User login
IM Residency Ultrasound
Although the advent of small ultraportable bedside ultrasound devices have heralded the age of the ultrasonic stethoscope,15 realizing the widespread potential of ultrasound‐assisted physical examination68 requires the creation of an imaging protocol that can be successfully taught to all physicians within the confines of accredited medical education. Prior feasibility studies of teaching internal medical residents are characterized by heterogeneity in imaging protocols, nonrandomized enrollment of a small number of trainees, and training that is short‐lived,6, 914 making their results difficult to generalize. Few data exist on the effects of sustained incorporation of a comprehensive, structured program within a conventional 3‐year internal medicine residency.
Over the past 14 years, we have developed cardiovascular limited ultrasound examination (CLUE), with the specific purpose of detecting prevalent cardiovascular pathologies that: (1) have been shown to affect morbidity and mortality in an adult population, (2) are often missed by physical examination, and (3) have been detected by medical residents who have been taught a simplified ultrasound examination. In this report, we will detail our observations regarding CLUE and its training curriculum with assessment of proficiency, program requirements, and the overall academic effect once firmly integrated into an internal medicine residency program.
METHODS
Setting and Participants
The ultrasound training program was created at Scripps Mercy Hospital San Diego Campus, a 500‐bed community hospital in San Diego, California, for integration into a 3‐year internal medicine residency program. It was accredited by the Accreditation Council for Graduate Medical Education (ACGME) and consisted of approximately 33 residents, and 23 full‐time and 82 part‐time faculty. Since 2005, all internal medicine residents have been participating in the ultrasound training program and their progress followed as a part of the ACGME Educational Innovation Project. Of the 41 consecutive graduating residents in whom performance data were collected, no resident had prior formal training in ultrasound.
Program Overview
Based upon initial studies of performing limited echo examination,1520 the following imaging protocols were combined to comprise CLUE, a brief, quick‐look two‐dimensional multi‐targeted ultrasound examination: (1) the extracranial carotid bulb for carotid atherosclerosis, (2) parasternal long‐axis view for left ventricular systolic dysfunction and left atrial enlargement, (3) apical lung views for interstitial edema, (4) basal lung views for pleural effusion, (5) a subcostal 4‐chamber view for isolated right ventricular enlargement or pericardial effusion, (6) the longitudinal view of the inferior vena cava for elevated central venous pressures, and (7) a mid‐abdominal longitudinal view for abdominal aortic aneurysm. Evidence‐basis for the exam targets and specifics of subjective diagnostic CLUE criteria (Table 1) have been published elsewhere.2130
| Disease | Diagnostic Criteria | Pitfalls |
|---|---|---|
| ||
| 1. Carotid atheroma | Focal thickened/calcified region of plaque22 | Reduced SN for isoechoic clot or dissection; not for use in acute neurologic syndromes |
| 2. LV systolic dysfunction | Mitral anterior leaflet tip does not approach septum (<1 cm) in diastole21, 23, 26 | Reduced SN for acute or apical wall motion abnormalities; FPs due to severe aortic regurgitation, mitral stenosis |
| 3. Left atrial enlargement | LA appears larger than aortic root (AP diameter) throughout the cardiac cycle21, 2426 | Reduced SN when LA asymmetrically enlarges (elongates); FPs due to far field artifact mistaken for posterior LA wall. |
| 4. Lung comet‐tail artifact | Three or more linear artifacts extending from pleura to the far field, moving with respiration26 | Reduced SN when probe not tilted to scan perpendicular to convex apical lung surface or imaging during inspiration only. Apical comets can be present in COPD with subclinical interstitial disease |
| 5. Pleural effusion | Anechoic region above the diaphragm and below lung27, 28 | Reduced SN for small effusions when probe not placed posterior enough. FPs of ascites or gastric fluid |
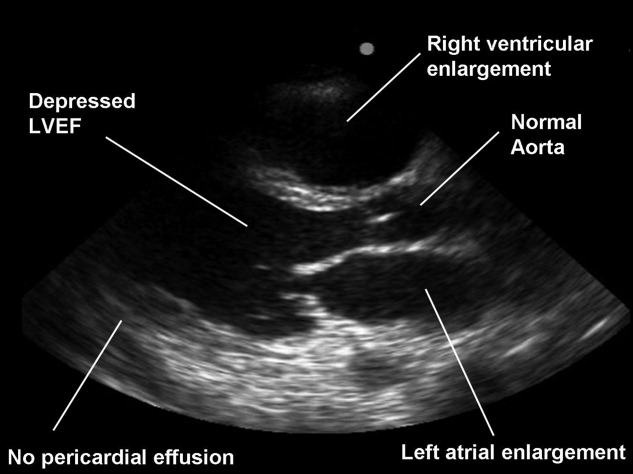
| 6. Pericardial effusion | Anechoic region seen deep to LV and above descending aorta in PLAX,15 or between the liver and RV in the subcostal view27 | FPs of an epicardial fat pad or right pleural effusion. A large effusion and dilated IVC are mandatory in the consideration of tamponade by the resident |
| 7. RV enlargement | Size (AP diameter) of the RV appears equal or greater than the LV29. | Reduced SN due to lack of imaging during a deep inspiration or due to off‐axis imaging |
| 8. IVC plethora | IVC AP diameter equals or exceeds the same‐level aortic diameter and fails to reduce size with respiration14, 26, 30 | Reduced SN when mistaking a hepatic vein for the IVC. FP when mistaking the descending aorta for a dilated IVC, particularly when IVC is collapsed. |
| 9. Abdominal aortic aneurysm | Focal dilation 1.5 the size of neighboring segment21 | Reduced SN due to bowel gas or mistaking a normal IVC for the aorta. FPs of cysts identified as aneurysmal disease |
Two useful mnemonics were created to teach the imaging protocol. If using only the 3 MHz cardiac probe, residents were taught to work backward against the flow of blood, in regards to physiologic effects and the sequence of CLUE views. Starting in the left ventricle, systolic function was first evaluated, followed by left atrial enlargement, the presence of lung comets, then lung effusions, then right ventricular enlargement, the presence of pericardial effusion, then elevation of central venous pressures. If the high‐frequency 5 MHz linear probe was available for carotid imaging, then an additional mnemonic was remembered that atherosclerotic progression increased from top to bottom in CLUE, typified by the frequent detection of early disease in the carotid bulb, then occasional cardiac manifestations, followed by the infrequent late manifestation of an abdominal aortic aneurysm. In our practice, performance of the complete CLUE starting at the top (carotids), changing transducers to work backward in the thorax (cardiac, lung, and inferior vena cava), and finishing with the bottom (aorta) was often dependent upon equipment and linear probe availability at the point‐of‐care.
A formalized CLUE curriculum was implemented into the residency in 2006. Twelve monthly 1‐hour CLUE lectures were given per year. Most lectures were 3045 minutes in length, leaving 1530 minutes for imaging resident or patient volunteers. All forms of ultrasound devices available to the residents, including pocket‐sized, hand‐carried, cart‐based, and standard ultrasound machines, were used in this forum. To learn the fundamentals of imaging technique, the intern during the cardiology consultation month rotation was first expected to image 1030 patients in the echocardiography and vascular ultrasound labs under the tutelage of the sonographers. Once weekly, 1‐hour bedside teaching was given to junior and senior residents on the intensive care unit (ICU) and cardiology consult rotations, in a traditional case‐based format. Over the ICU month rotation, junior and senior residents could each image an additional 1030 patients, resulting in a minimum of 30 studies obtained on acutely ill patients during the ICU rotations of residency. During clinical care rotations over the 3‐year residency, all residents imaged a minimum of 30 patients (at least 10 proctored studies during their internship cardiology consultation month, 10 proctored during ICU junior year rotations, and 10 proctored during ICU senior year rotations), with some residents imaging over a hundred patients (Table 2). To assist their education in CLUE, multiple learning aides were made available, including instructional how‐to‐image videos, a 200‐page syllabus, self‐assessment tests, and an instructional web site. Overall, the independent study and performance of CLUE was encouraged, but without formal performance incentives, monitoring, or effect upon residency evaluations.
| Lecture | Imaging | Other | |
|---|---|---|---|
| |||
| PGY‐1 (intern) | 12 (1‐hr) conferences; Web site instruction; syllabus; 12 (1‐hr) bedside ICU rounds | Echo lab imaging with 20 (10 proctored) studies on cardiology consults; outpatient cardiology clinics | Research; imaging in ICU, CHF, and medical clinics; ED |
| PGY‐2 (junior) | 12 (1‐hr) conferences; Web site instruction; syllabus; 8 (1‐hr) bedside ICU rounds | 20 (10 proctored) during 2 ICU rotations | Research; teaching others; imaging in CHF and medical clinics; ED; echo lab |
| PGY‐3 (senior) | 12 (1‐hr) conferences; Web site instruction; syllabus; 12 (1‐hr) bedside ICU rounds | 20 (10 proctored) during 2 ICU rotations, cardiology consults, echo lab | Research; teaching others; imaging in CHF and medical clinics; ED; CLUE‐CEX |
| Time completed (estimate) | 50 hr | 60 cases (30 proctored) | |
At our institution, the medical director of the Echocardiography and Vascular ultrasound laboratory was a cardiologist (B.J.K.) who directed the CLUE training program. The Director provided the monthly lecture series to the entire residency and was responsible for weekly 1‐hour bedside ICU rounds. If given maintenance responsibilities of weekly bedside ICU rounds (1 hour/week), monthly lecture and preparation (5 hours/month), and availability to teach the cardiology intern (3 hours/month) and maintain the Web site (4 hours/month), the program required 4 hours/week of the Director's time. The program used 3 dedicated devices: the SonoSite 180 (SonoSite, Inc, Bothell, WA), the MicroMaxx (SonoSite, Inc) and, in 2010, a pocket‐sized cardiac ultrasound stethoscope, the Vscan (GE Healthcare, Wauwatosa, WI). No patient charges were submitted for performance or interpretation of any CLUE.
Assessment and Follow‐Up
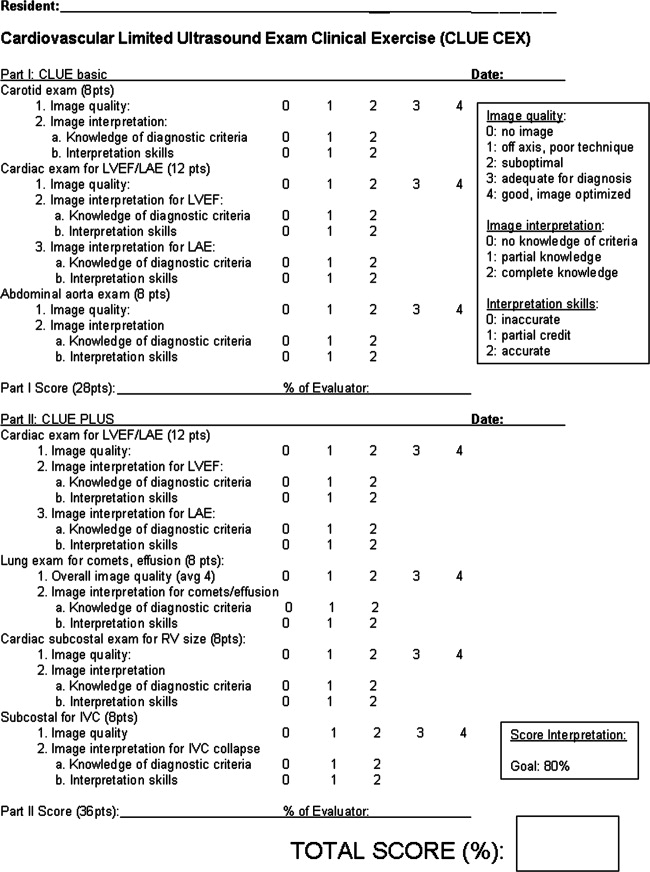
A proficiency test was performed at the end of each resident's senior year. The test, cardiovascular limited ultrasound exam‐clinical exercise (CLUE‐CEX), involved imaging any available, consenting patient and assessing the resident's technical skills by image quality, knowledge of diagnostic criteria, and ability to discuss the clinical aspects of potential findings in a question‐and‐answer oral interview format, typically requiring 2030 minutes to perform. Each resident CLUE view was rated for: (1) image quality which accounted for 44% of total exam points, (2) specific knowledge related to each view which accounted for 28% of total exam points, and (3) diagnostic accuracy of the interpretation of each view which accounted for 28% of total exam points (see Figure 1). CLUE‐CEX scores were recorded as a percentage of total possible points, normalized to the difficulty of imaging the individual patient as determined by the Director's imaging. The test encompassed performance of all 7 views, demonstrated in 2 exams employing 2 transducers (cardiac and vascular) on the same patient (Figure 1). A passing threshold had been empirically derived at >80% of the total available points, a value that: (1) required performance in all 3 categories, (2) subjectively correlated to competency when assessed by the Director, and (3) had parity with other thresholds of clinical skill assessment by faculty and in graduate education. The Director had no knowledge of non‐CLUE resident evaluations, In‐training scores, or academic performance outside of CLUE. Residents were not remanded for CLUE‐CEX failure.
