User login
Care Transitions from Hospital to Home
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
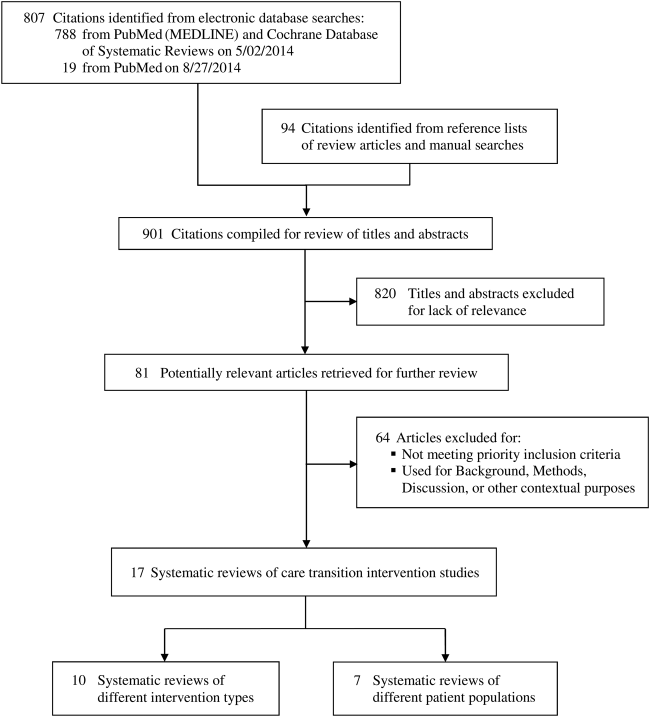
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as patients transfer between different locations or different levels of care within the same location.[1] Early studies showed that nurse‐led transitional care interventions beginning in the hospital and continuing after discharge had the potential to reduce the rate of hospital readmissions.[2, 3] Since then, the healthcare landscape has been evolving in important ways, with the spread of the electronic medical record, the patient‐centered medical home, and an increased push to health systems integration.[4, 5, 6]
The potential success or failure of transitional care interventions, which are inherently complex and can involve multiple components, may depend on the nature of the interventions themselves, the settings in which they were implemented, and/or the populations included. Health systems are faced with a large array of transitional care interventions and patient populations to whom such activities might apply.
The main aim of this article, culled from a larger report commissioned by the Veterans Health Administration (VHA)[7] was to catalogue which types of transitional care interventions hold promise and which populations have been best studied, to help health systems guide prioritization and adaptation of the most relevant transitional care activities and help focus future research efforts.
METHODS
We conducted a review of systematic reviews published in English, following Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline for systematic reviews.[8] A protocol describing the review plan was posted to a public website before the study was initiated.[9] From an initial review of the literature, we recognized that most systematic reviews typically either examined different transitional care intervention types in a given patient population, or examined a given intervention type in a variety of patient populations. We use the term intervention type to refer to single‐ or multicomponent interventions that used a similar approach or bundle of care processes (eg, telemonitoring, hospital‐at‐home), or addressed a similar key process of the care transition (eg, medication reconciliation). Patient populations are defined according to clinical condition (eg, congestive heart failure) or demographic characteristics (eg, geriatric). Given that the review was originally commissioned by the VHA, we excluded pediatric and obstetric patient populations.
We identified categories of patient populations and intervention types with input from a panel of content experts, an initial scan of the literature, and with input from our study team to help guide our literature search (see Supporting Information, Appendix A, in the online version of this article). We searched PubMed and Cochrane databases of systematic reviews from database inception through May 2014.
We selected reviews that reported hospital readmissions as an outcome, regardless of whether it was the primary outcome. However, we summarized other outcomes reported by each review. Within each patient population or intervention type of interest, we first identified reviews that fulfilled key quality criteria: (1) clearly reported their search strategy, (2) reported inclusion and exclusion criteria, and (3) conducted an appraisal of the internal validity of the included trials.[10, 11] If there was more than 1 review within each category fulfilling these criteria, we prioritized the most recent review and those with the broadest scope. We discussed the ultimate choice of review as a group and resolved any disagreements through consensus. One author abstracted prespecified data from each review and a second author checked entries for accuracy (see Supporting Information, Appendix B, in the online version of this article).
We qualitatively synthesized the literature, using the categories of intervention type, patient population, and healthcare setting to organize our synthesis. We further identified common themes that cut across different intervention types and patient populations related to the following characteristics (derived from an existing taxonomy):[12] transition type (hospital to home, hospital to nursing facility), intervention target (patient, caregiver), key processes (education, personal health record), key personnel involved (nurse, social worker), method of postdischarge follow‐up (phone, home visits), and intensity and complexity. We developed brief narrative summaries of findings for each review. These narratives were compiled into a single document and reviewed independently by each of the authors of this report, who then compiled a brief list of key cross‐cutting themes in the evidence.
RESULTS
We reviewed 807 titles and abstracts from the electronic search, and identified an additional 94 from reviewing reference lists and performing manual searches for recently published and unpublished or ongoing studies (Figure 1). Eighty‐one systematic reviews met our inclusion criteria and, of these, we selected 17 that were the most recent and broadly scoped: 10 of intervention types (Table 1) and 7 of patient populations (Table 2).
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) | Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Geriatric case management (community dwelling, age 65+ years), Huntley, 2013,[34] 19502010 |
11 RCTs (11 studies total), N = 4318 |
0.71 (0.49 to 1.03) |
Combined estimate NR. Mortality (5 studies) was not significantly different based on case management. |
Clinical: NR. Other utilization: ED visits, GP visits, specialist clinic/outpatient visits, and LOS were not improved by case management in all but 1 study. | Cochrane ROB. Risk of bias was generally low. Most studies had low or unclear ROB in all categories except 1 study that had high ROB in 3 categories. |
| Geriatric case assessment (age 65+ years), Ellis, 2011,[26]
19662010 |
22 RCTs (22 studies total), N = 10,315 |
No difference between groups, N = 3822. OR 1.03 (0.89 to 1.18) |
Death or functional decline, combined outcome: 0.76 (0.64 to 0.90, P = 0.001) based on data from 5 RCTs, N = 2622 |
Clinical: significant improvement in cognitive function associated with CGA based on 5 trials. There were nonsignificant differences for dependence. Other utilization: costs were mixed. Few trials accounted for nursing home costs; those that did suggested that CGA might be associated with overall reduced cost. | Cochrane ROB. The studies identified were heterogeneous in quality. All used some method of individual patient randomization, though reporting of key issues such as allocation concealment varied. Outcome assessment was seldom blinded though this is less of an issue for hard outcomes such as death or institutionalization. Some trials noted attrition for functional or cognitive outcomes. |
| Discharge planning (mostly older medical, though some studies included surgery, psych), Shepperd, 2013,[13] 19462012 | 24 RCTs (24 studies total), N = 8098 | Within 3 months of discharge: 0.82 (0.73 to 0.92) for older patients with a medical condition. No difference was found when mixed medical and surgical populations were included. |
At 69 months: 0.99 (0.78 to 1.25) |
Clinical: QOL outcomes were mixed. Other utilization: lower medical LOS in 10 trials. No change in surgical LOS (2 trials) | Cochrane ROB. Low ROB: n = 9, medium ROB: n = 9, high ROB: n = 5, unclear ROB: n = 1 |
| ERAS/fast track (postpancreatic surgery), Kagedan, 2015,[35] 20002013 | 0 trials or RCTs (10 studies total), N = 0 (no RCTs) | Range among studies in % of patients readmitted, ERAS vs UC: (3.515) vs (025) |
Range (% of patients), ERAS vs UC: (04) vs (03) |
Clinical: NR. Other utilization: 2/4 studies that examining costs showed reduction, 2/4 no change |
GRADE (low, moderate, high). No high‐quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single‐group prospective studies were graded as low quality. Moderate quality: n = 7, low quality: n = 3 |
| Hospital at home, Caplan, 2012,[14] database inception through 2012 | 61 RCTs (61 studies total), N = 6992 | 0.75 (0.59 to 0.95) | 0.81 (0.69 to 0.95) |
Clinical: consistent higher satisfaction (21/22 studies reporting patient satisfaction, 6/8 studies reporting CG satisfaction). No difference in caregiver burden (7 studies). Other utilization: mean cost lower (11 RCTS): 1567.11 (2069.53 to 1064.69, P 0.001). Average cost savings 26.5%, 32/34 studies concluded HAH was less expensive. |
EPOC criteria. Quality ratings not reported. Almost all studies were not blinded. However, many studies used blinded initial assessments before randomisation. Some outcome assessment was blinded. |
| Medication reconciliation, Kwan, 2013,[15] 19802012 | 5 RCTs (18 studies total), N = 1075 | ER visits and hospitalizations within 30 days of discharge in 3 RCTs, HR: 0.77 (0.63 to 0.95) | NR | Clinical: NR. Other utilization: NR | Cochrane ROB. Low ROB: n = 5 RCTs |
| PCMH, Jackson, 2013,[36] database inception through June 2012 | 9 RCTs (19 studies total), N = 54,465 | 0.96 (0.84 to 1.10) | NR | Clinical: NR. Other utilization: 3 RCTs reporting ED utilization found no effect. Combined RR: 0.93 (95% CI: 0.72 to 1.20). | AHRQ (good, fair, poor quality). All but 1 study were rated as being good or fair quality. |
