User login
Vitamin supplementation in healthy patients: What does the evidence support?
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
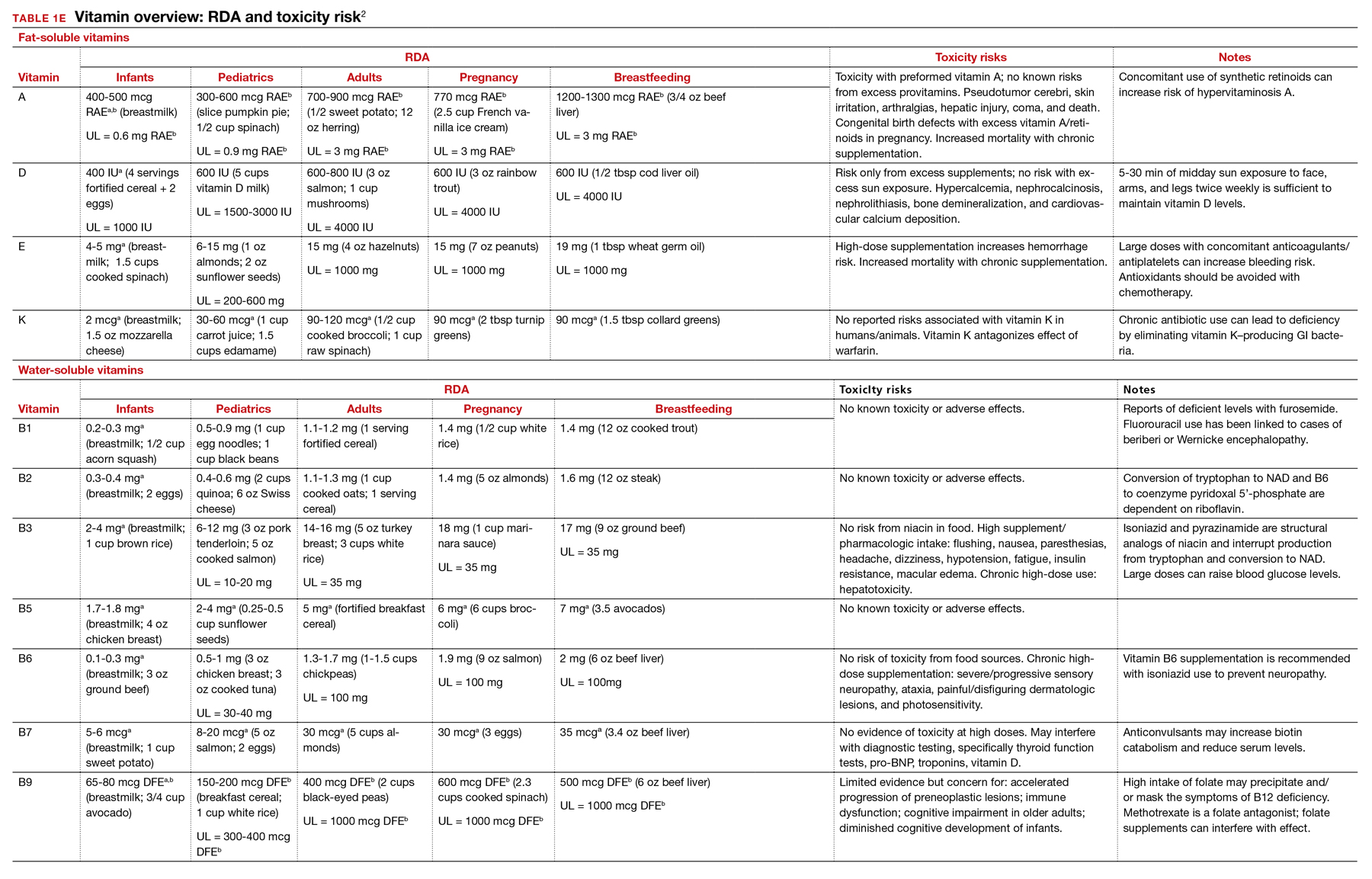
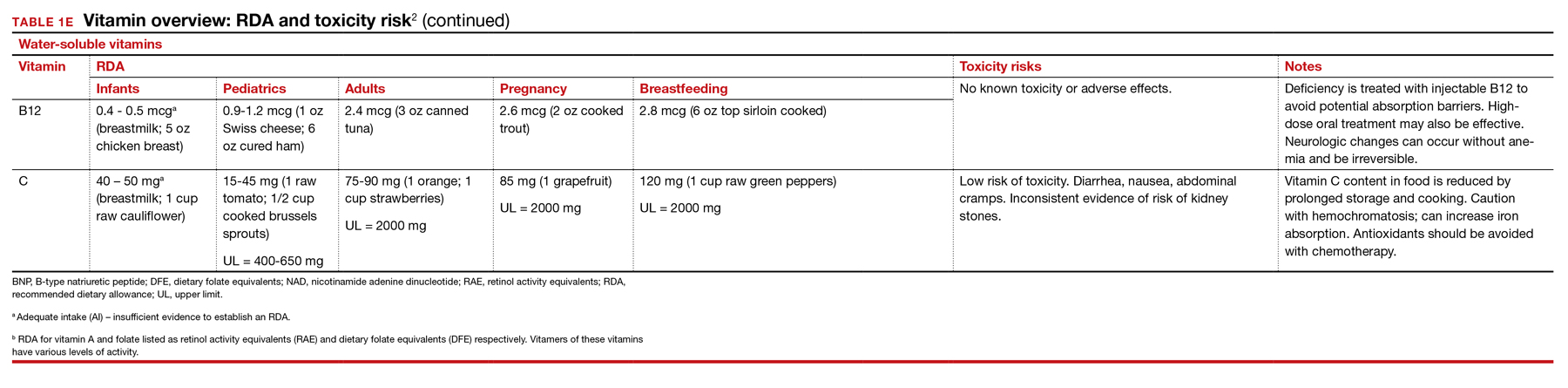
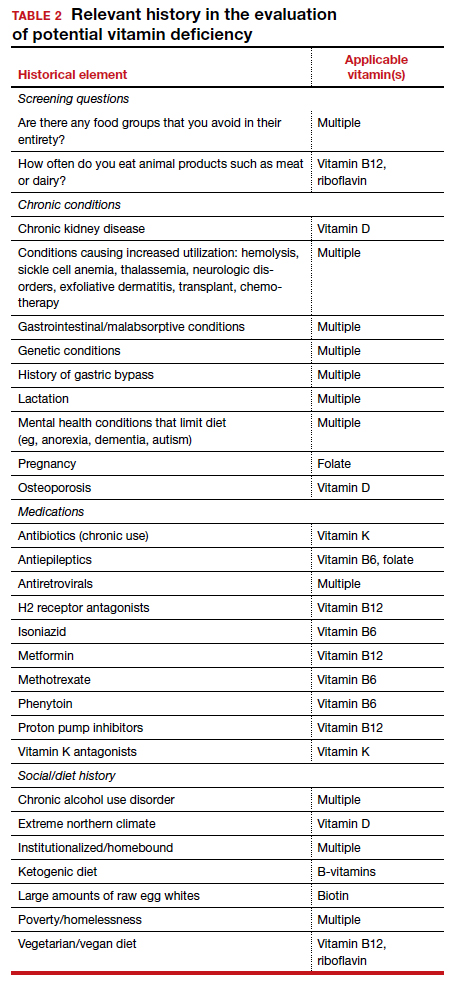
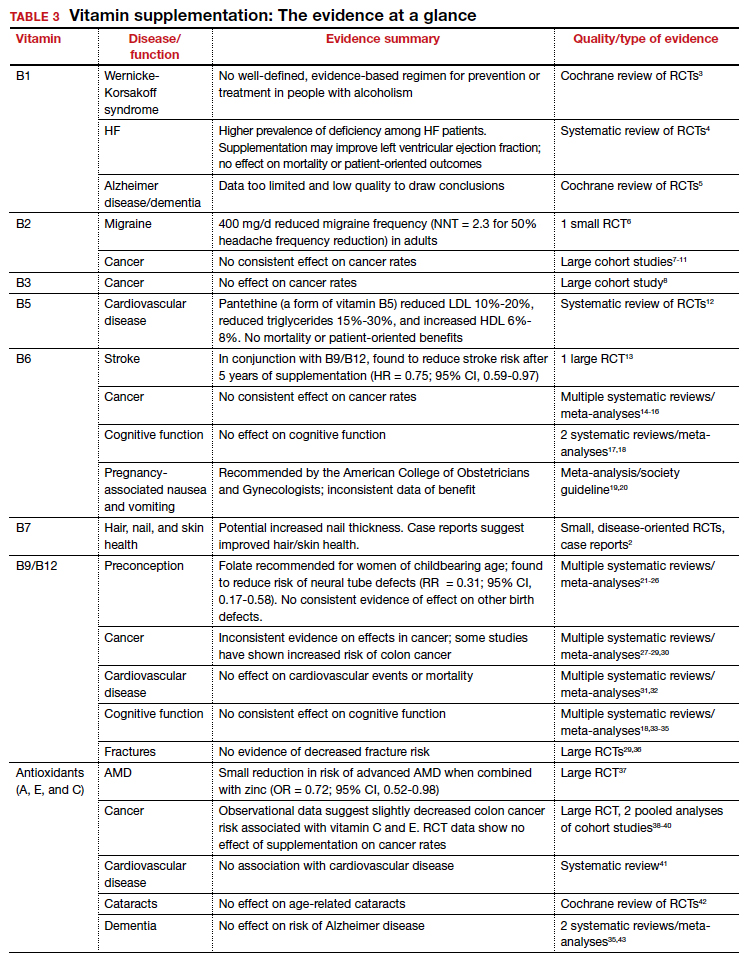
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE E12 lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96
B Complex vitamins
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
The takeaway: Given the lack of evidence, supplementation in the general population is not recommended.
Continue to: Vitamin B2...
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains Riboflavin is essential to energy production, cellular growth, and metabolism.2
The takeaway: Its use as migraine prophylaxis has limited data,97 but there is otherwise no evidence to support health benefits of riboflavin supplementation.
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
The takeaway: There is no evidence supporting a clinical benefit from niacin supplementation.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
The takeaway: There is no data that supplementation of any form of vitamin B5 has any patient-oriented clinical benefits.
Continue to: Vitamin B6...
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
The takeaway: Vitamin B6 is recommended as a potential treatment option for nausea in pregnancy.19 Otherwise, vitamin B6 is readily available in food, deficiency is rare, and no patient-oriented evidence supports supplementation in the general population.
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
The takeaway: No evidence supports the health benefits of biotin supplementation.
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12...
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
While observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation.
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
The takeaway: High-quality RCT evidence demonstrates a protective effect of preconceptual daily folate supplementation in preventing neural tube defects.22 The USPSTF recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects.
Antioxidants
In addition to their individual roles, vitamins A, E, and C are antioxidants, functioning to protect cells from oxidative damage by free radical species.2 Due to this shared role, these vitamins are commonly studied together. Antioxidants are hypothesized to protect from various diseases, including cancer, cardiovascular disease, dementia, autoimmune disorders, depression, cataracts, and age-related vision decline.2,37,107-112
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Continue to: Vitamin A...
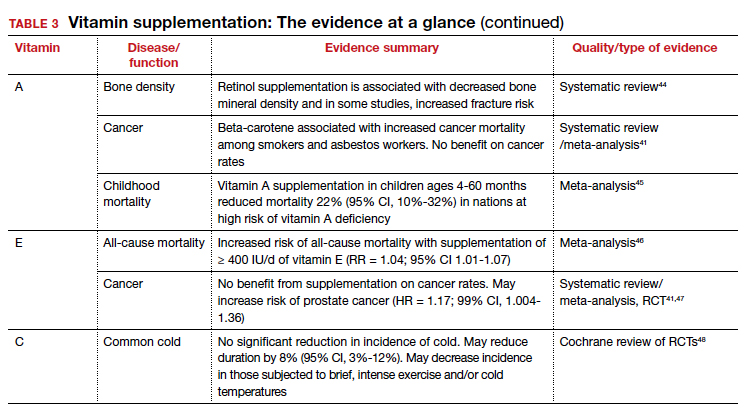
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
The takeaway: Given the USPSTF grade “D” recommendation and concern for potential harms, supplementation is not recommended in healthy patients without risk factors for deficiency.2
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
The takeaway: Vitamin E supplementation’s effects on cancer, cardiovascular disease, ophthalmologic disorders, and cognition have been investigated; data is either lacking to support a benefit or demonstrates harms as outlined above. Given this and the USPSTF grade “D” recommendation, supplementation is not recommended in healthy patients.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Vitamin C supplementation at the onset of illness does not seem to have benefit.