The graduating class of 2011 was the first class to initially enter into an entire residency program fully immersed in the CLUE curriculum, and was therefore specifically asked to report their impression of the CLUE program after graduation through a post‐residency questionnaire. A Likert‐type scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree) was used to assess the perceived validity of the following statements: (1) CLUE improved my own bedside cardiovascular evaluation; and (2) I would use CLUE if ultrasound were available in my future position. Each resident was then asked if too much, not enough, or an appropriate amount of time was spent to learn CLUE, and to choose the most effective form of CLUE teaching to which they were exposed: didactic lectures, bedside ICU teaching, Web site/syllabus, and one‐to‐one training with the Director or sonographer.
Statistical Analysis
The CLUE experience was divided into 3 phases: (1) pre‐CLUE era, the 4‐year period (classes graduating 20022005) prior to the institution of the formal CLUE curriculum; (2) the 2‐year CLUE phase‐in period (classes graduating 20062007), in which portions of the residency were undergoing the 3‐year curriculum; (3) the 4‐year CLUE‐CEX era (classes graduating 20082011) when all residency classes were trained in the standardized fashion and underwent CLUE‐CEX assessment. In‐training postgraduate year‐3 (PGY‐3) scores, the result of a nationwide standardized test developed by the American College of Physicians, were used as representative of senior resident academic knowledge. A percentile rank score is provided to compare residents to nationwide data. The group of residents who had been selected to be the following year's chief residents had their CLUE‐CEX scores analyzed as a subgroup.
Data are presented as mean standard deviation and analyzed in SPSS, version 12.0 (SPSS, Inc, Chicago, IL). Linear regression was used to investigate the relationship between In‐training percentile ranks and CLUE‐CEX scores. Analysis of variance was used to determine any effect of gender and chief resident selection on CLUE‐CEX, and to assess average resident In‐training percentile ranks during the pre‐CLUE and CLUE‐CEX periods. Subset analysis of individual CLUE‐CEX scores was performed in regards to image quality, diagnostic knowledge, and interpretative skills. A value of P < 0.05 was considered significant.
RESULTS
Observations During CLUE Program Development
CLUE‐CEX scores (20082011) included data from 41 residents; 51% were male. In the class of 2009, one second‐year male resident transferred to another program for nonacademic reasons, reducing its number to 9. We observed that the impact of the CLUE program depended in part upon resident‐to‐resident teaching and required a critical mass of residents to be trained during a phase‐in period before a maximal effect could be appreciated. We observed that didactic knowledge occurred before imaging skills and remained dominant by graduation, with mean percentile CLUE‐CEX scores for image quality, knowledge, and interpretative accuracy at 82% 5%, 91% 3%, and 91% 8%, respectively. Residents typically found apical lung imaging the easiest to perform (CLUE‐CEX score of 89% 19%), followed by carotid (84% 18%), inferior vena cava (IVC) imaging (84% 26%), screening for abdominal aortic aneurysm (AAA) (83% 2 4%), parasternal long‐axis (79% 30%), and subcostal cardiac 4‐chamber imaging (73% 33%). Each view had technical and diagnostic pitfalls that were noted during resident practice (see Table 1), resulting in changes in our teaching and case review in subsequent years.
Residency and CLUE Performance
In attempting to achieve a CLUE proficiency score of >80% on the CLUE‐CEX in their graduating year, 8/41 (19.5%) senior residents failed. In these 8 residents, imaging quality, knowledge, and interpretative accuracy were all depressed: 55% 19%, 79% 11%, and 75% 11%, respectively. Two of these 8 had been selected as future chief residents over the 4‐year period, positions typically awarded to 2 residents per graduating year. The performance of the residents is seen in Table 3. The CLUE program did not exert a negative effect upon the academic performance of the residency, as evidenced by the lack of a significant difference in the Pre‐CLUE, 2‐year CLUE, and CLUE‐CEX periods in regards to average resident In‐training percentile rank scores (67.5 20.1, 62.3 20.5, 69.4 16.9, respectively; P = 0.37).
| Time Era (Year of Graduation) | n | Fail Rate | CLUE‐CEX (Mean SD) | Resident IT Percentile Rank (Mean SD) (Range) |
|---|---|---|---|---|
| ||||
| Pre‐CLUE (20022005) | 39 | 67.5 20.1 (2099) | ||
| Phase‐in CLUE (20062007) | 19 | 62.3 20.5 (2097) | ||
| CLUE‐CEX (20082011) | 41 | 19% | 87.4 11.9 | 69.4 16.9 (3499) |
| Year 2008 | 11 | 36% | 84.3 13.9 | 74.7 17.9 (4599) |
| Year 2009 | 9 | 11% | 89.1 7.0 | 73.0 16.6 (3493) |
| Year 2010 | 10 | 30% | 84.2 16.9 | 57.1 12.7 (4287) |
| Year 2011 | 11 | 0% | 92.1 5.7 | 72.4 15.9 (3499) |
Figure 2 shows the relationship between CLUE‐CEX scores and In‐training PGY‐3 scores. There was no significant relationship between resident academic performance and CLUE capabilities (r = 0.05, P = 0.75). Similarly, chief resident performance (n = 14) was not significantly associated with CLUE‐CEX scores (r = 0.15, P = 0.37), nor was male gender (P = 0.07). Approximately one‐half (49%) of the residents in the 4‐year CLUE‐CEX era entered fellowships, unchanged from historic rates, with only 1 resident during this era entering into a cardiology fellowship.
The Likert‐type questionnaire was returned by 11/11 graduating residents in 2011. Mean score of 4.3 0.6 (range: 35), with 6/11 responding agree, was given for the statement of whether CLUE improved the resident's own bedside exam. A score of 4.5 0.7 (range: 35), with 7/11 responding strongly agree, was given for whether the resident would use CLUE in the future if ultrasound were available. The majority (9/11) of residents felt that the time spent on CLUE was appropriate, with 2 residents responding not enough. Residents ranked one‐to‐one training with the Director(n = 6), followed by bedside ICU rounds (n = 5) as the preferred teaching methods to learn CLUE.
DISCUSSION
We report the experience of enrolling 6 consecutive classes, in an internal medicine residency, to test the feasibility of incorporating ongoing training in a specific, evidence‐based cardiovascular limited ultrasound examination within an already existing 3‐year curriculum. Using unbiased and complete enrollment, we found that residents who perform well on standardized academic testing or who are selected as chief residents do not necessarily perform more competently in CLUE, and that a significant overall initial resident failure rate can be anticipated. By questionnaire, residents felt confident in using the technique to improve their future bedside exams.
Burgeoning interest in the limited or focused application of ultrasound during bedside evaluation has already resulted in the incorporation of ultrasound training into emergency medicine residencies and critical care fellowships, with minimal standardization on curriculum, teaching methodology, or competency requirements. Given the multiple subspecialty applications for ultrasound, the potential exists of excessive diversity in bedside ultrasound practice, weakening the development of a single, simplified exam technique as a clinical tool for all physicians.31 Prior feasibility studies914 have evaluated the learning curve of internal medicine or primary care residents in performing various limited exams, but have not provided the rationale regarding the imaging protocols, the methods used for teaching, and the assessment of the program results over a sustained period of time. Furthermore, prior studies have not randomized subject trainees, likely resulting in the selected enrollment of highly motivated or skilled residents who want to perform a particular technique or have a bias to learn it. Our reported 19% unremanded failure rate on CLUE‐CEX will likely be more reflective of the general experience when initially integrating entire classes of internal medicine residents into a standard curriculum. The feasibility of introducing ultrasound at an earlier stage than residency may improve familiarity with the modality, and a 4‐year medical‐student curriculum has been recently described.32 Although introduction in medical school could allow for more adept and specific clinical training during residency, the optimal time for education in bedside ultrasound remains unclear.
Critical to the development of our program was the necessity to commit to teaching a single exam, the CLUE. We derived CLUE to quickly screen for important targets that had evidence‐basis to affect outcome, such as manifestations of subclinical atherosclerosis or chamber enlargement due to elevated filling pressures. Subsequent CLUE outcome studies have demonstrated diagnostic accuracy and prognostic value in its components,18, 26, 29, 30 and an effect upon medical decision‐making,21 even when performed by briefly trained novices.18, 21, 30 It is anticipated that this cardiovascular examination will later expand to a more advanced version or become a component of a full‐body ultrasound‐assisted physical. Therefore, evidence‐basis and brevity governed the development of a practical and teachable fundamental CLUE, and our skill assessment results are likely specific to CLUE itself.
This report contains primarily observations noted during the development of our program, written in retrospect with emphasis on real world feasibility. It was not a rigorous evaluation of specific ultrasound teaching methods. We found that training is feasible, at modest costs, when existing in‐hospital resources are utilized and include a part‐time faculty appointment and shared devices. Training the sonographers to perform CLUE as a part of the standard echocardiogram was a trivial task, but created the great benefit of being able to retrospectively review both the CLUE and formal echo in case review and teaching. Monthly CLUE lectures in the daily noon conference docket, and the use of the cardiology consultation and ICU rotations, allowed integration of the CLUE curriculum into preexisting venues and persistent practice opportunities within the residency. To prevent bias, we intentionally did not track, bring attention to, or incentivize resident performance in CLUE over any other topic; therefore, we can only approximate lecture and bedside teaching hours spent by each resident in light of detractions due to residency hour restrictions, vacations, and away rotations (Table 2). The CLUE‐CEX, although subject to the biases of any subjective resident skill assessment, was easily accomplished using a single form and faculty member, and was an efficient tool for program feedback and development.
In conclusion, we report the feasibility of sustained incorporation of an ultrasound training program in an internal medicine residency. We await studies regarding clinical outcome and validation of similar experiences in larger, multicenter programs.
Acknowledgements
The authors acknowledge the sonographers of the Scripps Mercy Cardiovascular Ultrasound Laboratory and Dudie Keane, for their dedication and assistance in the implementation of the CLUE program.
Disclosure: Nothing to report.
Note: The correction that was made, was the text in Fig. 1 and Fig. 2 were reversed. This article was published online on May 17, 2012. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected on May 22, 2012.
