User login
The Paradox of Achalasia Symptoms
In contrast to most diseases, as achalasia progresses, the symptoms improve. Specifically, reduction of symptoms of dysphagia lulls the gastroenterologist into thinking their patients are doing well.
This improvement in dysphagia is likely due to two mechanisms. The first is that as the esophagus dilates, there is a greater capacity for food accumulation before sensation occurs. Whether this is completely a volume issue or whether there is a contribution from increased esophageal body distensibility is unclear. Similarly, as achalasia results from inflammation and destruction of the motor neurons of the myenteric plexus, sensory neurons are also damaged. As a result, the patient’s ability to sense food retention lessens. To some degree, this explains the phenomenon of patients presenting with megaesophagus; after years of initially diminishing or stable symptoms managed with patient accommodation, patients present with end-stage disease manifested by a food-impacted esophagus, nocturnal aspiration, and weight loss.
This aspect of the natural history of achalasia has led esophagologists to follow patients with achalasia after treatment at regular intervals with objective examinations such as timed esophagography to mitigate against this worsening yet symptomatically stable course.
Dr. Katzka is based in the Division of Digestive and Liver Diseases, Columbia University Medical Center, New York. He receives research support from Medtronic and is an associate editor for GI & Hepatology News. Previously published in Gastro Hep Advances. 2024 Jan 19. doi: 10.1016/j.gastha.2024.01.006.
In contrast to most diseases, as achalasia progresses, the symptoms improve. Specifically, reduction of symptoms of dysphagia lulls the gastroenterologist into thinking their patients are doing well.
This improvement in dysphagia is likely due to two mechanisms. The first is that as the esophagus dilates, there is a greater capacity for food accumulation before sensation occurs. Whether this is completely a volume issue or whether there is a contribution from increased esophageal body distensibility is unclear. Similarly, as achalasia results from inflammation and destruction of the motor neurons of the myenteric plexus, sensory neurons are also damaged. As a result, the patient’s ability to sense food retention lessens. To some degree, this explains the phenomenon of patients presenting with megaesophagus; after years of initially diminishing or stable symptoms managed with patient accommodation, patients present with end-stage disease manifested by a food-impacted esophagus, nocturnal aspiration, and weight loss.
This aspect of the natural history of achalasia has led esophagologists to follow patients with achalasia after treatment at regular intervals with objective examinations such as timed esophagography to mitigate against this worsening yet symptomatically stable course.
Dr. Katzka is based in the Division of Digestive and Liver Diseases, Columbia University Medical Center, New York. He receives research support from Medtronic and is an associate editor for GI & Hepatology News. Previously published in Gastro Hep Advances. 2024 Jan 19. doi: 10.1016/j.gastha.2024.01.006.
In contrast to most diseases, as achalasia progresses, the symptoms improve. Specifically, reduction of symptoms of dysphagia lulls the gastroenterologist into thinking their patients are doing well.
This improvement in dysphagia is likely due to two mechanisms. The first is that as the esophagus dilates, there is a greater capacity for food accumulation before sensation occurs. Whether this is completely a volume issue or whether there is a contribution from increased esophageal body distensibility is unclear. Similarly, as achalasia results from inflammation and destruction of the motor neurons of the myenteric plexus, sensory neurons are also damaged. As a result, the patient’s ability to sense food retention lessens. To some degree, this explains the phenomenon of patients presenting with megaesophagus; after years of initially diminishing or stable symptoms managed with patient accommodation, patients present with end-stage disease manifested by a food-impacted esophagus, nocturnal aspiration, and weight loss.
This aspect of the natural history of achalasia has led esophagologists to follow patients with achalasia after treatment at regular intervals with objective examinations such as timed esophagography to mitigate against this worsening yet symptomatically stable course.
Dr. Katzka is based in the Division of Digestive and Liver Diseases, Columbia University Medical Center, New York. He receives research support from Medtronic and is an associate editor for GI & Hepatology News. Previously published in Gastro Hep Advances. 2024 Jan 19. doi: 10.1016/j.gastha.2024.01.006.
Then and Now: A ‘lifetime’ of advancement in upper GI tract
Fifteen years is a lifetime for the advancement of medical research. This seems particularly true for upper GI tract disorders.
In 2007, eosinophilic esophagitis was a rare disease; limited clinical data were available describing the symptoms, demographic characteristics, and endoscopic findings. Treatment was guided mostly by uncontrolled patient series for topical steroids and comprehensive diet exclusion therapy. Today, the molecular, genetic, and evolving microbiome’s contributions to EoE are being elucidated. EoE is recognized as one of the most common diseases in our practice, and rigorously performed controlled trials of steroids and biologics (including Food and Drug Administration–approved dupilumab) guide our treatment. Diet has also become easier with the identification of a single food antigen as the cause in 40% of EoE patients. The most pressing need is for a test that’s reliable and less invasive than endoscopy to assess and monitor treatment.
Barrett’s esophagus was of great concern 15 years ago and has surged in importance because of the increasing incidence of Barrett’s and esophageal adenocarcinoma, likely emphasized by the obesity epidemic. Sadly, survival with esophageal adenocarcinoma has changed little because most patients present with advanced stages. Multiple studies are questioning guideline recommendations because of their low yield and high expense. Fortunately, a range of easier screening tools is being tested, including sponge on string devices, video capsules, transnasal endoscopy, and the electronic “nose.” These can provide more widespread screening in broader populations of patients at risk who may lack heartburn or classic demographics. In 2007 there was little endoscopic therapy; now, the gastroenterologist has a robust armamentarium with multiple methods for mucosal ablation and resection achieving cure and sparing the patient an esophagectomy. Tissue biomarkers continue to be elucidated and are being applied to clinical practice.
For esophageal motility disorders, manometric data were obtained through a primitive water-infused system. With high-resolution manometry, the Chicago Classification, and impedance planimetry, our ability to precisely define, understand, and treat these disorders has been greatly enhanced.
In prior decades, the association of H. pylori to gastric cancer was noted but landmark trials and meta-analyses have strongly linked eradication of H. pylori with reduction in gastric cancer. These include broad population studies from Taiwan and the U.S. Veterans Health Administration, as well as a Cochrane review. These data have reinforced the need to search for and eradicate H. pylori infection. Although antibiotic resistance is rampant, newer antibiotic combinations including nitazoxanide, levofloxacin, rifabutin, and tinidazole have been proven effective. Potassium-competitive acid blockers may also augment effective eradication.
