User login
Things We Do For No Reason: Routine Blood Culture Acquisition for Children Hospitalized with Community-Acquired Pneumonia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing [email protected].
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing [email protected].

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing [email protected].
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
© 2020 Society of Hospital Medicine
Home Smoke Exposure and Health-Related Quality of Life in Children with Acute Respiratory Illness
Acute respiratory illnesses (ARIs), including acute exacerbations of asthma, croup, pneumonia, and bronchiolitis, are among the most common illnesses in childhood.1 Although most ARIs can be managed in the outpatient setting,
Exposure to secondhand smoke (SHS) is a preventable risk factor for ARI in children, particularly when there is regular exposure in the home.2 Chronic exposure to SHS impacts systemic inflammation by suppressing serum interferon-gamma,3 which can lead to increased susceptibility to viral and bacterial infections,4 and increasing Th2 (atopic) cytokine expression, which is associated with asthma.5 SHS exposure in children has also been linked to diminished lung function.6 As a result, SHS exposure is associated with increased ARI susceptibility and severity in children.7-10
Much research has focused on the clinical impact of SHS exposure on respiratory health in children, but little is known about the impact on patient-reported outcomes, such as health-related quality of life (HRQOL). Patient-reported outcomes help provide a comprehensive evaluation of the effectiveness of healthcare delivery systems. These outcomes are increasingly used by health service researchers to better understand patient and caregiver perspectives.11 Given the known associations between SHS exposure and ARI morbidity, we postulated that regular SHS exposure would also impact HRQOL in children. In this study, we assessed the relationship between SHS exposure and HRQOL within a large, multicenter, prospective cohort of children presenting to the emergency department (ED) and/or hospital with ARI.
METHODS
Study Population
This study was nested within the Pediatric Respiratory Illness Measurement System (PRIMES) study, a prospective cohort study of children with ARI in the ED and inpatient settings at five tertiary care children’s hospitals within the Pediatric Research in Inpatient Settings Network in Colorado, Pennsylvania, Tennessee, Texas, and Washington. Eligible children were two weeks to 16 years of age hospitalized after presenting to the ED with a primary diagnosis of asthma, croup, bronchiolitis, or pneumonia between July 1, 2014 and June 30, 2016. Because of an anticipated low frequency of croup hospitalizations, we also included children presenting to the ED and then discharged to home with this diagnosis. Children were assigned to a PRIMES diagnosis group based on their final discharge diagnosis. If there was a discrepancy between admission and discharge diagnoses, the discharge diagnosis was used. If a child had more than one discharge diagnosis for a PRIMES condition (eg, acute asthma and pneumonia), we chose the PRIMES condition with the lowest total enrollments overall. If the final discharge diagnosis was not a PRIMES condition, the case was excluded from further analysis. Patients with immunodeficiency, cystic fibrosis, a history of prematurity <32 weeks, chronic neuromuscular disease, cardiovascular disease, pulmonary diseases (other than asthma), and moderate to severe developmental delay were also excluded. Children admitted to intensive care were eligible only if they were transferred to an acute care ward <72 hours following admission. A survey was administered at the time of enrollment that collected information on SHS exposure, HRQOL, healthcare utilization, and demographics. All study procedures were reviewed and approved by the institutional review boards at each of the participating hospitals.
SECONDHAND SMOKE EXPOSURE
To ascertain SHS exposure, we asked caregivers, “How many persons living in the child’s home smoke?” Responses were dichotomized into non-SHS exposed (0 smokers) and SHS exposed (≥1 smokers). Children with missing data on home SHS exposure were excluded.
Health-Related Quality of Life Outcomes
We estimated HRQOL using the Pediatric Quality of Life (PedsQLTM) 4.0 Generic Core and Infant Scales. The PedsQL instruments are validated, population HRQOL measures that evaluate the physical, mental, emotional, and social functioning of children two to 18 years old based on self- or caregiver-proxy report.12-15 These instruments have also shown responsiveness as well as construct and predictive validity in hospitalized children.11 For this study, we focused on the PedsQL physical functioning subscale, which assesses for problems with physical activities (eg, sports activity or exercise, low energy, and hurts or aches) on a five-point Likert scale (never to almost always a problem). Scores range from 0 to 100 with higher scores indicating a better HRQOL. The reported minimal clinically important difference (MCID), defined as the smallest difference in which individuals would perceive a benefit or would necessitate a change in management, for this scale is 4.5 points.16,17
Children >8 years old were invited to complete the self-report version of the PedsQL. For children <8 years old, and for older children who were unable to complete them, surveys were completed by a parent or legal guardian. Respondents were asked to assess perceptions of their (or their child’s) HRQOL during periods of baseline health (the child’s usual state of health in the month preceding the current illness) and during the acute illness (the child’s state of health at the time of admission) as SHS exposure may influence perceptions of general health and/or contribute to worse outcomes during periods of acute illness.
Covariates collected at the time of enrollment included sociodemographics (child age, gender, race/ethnicity, and caregiver education), and healthcare utilization (caregiver-reported patient visits to a healthcare provider in the preceding six months). Insurance status and level of medical complexity (using the Pediatric Medical Complexity Algorithm)18 were obtained using the Pediatric Hospital Information System database, an administrative database containing clinical and resource utilization data from >45 children’s hospitals in the United States including all of the PRIMES study hospitals.13
Analysis
Descriptive statistics included frequency (%) and mean (standard deviation). Bivariate comparisons according to SHS exposure status were analyzed using chi-squared tests for categorical variables and analysis of variance for continuous variables. Multivariable linear mixed regression models were used to examine associations between home SHS exposure and HRQOL for baseline health and during admission, overall and stratified by diagnosis. Covariates in each model included age, sex, race/ethnicity, caregiver education, and healthcare visits in the preceding six months. We also included a hospital random effect to account for clustering of patients within hospitals and used robust standard errors for inference.
In a secondary analysis to explore potential dose-response effects of SHS exposure, we examined associations between an ordinal exposure variable (0 smokers, 1 smoker, ≥2 smokers) and HRQOL for baseline health and during admission for the acute illness. Because of sample size limitations, diagnosis-specific analyses examining dose-response effects were not conducted.
RESULTS
Study Population
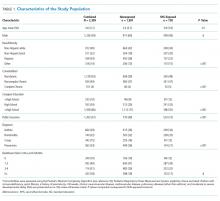
Of the 2,334 children enrolled in the PRIMES study, 25 (1%) respondents did not report on home SHS exposure and were excluded, yielding a final study population of 2,309 children, of whom 728 (32%) had reported home SHS exposure. The study population included 664 children with asthma (mean age seven years [3.5]; 38% with home SHS exposure), 740 with bronchiolitis (mean age 0.7 years [0.5]; 32% with home SHS exposure), 342 with croup (mean age 1.7 [1.1]; 25% with home SHS exposure), and 563 with pneumonia (mean age 4.4 [3.8]; 27% with home SHS exposure; Table 1). Compared with non-SHS-exposed children, those with home SHS exposure tend to be slightly older (3.9 vs 3.4 years, P = .01), more likely to be non-Hispanic Black (29% vs 19%, P < .001), to have a chronic condition (52% vs 41%, P < .001), to come from a household where caregiver(s) did not graduate from college (45% vs 29%, P < .001), and to have public insurance (73% vs 49%, P < .001).
