User login
Should patients stop taking aspirin for primary prevention?
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
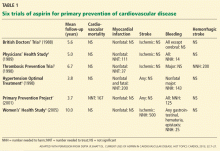
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.
WHAT ABOUT PATIENTS WITH DIABETES?
When considering whether to prescribe aspirin for primary prevention, the individual patient’s risks of cardiovascular disease and bleeding must be carefully assessed. Those at highest risk of cardiovascular disease and at low risk of bleeding may still benefit, but current evidence does not clearly support this strategy.
For example, diabetes mellitus has traditionally been considered a coronary heart disease equivalent, and aspirin was routinely prescribed as “secondary prevention.”11 In the six trials of aspirin for primary prevention, the prevalence of diabetic patients ranged from 1% to 17%, the efficacy of aspirin in this subgroup was inconsistent among the trials, and aspirin did not confer a net clinical benefit according to the 2009 Antithrombotic Trialists’ Collaboration meta-analysis.1,3–8,10
Additionally, two trials of aspirin for primary prevention in diabetes12,13 failed to demonstrate significant efficacy for aspirin compared with no aspirin, either in Japanese patients with type 2 diabetes and no history of cardiovascular disease12 or in patients with asymptomatic peripheral artery disease.13
Thus, the current evidence for aspirin for primary prevention in diabetes does not demonstrate a net clinical benefit, but ongoing trials (Table 2) may provide evidence for the use of aspirin in this important subgroup.
An important finding from the 2009 Antithrombotic Trialists’ Collaboration was that traditional risk factors for cardiovascular disease also increase the risk of major bleeding, thus making it difficult to determine who will receive the maximum net clinical benefit.10 Additionally, many of the aspirin primary prevention trials predated the widespread use of statins and the current lower prevalence of smoking, which may further limit the generalizability of the positive signals seen in earlier trials.
THE DATA ARE MIXED, BUT ONE MESSAGE IS CLEAR
Based on the current available evidence, the US Food and Drug Administration recently issued a Consumer Update that does not support aspirin for primary prevention and warns patients about the risk of serious bleeding complications.14 Moreover, current guidelines and consensus panels (Table 3) for aspirin in primary prevention differ from one another,15–21 making it challenging for clinicians to determine which patients would benefit. One message is clear in the most current clinical guidelines, namely, that routine use of aspirin for primary prevention is not recommended.15–21 Several ongoing trials may resolve this important clinical dilemma.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
- Depta JP, Bhatt DL. Current uses of aspirin in cardiovascular disease. Hot Topics Cardiol 2013; 32:7–21.
- Nemerovski CW, Salinitri FD, Morbitzer KA, Moser LR. Aspirin for primary prevention of cardiovascular disease events. Pharmacotherapy 2012; 32:1020–1035.
- Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988; 296:313–316.
- Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 1989; 321:129–135.
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet 1998; 351:233–241.
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998; 351:1755–1762.
- de Gaetano G; Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001; 357:89–95.
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005; 352:1293–1304.
- Kjeldsen SE, Kolloch RE, Leonetti G, et al. Influence of gender and age on preventing cardiovascular disease by antihypertensive treatment and acetylsalicylic acid. The HOT study. Hypertension Optimal Treatment. J Hypertens 2000; 18:629–642.
- Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373:1849–1860.
- Buse JB, Ginsberg HN, Bakris GL, et al; American Heart Association; American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007; 115:114–126.
- Ogawa H, Nakayama M, Morimoto T, et al; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008; 300:2134–2141.
- Belch J, MacCuish A, Campbell I, et al; Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008; 337:a1840.
- US Food and Drug Administration (FDA). Use of aspirin for primary prevention of heart attack and stroke. http://www.fda.gov/drugs/resourcesforyou/consumers/ucm390574.htm. Accessed January 9, 2015.
- Vandvik PO, Lincoff AM, Gore JM, et al; American College of Chest Physicians. Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e637S–e668S.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013; 36(suppl 1):S11–S66.
- Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 2002; 106:388–391.
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update: a Guideline from the American Heart Association. Circulation 2011; 123:1243–1262.
- Bell AD, Roussin A, Cartier R, et al; Canadian Cardiovascular Society. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2011; 27(suppl A):S1–S59.
- Perk J, De Backer G, Gohlke H, et al; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012; 33:1635–1701.
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150:396–404.
The promise of renal denervation
Resistant hypertension has become the focus of intense medical interest. The most commonly accepted definition of resistant hypertension is uncontrolled blood pressure despite the use of drugs from three or more antihypertensive classes, one of which is a diuretic, at maximally tolerated doses. About 1 in 50 patients with a new diagnosis of hypertension will develop resistant hypertension.1
In the 1950s, surgical renal denervation was shown to be a highly effective treatment for resistant hypertension, but the procedure was abandoned because of intolerable side effects such as bladder dysfunction and orthostasis. More recently, carotid baroreceptor surgery for resistant hypertension was investigated; results were encouraging, but this currently remains a surgical procedure.2 Now, catheter-based renal denervation has emerged as a potential minimally invasive strategy to treat resistant hypertension.