Endoscopy itself is one of the greatest areas of advancement in upper GI disease since 2007. What was once limited to biopsy, removal of polyps, and control of gastrointestinal bleeding, now has a breathtaking range of diagnostic and therapeutic capabilities. Who could imagine being able to perform bariatric procedures, create a gastrojejunostomy, treat a Zenker’s diverticulum, or drain extraluminal abscesses through an endoscope? With description of the technique of submucosal tunneling, endoscopic mucosal resection has been extended to submucosal dissection for more advanced cancers and benign tumors. This technique has also revolutionized the treatment of achalasia with peroral endoscopic myotomy, a procedure found equivalent to laparoscopic myotomy in controlled trials. Finally, artificial intelligence has taken endoscopic imaging by storm, and the accuracy with which we will diagnose premalignant lesions of the esophagus and stomach should significantly increase our abilities to prevent and treat early cancers.
Dr. Katzka is professor of medicine at Columbia University, New York. He reports consulting for Takeda and Celgene.
This article was updated July 7, 2022.
Fifteen years is a lifetime for the advancement of medical research. This seems particularly true for upper GI tract disorders.
In 2007, eosinophilic esophagitis was a rare disease; limited clinical data were available describing the symptoms, demographic characteristics, and endoscopic findings. Treatment was guided mostly by uncontrolled patient series for topical steroids and comprehensive diet exclusion therapy. Today, the molecular, genetic, and evolving microbiome’s contributions to EoE are being elucidated. EoE is recognized as one of the most common diseases in our practice, and rigorously performed controlled trials of steroids and biologics (including Food and Drug Administration–approved dupilumab) guide our treatment. Diet has also become easier with the identification of a single food antigen as the cause in 40% of EoE patients. The most pressing need is for a test that’s reliable and less invasive than endoscopy to assess and monitor treatment.
Barrett’s esophagus was of great concern 15 years ago and has surged in importance because of the increasing incidence of Barrett’s and esophageal adenocarcinoma, likely emphasized by the obesity epidemic. Sadly, survival with esophageal adenocarcinoma has changed little because most patients present with advanced stages. Multiple studies are questioning guideline recommendations because of their low yield and high expense. Fortunately, a range of easier screening tools is being tested, including sponge on string devices, video capsules, transnasal endoscopy, and the electronic “nose.” These can provide more widespread screening in broader populations of patients at risk who may lack heartburn or classic demographics. In 2007 there was little endoscopic therapy; now, the gastroenterologist has a robust armamentarium with multiple methods for mucosal ablation and resection achieving cure and sparing the patient an esophagectomy. Tissue biomarkers continue to be elucidated and are being applied to clinical practice.
For esophageal motility disorders, manometric data were obtained through a primitive water-infused system. With high-resolution manometry, the Chicago Classification, and impedance planimetry, our ability to precisely define, understand, and treat these disorders has been greatly enhanced.
In prior decades, the association of H. pylori to gastric cancer was noted but landmark trials and meta-analyses have strongly linked eradication of H. pylori with reduction in gastric cancer. These include broad population studies from Taiwan and the U.S. Veterans Health Administration, as well as a Cochrane review. These data have reinforced the need to search for and eradicate H. pylori infection. Although antibiotic resistance is rampant, newer antibiotic combinations including nitazoxanide, levofloxacin, rifabutin, and tinidazole have been proven effective. Potassium-competitive acid blockers may also augment effective eradication.
Endoscopy itself is one of the greatest areas of advancement in upper GI disease since 2007. What was once limited to biopsy, removal of polyps, and control of gastrointestinal bleeding, now has a breathtaking range of diagnostic and therapeutic capabilities. Who could imagine being able to perform bariatric procedures, create a gastrojejunostomy, treat a Zenker’s diverticulum, or drain extraluminal abscesses through an endoscope? With description of the technique of submucosal tunneling, endoscopic mucosal resection has been extended to submucosal dissection for more advanced cancers and benign tumors. This technique has also revolutionized the treatment of achalasia with peroral endoscopic myotomy, a procedure found equivalent to laparoscopic myotomy in controlled trials. Finally, artificial intelligence has taken endoscopic imaging by storm, and the accuracy with which we will diagnose premalignant lesions of the esophagus and stomach should significantly increase our abilities to prevent and treat early cancers.
Dr. Katzka is professor of medicine at Columbia University, New York. He reports consulting for Takeda and Celgene.
This article was updated July 7, 2022.
Fifteen years is a lifetime for the advancement of medical research. This seems particularly true for upper GI tract disorders.
In 2007, eosinophilic esophagitis was a rare disease; limited clinical data were available describing the symptoms, demographic characteristics, and endoscopic findings. Treatment was guided mostly by uncontrolled patient series for topical steroids and comprehensive diet exclusion therapy. Today, the molecular, genetic, and evolving microbiome’s contributions to EoE are being elucidated. EoE is recognized as one of the most common diseases in our practice, and rigorously performed controlled trials of steroids and biologics (including Food and Drug Administration–approved dupilumab) guide our treatment. Diet has also become easier with the identification of a single food antigen as the cause in 40% of EoE patients. The most pressing need is for a test that’s reliable and less invasive than endoscopy to assess and monitor treatment.
Barrett’s esophagus was of great concern 15 years ago and has surged in importance because of the increasing incidence of Barrett’s and esophageal adenocarcinoma, likely emphasized by the obesity epidemic. Sadly, survival with esophageal adenocarcinoma has changed little because most patients present with advanced stages. Multiple studies are questioning guideline recommendations because of their low yield and high expense. Fortunately, a range of easier screening tools is being tested, including sponge on string devices, video capsules, transnasal endoscopy, and the electronic “nose.” These can provide more widespread screening in broader populations of patients at risk who may lack heartburn or classic demographics. In 2007 there was little endoscopic therapy; now, the gastroenterologist has a robust armamentarium with multiple methods for mucosal ablation and resection achieving cure and sparing the patient an esophagectomy. Tissue biomarkers continue to be elucidated and are being applied to clinical practice.
For esophageal motility disorders, manometric data were obtained through a primitive water-infused system. With high-resolution manometry, the Chicago Classification, and impedance planimetry, our ability to precisely define, understand, and treat these disorders has been greatly enhanced.
