 2 years.22 In a similar trial of rivaroxaban vs warfarin in nonvalvular atrial fibrillation (the ROCKET-AF trial), 24% of those treated with rivaroxaban and 22% of those treated with warfarin stopped treatment during the study.19 In the ARISTOTLE trial (Apixaban vs Warfarin in Patients With Atrial Fibrillation), 25% of patients discontinued apixaban and 28% discontinued warfarin.20
The results of these trials show a clear need for treatments without high attrition rates, since patients with atrial fibrillation need protection from stroke for the rest of their life.
SURGICAL CLOSURE AS AN ADD-ON TO OTHER PROCEDURES
If the patient is undergoing cardiac surgery for another reason, the surgeon can excise, suture, staple, or clip the left atrial appendage shut at the same time. Closure is recommended as part of valve replacement.8 In a 2008 retrospective study, left atrial appendage closure was successfully performed in 40% of those undergoing the procedure during cardiac surgery, and complete closure occurred more often with excision than with suture exclusion and stapler exclusion.23 A study of patients who underwent ligation of the left atrial appendage during mitral value replacement found that 35% demonstrated incomplete closure as determined by transesophageal echocardiography.24
Newer devices have shown more success. The AtriClip (AtriCure Inc., West Chester, OH) is a self-closing, implantable clip applied epicardially by either an open surgical or a minimally invasive technique.25 Successful closure was confirmed in 60 of 61 patients at 90 days as determined by computed tomography or transesophageal echocardiography, and there were no adverse events related to implantation of the device.25 The TigerPaw system (Terumo Cardiovascular Systems, Ann Arbor, MI)26 is a fastener delivered surgically around the base of the ostium of the left atrial appendage. In an initial trial, 90 days after the procedure, transesophageal echocardiography showed no leaks in any of those who were examined (54 of 60 patients).
Amputation of the left atrial appendage is also considered part of the maze procedure for atrial fibrillation, in which the operator creates multiple small scars in the atria to prevent irregular impulses from being conducted.27
Results of these surgical approaches have been mixed, as incomplete closure or clipping actually increases the risk of left atrial thrombus formation and embolization.28 Moreover, these invasive surgical techniques are associated with significant periprocedural morbidity.29 Because of the high risk of surgical complications, cardiac specialists have sought less invasive percutaneous procedures to manage stroke risk in patients with atrial fibrillation.
PERCUTANEOUS OCCLUSION
One option for closing the left atrial appendage is a percutaneous transseptal approach in which a plug is placed in the opening connecting the appendage to the rest of the atrium.
The PLAATO device
The Percutaneous LAA Transcatheter Occlusion (PLAATO) device (Appriva Medical Inc., Sunnyvale, CA) contains an expandable nitinol-covered cage designed to be placed in the orifice of the left atrial appendage. Over time, tissue grows into the device, entirely isolating the appendage from the rest of the atrium.
In 2002, Sievert et al30 reported using this device in 15 patients. Subsequently, in a nonrandomized trial in patients with contraindications to lifelong anticoagulation, total occlusion was achieved in 108 of 111 patients, with no thrombosis or migration of the device at 6 months. The annual risk of stroke was 2.2%, a reduction in relative risk of 65% based on the CHADS2 score.31
But despite this apparent success, the PLAATO device was discontinued for unspecified commercial reasons.
Amplatzer cardiac plug
Modeled after an atrial septal occluder, the Amplatzer cardiac plug (St. Jude Medical, St. Paul, MN) consists of a lobe and a disk connected by a central waist.
In 2011, Park et al32 published their initial experience implanting this device in patients who either could not tolerate or did not desire long-term anticoagulation. They reported a 96% closure rate (137 of 143 patients), but there were serious complications in 10 patients: 3 with ischemic stroke, 2 with device embolism, and 5 with pericardial effusions.
Urena et al33 reported similar results in 52 patients with absolute contraindications to warfarin, with a 98.1% implantation rate. Patients were then maintained on either single or dual antiplatelet therapy at the discretion of the operator. At 20-month follow-up, there had been one stroke, one transient ischemic attack, and one major bleeding event. The leakage rate was 16.2% as determined by transesophageal echocardiography.
While initial results were promising, a clinical trial comparing this device and optimal medical treatment is currently on hold. Thus, there are no clear data comparing the Amplatzer device with oral anticoagulation.34
The Watchman device
The Watchman device (Boston Scientific, Marlborough, MA), an evolution of the PLAATO device, is a self-expanding nitinol structure with fixation barbs and a polyethylene membrane to protect the atrium-facing side of the device (Figure 1).
A pilot trial reported successful implantation in 66 of 75 patients, though the device was found to migrate after placement in 5 of the first 16 patients using the original device and delivery system. The device was modified, and no further embolization of the device occurred.35
The PROTECT-AF trial (Protection in Patients With Atrial Fibrillation)36 was the first completed and published randomized controlled trial evaluating left atrial appendage closure using a device vs long-term warfarin therapy. This study randomized 707 people with nonvalvular atrial fibrillation from 59 centers worldwide to receive the Watchman device or a control treatment. The study included patients age 18 or older with nonvalvular atrial fibrillation who were able to tolerate warfarin therapy. Patients in the control group received warfarin for the duration of the study and were monitored every 2 weeks for a goal INR of 2 to 3, achieving a therapeutic INR 66% of the time. The device group was also treated with warfarin for 45 days to allow device endothelialization. Warfarin was discontinued if transesophageal echocardiography showed complete closure or significantly decreased flow around the device. Patients in the device group were then treated with aspirin and clopidogrel for 6 months, and then with aspirin indefinitely.