- ,,,,.Diagnostic performance of a pocket‐sized ultrasound device for quick‐look cardiac imaging.Am J Emerg Med. 2012;30(1):32–36.
- ,,,,,.New pocket echocardiography device is interchangeable with high‐end portable system when performed by experienced examiners.Acta Anaesthesiol Scand.2010;54(10):1217–1223.
- ,,, et al.Usefulness of a new miniaturized echocardiographic system in outpatient cardiology consultations as an extension of physical examination.J Am Soc Echocardiogr.2011;24(2):117–124.
- ,.Diagnostic accuracy of a hand‐held ultrasound scanner in routine patients referred for echocardiography.J Am Soc Echocardiogr.2011;24(2):111–116.
- ,,,,,.is pocket mobile echocardiography the next‐generation stethoscope? A cross‐sectional comparison of rapidly acquired images with standard transthoracic echocardiography.Ann Intern Med.2011;155(1):33–38.
- ,.Hand‐carried ultrasound: evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2:217–223.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr Rev.1998;7:79–81.
- .A personal ultrasound imager (ultrasound stethoscope): a revolution in the physical cardiac diagnosis!Eur Heart J.2002;23:523–527.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Ultrasonography performed by primary care residents for abdominal aortic ultrasound screening.J Gen Intern Med.2001;16:845–849.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126:693–701.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Med.2000;108:331–333.
- ,.Indications for limited echo imaging: a mathematical model.J Am Soc Echocardiogr.2000;13:855–861.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,.Time requirements of the standard echocardiogram: implications regarding “limited” studies.J Am Soc Echocardiogr.2003;16:1015–1018.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100:321–325.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol2002;90(9):1038–1039.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices: implications for bedside examination.Am J Med.2005;118(8):912–916.
- ,,,,.A hand‐carried ultrasound sign of cardiac disease: the left atrium‐to‐aorta diastolic ratio.Am J Emerg Med.2010;28(2):203–207.
- ,,,,,.A cardiopulmonary limited ultrasound examination for “quick‐look” bedside application.Am J Cardiol.2011;108:586–590.
- ,,, et al.Focused Assessment with Sonography for Trauma (FAST): results from an international consensus conference.J Trauma.1999;46:466–472.
- ,.The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure.J Am Coll Cardiol.2000;35:1638–1646.
- ,,,,,.Prognostic value of echocardiographic right/left ventricular end‐diastolic diameter ration in patients with acute pulmonary embolism.Chest.2008;133:358–362.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,,.Hospitalist use of hand‐carried ultrasound: preparing for battle.J Hosp Med.2010;5:163–167.
- ,,, et al.An integrated ultrasound curriculum (iUSC) for medical students: 4‐year experience.Crit Ultrasound J.2011;3(1):1–12.
Although the advent of small ultraportable bedside ultrasound devices have heralded the age of the ultrasonic stethoscope,15 realizing the widespread potential of ultrasound‐assisted physical examination68 requires the creation of an imaging protocol that can be successfully taught to all physicians within the confines of accredited medical education. Prior feasibility studies of teaching internal medical residents are characterized by heterogeneity in imaging protocols, nonrandomized enrollment of a small number of trainees, and training that is short‐lived,6, 914 making their results difficult to generalize. Few data exist on the effects of sustained incorporation of a comprehensive, structured program within a conventional 3‐year internal medicine residency.
Over the past 14 years, we have developed cardiovascular limited ultrasound examination (CLUE), with the specific purpose of detecting prevalent cardiovascular pathologies that: (1) have been shown to affect morbidity and mortality in an adult population, (2) are often missed by physical examination, and (3) have been detected by medical residents who have been taught a simplified ultrasound examination. In this report, we will detail our observations regarding CLUE and its training curriculum with assessment of proficiency, program requirements, and the overall academic effect once firmly integrated into an internal medicine residency program.
METHODS
Setting and Participants
The ultrasound training program was created at Scripps Mercy Hospital San Diego Campus, a 500‐bed community hospital in San Diego, California, for integration into a 3‐year internal medicine residency program. It was accredited by the Accreditation Council for Graduate Medical Education (ACGME) and consisted of approximately 33 residents, and 23 full‐time and 82 part‐time faculty. Since 2005, all internal medicine residents have been participating in the ultrasound training program and their progress followed as a part of the ACGME Educational Innovation Project. Of the 41 consecutive graduating residents in whom performance data were collected, no resident had prior formal training in ultrasound.
Program Overview
Based upon initial studies of performing limited echo examination,1520 the following imaging protocols were combined to comprise CLUE, a brief, quick‐look two‐dimensional multi‐targeted ultrasound examination: (1) the extracranial carotid bulb for carotid atherosclerosis, (2) parasternal long‐axis view for left ventricular systolic dysfunction and left atrial enlargement, (3) apical lung views for interstitial edema, (4) basal lung views for pleural effusion, (5) a subcostal 4‐chamber view for isolated right ventricular enlargement or pericardial effusion, (6) the longitudinal view of the inferior vena cava for elevated central venous pressures, and (7) a mid‐abdominal longitudinal view for abdominal aortic aneurysm. Evidence‐basis for the exam targets and specifics of subjective diagnostic CLUE criteria (Table 1) have been published elsewhere.2130
| Disease | Diagnostic Criteria | Pitfalls |
|---|---|---|
| ||
| 1. Carotid atheroma | Focal thickened/calcified region of plaque22 | Reduced SN for isoechoic clot or dissection; not for use in acute neurologic syndromes |
| 2. LV systolic dysfunction | Mitral anterior leaflet tip does not approach septum (<1 cm) in diastole21, 23, 26 | Reduced SN for acute or apical wall motion abnormalities; FPs due to severe aortic regurgitation, mitral stenosis |
| 3. Left atrial enlargement | LA appears larger than aortic root (AP diameter) throughout the cardiac cycle21, 2426 | Reduced SN when LA asymmetrically enlarges (elongates); FPs due to far field artifact mistaken for posterior LA wall. |
| 4. Lung comet‐tail artifact | Three or more linear artifacts extending from pleura to the far field, moving with respiration26 | Reduced SN when probe not tilted to scan perpendicular to convex apical lung surface or imaging during inspiration only. Apical comets can be present in COPD with subclinical interstitial disease |
| 5. Pleural effusion | Anechoic region above the diaphragm and below lung27, 28 | Reduced SN for small effusions when probe not placed posterior enough. FPs of ascites or gastric fluid |
| 6. Pericardial effusion | Anechoic region seen deep to LV and above descending aorta in PLAX,15 or between the liver and RV in the subcostal view27 | FPs of an epicardial fat pad or right pleural effusion. A large effusion and dilated IVC are mandatory in the consideration of tamponade by the resident |
| 7. RV enlargement | Size (AP diameter) of the RV appears equal or greater than the LV29. | Reduced SN due to lack of imaging during a deep inspiration or due to off‐axis imaging |
| 8. IVC plethora | IVC AP diameter equals or exceeds the same‐level aortic diameter and fails to reduce size with respiration14, 26, 30 | Reduced SN when mistaking a hepatic vein for the IVC. FP when mistaking the descending aorta for a dilated IVC, particularly when IVC is collapsed. |
| 9. Abdominal aortic aneurysm | Focal dilation 1.5 the size of neighboring segment21 | Reduced SN due to bowel gas or mistaking a normal IVC for the aorta. FPs of cysts identified as aneurysmal disease |
Two useful mnemonics were created to teach the imaging protocol. If using only the 3 MHz cardiac probe, residents were taught to work backward against the flow of blood, in regards to physiologic effects and the sequence of CLUE views. Starting in the left ventricle, systolic function was first evaluated, followed by left atrial enlargement, the presence of lung comets, then lung effusions, then right ventricular enlargement, the presence of pericardial effusion, then elevation of central venous pressures. If the high‐frequency 5 MHz linear probe was available for carotid imaging, then an additional mnemonic was remembered that atherosclerotic progression increased from top to bottom in CLUE, typified by the frequent detection of early disease in the carotid bulb, then occasional cardiac manifestations, followed by the infrequent late manifestation of an abdominal aortic aneurysm. In our practice, performance of the complete CLUE starting at the top (carotids), changing transducers to work backward in the thorax (cardiac, lung, and inferior vena cava), and finishing with the bottom (aorta) was often dependent upon equipment and linear probe availability at the point‐of‐care.
A formalized CLUE curriculum was implemented into the residency in 2006. Twelve monthly 1‐hour CLUE lectures were given per year. Most lectures were 3045 minutes in length, leaving 1530 minutes for imaging resident or patient volunteers. All forms of ultrasound devices available to the residents, including pocket‐sized, hand‐carried, cart‐based, and standard ultrasound machines, were used in this forum. To learn the fundamentals of imaging technique, the intern during the cardiology consultation month rotation was first expected to image 1030 patients in the echocardiography and vascular ultrasound labs under the tutelage of the sonographers. Once weekly, 1‐hour bedside teaching was given to junior and senior residents on the intensive care unit (ICU) and cardiology consult rotations, in a traditional case‐based format. Over the ICU month rotation, junior and senior residents could each image an additional 1030 patients, resulting in a minimum of 30 studies obtained on acutely ill patients during the ICU rotations of residency. During clinical care rotations over the 3‐year residency, all residents imaged a minimum of 30 patients (at least 10 proctored studies during their internship cardiology consultation month, 10 proctored during ICU junior year rotations, and 10 proctored during ICU senior year rotations), with some residents imaging over a hundred patients (Table 2). To assist their education in CLUE, multiple learning aides were made available, including instructional how‐to‐image videos, a 200‐page syllabus, self‐assessment tests, and an instructional web site. Overall, the independent study and performance of CLUE was encouraged, but without formal performance incentives, monitoring, or effect upon residency evaluations.
| Lecture | Imaging | Other | |
|---|---|---|---|
| |||
| PGY‐1 (intern) | 12 (1‐hr) conferences; Web site instruction; syllabus; 12 (1‐hr) bedside ICU rounds | Echo lab imaging with 20 (10 proctored) studies on cardiology consults; outpatient cardiology clinics | Research; imaging in ICU, CHF, and medical clinics; ED |
| PGY‐2 (junior) | 12 (1‐hr) conferences; Web site instruction; syllabus; 8 (1‐hr) bedside ICU rounds | 20 (10 proctored) during 2 ICU rotations | Research; teaching others; imaging in CHF and medical clinics; ED; echo lab |
| PGY‐3 (senior) | 12 (1‐hr) conferences; Web site instruction; syllabus; 12 (1‐hr) bedside ICU rounds | 20 (10 proctored) during 2 ICU rotations, cardiology consults, echo lab | Research; teaching others; imaging in CHF and medical clinics; ED; CLUE‐CEX |
| Time completed (estimate) | 50 hr | 60 cases (30 proctored) | |
At our institution, the medical director of the Echocardiography and Vascular ultrasound laboratory was a cardiologist (B.J.K.) who directed the CLUE training program. The Director provided the monthly lecture series to the entire residency and was responsible for weekly 1‐hour bedside ICU rounds. If given maintenance responsibilities of weekly bedside ICU rounds (1 hour/week), monthly lecture and preparation (5 hours/month), and availability to teach the cardiology intern (3 hours/month) and maintain the Web site (4 hours/month), the program required 4 hours/week of the Director's time. The program used 3 dedicated devices: the SonoSite 180 (SonoSite, Inc, Bothell, WA), the MicroMaxx (SonoSite, Inc) and, in 2010, a pocket‐sized cardiac ultrasound stethoscope, the Vscan (GE Healthcare, Wauwatosa, WI). No patient charges were submitted for performance or interpretation of any CLUE.