In prior decades, the association of H. pylori to gastric cancer was noted but landmark trials and meta-analyses have strongly linked eradication of H. pylori with reduction in gastric cancer. These include broad population studies from Taiwan and the U.S. Veterans Health Administration, as well as a Cochrane review. These data have reinforced the need to search for and eradicate H. pylori infection. Although antibiotic resistance is rampant, newer antibiotic combinations including nitazoxanide, levofloxacin, rifabutin, and tinidazole have been proven effective. Potassium-competitive acid blockers may also augment effective eradication.
Endoscopy itself is one of the greatest areas of advancement in upper GI disease since 2007. What was once limited to biopsy, removal of polyps, and control of gastrointestinal bleeding, now has a breathtaking range of diagnostic and therapeutic capabilities. Who could imagine being able to perform bariatric procedures, create a gastrojejunostomy, treat a Zenker’s diverticulum, or drain extraluminal abscesses through an endoscope? With description of the technique of submucosal tunneling, endoscopic mucosal resection has been extended to submucosal dissection for more advanced cancers and benign tumors. This technique has also revolutionized the treatment of achalasia with peroral endoscopic myotomy, a procedure found equivalent to laparoscopic myotomy in controlled trials. Finally, artificial intelligence has taken endoscopic imaging by storm, and the accuracy with which we will diagnose premalignant lesions of the esophagus and stomach should significantly increase our abilities to prevent and treat early cancers.
Dr. Katzka is professor of medicine at Columbia University, New York. He reports consulting for Takeda and Celgene.
This article was updated July 7, 2022.
Upper and lower gastroenterology – the state of the art
In the upper GI section of the Postgraduate course program, Ikuo Hirano, MD, educated us on the refractory patient with eosinophilic esophagitis, reinforcing the need for chronic maintenance treatment and the complementary role of dilation. Gregory Ginsberg, MD, elucidated the specific strategies needed for gastric polyps with advice on which to leave and which to resect. Sachin Wani, MD, carefully outlined the changing landscape of Barrett’s esophagus with emphasis on our move to ablate rather than observe low-grade dysplasia. In the difficult area of treating gastroparesis, Linda Nguyen, MD, acquainted us with some of the newer medications for this disorder and discussed the emerging role of endoscopic pyloromyotomy. Michael Camilleri, MD, delivered a thorough analysis on the concept of leaky gut with data-driven recommendations on testing and the lack of adequate treatment. Finally, William Chey, MD, gave perspective to diagnosis and treatment of small-bowel bacterial overgrowth, particularly with its role in irritable bowel syndrome.
In the lower GI section of the course, Sunanda Kane, MD, gave a wonderful overview the present and emerging biologics for treatment of inflammatory bowel disease (IBD). David Rubin, MD, shared his expertise and vast experience for best management of ulcerative colitis while Edward Loftus Jr., MD, discussed the fact and fiction of diet-based therapy in IBD. This was followed by a timely lecture by Christina Ha, MD, on the need to think well outside the GI tract in IBD, discussing infections, cancers, and vaccinations in patients with IBD. The IBD section finished with an erudite and timely lecture by Marla Dubinsky, MD, evaluating the controversy over use of biosimilars in our clinical practice. The remainder of the lower GI section started with AGA President David Lieberman, MD, analyzing recent data on the need to move the colonic cancer screening age to 45 years, particularly in African Americans. Following this was a timely talk by Xavie Llor, MD, PhD, on when to suspect and how to test for the expanding definition of Lynch syndrome. Lin Chang, MD, delivered the penultimate clinical lecture on management of irritable bowel syndrome based on her many years of clinical expertise in this area. Finally, Gail Hecht, MD, AGAF, a former AGA president, summarized the exciting world of microbiome research from the recent annual Gut Microbiota for Health World Summit. All in all it was considered one of the best AGA Postgraduate courses by many and we look forward to even greater improvements for 2020.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Katzka is professor of medicine and head of the Esophageal Interest Group at the Mayo Clinic in Rochester, Minn. He is on the advisory boards for Shire and Celgene.
In the upper GI section of the Postgraduate course program, Ikuo Hirano, MD, educated us on the refractory patient with eosinophilic esophagitis, reinforcing the need for chronic maintenance treatment and the complementary role of dilation. Gregory Ginsberg, MD, elucidated the specific strategies needed for gastric polyps with advice on which to leave and which to resect. Sachin Wani, MD, carefully outlined the changing landscape of Barrett’s esophagus with emphasis on our move to ablate rather than observe low-grade dysplasia. In the difficult area of treating gastroparesis, Linda Nguyen, MD, acquainted us with some of the newer medications for this disorder and discussed the emerging role of endoscopic pyloromyotomy. Michael Camilleri, MD, delivered a thorough analysis on the concept of leaky gut with data-driven recommendations on testing and the lack of adequate treatment. Finally, William Chey, MD, gave perspective to diagnosis and treatment of small-bowel bacterial overgrowth, particularly with its role in irritable bowel syndrome.
In the lower GI section of the course, Sunanda Kane, MD, gave a wonderful overview the present and emerging biologics for treatment of inflammatory bowel disease (IBD). David Rubin, MD, shared his expertise and vast experience for best management of ulcerative colitis while Edward Loftus Jr., MD, discussed the fact and fiction of diet-based therapy in IBD. This was followed by a timely lecture by Christina Ha, MD, on the need to think well outside the GI tract in IBD, discussing infections, cancers, and vaccinations in patients with IBD. The IBD section finished with an erudite and timely lecture by Marla Dubinsky, MD, evaluating the controversy over use of biosimilars in our clinical practice. The remainder of the lower GI section started with AGA President David Lieberman, MD, analyzing recent data on the need to move the colonic cancer screening age to 45 years, particularly in African Americans. Following this was a timely talk by Xavie Llor, MD, PhD, on when to suspect and how to test for the expanding definition of Lynch syndrome. Lin Chang, MD, delivered the penultimate clinical lecture on management of irritable bowel syndrome based on her many years of clinical expertise in this area. Finally, Gail Hecht, MD, AGAF, a former AGA president, summarized the exciting world of microbiome research from the recent annual Gut Microbiota for Health World Summit. All in all it was considered one of the best AGA Postgraduate courses by many and we look forward to even greater improvements for 2020.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Katzka is professor of medicine and head of the Esophageal Interest Group at the Mayo Clinic in Rochester, Minn. He is on the advisory boards for Shire and Celgene.