At 1,065 patient-years of follow-up, PROTECT-AF showed that in patients with atrial fibrillation who were candidates for warfarin therapy, percutaneous left atrial appendage closure using the Watchman device reduced the rate of hemorrhagic stroke compared with warfarin and was noninferior to warfarin in terms of all-cause mortality and stroke. A 4-year follow-up to the PROTECT-AF trial found that receiving the Watchman was better than taking warfarin in terms of risk of cardiovascular death, stroke and other systemic embolization, and all-cause mortality. The adverse event rates were 2.3% in the device group and 3.8% in the control group, a 40% relative risk reduction in the Watchman group.37
The PREVAIL trial (Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation vs Long-Term Warfarin Therapy) aimed to confirm the safety and efficacy of the Watchman device compared with long-term warfarin therapy.38 The event rate (defined as 7-day occurrence of death, ischemic stroke, systemic embolism, and procedure- or device-related complications requiring major cardiovascular or endovascular intervention) was 2.2%. But the PREVAIL trial was unable to show that the device was noninferior to warfarin in terms of its second primary end point of stroke, systemic embolism, and cardiovascular or unexplained death at 18 months. When performed by physicians who were new to the procedure, the procedure was successful (ie, the device was successfully implanted) in 93.2%; the rate was slightly higher (96.3%) when performed by experienced implanters.
Safety data gathered in PREVAIL in conjunction with demonstrated efficacy from PROTECT-AF suggest that the Watchman device may be a safe and effective alternative to long-term oral anticoagulation in patients with nonvalvular atrial fibrillation.
In patients with contraindications to warfarin
Most of the published data have been about the efficacy of occlusion devices compared with long-term warfarin therapy. Unfortunately, the population that has not been studied extensively is patients who have contraindications to long-term oral anticoagulation, who would benefit the most from an occlusive device.
The ASA Plavix Feasibility Study (ASAP) focused on this population, specifically those who had a CHADS2 score of 1 or higher and who were considered ineligible for warfarin, to determine whether closure using the Watchman device could be safely performed without a transition period with warfarin.39 After device implantation, trial participants were given clopidogrel for 6 months and aspirin indefinitely. The trial enrolled 150 patients and followed them for a mean of 14.4 (± 8.6) months. In that time, there were four strokes, five pericardial effusions, and six instances of device-related thrombus by transesophageal echocardiography. Three of the strokes were ischemic (1.7% per year), which is a 77% reduction from the expected rate of 7.3% based on the CHADS2 scores of the patient cohort.
These data suggest that implantation of the Watchman device may be appropriate for those who cannot tolerate warfarin even in the short term.
The Lariat system
The Lariat suture delivery device (SentreHeart, Inc., Redwood City, CA) is approved by the US Food and Drug Administration (FDA) for soft-tissue closure and has been used for percutaneous left atrial appendage closure. It uses a magnet-tipped wire that is passed to the epicardial side of the left atrial appendage via pericardial access to meet a second magnet-tipped wire introduced into the appendage via transseptal access. A “lasso” is then advanced over the epicardial guide wire and tightened down around the ostium of the left atrial appendage. This tool facilitates deployment of a nonabsorbable polyester suture, which effectively ligates off the appendage from the rest of the left atrium (Figure 2).40 In theory, the Lariat’s epicardial approach could eliminate the need for short- and long-term anticoagulation, as there would be no foreign body left within the heart.
In an initial cohort of 89 patients in Poland,41 the investigators reported a 96% closure rate as determined by transesophageal echocardiography immediately after the procedure. At 1-year follow-up, there was 98% complete closure, including cases of incomplete closure detected earlier.41 Adverse events were limited, with only two cases of severe pericarditis, two strokes, and one pericardial effusion. These results were replicated in the United States in a cohort of 25 patients, with a 100% closure rate and no stroke events.42
There have been three published case reports of left atrial clot formation after successful left atrial appendage ligation using the Lariat device.43–45 These experiences further emphasize that closure does not necessarily confer instant stroke prevention, and there remains a need to investigate the need for routine imaging and possibly periprocedural anticoagulation after ligation.
More recently, Pillai et al46 published their initial experience following 71 patients with echocardiograms 3 months after left atrial appendage closure using the Lariat device. They reported leaks in 6 of the 71 patients; five of the leaks were successfully closed using the Amplatzer Septal Occluder, and one was closed with a repeat Lariat procedure.
Although the Lariat system has been used in more than 2,000 patients worldwide (SentreHeart, personal communication), there has been no published systematic comparison between it and oral anticoagulation to date.
AN EMERGING OPTION
Established guidelines help determine which patients with atrial fibrillation should receive oral anticoagulant therapy. For patients who have absolute contraindications to oral anticoagulants or who are undergoing cardiac surgery, surgical ligation of the left atrial appendage is an option. But for those with contraindications to oral anticoagulation in both the short term and the long term, there is a growing body of evidence suggesting that a percutaneous intervention is at least noninferior to—and in some cases is superior to—warfarin. Figure 3 shows our recommendations for the steps to determine which patients would be most appropriate to consider for left atrial appendage closure.
Holmes et al47 propose that we may now have enough evidence to support an expedited regulatory approval process of these occlusion devices. But there are still a number of areas in which further investigation is clearly needed before left atrial appendage occlusion devices can be widely adopted.