Assessment and Follow‐Up
A proficiency test was performed at the end of each resident's senior year. The test, cardiovascular limited ultrasound exam‐clinical exercise (CLUE‐CEX), involved imaging any available, consenting patient and assessing the resident's technical skills by image quality, knowledge of diagnostic criteria, and ability to discuss the clinical aspects of potential findings in a question‐and‐answer oral interview format, typically requiring 2030 minutes to perform. Each resident CLUE view was rated for: (1) image quality which accounted for 44% of total exam points, (2) specific knowledge related to each view which accounted for 28% of total exam points, and (3) diagnostic accuracy of the interpretation of each view which accounted for 28% of total exam points (see Figure 1). CLUE‐CEX scores were recorded as a percentage of total possible points, normalized to the difficulty of imaging the individual patient as determined by the Director's imaging. The test encompassed performance of all 7 views, demonstrated in 2 exams employing 2 transducers (cardiac and vascular) on the same patient (Figure 1). A passing threshold had been empirically derived at >80% of the total available points, a value that: (1) required performance in all 3 categories, (2) subjectively correlated to competency when assessed by the Director, and (3) had parity with other thresholds of clinical skill assessment by faculty and in graduate education. The Director had no knowledge of non‐CLUE resident evaluations, In‐training scores, or academic performance outside of CLUE. Residents were not remanded for CLUE‐CEX failure.

The graduating class of 2011 was the first class to initially enter into an entire residency program fully immersed in the CLUE curriculum, and was therefore specifically asked to report their impression of the CLUE program after graduation through a post‐residency questionnaire. A Likert‐type scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree) was used to assess the perceived validity of the following statements: (1) CLUE improved my own bedside cardiovascular evaluation; and (2) I would use CLUE if ultrasound were available in my future position. Each resident was then asked if too much, not enough, or an appropriate amount of time was spent to learn CLUE, and to choose the most effective form of CLUE teaching to which they were exposed: didactic lectures, bedside ICU teaching, Web site/syllabus, and one‐to‐one training with the Director or sonographer.
Statistical Analysis
The CLUE experience was divided into 3 phases: (1) pre‐CLUE era, the 4‐year period (classes graduating 20022005) prior to the institution of the formal CLUE curriculum; (2) the 2‐year CLUE phase‐in period (classes graduating 20062007), in which portions of the residency were undergoing the 3‐year curriculum; (3) the 4‐year CLUE‐CEX era (classes graduating 20082011) when all residency classes were trained in the standardized fashion and underwent CLUE‐CEX assessment. In‐training postgraduate year‐3 (PGY‐3) scores, the result of a nationwide standardized test developed by the American College of Physicians, were used as representative of senior resident academic knowledge. A percentile rank score is provided to compare residents to nationwide data. The group of residents who had been selected to be the following year's chief residents had their CLUE‐CEX scores analyzed as a subgroup.
Data are presented as mean standard deviation and analyzed in SPSS, version 12.0 (SPSS, Inc, Chicago, IL). Linear regression was used to investigate the relationship between In‐training percentile ranks and CLUE‐CEX scores. Analysis of variance was used to determine any effect of gender and chief resident selection on CLUE‐CEX, and to assess average resident In‐training percentile ranks during the pre‐CLUE and CLUE‐CEX periods. Subset analysis of individual CLUE‐CEX scores was performed in regards to image quality, diagnostic knowledge, and interpretative skills. A value of P < 0.05 was considered significant.
RESULTS
Observations During CLUE Program Development
CLUE‐CEX scores (20082011) included data from 41 residents; 51% were male. In the class of 2009, one second‐year male resident transferred to another program for nonacademic reasons, reducing its number to 9. We observed that the impact of the CLUE program depended in part upon resident‐to‐resident teaching and required a critical mass of residents to be trained during a phase‐in period before a maximal effect could be appreciated. We observed that didactic knowledge occurred before imaging skills and remained dominant by graduation, with mean percentile CLUE‐CEX scores for image quality, knowledge, and interpretative accuracy at 82% 5%, 91% 3%, and 91% 8%, respectively. Residents typically found apical lung imaging the easiest to perform (CLUE‐CEX score of 89% 19%), followed by carotid (84% 18%), inferior vena cava (IVC) imaging (84% 26%), screening for abdominal aortic aneurysm (AAA) (83% 2 4%), parasternal long‐axis (79% 30%), and subcostal cardiac 4‐chamber imaging (73% 33%). Each view had technical and diagnostic pitfalls that were noted during resident practice (see Table 1), resulting in changes in our teaching and case review in subsequent years.
Residency and CLUE Performance
In attempting to achieve a CLUE proficiency score of >80% on the CLUE‐CEX in their graduating year, 8/41 (19.5%) senior residents failed. In these 8 residents, imaging quality, knowledge, and interpretative accuracy were all depressed: 55% 19%, 79% 11%, and 75% 11%, respectively. Two of these 8 had been selected as future chief residents over the 4‐year period, positions typically awarded to 2 residents per graduating year. The performance of the residents is seen in Table 3. The CLUE program did not exert a negative effect upon the academic performance of the residency, as evidenced by the lack of a significant difference in the Pre‐CLUE, 2‐year CLUE, and CLUE‐CEX periods in regards to average resident In‐training percentile rank scores (67.5 20.1, 62.3 20.5, 69.4 16.9, respectively; P = 0.37).
| Time Era (Year of Graduation) | n | Fail Rate | CLUE‐CEX (Mean SD) | Resident IT Percentile Rank (Mean SD) (Range) |
|---|---|---|---|---|
| ||||
| Pre‐CLUE (20022005) | 39 | 67.5 20.1 (2099) | ||
| Phase‐in CLUE (20062007) | 19 | 62.3 20.5 (2097) | ||
| CLUE‐CEX (20082011) | 41 | 19% | 87.4 11.9 | 69.4 16.9 (3499) |
| Year 2008 | 11 | 36% | 84.3 13.9 | 74.7 17.9 (4599) |
| Year 2009 | 9 | 11% | 89.1 7.0 | 73.0 16.6 (3493) |
| Year 2010 | 10 | 30% | 84.2 16.9 | 57.1 12.7 (4287) |
| Year 2011 | 11 | 0% | 92.1 5.7 | 72.4 15.9 (3499) |
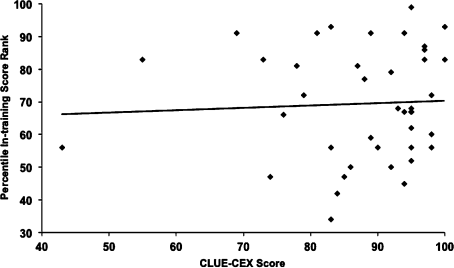
Figure 2 shows the relationship between CLUE‐CEX scores and In‐training PGY‐3 scores. There was no significant relationship between resident academic performance and CLUE capabilities (r = 0.05, P = 0.75). Similarly, chief resident performance (n = 14) was not significantly associated with CLUE‐CEX scores (r = 0.15, P = 0.37), nor was male gender (P = 0.07). Approximately one‐half (49%) of the residents in the 4‐year CLUE‐CEX era entered fellowships, unchanged from historic rates, with only 1 resident during this era entering into a cardiology fellowship.

The Likert‐type questionnaire was returned by 11/11 graduating residents in 2011. Mean score of 4.3 0.6 (range: 35), with 6/11 responding agree, was given for the statement of whether CLUE improved the resident's own bedside exam. A score of 4.5 0.7 (range: 35), with 7/11 responding strongly agree, was given for whether the resident would use CLUE in the future if ultrasound were available. The majority (9/11) of residents felt that the time spent on CLUE was appropriate, with 2 residents responding not enough. Residents ranked one‐to‐one training with the Director(n = 6), followed by bedside ICU rounds (n = 5) as the preferred teaching methods to learn CLUE.
DISCUSSION
We report the experience of enrolling 6 consecutive classes, in an internal medicine residency, to test the feasibility of incorporating ongoing training in a specific, evidence‐based cardiovascular limited ultrasound examination within an already existing 3‐year curriculum. Using unbiased and complete enrollment, we found that residents who perform well on standardized academic testing or who are selected as chief residents do not necessarily perform more competently in CLUE, and that a significant overall initial resident failure rate can be anticipated. By questionnaire, residents felt confident in using the technique to improve their future bedside exams.
Burgeoning interest in the limited or focused application of ultrasound during bedside evaluation has already resulted in the incorporation of ultrasound training into emergency medicine residencies and critical care fellowships, with minimal standardization on curriculum, teaching methodology, or competency requirements. Given the multiple subspecialty applications for ultrasound, the potential exists of excessive diversity in bedside ultrasound practice, weakening the development of a single, simplified exam technique as a clinical tool for all physicians.31 Prior feasibility studies914 have evaluated the learning curve of internal medicine or primary care residents in performing various limited exams, but have not provided the rationale regarding the imaging protocols, the methods used for teaching, and the assessment of the program results over a sustained period of time. Furthermore, prior studies have not randomized subject trainees, likely resulting in the selected enrollment of highly motivated or skilled residents who want to perform a particular technique or have a bias to learn it. Our reported 19% unremanded failure rate on CLUE‐CEX will likely be more reflective of the general experience when initially integrating entire classes of internal medicine residents into a standard curriculum. The feasibility of introducing ultrasound at an earlier stage than residency may improve familiarity with the modality, and a 4‐year medical‐student curriculum has been recently described.32 Although introduction in medical school could allow for more adept and specific clinical training during residency, the optimal time for education in bedside ultrasound remains unclear.
Critical to the development of our program was the necessity to commit to teaching a single exam, the CLUE. We derived CLUE to quickly screen for important targets that had evidence‐basis to affect outcome, such as manifestations of subclinical atherosclerosis or chamber enlargement due to elevated filling pressures. Subsequent CLUE outcome studies have demonstrated diagnostic accuracy and prognostic value in its components,18, 26, 29, 30 and an effect upon medical decision‐making,21 even when performed by briefly trained novices.18, 21, 30 It is anticipated that this cardiovascular examination will later expand to a more advanced version or become a component of a full‐body ultrasound‐assisted physical. Therefore, evidence‐basis and brevity governed the development of a practical and teachable fundamental CLUE, and our skill assessment results are likely specific to CLUE itself.
This report contains primarily observations noted during the development of our program, written in retrospect with emphasis on real world feasibility. It was not a rigorous evaluation of specific ultrasound teaching methods. We found that training is feasible, at modest costs, when existing in‐hospital resources are utilized and include a part‐time faculty appointment and shared devices. Training the sonographers to perform CLUE as a part of the standard echocardiogram was a trivial task, but created the great benefit of being able to retrospectively review both the CLUE and formal echo in case review and teaching. Monthly CLUE lectures in the daily noon conference docket, and the use of the cardiology consultation and ICU rotations, allowed integration of the CLUE curriculum into preexisting venues and persistent practice opportunities within the residency. To prevent bias, we intentionally did not track, bring attention to, or incentivize resident performance in CLUE over any other topic; therefore, we can only approximate lecture and bedside teaching hours spent by each resident in light of detractions due to residency hour restrictions, vacations, and away rotations (Table 2). The CLUE‐CEX, although subject to the biases of any subjective resident skill assessment, was easily accomplished using a single form and faculty member, and was an efficient tool for program feedback and development.
In conclusion, we report the feasibility of sustained incorporation of an ultrasound training program in an internal medicine residency. We await studies regarding clinical outcome and validation of similar experiences in larger, multicenter programs.
Acknowledgements
The authors acknowledge the sonographers of the Scripps Mercy Cardiovascular Ultrasound Laboratory and Dudie Keane, for their dedication and assistance in the implementation of the CLUE program.
Disclosure: Nothing to report.