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
The takeaway: Overall, data remain inconclusive as to potential benefits of vitamin C supplementation, although risks of potential harms are likely low.
Continue to: Vitamin D...
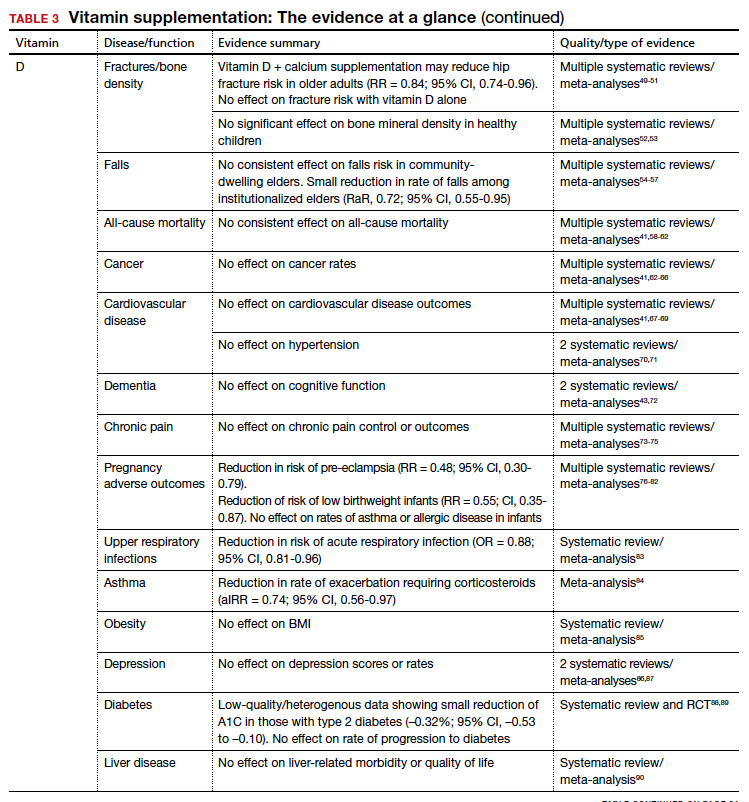
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
The AAP recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
The takeaway: Given these findings, the USPSTF has recommended against (grade D) vitamin D supplementation to prevent falls in community-dwelling elders.55
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
The takeaway: Due to poor availability in breastmilk, the American Academy of Pediatrics (AAP) recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.123 RCT data support high-dose supplementation of lactating women (6400 IU/d) as an alternative strategy to supplementation of the infant.124 The AAP recommends that all nonbreastfeeding infants and older children ingesting < 1000 mL/d of vitamin D–fortified formula or milk should also be supplemented with 400 IU/d of vitamin D.123 Despite these universal recommendations for supplementation, evidence is mixed on the effect of vitamin D supplementation on bone health in children.52,53
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
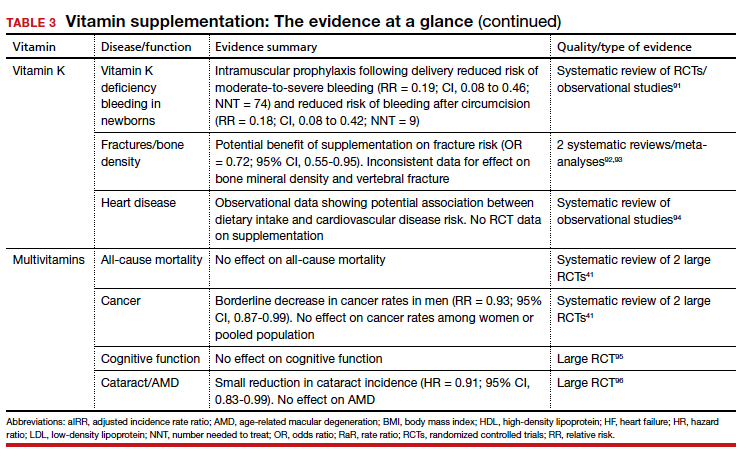
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Children taking multivitamins were often found to have excess levels of potentially harmful nutrients, such as retinol, zinc, and folic acid.
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
The takeaway: The administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of VKDB. Vitamin K may lead to a reduction in fracture risk, but the FDA considers the evidence insufficient. Vitamin K’s potential link to a lowered risk of cardiovascular disease has not been demonstrated with patient-oriented outcomes. Vitamin K has low potential for toxicity, although its interaction with vitamin K antagonists (ie, warfarin) is clinically relevant.
Multivitamins
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
Safe medication storage should be practiced, as multivitamins with iron are a leading cause of poisoning in children.
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
The takeaway: There is limited evidence to support any benefit from multivitamin supplementation. Prenatal multivitamins are generally recommended in the pregnancy and preconception period. Overall, the risks of multivitamins are minimal, although that risk is dependent on the multivitamin’s constituent components.143 Components such as vitamin K may interact with a patient’s medications, and multivitamins have been shown to reduce the circulating levels of antiretrovirals.144 Specifically, multivitamins with iron should be avoided in men and postmenopausal women, and safe medication storage should be practiced as multivitamins with iron are a leading cause of poisoning in children.2
Summary
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE E12 lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96