In the upper GI section of the Postgraduate course program, Ikuo Hirano, MD, educated us on the refractory patient with eosinophilic esophagitis, reinforcing the need for chronic maintenance treatment and the complementary role of dilation. Gregory Ginsberg, MD, elucidated the specific strategies needed for gastric polyps with advice on which to leave and which to resect. Sachin Wani, MD, carefully outlined the changing landscape of Barrett’s esophagus with emphasis on our move to ablate rather than observe low-grade dysplasia. In the difficult area of treating gastroparesis, Linda Nguyen, MD, acquainted us with some of the newer medications for this disorder and discussed the emerging role of endoscopic pyloromyotomy. Michael Camilleri, MD, delivered a thorough analysis on the concept of leaky gut with data-driven recommendations on testing and the lack of adequate treatment. Finally, William Chey, MD, gave perspective to diagnosis and treatment of small-bowel bacterial overgrowth, particularly with its role in irritable bowel syndrome.
In the lower GI section of the course, Sunanda Kane, MD, gave a wonderful overview the present and emerging biologics for treatment of inflammatory bowel disease (IBD). David Rubin, MD, shared his expertise and vast experience for best management of ulcerative colitis while Edward Loftus Jr., MD, discussed the fact and fiction of diet-based therapy in IBD. This was followed by a timely lecture by Christina Ha, MD, on the need to think well outside the GI tract in IBD, discussing infections, cancers, and vaccinations in patients with IBD. The IBD section finished with an erudite and timely lecture by Marla Dubinsky, MD, evaluating the controversy over use of biosimilars in our clinical practice. The remainder of the lower GI section started with AGA President David Lieberman, MD, analyzing recent data on the need to move the colonic cancer screening age to 45 years, particularly in African Americans. Following this was a timely talk by Xavie Llor, MD, PhD, on when to suspect and how to test for the expanding definition of Lynch syndrome. Lin Chang, MD, delivered the penultimate clinical lecture on management of irritable bowel syndrome based on her many years of clinical expertise in this area. Finally, Gail Hecht, MD, AGAF, a former AGA president, summarized the exciting world of microbiome research from the recent annual Gut Microbiota for Health World Summit. All in all it was considered one of the best AGA Postgraduate courses by many and we look forward to even greater improvements for 2020.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Katzka is professor of medicine and head of the Esophageal Interest Group at the Mayo Clinic in Rochester, Minn. He is on the advisory boards for Shire and Celgene.
Introduction to Clinical Practice Update Committee articles
In this issue of Gastroenterology and in an upcoming issue of Clinical Gastroenterology and Hepatology, an important behind-the-scenes change is occurring. Specifically, both journals will continue to publish important and timely reviews and commentaries, but the formulation and design of some of these articles will now be driven by a new committee. The newly formed American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee (CPUC) is charged with providing authoritative and balanced articles for the two journals that have the greatest impact on the clinical practice of gastroenterology and hepatology. Potential subjects for these articles will derive from several sources in coordination with the AGA Institute Clinical Guidelines Committee.
First, important topics that are proposed initially for an AGA guideline but do not meet rigid criteria for guideline grading will be sent to the CPUC for review and consideration. Second, ideas for topics will also be generated by the CPUC members and journal editors, as well as by other AGA committees and AGA members themselves. Third, summaries of important symposia such as the yearly Freston Conference will be included. Topics can be presented in the form of a comprehensive, narrative review or, for particularly timely issues in gastroenterology, hepatology, and health care in general, as a rapid commentary.
We believe that the vetting process of these articles is important. All prospective topics and selected authors will be approved by the CPUC with guidance from the AGA Institute Governing Board. At least 2 authors who are recognized authorities will be invited to write an article and will be chosen from different institutions. It is anticipated that the authors will often have differing opinions, to help ensure balance and achieve consensus. A member of the CPUC with expertise in the field will guide the writing to ensure the highest quality and greatest balance in the final product. Once an article is written, it will be submitted for evaluation by the Editors of Gastroenterology and Clinical Gastroenterology and Hepatology, who will decide whether to accept the paper or send it for further review before acceptance.
With seasoned and motivated authors and the guidance of the CPUC, we expect outstanding reviews that will be published in a timely manner and provide AGA members and readers of the journals the most up-to-date and scholarly information across the spectrum of gastroenterology, hepatology, and nutrition. We are excited about this new process as a means to enhance our already outstanding journals, which are dedicated to publishing the best research and practice information in our field. Because this is a new process, we welcome feedback and suggestions for improvement and look forward to providing content that helps your research and clinical practice.
Dr. Katzka is at Mayo Clinic, Rochester, Minn.; Dr. Friedman is at Newton-Wellesley Hospital, Newton, Mass.; Dr. Inadomi is at University of Washington School of Medicine, Seattle; Dr. Kalloo is at Johns Hopkins Hospital, Baltimore; Dr. Kim is at South Denver Gastroenterology, P.C., Littleton, Colo.; Dr. Koch is at Virginia Mason Medical Center, Seattle; Dr. Lieberman is at Oregon Health and Science University, Portland; Dr. Lichtenstein is at the Hospital of the University of Pennsylvania, Merion Station, Penn.: Dr. Lim is at Yale University School of Medicine, New Haven, Conn.; Dr. Pandolfino is at Northwestern University, Chicago; Dr. Shin is at Indiana University School of Medicine, Indianapolis; and Dr. Siedler is Committee Liaison, American Gastroenterological Association.
In this issue of Gastroenterology and in an upcoming issue of Clinical Gastroenterology and Hepatology, an important behind-the-scenes change is occurring. Specifically, both journals will continue to publish important and timely reviews and commentaries, but the formulation and design of some of these articles will now be driven by a new committee. The newly formed American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee (CPUC) is charged with providing authoritative and balanced articles for the two journals that have the greatest impact on the clinical practice of gastroenterology and hepatology. Potential subjects for these articles will derive from several sources in coordination with the AGA Institute Clinical Guidelines Committee.
First, important topics that are proposed initially for an AGA guideline but do not meet rigid criteria for guideline grading will be sent to the CPUC for review and consideration. Second, ideas for topics will also be generated by the CPUC members and journal editors, as well as by other AGA committees and AGA members themselves. Third, summaries of important symposia such as the yearly Freston Conference will be included. Topics can be presented in the form of a comprehensive, narrative review or, for particularly timely issues in gastroenterology, hepatology, and health care in general, as a rapid commentary.