Note: The correction that was made, was the text in Fig. 1 and Fig. 2 were reversed. This article was published online on May 17, 2012. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected on May 22, 2012.
Although the advent of small ultraportable bedside ultrasound devices have heralded the age of the ultrasonic stethoscope,15 realizing the widespread potential of ultrasound‐assisted physical examination68 requires the creation of an imaging protocol that can be successfully taught to all physicians within the confines of accredited medical education. Prior feasibility studies of teaching internal medical residents are characterized by heterogeneity in imaging protocols, nonrandomized enrollment of a small number of trainees, and training that is short‐lived,6, 914 making their results difficult to generalize. Few data exist on the effects of sustained incorporation of a comprehensive, structured program within a conventional 3‐year internal medicine residency.
Over the past 14 years, we have developed cardiovascular limited ultrasound examination (CLUE), with the specific purpose of detecting prevalent cardiovascular pathologies that: (1) have been shown to affect morbidity and mortality in an adult population, (2) are often missed by physical examination, and (3) have been detected by medical residents who have been taught a simplified ultrasound examination. In this report, we will detail our observations regarding CLUE and its training curriculum with assessment of proficiency, program requirements, and the overall academic effect once firmly integrated into an internal medicine residency program.
METHODS
Setting and Participants
The ultrasound training program was created at Scripps Mercy Hospital San Diego Campus, a 500‐bed community hospital in San Diego, California, for integration into a 3‐year internal medicine residency program. It was accredited by the Accreditation Council for Graduate Medical Education (ACGME) and consisted of approximately 33 residents, and 23 full‐time and 82 part‐time faculty. Since 2005, all internal medicine residents have been participating in the ultrasound training program and their progress followed as a part of the ACGME Educational Innovation Project. Of the 41 consecutive graduating residents in whom performance data were collected, no resident had prior formal training in ultrasound.
Program Overview
Based upon initial studies of performing limited echo examination,1520 the following imaging protocols were combined to comprise CLUE, a brief, quick‐look two‐dimensional multi‐targeted ultrasound examination: (1) the extracranial carotid bulb for carotid atherosclerosis, (2) parasternal long‐axis view for left ventricular systolic dysfunction and left atrial enlargement, (3) apical lung views for interstitial edema, (4) basal lung views for pleural effusion, (5) a subcostal 4‐chamber view for isolated right ventricular enlargement or pericardial effusion, (6) the longitudinal view of the inferior vena cava for elevated central venous pressures, and (7) a mid‐abdominal longitudinal view for abdominal aortic aneurysm. Evidence‐basis for the exam targets and specifics of subjective diagnostic CLUE criteria (Table 1) have been published elsewhere.2130
| Disease | Diagnostic Criteria | Pitfalls |
|---|---|---|
| ||
| 1. Carotid atheroma | Focal thickened/calcified region of plaque22 | Reduced SN for isoechoic clot or dissection; not for use in acute neurologic syndromes |
| 2. LV systolic dysfunction | Mitral anterior leaflet tip does not approach septum (<1 cm) in diastole21, 23, 26 | Reduced SN for acute or apical wall motion abnormalities; FPs due to severe aortic regurgitation, mitral stenosis |
| 3. Left atrial enlargement | LA appears larger than aortic root (AP diameter) throughout the cardiac cycle21, 2426 | Reduced SN when LA asymmetrically enlarges (elongates); FPs due to far field artifact mistaken for posterior LA wall. |
| 4. Lung comet‐tail artifact | Three or more linear artifacts extending from pleura to the far field, moving with respiration26 | Reduced SN when probe not tilted to scan perpendicular to convex apical lung surface or imaging during inspiration only. Apical comets can be present in COPD with subclinical interstitial disease |
| 5. Pleural effusion | Anechoic region above the diaphragm and below lung27, 28 | Reduced SN for small effusions when probe not placed posterior enough. FPs of ascites or gastric fluid |
| 6. Pericardial effusion | Anechoic region seen deep to LV and above descending aorta in PLAX,15 or between the liver and RV in the subcostal view27 | FPs of an epicardial fat pad or right pleural effusion. A large effusion and dilated IVC are mandatory in the consideration of tamponade by the resident |
| 7. RV enlargement | Size (AP diameter) of the RV appears equal or greater than the LV29. | Reduced SN due to lack of imaging during a deep inspiration or due to off‐axis imaging |
| 8. IVC plethora | IVC AP diameter equals or exceeds the same‐level aortic diameter and fails to reduce size with respiration14, 26, 30 | Reduced SN when mistaking a hepatic vein for the IVC. FP when mistaking the descending aorta for a dilated IVC, particularly when IVC is collapsed. |
| 9. Abdominal aortic aneurysm | Focal dilation 1.5 the size of neighboring segment21 | Reduced SN due to bowel gas or mistaking a normal IVC for the aorta. FPs of cysts identified as aneurysmal disease |
Two useful mnemonics were created to teach the imaging protocol. If using only the 3 MHz cardiac probe, residents were taught to work backward against the flow of blood, in regards to physiologic effects and the sequence of CLUE views. Starting in the left ventricle, systolic function was first evaluated, followed by left atrial enlargement, the presence of lung comets, then lung effusions, then right ventricular enlargement, the presence of pericardial effusion, then elevation of central venous pressures. If the high‐frequency 5 MHz linear probe was available for carotid imaging, then an additional mnemonic was remembered that atherosclerotic progression increased from top to bottom in CLUE, typified by the frequent detection of early disease in the carotid bulb, then occasional cardiac manifestations, followed by the infrequent late manifestation of an abdominal aortic aneurysm. In our practice, performance of the complete CLUE starting at the top (carotids), changing transducers to work backward in the thorax (cardiac, lung, and inferior vena cava), and finishing with the bottom (aorta) was often dependent upon equipment and linear probe availability at the point‐of‐care.
A formalized CLUE curriculum was implemented into the residency in 2006. Twelve monthly 1‐hour CLUE lectures were given per year. Most lectures were 3045 minutes in length, leaving 1530 minutes for imaging resident or patient volunteers. All forms of ultrasound devices available to the residents, including pocket‐sized, hand‐carried, cart‐based, and standard ultrasound machines, were used in this forum. To learn the fundamentals of imaging technique, the intern during the cardiology consultation month rotation was first expected to image 1030 patients in the echocardiography and vascular ultrasound labs under the tutelage of the sonographers. Once weekly, 1‐hour bedside teaching was given to junior and senior residents on the intensive care unit (ICU) and cardiology consult rotations, in a traditional case‐based format. Over the ICU month rotation, junior and senior residents could each image an additional 1030 patients, resulting in a minimum of 30 studies obtained on acutely ill patients during the ICU rotations of residency. During clinical care rotations over the 3‐year residency, all residents imaged a minimum of 30 patients (at least 10 proctored studies during their internship cardiology consultation month, 10 proctored during ICU junior year rotations, and 10 proctored during ICU senior year rotations), with some residents imaging over a hundred patients (Table 2). To assist their education in CLUE, multiple learning aides were made available, including instructional how‐to‐image videos, a 200‐page syllabus, self‐assessment tests, and an instructional web site. Overall, the independent study and performance of CLUE was encouraged, but without formal performance incentives, monitoring, or effect upon residency evaluations.
| Lecture | Imaging | Other | |
|---|---|---|---|
| |||
| PGY‐1 (intern) | 12 (1‐hr) conferences; Web site instruction; syllabus; 12 (1‐hr) bedside ICU rounds | Echo lab imaging with 20 (10 proctored) studies on cardiology consults; outpatient cardiology clinics | Research; imaging in ICU, CHF, and medical clinics; ED |
| PGY‐2 (junior) | 12 (1‐hr) conferences; Web site instruction; syllabus; 8 (1‐hr) bedside ICU rounds | 20 (10 proctored) during 2 ICU rotations | Research; teaching others; imaging in CHF and medical clinics; ED; echo lab |
| PGY‐3 (senior) | 12 (1‐hr) conferences; Web site instruction; syllabus; 12 (1‐hr) bedside ICU rounds | 20 (10 proctored) during 2 ICU rotations, cardiology consults, echo lab | Research; teaching others; imaging in CHF and medical clinics; ED; CLUE‐CEX |
| Time completed (estimate) | 50 hr | 60 cases (30 proctored) | |
At our institution, the medical director of the Echocardiography and Vascular ultrasound laboratory was a cardiologist (B.J.K.) who directed the CLUE training program. The Director provided the monthly lecture series to the entire residency and was responsible for weekly 1‐hour bedside ICU rounds. If given maintenance responsibilities of weekly bedside ICU rounds (1 hour/week), monthly lecture and preparation (5 hours/month), and availability to teach the cardiology intern (3 hours/month) and maintain the Web site (4 hours/month), the program required 4 hours/week of the Director's time. The program used 3 dedicated devices: the SonoSite 180 (SonoSite, Inc, Bothell, WA), the MicroMaxx (SonoSite, Inc) and, in 2010, a pocket‐sized cardiac ultrasound stethoscope, the Vscan (GE Healthcare, Wauwatosa, WI). No patient charges were submitted for performance or interpretation of any CLUE.
Assessment and Follow‐Up
A proficiency test was performed at the end of each resident's senior year. The test, cardiovascular limited ultrasound exam‐clinical exercise (CLUE‐CEX), involved imaging any available, consenting patient and assessing the resident's technical skills by image quality, knowledge of diagnostic criteria, and ability to discuss the clinical aspects of potential findings in a question‐and‐answer oral interview format, typically requiring 2030 minutes to perform. Each resident CLUE view was rated for: (1) image quality which accounted for 44% of total exam points, (2) specific knowledge related to each view which accounted for 28% of total exam points, and (3) diagnostic accuracy of the interpretation of each view which accounted for 28% of total exam points (see Figure 1). CLUE‐CEX scores were recorded as a percentage of total possible points, normalized to the difficulty of imaging the individual patient as determined by the Director's imaging. The test encompassed performance of all 7 views, demonstrated in 2 exams employing 2 transducers (cardiac and vascular) on the same patient (Figure 1). A passing threshold had been empirically derived at >80% of the total available points, a value that: (1) required performance in all 3 categories, (2) subjectively correlated to competency when assessed by the Director, and (3) had parity with other thresholds of clinical skill assessment by faculty and in graduate education. The Director had no knowledge of non‐CLUE resident evaluations, In‐training scores, or academic performance outside of CLUE. Residents were not remanded for CLUE‐CEX failure.

The graduating class of 2011 was the first class to initially enter into an entire residency program fully immersed in the CLUE curriculum, and was therefore specifically asked to report their impression of the CLUE program after graduation through a post‐residency questionnaire. A Likert‐type scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree) was used to assess the perceived validity of the following statements: (1) CLUE improved my own bedside cardiovascular evaluation; and (2) I would use CLUE if ultrasound were available in my future position. Each resident was then asked if too much, not enough, or an appropriate amount of time was spent to learn CLUE, and to choose the most effective form of CLUE teaching to which they were exposed: didactic lectures, bedside ICU teaching, Web site/syllabus, and one‐to‐one training with the Director or sonographer.
Statistical Analysis
The CLUE experience was divided into 3 phases: (1) pre‐CLUE era, the 4‐year period (classes graduating 20022005) prior to the institution of the formal CLUE curriculum; (2) the 2‐year CLUE phase‐in period (classes graduating 20062007), in which portions of the residency were undergoing the 3‐year curriculum; (3) the 4‐year CLUE‐CEX era (classes graduating 20082011) when all residency classes were trained in the standardized fashion and underwent CLUE‐CEX assessment. In‐training postgraduate year‐3 (PGY‐3) scores, the result of a nationwide standardized test developed by the American College of Physicians, were used as representative of senior resident academic knowledge. A percentile rank score is provided to compare residents to nationwide data. The group of residents who had been selected to be the following year's chief residents had their CLUE‐CEX scores analyzed as a subgroup.