B Complex vitamins
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
The takeaway: Given the lack of evidence, supplementation in the general population is not recommended.
Continue to: Vitamin B2...
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains Riboflavin is essential to energy production, cellular growth, and metabolism.2
The takeaway: Its use as migraine prophylaxis has limited data,97 but there is otherwise no evidence to support health benefits of riboflavin supplementation.
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
The takeaway: There is no evidence supporting a clinical benefit from niacin supplementation.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
The takeaway: There is no data that supplementation of any form of vitamin B5 has any patient-oriented clinical benefits.
Continue to: Vitamin B6...
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
The takeaway: Vitamin B6 is recommended as a potential treatment option for nausea in pregnancy.19 Otherwise, vitamin B6 is readily available in food, deficiency is rare, and no patient-oriented evidence supports supplementation in the general population.
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
The takeaway: No evidence supports the health benefits of biotin supplementation.
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12...
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
While observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation.
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
The takeaway: High-quality RCT evidence demonstrates a protective effect of preconceptual daily folate supplementation in preventing neural tube defects.22 The USPSTF recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects.
Antioxidants
In addition to their individual roles, vitamins A, E, and C are antioxidants, functioning to protect cells from oxidative damage by free radical species.2 Due to this shared role, these vitamins are commonly studied together. Antioxidants are hypothesized to protect from various diseases, including cancer, cardiovascular disease, dementia, autoimmune disorders, depression, cataracts, and age-related vision decline.2,37,107-112
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Continue to: Vitamin A...
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
The takeaway: Given the USPSTF grade “D” recommendation and concern for potential harms, supplementation is not recommended in healthy patients without risk factors for deficiency.2
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
The takeaway: Vitamin E supplementation’s effects on cancer, cardiovascular disease, ophthalmologic disorders, and cognition have been investigated; data is either lacking to support a benefit or demonstrates harms as outlined above. Given this and the USPSTF grade “D” recommendation, supplementation is not recommended in healthy patients.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Vitamin C supplementation at the onset of illness does not seem to have benefit.
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
The takeaway: Overall, data remain inconclusive as to potential benefits of vitamin C supplementation, although risks of potential harms are likely low.
Continue to: Vitamin D...
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
The AAP recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
The takeaway: Given these findings, the USPSTF has recommended against (grade D) vitamin D supplementation to prevent falls in community-dwelling elders.55
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
The takeaway: Due to poor availability in breastmilk, the American Academy of Pediatrics (AAP) recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.123 RCT data support high-dose supplementation of lactating women (6400 IU/d) as an alternative strategy to supplementation of the infant.124 The AAP recommends that all nonbreastfeeding infants and older children ingesting < 1000 mL/d of vitamin D–fortified formula or milk should also be supplemented with 400 IU/d of vitamin D.123 Despite these universal recommendations for supplementation, evidence is mixed on the effect of vitamin D supplementation on bone health in children.52,53
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Children taking multivitamins were often found to have excess levels of potentially harmful nutrients, such as retinol, zinc, and folic acid.
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
The takeaway: The administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of VKDB. Vitamin K may lead to a reduction in fracture risk, but the FDA considers the evidence insufficient. Vitamin K’s potential link to a lowered risk of cardiovascular disease has not been demonstrated with patient-oriented outcomes. Vitamin K has low potential for toxicity, although its interaction with vitamin K antagonists (ie, warfarin) is clinically relevant.
Multivitamins
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
Safe medication storage should be practiced, as multivitamins with iron are a leading cause of poisoning in children.
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
The takeaway: There is limited evidence to support any benefit from multivitamin supplementation. Prenatal multivitamins are generally recommended in the pregnancy and preconception period. Overall, the risks of multivitamins are minimal, although that risk is dependent on the multivitamin’s constituent components.143 Components such as vitamin K may interact with a patient’s medications, and multivitamins have been shown to reduce the circulating levels of antiretrovirals.144 Specifically, multivitamins with iron should be avoided in men and postmenopausal women, and safe medication storage should be practiced as multivitamins with iron are a leading cause of poisoning in children.2
Summary
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE E12 lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96