We believe that the vetting process of these articles is important. All prospective topics and selected authors will be approved by the CPUC with guidance from the AGA Institute Governing Board. At least 2 authors who are recognized authorities will be invited to write an article and will be chosen from different institutions. It is anticipated that the authors will often have differing opinions, to help ensure balance and achieve consensus. A member of the CPUC with expertise in the field will guide the writing to ensure the highest quality and greatest balance in the final product. Once an article is written, it will be submitted for evaluation by the Editors of Gastroenterology and Clinical Gastroenterology and Hepatology, who will decide whether to accept the paper or send it for further review before acceptance.
With seasoned and motivated authors and the guidance of the CPUC, we expect outstanding reviews that will be published in a timely manner and provide AGA members and readers of the journals the most up-to-date and scholarly information across the spectrum of gastroenterology, hepatology, and nutrition. We are excited about this new process as a means to enhance our already outstanding journals, which are dedicated to publishing the best research and practice information in our field. Because this is a new process, we welcome feedback and suggestions for improvement and look forward to providing content that helps your research and clinical practice.
Dr. Katzka is at Mayo Clinic, Rochester, Minn.; Dr. Friedman is at Newton-Wellesley Hospital, Newton, Mass.; Dr. Inadomi is at University of Washington School of Medicine, Seattle; Dr. Kalloo is at Johns Hopkins Hospital, Baltimore; Dr. Kim is at South Denver Gastroenterology, P.C., Littleton, Colo.; Dr. Koch is at Virginia Mason Medical Center, Seattle; Dr. Lieberman is at Oregon Health and Science University, Portland; Dr. Lichtenstein is at the Hospital of the University of Pennsylvania, Merion Station, Penn.: Dr. Lim is at Yale University School of Medicine, New Haven, Conn.; Dr. Pandolfino is at Northwestern University, Chicago; Dr. Shin is at Indiana University School of Medicine, Indianapolis; and Dr. Siedler is Committee Liaison, American Gastroenterological Association.
In this issue of Gastroenterology and in an upcoming issue of Clinical Gastroenterology and Hepatology, an important behind-the-scenes change is occurring. Specifically, both journals will continue to publish important and timely reviews and commentaries, but the formulation and design of some of these articles will now be driven by a new committee. The newly formed American Gastroenterological Association (AGA) Institute Clinical Practice Updates Committee (CPUC) is charged with providing authoritative and balanced articles for the two journals that have the greatest impact on the clinical practice of gastroenterology and hepatology. Potential subjects for these articles will derive from several sources in coordination with the AGA Institute Clinical Guidelines Committee.
First, important topics that are proposed initially for an AGA guideline but do not meet rigid criteria for guideline grading will be sent to the CPUC for review and consideration. Second, ideas for topics will also be generated by the CPUC members and journal editors, as well as by other AGA committees and AGA members themselves. Third, summaries of important symposia such as the yearly Freston Conference will be included. Topics can be presented in the form of a comprehensive, narrative review or, for particularly timely issues in gastroenterology, hepatology, and health care in general, as a rapid commentary.
We believe that the vetting process of these articles is important. All prospective topics and selected authors will be approved by the CPUC with guidance from the AGA Institute Governing Board. At least 2 authors who are recognized authorities will be invited to write an article and will be chosen from different institutions. It is anticipated that the authors will often have differing opinions, to help ensure balance and achieve consensus. A member of the CPUC with expertise in the field will guide the writing to ensure the highest quality and greatest balance in the final product. Once an article is written, it will be submitted for evaluation by the Editors of Gastroenterology and Clinical Gastroenterology and Hepatology, who will decide whether to accept the paper or send it for further review before acceptance.
With seasoned and motivated authors and the guidance of the CPUC, we expect outstanding reviews that will be published in a timely manner and provide AGA members and readers of the journals the most up-to-date and scholarly information across the spectrum of gastroenterology, hepatology, and nutrition. We are excited about this new process as a means to enhance our already outstanding journals, which are dedicated to publishing the best research and practice information in our field. Because this is a new process, we welcome feedback and suggestions for improvement and look forward to providing content that helps your research and clinical practice.
Dr. Katzka is at Mayo Clinic, Rochester, Minn.; Dr. Friedman is at Newton-Wellesley Hospital, Newton, Mass.; Dr. Inadomi is at University of Washington School of Medicine, Seattle; Dr. Kalloo is at Johns Hopkins Hospital, Baltimore; Dr. Kim is at South Denver Gastroenterology, P.C., Littleton, Colo.; Dr. Koch is at Virginia Mason Medical Center, Seattle; Dr. Lieberman is at Oregon Health and Science University, Portland; Dr. Lichtenstein is at the Hospital of the University of Pennsylvania, Merion Station, Penn.: Dr. Lim is at Yale University School of Medicine, New Haven, Conn.; Dr. Pandolfino is at Northwestern University, Chicago; Dr. Shin is at Indiana University School of Medicine, Indianapolis; and Dr. Siedler is Committee Liaison, American Gastroenterological Association.
In reply: Eosinophilic esophagitis
In Reply: I am most grateful to Drs. Theodoropoulos and Morris for their letter. I fully agree that we are getting smarter with diet elimination therapies by introducing more than one food at a time in the hope that we can lessen the number of endoscopies needed to isolate specific antigenic causes of eosinophilic esophagitis. This is not always successful, but in some of the more fortunate patients, we can get by with one or two endoscopies. It is my hope that with less-invasive tools such as the Cytosponge, the esophageal string test, and perhaps even serum evaluations, we can further embrace diet therapy as a standard treatment in more patients with eosinophilic esophagitis.
I think it is also important to note that although traditional radioallergosorbent and skin testing was only 45% accurate for eosinophilic esophagitis in the meta-analysis cited, this testing is still important, given the number of IgE-related allergies additionally uncovered in patients with eosinophilic esophagitis.
In Reply: I am most grateful to Drs. Theodoropoulos and Morris for their letter. I fully agree that we are getting smarter with diet elimination therapies by introducing more than one food at a time in the hope that we can lessen the number of endoscopies needed to isolate specific antigenic causes of eosinophilic esophagitis. This is not always successful, but in some of the more fortunate patients, we can get by with one or two endoscopies. It is my hope that with less-invasive tools such as the Cytosponge, the esophageal string test, and perhaps even serum evaluations, we can further embrace diet therapy as a standard treatment in more patients with eosinophilic esophagitis.