Data are presented as mean standard deviation and analyzed in SPSS, version 12.0 (SPSS, Inc, Chicago, IL). Linear regression was used to investigate the relationship between In‐training percentile ranks and CLUE‐CEX scores. Analysis of variance was used to determine any effect of gender and chief resident selection on CLUE‐CEX, and to assess average resident In‐training percentile ranks during the pre‐CLUE and CLUE‐CEX periods. Subset analysis of individual CLUE‐CEX scores was performed in regards to image quality, diagnostic knowledge, and interpretative skills. A value of P < 0.05 was considered significant.
RESULTS
Observations During CLUE Program Development
CLUE‐CEX scores (20082011) included data from 41 residents; 51% were male. In the class of 2009, one second‐year male resident transferred to another program for nonacademic reasons, reducing its number to 9. We observed that the impact of the CLUE program depended in part upon resident‐to‐resident teaching and required a critical mass of residents to be trained during a phase‐in period before a maximal effect could be appreciated. We observed that didactic knowledge occurred before imaging skills and remained dominant by graduation, with mean percentile CLUE‐CEX scores for image quality, knowledge, and interpretative accuracy at 82% 5%, 91% 3%, and 91% 8%, respectively. Residents typically found apical lung imaging the easiest to perform (CLUE‐CEX score of 89% 19%), followed by carotid (84% 18%), inferior vena cava (IVC) imaging (84% 26%), screening for abdominal aortic aneurysm (AAA) (83% 2 4%), parasternal long‐axis (79% 30%), and subcostal cardiac 4‐chamber imaging (73% 33%). Each view had technical and diagnostic pitfalls that were noted during resident practice (see Table 1), resulting in changes in our teaching and case review in subsequent years.
Residency and CLUE Performance
In attempting to achieve a CLUE proficiency score of >80% on the CLUE‐CEX in their graduating year, 8/41 (19.5%) senior residents failed. In these 8 residents, imaging quality, knowledge, and interpretative accuracy were all depressed: 55% 19%, 79% 11%, and 75% 11%, respectively. Two of these 8 had been selected as future chief residents over the 4‐year period, positions typically awarded to 2 residents per graduating year. The performance of the residents is seen in Table 3. The CLUE program did not exert a negative effect upon the academic performance of the residency, as evidenced by the lack of a significant difference in the Pre‐CLUE, 2‐year CLUE, and CLUE‐CEX periods in regards to average resident In‐training percentile rank scores (67.5 20.1, 62.3 20.5, 69.4 16.9, respectively; P = 0.37).
| Time Era (Year of Graduation) | n | Fail Rate | CLUE‐CEX (Mean SD) | Resident IT Percentile Rank (Mean SD) (Range) |
|---|---|---|---|---|
| ||||
| Pre‐CLUE (20022005) | 39 | 67.5 20.1 (2099) | ||
| Phase‐in CLUE (20062007) | 19 | 62.3 20.5 (2097) | ||
| CLUE‐CEX (20082011) | 41 | 19% | 87.4 11.9 | 69.4 16.9 (3499) |
| Year 2008 | 11 | 36% | 84.3 13.9 | 74.7 17.9 (4599) |
| Year 2009 | 9 | 11% | 89.1 7.0 | 73.0 16.6 (3493) |
| Year 2010 | 10 | 30% | 84.2 16.9 | 57.1 12.7 (4287) |
| Year 2011 | 11 | 0% | 92.1 5.7 | 72.4 15.9 (3499) |
Figure 2 shows the relationship between CLUE‐CEX scores and In‐training PGY‐3 scores. There was no significant relationship between resident academic performance and CLUE capabilities (r = 0.05, P = 0.75). Similarly, chief resident performance (n = 14) was not significantly associated with CLUE‐CEX scores (r = 0.15, P = 0.37), nor was male gender (P = 0.07). Approximately one‐half (49%) of the residents in the 4‐year CLUE‐CEX era entered fellowships, unchanged from historic rates, with only 1 resident during this era entering into a cardiology fellowship.

The Likert‐type questionnaire was returned by 11/11 graduating residents in 2011. Mean score of 4.3 0.6 (range: 35), with 6/11 responding agree, was given for the statement of whether CLUE improved the resident's own bedside exam. A score of 4.5 0.7 (range: 35), with 7/11 responding strongly agree, was given for whether the resident would use CLUE in the future if ultrasound were available. The majority (9/11) of residents felt that the time spent on CLUE was appropriate, with 2 residents responding not enough. Residents ranked one‐to‐one training with the Director(n = 6), followed by bedside ICU rounds (n = 5) as the preferred teaching methods to learn CLUE.
DISCUSSION
We report the experience of enrolling 6 consecutive classes, in an internal medicine residency, to test the feasibility of incorporating ongoing training in a specific, evidence‐based cardiovascular limited ultrasound examination within an already existing 3‐year curriculum. Using unbiased and complete enrollment, we found that residents who perform well on standardized academic testing or who are selected as chief residents do not necessarily perform more competently in CLUE, and that a significant overall initial resident failure rate can be anticipated. By questionnaire, residents felt confident in using the technique to improve their future bedside exams.
Burgeoning interest in the limited or focused application of ultrasound during bedside evaluation has already resulted in the incorporation of ultrasound training into emergency medicine residencies and critical care fellowships, with minimal standardization on curriculum, teaching methodology, or competency requirements. Given the multiple subspecialty applications for ultrasound, the potential exists of excessive diversity in bedside ultrasound practice, weakening the development of a single, simplified exam technique as a clinical tool for all physicians.31 Prior feasibility studies914 have evaluated the learning curve of internal medicine or primary care residents in performing various limited exams, but have not provided the rationale regarding the imaging protocols, the methods used for teaching, and the assessment of the program results over a sustained period of time. Furthermore, prior studies have not randomized subject trainees, likely resulting in the selected enrollment of highly motivated or skilled residents who want to perform a particular technique or have a bias to learn it. Our reported 19% unremanded failure rate on CLUE‐CEX will likely be more reflective of the general experience when initially integrating entire classes of internal medicine residents into a standard curriculum. The feasibility of introducing ultrasound at an earlier stage than residency may improve familiarity with the modality, and a 4‐year medical‐student curriculum has been recently described.32 Although introduction in medical school could allow for more adept and specific clinical training during residency, the optimal time for education in bedside ultrasound remains unclear.
Critical to the development of our program was the necessity to commit to teaching a single exam, the CLUE. We derived CLUE to quickly screen for important targets that had evidence‐basis to affect outcome, such as manifestations of subclinical atherosclerosis or chamber enlargement due to elevated filling pressures. Subsequent CLUE outcome studies have demonstrated diagnostic accuracy and prognostic value in its components,18, 26, 29, 30 and an effect upon medical decision‐making,21 even when performed by briefly trained novices.18, 21, 30 It is anticipated that this cardiovascular examination will later expand to a more advanced version or become a component of a full‐body ultrasound‐assisted physical. Therefore, evidence‐basis and brevity governed the development of a practical and teachable fundamental CLUE, and our skill assessment results are likely specific to CLUE itself.
This report contains primarily observations noted during the development of our program, written in retrospect with emphasis on real world feasibility. It was not a rigorous evaluation of specific ultrasound teaching methods. We found that training is feasible, at modest costs, when existing in‐hospital resources are utilized and include a part‐time faculty appointment and shared devices. Training the sonographers to perform CLUE as a part of the standard echocardiogram was a trivial task, but created the great benefit of being able to retrospectively review both the CLUE and formal echo in case review and teaching. Monthly CLUE lectures in the daily noon conference docket, and the use of the cardiology consultation and ICU rotations, allowed integration of the CLUE curriculum into preexisting venues and persistent practice opportunities within the residency. To prevent bias, we intentionally did not track, bring attention to, or incentivize resident performance in CLUE over any other topic; therefore, we can only approximate lecture and bedside teaching hours spent by each resident in light of detractions due to residency hour restrictions, vacations, and away rotations (Table 2). The CLUE‐CEX, although subject to the biases of any subjective resident skill assessment, was easily accomplished using a single form and faculty member, and was an efficient tool for program feedback and development.
In conclusion, we report the feasibility of sustained incorporation of an ultrasound training program in an internal medicine residency. We await studies regarding clinical outcome and validation of similar experiences in larger, multicenter programs.
Acknowledgements
The authors acknowledge the sonographers of the Scripps Mercy Cardiovascular Ultrasound Laboratory and Dudie Keane, for their dedication and assistance in the implementation of the CLUE program.
Disclosure: Nothing to report.
Note: The correction that was made, was the text in Fig. 1 and Fig. 2 were reversed. This article was published online on May 17, 2012. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected on May 22, 2012.
- ,,,,.Diagnostic performance of a pocket‐sized ultrasound device for quick‐look cardiac imaging.Am J Emerg Med. 2012;30(1):32–36.
- ,,,,,.New pocket echocardiography device is interchangeable with high‐end portable system when performed by experienced examiners.Acta Anaesthesiol Scand.2010;54(10):1217–1223.
- ,,, et al.Usefulness of a new miniaturized echocardiographic system in outpatient cardiology consultations as an extension of physical examination.J Am Soc Echocardiogr.2011;24(2):117–124.
- ,.Diagnostic accuracy of a hand‐held ultrasound scanner in routine patients referred for echocardiography.J Am Soc Echocardiogr.2011;24(2):111–116.
- ,,,,,.is pocket mobile echocardiography the next‐generation stethoscope? A cross‐sectional comparison of rapidly acquired images with standard transthoracic echocardiography.Ann Intern Med.2011;155(1):33–38.
- ,.Hand‐carried ultrasound: evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2:217–223.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr Rev.1998;7:79–81.
- .A personal ultrasound imager (ultrasound stethoscope): a revolution in the physical cardiac diagnosis!Eur Heart J.2002;23:523–527.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Ultrasonography performed by primary care residents for abdominal aortic ultrasound screening.J Gen Intern Med.2001;16:845–849.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126:693–701.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Med.2000;108:331–333.
- ,.Indications for limited echo imaging: a mathematical model.J Am Soc Echocardiogr.2000;13:855–861.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,.Time requirements of the standard echocardiogram: implications regarding “limited” studies.J Am Soc Echocardiogr.2003;16:1015–1018.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100:321–325.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol2002;90(9):1038–1039.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices: implications for bedside examination.Am J Med.2005;118(8):912–916.
- ,,,,.A hand‐carried ultrasound sign of cardiac disease: the left atrium‐to‐aorta diastolic ratio.Am J Emerg Med.2010;28(2):203–207.
- ,,,,,.A cardiopulmonary limited ultrasound examination for “quick‐look” bedside application.Am J Cardiol.2011;108:586–590.
- ,,, et al.Focused Assessment with Sonography for Trauma (FAST): results from an international consensus conference.J Trauma.1999;46:466–472.
- ,.The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure.J Am Coll Cardiol.2000;35:1638–1646.
- ,,,,,.Prognostic value of echocardiographic right/left ventricular end‐diastolic diameter ration in patients with acute pulmonary embolism.Chest.2008;133:358–362.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,,.Hospitalist use of hand‐carried ultrasound: preparing for battle.J Hosp Med.2010;5:163–167.
- ,,, et al.An integrated ultrasound curriculum (iUSC) for medical students: 4‐year experience.Crit Ultrasound J.2011;3(1):1–12.
- ,,,,.Diagnostic performance of a pocket‐sized ultrasound device for quick‐look cardiac imaging.Am J Emerg Med. 2012;30(1):32–36.