The trials discussed above had specific inclusion and exclusion criteria, and therefore, although they support percutaneous intervention, the generalizability of their results remains in question. Indeed, the patients in PROTECT-AF36 had an average CHADS2 score of only 2.2. This study also included only patients who were able to tolerate both aspirin and clopidogrel simultaneously for a significant amount of time. Hence, one cannot assume the results would be the same in patients who have strict contraindications to warfarin or any target-specific oral anticoagulant. Concern regarding the generalizability of the conclusions from PROTECT-AF and PREVAIL has led to mixed votes (three assessments to date) from the FDA Circulatory Device Panel.48
In an encouraging review of cases, Gafoor et al49 reported safe and efficacious occlusion in octogenarians using the devices mentioned above. These patients often pose the greatest challenge in initiating long-term anticoagulation because of the many drug-drug interactions and the risk of intracranial hemorrhage secondary to falls.
Further, while occlusion devices would clearly be useful for patients in whom traditional oral anticoagulation is not an option, the newer oral anticoagulants might complicate the picture somewhat. As shown by Ruff et al,21 the risk-benefit ratio of these target-specific oral anticoagulants is quite favorable and by some measurements is superior to that of warfarin. Could there be a group of patients who cannot take warfarin but could instead do well on one of the newer anticoagulants, thus alleviating the need for percutaneous intervention? As the newer oral anticoagulants become more commonly used, the cost-benefit analysis of implanting an occlusion device could shift.
Lastly, in this era of high-value medical care, one must consider the cost-effectiveness of these novel interventions. As with any new technology, the up-front cost of implantation is certainly greater than that of warfarin therapy. If device implantation can prevent a hospitalization from a major bleed secondary to warfarin use or prevent a catastrophic stroke due to untreated atrial fibrillation, then the cost-benefit analysis may be tipped in the other direction. As these devices become more widely available and physicians have more experience implanting them, the costs will likely decrease.
As with oral anticoagulation therapy, all interventions, whether surgical or percutaneous, carry a risk of bleeding and stroke. There remains no substitute for frank and clear discussions between the physician and patient regarding the risks and benefits of each approach.
While a growing body of evidence surrounds left atrial appendage occlusion devices, many questions remain. Notably, could these devices be used in patients who can tolerate oral anticoagulants? And if so, which subgroups would benefit most? Does occlusion or ligation of the left atrial appendage affect electrical connections between it and the left atrium, thereby lowering the burden of atrial fibrillation?
We expect that continued investigation of and experience with left atrial appendage closure devices will position them one day as a viable and equal option for preventing stroke in patients with atrial fibrillation.
Can patients with atrial fibrillation undergo a percutaneous procedure to reduce their risk of stroke, thereby eliminating the need for lifelong treatment with an oral anticoagulant drug? The data are preliminary, but this is an emerging option that physicians should be aware of.
We review here the current evidence and techniques aimed at isolating the left atrial appendage to prevent stroke, and we emphasize the need for continued systematic comparisons between oral anticoagulation and percutaneous treatment options.
NOVEL TREATMENTS ARE NEEDED
Atrial fibrillation is the most common cardiac arrhythmia,1 affecting an estimated 1% to 2% of people worldwide. In 2001, an estimated 2.3 million persons in the United States had atrial fibrillation, and that number is expected to more than double by 2050.2
Atrial fibrillation independently increases the risk of stroke by a factor of 4 to 5.3 The American Heart Association ranks stroke as the fourth most common cause of death and the leading cause of disability in the United States.4 Atrial fibrillation accounts for 15% of strokes in people of all ages and 30% in those over age 80.5 Untreated, 2% to 5% of patients with atrial fibrillation suffer a stroke in any given year.6 Most of these strokes are cardioembolic, with thrombi originating in the left atrial appendage.7 Furthermore, it has been estimated8,9 that patients with atrial fibrillation who have already had a stroke and cannot tolerate oral anticoagulants have an annual risk of stroke close to 12% and a relative risk of approximately 3.0 compared with those with atrial fibrillation and prior stroke who can tolerate anticoagulation.
Oral anticoagulation effectively prevents thromboembolic events associated with atrial fibrillation,10 but several factors limit its efficacy and applicability. The risk of bleeding complications, the need for frequent monitoring, and challenges with compliance create a large population of patients who would benefit from alternative approaches. Consequently, physicians have looked for other ways to prevent stroke—especially surgical and transcatheter procedures—that are not associated with an ongoing risk of hemorrhage and a lifelong need to take an anticoagulant.
THE LEFT ATRIAL APPENDAGE: A SITE OF CLOT FORMATION
The left atrial appendage is the most common site of thrombus formation, particularly in patients with nonvalvular atrial fibrillation. Nearly 90% of thrombi discovered in the left atrium form in the appendage.7 A study of 233 patients not on long-term anticoagulation revealed that after 48 hours of atrial fibrillation, 15% had a left atrial thrombus, and all but one of the thrombi were in the appendage.11
Believed to function as a decompression chamber during left ventricular systole, the left atrial appendage is embryologically derived from the left wall of the primary atrium. It is in close proximity to the free wall of the left ventricle, and therefore its flow can vary with left ventricular function. Relative stasis due to its location and extensive trabeculations, especially in times of poor forward flow, make it a high-risk site for clot formation.12
ANTICOAGULATION: EFFECTIVE BUT IMPERFECT
In deciding whether a patient with atrial fibrillation should be prescribed anticoagulation therapy, the physician must balance the risk of stroke against the risk of bleeding. Several tools for assessing these two risks have been developed. Of note, some of the risk factors for stroke are the same as some of the risk factors for bleeding.