| Telemonitoring and structured telephone support (heart failure)
Pandor, 2013,[22] 19992011 |
21 RCTs (21 studies total), N = 6317 |
Median HR (credible interval, 2.5% to 97.5%). All to cause: STS HH: 0.97 (0.70 to 1.31). TM office hours (transmitted data reviewed by medical staff during office hours): 0.75 (0.49 to 1.10). HF to related: STS HH: 0.77 (0.62 to 0.96). TM office hours: 0.95 (0.70 to 1.34) |
Median HR (credible interval, 2.5% to 97.5%): STS HH vs UC: 0.77 (0.55 to 1.08). TM office hours vs UC: 0.76 (0.49 to 1.18) |
Clinical: QOL improved in 3 of 4 studies of STS interventions, and 2 of 4 studies of telemonitoring interventions. Other utilization: HF‐related hospitalizations: no change for STS HM and TM office hours; reduced with STS HH 0.76 (0.61 to 0.94). Five of 6 studies found no change in LOS, 1 showed reduced. |
Study quality not reported individually. The methodological quality of the 21 included studies varied widely and reporting was generally poor on random sequence generation, allocation concealment, blinding of outcome assessment, definition and confirmation of HF diagnosis, and intention‐to‐treat analysis. |
| Telephone follow‐up, primary‐care based, Crocker 2012,[21] 19482011 | 3 RCTs (3 studies total), N = 1765 | Combined estimate NR. None of the 3 RCTs reported a statistically significant impact of telephone follow‐up on readmission or ER visits. | NR | Clinical: NR. Other utilization: In all 3 included studies, primary care contact improved with postdischarge telephone follow‐up. Two studies examining ED visits showed no effect. | Study quality not reported individually: assessed sequence generation, allocation concealment, blinding, follow‐up and intent to treat analysis, and publication bias. Most studies were high or unclear ROB based on poor reporting of sequence generation, allocation concealment; lack of blinding; and lack of information about attrition. |
| Telephone follow‐up, hospital‐based (unselected with cardiac and surgical subgroup analyses), Mistiaen, 2006,[20] database inception through July 2003 |
13 RCTs (33 studies total), N = 5110 |
Cardiac (3 RCTs, N = 616): 0.75 (0.41 to 1.36). Surgical (4 RCTs, N = 460): 0.65 (0.28 to 1.55) | NR | Clinical: No change in anxiety 1 month post‐DC in cardiac surgery patients in pooled effect from 3 studies. No change in depression based on 2 studies. Other utilization: no change in ED visits in surgery patients (pooled from 2 studies) | Cochrane ROB. Medium ROB: n = 7. High ROB: n = 26 |
| Systematic Review, Sample Characteristics, Search Dates |
N Controlled Trials (N Total Studies), N = Total Patients in RCTs |
Summary Estimate for Readmission Risk (95% CI) |
Summary Estimate for Mortality (95% CI) | Other Outcomes (Clinical and Utilization) | Quality Assessment Method, Range of Scores |
|---|---|---|---|---|---|
| |||||
| Acute MI/acute coronary syndrome, Auer, 2008,[25] 19662007 |
16 controlled trials, including 14 RCTs (26 studies total), N = 1910 from RCTs |
612 months: 0.96 (0.79 to 1.17) | All causes: 0.94 (0.63 to 1.40). All causes at 1 year: 0.94 (0.63 to 1.44) | Clinical: re‐infarction rates: RR 0.51 (95% CI: 0.23 to 1.1). Smoking cessation: RR 1.29 (1.02 to 1.63, I2 = 66%). Other utilization: NR |
Modified Jadad score 3 (lowest ROB category): n = 8, 2: n = 5; 1 (highest ROB category): n = 3. Before‐after designs: n = 12 (no formal ROB assessment) |
| Cancer, Smeenk, 1998,[37] 19851997 |
5 RCTs (9 studies total) N = 4249 |
Range of ratios for readmission (%) in intervention group/ control group: 0.621.12. Combined estimate NR. Timing of readmission assessment NR. | NR | Clinical: quality of life outcomes were positively associated with home‐care programs in 3 of 7 studies. Other utilization: NR |
Weighted methodological quality score (0100 max): Range: 4868. All considered moderate quality |
| CHF (moderate‐severe, geriatric), Feltner, 2014,[16] 19902013 |
47 RCTs (47 studies total) N = 8693 |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.75 (0.66 to 0.86). Structured telephone support, 36 months: 0.92 (0.77 to 1.10). Telemonitoring, 36 months: 1.11 (0.87 to 1.42). Clinic‐based (MDS‐HF), 6 months: 0.70 (0.55 to 0.89) |
Combined RR (95% CI) by intervention type; results from single studies per intervention type not included below: Home‐visiting program, 36 months: 0.77 (0.60 to 0.996). Structured telephone support, 3.6 months: 0.69 (0.51 to 0.92). Clinic‐based (MDS‐HF), 6 months: 0.56 (0.34 to 0.92) |
Clinical: NR. Other utilization: NR |
AHRQ ROB for trials. Low ROB: n = 6, medium ROB: n = 27, high ROB: n = 9, unclear ROB: n = 5 |
| COPD, Prieto‐Centurion, 2014,[27] 19662013 |
5 RCTs (5 studies total) N = 1393 |
2 studies found reduced 12‐month readmissions (mean number of hospitalizations per patient, 1.0 vs 1.8; P = 0.01; percent hospitalized, 45% vs 67%; P = 0.028). Three studies found no significant change in 6‐ or 12‐month readmissions. |
4 of 5 studies: no difference. 1 study: increased 12‐month mortality (17% vs 7%, P = 0.003) | Clinical: NR. Other utilization: NR | EPOC criteria (no. domains with low ROB: 17 max). 6: n = 4, 5: n = 1 |
| General/unselected, Leppin, 2014,[24] 19902013 | 42 RCTs (42 studies total), N = 17,273 | 30 days: 0.82 (0.73 to 0.91) | NR | Clinical: NR. Other utilization: NR | EPOC ROB (high, low, unclear). Most studies were at overall low risk of bias. The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data. Eight of 42 studies were rated as low ROB in all categories; all others were rated as high or unclear ROB in 1 or more categories. |
| Mental health admissions, Vigod, 2013,[38] database inception through 2012 |
13 controlled trials, including 8 RCTs (15 studies total) N = 1007 (RCTs) |
Range among studies in % of patients readmitted, intervention group vs control: 3 month: 7%23% vs 13%36%, 624 month: 0%63% vs 4%69% | NR | Clinical: NR. Other utilization: NR |
EPOC criteria. No. of domains with low ROB (19 max): range 38. Most included studies had small sample sizes, high dropout rates, and/or did not account for baseline differences between groups on key prognostic factors. |
| Stroke or ACS, Prvu Bettger, 2012,[18] 20002012 |
24 RCTs stroke, 8 RCTs MI (44 studies total: 27 stroke, 17 MI), N = 4307 stroke, N = 1062 MI |
Insufficient evidence for most intervention subtypes in both stroke and MI. Moderate strength evidence that hospital‐initiated support did not reduce readmissions in stroke patients. Timing of readmission assessment NR. | Low strength evidence in MI patients: reduced 3 month mortality (1 study), reduced 12 month mortality (2 studies) |
Clinical: No significant differences in ADLs. Inconsistent effects on caregiver strain, quality of life in 5 studies measuring caregiver outcomes. Other utilization: NR |
AHRQ (good, fair, poor quality). Good: n = 10, fair: n = 42, poor: n = 10. Strength of evidence insufficient for all intervention/population subgroups except as noted. |

Intervention Types
Among reviews focused on specific intervention types (Table 1), several show promise in reducing readmissions and/or mortality.[13, 14, 15, 16] There is moderate‐strength evidence that structured and individually tailored discharge planning reduces readmissions within 90 days (relative risk [RR]: 0.82, 95% confidence interval [CI]: 0.73 to 0.92) and hospital length of stay (0.91 days, 95% CI: 1.55 to 0.27).[13] However, most of the benefit was seen among studies of robust interventions that included a combination of care processes. In 9 of the interventions, a nurse advocate helped with discharge planning activities and care coordination. Twelve of the interventions included postdischarge follow‐up.
Moderate strength evidence from 61 trials found that hospital‐at‐home interventions were associated with reductions in 30‐day readmissions (RR: 0.75, 95% CI: 0.59 to 0.95) and mortality (RR: 0.81, 95% CI: 0.69 to 0.95).[14] Frequently, specific components of the included interventions were not well described, and periods of observation for outcomes were not specified. Interventions were associated with greater patient and caregiver satisfaction in the vast majority of studies reporting such outcomes.
The impact of medication reconciliation interventions on clinically significant adverse drug events was variable.[15] Readmissions and emergency room visits were reduced (RR: 0.77, 95% CI: 0.0.63 to 0.95) in 3 trials, but this reduction was driven by 1 intervention that included additional care processes such as postdischarge follow‐up.[17] Interventions focused solely on medication reconciliation around the time of discharge were not effective.
One review of patients with stroke or myocardial infarction (MI) described 5 intervention types: hospital‐based discharge preparation, hospital‐based patient and family education, community‐based patient and family education, community‐based models of support interventions, and chronic disease management models of care.[18] They found moderate‐strength evidence that early supported discharge of stroke patients (short hospital stay followed by intensive home care with a multidisciplinary team) shortened length of stay without adversely impacting readmissions or mortality. Specialty care after an MI was associated with reduced mortality, but the strength of evidence was low, being largely based on 1 Veterans Affairs observational study.[19] There was insufficient evidence examining the other types of interventions in this review.