Home SHS Exposure and Health-related Quality of Life
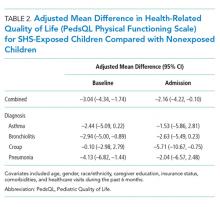
The overall mean HRQOL score for baseline health was 83 (15), with a range across diagnoses of 82 to 87. Compared with non-SHS-exposed children, children with home SHS exposure had a lower mean HRQOL score for baseline health (adjusted mean difference –3.04 [95% CI -4.34, –1.74]). In analyses stratified by diagnosis, baseline health scores were lower for SHS-exposed children for all four conditions, but differences were statistically significant only for bronchiolitis (adjusted mean difference –2.94 [–5.0, –0.89]) and pneumonia (adjusted mean value –4.13 [–6.82, –1.44]; Table 2); none of these differences met the MCID threshold.
The overall mean HRQOL score at the time of admission was 56 (23), with a range across diagnoses of 49 to 61, with lower scores noted among SHS-exposed children compared with non-SHS-exposed children (adjusted mean difference –2.16 [–4.22, –0.10]). Similar to scores representing baseline health, admission scores were lower across all four conditions for SHS-exposed children. Only children with croup, however, had significantly lower admission scores that also met the MCID threshold (adjusted mean difference –5.71 [–10.67, –0.75]; Table 2).
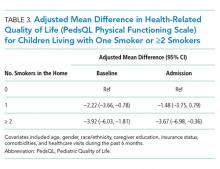
To assess for potential dose-response effects of SHS exposure on HRQOL, we stratified SHS-exposed children into those with one smoker in the home (n = 513) and those with ≥2 smokers in the home (n = 215). Compared with non-SHS-exposed children, both HRQOL scores (baseline health and admission) were lower for SHS-exposed children. Consistent with a dose-response association, scores were lowest for children with ≥2 smokers in the home, both at baseline health (adjusted mean difference –3.92 [–6.03, –1.81]) and on admission (adjusted mean difference –3.67 [–6.98, –0.36]; Table 3).
DISCUSSION
Within a multicenter cohort of 2,309 children hospitalized with ARI, we noted significantly lower HRQOL scores among children exposed to SHS in the home as compared with nonexposed children. Differences were greatest for children living with ≥2 smokers in the home. In analyses stratified by diagnosis, differences in baseline health HRQOL scores were greatest for children with bronchiolitis and pneumonia. Differences in acute illness scores were greatest for children with croup.16
Our study provides evidence for acute and chronic impacts of SHS on HRQOL in children hospitalized with ARI. Although several studies have linked SHS exposure to reduced HRQOL in adults,19,20 few similar studies have been conducted in children. Nonetheless, a wealth of studies have documented the negative impact of SHS exposure on clinical outcomes among children with ARI.8,10,21-23 Our findings that home SHS exposure was associated with reduced HRQOL among our cohort of children with ARI are therefore consistent with related findings in adults and children. The observation that the effects of SHS exposure on HRQOL were greatest among children living with ≥2 smokers provides further evidence of a potential causal link between regular SHS exposure and HRQOL.
Although the magnitude and significance of associations between SHS exposure and HRQOL varied for each of the four diagnoses for baseline health and the acute illness, it is important to note that the point estimates for the adjusted mean differences were uniformly lower for the SHS-exposed children in each subgroup. Even so, only acute illness scores for croup exceeded the MCID threshold.16 Croup is the only included condition of the upper airway and is characterized by laryngotracheal inflammation leading to the typical cough and, in moderate to severe cases, stridor. Given that chronic SHS exposure induces a proinflammatory state,3 it is possible that SHS-exposed children with croup had more severe illness compared with nonexposed children with croup resulting in lower HRQOL scores on admission. Further, perceived differences in illness severity and HRQOL may be more readily apparent in children with croup (eg, stridor at rest vs intermittent or no stridor) as compared with children with lower respiratory tract diseases.
Of the four included diagnoses, the link between SHS exposure and asthma outcomes has been most studied. Prior work has demonstrated more frequent and severe acute exacerbations, as well as worse long-term lung function among SHS-exposed children as compared with nonexposed children.22-24 It was, therefore, surprising that our study failed to demonstrate associations between SHS exposure and HRQOL among children with asthma. Reasons for this finding are unclear. One hypothesis is that caregivers of SHS-exposed children with asthma may be more aware of the impacts of SHS exposure on respiratory health (through prior education) and, thus, more likely to modify their smoking behaviors, or for their children to be on daily asthma controller therapy. Alternatively, caregivers of children with asthma may be more likely to underreport home SHS exposure. Thirty-eight percent of children with asthma, however, were classified as SHS-exposed. This percentage was greater than the other three conditions studied (25%-32%), suggesting that differential bias in underreporting was minimal. Given that children with asthma were older, on average, than children with the other three conditions, it may also be that these children spent more time in smoke-free environments (eg, school).
Nearly one-third of children in our study were exposed to SHS in the home. This is similar to the prevalence of exposure in other studies conducted among hospitalized children8,10,21,25 but higher than the national prevalence of home SHS exposure among children in the United States.26 Thus, hospitalized children represent a particularly vulnerable population and an important target for interventions aiming to reduce exposure to SHS. Although longitudinal interventions are likely necessary to affect long-term success, hospitalization for ARI may serve as a powerful teachable moment to begin cessation efforts. Hospitalization also offers time beyond a typical primary care outpatient encounter to focus on cessation counseling and may be the only opportunity to engage in counseling activities for some families with limited time or access. Further, prior studies have demonstrated both the feasibility and the effectiveness of smoking cessation interventions in hospitalized children.27-30 Unfortunately, however, SHS exposure is often not documented at the time of hospitalization, and many opportunities to intervene are missed.25,31 Thus, there is a need for improved strategies to reliably identify and intervene on SHS-exposed children in the hospital setting.
These findings should be considered in the context of several limitations. The observational nature of our study raises the potential for confounding, specifically with regard to socioeconomic status, as this is associated with both SHS exposure and lower HRQOL. Our modeling approach attempted to control for several factors associated with socioeconomic status, including caregiver education and insurance coverage, but there is potential for residual confounding. No single question is sufficient to fully assess SHS exposure as the intensity of home SHS exposure likely varies widely, and some children may be exposed to SHS outside of the home environment.32 The home, however, is often the most likely source of regular SHS exposure,33,34 especially among young children (our cohort’s mean age was 3.6 years). Misclassification of SHS exposure is also possible due to underreporting of smoking.35,36 As a result, some children regularly exposed to SHS may have been misclassified as nonexposed, and the observed associations between SHS exposure and HRQOL may be underestimated. Confirming our study’s findings using objective assessments of SHS exposure, such as cotinine, are warranted. Given the young age of our cohort, the PedsQL surveys were completed by the parent or legal guardian only in >90% of the enrolled subjects, and caregiver perceptions may not accurately reflect the child’s perceptions. Prior work, however, has demonstrated the validity of parent-proxy reporting of the PedsQL, including correlation with child self-report.37 In our study, correlation between child and caregiver reporting (when available) was also very good (r = 0.72, 95% CI 0.64, 0.77). It is also possible that the timing of the HRQOL assessments (on admission) may have biased perceptions of baseline HRQOL, although we anticipate any bias would likely be nondifferential between SHS-exposed and nonexposed children and across diagnoses.
Nearly one-third of children in our study were exposed to SHS exposure in the home, and SHS exposure was associated with lower HRQOL for baseline health and during acute illness, providing further evidence of the dangers of SHS. Much work is needed in order to eliminate the impact of SHS on child health and families of children hospitalized for respiratory illness should be considered a priority population for smoking cessation efforts.