B Complex vitamins
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
The takeaway: Given the lack of evidence, supplementation in the general population is not recommended.
Continue to: Vitamin B2...
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains Riboflavin is essential to energy production, cellular growth, and metabolism.2
The takeaway: Its use as migraine prophylaxis has limited data,97 but there is otherwise no evidence to support health benefits of riboflavin supplementation.
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
The takeaway: There is no evidence supporting a clinical benefit from niacin supplementation.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
The takeaway: There is no data that supplementation of any form of vitamin B5 has any patient-oriented clinical benefits.
Continue to: Vitamin B6...
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
The takeaway: Vitamin B6 is recommended as a potential treatment option for nausea in pregnancy.19 Otherwise, vitamin B6 is readily available in food, deficiency is rare, and no patient-oriented evidence supports supplementation in the general population.
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
The takeaway: No evidence supports the health benefits of biotin supplementation.
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12...
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
While observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation.
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
The takeaway: High-quality RCT evidence demonstrates a protective effect of preconceptual daily folate supplementation in preventing neural tube defects.22 The USPSTF recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects.
Antioxidants
In addition to their individual roles, vitamins A, E, and C are antioxidants, functioning to protect cells from oxidative damage by free radical species.2 Due to this shared role, these vitamins are commonly studied together. Antioxidants are hypothesized to protect from various diseases, including cancer, cardiovascular disease, dementia, autoimmune disorders, depression, cataracts, and age-related vision decline.2,37,107-112
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Continue to: Vitamin A...
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
The takeaway: Given the USPSTF grade “D” recommendation and concern for potential harms, supplementation is not recommended in healthy patients without risk factors for deficiency.2
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
The takeaway: Vitamin E supplementation’s effects on cancer, cardiovascular disease, ophthalmologic disorders, and cognition have been investigated; data is either lacking to support a benefit or demonstrates harms as outlined above. Given this and the USPSTF grade “D” recommendation, supplementation is not recommended in healthy patients.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Vitamin C supplementation at the onset of illness does not seem to have benefit.
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
The takeaway: Overall, data remain inconclusive as to potential benefits of vitamin C supplementation, although risks of potential harms are likely low.
Continue to: Vitamin D...
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
The AAP recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
The takeaway: Given these findings, the USPSTF has recommended against (grade D) vitamin D supplementation to prevent falls in community-dwelling elders.55
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
The takeaway: Due to poor availability in breastmilk, the American Academy of Pediatrics (AAP) recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.123 RCT data support high-dose supplementation of lactating women (6400 IU/d) as an alternative strategy to supplementation of the infant.124 The AAP recommends that all nonbreastfeeding infants and older children ingesting < 1000 mL/d of vitamin D–fortified formula or milk should also be supplemented with 400 IU/d of vitamin D.123 Despite these universal recommendations for supplementation, evidence is mixed on the effect of vitamin D supplementation on bone health in children.52,53
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Children taking multivitamins were often found to have excess levels of potentially harmful nutrients, such as retinol, zinc, and folic acid.
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
The takeaway: The administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of VKDB. Vitamin K may lead to a reduction in fracture risk, but the FDA considers the evidence insufficient. Vitamin K’s potential link to a lowered risk of cardiovascular disease has not been demonstrated with patient-oriented outcomes. Vitamin K has low potential for toxicity, although its interaction with vitamin K antagonists (ie, warfarin) is clinically relevant.
Multivitamins
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
Safe medication storage should be practiced, as multivitamins with iron are a leading cause of poisoning in children.
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
The takeaway: There is limited evidence to support any benefit from multivitamin supplementation. Prenatal multivitamins are generally recommended in the pregnancy and preconception period. Overall, the risks of multivitamins are minimal, although that risk is dependent on the multivitamin’s constituent components.143 Components such as vitamin K may interact with a patient’s medications, and multivitamins have been shown to reduce the circulating levels of antiretrovirals.144 Specifically, multivitamins with iron should be avoided in men and postmenopausal women, and safe medication storage should be practiced as multivitamins with iron are a leading cause of poisoning in children.2
Summary
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
Vitamin supplementation in healthy patients: What does the evidence support?
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
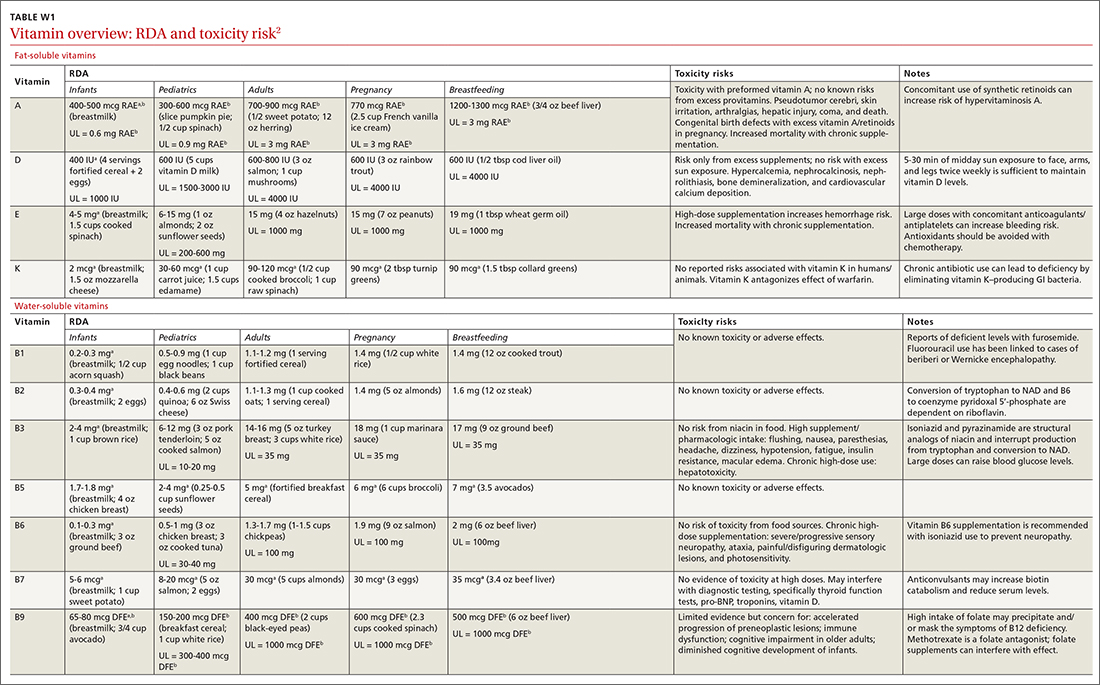
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
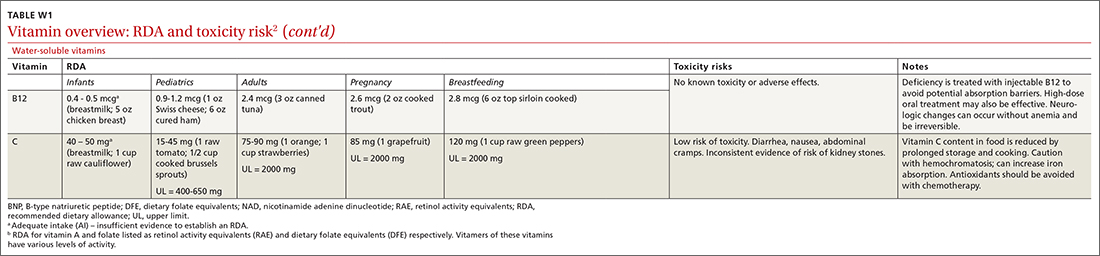
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96