In this issue of Cleveland Clinic Journal of Medicine, Thomas et al provide an elegant review of catheter-based renal denervation to treat resistant hypertension.3 The authors nicely summarize the available data for renal denervation for resistant hypertension. A reduction in office systolic blood pressure of about 30 mm Hg has been observed.4,5 In the published studies to date, there have been no major complications beyond those associated with any angiographic procedure.
Of note, this procedure is investigational in the United States, though it is available outside of research studies in other parts of the world. Symplicity HTN-3, a pivotal trial for potential US Food and Drug Administration approval of catheter-based renal denervation, is ongoing.6
The review by Thomas et al is relevant to primary care physicians, cardiologists, nephrologists, and endocrinologists, all of whom manage patients with resistant and refractory hypertension. It explains the potential indications and referral patterns for the procedure, if approved. This review brings clinicians quickly up to speed about the exciting developments in renal denervation.
UNANSWERED QUESTIONS
As should be evident, there are many unanswered questions about renal denervation.
The long-term durability of catheter-based renal denervation remains to be determined. The available data support a sustained effect out to at least 2 years.7 Further study will be necessary to determine if there are some patients in whom the effects wear out over time. But even if that happens, assuming the beneficial effect lasts at least a few years, it may be reasonable to repeat the procedure.
Another important question is whether the reductions in blood pressure with denervation translate into reductions in stroke, heart failure, renal failure, myocardial infarction, and death. It is logical to think that this relationship holds for catheter-based denervation as it does for medical therapy, though more study is needed to see if this is true.
CAVEATS
As with coronary artery disease, it will be important to ensure that patients labeled as having resistant hypertension truly have the disease. The diagnosis requires a careful history, evaluation of potential causes of secondary hypertension, and increased use of ambulatory blood pressure monitoring to rule out white-coat and masked hypertension.
If a patient truly has resistant hypertension, appropriate lifestyle modifications (primarily salt restriction to levels well below 2.4 g/day) and aggressive pharmacotherapy should first be attempted.8 Aldosterone blockade clearly has an important role, especially in obese patients, as it has been shown to markedly lower blood pressure in this phenotype.9
Imitation is the greatest form of flattery, and this is certainly true in the world of drugs and medical devices. Accordingly, a number of systems for renal denervation are being developed. This will likely spur further innovation and refinement in the technology.
On the other hand, as with coronary artery stents, it is important to realize that there is a fair amount of engineering sophistication behind catheter-based renal denervation. As has already happened in some parts of the world, taking a radiofrequency catheter designed for electrophysiology procedures and indiscriminately using it for renal denervation could be dangerous for patients.
Furthermore, if practitioners rapidly adopt this procedure but do not adhere to the indications and protocols used in the clinical trials, the outcomes could be worse, and the net result might be a setback for this promising field of research.
OTHER INDICATIONS AND BENEFITS?
As Thomas et al point out, in addition to resistant hypertension, renal denervation has also been studied in heart failure, chronic renal failure, diabetes mellitus, and sleep apnea.10–12 Sympathetic nerve overactivity appears to have a pathologic role in all these diseases. In small studies, renal denervation has already been shown to improve systolic and diastolic dysfunction, to cause regression of left ventricular hypertrophy, and to improve glycemic control. Since these cardiovascular risk factors often cluster in the same patient, a treatment that addresses several risk factors simultaneously would be expected to have a profound benefit on cardiovascular outcomes, though this remains to be established.
Several studies are under way. Symplicity-HF will study renal denervation in 40 patients with chronic heart failure and renal impairment. The Symplicity registry will follow more than 5,000 patients undergoing catheter-based renal denervation for resistant hypertension and other conditions marked by sympathetic nerve overactivity. If an important role for renal denervation is validated in Symplicity HTN-3, it would be easy to imagine trials of renal denervation in patients with lesser degrees of hypertension.
Only with further careful randomized trials of renal denervation will its full promise be realized.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; e-pub ahead of print.
- Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol 2011; 58:765–773.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension. Cleve Clin J Med 2012; 79:501–510.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012; in press.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
Resistant hypertension has become the focus of intense medical interest. The most commonly accepted definition of resistant hypertension is uncontrolled blood pressure despite the use of drugs from three or more antihypertensive classes, one of which is a diuretic, at maximally tolerated doses. About 1 in 50 patients with a new diagnosis of hypertension will develop resistant hypertension.1
In the 1950s, surgical renal denervation was shown to be a highly effective treatment for resistant hypertension, but the procedure was abandoned because of intolerable side effects such as bladder dysfunction and orthostasis. More recently, carotid baroreceptor surgery for resistant hypertension was investigated; results were encouraging, but this currently remains a surgical procedure.2 Now, catheter-based renal denervation has emerged as a potential minimally invasive strategy to treat resistant hypertension.
In this issue of Cleveland Clinic Journal of Medicine, Thomas et al provide an elegant review of catheter-based renal denervation to treat resistant hypertension.3 The authors nicely summarize the available data for renal denervation for resistant hypertension. A reduction in office systolic blood pressure of about 30 mm Hg has been observed.4,5 In the published studies to date, there have been no major complications beyond those associated with any angiographic procedure.
Of note, this procedure is investigational in the United States, though it is available outside of research studies in other parts of the world. Symplicity HTN-3, a pivotal trial for potential US Food and Drug Administration approval of catheter-based renal denervation, is ongoing.6
The review by Thomas et al is relevant to primary care physicians, cardiologists, nephrologists, and endocrinologists, all of whom manage patients with resistant and refractory hypertension. It explains the potential indications and referral patterns for the procedure, if approved. This review brings clinicians quickly up to speed about the exciting developments in renal denervation.
UNANSWERED QUESTIONS
As should be evident, there are many unanswered questions about renal denervation.
The long-term durability of catheter-based renal denervation remains to be determined. The available data support a sustained effect out to at least 2 years.7 Further study will be necessary to determine if there are some patients in whom the effects wear out over time. But even if that happens, assuming the beneficial effect lasts at least a few years, it may be reasonable to repeat the procedure.
Another important question is whether the reductions in blood pressure with denervation translate into reductions in stroke, heart failure, renal failure, myocardial infarction, and death. It is logical to think that this relationship holds for catheter-based denervation as it does for medical therapy, though more study is needed to see if this is true.
CAVEATS
As with coronary artery disease, it will be important to ensure that patients labeled as having resistant hypertension truly have the disease. The diagnosis requires a careful history, evaluation of potential causes of secondary hypertension, and increased use of ambulatory blood pressure monitoring to rule out white-coat and masked hypertension.
If a patient truly has resistant hypertension, appropriate lifestyle modifications (primarily salt restriction to levels well below 2.4 g/day) and aggressive pharmacotherapy should first be attempted.8 Aldosterone blockade clearly has an important role, especially in obese patients, as it has been shown to markedly lower blood pressure in this phenotype.9
Imitation is the greatest form of flattery, and this is certainly true in the world of drugs and medical devices. Accordingly, a number of systems for renal denervation are being developed. This will likely spur further innovation and refinement in the technology.
On the other hand, as with coronary artery stents, it is important to realize that there is a fair amount of engineering sophistication behind catheter-based renal denervation. As has already happened in some parts of the world, taking a radiofrequency catheter designed for electrophysiology procedures and indiscriminately using it for renal denervation could be dangerous for patients.
Furthermore, if practitioners rapidly adopt this procedure but do not adhere to the indications and protocols used in the clinical trials, the outcomes could be worse, and the net result might be a setback for this promising field of research.
OTHER INDICATIONS AND BENEFITS?
As Thomas et al point out, in addition to resistant hypertension, renal denervation has also been studied in heart failure, chronic renal failure, diabetes mellitus, and sleep apnea.10–12 Sympathetic nerve overactivity appears to have a pathologic role in all these diseases. In small studies, renal denervation has already been shown to improve systolic and diastolic dysfunction, to cause regression of left ventricular hypertrophy, and to improve glycemic control. Since these cardiovascular risk factors often cluster in the same patient, a treatment that addresses several risk factors simultaneously would be expected to have a profound benefit on cardiovascular outcomes, though this remains to be established.
Several studies are under way. Symplicity-HF will study renal denervation in 40 patients with chronic heart failure and renal impairment. The Symplicity registry will follow more than 5,000 patients undergoing catheter-based renal denervation for resistant hypertension and other conditions marked by sympathetic nerve overactivity. If an important role for renal denervation is validated in Symplicity HTN-3, it would be easy to imagine trials of renal denervation in patients with lesser degrees of hypertension.
Only with further careful randomized trials of renal denervation will its full promise be realized.