Calculating the risk of stroke
CHADS2 and CHA2DS2-VASc are the two most commonly used tools for assessing the risk of stroke, but only the newer CHA2DS2-VASc has received a class I recommendation (the highest) from the European Society of Cardiology (ESC).13
CHADS2 risk factors are Congestive heart failure (1 point), Hypertension (1 point), Age 75 or older (1 point), Diabetes (1 point), and Stroke or transient ischemic attack (2 points). Risk of stroke is considered low with a score of 0, intermediate with a score of 1, and high with a score of 2 or more.
CHA2DS2-VASc risk factors are Congestive heart failure or left ventricular ejection fraction ≤ 40% (1 point), Hypertension (1 point), Age ≥ 75 (2 points), Age 65–74 (1 point), Diabetes mellitus (1 point), Stroke, transient ischemic attack, or thromboembolism (2 points), Vascular disease (1 point), and female Sex (1 point). Low risk is defined as a score of 0 for a man or, for a woman with no other risk factors, a score of 1. A score of 1 for a man indicates moderate risk, and a score of 2 or more is high risk.
Calculating the risk of bleeding
Tools for assessing bleeding risk include ATRIA2 and HAS-BLED,14 the latter carrying a class I recommendation from the ESC.13
HAS-BLED risk factors are Hypertension (1 point), Abnormal renal or liver function (1 point each), Stroke (1 point), Bleeding (1 point), Labile international normalized ratio (INR) (1 point), Elderly (age > 65) (1 point), and Drug or alcohol use (1 point each). The risk of bleeding is considered high with a score of 3 or higher.
Disadvantages of oral anticoagulation
Oral anticoagulation is the standard treatment for preventing stroke in patients with atrial fibrillation, and the vitamin K antagonist warfarin remains the foundation.
Though highly effective, warfarin requires close monitoring and frequent dose adjustments because of its numerous food and drug interactions. Bleeding risk and the challenge of frequent monitoring rule out treatment with warfarin in 14% to 44% of patients with atrial fibrillation.15 Even in “ideal” candidates, warfarin is underused, with one study reporting that only 38% of those with clinical indications for it had been prescribed warfarin, and of those for whom it had not been prescribed, 63% were also not taking aspirin.16 Moreover, a meta-analysis suggested that the average patient treated with warfarin has his or her INR in the therapeutic range only about 55% of the time.17
Newer, target-specific oral anticoagulants such as dabigatran (a direct thrombin inhibitor) and rivaroxaban and apixaban (both factor Xa inhibitors) do not require monitoring and have fewer drug interactions. But like warfarin, they also confer a risk of serious bleeding.18–20 Most of the studies of these newer drugs have compared them with warfarin, with the preponderance of evidence showing them to be either noninferior or superior to warfarin for stroke reduction. But bleeding complication rates remain significant, apixaban having lower rates of major bleeding than dabigatran and rivaroxaban.
In a meta-analysis, Ruff et al21 concluded that the target-specific oral anticoagulants provide a favorable balance of risk and benefit. Compared with warfarin, these new drugs reduced the rate of stroke or systemic embolic events by 19%. There was also a significant reduction in rates of intracranial hemorrhage and all-cause mortality. The risk of major bleeding was similar to that with warfarin, though there was a higher risk of gastrointestinal bleeding with target-specific agents. These effects were consistent across a wide range of patients.
Given the difficulties, risks, and serious side effects of anticoagulant therapy, many patients stop taking these drugs soon after starting them, either on their own or on their physician’s recommendation. In the RE-LY trial (Dabigatran vs Warfarin in Patients With Atrial Fibrillation), 10% of patients receiving dabigatran and 17% of those receiving warfarin stopped the treatment within 1 to 2 years.22 In a similar trial of rivaroxaban vs warfarin in nonvalvular atrial fibrillation (the ROCKET-AF trial), 24% of those treated with rivaroxaban and 22% of those treated with warfarin stopped treatment during the study.19 In the ARISTOTLE trial (Apixaban vs Warfarin in Patients With Atrial Fibrillation), 25% of patients discontinued apixaban and 28% discontinued warfarin.20
The results of these trials show a clear need for treatments without high attrition rates, since patients with atrial fibrillation need protection from stroke for the rest of their life.
SURGICAL CLOSURE AS AN ADD-ON TO OTHER PROCEDURES
If the patient is undergoing cardiac surgery for another reason, the surgeon can excise, suture, staple, or clip the left atrial appendage shut at the same time. Closure is recommended as part of valve replacement.8 In a 2008 retrospective study, left atrial appendage closure was successfully performed in 40% of those undergoing the procedure during cardiac surgery, and complete closure occurred more often with excision than with suture exclusion and stapler exclusion.23 A study of patients who underwent ligation of the left atrial appendage during mitral value replacement found that 35% demonstrated incomplete closure as determined by transesophageal echocardiography.24
Newer devices have shown more success. The AtriClip (AtriCure Inc., West Chester, OH) is a self-closing, implantable clip applied epicardially by either an open surgical or a minimally invasive technique.25 Successful closure was confirmed in 60 of 61 patients at 90 days as determined by computed tomography or transesophageal echocardiography, and there were no adverse events related to implantation of the device.25 The TigerPaw system (Terumo Cardiovascular Systems, Ann Arbor, MI)26 is a fastener delivered surgically around the base of the ostium of the left atrial appendage. In an initial trial, 90 days after the procedure, transesophageal echocardiography showed no leaks in any of those who were examined (54 of 60 patients).