I think it is also important to note that although traditional radioallergosorbent and skin testing was only 45% accurate for eosinophilic esophagitis in the meta-analysis cited, this testing is still important, given the number of IgE-related allergies additionally uncovered in patients with eosinophilic esophagitis.
In Reply: I am most grateful to Drs. Theodoropoulos and Morris for their letter. I fully agree that we are getting smarter with diet elimination therapies by introducing more than one food at a time in the hope that we can lessen the number of endoscopies needed to isolate specific antigenic causes of eosinophilic esophagitis. This is not always successful, but in some of the more fortunate patients, we can get by with one or two endoscopies. It is my hope that with less-invasive tools such as the Cytosponge, the esophageal string test, and perhaps even serum evaluations, we can further embrace diet therapy as a standard treatment in more patients with eosinophilic esophagitis.
I think it is also important to note that although traditional radioallergosorbent and skin testing was only 45% accurate for eosinophilic esophagitis in the meta-analysis cited, this testing is still important, given the number of IgE-related allergies additionally uncovered in patients with eosinophilic esophagitis.
The ‘skinny’ on eosinophilic esophagitis
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
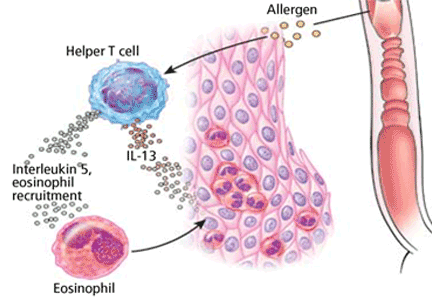
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
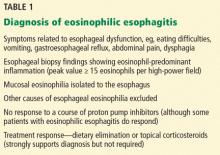
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
Eosinophilic esophagitis is a new disease defined by specific criteria that include a constellation of symptoms. Consensus guidelines define it as a chronic antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil-predominant inflammation.1
Ten years ago, a biopsy that revealed eosinophils in the esophagus was diagnostic, because normally eosinophils are not seen in the esophagus. The current definition has evolved to become more comprehensive and includes clinical, demographic, and radiographic criteria.
This article presents an overview of eosinophilic esophagitis—its pathogenesis, epidemiology, clinical presentation, diagnosis, and management.
ALLERGIC ORIGIN
Eosinophilic esophagitis is best regarded as a systemic rather than a single-organ disease, although current treatments are mostly directed specifically at esophageal inflammation. Evidence is clear that eosinophilic esophagitis is allergy-mediated.
The current “two-hit” etiologic model involves exposure first to aeroallergens that prime the esophagus, followed by food allergens that cause an eosinophilic response with antigen recognition and stimulation of immune cells from the bone marrow. Other allergic avenues may also be present, including those involved with atopy, asthma, eczema, and food allergies, which stimulate the Th2 pathway and lead to esophageal eosinophilia and inflammation.2
The two-hit model is supported experimentally: the disease can be induced in mice by injecting ovalbumin under the skin as a sensitizing agent, then exposing the airway to an aerosol of Aspergillus fumigatus, producing an allergic reaction involving classic Th2 allergy pathways.3 Further evidence is that many patients report that asthma or rhinitis developed years before esophageal disease began.
Patients with eosinophilic esophagitis and their family members have a high prevalence of allergies, and the disease frequently flares up during allergy season. Endoscopic biopsy specimens from patients often reveal increased T cells, mast cells, interleukin (IL)-5, and tumor necrosis factor alpha, all of which stimulate eotaxin and are essential to an allergic reaction. They also have high levels of CD3, CDA, and CD1A antigen-presenting lymphocytes, which are all associated with allergy.
Eosinophilic esophagitis responds to allergy medications, including corticosteroids and IL-5 or IL-13 mast-cell inhibitors. The strongest evidence for an allergic etiology is that withdrawing culpable food allergens leads to resolution of the disease. Peterson et al4 gave 18 adults with eosinophilic esophagitis an elemental diet (ie, a pure amino acid, carbohydrate-based diet in which all suspected allergens have been removed), and in 2 to 4 weeks, the mean number of eosinophils seen histologically fell from 54 to 10 cells per high-power field. The response was nearly complete (≤ 10 eosinophils per high-power field) in 72% of patients. When patients resumed a normal diet, the eosinophil content increased substantially within a few days.
Role of leaky tight junctions
Normally, the junctions between epithelial cells are tight, but many conditions, including allergic and autoimmune diseases, are now believed to involve altered permeability of this tissue. Tight-junction proteins play an important role in regulating antigen delivery and are modulated by cytokines. Activation of cytokines causes the membrane to become more permeable, allowing antigens to get through, leading to an enhanced reaction. In eosinophilic esophagitis, it is postulated that food antigens that pass through the leaky membrane activate CD1-antigen-presenting cells, which then initiate an allergic reaction.5–9
PREVALENCE IS INCREASING
Eosinophilic esophagitis was first described in 1993 with a report of 12 patients who had dysphagia, normal endoscopy, no acid reflux, and intraepithelial eosinophilia.10 The authors recognized that these patients had a distinct disease.
Since then, the disease has increased in prevalence. Kapel et al11 reviewed more than 74,000 endoscopy slides from a national pathology database and found 363 cases, with increasing prevalence during the study period from 2002 to 2005. Looking back further in a similar study, Whitney-Miller et al12 found a 0.3% prevalence from the years 1992 to 2000 vs 3.8% from 2001 to 2004.
Sealock et al13 reviewed the literature to assess the prevalence of eosinophilic esophagitis and found considerable variation depending on the populations sampled. One study from Sweden14 found a prevalence of 0.4% by performing endoscopy in 1,000 randomly selected people from nearly 3,000 responders to a questionnaire on abdominal symptoms. A study based on a Swiss database15 found only a 0.02% prevalence. Other studies show higher rates: a study from Florida that examined biopsy specimens from patients who underwent endoscopy for any reason found a prevalence of 1%.16 Another US study found a 15% prevalence in patients with dysphagia.17 Since these studies were done nearly a decade ago, we can expect the prevalence to be higher today.