Two reviews examined the effects of postdischarge follow‐up calls in unselected populations. An older Cochrane review from 2006 focused on calls performed by hospital‐based personnel.[20] Though 33 studies including 5110 patients were included in this review, there was inconclusive evidence of the effectiveness of these interventions, largely because of methodological limitations in most included studies. A more recent review similarly concluded there was insufficient evidence of the effects of postdischarge calls on utilization in 3 studies, though they did find that the interventions were associated with higher rates of primary care engagement.[21]
One review focused on postdischarge remote monitoring in patients with congestive heart failure (CHF)[22, 23] via structured telephone support (STS) or telemonitoring. STS interventions typically included periodic scripted telephone calls from nurses to review symptoms, interval physiologic data such as weight, and self‐management skills. Telemonitoring focused on remote transfer of physiologic data, with phone contact when abnormal vital signs or weights occurred. STS interventions reduced long‐term (6 months), but not short‐term (23 months) heart failure readmissions, and were associated with reduced long‐term mortality.[16, 23] Though 1 review noted a trend toward reduced mortality with telemonitoring interventions, both reviews noted the substantial methodological shortcomings of this literature and the inconsistency of results across studies. There was insufficient evidence of the comparative effectiveness between STS and telemonitoring interventions.[16]
One review of CHF patients categorized interventions into 6 types: home‐visiting programs, STS, telemonitoring, outpatient clinic‐based (including multidisciplinary CHF clinics), primarily educational, and other.[16] This review found moderate‐strength evidence that interventions with multidisciplinary heart failure (HF) clinic visits or home visits reduced both all‐cause readmissions and mortality, with number needed to treat below 10 for readmission and 18 to 33 for mortality (for multidisciplinary heart failure clinic and home visiting programs, respectively). STS interventions produced a similar mortality benefit but did not reduce all‐cause readmissions.
Healthcare Setting
We found no evidence directly examining whether intervention effectiveness depends on factors such as the presence of a shared electronic medical record, access to community resources, integration of primary and hospital care, and the presence of a medical home. Moreover, the transitional care literature generally has provided only scant descriptions of the health system context of the interventions.
Patient Population
The relative importance of careful patient selection, as compared to intervening on an unselected group of patients, is unclear. Many studies in these reviews used inclusion criteria that selected patients who were at high risk for readmission because of older age, significant medical comorbidity, and/or a history of high utilization. However, few reviews explicitly examined variation of intervention effects based on patient criteria.
The characteristics and findings of reviews of specific patient populations are shown in Table 2. One review found studies that did and did not use high‐risk patient selection criteria had similar results.[15] A metaregression of trials including general medical or CHF populations did not find significantly different effects between studies without age restrictions and those that included only patients over 65 years of age (interaction P = 0.24).[24] Similarly, a review of hospital‐at‐home studies did not find a clear difference in effects among studies in patients younger than 70 years old, between ages 70 and 73 years, and older than 74 years.[14]
Some of the reviews also speculated that focusing on specific groups of patients allowed disease‐specific customization of interventions and supported expertise development. For example, 1 review found that interventions in acute MI patients, which focused on effective use of disease‐specific medications, were associated with a mortality benefit, though this was largely driven by 1 study.[25] Another review examining comprehensive geriatric assessment interventions found that gains in the combined outcome of mortality and functional decline were only associated with interventions delivered in a geriatric ward setting.[26] The authors speculate that the multidisciplinary team of providers developed more expertise and facility with the patient population.
We found insufficient evidence to determine whether transitional care affects specific patient populations differently. Although there were successful interventions in CHF patients and no consistent evidence of benefit in chronic obstructive pulmonary disease (COPD) patients, it is unclear whether these differences were due to the markedly different types of interventions examined or to the choice of population itself.[16, 27] Populations with chronic medical illnesses were well represented in the literature, although there was a dearth of evidence in mental illness or surgical populations.
Cross‐cutting Themes
Across different intervention types, patient populations, and settings, successful interventions tended to be more comprehensive, involve more aspects of the care transition, and include components before and after hospital discharge. Successful interventions also tended to be flexible enough to accommodate individual patient needs. However, the strength of evidence supporting these overarching conclusions should be considered low because these are indirect, post hoc comparisons across literature that includes many different intervention types, studied in varied populations and clinical settings, and implemented in different ways. We found very few comparative effectiveness studies among the included reviews.
As noted above, the effective discharge planning and medication reconciliation interventions were those that included additional personnel and spanned care settings.[13, 17] In contrast, interventions in COPD populations did not consistently reduce readmissions or mortality, but the interventions began after hospital discharge and frequently omitted some care processes such as discharge planning that are often 1 component of successful interventions in other populations.[27]
One review created a comprehensive support variable that was based on number of patient interactions, number of personnel involved, number of intervention components, and the ability of the intervention to address self‐management needs.[24] A metaregression including 42 trials, the vast majority of which included general medical patients or patients with CHF and were considered to be methodologically sound, found interventions were overall associated with reductions in readmissions (pooled RR: 0.82, 95% CI: 0.73 to 0.91), and interventions with the most comprehensive support accounted for most of the benefit (RR readmission in the 7 studies with highest comprehensive support scores compared to 15 studies with the lowest scores: 0.63, 95% CI: 0.43 to 0.91).[24]
In a review of 47 trials in CHF patients, the key processes of care that seemed to be associated with reduced readmissions included: self‐management education delivered in person, early postdischarge contact, a point of postdischarge contact, and the ability to individually tailor the intervention.[16]
It is unclear whether home visits are a necessary component of transitional care interventions. A meta‐analysis of trials including general medicine or CHF patients did not find that the setting of care delivery influenced outcomes; however, all but 1 of the most comprehensive interventions included home visits in their model.[24] A review of CHF populations found interventions with multidisciplinary HF clinic visits or home visits reduced all‐cause readmissions and mortality, but found insufficient evidence directly comparing interventions with and without home visits.[16]
We found little evidence examining the impact of different transition types (most studies focused on hospital‐to‐home transitions), intervention targets (most studies focused on patients rather than caregivers), or key personnel involved.
DISCUSSION
We examined 17 systematic reviews across different patient populations representing a variety of intervention types to provide a broad overview of the care transitions literature. Variations in population studied, intervention definition, personnel, outcome definition, and setting make it difficult to identify strong evidence in support of a specific intervention type that should be broadly implemented. There were, however, some common themes that emerged across the literature suggesting that successful interventions addressed more aspects of the care transition, included the means to assess and respond to individual peridischarge needs, and included components that spanned care settings. In practical terms, the actualization of these themes has been accomplished in many interventions with the addition of transitional care personnel such as nurses and/or pharmacists. Additionally, interventions have often been tailored to the needs of individual patients with the use of needs assessment and patient‐centered personalized health records.[1]
Because there are many potential steps in the care transition, focusing on only 1 of these steps, such as medication reconciliation, is unlikely to have significant benefit on risk of readmission.[15] The pathways to readmission vary, as suggested both by the inability to accurately anticipate which patients will be readmitted,[28] and by case review studies characterizing underlying factors contributing to preventable readmissions.[29]
The problems with recommending that a specific intervention be broadly implemented include both the lack of evidence supporting such a recommendation and the likelihood that the transitional care gaps are not the same in all settings, or for all populations of patients treated. As health systems rapidly evolve, it may be useful for them to inventory strengths and weaknesses of their current approach to transitional care both to identify critical care gaps and to avoid investment in resource‐intensive transitional care interventions that may be redundant with existing activities.
Indeed, transitional care gaps may have changed over the last decade. Two large reviews showed that more recently published studies were less likely to have found an improvement in outcomes.[14, 24] In the years since some of the most successful and widely cited transitional care interventions were developed and evaluated, many health systems have undertaken major transformations, including the adoption of the patient‐centered medical home model and integration of electronic health records, which may implicitly address some earlier gaps. For instance, foundational qualitative work for the Care Transitions Measure identified discontinuities in information transfer as 1 of 4 major transitional care barriers identified by patients, and the personal health record was created, in part, to address this gap.[30] A shared electronic health record across healthcare settings has the potential to mitigate some of these concerns.
In general, there is an overarching need for better evidence to guide selection and implementation of complex, multicomponent transitional care interventions in different settings. There remain a number of gaps regarding the operationalization of interventions. For instance, the optimal choice of personnel, the comparative effects of home visits and other forms of postdischarge follow‐up, and the best approach to patient selection (whether through use of a formal readmission risk assessment model or a focus on populations with high‐risk comorbidities) are unknown.
One of the major weaknesses of the transitional care literature is the marked variation in intervention definitions, timing of outcome follow‐up, and descriptions of interventions and usual care. Use of taxonomies to guide study design and description may help standardize reporting.
Most of the care transitions literature has been hospital‐focused, and the interventions often extend hospital services beyond hospitalization. Given the growth of medical homes, it will be important to examine the effectiveness of outpatient‐based care transitions models that reach‐in to the hospital. Studies comparing approaches such as home‐visit and telephone‐based interventions, different risk‐prioritization schemes, and the use of different types of personnel are also needed.
There is very little literature examining transitional care interventions in patients with mental health conditions or undergoing surgery. A recent report for the Veterans Health Administration found that 24% of patients with chronic mental health conditions are readmitted within 30 days of discharge.[31] About 1 in 7 Medicare patients admitted to a surgical service is readmitted within 30 days.[32] The transitional care needs of these populations may differ substantially from medical populations and warrant further study.