B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
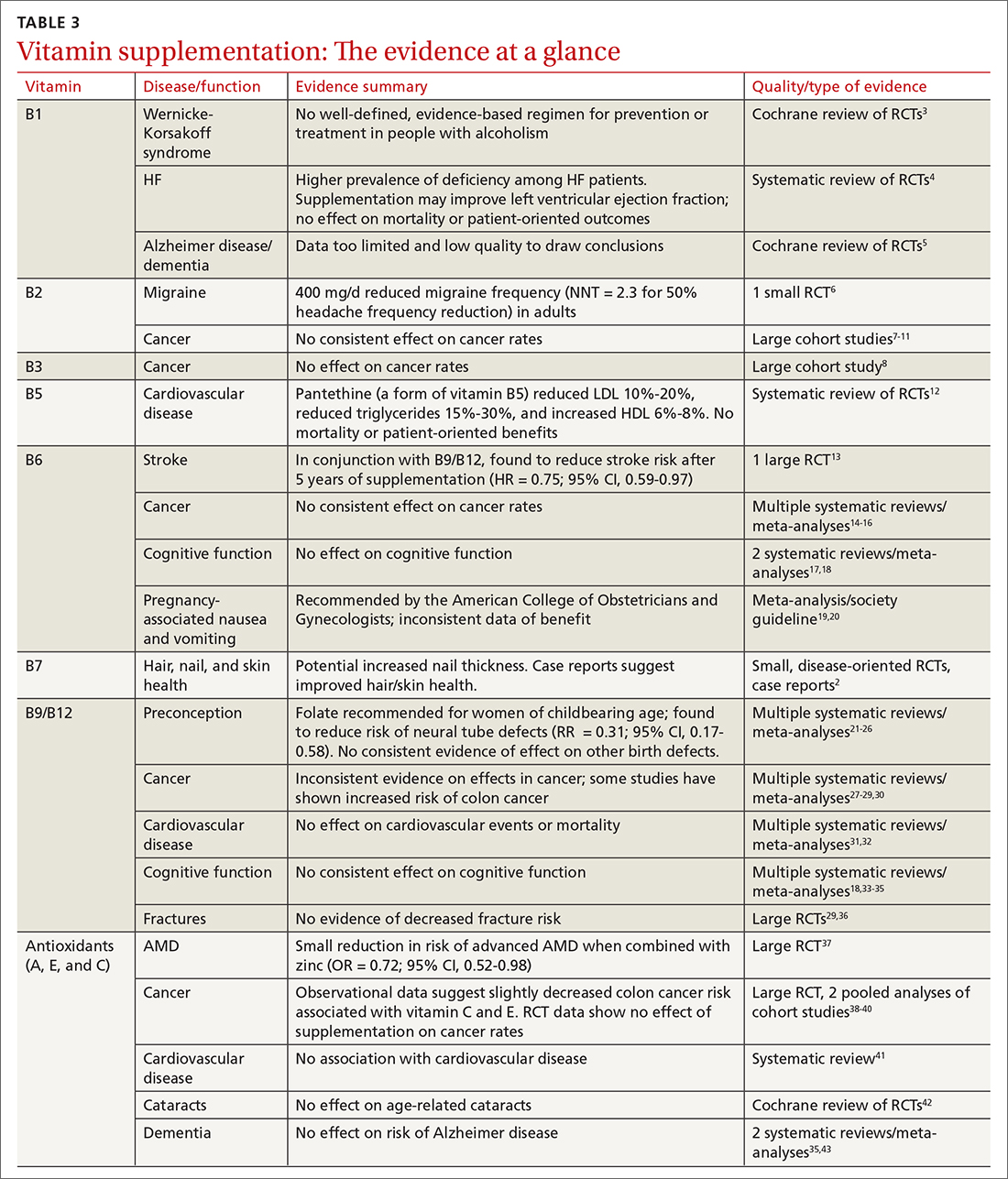
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2
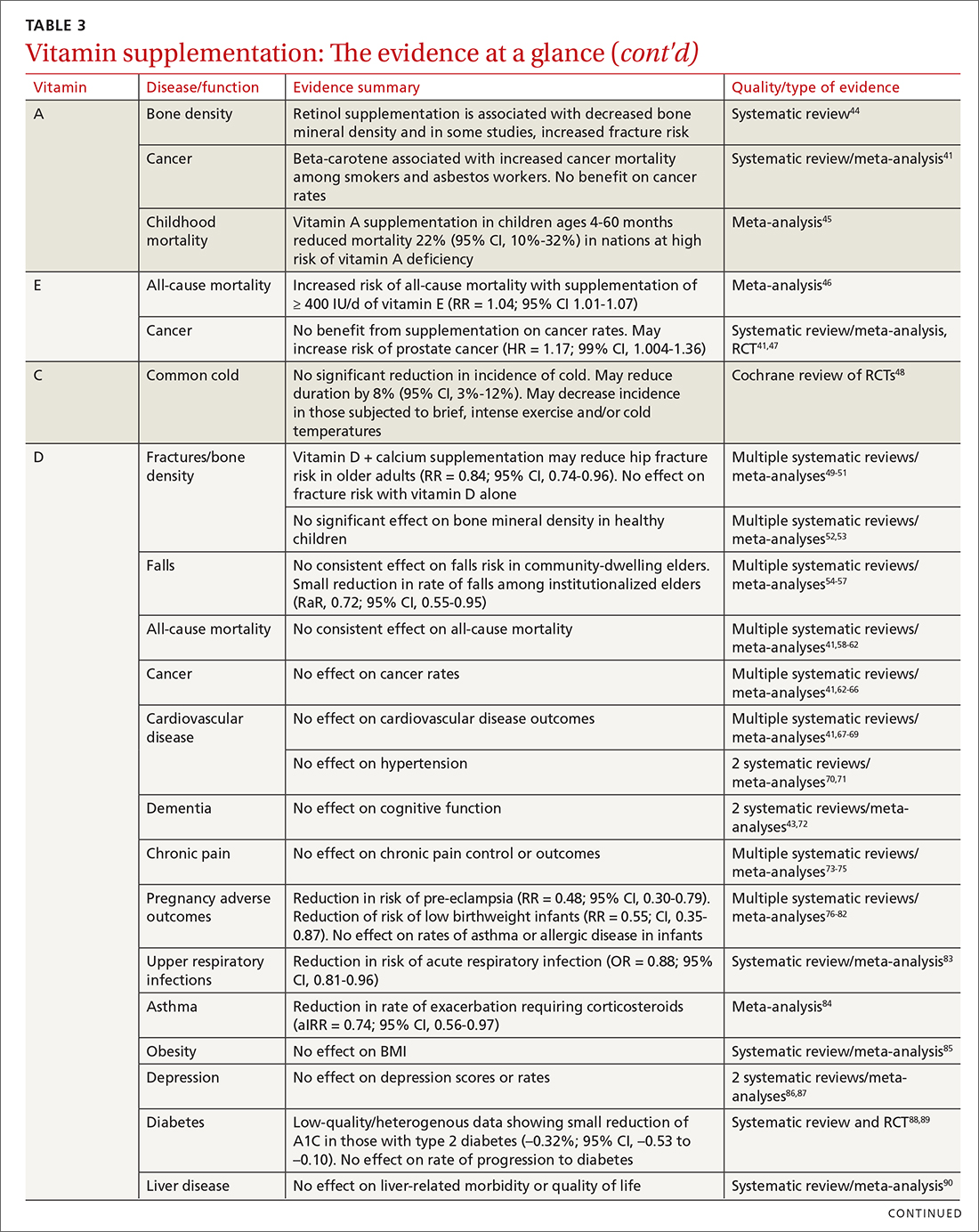
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
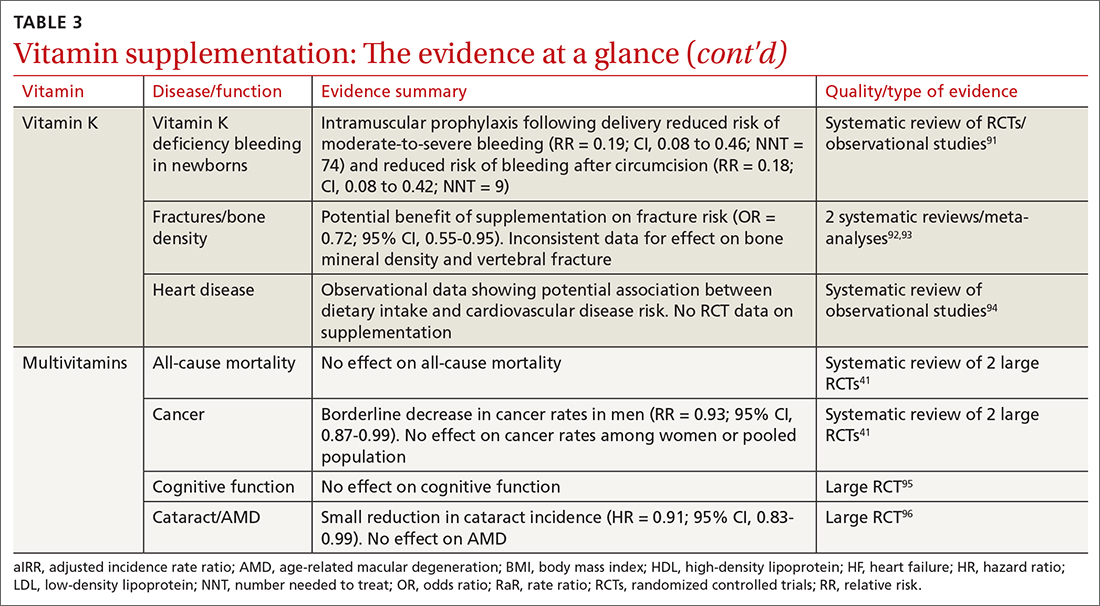
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1