Acknowledgment
The authors wish to acknowledge the efforts of PRIS-PRIMES study team. The authors also wish to thank the children and families who consented to be a part of the PRIMES study.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
Funding
This study was supported by NIH-NHLBI 1R01HL121067 to RMS.
1. Witt WP, Weiss AJ, Elixhauser A. Overview of Hospital Stays for Children in the United States, 2012: Statistical Brief #187. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006. PubMed
2. Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735-744. PubMed
3. Jinot J, Bayard S. Respiratory health effects of exposure to environmental tobacco smoke. Rev Environ Health. 1996;11(3):89-100. PubMed
4. Wilson KM, Wesgate SC, Pier J, et al. Secondhand smoke exposure and serum cytokine levels in healthy children. Cytokine. 2012;60(1):34-37. PubMed
5. Feleszko W, Zawadzka-Krajewska A, Matysiak K, et al. Parental tobacco smoking is associated with augmented IL-13 secretion in children with allergic asthma. J Allergy Clin Immunol. 2006;117(1):97-102. PubMed
6. Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357-366. PubMed
7. Merianos AL, Dixon CA, Mahabee-Gittens EM. Secondhand smoke exposure, illness severity, and resource utilization in pediatric emergency department patients with respiratory illnesses. J Asthma. 2017;54(8):798-806. PubMed
8. Ahn A, Edwards KM, Grijalva CG, et al. Secondhand Smoke Exposure and Illness Severity among Children Hospitalized with Pneumonia. J Pediatr. 2015;167(4):869-874 e861. PubMed
9. Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168(8):897-905. PubMed
10. Bradley JP, Bacharier LB, Bonfiglio J, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115(1):e7-e14. PubMed
11. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168(12):1114-1121. PubMed
12. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. PubMed
13. Varni JW, Limbers CA, Neighbors K, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011;20(1):45-55.
14. Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res (Hoboken). 2011;63(11):S420-S430. PubMed
15. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126-139. PubMed
16. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329-341. PubMed
17. Varni JW, Burwinkle TM, Seid M. The PedsQL 4.0 as a school population health measure: feasibility, reliability, and validity. Qual Life Res. 2006;15(2):203-215. PubMed
18. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. PubMed
19. Chen J, Wang MP, Wang X, Viswanath K, Lam TH, Chan SS. Secondhand smoke exposure (SHS) and health-related quality of life (HRQoL) in Chinese never smokers in Hong Kong. BMJ Open. 2015;5(9):e007694. PubMed
20. Bridevaux PO, Cornuz J, Gaspoz JM, et al. Secondhand smoke and health-related quality of life in never smokers: results from the SAPALDIA cohort study 2. Arch Intern Med. 2007;167(22):2516-2523. PubMed
21. Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. J Pediatr. 2013;162(1):16-21. PubMed
22. LeSon S, Gershwin ME. Risk factors for asthmatic patients requiring intubation. I. Observations in children. J Asthma. 1995;32(4):285-294. PubMed
23. Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328(23):1665-1669. PubMed
24. Evans D, Levison MJ, Feldman CH, et al. The impact of passive smoking on emergency room visits of urban children with asthma. Am Rev Respir Dis. 1987;135(3):567-572. PubMed
25. Wilson KM, Wesgate SC, Best D, Blumkin AK, Klein JD. Admission screening for secondhand tobacco smoke exposure. Hosp Pediatr. 2012;2(1):26-33. PubMed
26. Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003-2006. Pediatrics. 2009;124(5):1299-1305. PubMed
27. Ralston S, Roohi M. A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561-566. PubMed
28. Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140-145. PubMed
29. Torok MR, Lowary M, Ziniel SI, et al. Perceptions of parental tobacco dependence treatment among a children’s hospital staff. Hosp Pediatr. 2018;8(11):724-728. PubMed
30. Jenssen BP, Shelov ED, Bonafide CP, Bernstein SL, Fiks AG, Bryant-Stephens T. Clinical decision support tool for parental tobacco treatment in hospitalized children. Appl Clin Inform. 2016;7(2):399-411. PubMed
31. Lustre BL, Dixon CA, Merianos AL, Gordon JS, Zhang B, Mahabee-Gittens EM. Assessment of tobacco smoke exposure in the pediatric emergency department. Prev Med. 2016;85:42-46. PubMed
32. Groner JA, Rule AM, McGrath-Morrow SA, et al. Assessing pediatric tobacco exposure using parent report: comparison with hair nicotine. J Expo Sci Environ Epidemiol. 2018;28(6):530-537. PubMed
33. Gergen PJ. Environmental tobacco smoke as a risk factor for respiratory disease in children. Respir Physiol. 2001;128(1):39-46. PubMed
34. Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231-252. PubMed
35. Couluris M, Schnapf BM, Casey A, Xu P, Gross-King M, Krischer J. How to measure secondhand smoke exposure in a pediatric clinic setting. Arch Pediatr Adolesc Med. 2011;165(7):670-671. PubMed
36. Boyaci H, Etiler N, Duman C, Basyigit I, Pala A. Environmental tobacco smoke exposure in school children: parent report and urine cotinine measures. Pediatr Int. 2006;48(4):382-389. PubMed
37. Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5(1):2. PubMed
Acute respiratory illnesses (ARIs), including acute exacerbations of asthma, croup, pneumonia, and bronchiolitis, are among the most common illnesses in childhood.1 Although most ARIs can be managed in the outpatient setting,
Exposure to secondhand smoke (SHS) is a preventable risk factor for ARI in children, particularly when there is regular exposure in the home.2 Chronic exposure to SHS impacts systemic inflammation by suppressing serum interferon-gamma,3 which can lead to increased susceptibility to viral and bacterial infections,4 and increasing Th2 (atopic) cytokine expression, which is associated with asthma.5 SHS exposure in children has also been linked to diminished lung function.6 As a result, SHS exposure is associated with increased ARI susceptibility and severity in children.7-10
Much research has focused on the clinical impact of SHS exposure on respiratory health in children, but little is known about the impact on patient-reported outcomes, such as health-related quality of life (HRQOL). Patient-reported outcomes help provide a comprehensive evaluation of the effectiveness of healthcare delivery systems. These outcomes are increasingly used by health service researchers to better understand patient and caregiver perspectives.11 Given the known associations between SHS exposure and ARI morbidity, we postulated that regular SHS exposure would also impact HRQOL in children. In this study, we assessed the relationship between SHS exposure and HRQOL within a large, multicenter, prospective cohort of children presenting to the emergency department (ED) and/or hospital with ARI.
METHODS
Study Population
This study was nested within the Pediatric Respiratory Illness Measurement System (PRIMES) study, a prospective cohort study of children with ARI in the ED and inpatient settings at five tertiary care children’s hospitals within the Pediatric Research in Inpatient Settings Network in Colorado, Pennsylvania, Tennessee, Texas, and Washington. Eligible children were two weeks to 16 years of age hospitalized after presenting to the ED with a primary diagnosis of asthma, croup, bronchiolitis, or pneumonia between July 1, 2014 and June 30, 2016. Because of an anticipated low frequency of croup hospitalizations, we also included children presenting to the ED and then discharged to home with this diagnosis. Children were assigned to a PRIMES diagnosis group based on their final discharge diagnosis. If there was a discrepancy between admission and discharge diagnoses, the discharge diagnosis was used. If a child had more than one discharge diagnosis for a PRIMES condition (eg, acute asthma and pneumonia), we chose the PRIMES condition with the lowest total enrollments overall. If the final discharge diagnosis was not a PRIMES condition, the case was excluded from further analysis. Patients with immunodeficiency, cystic fibrosis, a history of prematurity <32 weeks, chronic neuromuscular disease, cardiovascular disease, pulmonary diseases (other than asthma), and moderate to severe developmental delay were also excluded. Children admitted to intensive care were eligible only if they were transferred to an acute care ward <72 hours following admission. A survey was administered at the time of enrollment that collected information on SHS exposure, HRQOL, healthcare utilization, and demographics. All study procedures were reviewed and approved by the institutional review boards at each of the participating hospitals.