Our review has a number of important limitations. Our overview of the literature was necessarily broad rather than in‐depth. There are many nuances in the results, internal validity, and generalizability of studies that are not represented in our overview. It was difficult to use established criteria to formally rate the strength of evidence for each of our conclusions, but we indicated strength of evidence ratings when reported in reviews. As we note in the results, our assessment of cross‐cutting themes is based largely on low‐strength evidence, given the indirect comparisons and the many factors that varied among the included studies. Our inclusion criteria specified readmissions as an outcome, but there are care transitions that focus exclusively on other outcomes, such as smoking cessation interventions around the time of discharge.[33] Furthermore, there are many outpatient‐based interventions designed to affect emergency room and hospital utilization that are not captured in our review, but may nevertheless be important to understanding the role of care coordination in the context of the medical home. We did not systematically update the included reviews' searches, and there may be more recent studies not represented here, though we are not aware of newer studies that would substantively change our summary of findings.
CONCLUSIONS
The literature includes many different types of interventions, studied in varied populations and clinical settings, and implemented in different ways. Furthermore, there are very little comparative effectiveness data. It is therefore difficult to conclusively identify specific intervention components and characteristics that are necessary for successful care transitions. Effective interventions are generally more comprehensive, address more aspects of the care transition, extend beyond the hospital stay, and have the flexibility to respond to individual patient needs. Transitional care interventions have not been well studied in integrated health system settings, or in mental health and surgical populations.
Disclosures: The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs or the US government.
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration (VHA) Project ESP 05‐225, VA#01‐0206. Dr. Jencks' work on this project was supported in part by a grant from the Quality Enhancement Research Initiative (05‐225), Department of Veterans Affairs. Dr. Jencks has reported prior consulting work with the following entities: Inovalon, Care Centrix, Affymax, Curaspan, Reinforced Care, Health Services Advisory Group, Delmarva Foundation, Connecticut Peer Review Organization, Maryland Health Services Cost Review Commission, Institute for Healthcare Improvement, American Association for Respiratory Care, Monaghan Medical, Iowa Society for Respiratory Care.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
- . Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51(4):549–555.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- , . Use and characteristics of electronic health record systems among office‐based physician practices: United States, 2001–2013. NCHS Data Brief. 2014(143):1–8.
- . Reform Update: Medical‐home adoption growing; evidence of effectiveness still elusive. Modern Healthcare website. Available at: http://www.modernhealthcare.com/article/20140818/NEWS/308189963. Published August 18, 2014. Accessed April 14, 2015.
- . Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 suppl):S284–S290.
- , , , et al. Transitions of care from hospital to home: a summary of systematic evidence reviews and recommendations for transitional care in the Veterans Health Administration. VA‐ESP Project #05–225. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0078978. Accessed August 1, 2015.
- , , , , The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Health Services Research 7(1):10.
- , , , , . Using existing systematic reviews in complex systematic reviews. Ann Intern Med. 2008;148(10):776–782.
- , , , et al. Transition of care for acute stroke and myocardial infarction patients from hospitalization to rehabilitation, recovery, and secondary prevention. Evidence Reports/Technology Assessments, No. 202. Report No.: 11(12)‐E011. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82455. Accessed August 1, 2015.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
- , , , , , . A meta‐analysis of “hospital in the home”. Med J Aust. 2012;197(9):512–519.
- , , , . Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 pt 2):397–403.
- , , , et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta‐analysis. Ann Intern Med. 2014;160(11):774–784.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407–416.
- , , , et al. Inpatient and follow‐up cardiology care and mortality for acute coronary syndrome patients in the Veterans Health Administration. Am Heart J. 2007;154(3):489–494.
- , . Telephone follow‐up, initiated by a hospital‐based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database Syst Rev. 2006(4):CD004510.
- , , . Telephone follow‐up as a primary care intervention for postdischarge outcomes improvement: a systematic review. Am J Med. 2012;125(9):915–921.
- , , , et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta‐analysis. Heart. 2013;99(23):1717–1726.
- , , , et al. Home telemonitoring or structured telephone support programmes after recent discharge in patients with heart failure: systematic review and economic evaluation. Health Technol Assess. 2013;17(32):1–207, v‐vi.
- , , , et al. Preventing 30‐day hospital readmissions: a systematic review and meta‐analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107.
- , , , , . Efficacy of in‐hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta‐analysis. Circulation. 2008;117(24):3109–3117.
- , , , , . Comprehensive geriatric assessment for older adults admitted to hospital: meta‐analysis of randomised controlled trials. BMJ. 2011;343:d6553.
- , , et al. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424.
- , , , et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698.
- , , , et al. Classifying general medicine readmissions. J Gen Intern Med. 1996;11(10):597–607.
- , , . Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure. Med Care. 2005;43(3):246–255.
- , . An Investigation Into the Cost of VA Hospital Readmissions. Washington DC: US Department of Veterans Affairs, Office of Quality, Safety and Value; 2014.
- , , , , . Variation in surgical‐readmission rates and quality of hospital care. N Engl J Med. 2013;369(12):1134–1142.
- , , , et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312(7):719–728.
- , , , et al. Is case management effective in reducing the risk of unplanned hospital admissions for older people? A systematic review and meta‐analysis. Fam Pract. 2013;30(3):266–275.
- , , , . Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford). 2015;17(1):11–16.
- , , , et al. Improving patient care. The patient centered medical home. A systematic review. Ann Intern Med. 2013;158(3):169–178.
- , , , . Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ. 1998;316(7149):1939–1944.
- , , , et al. Transitional interventions to reduce early psychiatric readmissions in adults: systematic review. Br J Psychiatry. 2013;202(3):187–194.
Care Transitions for the Underserved
Hospital readmissions are common and costly, and represent a significant burden to the healthcare system. The challenges of postdischarge medication uncertainty, lack of self‐management support, and lack of timely access to health professionals1 are compounded in uninsured and Medicaid individuals by limited access to medications and primary care, financial strain, insecure housing, and limited social support.2
Our hospital cares for a large number of uninsured and low‐income publicly insured patients. The Portland area safety‐net, which consists of a network of 14 federally qualified health centers and free clinics, has limited capacity for uncompensated care. Uninsured patientsand to a lesser degree, Medicaid patientshave difficulty establishing primary care. Prior to the implementation of our program, uninsured and Medicaid patients without a usual source of care were given a list of safety‐net clinics at discharge, but frequently could not access appointments or navigate the complex system. There were no well‐developed partnerships between hospital and outpatient clinics for uninsured or Medicaid patients. The hospital lacked a systematic approach to securing postdischarge follow‐up and peridischarge patient education, and uninsured patients were financially responsible for most medications upon discharge. The costs of uncompensated or undercompensated potentially preventable readmissions for these patients, along with the recognition of gaps in quality, ultimately provided the rationale for a medical center‐funded transitional care intervention for uninsured and low‐income publicly insured patients.
Several transitional care improvement programs have shown effectiveness in reducing hospital readmissions,1, 35 but most have been conducted in settings where patients have secure access to outpatient care, and none have focused specifically on uninsured or Medicaid patients. Moreover, the development of these programs requires time and capital. Transitional care programs that have published results, to date, have been funded through government or private foundation grants1, 35; however, broader implementation of transitional care innovations will require financial and intellectual engagement of healthcare institutions themselves.
This report describes development of the Care Transitions Innovation (C‐TraIn), a multicomponent transitional care intervention for uninsured and low‐income publicly insured adults at a large, urban academic medical center, Oregon Health & Science University (OHSU). Because institutional funding and engagement is critical to the sustainability and scalability of similar programs, we also describe our process for gaining institutional support. Our hypothesis is that C‐TraIn can reduce readmissions and emergency department (ED) use at 30 days after hospital discharge, compared with usual care.
METHODS
Engaging Institutional Leaders
Early and continued efforts to engage hospital administrators were integral to our ultimate success in gaining institutional funding and leadership support. Initially, we convened what we called a Health Systems Morbidity and Mortality conference, featuring an uninsured patient who told of his postdischarge experiences and costly, potentially preventable readmission. We invited a broad array of potential stakeholders, including representatives from hospital administration, hospital case managers and social workers, community safety‐net providers, inpatient and outpatient physicians, residents, and medical students. Our patient was previously admitted to OHSU and diagnosed with pneumonia, hypothyroidism, sleep apnea, and depression. At discharge, he was given a list of low‐cost clinics; however, he was unable to arrange follow‐up, could not afford prescriptions, and felt overwhelmed trying to navigate a complex system. Consequently, he received no outpatient healthcare and his illnesses progressed. Unable to stay awake as a long‐haul trucker, he lost his job and subsequently his housing, and was readmitted to the intensive care unit with severe hypercarbic respiratory failure, volume overload, and hypothyroidism. The $130,000 charge for his 19‐day rehospitalization was largely un‐recuperated by the hospital. The case was a stark example of the patient‐safety and financial costs of fragmented care, and the conference was a nidus for further institutional engagement and program development, the key steps of which are described in Table 1.