While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.

Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96

B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5

Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2

Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.

Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1

While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.

Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE W12 (available at mdedge.com/familymedicine) lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96

B COMPLEX VITAMINS
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5

Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains
Riboflavin is essential to energy production, cellular growth, and metabolism.2

Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.

Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
Continue to: Vitamin B6
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
Continue to: ANTIOXIDANTS
ANTIOXIDANTS
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils
Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
Continue to: Vitamin D
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
Continue to: MULTIVITAMINS
MULTIVITAMINS
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
SUMMARY
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
CORRESPONDENCE
Joel Herness, MD, 4700 North Las Vegas Boulevard, Nellis AFB, NV 89191; [email protected]
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
GRADE DEFINITIONS
For an explanation of USPSTF grade definitions, see www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/grade-definitions
Prescribe an SGLT2 inhibitor for heart failure in the absence of diabetes?
ILLUSTRATIVE CASE
A 64-year-old overweight White man with a history of hypertension, hyperlipidemia, and HF with an ejection fraction (EF) of 40% presents for primary care follow-up after a recent inpatient admission for worsened HF symptoms. At baseline, he is comfortable at rest but becomes dyspneic upon walking to another room within his home. He is already taking a mineralocorticoid receptor antagonist, a high-intensity statin, a beta-blocker, and an angiotensin-converting enzyme (ACE) inhibitor. What other medication should be considered to minimize his cardiovascular (CV)risk?
An estimated 1% to 2% of the world’s adult population has HF.2 Although the exact prevalence is difficult to quantify due to variations in definitions and diagnostic methods, the American Heart Association (AHA) estimated that 6.2 million Americans had HF between 2013 and 2016.3 Prevalence increases with age, with an annual incidence of approximately 35 per 1000 by age 85.4 Due to the significant morbidity and mortality associated with HF, advancements in treatment are needed.
SGLT2 inhibitors work within the proximal tubule of the kidneys, resulting in increased glucose and sodium excretion with secondary osmotic diuresis and therefore a modest reduction in serum glucose.1,2,5,6 SGLT2 inhibitors are classically prescribed for hyperglycemia treatment in type 2 diabetes. However, preliminary data suggest that this class of medication also positively impacts cardiac function. The diuresis and natriuresis effects of SGLT2 inhibitors appear to optimize cardiac output and subsequent oxygen consumption through a reduction of afterload and preload.1,2,5,6 Further, SGLT2 inhibitors may decrease inflammatory pathways and lead to a secondary reduction of cardiac remodeling via a reduction and modulation of inflammatory pathways. This reduction and modulation may also be associated with a reduction in development, and possibly a reversal, of hypertrophic cardiomyopathy, cardiac fibrosis, and atherosclerosis.5,6 Some of the previously reported adverse effects of SGLT2 inhibitors include urinary tract infection, acute kidney injury, lower extremity amputation, bone fracture, and diabetic ketoacidosis.2
In several studies of patients with type 2 diabetes, SGLT2 inhibitors have shown benefit in reducing CV disease–related death and hospitalization for HF.1,2,5,6 A recent expert consensus from the American College of Cardiology (ACC) states that SGLT2 therapy should be considered for any patient with type 2 diabetes who also has established atherosclerotic CV disease, HF (a clinical syndrome as defined in ACC/AHA guidelines), or diabetic kidney disease, or who is at a high risk for atherosclerotic CV disease (ie, has signs of end-organ damage, such as left ventricular hypertrophy or retinopathy, or multiple risk factors such as advanced age, smoking, hypertension, and family history).7,8
Additionally, a 2019 randomized controlled trial (RCT) by Nassif et al showed that, compared to placebo, dapagliflozin significantly improved both patient-reported HF symptoms and cardiac natriuretic peptide levels over 12 weeks in patients with and without diabetes.9 In September 2020, UpToDate added SGLT2 inhibitors as an option for patients with continued symptoms of HF despite use of appropriate primary agents and mineralocorticoid receptor antagonists, whether or not they have type 2 diabetes; this update was based on 2 studies, 1 of which is reviewed here.10
STUDY SUMMARY
Dapagliflozin demonstrated better CV outcomes than placebo
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) study is an RCT that compared dapagliflozin to placebo among 4744 patients ages 18 years and older who had HF with an EF ≤ 40% and NYHA class II, III, or IV symptoms. The study included patients with (41.8%) and without diabetes. Most patients were male (76.2%-77%), White (70%), and European (44.7%-46.1%).
Patients were randomized to receive either dapagliflozin 10 mg/d or a matching placebo in addition to standard HF therapy (including an ACE inhibitor, angiotensin receptor blocker, or sacubitril-valsartan plus a beta-blocker unless contraindicated; mineralocorticoid antagonist use was encouraged). Follow-up occurred at 14 days, 60 days, 4 months, and then every 4 months, for an average of about 18 months. Patients with diabetes continued to use their glucose-lowering therapies, with dose adjustments, as needed.
Continue to: The primary outcome...
The primary outcome was a composite of worsening HF (hospitalization or urgent visit requiring intravenous HF therapy) or death from a CV cause. Secondary outcomes included a composite of hospitalization for HF or CV death; total number of hospitalizations for HF (including repeat admissions) and CV death; a change in Kansas City Cardiomyopathy Questionnaire symptom score; a composite of worsening renal function including a sustained (≥ 28 d) decline in the estimated glomerular filtration rate (eGFR) of ≥ 50%, end-stage renal disease (defined as sustained eGFR of < 15 mL/min/1.73 m2, sustained dialysis, or renal transplantation), or renal death; and death from any cause.
The primary outcome of worsening HF or death from CV causes occurred in 386 of 2373 patients (16.3%) in the dapagliflozin group and in 502 of 2371 patients (21.2%) in the placebo group (hazard ratio [HR] = 0.74; 95% CI, 0.65-0.85; P < .001). The composite score of hospitalizations for HF plus death from a CV cause was lower in the dapagliflozin group compared to the placebo group (HR = 0.75; 95% CI, 0.65-0.85; P < .001).
A total of 276 patients (11.6%) in the dapagliflozin group and 329 patients (13.9%) in the placebo group died from any cause (HR = 0.83; 95% CI, 0.71-0.97). More patients in the dapagliflozin group than in the placebo group had an improvement in symptom score (58.3% vs 50.9%; odds ratio = 1.15; 95% CI, 1.08-1.23; P < .001). Renal composite outcome did not differ between the 2 treatment groups. Potential adverse effects included volume depletion, renal adverse event, and major hypoglycemia, which occurred at the same rate in the treatment and placebo groups. There was no difference in outcomes or adverse effects between patients with and without diabetes.1
WHAT'S NEW
Evidence supports dapagliflozin use in a new patient population
The DAPA-HF study compared dapagliflozin to placebo in HF patients both with and without diabetes and demonstrated decreased HF exacerbations and CV deaths, improved patient-reported HF symptoms, and lower all-cause mortality in the treatment group. This study supports use of dapagliflozin in a new patient population—those with HF—rather than solely in patients with diabetes, as the drug was originally marketed.
CAVEATS
Specific study population may limit generalizability
The DAPA-HF study included mostly male, White, European patients followed for an average of 18.2 months as part of initial Phase III studies funded by AstraZeneca (the pharmaceutical company that developed dapagliflozin). Given the potential conflict due to funding, all statistical results were verified by an independent academic group, and analyses were completed with an intention-to-treat model. The outlined benefits described here were only studied in a population of patients with reduced EF (≤ 40%), so the impact remains unclear for patients with preserved EF. Safety and benefits beyond 24 months were not studied in this RCT; therefore long-term data are still unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Adding an SGLT2 inhibitor may be cost prohibitive for some patients
An SGLT2 inhibitor costs, on average, $500 to $600 for a 30-day supply, which may be prohibitive for some patients.11 Integration of SGLT2 inhibitors into a patient’s medication regimen may require dose adjustments of other medications, particularly glucose-lowering therapies, and the optimal prioritization of medications is not yet known.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008. doi: 10.1056/NEJMoa1911303
2. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012
3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139-e596. doi: 10.1161/CIR.0000000000000757
4. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072. doi: 10.1161/01.cir.0000039105.49749.6f