Amputation of the left atrial appendage is also considered part of the maze procedure for atrial fibrillation, in which the operator creates multiple small scars in the atria to prevent irregular impulses from being conducted.27
Results of these surgical approaches have been mixed, as incomplete closure or clipping actually increases the risk of left atrial thrombus formation and embolization.28 Moreover, these invasive surgical techniques are associated with significant periprocedural morbidity.29 Because of the high risk of surgical complications, cardiac specialists have sought less invasive percutaneous procedures to manage stroke risk in patients with atrial fibrillation.
PERCUTANEOUS OCCLUSION
One option for closing the left atrial appendage is a percutaneous transseptal approach in which a plug is placed in the opening connecting the appendage to the rest of the atrium.
The PLAATO device
The Percutaneous LAA Transcatheter Occlusion (PLAATO) device (Appriva Medical Inc., Sunnyvale, CA) contains an expandable nitinol-covered cage designed to be placed in the orifice of the left atrial appendage. Over time, tissue grows into the device, entirely isolating the appendage from the rest of the atrium.
In 2002, Sievert et al30 reported using this device in 15 patients. Subsequently, in a nonrandomized trial in patients with contraindications to lifelong anticoagulation, total occlusion was achieved in 108 of 111 patients, with no thrombosis or migration of the device at 6 months. The annual risk of stroke was 2.2%, a reduction in relative risk of 65% based on the CHADS2 score.31
But despite this apparent success, the PLAATO device was discontinued for unspecified commercial reasons.
Amplatzer cardiac plug
Modeled after an atrial septal occluder, the Amplatzer cardiac plug (St. Jude Medical, St. Paul, MN) consists of a lobe and a disk connected by a central waist.
In 2011, Park et al32 published their initial experience implanting this device in patients who either could not tolerate or did not desire long-term anticoagulation. They reported a 96% closure rate (137 of 143 patients), but there were serious complications in 10 patients: 3 with ischemic stroke, 2 with device embolism, and 5 with pericardial effusions.
Urena et al33 reported similar results in 52 patients with absolute contraindications to warfarin, with a 98.1% implantation rate. Patients were then maintained on either single or dual antiplatelet therapy at the discretion of the operator. At 20-month follow-up, there had been one stroke, one transient ischemic attack, and one major bleeding event. The leakage rate was 16.2% as determined by transesophageal echocardiography.
While initial results were promising, a clinical trial comparing this device and optimal medical treatment is currently on hold. Thus, there are no clear data comparing the Amplatzer device with oral anticoagulation.34
The Watchman device
The Watchman device (Boston Scientific, Marlborough, MA), an evolution of the PLAATO device, is a self-expanding nitinol structure with fixation barbs and a polyethylene membrane to protect the atrium-facing side of the device (Figure 1).
A pilot trial reported successful implantation in 66 of 75 patients, though the device was found to migrate after placement in 5 of the first 16 patients using the original device and delivery system. The device was modified, and no further embolization of the device occurred.35
The PROTECT-AF trial (Protection in Patients With Atrial Fibrillation)36 was the first completed and published randomized controlled trial evaluating left atrial appendage closure using a device vs long-term warfarin therapy. This study randomized 707 people with nonvalvular atrial fibrillation from 59 centers worldwide to receive the Watchman device or a control treatment. The study included patients age 18 or older with nonvalvular atrial fibrillation who were able to tolerate warfarin therapy. Patients in the control group received warfarin for the duration of the study and were monitored every 2 weeks for a goal INR of 2 to 3, achieving a therapeutic INR 66% of the time. The device group was also treated with warfarin for 45 days to allow device endothelialization. Warfarin was discontinued if transesophageal echocardiography showed complete closure or significantly decreased flow around the device. Patients in the device group were then treated with aspirin and clopidogrel for 6 months, and then with aspirin indefinitely.
At 1,065 patient-years of follow-up, PROTECT-AF showed that in patients with atrial fibrillation who were candidates for warfarin therapy, percutaneous left atrial appendage closure using the Watchman device reduced the rate of hemorrhagic stroke compared with warfarin and was noninferior to warfarin in terms of all-cause mortality and stroke. A 4-year follow-up to the PROTECT-AF trial found that receiving the Watchman was better than taking warfarin in terms of risk of cardiovascular death, stroke and other systemic embolization, and all-cause mortality. The adverse event rates were 2.3% in the device group and 3.8% in the control group, a 40% relative risk reduction in the Watchman group.37
The PREVAIL trial (Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation vs Long-Term Warfarin Therapy) aimed to confirm the safety and efficacy of the Watchman device compared with long-term warfarin therapy.38 The event rate (defined as 7-day occurrence of death, ischemic stroke, systemic embolism, and procedure- or device-related complications requiring major cardiovascular or endovascular intervention) was 2.2%. But the PREVAIL trial was unable to show that the device was noninferior to warfarin in terms of its second primary end point of stroke, systemic embolism, and cardiovascular or unexplained death at 18 months. When performed by physicians who were new to the procedure, the procedure was successful (ie, the device was successfully implanted) in 93.2%; the rate was slightly higher (96.3%) when performed by experienced implanters.