Celiac disease has also been increasing in recent decades, as has gluten sensitivity. Allergies in general are on the rise worldwide, including asthma and atopic dermatitis. Theories as to the cause of these increases have focused on ambient antigens, food additives, proton pump inhibitors (PPIs), and the microbiome.18,19
DIAGNOSING EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis is diagnosed with a combination of symptomatic, histologic, and radiographic findings (Table 1). The classic patient is a white male—a child, teenager, or young adult—with dysphagia.
A case series of 23 adult patients20 found a mean age of 35 (age range 18 to 57), with a male preponderance (14:9). There is commonly a history of other allergies, including asthma, allergic rhinitis, and atopic dermatitis. Patients more commonly present with dysphagia than heartburn or other esophageal symptoms.11
Endoscopic findings—eosinophils, later fibrosis
Finding eosinophils in the esophagus is nonspecific and is not sufficient to make the diagnosis. Other systemic diseases can involve esophageal eosinophilia, including Churg-Strauss syndrome, Crohn disease, and helminthic diseases. Whether some are related to eosinophilic esophagitis or are independent is not well understood.
Characteristic findings on endoscopy include a corrugated or ringed appearance and linear furrows, resulting from fibrosis and scarring. “Micro-tears” may also be visible projecting linearly up the esophagus. Multiple white specks are signs of conglomerations of eosinophils and are easily confused with yeast infection. Strictures from scar tissue cause the mucosa to be tight and fragile, making the esophagus very susceptible to tearing during endoscopy.
After years of untreated disease, the esophagus becomes increasingly inflamed and fibrotic. Adult patients with eosinophilic esophagitis who were followed for a decade were found to develop increasing collagen deposition in which the submucosa or even the entire esophageal wall was diffusely fibrotic.21
Radiographic findings—a narrow esophagus
On radiography, the esophagus may appear narrow—not uncommonly one-third to one-quarter the caliber of a normal esophagus. As the esophagus progressively narrows, both eating and treatment become extremely difficult.
Symptoms are different in children and adults
Symptoms reflect the endoscopic changes over time. In children, the condition manifests with feeding difficulties, vomiting, symptoms of gastroesophageal reflux, and abdominal pain as signs of inflammation. As the esophagus becomes fibrotic, teenagers and young adults tend to present with strictures, dysphagia, and food impaction. Of patients who present to an emergency department with food impaction, the major cause is now eosinophilic esophagitis.22
It is important to pay attention to symptoms in children to diagnose the condition and start treatment early to prevent or postpone disease advancement. Medical therapy does not clearly reverse the fibrosis.
As in many chronic benign diseases, patients learn to compensate, so a careful history is essential. Many deny having a swallowing problem, but questioning may reveal that they have always been slow, picky eaters, consuming mostly soft foods and drinking fluids with every bite.
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease
Distinguishing eosinophilic esophagitis from gastroesophageal reflux disease can be a challenge, as signs and symptoms overlap.
Veerappan et al23 looked for predictors of eosinophilic esophagitis in 400 adults who underwent routine upper endoscopy, 6.5% of whom had eosinophilic esophagitis. They found significant overlap in medical history for patients with and without the disease; while a higher proportion of patients with eosinophilic esophagitis had a history of asthma, dysphagia, food impactions, dermatitis, and food allergies, these conditions also occurred in other patients.
Similarly, the classic endoscopic findings of eosinophilic esophagitis—rings, furrows, strictures, and plaques—also occur in other conditions.23 Reflux disease can cause scarring from excess acid and may even be associated with eosinophils in the esophagus, indicative of a combination of allergy and reflux. A small-caliber esophagus is also occasionally present in patients with reflux disease.
Ambulatory pH monitoring has been recommended to help determine if gastroesophageal reflux is the cause of esophageal eosinophilia and to guide therapy. However, in a prospective study of 51 patients,24 neither positive nor negative results of initial pH monitoring accurately predicted response to PPIs or steroid therapy. Another study found that half of patients with an eosinophilic esophagitis profile without evidence of acid reflux by pH monitoring responded to treatment with a PPI.25
This raises the question of whether some patients with eosinophilic esophagitis have more acid reflux than is detected by pH monitoring, or alternatively, whether PPIs have other, less-recognized effects besides reducing acidity. Investigators are now ascribing a host of anti-inflammatory actions to PPIs, including effects on antioxidants, inflammatory cells, endothelial cells, and the gut microflora.26 And PPIs may alleviate eosinophilic esophagitis through anti-inflammatory effects rather than by inhibiting secretion of gastric acid.
THREE TYPES OF THERAPY
In general, three types of therapy are available for patients with eosinophilic esophagitis: medications, allergen avoidance, and esophageal dilation (Table 2).
Medications: Try a PPI first, then a corticosteroid
A PPI should be tried even for patients with a classic presentation of eosinophilic esophagitis because some will respond, and long-term PPI therapy is preferable to long-term steroid treatment. Patients should be put on a 2-month course and should then undergo repeat biopsy.
For patients who do not respond to a PPI, a corticosteroid or montelukast can be tried. Topical therapy is showing promise as both a short- and long-term option to bring about remission.27 For administration, a corticosteroid (budesonide or fluticasone) is mixed with a viscous solution, such as water with honey or chocolate syrup, making it thick so it better coats the esophagus. The therapy can be very effective: in up to 8 weeks some patients have a 90% resolution of esophageal eosinophilia. However, about 5% of patients develop a yeast infection, and adrenal suppression is a concern but appears to be uncommon.
Avoidance of allergens
Because eosinophilic esophagitis is an allergic disease, eliminating allergens should be an effective treatment. Unfortunately, from a practical standpoint, elimination is very difficult. The elemental diet formula is expensive and unpalatable, making it impractical for a prolonged period.
Gonsalves et al28 put 50 adult patients with eosinophilic esophagitis on a diet eliminating the six most common foods believed to trigger the disease—wheat, milk, nuts, eggs, soy, and seafood—and found a marked reduction in eosinophils in the proximal and distal esophagus after 6 weeks. Additional triggers that have been identified include rice, corn, and legumes.29
Unfortunately, maintaining a diet without the most commonly identified allergens is not easy. Although some very motivated patients can do it, it is especially hard for teens and young adults. Variations of the diet, such as eliminating just two foods, make following a plan easier. Omitting milk alone would benefit an estimated 20% of patients with eosinophilic esophagitis.