Resistant hypertension has become the focus of intense medical interest. The most commonly accepted definition of resistant hypertension is uncontrolled blood pressure despite the use of drugs from three or more antihypertensive classes, one of which is a diuretic, at maximally tolerated doses. About 1 in 50 patients with a new diagnosis of hypertension will develop resistant hypertension.1
In the 1950s, surgical renal denervation was shown to be a highly effective treatment for resistant hypertension, but the procedure was abandoned because of intolerable side effects such as bladder dysfunction and orthostasis. More recently, carotid baroreceptor surgery for resistant hypertension was investigated; results were encouraging, but this currently remains a surgical procedure.2 Now, catheter-based renal denervation has emerged as a potential minimally invasive strategy to treat resistant hypertension.
In this issue of Cleveland Clinic Journal of Medicine, Thomas et al provide an elegant review of catheter-based renal denervation to treat resistant hypertension.3 The authors nicely summarize the available data for renal denervation for resistant hypertension. A reduction in office systolic blood pressure of about 30 mm Hg has been observed.4,5 In the published studies to date, there have been no major complications beyond those associated with any angiographic procedure.
Of note, this procedure is investigational in the United States, though it is available outside of research studies in other parts of the world. Symplicity HTN-3, a pivotal trial for potential US Food and Drug Administration approval of catheter-based renal denervation, is ongoing.6
The review by Thomas et al is relevant to primary care physicians, cardiologists, nephrologists, and endocrinologists, all of whom manage patients with resistant and refractory hypertension. It explains the potential indications and referral patterns for the procedure, if approved. This review brings clinicians quickly up to speed about the exciting developments in renal denervation.
UNANSWERED QUESTIONS
As should be evident, there are many unanswered questions about renal denervation.
The long-term durability of catheter-based renal denervation remains to be determined. The available data support a sustained effect out to at least 2 years.7 Further study will be necessary to determine if there are some patients in whom the effects wear out over time. But even if that happens, assuming the beneficial effect lasts at least a few years, it may be reasonable to repeat the procedure.
Another important question is whether the reductions in blood pressure with denervation translate into reductions in stroke, heart failure, renal failure, myocardial infarction, and death. It is logical to think that this relationship holds for catheter-based denervation as it does for medical therapy, though more study is needed to see if this is true.
CAVEATS
As with coronary artery disease, it will be important to ensure that patients labeled as having resistant hypertension truly have the disease. The diagnosis requires a careful history, evaluation of potential causes of secondary hypertension, and increased use of ambulatory blood pressure monitoring to rule out white-coat and masked hypertension.
If a patient truly has resistant hypertension, appropriate lifestyle modifications (primarily salt restriction to levels well below 2.4 g/day) and aggressive pharmacotherapy should first be attempted.8 Aldosterone blockade clearly has an important role, especially in obese patients, as it has been shown to markedly lower blood pressure in this phenotype.9
Imitation is the greatest form of flattery, and this is certainly true in the world of drugs and medical devices. Accordingly, a number of systems for renal denervation are being developed. This will likely spur further innovation and refinement in the technology.
On the other hand, as with coronary artery stents, it is important to realize that there is a fair amount of engineering sophistication behind catheter-based renal denervation. As has already happened in some parts of the world, taking a radiofrequency catheter designed for electrophysiology procedures and indiscriminately using it for renal denervation could be dangerous for patients.
Furthermore, if practitioners rapidly adopt this procedure but do not adhere to the indications and protocols used in the clinical trials, the outcomes could be worse, and the net result might be a setback for this promising field of research.
OTHER INDICATIONS AND BENEFITS?
As Thomas et al point out, in addition to resistant hypertension, renal denervation has also been studied in heart failure, chronic renal failure, diabetes mellitus, and sleep apnea.10–12 Sympathetic nerve overactivity appears to have a pathologic role in all these diseases. In small studies, renal denervation has already been shown to improve systolic and diastolic dysfunction, to cause regression of left ventricular hypertrophy, and to improve glycemic control. Since these cardiovascular risk factors often cluster in the same patient, a treatment that addresses several risk factors simultaneously would be expected to have a profound benefit on cardiovascular outcomes, though this remains to be established.
Several studies are under way. Symplicity-HF will study renal denervation in 40 patients with chronic heart failure and renal impairment. The Symplicity registry will follow more than 5,000 patients undergoing catheter-based renal denervation for resistant hypertension and other conditions marked by sympathetic nerve overactivity. If an important role for renal denervation is validated in Symplicity HTN-3, it would be easy to imagine trials of renal denervation in patients with lesser degrees of hypertension.
Only with further careful randomized trials of renal denervation will its full promise be realized.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; e-pub ahead of print.
- Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol 2011; 58:765–773.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension. Cleve Clin J Med 2012; 79:501–510.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012; in press.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation 2012; e-pub ahead of print.
- Bisognano JD, Bakris G, Nadim MK, et al. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal Trial. J Am Coll Cardiol 2011; 58:765–773.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension. Cleve Clin J Med 2012; 79:501–510.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol 2012; in press.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.