| Time | Key Step | How Step Was Achieved | Take Home Points |
|---|---|---|---|
| |||
| July 2008July 2009 | 1. Identified key stakeholders | Considered varied stakeholders impacted by transitional care gaps for uninsured and Medicaid patients | Casting a wide net early in the process promoted high level of engagement and allowed self‐identification of some stakeholders |
| 2. Framed problems and opportunities; exposed costs of existing system shortcomings | Educational conference (that we called a Health Systems M&M) fostered a blame‐free environment to explore varied perspectives | Individual patient story made policy issue more accessible to a wide range of stakeholders | |
| Discussion of exposed drivers and costs of misaligned incentives; highlighted inroads to developing a business case for change | |||
| Oct 2008June 2009 | 3. Identified administrative allies and leaders with high bridging capital | Follow‐up with administrator after Health System M&M allowed further identification of key administrative stakeholders | Administrator insight highlighted institutional priorities and strategic plans |
| Ongoing meetings over 9 moto advocate for change, explore support for program development | Key ally within administration facilitated conversation with executive leadership whose support was a critical for program success | ||
| July 2009June 2010 | 4. Framed processes locally with continued involvement from multiple stakeholders | Performed multicomponent needs assessment | Patient assessment included inpatients for ease of survey administration |
| Utilized efforts of student volunteers for low‐budget option | |||
| Existing administrative support aided patient tracking | |||
| Non‐integrated health system and lack of claims data for uninsured limited usefulness of administrative utilization data | |||
| 5. Performed cost analysis to further support the business and quality case | Used OHSU data from needs assessment patient sample to estimate potential costs and savings of saved readmissions and avoided ED visits | Business case highlighted existing costs to OHSU for uncompensated care; program presented a solution to realign incentives and better allocate existing hospital expenditures | |
| Qualitative patient interviews exposed opportunity for quality improvement | Highlighted pilot as an opportunity for institutional learning about transitional care improvements | ||
| 6. Use needs assessment to map intervention | Drew upon local and national health systems expertise through literature review and consultation with local and national program leaders | OHSU's Care Transitions Innovation (C‐TraIn) includes elements aimed at improving access, patient education, care coordination, and systems integration (Table 2) | |
| Matched patient needs to specific elements of program design | |||
Planning the Intervention
Findings from a patient needs assessment and community stakeholder meetingsdescribed belowdirectly informed a multicomponent intervention that includes linkages and payment for medical homes for uninsured patients who lack access to outpatient care, a transitional care nurse whose care bridges inpatient and outpatient settings, inpatient pharmacy consultation, and provision of 30 days of medications at hospital discharge for uninsured patients (Table 2).
| Program Element | Description | Resources per 200 Patients |
|---|---|---|
| ||
| Transitional care RN | Augments patient education and care coordination in the hospital until 30 days after discharge. Tasks include: | 1.0 FTE nurse salary* |
| developing a personal health record with inpatients | ||
| completing a home visit within 72 hr of discharge to focus on medication reconciliation and patient self‐management | ||
| low‐risk patients receive 3 calls and no home visit (see Supporting Information, Appendix 1, in the online version of this article) | ||
| 2 subsequent phone calls to provide additional coaching, identify unmet needs, and close the loop on incomplete financial paperwork | ||
| The nurse provides a warm handoff with clinic staff, assists in scheduling timely posthospital follow‐up, and assures timely transfer of DC summaries. She coordinates posthospital care management with Medicaid case‐workers when available. | ||
| Pharmacy | Consultation: Inpatient pharmacists reconcile and simplify medication regimens, educate patients, and assess adherence barriers. | 0.4 FTE inpatient pharmacist salary |
| Prescription support: For uninsured patients, pharmacists guide MD prescribing towards medications available on the C‐TraIn value‐based formulary, a low‐cost formulary that reflects medications available through $4 plans, a Medicaid formulary, and FQHC on‐site pharmacies. | Estimated $12/prescription; 6.5 prescriptions/patient | |
| Uninsured patients are given 30 days of bridging prescription medications at hospital discharge free of charge. | ||
| Outpatient medical home and specialty care linkages | OHSU has partnered with outpatient clinics on a per‐patient basis to support funding of primary care for uninsured patients who lack a usual source of care. Clinics also provide coordinated care for Medicaid patients without assigned primary care, and have committed to engaging in continuous quality improvement. Clinics include an academic general internal medicine practice, an FQHC specializing in addiction and care for the homeless, and an FQHC that serves a low‐income rural population. | Estimated 8 primary care visits/yr at $205/visit (FQHC reimbursement rate) equates to $1640/ patient/yr. |
| Timely posthospital specialty care related to index admission diagnoses is coordinated through OHSU's outpatient specialty clinics. | ||
| Monthly care coordination meetings | We convene a diverse team of community clinic champions, OHSU inpatient and outpatient pharmacy and nurse representatives, hospital administrative support, and a CareOregon representive. | |
| At each meeting, we review individual patient cases, seek feedback from diverse, and previously siloed, team members, and engage in ongoing quality improvement. | ||
Needs Assessment
We conducted a mixed‐methods needs assessment of consecutive nonelderly adult inpatients (65 years old) admitted to general medicine and cardiology, between July and October 2009, with no insurance, Medicaid, or MedicareMedicaid. Five volunteer medical and pre‐medical students surveyed 116 patients (see Supporting Information survey, Appendix 2, in the online version of this article). Forty patients reported prior admission within the last 6 months. With these participants, we conducted in‐depth semi‐structured interviews assessing self‐perceived transitional care barriers. Investigators drew preliminary themes from the interviews but delayed a scientifically rigorous qualitative analysis, given a compressed timeline in which to meet program development needs. Of the 116 patients surveyed, 22 had MedicareMedicaid. Given that many of these patients discharged to skilled nursing facilities, we focused program development using data from the 94 uninsured and Medicaid patients (Table 3).
| Uninsured (n = 43 patients) | Medicaid (n = 51 patients) | |
|---|---|---|
| ||
| Lack usual source of care (%) | 33.3 | 11.1* |
| Self‐reported 6 mo rehospitalization (%) | 60.0 | 48.6 |
| Average no. Rx prior to hospitalization | 4.4 | 13.8 |
| Barriers to taking meds as prescribed (%) | 42.9 | 21.6* |
| Cost of meds as leading barrier (%) | 30.0 | 2.9* |
| Marginal housing (%) | 40.5 | 32.4 |
| Low health literacy (%) | 41.5 | 41.7 |
| Transportation barrier (%) | 11.9 | 31.4* |
| Comorbid depression (%) | 54.8 | 45.9 |
| Income 30 K (%) | 79.5 | 96.8 |
Finding 1: Thirty‐three percent of uninsured and 11% of Medicaid patients lacked a usual source of care. This was highest among Portland‐area residents (45%). Program element: We forged relationships with 3 outpatient clinics and developed a contractual relationship whereby OHSU pays for medical homes for uninsured patients lacking usual care. Finding 2: Patients were unclear as to how to self‐manage care or who to contact with questions after hospitalization. Program element: Transitional care nurse provides intensive peridischarge education, performs home visits within 3 days of discharge, and serves as a point person for patients during the peridischarge period. Finding 3: Among uninsured patients, cost was the leading barrier to taking medications as prescribed and often led to self‐rationing of medications without provider input. Program element: We developed a low‐cost, value‐based formulary for uninsured patients that parallels partnering clinic formularies, $4 plans, and medication assistance programs. After 30 days of program‐funded medications, patients then get medications through these other sources. Inpatient pharmacists consult on all patients to reconcile medications, identify access and adherence gaps, provide patient education, and communicate across settings. Finding 4: Comorbid depression was common. Program element: We sought partnerships with clinics with integrated mental health services. Finding 5: Over half of patients live in 3 counties surrounding Portland. Program element: We restricted our intervention to patients residing in local counties and included postdischarge home visits in our model. Partnering clinics match patient geographic distribution. Finding 6: Self‐ reported 6‐month readmission (60%) rates exceeded rates estimated by hospital administrative data (18%), supporting qualitative findings that patients seek care at numerous hospitals. Program element: Given that utilization claims data are unavailable for the uninsured, we included phone follow‐up surveys to assess self‐reported utilization 30 days postdischarge. Finding 7: Using administrative data, we estimated that the hospital loses an average of $11,000 per readmission per patient in direct, unremunerated costs. Indirect costs (such as costs of hospital staff) and opportunity costs (of potential revenue from an insured patient occupying the bed) were excluded, thus presenting a conservative estimate of cost savings. Program element: We used local cost data to support the business case and emphasize potential value of an up‐front investment in transitional care.
Defining the Setting
We convened a series of 3 work group meetings with diverse internal and external stakeholders (Table 4) to further define an intervention in the context of local health system realities. Work groups shaped the program in several specific ways. First, community clinic leaders emphasized that limited specialty access is an important barrier when caring for recently hospitalized uninsured and Medicaid patients. They felt expanded postdischarge access to specialists would be important to increase their capacity for recently discharged patients. Thus, we streamlined patients' posthospital specialty access for conditions treated during hospitalization. Second, initially we considered linking with 1 clinic; however, health systems researchers and clinic providers cautioned us, suggesting that partnering with multiple clinics would make our work more broadly applicable. Finally, pharmacists and financial assistance staff revealed that financial assistance forms are often not completed during hospitalization because inpatients lack access to income documentation. This led us to incorporate help with financial paperwork into the postdischarge intervention.
| Clinical staff |
| Hospital medicine physician |
| General internal medicine physician |
| Hospital ward nurse staff |
| Pharmacy (inpatient, outpatient, medication assistance programs) |
| Care management/social work |
| Emergency medicine |
| Health system leadership |
| Hospital administrative leadership |
| Primary care clinic leadership |
| Safety‐net clinic leadership |
| Specialty clinic leadership |
| Hospital business development and strategic planning |
| CareOregon (Medicaid managed care) leadership |
| Other |
| Patients |
| Health systems researchers |
| Clinical informatics |
| Hospital financials (billing, financial screening, admitting) |
Pilot Testing
We conducted pilot testing over 4 weeks, incorporating a Plan‐Do‐Study‐Act approach. For example, our transitional care nurse initially used an intervention guide with a list of steps outlined; however, we quickly discovered that the multiple and varied needs of this patient populationincluding housing, transportation, and foodwere overwhelming and pulled the nurse in many directions. In consultation with our quality improvement experts, we reframed the intervention guide as a checklist to be completed for each patient.