5. Ghosh RK, Ghosh GC, Gupta M, et al. Sodium glucose co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2019;124:1790-1796. doi: 10.1016/j.amjcard.2019.08.038
6. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108-2117. doi: 10.1007/s00125-018-4670-7
7. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117-1145. doi: 10.1016/j.jacc.2020.05.037
8. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327. doi: 10.1161/CIR.0b013e31829e8776
9. Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929
10. Colucci WS. Secondary pharmacologic therapy in heart failure with reduced ejection fraction (HFrEF) in adults. UpToDate. Published October 9, 2020. Accessed June 23, 2021. www.uptodate.com/contents/secondary-pharmacologic-therapy-in-heart-failure-with-reduced-ejection-fraction-hfref-in-adults
11. Dapagliflozin. GoodRx. Accessed June 23, 2021. www.goodrx.com/dapagliflozin
ILLUSTRATIVE CASE
A 64-year-old overweight White man with a history of hypertension, hyperlipidemia, and HF with an ejection fraction (EF) of 40% presents for primary care follow-up after a recent inpatient admission for worsened HF symptoms. At baseline, he is comfortable at rest but becomes dyspneic upon walking to another room within his home. He is already taking a mineralocorticoid receptor antagonist, a high-intensity statin, a beta-blocker, and an angiotensin-converting enzyme (ACE) inhibitor. What other medication should be considered to minimize his cardiovascular (CV)risk?
An estimated 1% to 2% of the world’s adult population has HF.2 Although the exact prevalence is difficult to quantify due to variations in definitions and diagnostic methods, the American Heart Association (AHA) estimated that 6.2 million Americans had HF between 2013 and 2016.3 Prevalence increases with age, with an annual incidence of approximately 35 per 1000 by age 85.4 Due to the significant morbidity and mortality associated with HF, advancements in treatment are needed.
SGLT2 inhibitors work within the proximal tubule of the kidneys, resulting in increased glucose and sodium excretion with secondary osmotic diuresis and therefore a modest reduction in serum glucose.1,2,5,6 SGLT2 inhibitors are classically prescribed for hyperglycemia treatment in type 2 diabetes. However, preliminary data suggest that this class of medication also positively impacts cardiac function. The diuresis and natriuresis effects of SGLT2 inhibitors appear to optimize cardiac output and subsequent oxygen consumption through a reduction of afterload and preload.1,2,5,6 Further, SGLT2 inhibitors may decrease inflammatory pathways and lead to a secondary reduction of cardiac remodeling via a reduction and modulation of inflammatory pathways. This reduction and modulation may also be associated with a reduction in development, and possibly a reversal, of hypertrophic cardiomyopathy, cardiac fibrosis, and atherosclerosis.5,6 Some of the previously reported adverse effects of SGLT2 inhibitors include urinary tract infection, acute kidney injury, lower extremity amputation, bone fracture, and diabetic ketoacidosis.2
In several studies of patients with type 2 diabetes, SGLT2 inhibitors have shown benefit in reducing CV disease–related death and hospitalization for HF.1,2,5,6 A recent expert consensus from the American College of Cardiology (ACC) states that SGLT2 therapy should be considered for any patient with type 2 diabetes who also has established atherosclerotic CV disease, HF (a clinical syndrome as defined in ACC/AHA guidelines), or diabetic kidney disease, or who is at a high risk for atherosclerotic CV disease (ie, has signs of end-organ damage, such as left ventricular hypertrophy or retinopathy, or multiple risk factors such as advanced age, smoking, hypertension, and family history).7,8
Additionally, a 2019 randomized controlled trial (RCT) by Nassif et al showed that, compared to placebo, dapagliflozin significantly improved both patient-reported HF symptoms and cardiac natriuretic peptide levels over 12 weeks in patients with and without diabetes.9 In September 2020, UpToDate added SGLT2 inhibitors as an option for patients with continued symptoms of HF despite use of appropriate primary agents and mineralocorticoid receptor antagonists, whether or not they have type 2 diabetes; this update was based on 2 studies, 1 of which is reviewed here.10
STUDY SUMMARY
Dapagliflozin demonstrated better CV outcomes than placebo
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) study is an RCT that compared dapagliflozin to placebo among 4744 patients ages 18 years and older who had HF with an EF ≤ 40% and NYHA class II, III, or IV symptoms. The study included patients with (41.8%) and without diabetes. Most patients were male (76.2%-77%), White (70%), and European (44.7%-46.1%).
Patients were randomized to receive either dapagliflozin 10 mg/d or a matching placebo in addition to standard HF therapy (including an ACE inhibitor, angiotensin receptor blocker, or sacubitril-valsartan plus a beta-blocker unless contraindicated; mineralocorticoid antagonist use was encouraged). Follow-up occurred at 14 days, 60 days, 4 months, and then every 4 months, for an average of about 18 months. Patients with diabetes continued to use their glucose-lowering therapies, with dose adjustments, as needed.
Continue to: The primary outcome...
The primary outcome was a composite of worsening HF (hospitalization or urgent visit requiring intravenous HF therapy) or death from a CV cause. Secondary outcomes included a composite of hospitalization for HF or CV death; total number of hospitalizations for HF (including repeat admissions) and CV death; a change in Kansas City Cardiomyopathy Questionnaire symptom score; a composite of worsening renal function including a sustained (≥ 28 d) decline in the estimated glomerular filtration rate (eGFR) of ≥ 50%, end-stage renal disease (defined as sustained eGFR of < 15 mL/min/1.73 m2, sustained dialysis, or renal transplantation), or renal death; and death from any cause.
The primary outcome of worsening HF or death from CV causes occurred in 386 of 2373 patients (16.3%) in the dapagliflozin group and in 502 of 2371 patients (21.2%) in the placebo group (hazard ratio [HR] = 0.74; 95% CI, 0.65-0.85; P < .001). The composite score of hospitalizations for HF plus death from a CV cause was lower in the dapagliflozin group compared to the placebo group (HR = 0.75; 95% CI, 0.65-0.85; P < .001).
A total of 276 patients (11.6%) in the dapagliflozin group and 329 patients (13.9%) in the placebo group died from any cause (HR = 0.83; 95% CI, 0.71-0.97). More patients in the dapagliflozin group than in the placebo group had an improvement in symptom score (58.3% vs 50.9%; odds ratio = 1.15; 95% CI, 1.08-1.23; P < .001). Renal composite outcome did not differ between the 2 treatment groups. Potential adverse effects included volume depletion, renal adverse event, and major hypoglycemia, which occurred at the same rate in the treatment and placebo groups. There was no difference in outcomes or adverse effects between patients with and without diabetes.1
WHAT'S NEW
Evidence supports dapagliflozin use in a new patient population
The DAPA-HF study compared dapagliflozin to placebo in HF patients both with and without diabetes and demonstrated decreased HF exacerbations and CV deaths, improved patient-reported HF symptoms, and lower all-cause mortality in the treatment group. This study supports use of dapagliflozin in a new patient population—those with HF—rather than solely in patients with diabetes, as the drug was originally marketed.
CAVEATS
Specific study population may limit generalizability
The DAPA-HF study included mostly male, White, European patients followed for an average of 18.2 months as part of initial Phase III studies funded by AstraZeneca (the pharmaceutical company that developed dapagliflozin). Given the potential conflict due to funding, all statistical results were verified by an independent academic group, and analyses were completed with an intention-to-treat model. The outlined benefits described here were only studied in a population of patients with reduced EF (≤ 40%), so the impact remains unclear for patients with preserved EF. Safety and benefits beyond 24 months were not studied in this RCT; therefore long-term data are still unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Adding an SGLT2 inhibitor may be cost prohibitive for some patients
An SGLT2 inhibitor costs, on average, $500 to $600 for a 30-day supply, which may be prohibitive for some patients.11 Integration of SGLT2 inhibitors into a patient’s medication regimen may require dose adjustments of other medications, particularly glucose-lowering therapies, and the optimal prioritization of medications is not yet known.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 64-year-old overweight White man with a history of hypertension, hyperlipidemia, and HF with an ejection fraction (EF) of 40% presents for primary care follow-up after a recent inpatient admission for worsened HF symptoms. At baseline, he is comfortable at rest but becomes dyspneic upon walking to another room within his home. He is already taking a mineralocorticoid receptor antagonist, a high-intensity statin, a beta-blocker, and an angiotensin-converting enzyme (ACE) inhibitor. What other medication should be considered to minimize his cardiovascular (CV)risk?
An estimated 1% to 2% of the world’s adult population has HF.2 Although the exact prevalence is difficult to quantify due to variations in definitions and diagnostic methods, the American Heart Association (AHA) estimated that 6.2 million Americans had HF between 2013 and 2016.3 Prevalence increases with age, with an annual incidence of approximately 35 per 1000 by age 85.4 Due to the significant morbidity and mortality associated with HF, advancements in treatment are needed.