Safety data gathered in PREVAIL in conjunction with demonstrated efficacy from PROTECT-AF suggest that the Watchman device may be a safe and effective alternative to long-term oral anticoagulation in patients with nonvalvular atrial fibrillation.
In patients with contraindications to warfarin
Most of the published data have been about the efficacy of occlusion devices compared with long-term warfarin therapy. Unfortunately, the population that has not been studied extensively is patients who have contraindications to long-term oral anticoagulation, who would benefit the most from an occlusive device.
The ASA Plavix Feasibility Study (ASAP) focused on this population, specifically those who had a CHADS2 score of 1 or higher and who were considered ineligible for warfarin, to determine whether closure using the Watchman device could be safely performed without a transition period with warfarin.39 After device implantation, trial participants were given clopidogrel for 6 months and aspirin indefinitely. The trial enrolled 150 patients and followed them for a mean of 14.4 (± 8.6) months. In that time, there were four strokes, five pericardial effusions, and six instances of device-related thrombus by transesophageal echocardiography. Three of the strokes were ischemic (1.7% per year), which is a 77% reduction from the expected rate of 7.3% based on the CHADS2 scores of the patient cohort.
These data suggest that implantation of the Watchman device may be appropriate for those who cannot tolerate warfarin even in the short term.
The Lariat system
The Lariat suture delivery device (SentreHeart, Inc., Redwood City, CA) is approved by the US Food and Drug Administration (FDA) for soft-tissue closure and has been used for percutaneous left atrial appendage closure. It uses a magnet-tipped wire that is passed to the epicardial side of the left atrial appendage via pericardial access to meet a second magnet-tipped wire introduced into the appendage via transseptal access. A “lasso” is then advanced over the epicardial guide wire and tightened down around the ostium of the left atrial appendage. This tool facilitates deployment of a nonabsorbable polyester suture, which effectively ligates off the appendage from the rest of the left atrium (Figure 2).40 In theory, the Lariat’s epicardial approach could eliminate the need for short- and long-term anticoagulation, as there would be no foreign body left within the heart.
In an initial cohort of 89 patients in Poland,41 the investigators reported a 96% closure rate as determined by transesophageal echocardiography immediately after the procedure. At 1-year follow-up, there was 98% complete closure, including cases of incomplete closure detected earlier.41 Adverse events were limited, with only two cases of severe pericarditis, two strokes, and one pericardial effusion. These results were replicated in the United States in a cohort of 25 patients, with a 100% closure rate and no stroke events.42
There have been three published case reports of left atrial clot formation after successful left atrial appendage ligation using the Lariat device.43–45 These experiences further emphasize that closure does not necessarily confer instant stroke prevention, and there remains a need to investigate the need for routine imaging and possibly periprocedural anticoagulation after ligation.
More recently, Pillai et al46 published their initial experience following 71 patients with echocardiograms 3 months after left atrial appendage closure using the Lariat device. They reported leaks in 6 of the 71 patients; five of the leaks were successfully closed using the Amplatzer Septal Occluder, and one was closed with a repeat Lariat procedure.
Although the Lariat system has been used in more than 2,000 patients worldwide (SentreHeart, personal communication), there has been no published systematic comparison between it and oral anticoagulation to date.
AN EMERGING OPTION
Established guidelines help determine which patients with atrial fibrillation should receive oral anticoagulant therapy. For patients who have absolute contraindications to oral anticoagulants or who are undergoing cardiac surgery, surgical ligation of the left atrial appendage is an option. But for those with contraindications to oral anticoagulation in both the short term and the long term, there is a growing body of evidence suggesting that a percutaneous intervention is at least noninferior to—and in some cases is superior to—warfarin. Figure 3 shows our recommendations for the steps to determine which patients would be most appropriate to consider for left atrial appendage closure.
Holmes et al47 propose that we may now have enough evidence to support an expedited regulatory approval process of these occlusion devices. But there are still a number of areas in which further investigation is clearly needed before left atrial appendage occlusion devices can be widely adopted.
The trials discussed above had specific inclusion and exclusion criteria, and therefore, although they support percutaneous intervention, the generalizability of their results remains in question. Indeed, the patients in PROTECT-AF36 had an average CHADS2 score of only 2.2. This study also included only patients who were able to tolerate both aspirin and clopidogrel simultaneously for a significant amount of time. Hence, one cannot assume the results would be the same in patients who have strict contraindications to warfarin or any target-specific oral anticoagulant. Concern regarding the generalizability of the conclusions from PROTECT-AF and PREVAIL has led to mixed votes (three assessments to date) from the FDA Circulatory Device Panel.48
In an encouraging review of cases, Gafoor et al49 reported safe and efficacious occlusion in octogenarians using the devices mentioned above. These patients often pose the greatest challenge in initiating long-term anticoagulation because of the many drug-drug interactions and the risk of intracranial hemorrhage secondary to falls.
Further, while occlusion devices would clearly be useful for patients in whom traditional oral anticoagulation is not an option, the newer oral anticoagulants might complicate the picture somewhat. As shown by Ruff et al,21 the risk-benefit ratio of these target-specific oral anticoagulants is quite favorable and by some measurements is superior to that of warfarin. Could there be a group of patients who cannot take warfarin but could instead do well on one of the newer anticoagulants, thus alleviating the need for percutaneous intervention? As the newer oral anticoagulants become more commonly used, the cost-benefit analysis of implanting an occlusion device could shift.