Pilot testing also underscored the importance of monthly meetings to promote shared learning and create a forum for communication and problem solving across settings. During these meetings, patient case discussions inform continuous quality improvement and promote energy‐sustaining team‐building. Information is then disseminated to each clinic site and arm of the intervention through a designated champion from each group. We also planned to meet monthly with the hospital executive director to balance service and research needs, and engage in rapid‐cycle change throughout our 1‐year demonstration project.
Funding the Program
We talked to others with experience implementing nurse‐led transitional care interventions. Based on these discussions, we anticipated our nurse would be able to see 200 patients over the course of 1 year, and we developed our budget accordingly (Table 2). From our needs assessment, we knew 60% of patients reported at least 1 hospitalization in the 6 months prior. If we assumed that 60% (120) of the 200 patients randomized to our intervention would get readmitted, then a 20% reduction would lead to 24 avoided readmissions and translate into $264,000 in savings for the health system. Even though the hospital would not reap all of these savings, as patients get admitted to other area hospitals, hospital administration acknowledged the value of setting the stage for community‐wide solutions. Moreover, the benefit was felt to extend beyond financial savings to improved quality and institutional learning around transitional care.
PROGRAM EVALUATION
We are conducting a clustered, randomized controlled trial to evaluate C‐TraIn's impact on quality, access, and high‐cost utilization at 30 days after hospital discharge. Results are anticipated in mid‐2012. We chose to perform an analysis clustered by admitting team, because communication between the C‐TraIn nurse, physician team, and pharmacist consult services could introduce secular change effects that could impact the care received by other patients on a given team. There are 5 general medicine resident teams, 1 hospitalist service, and 1 cardiology service, and the physician personnel for each team changes from month to month. Because the cardiology and hospitalist services differ slightly from resident teams, we chose a randomized cross‐over design such that intervention and control teams are redesignated every 3 months. To enhance internal validity, study personnel who enroll patients and administer baseline and 30‐day surveys are blinded to intervention status. We are collecting data on prior utilization, usual source of care, outpatient access, insurance, patient activation,6 functional status,7, 8 self‐rated health,7 health literacy, care transitions education,9 alcohol and substance abuse, and social support.10 Our primary outcome will be self‐reported 30‐day hospital readmission and ED use. We will also evaluate administrative claims data to identify 30‐day OHSU readmission and ED utilization rates. We will assess whether improved access to medications, rates of outpatient follow‐up and time to follow‐up mediate any effect on primary outcomes. Secondary outcomes will include outpatient utilization, patient activation, self‐rated health, and functional status.
Given limited experience with transitional care programs in socioeconomically disadvantaged patients, we are measuring acceptability and feasibility by tracking rates of those declining the intervention, and through semi‐structured interviews at 30 days. We are monitoring fidelity to core elements of the program through chart and checklist reviews, and seeking provider feedback through in‐person meetings with key implementers. To ensure possibility of broader adoption beyond OHSU, we are developing a toolkit that defines core program elements and can be adapted for use in various settings.
DISCUSSION
Using a process of broad stakeholder engagement, exposure of financial incentives, and data‐driven understanding of institutional and population needs, we built consensus and gained institutional financial commitment for implementation of a multicomponent transitional care program for uninsured and Medicaid patients. Our experience is relevant to other hospital systems, and may have particular relevance to academic medical centers, whose tripartite mission of clinical care, research, and education make them a natural place for healthcare reform.11
Several key lessons from our experience may be widely applicable. First, key administrative allies helped us understand institutional priorities and identify key institutional change‐agents. Though initial attempts to gain support were met cautiously, persistent advocacy, development of a strong business case, and support from several administrative allies compelled further leadership support. Second, unlike traditional grant funding cycles, hospital budgets operate in real‐time rapid‐change cycles, necessitating rapid data collection, analysis, and program design. Such demands could potentially threaten the viability of the program itself, or result in premature diffusion of novel practices into disparate populations. Communication with administrative leadership about the value of sound research design within the context of faster‐paced institutional needs was important and allowed time for data‐driven program development and diffusion. Simultaneously, we recognized the need to move quickly, provide regular progress updates, and use existing institutional resources, such as volunteer students and business development office, when possible.
We found that cross‐site hospitalcommunity partnerships are an essential program element. Partnership occurs through a payment agreement and through active engagement in ongoing quality improvement, including clinic representation at monthly team meetings. Clinic partnerships have enabled multidisciplinary cross‐site communication and relationships that facilitate innovation across routinely siloed elements of the system, allowing the team to anticipate and respond to patient problems before they lead to readmissions or poor outcomes. Our experience matches findings from recent program evaluations that found that care coordination attempts are unsuccessful without strong cross‐site linkages.12 These linkages are especially challenging and needed for uninsured and Medicaid patients, given their traditional lack of access and the additional social and financial barriers that influence their care.13
Limitations of our study include: implementation at a single, academic medical center; secular changes (which we mitigate against using randomized trial design); and potential for low power, if readmission rates are lower than anticipated from needs assessment data. Additionally, the need for a willing and invested program champion to coordinate an often messy, complex intervention may limit generalizability.
While transitional care programs continue to proliferate in response to increasingly recognized gaps in a fragmented care system,14, 15 few interventions specifically address the needs of socioeconomically disadvantaged patients. The major study that did5 was conducted in Massachusetts, where many patients received care through a state Free Care program and robust local safety‐net. Others have largely been tested in integrated care settings,1 and target patients who are part of managed care programs.1, 4, 16
To our knowledge, there are no well‐described programs that include explicit purchasing of outpatient medical homes for uninsured patients who would not otherwise have access to care. Our experience shifts the paradigm of the role of hospitals in care for the uninsured and underinsured: instead of a reactive, uncoordinated role, we assert that the hospital's strategic up‐front allocation of resources has a sound business, quality, and ethical foundation. This is especially important, given a new era of payment reform and coordinated care organizations. There is an opportunity to both improve quality for the uninsured and Medicaid patients, control costs, and gain valuable experience that can inform transitional care improvements for broader patient populations. If our study is successful in reducing readmissions, there may be important implications as to how to redefine the hospital's role in outpatient access to care linkages, especially for uninsured and Medicaid patients.
Acknowledgements
The authors acknowledge Char Riley, Dawn Whitney, and Tara Harben of OHSU, as well as volunteer research assistants Amie Leaverton, Molly McClain, Emily Johnson, Travis Geraci, and Claudia Sells.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- ,,,,.Medicaid patients at high risk for frequent hospital admission: real‐time identification and remediable risks.J Urban Health.2009;86(2):230–241.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- ,,,,.The effect of Evercare on hospital use.J Am Geriatr Soc.2003;51(10):1427–1434.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,,.Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers.Health Serv Res.2004;39(4 pt 1):1005–1026.
- The EuroQol Group.EuroQol—a new facility for the measurement of health‐related quality of life.Health Policy.1990;16(3):199–208.
- ,,,,,.Trajectories of life‐space mobility after hospitalization.Ann Intern Med.2009;150(6):372–378.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,,,.Assessing social support: the social support questionnaire.J Pers Soc Psychol.1983;44(1):127–139.
- .Payment reform and the mission of academic medical centers.N Engl J Med.2010;363(19):1784–1786.
- ,,,.Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials.JAMA.2009;301(6):603–618.
- ,,,.Post‐discharge intervention in vulnerable, chronically ill patients.J Hosp Med.2012;7(2):124–130.
- ,,, et al.Discharge planning from hospital to home.Cochrane Database Syst Rev.2010(1):000313.
- .Preventing the rebound: improving care transition in hospital discharge processes.Aust Health Rev.2010;34(4):445–451.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
Hospital readmissions are common and costly, and represent a significant burden to the healthcare system. The challenges of postdischarge medication uncertainty, lack of self‐management support, and lack of timely access to health professionals1 are compounded in uninsured and Medicaid individuals by limited access to medications and primary care, financial strain, insecure housing, and limited social support.2
Our hospital cares for a large number of uninsured and low‐income publicly insured patients. The Portland area safety‐net, which consists of a network of 14 federally qualified health centers and free clinics, has limited capacity for uncompensated care. Uninsured patientsand to a lesser degree, Medicaid patientshave difficulty establishing primary care. Prior to the implementation of our program, uninsured and Medicaid patients without a usual source of care were given a list of safety‐net clinics at discharge, but frequently could not access appointments or navigate the complex system. There were no well‐developed partnerships between hospital and outpatient clinics for uninsured or Medicaid patients. The hospital lacked a systematic approach to securing postdischarge follow‐up and peridischarge patient education, and uninsured patients were financially responsible for most medications upon discharge. The costs of uncompensated or undercompensated potentially preventable readmissions for these patients, along with the recognition of gaps in quality, ultimately provided the rationale for a medical center‐funded transitional care intervention for uninsured and low‐income publicly insured patients.
Several transitional care improvement programs have shown effectiveness in reducing hospital readmissions,1, 35 but most have been conducted in settings where patients have secure access to outpatient care, and none have focused specifically on uninsured or Medicaid patients. Moreover, the development of these programs requires time and capital. Transitional care programs that have published results, to date, have been funded through government or private foundation grants1, 35; however, broader implementation of transitional care innovations will require financial and intellectual engagement of healthcare institutions themselves.
This report describes development of the Care Transitions Innovation (C‐TraIn), a multicomponent transitional care intervention for uninsured and low‐income publicly insured adults at a large, urban academic medical center, Oregon Health & Science University (OHSU). Because institutional funding and engagement is critical to the sustainability and scalability of similar programs, we also describe our process for gaining institutional support. Our hypothesis is that C‐TraIn can reduce readmissions and emergency department (ED) use at 30 days after hospital discharge, compared with usual care.