- ,,,,,.New pocket echocardiography device is interchangeable with high‐end portable system when performed by experienced examiners.Acta Anaesthesiol Scand.2010;54(10):1217–1223.
- ,,, et al.Usefulness of a new miniaturized echocardiographic system in outpatient cardiology consultations as an extension of physical examination.J Am Soc Echocardiogr.2011;24(2):117–124.
- ,.Diagnostic accuracy of a hand‐held ultrasound scanner in routine patients referred for echocardiography.J Am Soc Echocardiogr.2011;24(2):111–116.
- ,,,,,.is pocket mobile echocardiography the next‐generation stethoscope? A cross‐sectional comparison of rapidly acquired images with standard transthoracic echocardiography.Ann Intern Med.2011;155(1):33–38.
- ,.Hand‐carried ultrasound: evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2:217–223.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr Rev.1998;7:79–81.
- .A personal ultrasound imager (ultrasound stethoscope): a revolution in the physical cardiac diagnosis!Eur Heart J.2002;23:523–527.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Ultrasonography performed by primary care residents for abdominal aortic ultrasound screening.J Gen Intern Med.2001;16:845–849.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126:693–701.
- ,,, et al.A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Med.2000;108:331–333.
- ,.Indications for limited echo imaging: a mathematical model.J Am Soc Echocardiogr.2000;13:855–861.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,.Time requirements of the standard echocardiogram: implications regarding “limited” studies.J Am Soc Echocardiogr.2003;16:1015–1018.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100:321–325.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol2002;90(9):1038–1039.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices: implications for bedside examination.Am J Med.2005;118(8):912–916.
- ,,,,.A hand‐carried ultrasound sign of cardiac disease: the left atrium‐to‐aorta diastolic ratio.Am J Emerg Med.2010;28(2):203–207.
- ,,,,,.A cardiopulmonary limited ultrasound examination for “quick‐look” bedside application.Am J Cardiol.2011;108:586–590.
- ,,, et al.Focused Assessment with Sonography for Trauma (FAST): results from an international consensus conference.J Trauma.1999;46:466–472.
- ,.The role of thoracic ultrasonography for evaluation of patients with decompensated chronic heart failure.J Am Coll Cardiol.2000;35:1638–1646.
- ,,,,,.Prognostic value of echocardiographic right/left ventricular end‐diastolic diameter ration in patients with acute pulmonary embolism.Chest.2008;133:358–362.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,,.Hospitalist use of hand‐carried ultrasound: preparing for battle.J Hosp Med.2010;5:163–167.
- ,,, et al.An integrated ultrasound curriculum (iUSC) for medical students: 4‐year experience.Crit Ultrasound J.2011;3(1):1–12.
Copyright © 2012 Society of Hospital Medicine
Hospitalist Use of HCU
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.
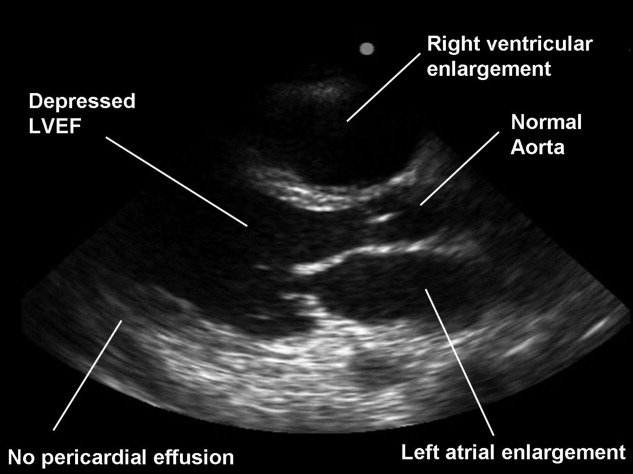
Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.

Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
Hand‐carried ultrasound (HCU) is a field technique. Originally intended for military triage, the advent of small, portable, ultrasound devices has brought ultrasound imaging to the patient's bedside to guide procedures and evaluate life‐threatening conditions. Although many recently‐trained physicians in emergency or critical care medicine now routinely use HCU to place central lines1 and tap effusions,2, 3 the capability of this technique to augment physical examination by all physicians has far greater potential value in medicine. When applied in acute critical scenarios, HCU techniques can quickly demonstrate findings regarding abdominal aortic aneurysm,4 deep vein thrombosis,5 pericardial fluid, or hemoperitoneum6 in patients with unexplained hypotension, and examine inferior vena cava collapsibility7 or brachial artery velocity variation8 to help determine the need for volume resuscitation in sepsis. In patients with unexplained dyspnea, HCU can search for ultrasound lung comet‐tail artifacts as a sign of pulmonary edema,9 or use the presence of pleural sliding to exclude pneumothorax.10 In addition, numerous less urgent applications for HCU imaging are emerging such as cardiac, lung, vascular, musculoskeletal, nerve, thyroid, gallbladder, liver, spleen, renal, testicular, and bladder imaging.
Medical or surgical subspecialties familiar with ultrasound have developed limited HCU examinations that serve specific purposes within the relatively narrow clinical indications encountered by these specialties. As a consequence, overall expertise in bedside HCU currently requires the mastery of multiple unrelated ultrasound views and diagnostic criteria. Without central leadership within this burgeoning field, HCU has found no consensus on its use or development within general medical practice. No one has yet validated a single ultrasound imaging protocol for augmenting the physical examination on all patients akin to the use of the stethoscope. This review discusses the importance of the internisthospitalist at this critical point in the early development of bedside HCU examination, focusing on the cardiopulmonary component as a prototype that has universal application across medical practice. Involvement by hospitalists in pioneering the overall technique will direct research in clinical outcome, restructure internal medicine education, change perception of the physical examination, and spur industry in device development specific for general medicine.
The role of the hospitalist as the leading in‐house diagnostician is unique in medicine, requiring breadth in medical knowledge and unprecedented communication skills in the seamless care of the most medically ill patients in the community.11 Ideally, the hospitalist quickly recognizes disease, discriminately uses consultation or expensive diagnostic testing, chooses cost‐effective therapies, and shortens length of hospital stay. Early accurate diagnosis afforded by HCU imaging has the potential to improve efficiency of medical care across a wide spectrum of clinical presentations. Although to date there are no outcome studies using a mortality endpoint, small individual studies have demonstrated that specific HCU findings improve diagnostic accuracy and relate to hospital stay length12 and readmission.13 The hospitalist position is in theory well‐suited for learning and applying bedside ultrasound, having both expert resources in the hospital to guide training and a clinical objective to reduce unnecessary hospital costs.
Saving the Bedside Examination: The Laying‐on of Ultrasound
Bedside examination is a vital component of the initial hospitalist‐patient interaction, adding objective data to the patient's history. In this era of physician surrogates and telemedicine, physical examination remains a nonnegotiable reason why physicians must appear in person at the patient's bedside to lay on hands. However, bedside cardiovascular examination skills have greatly diminished over the past decade for a variety of reasons.14 In particular, physical examination is impaired in the environment in which the hospitalist must practice. The admitting physician must oftentimes hurriedly examine the patient on the gurney in the noisy emergency department or in bed in an alarm‐filled intensive care unit (ICU) or hospital room. Ambient noise levels often preclude auscultation of acute aortic and mitral valve regurgitation, splitting of valve sounds, low diastolic rumbles, soft gallops, and fine rales. Patient positioning is limited in ventilated patients or those in respiratory or circulatory distress. Although medical education still honors the value of teaching the traditional cardiac examination, no outcome data exist to justify the application of the various maneuvers and techniques learned in medical school to contemporary, commonly encountered inpatient care scenarios. For example, few physical examination data exist on how to evaluate central venous pressures of an obese patient on the ventilator or assess the severity of aortic stenosis in the elderly hypertensive patient. Furthermore, many important cardiopulmonary abnormalities that are easily detected by ultrasound, such as pericardial fluid, well‐compensated left ventricular systolic dysfunction, small pleural effusion, and left atrial enlargement, make no characteristic sound for auscultation. The effect of undiagnosed cardiac abnormalities on the patient's immediate hospital course is unknown, but is likely related to the clinical presentation and long‐term outcome. Today, the hospitalist's suspicion of cardiovascular abnormalities is more often generated from elements in the patient's initial history, serum biomarkers, chest radiography, or electrocardiogram, and less from auscultation. Accordingly, cardiac physical examination is only adjunctively used in determining the general direction of the ensuing evaluation and when abnormal, often generates additional diagnostic testing for confirmation.
The optimal role of HCU for the internist‐hospitalist is in augmentation of bedside physical diagnosis.15, 16 Unlike x‐ray or even rapid serum biomarkers, ultrasound is a safe, immediate, noninvasive modality and has been particularly effective in delineating cardiac structure and physiology. Accurate HCU estimation of a patient's central venous pressure,17 left atrial size,18 or left ventricular ejection fraction19, 20 is of particular value in those with unexplained respiratory distress or circulatory collapse, or in those in whom referral for echocardiography or cardiac consultation is not obvious. Asymptomatic left ventricular systolic dysfunction has an estimated prevalence of 5% in adult populations,21 and its detection would have immediate implications in regard to etiology, volume management, and drug therapy. Multiple studies have shown the prognostic importance of left atrial enlargement in ischemic cardiac disease, congestive heart failure, atrial arrhythmias, and stroke.22 The inferior vena cava diameter has been related to central venous pressure and prognosis in congestive heart failure. A recent study13 using medical residents employing HCU demonstrated that persistent dilatation of the inferior vena cava at discharge related to a higher readmission rate in patients with congestive heart failure. The potential exists to follow and guide a patient's response to therapy with HCU during daily rounds. Comparative studies2325 confirm that HCU examinations are better than expert auscultation and improve overall exam accuracy when added to traditional physical exam techniques. Entering into the modern‐day emergency room with a pocket‐sized ultrasound device that provides the immediate capability of detecting left ventricular dysfunction, left atrial enlargement, pericardial effusion, or abnormalities in volume status, provides an additional sense of being prepared for battle.
Deriving Limited Ultrasound Applications: Time Well Spent
However, in order for a hospitalist to use HCU, easily applied limited imaging protocols must be derived from standard ultrasound examination techniques for each organ. For the heart, studies from our laboratory have shown that it is feasible to distill the comprehensive echocardiogram down to simple cardiac screening examinations for rapid bedside HCU use.2628 We found that a limited cardiac ultrasound study consisting of a single parasternal long‐axis (PLAX) view (Figure 1) requires only seconds to perform and can identify those patients who have significant cardiac abnormalities. In an outpatient population (n = 196) followed in an internal medicine clinic, the PLAX component of an HCU cardiac screening protocol uncovered left atrial enlargement in 4 patients and left ventricular systolic dysfunction in 4 patients that had not been suspected by the patients' primary physicians.29 In a study of 124 patients in the emergency department with suspected cardiac disease,12 abnormal cardiac findings were noted 3 times more frequently by PLAX than by clinical evaluation, and an abnormal PLAX was significantly associated with a longer hospital length of stay. In other preliminary studies using cardiologists, limited imaging has been shown to reduce costs of unnecessary echo referral.28, 3032 Cost analysis has yet to be performed in nonexpert HCU users, but benefit is likely related to the difference between the user's own accuracy with the stethoscope and the HCU device.