SECONDHAND SMOKE EXPOSURE
To ascertain SHS exposure, we asked caregivers, “How many persons living in the child’s home smoke?” Responses were dichotomized into non-SHS exposed (0 smokers) and SHS exposed (≥1 smokers). Children with missing data on home SHS exposure were excluded.
Health-Related Quality of Life Outcomes
We estimated HRQOL using the Pediatric Quality of Life (PedsQLTM) 4.0 Generic Core and Infant Scales. The PedsQL instruments are validated, population HRQOL measures that evaluate the physical, mental, emotional, and social functioning of children two to 18 years old based on self- or caregiver-proxy report.12-15 These instruments have also shown responsiveness as well as construct and predictive validity in hospitalized children.11 For this study, we focused on the PedsQL physical functioning subscale, which assesses for problems with physical activities (eg, sports activity or exercise, low energy, and hurts or aches) on a five-point Likert scale (never to almost always a problem). Scores range from 0 to 100 with higher scores indicating a better HRQOL. The reported minimal clinically important difference (MCID), defined as the smallest difference in which individuals would perceive a benefit or would necessitate a change in management, for this scale is 4.5 points.16,17
Children >8 years old were invited to complete the self-report version of the PedsQL. For children <8 years old, and for older children who were unable to complete them, surveys were completed by a parent or legal guardian. Respondents were asked to assess perceptions of their (or their child’s) HRQOL during periods of baseline health (the child’s usual state of health in the month preceding the current illness) and during the acute illness (the child’s state of health at the time of admission) as SHS exposure may influence perceptions of general health and/or contribute to worse outcomes during periods of acute illness.
Covariates collected at the time of enrollment included sociodemographics (child age, gender, race/ethnicity, and caregiver education), and healthcare utilization (caregiver-reported patient visits to a healthcare provider in the preceding six months). Insurance status and level of medical complexity (using the Pediatric Medical Complexity Algorithm)18 were obtained using the Pediatric Hospital Information System database, an administrative database containing clinical and resource utilization data from >45 children’s hospitals in the United States including all of the PRIMES study hospitals.13
Analysis
Descriptive statistics included frequency (%) and mean (standard deviation). Bivariate comparisons according to SHS exposure status were analyzed using chi-squared tests for categorical variables and analysis of variance for continuous variables. Multivariable linear mixed regression models were used to examine associations between home SHS exposure and HRQOL for baseline health and during admission, overall and stratified by diagnosis. Covariates in each model included age, sex, race/ethnicity, caregiver education, and healthcare visits in the preceding six months. We also included a hospital random effect to account for clustering of patients within hospitals and used robust standard errors for inference.
In a secondary analysis to explore potential dose-response effects of SHS exposure, we examined associations between an ordinal exposure variable (0 smokers, 1 smoker, ≥2 smokers) and HRQOL for baseline health and during admission for the acute illness. Because of sample size limitations, diagnosis-specific analyses examining dose-response effects were not conducted.
RESULTS
Study Population
Of the 2,334 children enrolled in the PRIMES study, 25 (1%) respondents did not report on home SHS exposure and were excluded, yielding a final study population of 2,309 children, of whom 728 (32%) had reported home SHS exposure. The study population included 664 children with asthma (mean age seven years [3.5]; 38% with home SHS exposure), 740 with bronchiolitis (mean age 0.7 years [0.5]; 32% with home SHS exposure), 342 with croup (mean age 1.7 [1.1]; 25% with home SHS exposure), and 563 with pneumonia (mean age 4.4 [3.8]; 27% with home SHS exposure; Table 1). Compared with non-SHS-exposed children, those with home SHS exposure tend to be slightly older (3.9 vs 3.4 years, P = .01), more likely to be non-Hispanic Black (29% vs 19%, P < .001), to have a chronic condition (52% vs 41%, P < .001), to come from a household where caregiver(s) did not graduate from college (45% vs 29%, P < .001), and to have public insurance (73% vs 49%, P < .001).
Home SHS Exposure and Health-related Quality of Life
The overall mean HRQOL score for baseline health was 83 (15), with a range across diagnoses of 82 to 87. Compared with non-SHS-exposed children, children with home SHS exposure had a lower mean HRQOL score for baseline health (adjusted mean difference –3.04 [95% CI -4.34, –1.74]). In analyses stratified by diagnosis, baseline health scores were lower for SHS-exposed children for all four conditions, but differences were statistically significant only for bronchiolitis (adjusted mean difference –2.94 [–5.0, –0.89]) and pneumonia (adjusted mean value –4.13 [–6.82, –1.44]; Table 2); none of these differences met the MCID threshold.
The overall mean HRQOL score at the time of admission was 56 (23), with a range across diagnoses of 49 to 61, with lower scores noted among SHS-exposed children compared with non-SHS-exposed children (adjusted mean difference –2.16 [–4.22, –0.10]). Similar to scores representing baseline health, admission scores were lower across all four conditions for SHS-exposed children. Only children with croup, however, had significantly lower admission scores that also met the MCID threshold (adjusted mean difference –5.71 [–10.67, –0.75]; Table 2).
To assess for potential dose-response effects of SHS exposure on HRQOL, we stratified SHS-exposed children into those with one smoker in the home (n = 513) and those with ≥2 smokers in the home (n = 215). Compared with non-SHS-exposed children, both HRQOL scores (baseline health and admission) were lower for SHS-exposed children. Consistent with a dose-response association, scores were lowest for children with ≥2 smokers in the home, both at baseline health (adjusted mean difference –3.92 [–6.03, –1.81]) and on admission (adjusted mean difference –3.67 [–6.98, –0.36]; Table 3).
DISCUSSION
Within a multicenter cohort of 2,309 children hospitalized with ARI, we noted significantly lower HRQOL scores among children exposed to SHS in the home as compared with nonexposed children. Differences were greatest for children living with ≥2 smokers in the home. In analyses stratified by diagnosis, differences in baseline health HRQOL scores were greatest for children with bronchiolitis and pneumonia. Differences in acute illness scores were greatest for children with croup.16
Our study provides evidence for acute and chronic impacts of SHS on HRQOL in children hospitalized with ARI. Although several studies have linked SHS exposure to reduced HRQOL in adults,19,20 few similar studies have been conducted in children. Nonetheless, a wealth of studies have documented the negative impact of SHS exposure on clinical outcomes among children with ARI.8,10,21-23 Our findings that home SHS exposure was associated with reduced HRQOL among our cohort of children with ARI are therefore consistent with related findings in adults and children. The observation that the effects of SHS exposure on HRQOL were greatest among children living with ≥2 smokers provides further evidence of a potential causal link between regular SHS exposure and HRQOL.