Lastly, in this era of high-value medical care, one must consider the cost-effectiveness of these novel interventions. As with any new technology, the up-front cost of implantation is certainly greater than that of warfarin therapy. If device implantation can prevent a hospitalization from a major bleed secondary to warfarin use or prevent a catastrophic stroke due to untreated atrial fibrillation, then the cost-benefit analysis may be tipped in the other direction. As these devices become more widely available and physicians have more experience implanting them, the costs will likely decrease.
As with oral anticoagulation therapy, all interventions, whether surgical or percutaneous, carry a risk of bleeding and stroke. There remains no substitute for frank and clear discussions between the physician and patient regarding the risks and benefits of each approach.
While a growing body of evidence surrounds left atrial appendage occlusion devices, many questions remain. Notably, could these devices be used in patients who can tolerate oral anticoagulants? And if so, which subgroups would benefit most? Does occlusion or ligation of the left atrial appendage affect electrical connections between it and the left atrium, thereby lowering the burden of atrial fibrillation?
We expect that continued investigation of and experience with left atrial appendage closure devices will position them one day as a viable and equal option for preventing stroke in patients with atrial fibrillation.
- Rosamond W, Flegal K, Furie K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25–146.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987; 147:1561–1564.
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154:1449–1457.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996; 61:755–759.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Odell JA, Blackshear JL, Davies E, et al. Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann Thorac Surg 1996; 61:565–569.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999; 131:492–501.
- Manning WJ, Silverman DI, Keighley CS, Oettgen P, Douglas PS. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol 1995; 25:1354–1361.
- Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart 1999; 82:547–554.
- Lip GY. Recommendations for thromboprophylaxis in the 2012 focused update of the ESC guidelines on atrial fibrillation: a commentary. J Thromb Haemost 2013; 11:615–626.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Onalan O, Lashevsky I, Hamad A, Crystal E. Nonpharmacologic stroke prevention in atrial fibrillation. Expert Rev Cardiovasc Ther 2005; 3:619–633.
- Brass LM, Krumholz HM, Scinto JM, Radford M. Warfarin use among patients with atrial fibrillation. Stroke 1997; 28:2382–2389.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm 2009; 15:244–252.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost 2014; 111:933–942.
- Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008; 52:924–929.
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000; 36:468–471.
- Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011; 142:1002–1009.e1.
- Slater AD, Tatooles AJ, Coffey A, et al. Prospective clinical study of a novel left atrial appendage occlusion device. Ann Thorac Surg 2012; 93:2035-2040.
- Pinho-Gomes AC, Amorim MJ, Oliveira SM, Leite-Moreira AF. Surgical treatment of atrial fibrillation: an updated review. Eur J Cardiothorac Surg 2014; 46:167–178.
- Aryana A, Cavaco D, Arthur A, O’Neill PG, Adragão P, D’Avila A. Percutaneous endocardial occlusion of incompletely surgically ligated left atrial appendage. J Cardiovasc Electrophysiol 2013; 24:968–974.
- García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 2002; 105:1887–1889.
- Ostermayer SH, Reisman M, Kramer PH, et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol 2005; 46:9–14.
- Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011; 77:700–706.
- Urena M, Rodés-Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol 2013; 62:96–102.
- ClinicalTrials.gov. http://clinicaltrials.gov/show/NCT01118299. Accessed January 30, 2015.
- Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007; 49:1490–1495.
- Holmes DR, Reddy VY, Turi ZG, et al; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
- Boston Scientific. WATCHMAN™ Left Atrial Appendage Closure Device. http://www.bostonscientific.com/watchman-eu/assets/pdf/SH-158101-AA-PROTECT-AF-Reddy-HRS-2013.pdf. Accessed January 30, 2015.
- David Holmes M. Boston Scientific. March 9, 2013. Available at: http://www.bostonscientific.com/watchman-eu/assets/downloads/PREVAIL-Clinical-Results.ppt.pdf. Accessed January 30, 2015.
- Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Koneru JN, Badhwar N, Ellenbogen KA, Lee RJ. LAA ligation using the LARIAT suture delivery device: tips and tricks for a successful procedure. Heart Rhythm 2014; 11:911–921.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Massumi A, Chelu MG, Nazeri A, et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol 2013; 111:869–873.
- Giedrimas E, Lin AC, Knight BP. Left atrial thrombus after appendage closure using LARIAT. Circ Arrhythm Electrophysiol 2013; 6:e52–e53.
- Briceno DF, Fernando RR, Laing ST. Left atrial appendage thrombus post LARIAT closure device. Heart Rhythm 2014; 11:1600–1601.
- Baker MS, Paul Mounsey J, Gehi AK, Chung EH. Left atrial thrombus after appendage ligation with LARIAT. Heart Rhythm 2014; 11:1489.
- Pillai AM, Kanmanthareddy A, Earnest M, et al. Initial experience with post Lariat left atrial appendage leak closure with Amplatzer septal occluder device and repeat Lariat application. Heart Rhythm 2014; 11:1877–1883.
- Holmes DR Jr, Lakkireddy DR, Whitlock RP, Waksman R, Mack MJ. Left atrial appendage occlusion: opportunities and challenges. J Am Coll Cardiol 2014; 63:291–298.
- Wood S. FDA Advisors cool on Watchman approval amid ischemic-stroke data. Medscape Multispecialty October 8, 2014. www.medscape.com/viewarticle/832993.