Although experts in ultrasound exist in radiology and cardiology, it is unlikely these subspecialists will spontaneously create and optimize a full‐body HCU imaging protocol for hospitalists. Similar to the use of ultrasound in emergency medicine, anesthesiology, and critical care medicine, the derivation of a bedside ultrasound exam appropriate for the in‐hospital physical examination should be developed within the specialty itself, by those acquainted with the clinical scenarios in which HCU would be deployed. For example, the question of whether the gallbladder should be routinely imaged by a quick HCU exam in the evaluation of chest pain is similar to the question of whether the Valsalva maneuver should be performed in the evaluation of every murmurboth require Bayesian knowledge of disease prevalence, exam difficulty, and test accuracy. With the collaboration of experts in ultrasound, internists can derive brief, easily learned, limited ultrasound exams for left ventricular dysfunction, left atrial enlargement, carotid atherosclerosis, interstitial lung disease, hepatosplenomegaly, cholelithiasis, hydronephrosis, renal atrophy, pleural or pericardial effusion, ascites, deep vein thrombosis, and abdominal aortic aneurysm. The discovery of these disease states has clinical value for long‐term care, even if incidental to the patient's acute presentation. The lasting implications of a more comprehensive general examination will likely differentiate the use of HCU in internal medicine practice from that of emergency medicine.
Basic Training in HCU
A significant challenge to medical education will be in physician training in HCU. Over 15 studies12, 13, 15, 1720, 22, 23, 3343 have now shown the ability of briefly trained medical students, residents, and physicians in internal medicine to perform a limited cardiovascular ultrasound examination. Not surprisingly, these studies show variable degrees of training proficiency, apparently dependent upon the complexity of the imaging protocol. In a recent pair of studies from 1 institution,42, 43 10 hospitalists were trained to perform an extensive HCU echocardiogram including 4 views, color and spectral Doppler, and interpret severity of valvular disease, ventricular function, pericardial effusion. In 345 patients already referred for formal echocardiography, which later served as the gold standard, HCU improved the hospitalists' physical examination for left ventricular dysfunction, cardiomegaly, and pericardial effusion, but not for valvular disease. Notably, despite a focused training program including didactic teaching, self‐study cases, 5 training studies, and the imaging of 35 patients with assistance as needed, image acquisition was inferior to standard examination and image interpretation was inferior to that of cardiology fellows. Such data reemphasize the fact that the scope of each body‐system imaging protocol must be narrow in order to make the learning of a full‐body HCU exam feasible and to incorporate training into time already allocated to the bedside physical examination curriculum or continuing medical education activities.
At our institution, internal medical residents are trained in bedside cardiovascular ultrasound to blend results with their auscultative findings during bedside examination. We have developed 2 cardiovascular limited ultrasound examinations (CLUEs) that can be performed in 5 minutes and have evidence‐basis for their clinical use through pilot training studies.18, 19, 29, 35 Our basic CLUE, designed for general cardiovascular examination, includes screening the carotid bulb for subclinical atherosclerosis, PLAX imaging for left atrial enlargement and systolic dysfunction of the left ventricle, and abdominal scanning for abdominal aortic aneurysm. In this imaging protocol consisting of only 4 targets, atherosclerotic risk increases from top to bottom (cephalad to caudal), making the exam easy to remember. The CLUEparasternal, lung, and subcostal (CLUE‐PLUS), designed for the urgent evaluation of unexplained dyspnea or hypotension, uses a work backward imaging format (from left ventricle to right atrium) and a single cardiac transducer for simplicity. The PLAX view screens for left ventricular systolic dysfunction and then left atrial enlargement. Next, a brief 4‐point lung exam screens for ultrasonic lung comets and pleural effusion. A subcostal view of the heart is used to evaluate right ventricular size and pericardial effusion, and finally the inferior vena cava is evaluated for central venous pressures. CLUEs are taught in bedside and didactic formats over the 3 years of residency with formal competency testing after lecture attendance, practice imaging in our echo‐vascular laboratories, participation in rounds, and completion of at least 30 supervised examinations.
Reaffirming the Role of the Internist
Although emergency44 and critical care45 medical subspecialties have begun to train their constituencies in HCU, general diagnostic techniques that have wide‐ranging application in medical illness should be the evidence‐based tools of the internist. The rejuvenation of bedside examination using HCU on multiple organ systems should be orchestrated within internal medicine and not simply evolve as an unedited collection of all subspecialty organ ultrasound examinations. Device development can then be customized and made affordable for use in general internal medicine, perhaps limiting the unnecessary production costs and training requirements for advanced Doppler or multiple transducers.
Concern has been raised about the medical and economic impact of training internists in HCU. Although training costs can be incorporated in residency or hospital‐based continuing medical education, discussions regarding reimbursement for cardiac imaging require a distinction between the brief application of ultrasound using a small device by a nontraditional user and a limited echocardiogram as defined by payers and professional societies.46 To date, no procedural code or reimbursement has yet been approved for ultrasound‐assisted physical examination using HCU devices and likely awaits outcome data. There is also concern about the possibility of errors being made by HCU use by briefly trained physicians. Patient care and cost‐savings depend on HCU accuracy, being liable both for unnecessary referrals due to false‐positive screening HCU exams and delays in diagnosis due to false‐negative examinations. However, such errors are commonplace and accepted with standard physical examination techniques and the current use of the stethoscope, both of which lack sensitivity when compared to HCU.
HCU is a disruptive technology.47 However, unlike the successful disruption that small desktop computers had on their mainframe counterparts, HCU devices appeared before the operating system of their clinical application had been formulated, making dissemination to new users nearly impossible. Furthermore, placing ultrasound transducers into the hands of nontraditional users often alienates or displaces established users of ultrasound as well as established untrained members within the profession. Competency requirements will have to be derived, preferably from studies performed within the profession for specific uses in internal medicine. Perhaps championed by hospitalists and driven by hospital‐based outcome studies, the use of HCU by internists as a physical exam technique will require advocacy by internists themselves. The alternative, having the hospitalist ask the emergency department physician for help in examining the patient, is difficult to imagine. The answer to whether the hospitalist should use HCU should be a resounding yesbased upon the benefit of earlier, more accurate examination and the value of preserving the diagnostic role of the internist at the bedside. In regard to the latter, it is a concept worth fighting for.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.
- ,,,.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24(12):2053–2058.
- .Ultrasound‐guided thoracentesis.Chest.2006;129(6):1709–1714.
- ,,, et al.Hand‐carried ultrasound‐guided pericardiocentesis and thoracentesis.J Am Soc Echocardogr.2003;16(5):480–484.
- ,,, et al.A prospective study of a hand‐held ultrasound device in abdominal aortic aneurysm evaluation.Am J Surg.2003;186(5):455–459.
- ,,.Emergency department compression ultrasound to diagnose proximal deep vein thrombosis.J Emerg Med.2001;20(2):107–112.
- ,,,,.The hand‐held FAST: experience with hand‐held trauma sonography in a level‐I urban trauma center.Injury.2002;33(4):303–308.
- ,,, et al.Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients.Intensive Care Med.2004;30(9):1740–1746.
- ,,, et al.Radial artery pulse pressure variation correlates with brachial artery peak velocity variation in ventilated subjects when measured by internal medicine residents using hand‐carried ultrasound devices.Chest.2007;131(5):1301–1307.
- ,,,,,.Evaluation of ultrasound lung comets by hand‐held echocardiography.Cardiovasc Ultrasound.2006;4:34.
- ,.A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding.Chest.1995;108(5):1345–1348.
- ,The hospitalist movement 5 years later.JAMA.2002;287(4):487–494.
- ,,,.Screening cardiac ultrasound examination in patients with suspected cardiac disease in the emergency room setting.Am Heart J.2001;142:324–330.
- ,,, et al.Comparison of hand‐carried ultrasound assessment of the inferior vena cava and N‐terminal pro‐brain natriuretic peptide for predicting readmission after hospitalization for acute decompensated heart failure.J Am Coll Cardiol Img.2008;1:595–601.
- ,.Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency.JAMA.1997;278(9):717–722.
- ,.Technology insight: hand‐carried ultrasound cardiac assessment—evolution, not revolution.Nat Clin Pract Cardiovasc Med.2005;2(4):217–223.
- ,,.Hand‐carried ultrasound improves the bedside cardiovascular examination.Chest.2004;126(3):693–701.
- ,,, et al.A comparison of medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure.Am J Cardiol.2007;99(11):1614–1616.
- ,,, et al.Detection of left atrial enlargement using hand‐carried ultrasound devices to screen for cardiac abnormalities.Am J Med.2005;118(8):912–916.
- ,,,,.Usefulness of a hand‐held ultrasound device for the bedside examination of left ventricular function.Am J Cardiol.2002;90(9):1038–1039.
- ,,,.A hand‐carried personal ultrasound device for rapid evaluation of left ventricular function: use after limited echo training.Echocardiography.2003;20(4):309–312.
- ,.Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction.Circulation.2006;113:2851–2860.
- .The left atrium. A biomarker of chronic diastolic dysfunction and cardiovascular disease risk.J Am Coll Cardiol.2003;42:1206–1207.
- ,,, et al.Physician‐performed point‐of‐care echocardiography using a laptop platform compared with physical examination in the cardiovascular patient.J Am Coll Cardiol.2001;3(8):2013–2018.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20(5):477–485.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96(7):1002–1006.
- ,,,.Feasibility of “limited” echo imaging: characterization of incidental findings.J Am Soc Echocardiogr.1998;11:746–750.
- ,.Indications for limited echocardiographic imaging: a mathematical model.J Am Soc Echocardiogr.2000;13(9):855–861.
- ,,,.Limited cardiac ultrasound examination for cost‐effective echo referral.J Am Soc Echocardiogr.2002;15:640–646.
- ,,,,,.Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic.Am J Cardiol.2007;100(2):321–325.
- ,,,.Diagnostic accuracy and cost‐effective implications of an ultrasound screening strategy in suspected mitral valve prolapse.Am J Medicine.2000;108:331–333.
- ,,, et al.The use of hand‐carried ultrasound in the hospital setting—a cost‐effective analysis.J Am Soc Echocardiogr.2005;18(6):620–625.
- ,,, et al.A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography.Heart.2007;93(4):470–475.
- ,,, et al.Teaching cardiovascular anatomy to medical students by using a handheld ultrasound device.JAMA.2002;288(9):1062–1063.
- ,,,,.The use of small personal ultrasound devices by internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,,,.Briefly‐trained physicians can screen for early atherosclerosis at the bedside using hand‐held ultrasound.Am J Cardiol.2003;92:239–240.
- ,,,,,.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147(3):476–481.
- ,,, et al.Hand‐carried cardiac ultrasound as a tool to screen for important cardiovascular disease in an underserved minority health care clinic.J Am Soc Echocardiogr.2004;17(5):339–403.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118(9):1010–1018.
- ,,, et al.Use of hand‐carried ultrasound devices to augment the accuracy of medical student bedside cardiac diagnoses.J Am Soc Echocardiogr.2005;18(3):257–263.
- ,,, et al.Focused training for goal‐oriented hand‐held echocardiography performed by noncardiologist residents in the intensive care unit.Intensive Care Med.2007;33(10):1795–1799.
- ,,.A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic.Echocardiography.2006;23(6):439–446.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120(11):1000–1004.
- ,,, et al.Hand‐carried ultrasound performed by hospitalist: does it improve the cardiac physical examination?Am J Med.2009;122(1):35–41.
- ,,, et al.Usefulness of hand‐held ultrasound devices in out‐of‐hospital diagnosis performed by emergency physicians.Am J Emerg Med.2006;24(2):237–242.
- ,,, et al.Feasibility and potential clinical utility of goal‐directed transthoracic echocardiography performed by noncardiologist intensivists using a small hand‐carried device (SonoHeart) in critically ill patients.J Cardiothorac Vasc Anesth.2005;19(2):155–159.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the Echocardiography Task Force on New Technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc of Echocardiogr.2002;15(4):369–373.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78(5):102–112,199.