SGLT2 inhibitors work within the proximal tubule of the kidneys, resulting in increased glucose and sodium excretion with secondary osmotic diuresis and therefore a modest reduction in serum glucose.1,2,5,6 SGLT2 inhibitors are classically prescribed for hyperglycemia treatment in type 2 diabetes. However, preliminary data suggest that this class of medication also positively impacts cardiac function. The diuresis and natriuresis effects of SGLT2 inhibitors appear to optimize cardiac output and subsequent oxygen consumption through a reduction of afterload and preload.1,2,5,6 Further, SGLT2 inhibitors may decrease inflammatory pathways and lead to a secondary reduction of cardiac remodeling via a reduction and modulation of inflammatory pathways. This reduction and modulation may also be associated with a reduction in development, and possibly a reversal, of hypertrophic cardiomyopathy, cardiac fibrosis, and atherosclerosis.5,6 Some of the previously reported adverse effects of SGLT2 inhibitors include urinary tract infection, acute kidney injury, lower extremity amputation, bone fracture, and diabetic ketoacidosis.2
In several studies of patients with type 2 diabetes, SGLT2 inhibitors have shown benefit in reducing CV disease–related death and hospitalization for HF.1,2,5,6 A recent expert consensus from the American College of Cardiology (ACC) states that SGLT2 therapy should be considered for any patient with type 2 diabetes who also has established atherosclerotic CV disease, HF (a clinical syndrome as defined in ACC/AHA guidelines), or diabetic kidney disease, or who is at a high risk for atherosclerotic CV disease (ie, has signs of end-organ damage, such as left ventricular hypertrophy or retinopathy, or multiple risk factors such as advanced age, smoking, hypertension, and family history).7,8
Additionally, a 2019 randomized controlled trial (RCT) by Nassif et al showed that, compared to placebo, dapagliflozin significantly improved both patient-reported HF symptoms and cardiac natriuretic peptide levels over 12 weeks in patients with and without diabetes.9 In September 2020, UpToDate added SGLT2 inhibitors as an option for patients with continued symptoms of HF despite use of appropriate primary agents and mineralocorticoid receptor antagonists, whether or not they have type 2 diabetes; this update was based on 2 studies, 1 of which is reviewed here.10
STUDY SUMMARY
Dapagliflozin demonstrated better CV outcomes than placebo
The Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) study is an RCT that compared dapagliflozin to placebo among 4744 patients ages 18 years and older who had HF with an EF ≤ 40% and NYHA class II, III, or IV symptoms. The study included patients with (41.8%) and without diabetes. Most patients were male (76.2%-77%), White (70%), and European (44.7%-46.1%).
Patients were randomized to receive either dapagliflozin 10 mg/d or a matching placebo in addition to standard HF therapy (including an ACE inhibitor, angiotensin receptor blocker, or sacubitril-valsartan plus a beta-blocker unless contraindicated; mineralocorticoid antagonist use was encouraged). Follow-up occurred at 14 days, 60 days, 4 months, and then every 4 months, for an average of about 18 months. Patients with diabetes continued to use their glucose-lowering therapies, with dose adjustments, as needed.
Continue to: The primary outcome...
The primary outcome was a composite of worsening HF (hospitalization or urgent visit requiring intravenous HF therapy) or death from a CV cause. Secondary outcomes included a composite of hospitalization for HF or CV death; total number of hospitalizations for HF (including repeat admissions) and CV death; a change in Kansas City Cardiomyopathy Questionnaire symptom score; a composite of worsening renal function including a sustained (≥ 28 d) decline in the estimated glomerular filtration rate (eGFR) of ≥ 50%, end-stage renal disease (defined as sustained eGFR of < 15 mL/min/1.73 m2, sustained dialysis, or renal transplantation), or renal death; and death from any cause.
The primary outcome of worsening HF or death from CV causes occurred in 386 of 2373 patients (16.3%) in the dapagliflozin group and in 502 of 2371 patients (21.2%) in the placebo group (hazard ratio [HR] = 0.74; 95% CI, 0.65-0.85; P < .001). The composite score of hospitalizations for HF plus death from a CV cause was lower in the dapagliflozin group compared to the placebo group (HR = 0.75; 95% CI, 0.65-0.85; P < .001).
A total of 276 patients (11.6%) in the dapagliflozin group and 329 patients (13.9%) in the placebo group died from any cause (HR = 0.83; 95% CI, 0.71-0.97). More patients in the dapagliflozin group than in the placebo group had an improvement in symptom score (58.3% vs 50.9%; odds ratio = 1.15; 95% CI, 1.08-1.23; P < .001). Renal composite outcome did not differ between the 2 treatment groups. Potential adverse effects included volume depletion, renal adverse event, and major hypoglycemia, which occurred at the same rate in the treatment and placebo groups. There was no difference in outcomes or adverse effects between patients with and without diabetes.1
WHAT'S NEW
Evidence supports dapagliflozin use in a new patient population
The DAPA-HF study compared dapagliflozin to placebo in HF patients both with and without diabetes and demonstrated decreased HF exacerbations and CV deaths, improved patient-reported HF symptoms, and lower all-cause mortality in the treatment group. This study supports use of dapagliflozin in a new patient population—those with HF—rather than solely in patients with diabetes, as the drug was originally marketed.
CAVEATS
Specific study population may limit generalizability
The DAPA-HF study included mostly male, White, European patients followed for an average of 18.2 months as part of initial Phase III studies funded by AstraZeneca (the pharmaceutical company that developed dapagliflozin). Given the potential conflict due to funding, all statistical results were verified by an independent academic group, and analyses were completed with an intention-to-treat model. The outlined benefits described here were only studied in a population of patients with reduced EF (≤ 40%), so the impact remains unclear for patients with preserved EF. Safety and benefits beyond 24 months were not studied in this RCT; therefore long-term data are still unknown.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Adding an SGLT2 inhibitor may be cost prohibitive for some patients
An SGLT2 inhibitor costs, on average, $500 to $600 for a 30-day supply, which may be prohibitive for some patients.11 Integration of SGLT2 inhibitors into a patient’s medication regimen may require dose adjustments of other medications, particularly glucose-lowering therapies, and the optimal prioritization of medications is not yet known.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008. doi: 10.1056/NEJMoa1911303
2. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012
3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139-e596. doi: 10.1161/CIR.0000000000000757
4. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072. doi: 10.1161/01.cir.0000039105.49749.6f
5. Ghosh RK, Ghosh GC, Gupta M, et al. Sodium glucose co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2019;124:1790-1796. doi: 10.1016/j.amjcard.2019.08.038
6. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108-2117. doi: 10.1007/s00125-018-4670-7
7. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117-1145. doi: 10.1016/j.jacc.2020.05.037
8. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327. doi: 10.1161/CIR.0b013e31829e8776
9. Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929
10. Colucci WS. Secondary pharmacologic therapy in heart failure with reduced ejection fraction (HFrEF) in adults. UpToDate. Published October 9, 2020. Accessed June 23, 2021. www.uptodate.com/contents/secondary-pharmacologic-therapy-in-heart-failure-with-reduced-ejection-fraction-hfref-in-adults
11. Dapagliflozin. GoodRx. Accessed June 23, 2021. www.goodrx.com/dapagliflozin
1. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995-2008. doi: 10.1056/NEJMoa1911303
2. Lytvyn Y, Bjornstad P, Udell JA, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136:1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012
3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139-e596. doi: 10.1161/CIR.0000000000000757
4. Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068-3072. doi: 10.1161/01.cir.0000039105.49749.6f
5. Ghosh RK, Ghosh GC, Gupta M, et al. Sodium glucose co-transporter 2 inhibitors and heart failure. Am J Cardiol. 2019;124:1790-1796. doi: 10.1016/j.amjcard.2019.08.038
6. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108-2117. doi: 10.1007/s00125-018-4670-7
7. Das SR, Everett BM, Birtcher KK, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76:1117-1145. doi: 10.1016/j.jacc.2020.05.037
8. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327. doi: 10.1161/CIR.0b013e31829e8776
9. Nassif ME, Windsor SL, Tang F, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: The DEFINE-HF Trial. Circulation. 2019;140:1463-1476. doi: 10.1161/CIRCULATIONAHA.119.042929
10. Colucci WS. Secondary pharmacologic therapy in heart failure with reduced ejection fraction (HFrEF) in adults. UpToDate. Published October 9, 2020. Accessed June 23, 2021. www.uptodate.com/contents/secondary-pharmacologic-therapy-in-heart-failure-with-reduced-ejection-fraction-hfref-in-adults
11. Dapagliflozin. GoodRx. Accessed June 23, 2021. www.goodrx.com/dapagliflozin
PRACTICE CHANGER
Prescribe dapagliflozin, a sodium-glucose cotransporter-2 (SGLT2) inhibitor, 10 mg/d in addition to standard therapies for adult patients with heart failure (HF) with a reduced ejection fraction (≤ 40%) and New York Heart Association (NYHA) class II or greater, regardless of type 2 diabetes history, due to improved heart failure and cardiovascular outcomes.1
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial.1
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995‐2008.