METHODS
Engaging Institutional Leaders
Early and continued efforts to engage hospital administrators were integral to our ultimate success in gaining institutional funding and leadership support. Initially, we convened what we called a Health Systems Morbidity and Mortality conference, featuring an uninsured patient who told of his postdischarge experiences and costly, potentially preventable readmission. We invited a broad array of potential stakeholders, including representatives from hospital administration, hospital case managers and social workers, community safety‐net providers, inpatient and outpatient physicians, residents, and medical students. Our patient was previously admitted to OHSU and diagnosed with pneumonia, hypothyroidism, sleep apnea, and depression. At discharge, he was given a list of low‐cost clinics; however, he was unable to arrange follow‐up, could not afford prescriptions, and felt overwhelmed trying to navigate a complex system. Consequently, he received no outpatient healthcare and his illnesses progressed. Unable to stay awake as a long‐haul trucker, he lost his job and subsequently his housing, and was readmitted to the intensive care unit with severe hypercarbic respiratory failure, volume overload, and hypothyroidism. The $130,000 charge for his 19‐day rehospitalization was largely un‐recuperated by the hospital. The case was a stark example of the patient‐safety and financial costs of fragmented care, and the conference was a nidus for further institutional engagement and program development, the key steps of which are described in Table 1.
| Time | Key Step | How Step Was Achieved | Take Home Points |
|---|---|---|---|
| |||
| July 2008July 2009 | 1. Identified key stakeholders | Considered varied stakeholders impacted by transitional care gaps for uninsured and Medicaid patients | Casting a wide net early in the process promoted high level of engagement and allowed self‐identification of some stakeholders |
| 2. Framed problems and opportunities; exposed costs of existing system shortcomings | Educational conference (that we called a Health Systems M&M) fostered a blame‐free environment to explore varied perspectives | Individual patient story made policy issue more accessible to a wide range of stakeholders | |
| Discussion of exposed drivers and costs of misaligned incentives; highlighted inroads to developing a business case for change | |||
| Oct 2008June 2009 | 3. Identified administrative allies and leaders with high bridging capital | Follow‐up with administrator after Health System M&M allowed further identification of key administrative stakeholders | Administrator insight highlighted institutional priorities and strategic plans |
| Ongoing meetings over 9 moto advocate for change, explore support for program development | Key ally within administration facilitated conversation with executive leadership whose support was a critical for program success | ||
| July 2009June 2010 | 4. Framed processes locally with continued involvement from multiple stakeholders | Performed multicomponent needs assessment | Patient assessment included inpatients for ease of survey administration |
| Utilized efforts of student volunteers for low‐budget option | |||
| Existing administrative support aided patient tracking | |||
| Non‐integrated health system and lack of claims data for uninsured limited usefulness of administrative utilization data | |||
| 5. Performed cost analysis to further support the business and quality case | Used OHSU data from needs assessment patient sample to estimate potential costs and savings of saved readmissions and avoided ED visits | Business case highlighted existing costs to OHSU for uncompensated care; program presented a solution to realign incentives and better allocate existing hospital expenditures | |
| Qualitative patient interviews exposed opportunity for quality improvement | Highlighted pilot as an opportunity for institutional learning about transitional care improvements | ||
| 6. Use needs assessment to map intervention | Drew upon local and national health systems expertise through literature review and consultation with local and national program leaders | OHSU's Care Transitions Innovation (C‐TraIn) includes elements aimed at improving access, patient education, care coordination, and systems integration (Table 2) | |
| Matched patient needs to specific elements of program design | |||
Planning the Intervention
Findings from a patient needs assessment and community stakeholder meetingsdescribed belowdirectly informed a multicomponent intervention that includes linkages and payment for medical homes for uninsured patients who lack access to outpatient care, a transitional care nurse whose care bridges inpatient and outpatient settings, inpatient pharmacy consultation, and provision of 30 days of medications at hospital discharge for uninsured patients (Table 2).
| Program Element | Description | Resources per 200 Patients |
|---|---|---|
| ||
| Transitional care RN | Augments patient education and care coordination in the hospital until 30 days after discharge. Tasks include: | 1.0 FTE nurse salary* |
| developing a personal health record with inpatients | ||
| completing a home visit within 72 hr of discharge to focus on medication reconciliation and patient self‐management | ||
| low‐risk patients receive 3 calls and no home visit (see Supporting Information, Appendix 1, in the online version of this article) | ||
| 2 subsequent phone calls to provide additional coaching, identify unmet needs, and close the loop on incomplete financial paperwork | ||
| The nurse provides a warm handoff with clinic staff, assists in scheduling timely posthospital follow‐up, and assures timely transfer of DC summaries. She coordinates posthospital care management with Medicaid case‐workers when available. | ||
| Pharmacy | Consultation: Inpatient pharmacists reconcile and simplify medication regimens, educate patients, and assess adherence barriers. | 0.4 FTE inpatient pharmacist salary |
| Prescription support: For uninsured patients, pharmacists guide MD prescribing towards medications available on the C‐TraIn value‐based formulary, a low‐cost formulary that reflects medications available through $4 plans, a Medicaid formulary, and FQHC on‐site pharmacies. | Estimated $12/prescription; 6.5 prescriptions/patient | |
| Uninsured patients are given 30 days of bridging prescription medications at hospital discharge free of charge. | ||
| Outpatient medical home and specialty care linkages | OHSU has partnered with outpatient clinics on a per‐patient basis to support funding of primary care for uninsured patients who lack a usual source of care. Clinics also provide coordinated care for Medicaid patients without assigned primary care, and have committed to engaging in continuous quality improvement. Clinics include an academic general internal medicine practice, an FQHC specializing in addiction and care for the homeless, and an FQHC that serves a low‐income rural population. | Estimated 8 primary care visits/yr at $205/visit (FQHC reimbursement rate) equates to $1640/ patient/yr. |
| Timely posthospital specialty care related to index admission diagnoses is coordinated through OHSU's outpatient specialty clinics. | ||
| Monthly care coordination meetings | We convene a diverse team of community clinic champions, OHSU inpatient and outpatient pharmacy and nurse representatives, hospital administrative support, and a CareOregon representive. | |
| At each meeting, we review individual patient cases, seek feedback from diverse, and previously siloed, team members, and engage in ongoing quality improvement. | ||
Needs Assessment
We conducted a mixed‐methods needs assessment of consecutive nonelderly adult inpatients (65 years old) admitted to general medicine and cardiology, between July and October 2009, with no insurance, Medicaid, or MedicareMedicaid. Five volunteer medical and pre‐medical students surveyed 116 patients (see Supporting Information survey, Appendix 2, in the online version of this article). Forty patients reported prior admission within the last 6 months. With these participants, we conducted in‐depth semi‐structured interviews assessing self‐perceived transitional care barriers. Investigators drew preliminary themes from the interviews but delayed a scientifically rigorous qualitative analysis, given a compressed timeline in which to meet program development needs. Of the 116 patients surveyed, 22 had MedicareMedicaid. Given that many of these patients discharged to skilled nursing facilities, we focused program development using data from the 94 uninsured and Medicaid patients (Table 3).
| Uninsured (n = 43 patients) | Medicaid (n = 51 patients) | |
|---|---|---|
| ||
| Lack usual source of care (%) | 33.3 | 11.1* |
| Self‐reported 6 mo rehospitalization (%) | 60.0 | 48.6 |
| Average no. Rx prior to hospitalization | 4.4 | 13.8 |
| Barriers to taking meds as prescribed (%) | 42.9 | 21.6* |
| Cost of meds as leading barrier (%) | 30.0 | 2.9* |
| Marginal housing (%) | 40.5 | 32.4 |
| Low health literacy (%) | 41.5 | 41.7 |
| Transportation barrier (%) | 11.9 | 31.4* |
| Comorbid depression (%) | 54.8 | 45.9 |
| Income 30 K (%) | 79.5 | 96.8 |
Finding 1: Thirty‐three percent of uninsured and 11% of Medicaid patients lacked a usual source of care. This was highest among Portland‐area residents (45%). Program element: We forged relationships with 3 outpatient clinics and developed a contractual relationship whereby OHSU pays for medical homes for uninsured patients lacking usual care. Finding 2: Patients were unclear as to how to self‐manage care or who to contact with questions after hospitalization. Program element: Transitional care nurse provides intensive peridischarge education, performs home visits within 3 days of discharge, and serves as a point person for patients during the peridischarge period. Finding 3: Among uninsured patients, cost was the leading barrier to taking medications as prescribed and often led to self‐rationing of medications without provider input. Program element: We developed a low‐cost, value‐based formulary for uninsured patients that parallels partnering clinic formularies, $4 plans, and medication assistance programs. After 30 days of program‐funded medications, patients then get medications through these other sources. Inpatient pharmacists consult on all patients to reconcile medications, identify access and adherence gaps, provide patient education, and communicate across settings. Finding 4: Comorbid depression was common. Program element: We sought partnerships with clinics with integrated mental health services. Finding 5: Over half of patients live in 3 counties surrounding Portland. Program element: We restricted our intervention to patients residing in local counties and included postdischarge home visits in our model. Partnering clinics match patient geographic distribution. Finding 6: Self‐ reported 6‐month readmission (60%) rates exceeded rates estimated by hospital administrative data (18%), supporting qualitative findings that patients seek care at numerous hospitals. Program element: Given that utilization claims data are unavailable for the uninsured, we included phone follow‐up surveys to assess self‐reported utilization 30 days postdischarge. Finding 7: Using administrative data, we estimated that the hospital loses an average of $11,000 per readmission per patient in direct, unremunerated costs. Indirect costs (such as costs of hospital staff) and opportunity costs (of potential revenue from an insured patient occupying the bed) were excluded, thus presenting a conservative estimate of cost savings. Program element: We used local cost data to support the business case and emphasize potential value of an up‐front investment in transitional care.