Although the magnitude and significance of associations between SHS exposure and HRQOL varied for each of the four diagnoses for baseline health and the acute illness, it is important to note that the point estimates for the adjusted mean differences were uniformly lower for the SHS-exposed children in each subgroup. Even so, only acute illness scores for croup exceeded the MCID threshold.16 Croup is the only included condition of the upper airway and is characterized by laryngotracheal inflammation leading to the typical cough and, in moderate to severe cases, stridor. Given that chronic SHS exposure induces a proinflammatory state,3 it is possible that SHS-exposed children with croup had more severe illness compared with nonexposed children with croup resulting in lower HRQOL scores on admission. Further, perceived differences in illness severity and HRQOL may be more readily apparent in children with croup (eg, stridor at rest vs intermittent or no stridor) as compared with children with lower respiratory tract diseases.
Of the four included diagnoses, the link between SHS exposure and asthma outcomes has been most studied. Prior work has demonstrated more frequent and severe acute exacerbations, as well as worse long-term lung function among SHS-exposed children as compared with nonexposed children.22-24 It was, therefore, surprising that our study failed to demonstrate associations between SHS exposure and HRQOL among children with asthma. Reasons for this finding are unclear. One hypothesis is that caregivers of SHS-exposed children with asthma may be more aware of the impacts of SHS exposure on respiratory health (through prior education) and, thus, more likely to modify their smoking behaviors, or for their children to be on daily asthma controller therapy. Alternatively, caregivers of children with asthma may be more likely to underreport home SHS exposure. Thirty-eight percent of children with asthma, however, were classified as SHS-exposed. This percentage was greater than the other three conditions studied (25%-32%), suggesting that differential bias in underreporting was minimal. Given that children with asthma were older, on average, than children with the other three conditions, it may also be that these children spent more time in smoke-free environments (eg, school).
Nearly one-third of children in our study were exposed to SHS in the home. This is similar to the prevalence of exposure in other studies conducted among hospitalized children8,10,21,25 but higher than the national prevalence of home SHS exposure among children in the United States.26 Thus, hospitalized children represent a particularly vulnerable population and an important target for interventions aiming to reduce exposure to SHS. Although longitudinal interventions are likely necessary to affect long-term success, hospitalization for ARI may serve as a powerful teachable moment to begin cessation efforts. Hospitalization also offers time beyond a typical primary care outpatient encounter to focus on cessation counseling and may be the only opportunity to engage in counseling activities for some families with limited time or access. Further, prior studies have demonstrated both the feasibility and the effectiveness of smoking cessation interventions in hospitalized children.27-30 Unfortunately, however, SHS exposure is often not documented at the time of hospitalization, and many opportunities to intervene are missed.25,31 Thus, there is a need for improved strategies to reliably identify and intervene on SHS-exposed children in the hospital setting.
These findings should be considered in the context of several limitations. The observational nature of our study raises the potential for confounding, specifically with regard to socioeconomic status, as this is associated with both SHS exposure and lower HRQOL. Our modeling approach attempted to control for several factors associated with socioeconomic status, including caregiver education and insurance coverage, but there is potential for residual confounding. No single question is sufficient to fully assess SHS exposure as the intensity of home SHS exposure likely varies widely, and some children may be exposed to SHS outside of the home environment.32 The home, however, is often the most likely source of regular SHS exposure,33,34 especially among young children (our cohort’s mean age was 3.6 years). Misclassification of SHS exposure is also possible due to underreporting of smoking.35,36 As a result, some children regularly exposed to SHS may have been misclassified as nonexposed, and the observed associations between SHS exposure and HRQOL may be underestimated. Confirming our study’s findings using objective assessments of SHS exposure, such as cotinine, are warranted. Given the young age of our cohort, the PedsQL surveys were completed by the parent or legal guardian only in >90% of the enrolled subjects, and caregiver perceptions may not accurately reflect the child’s perceptions. Prior work, however, has demonstrated the validity of parent-proxy reporting of the PedsQL, including correlation with child self-report.37 In our study, correlation between child and caregiver reporting (when available) was also very good (r = 0.72, 95% CI 0.64, 0.77). It is also possible that the timing of the HRQOL assessments (on admission) may have biased perceptions of baseline HRQOL, although we anticipate any bias would likely be nondifferential between SHS-exposed and nonexposed children and across diagnoses.
Nearly one-third of children in our study were exposed to SHS exposure in the home, and SHS exposure was associated with lower HRQOL for baseline health and during acute illness, providing further evidence of the dangers of SHS. Much work is needed in order to eliminate the impact of SHS on child health and families of children hospitalized for respiratory illness should be considered a priority population for smoking cessation efforts.
Acknowledgment
The authors wish to acknowledge the efforts of PRIS-PRIMES study team. The authors also wish to thank the children and families who consented to be a part of the PRIMES study.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
Funding
This study was supported by NIH-NHLBI 1R01HL121067 to RMS.
Acute respiratory illnesses (ARIs), including acute exacerbations of asthma, croup, pneumonia, and bronchiolitis, are among the most common illnesses in childhood.1 Although most ARIs can be managed in the outpatient setting,
Exposure to secondhand smoke (SHS) is a preventable risk factor for ARI in children, particularly when there is regular exposure in the home.2 Chronic exposure to SHS impacts systemic inflammation by suppressing serum interferon-gamma,3 which can lead to increased susceptibility to viral and bacterial infections,4 and increasing Th2 (atopic) cytokine expression, which is associated with asthma.5 SHS exposure in children has also been linked to diminished lung function.6 As a result, SHS exposure is associated with increased ARI susceptibility and severity in children.7-10
Much research has focused on the clinical impact of SHS exposure on respiratory health in children, but little is known about the impact on patient-reported outcomes, such as health-related quality of life (HRQOL). Patient-reported outcomes help provide a comprehensive evaluation of the effectiveness of healthcare delivery systems. These outcomes are increasingly used by health service researchers to better understand patient and caregiver perspectives.11 Given the known associations between SHS exposure and ARI morbidity, we postulated that regular SHS exposure would also impact HRQOL in children. In this study, we assessed the relationship between SHS exposure and HRQOL within a large, multicenter, prospective cohort of children presenting to the emergency department (ED) and/or hospital with ARI.
METHODS
Study Population
This study was nested within the Pediatric Respiratory Illness Measurement System (PRIMES) study, a prospective cohort study of children with ARI in the ED and inpatient settings at five tertiary care children’s hospitals within the Pediatric Research in Inpatient Settings Network in Colorado, Pennsylvania, Tennessee, Texas, and Washington. Eligible children were two weeks to 16 years of age hospitalized after presenting to the ED with a primary diagnosis of asthma, croup, bronchiolitis, or pneumonia between July 1, 2014 and June 30, 2016. Because of an anticipated low frequency of croup hospitalizations, we also included children presenting to the ED and then discharged to home with this diagnosis. Children were assigned to a PRIMES diagnosis group based on their final discharge diagnosis. If there was a discrepancy between admission and discharge diagnoses, the discharge diagnosis was used. If a child had more than one discharge diagnosis for a PRIMES condition (eg, acute asthma and pneumonia), we chose the PRIMES condition with the lowest total enrollments overall. If the final discharge diagnosis was not a PRIMES condition, the case was excluded from further analysis. Patients with immunodeficiency, cystic fibrosis, a history of prematurity <32 weeks, chronic neuromuscular disease, cardiovascular disease, pulmonary diseases (other than asthma), and moderate to severe developmental delay were also excluded. Children admitted to intensive care were eligible only if they were transferred to an acute care ward <72 hours following admission. A survey was administered at the time of enrollment that collected information on SHS exposure, HRQOL, healthcare utilization, and demographics. All study procedures were reviewed and approved by the institutional review boards at each of the participating hospitals.