Identifying food triggers is a challenge in itself as there is no good noninvasive method of identifying the allergens. The radioallergosorbent test measures immunoglobulin (Ig) E, and the skin-prick test measures acute hypersensitivity, but neither is very sensitive for the Th2-mediated reaction involved in eosinophilic esophagitis. In early trials, endoscopy and biopsy were painstakingly performed with the removal and reintroduction of every suspected food allergen, requiring multiple biopsies weekly, which is impractical for safety and economic reasons.
Attempts are being made to devise less invasive methods of sampling the esophageal mucosa. Transnasal endoscopy—done as an outpatient procedure with topical anesthesia—is a possibility. Another possibility is the esophageal string test,30 which involves swalling a weighted capsule on a string and then, after an hour, pulling it up again and testing the tissue on the string.
The “cytosponge,” a new device currently under investigation, also uses a string delivery system. The patient swallows a sponge contained in a gelatin capsule and attached to a string. When the capsule dissolves in the stomach—a process that takes only a few minutes—the sponge expands. The string is then pulled up, causing the sponge to sample the esophageal mucosa and thus obtaining a histologic specimen. This method shows promise as an inexpensive and noninvasive way to monitor the disease, although larger studies are needed to establish efficacy.31
Dilation—proceed with caution
Dilation can be an important therapy, especially in teenagers and adults with a fibrotic, narrowed esophagus.
Early on, the procedure often resulted in complications such as deep mucosal tears and perforations. Jung et al32 retrospectively analyzed 293 dilations in 161 patients with eosinophilic esophagitis and found a deep mucosal tear in 27 patients (9%), three perforations, and one incidence of major bleeding. All complications resolved without surgery. Factors associated with increased risk of complications were luminal narrowing in the upper and middle third of the esophagus, a luminal stricture that could not be traversed with a standard upper endoscope, and use of a Savary dilator.
It is critical that dilation be done slowly—a few millimeters at a time. Several sessions may be needed.
TREATMENT DURING REMISSION IS CONTROVERSIAL
Unless the patient with eosinophilic esophagitis can consistently control the disease by avoiding allergens, the question arises of whether to continue treating a patient who is in remission.
On the one hand, there is no known risk of Barrett esophagus or malignancy when the condition is not treated, and weight loss is uncommon because patients tend to accommodate to the condition. However, the long-term consequences are uncertain. Allergies are chronic, and disease progression with more fibrosis should be prevented. Also, food impaction commonly occurs and this requires aggressive dilation, which is risky.
On the other hand, chronic steroid therapy involves risk. The optimum steroid dosage during remission and whether alternate-day dosing is adequate have yet to be determined.
Long-term trials are needed to answer these questions. In the meantime, most physicians tend to aggressively treat this disease, if not with specific food avoidance, then with steroid maintenance therapy.
MONITORING THE DISEASE
Monitoring eosinophilic esophagitis by clinical indicators is difficult. Once fibrosis develops, symptoms often do not reflect underlying pathology. It may turn out that, as in Crohn disease, monitoring mucosal healing rather than symptoms may be best.
Until we know more about this condition, careful monitoring of patients is important. However, it is too early to give specific guidance, such as endoscopy every 2 months or annually. Whether the eosinophil count should be the critical consideration is also unknown.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011; 128:3–20.
- Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology 2009; 137:1238–1249.
- Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest 2001; 107:83–90.
- Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013; 108:759–766.
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 2010; 20:142–149.
- Chang F, Anderson S. Clinical and pathological features of eosinophilic oesophagitis: a review. Pathology 2008; 40:3–8.
- Orlando LA, Orlando RC. Dilated intercellular spaces as a marker of GERD. Curr Gastroenterol Rep 2009; 11:190–194.
- Blanchard C, Wang N, Stringer KF, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest 2006; 116:536–547.
- Rothenberg ME, Spergel JM, Sherrill JD, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet 2010; 42:289–291.
- Attwood SE, Smyrk TC, Demeester TR, Jones JB. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993; 38:109–116.
- Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008; 134:1316–1321.
- Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis: a retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol 2009; 131:788–792.
- Sealock RJ, Rendon G, El-Serag HB. Systematic review: the epidemiology of eosinophilic oesophagitis in adults. Aliment Pharmacol Ther 2010; 32:712–719.
- Ronkainen J, Talley NJ, Aro P, et al. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut 2007; 56:615–620.
- Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005; 115:418–419.
- Almansa C, Krishna M, Buchner AM, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol 2009; 104:828–833.
- Prasad GA, Talley NJ, Romero Y, et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol 2007; 102:2627–2632.
- Dellon ES, Peery AF, Shaheen NJ, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology 2011; 141:1586–1592.
- Björkstén B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 1999; 29:342–346.
- Roy-Ghanta S, Larosa DF, Katzka DA. Atopic characteristics of adult patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2008; 6:531–535.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology 2003; 125:1660–1669.
- Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophiic inflammation with esophageal food impaction in adults. Gastrointest Endosc 2005; 61:795–801.
- Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol 2009; 7:420–426.
- Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012; 35:300–307.
- Molina-Infante J, Ferrando-Lamana L, Ripoll C, et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 2011; 9:110–117.
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci 2009; 54:2312–2317.
- Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 2010; 139:1526–1537.
- Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459.
- Lucendo AJ, Arias Á, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804.
- Fillon SA, Harris JK, Wagner BD, et al. Novel device to sample the esophageal microbiome—the esophageal string test. PLoS One 2012; 7:e42938.
- Katzka DA, Geno DM, Ravi A, et al. Accuracy, safety, and tolerability of tissue collection by Cytosponge vs endoscopy for evaluation of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2014. pii: S1542-3565(14)00933-1. doi: 10.1016/j.cgh.2014.06.026. [Epub ahead of print]
- Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011; 73:15–21.
KEY POINTS
- Eosinophilic esophagitis is an allergy-mediated, systemic disease.
- It is diagnosed by characteristic symptoms, esophageal biopsy (peak value ≥ 15 eosinophils per high-power field), and response to allergen avoidance or treatment with steroids.
- Therapy with a proton pump inhibitor should be tried even for patients with a classic presentation.
- Strict dietary avoidance of allergens has been shown to resolve the disease but is often impractical.
- Dilation is indicated for a narrowed esophagus but must be done cautiously because of the risk of tearing.
- How best to monitor the disease (eg, by annual endoscopy) is still uncertain.