- Gafoor S, Franke J, Bertog S, et al. Left atrial appendage occlusion in octogenarians: short-term and 1-year follow-up. Catheter Cardiovasc Interv 2014; 83:805–810.
- Rosamond W, Flegal K, Furie K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117:e25–146.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987; 147:1561–1564.
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154:1449–1457.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996; 61:755–759.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Odell JA, Blackshear JL, Davies E, et al. Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann Thorac Surg 1996; 61:565–569.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999; 131:492–501.
- Manning WJ, Silverman DI, Keighley CS, Oettgen P, Douglas PS. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol 1995; 25:1354–1361.
- Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart 1999; 82:547–554.
- Lip GY. Recommendations for thromboprophylaxis in the 2012 focused update of the ESC guidelines on atrial fibrillation: a commentary. J Thromb Haemost 2013; 11:615–626.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Onalan O, Lashevsky I, Hamad A, Crystal E. Nonpharmacologic stroke prevention in atrial fibrillation. Expert Rev Cardiovasc Ther 2005; 3:619–633.
- Brass LM, Krumholz HM, Scinto JM, Radford M. Warfarin use among patients with atrial fibrillation. Stroke 1997; 28:2382–2389.
- Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm 2009; 15:244–252.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost 2014; 111:933–942.
- Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008; 52:924–929.
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J Am Coll Cardiol 2000; 36:468–471.
- Ailawadi G, Gerdisch MW, Harvey RL, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg 2011; 142:1002–1009.e1.
- Slater AD, Tatooles AJ, Coffey A, et al. Prospective clinical study of a novel left atrial appendage occlusion device. Ann Thorac Surg 2012; 93:2035-2040.
- Pinho-Gomes AC, Amorim MJ, Oliveira SM, Leite-Moreira AF. Surgical treatment of atrial fibrillation: an updated review. Eur J Cardiothorac Surg 2014; 46:167–178.
- Aryana A, Cavaco D, Arthur A, O’Neill PG, Adragão P, D’Avila A. Percutaneous endocardial occlusion of incompletely surgically ligated left atrial appendage. J Cardiovasc Electrophysiol 2013; 24:968–974.
- García-Fernández MA, Pérez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258.
- Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 2002; 105:1887–1889.
- Ostermayer SH, Reisman M, Kramer PH, et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol 2005; 46:9–14.
- Park JW, Bethencourt A, Sievert H, et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011; 77:700–706.
- Urena M, Rodés-Cabau J, Freixa X, et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol 2013; 62:96–102.
- ClinicalTrials.gov. http://clinicaltrials.gov/show/NCT01118299. Accessed January 30, 2015.
- Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2007; 49:1490–1495.
- Holmes DR, Reddy VY, Turi ZG, et al; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
- Boston Scientific. WATCHMAN™ Left Atrial Appendage Closure Device. http://www.bostonscientific.com/watchman-eu/assets/pdf/SH-158101-AA-PROTECT-AF-Reddy-HRS-2013.pdf. Accessed January 30, 2015.
- David Holmes M. Boston Scientific. March 9, 2013. Available at: http://www.bostonscientific.com/watchman-eu/assets/downloads/PREVAIL-Clinical-Results.ppt.pdf. Accessed January 30, 2015.
- Reddy VY, Möbius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol 2013; 61:2551–2556.
- Koneru JN, Badhwar N, Ellenbogen KA, Lee RJ. LAA ligation using the LARIAT suture delivery device: tips and tricks for a successful procedure. Heart Rhythm 2014; 11:911–921.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013; 62:108–118.
- Massumi A, Chelu MG, Nazeri A, et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol 2013; 111:869–873.
- Giedrimas E, Lin AC, Knight BP. Left atrial thrombus after appendage closure using LARIAT. Circ Arrhythm Electrophysiol 2013; 6:e52–e53.
- Briceno DF, Fernando RR, Laing ST. Left atrial appendage thrombus post LARIAT closure device. Heart Rhythm 2014; 11:1600–1601.
- Baker MS, Paul Mounsey J, Gehi AK, Chung EH. Left atrial thrombus after appendage ligation with LARIAT. Heart Rhythm 2014; 11:1489.
- Pillai AM, Kanmanthareddy A, Earnest M, et al. Initial experience with post Lariat left atrial appendage leak closure with Amplatzer septal occluder device and repeat Lariat application. Heart Rhythm 2014; 11:1877–1883.
- Holmes DR Jr, Lakkireddy DR, Whitlock RP, Waksman R, Mack MJ. Left atrial appendage occlusion: opportunities and challenges. J Am Coll Cardiol 2014; 63:291–298.
- Wood S. FDA Advisors cool on Watchman approval amid ischemic-stroke data. Medscape Multispecialty October 8, 2014. www.medscape.com/viewarticle/832993.
- Gafoor S, Franke J, Bertog S, et al. Left atrial appendage occlusion in octogenarians: short-term and 1-year follow-up. Catheter Cardiovasc Interv 2014; 83:805–810.
KEY POINTS
- Few well-designed studies of surgical closure have been done.
- The Watchman percutaneous device was shown to be noninferior to warfarin in certain patients. Other closure devices demonstrate similar success, though trials have not compared them with warfarin.
- Occlusion of the left atrial appendage is an emerging option for general internists to be aware of when treating those with atrial fibrillation who cannot tolerate oral anticoagulation.