SECONDHAND SMOKE EXPOSURE
To ascertain SHS exposure, we asked caregivers, “How many persons living in the child’s home smoke?” Responses were dichotomized into non-SHS exposed (0 smokers) and SHS exposed (≥1 smokers). Children with missing data on home SHS exposure were excluded.
Health-Related Quality of Life Outcomes
We estimated HRQOL using the Pediatric Quality of Life (PedsQLTM) 4.0 Generic Core and Infant Scales. The PedsQL instruments are validated, population HRQOL measures that evaluate the physical, mental, emotional, and social functioning of children two to 18 years old based on self- or caregiver-proxy report.12-15 These instruments have also shown responsiveness as well as construct and predictive validity in hospitalized children.11 For this study, we focused on the PedsQL physical functioning subscale, which assesses for problems with physical activities (eg, sports activity or exercise, low energy, and hurts or aches) on a five-point Likert scale (never to almost always a problem). Scores range from 0 to 100 with higher scores indicating a better HRQOL. The reported minimal clinically important difference (MCID), defined as the smallest difference in which individuals would perceive a benefit or would necessitate a change in management, for this scale is 4.5 points.16,17
Children >8 years old were invited to complete the self-report version of the PedsQL. For children <8 years old, and for older children who were unable to complete them, surveys were completed by a parent or legal guardian. Respondents were asked to assess perceptions of their (or their child’s) HRQOL during periods of baseline health (the child’s usual state of health in the month preceding the current illness) and during the acute illness (the child’s state of health at the time of admission) as SHS exposure may influence perceptions of general health and/or contribute to worse outcomes during periods of acute illness.
Covariates collected at the time of enrollment included sociodemographics (child age, gender, race/ethnicity, and caregiver education), and healthcare utilization (caregiver-reported patient visits to a healthcare provider in the preceding six months). Insurance status and level of medical complexity (using the Pediatric Medical Complexity Algorithm)18 were obtained using the Pediatric Hospital Information System database, an administrative database containing clinical and resource utilization data from >45 children’s hospitals in the United States including all of the PRIMES study hospitals.13
Analysis
Descriptive statistics included frequency (%) and mean (standard deviation). Bivariate comparisons according to SHS exposure status were analyzed using chi-squared tests for categorical variables and analysis of variance for continuous variables. Multivariable linear mixed regression models were used to examine associations between home SHS exposure and HRQOL for baseline health and during admission, overall and stratified by diagnosis. Covariates in each model included age, sex, race/ethnicity, caregiver education, and healthcare visits in the preceding six months. We also included a hospital random effect to account for clustering of patients within hospitals and used robust standard errors for inference.
In a secondary analysis to explore potential dose-response effects of SHS exposure, we examined associations between an ordinal exposure variable (0 smokers, 1 smoker, ≥2 smokers) and HRQOL for baseline health and during admission for the acute illness. Because of sample size limitations, diagnosis-specific analyses examining dose-response effects were not conducted.
RESULTS
Study Population
Of the 2,334 children enrolled in the PRIMES study, 25 (1%) respondents did not report on home SHS exposure and were excluded, yielding a final study population of 2,309 children, of whom 728 (32%) had reported home SHS exposure. The study population included 664 children with asthma (mean age seven years [3.5]; 38% with home SHS exposure), 740 with bronchiolitis (mean age 0.7 years [0.5]; 32% with home SHS exposure), 342 with croup (mean age 1.7 [1.1]; 25% with home SHS exposure), and 563 with pneumonia (mean age 4.4 [3.8]; 27% with home SHS exposure; Table 1). Compared with non-SHS-exposed children, those with home SHS exposure tend to be slightly older (3.9 vs 3.4 years, P = .01), more likely to be non-Hispanic Black (29% vs 19%, P < .001), to have a chronic condition (52% vs 41%, P < .001), to come from a household where caregiver(s) did not graduate from college (45% vs 29%, P < .001), and to have public insurance (73% vs 49%, P < .001).
Home SHS Exposure and Health-related Quality of Life
The overall mean HRQOL score for baseline health was 83 (15), with a range across diagnoses of 82 to 87. Compared with non-SHS-exposed children, children with home SHS exposure had a lower mean HRQOL score for baseline health (adjusted mean difference –3.04 [95% CI -4.34, –1.74]). In analyses stratified by diagnosis, baseline health scores were lower for SHS-exposed children for all four conditions, but differences were statistically significant only for bronchiolitis (adjusted mean difference –2.94 [–5.0, –0.89]) and pneumonia (adjusted mean value –4.13 [–6.82, –1.44]; Table 2); none of these differences met the MCID threshold.
The overall mean HRQOL score at the time of admission was 56 (23), with a range across diagnoses of 49 to 61, with lower scores noted among SHS-exposed children compared with non-SHS-exposed children (adjusted mean difference –2.16 [–4.22, –0.10]). Similar to scores representing baseline health, admission scores were lower across all four conditions for SHS-exposed children. Only children with croup, however, had significantly lower admission scores that also met the MCID threshold (adjusted mean difference –5.71 [–10.67, –0.75]; Table 2).
To assess for potential dose-response effects of SHS exposure on HRQOL, we stratified SHS-exposed children into those with one smoker in the home (n = 513) and those with ≥2 smokers in the home (n = 215). Compared with non-SHS-exposed children, both HRQOL scores (baseline health and admission) were lower for SHS-exposed children. Consistent with a dose-response association, scores were lowest for children with ≥2 smokers in the home, both at baseline health (adjusted mean difference –3.92 [–6.03, –1.81]) and on admission (adjusted mean difference –3.67 [–6.98, –0.36]; Table 3).
DISCUSSION
Within a multicenter cohort of 2,309 children hospitalized with ARI, we noted significantly lower HRQOL scores among children exposed to SHS in the home as compared with nonexposed children. Differences were greatest for children living with ≥2 smokers in the home. In analyses stratified by diagnosis, differences in baseline health HRQOL scores were greatest for children with bronchiolitis and pneumonia. Differences in acute illness scores were greatest for children with croup.16
Our study provides evidence for acute and chronic impacts of SHS on HRQOL in children hospitalized with ARI. Although several studies have linked SHS exposure to reduced HRQOL in adults,19,20 few similar studies have been conducted in children. Nonetheless, a wealth of studies have documented the negative impact of SHS exposure on clinical outcomes among children with ARI.8,10,21-23 Our findings that home SHS exposure was associated with reduced HRQOL among our cohort of children with ARI are therefore consistent with related findings in adults and children. The observation that the effects of SHS exposure on HRQOL were greatest among children living with ≥2 smokers provides further evidence of a potential causal link between regular SHS exposure and HRQOL.
Although the magnitude and significance of associations between SHS exposure and HRQOL varied for each of the four diagnoses for baseline health and the acute illness, it is important to note that the point estimates for the adjusted mean differences were uniformly lower for the SHS-exposed children in each subgroup. Even so, only acute illness scores for croup exceeded the MCID threshold.16 Croup is the only included condition of the upper airway and is characterized by laryngotracheal inflammation leading to the typical cough and, in moderate to severe cases, stridor. Given that chronic SHS exposure induces a proinflammatory state,3 it is possible that SHS-exposed children with croup had more severe illness compared with nonexposed children with croup resulting in lower HRQOL scores on admission. Further, perceived differences in illness severity and HRQOL may be more readily apparent in children with croup (eg, stridor at rest vs intermittent or no stridor) as compared with children with lower respiratory tract diseases.
Of the four included diagnoses, the link between SHS exposure and asthma outcomes has been most studied. Prior work has demonstrated more frequent and severe acute exacerbations, as well as worse long-term lung function among SHS-exposed children as compared with nonexposed children.22-24 It was, therefore, surprising that our study failed to demonstrate associations between SHS exposure and HRQOL among children with asthma. Reasons for this finding are unclear. One hypothesis is that caregivers of SHS-exposed children with asthma may be more aware of the impacts of SHS exposure on respiratory health (through prior education) and, thus, more likely to modify their smoking behaviors, or for their children to be on daily asthma controller therapy. Alternatively, caregivers of children with asthma may be more likely to underreport home SHS exposure. Thirty-eight percent of children with asthma, however, were classified as SHS-exposed. This percentage was greater than the other three conditions studied (25%-32%), suggesting that differential bias in underreporting was minimal. Given that children with asthma were older, on average, than children with the other three conditions, it may also be that these children spent more time in smoke-free environments (eg, school).
Nearly one-third of children in our study were exposed to SHS in the home. This is similar to the prevalence of exposure in other studies conducted among hospitalized children8,10,21,25 but higher than the national prevalence of home SHS exposure among children in the United States.26 Thus, hospitalized children represent a particularly vulnerable population and an important target for interventions aiming to reduce exposure to SHS. Although longitudinal interventions are likely necessary to affect long-term success, hospitalization for ARI may serve as a powerful teachable moment to begin cessation efforts. Hospitalization also offers time beyond a typical primary care outpatient encounter to focus on cessation counseling and may be the only opportunity to engage in counseling activities for some families with limited time or access. Further, prior studies have demonstrated both the feasibility and the effectiveness of smoking cessation interventions in hospitalized children.27-30 Unfortunately, however, SHS exposure is often not documented at the time of hospitalization, and many opportunities to intervene are missed.25,31 Thus, there is a need for improved strategies to reliably identify and intervene on SHS-exposed children in the hospital setting.
These findings should be considered in the context of several limitations. The observational nature of our study raises the potential for confounding, specifically with regard to socioeconomic status, as this is associated with both SHS exposure and lower HRQOL. Our modeling approach attempted to control for several factors associated with socioeconomic status, including caregiver education and insurance coverage, but there is potential for residual confounding. No single question is sufficient to fully assess SHS exposure as the intensity of home SHS exposure likely varies widely, and some children may be exposed to SHS outside of the home environment.32 The home, however, is often the most likely source of regular SHS exposure,33,34 especially among young children (our cohort’s mean age was 3.6 years). Misclassification of SHS exposure is also possible due to underreporting of smoking.35,36 As a result, some children regularly exposed to SHS may have been misclassified as nonexposed, and the observed associations between SHS exposure and HRQOL may be underestimated. Confirming our study’s findings using objective assessments of SHS exposure, such as cotinine, are warranted. Given the young age of our cohort, the PedsQL surveys were completed by the parent or legal guardian only in >90% of the enrolled subjects, and caregiver perceptions may not accurately reflect the child’s perceptions. Prior work, however, has demonstrated the validity of parent-proxy reporting of the PedsQL, including correlation with child self-report.37 In our study, correlation between child and caregiver reporting (when available) was also very good (r = 0.72, 95% CI 0.64, 0.77). It is also possible that the timing of the HRQOL assessments (on admission) may have biased perceptions of baseline HRQOL, although we anticipate any bias would likely be nondifferential between SHS-exposed and nonexposed children and across diagnoses.
Nearly one-third of children in our study were exposed to SHS exposure in the home, and SHS exposure was associated with lower HRQOL for baseline health and during acute illness, providing further evidence of the dangers of SHS. Much work is needed in order to eliminate the impact of SHS on child health and families of children hospitalized for respiratory illness should be considered a priority population for smoking cessation efforts.
Acknowledgment
The authors wish to acknowledge the efforts of PRIS-PRIMES study team. The authors also wish to thank the children and families who consented to be a part of the PRIMES study.
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
Funding
This study was supported by NIH-NHLBI 1R01HL121067 to RMS.
1. Witt WP, Weiss AJ, Elixhauser A. Overview of Hospital Stays for Children in the United States, 2012: Statistical Brief #187. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006. PubMed
2. Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735-744. PubMed
3. Jinot J, Bayard S. Respiratory health effects of exposure to environmental tobacco smoke. Rev Environ Health. 1996;11(3):89-100. PubMed
4. Wilson KM, Wesgate SC, Pier J, et al. Secondhand smoke exposure and serum cytokine levels in healthy children. Cytokine. 2012;60(1):34-37. PubMed
5. Feleszko W, Zawadzka-Krajewska A, Matysiak K, et al. Parental tobacco smoking is associated with augmented IL-13 secretion in children with allergic asthma. J Allergy Clin Immunol. 2006;117(1):97-102. PubMed
6. Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357-366. PubMed
7. Merianos AL, Dixon CA, Mahabee-Gittens EM. Secondhand smoke exposure, illness severity, and resource utilization in pediatric emergency department patients with respiratory illnesses. J Asthma. 2017;54(8):798-806. PubMed
8. Ahn A, Edwards KM, Grijalva CG, et al. Secondhand Smoke Exposure and Illness Severity among Children Hospitalized with Pneumonia. J Pediatr. 2015;167(4):869-874 e861. PubMed
9. Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168(8):897-905. PubMed
10. Bradley JP, Bacharier LB, Bonfiglio J, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115(1):e7-e14. PubMed
11. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168(12):1114-1121. PubMed
12. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. PubMed
13. Varni JW, Limbers CA, Neighbors K, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011;20(1):45-55.
14. Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res (Hoboken). 2011;63(11):S420-S430. PubMed
15. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126-139. PubMed
16. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329-341. PubMed
17. Varni JW, Burwinkle TM, Seid M. The PedsQL 4.0 as a school population health measure: feasibility, reliability, and validity. Qual Life Res. 2006;15(2):203-215. PubMed
18. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. PubMed
19. Chen J, Wang MP, Wang X, Viswanath K, Lam TH, Chan SS. Secondhand smoke exposure (SHS) and health-related quality of life (HRQoL) in Chinese never smokers in Hong Kong. BMJ Open. 2015;5(9):e007694. PubMed
20. Bridevaux PO, Cornuz J, Gaspoz JM, et al. Secondhand smoke and health-related quality of life in never smokers: results from the SAPALDIA cohort study 2. Arch Intern Med. 2007;167(22):2516-2523. PubMed
21. Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. J Pediatr. 2013;162(1):16-21. PubMed
22. LeSon S, Gershwin ME. Risk factors for asthmatic patients requiring intubation. I. Observations in children. J Asthma. 1995;32(4):285-294. PubMed
23. Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328(23):1665-1669. PubMed
24. Evans D, Levison MJ, Feldman CH, et al. The impact of passive smoking on emergency room visits of urban children with asthma. Am Rev Respir Dis. 1987;135(3):567-572. PubMed
25. Wilson KM, Wesgate SC, Best D, Blumkin AK, Klein JD. Admission screening for secondhand tobacco smoke exposure. Hosp Pediatr. 2012;2(1):26-33. PubMed
26. Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003-2006. Pediatrics. 2009;124(5):1299-1305. PubMed
27. Ralston S, Roohi M. A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561-566. PubMed
28. Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140-145. PubMed
29. Torok MR, Lowary M, Ziniel SI, et al. Perceptions of parental tobacco dependence treatment among a children’s hospital staff. Hosp Pediatr. 2018;8(11):724-728. PubMed
30. Jenssen BP, Shelov ED, Bonafide CP, Bernstein SL, Fiks AG, Bryant-Stephens T. Clinical decision support tool for parental tobacco treatment in hospitalized children. Appl Clin Inform. 2016;7(2):399-411. PubMed
31. Lustre BL, Dixon CA, Merianos AL, Gordon JS, Zhang B, Mahabee-Gittens EM. Assessment of tobacco smoke exposure in the pediatric emergency department. Prev Med. 2016;85:42-46. PubMed
32. Groner JA, Rule AM, McGrath-Morrow SA, et al. Assessing pediatric tobacco exposure using parent report: comparison with hair nicotine. J Expo Sci Environ Epidemiol. 2018;28(6):530-537. PubMed
33. Gergen PJ. Environmental tobacco smoke as a risk factor for respiratory disease in children. Respir Physiol. 2001;128(1):39-46. PubMed
34. Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231-252. PubMed
35. Couluris M, Schnapf BM, Casey A, Xu P, Gross-King M, Krischer J. How to measure secondhand smoke exposure in a pediatric clinic setting. Arch Pediatr Adolesc Med. 2011;165(7):670-671. PubMed
36. Boyaci H, Etiler N, Duman C, Basyigit I, Pala A. Environmental tobacco smoke exposure in school children: parent report and urine cotinine measures. Pediatr Int. 2006;48(4):382-389. PubMed
37. Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5(1):2. PubMed
1. Witt WP, Weiss AJ, Elixhauser A. Overview of Hospital Stays for Children in the United States, 2012: Statistical Brief #187. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006. PubMed
2. Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735-744. PubMed
3. Jinot J, Bayard S. Respiratory health effects of exposure to environmental tobacco smoke. Rev Environ Health. 1996;11(3):89-100. PubMed
4. Wilson KM, Wesgate SC, Pier J, et al. Secondhand smoke exposure and serum cytokine levels in healthy children. Cytokine. 2012;60(1):34-37. PubMed
5. Feleszko W, Zawadzka-Krajewska A, Matysiak K, et al. Parental tobacco smoking is associated with augmented IL-13 secretion in children with allergic asthma. J Allergy Clin Immunol. 2006;117(1):97-102. PubMed
6. Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54(4):357-366. PubMed
7. Merianos AL, Dixon CA, Mahabee-Gittens EM. Secondhand smoke exposure, illness severity, and resource utilization in pediatric emergency department patients with respiratory illnesses. J Asthma. 2017;54(8):798-806. PubMed
8. Ahn A, Edwards KM, Grijalva CG, et al. Secondhand Smoke Exposure and Illness Severity among Children Hospitalized with Pneumonia. J Pediatr. 2015;167(4):869-874 e861. PubMed
9. Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168(8):897-905. PubMed
10. Bradley JP, Bacharier LB, Bonfiglio J, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115(1):e7-e14. PubMed
11. Desai AD, Zhou C, Stanford S, Haaland W, Varni JW, Mangione-Smith RM. Validity and responsiveness of the pediatric quality of life inventory (PedsQL) 4.0 generic core scales in the pediatric inpatient setting. JAMA Pediatr. 2014;168(12):1114-1121. PubMed
12. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. PubMed
13. Varni JW, Limbers CA, Neighbors K, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011;20(1):45-55.
14. Hullmann SE, Ryan JL, Ramsey RR, Chaney JM, Mullins LL. Measures of general pediatric quality of life: Child Health Questionnaire (CHQ), DISABKIDS Chronic Generic Measure (DCGM), KINDL-R, Pediatric Quality of Life Inventory (PedsQL) 4.0 Generic Core Scales, and Quality of My Life Questionnaire (QoML). Arthritis Care Res (Hoboken). 2011;63(11):S420-S430. PubMed
15. Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126-139. PubMed
16. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3(6):329-341. PubMed
17. Varni JW, Burwinkle TM, Seid M. The PedsQL 4.0 as a school population health measure: feasibility, reliability, and validity. Qual Life Res. 2006;15(2):203-215. PubMed
18. Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647-e1654. PubMed
19. Chen J, Wang MP, Wang X, Viswanath K, Lam TH, Chan SS. Secondhand smoke exposure (SHS) and health-related quality of life (HRQoL) in Chinese never smokers in Hong Kong. BMJ Open. 2015;5(9):e007694. PubMed
20. Bridevaux PO, Cornuz J, Gaspoz JM, et al. Secondhand smoke and health-related quality of life in never smokers: results from the SAPALDIA cohort study 2. Arch Intern Med. 2007;167(22):2516-2523. PubMed
21. Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. J Pediatr. 2013;162(1):16-21. PubMed
22. LeSon S, Gershwin ME. Risk factors for asthmatic patients requiring intubation. I. Observations in children. J Asthma. 1995;32(4):285-294. PubMed
23. Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328(23):1665-1669. PubMed
24. Evans D, Levison MJ, Feldman CH, et al. The impact of passive smoking on emergency room visits of urban children with asthma. Am Rev Respir Dis. 1987;135(3):567-572. PubMed
25. Wilson KM, Wesgate SC, Best D, Blumkin AK, Klein JD. Admission screening for secondhand tobacco smoke exposure. Hosp Pediatr. 2012;2(1):26-33. PubMed
26. Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003-2006. Pediatrics. 2009;124(5):1299-1305. PubMed
27. Ralston S, Roohi M. A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561-566. PubMed
28. Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140-145. PubMed
29. Torok MR, Lowary M, Ziniel SI, et al. Perceptions of parental tobacco dependence treatment among a children’s hospital staff. Hosp Pediatr. 2018;8(11):724-728. PubMed
30. Jenssen BP, Shelov ED, Bonafide CP, Bernstein SL, Fiks AG, Bryant-Stephens T. Clinical decision support tool for parental tobacco treatment in hospitalized children. Appl Clin Inform. 2016;7(2):399-411. PubMed
31. Lustre BL, Dixon CA, Merianos AL, Gordon JS, Zhang B, Mahabee-Gittens EM. Assessment of tobacco smoke exposure in the pediatric emergency department. Prev Med. 2016;85:42-46. PubMed
32. Groner JA, Rule AM, McGrath-Morrow SA, et al. Assessing pediatric tobacco exposure using parent report: comparison with hair nicotine. J Expo Sci Environ Epidemiol. 2018;28(6):530-537. PubMed
33. Gergen PJ. Environmental tobacco smoke as a risk factor for respiratory disease in children. Respir Physiol. 2001;128(1):39-46. PubMed
34. Klepeis NE, Nelson WC, Ott WR, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231-252. PubMed
35. Couluris M, Schnapf BM, Casey A, Xu P, Gross-King M, Krischer J. How to measure secondhand smoke exposure in a pediatric clinic setting. Arch Pediatr Adolesc Med. 2011;165(7):670-671. PubMed
36. Boyaci H, Etiler N, Duman C, Basyigit I, Pala A. Environmental tobacco smoke exposure in school children: parent report and urine cotinine measures. Pediatr Int. 2006;48(4):382-389. PubMed
37. Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5(1):2. PubMed
© 2019 Society of Hospital Medicine