Defining the Setting
We convened a series of 3 work group meetings with diverse internal and external stakeholders (Table 4) to further define an intervention in the context of local health system realities. Work groups shaped the program in several specific ways. First, community clinic leaders emphasized that limited specialty access is an important barrier when caring for recently hospitalized uninsured and Medicaid patients. They felt expanded postdischarge access to specialists would be important to increase their capacity for recently discharged patients. Thus, we streamlined patients' posthospital specialty access for conditions treated during hospitalization. Second, initially we considered linking with 1 clinic; however, health systems researchers and clinic providers cautioned us, suggesting that partnering with multiple clinics would make our work more broadly applicable. Finally, pharmacists and financial assistance staff revealed that financial assistance forms are often not completed during hospitalization because inpatients lack access to income documentation. This led us to incorporate help with financial paperwork into the postdischarge intervention.
| Clinical staff |
| Hospital medicine physician |
| General internal medicine physician |
| Hospital ward nurse staff |
| Pharmacy (inpatient, outpatient, medication assistance programs) |
| Care management/social work |
| Emergency medicine |
| Health system leadership |
| Hospital administrative leadership |
| Primary care clinic leadership |
| Safety‐net clinic leadership |
| Specialty clinic leadership |
| Hospital business development and strategic planning |
| CareOregon (Medicaid managed care) leadership |
| Other |
| Patients |
| Health systems researchers |
| Clinical informatics |
| Hospital financials (billing, financial screening, admitting) |
Pilot Testing
We conducted pilot testing over 4 weeks, incorporating a Plan‐Do‐Study‐Act approach. For example, our transitional care nurse initially used an intervention guide with a list of steps outlined; however, we quickly discovered that the multiple and varied needs of this patient populationincluding housing, transportation, and foodwere overwhelming and pulled the nurse in many directions. In consultation with our quality improvement experts, we reframed the intervention guide as a checklist to be completed for each patient.
Pilot testing also underscored the importance of monthly meetings to promote shared learning and create a forum for communication and problem solving across settings. During these meetings, patient case discussions inform continuous quality improvement and promote energy‐sustaining team‐building. Information is then disseminated to each clinic site and arm of the intervention through a designated champion from each group. We also planned to meet monthly with the hospital executive director to balance service and research needs, and engage in rapid‐cycle change throughout our 1‐year demonstration project.
Funding the Program
We talked to others with experience implementing nurse‐led transitional care interventions. Based on these discussions, we anticipated our nurse would be able to see 200 patients over the course of 1 year, and we developed our budget accordingly (Table 2). From our needs assessment, we knew 60% of patients reported at least 1 hospitalization in the 6 months prior. If we assumed that 60% (120) of the 200 patients randomized to our intervention would get readmitted, then a 20% reduction would lead to 24 avoided readmissions and translate into $264,000 in savings for the health system. Even though the hospital would not reap all of these savings, as patients get admitted to other area hospitals, hospital administration acknowledged the value of setting the stage for community‐wide solutions. Moreover, the benefit was felt to extend beyond financial savings to improved quality and institutional learning around transitional care.
PROGRAM EVALUATION
We are conducting a clustered, randomized controlled trial to evaluate C‐TraIn's impact on quality, access, and high‐cost utilization at 30 days after hospital discharge. Results are anticipated in mid‐2012. We chose to perform an analysis clustered by admitting team, because communication between the C‐TraIn nurse, physician team, and pharmacist consult services could introduce secular change effects that could impact the care received by other patients on a given team. There are 5 general medicine resident teams, 1 hospitalist service, and 1 cardiology service, and the physician personnel for each team changes from month to month. Because the cardiology and hospitalist services differ slightly from resident teams, we chose a randomized cross‐over design such that intervention and control teams are redesignated every 3 months. To enhance internal validity, study personnel who enroll patients and administer baseline and 30‐day surveys are blinded to intervention status. We are collecting data on prior utilization, usual source of care, outpatient access, insurance, patient activation,6 functional status,7, 8 self‐rated health,7 health literacy, care transitions education,9 alcohol and substance abuse, and social support.10 Our primary outcome will be self‐reported 30‐day hospital readmission and ED use. We will also evaluate administrative claims data to identify 30‐day OHSU readmission and ED utilization rates. We will assess whether improved access to medications, rates of outpatient follow‐up and time to follow‐up mediate any effect on primary outcomes. Secondary outcomes will include outpatient utilization, patient activation, self‐rated health, and functional status.
Given limited experience with transitional care programs in socioeconomically disadvantaged patients, we are measuring acceptability and feasibility by tracking rates of those declining the intervention, and through semi‐structured interviews at 30 days. We are monitoring fidelity to core elements of the program through chart and checklist reviews, and seeking provider feedback through in‐person meetings with key implementers. To ensure possibility of broader adoption beyond OHSU, we are developing a toolkit that defines core program elements and can be adapted for use in various settings.
DISCUSSION
Using a process of broad stakeholder engagement, exposure of financial incentives, and data‐driven understanding of institutional and population needs, we built consensus and gained institutional financial commitment for implementation of a multicomponent transitional care program for uninsured and Medicaid patients. Our experience is relevant to other hospital systems, and may have particular relevance to academic medical centers, whose tripartite mission of clinical care, research, and education make them a natural place for healthcare reform.11
Several key lessons from our experience may be widely applicable. First, key administrative allies helped us understand institutional priorities and identify key institutional change‐agents. Though initial attempts to gain support were met cautiously, persistent advocacy, development of a strong business case, and support from several administrative allies compelled further leadership support. Second, unlike traditional grant funding cycles, hospital budgets operate in real‐time rapid‐change cycles, necessitating rapid data collection, analysis, and program design. Such demands could potentially threaten the viability of the program itself, or result in premature diffusion of novel practices into disparate populations. Communication with administrative leadership about the value of sound research design within the context of faster‐paced institutional needs was important and allowed time for data‐driven program development and diffusion. Simultaneously, we recognized the need to move quickly, provide regular progress updates, and use existing institutional resources, such as volunteer students and business development office, when possible.
We found that cross‐site hospitalcommunity partnerships are an essential program element. Partnership occurs through a payment agreement and through active engagement in ongoing quality improvement, including clinic representation at monthly team meetings. Clinic partnerships have enabled multidisciplinary cross‐site communication and relationships that facilitate innovation across routinely siloed elements of the system, allowing the team to anticipate and respond to patient problems before they lead to readmissions or poor outcomes. Our experience matches findings from recent program evaluations that found that care coordination attempts are unsuccessful without strong cross‐site linkages.12 These linkages are especially challenging and needed for uninsured and Medicaid patients, given their traditional lack of access and the additional social and financial barriers that influence their care.13
Limitations of our study include: implementation at a single, academic medical center; secular changes (which we mitigate against using randomized trial design); and potential for low power, if readmission rates are lower than anticipated from needs assessment data. Additionally, the need for a willing and invested program champion to coordinate an often messy, complex intervention may limit generalizability.
While transitional care programs continue to proliferate in response to increasingly recognized gaps in a fragmented care system,14, 15 few interventions specifically address the needs of socioeconomically disadvantaged patients. The major study that did5 was conducted in Massachusetts, where many patients received care through a state Free Care program and robust local safety‐net. Others have largely been tested in integrated care settings,1 and target patients who are part of managed care programs.1, 4, 16
To our knowledge, there are no well‐described programs that include explicit purchasing of outpatient medical homes for uninsured patients who would not otherwise have access to care. Our experience shifts the paradigm of the role of hospitals in care for the uninsured and underinsured: instead of a reactive, uncoordinated role, we assert that the hospital's strategic up‐front allocation of resources has a sound business, quality, and ethical foundation. This is especially important, given a new era of payment reform and coordinated care organizations. There is an opportunity to both improve quality for the uninsured and Medicaid patients, control costs, and gain valuable experience that can inform transitional care improvements for broader patient populations. If our study is successful in reducing readmissions, there may be important implications as to how to redefine the hospital's role in outpatient access to care linkages, especially for uninsured and Medicaid patients.
Acknowledgements
The authors acknowledge Char Riley, Dawn Whitney, and Tara Harben of OHSU, as well as volunteer research assistants Amie Leaverton, Molly McClain, Emily Johnson, Travis Geraci, and Claudia Sells.
Hospital readmissions are common and costly, and represent a significant burden to the healthcare system. The challenges of postdischarge medication uncertainty, lack of self‐management support, and lack of timely access to health professionals1 are compounded in uninsured and Medicaid individuals by limited access to medications and primary care, financial strain, insecure housing, and limited social support.2
Our hospital cares for a large number of uninsured and low‐income publicly insured patients. The Portland area safety‐net, which consists of a network of 14 federally qualified health centers and free clinics, has limited capacity for uncompensated care. Uninsured patientsand to a lesser degree, Medicaid patientshave difficulty establishing primary care. Prior to the implementation of our program, uninsured and Medicaid patients without a usual source of care were given a list of safety‐net clinics at discharge, but frequently could not access appointments or navigate the complex system. There were no well‐developed partnerships between hospital and outpatient clinics for uninsured or Medicaid patients. The hospital lacked a systematic approach to securing postdischarge follow‐up and peridischarge patient education, and uninsured patients were financially responsible for most medications upon discharge. The costs of uncompensated or undercompensated potentially preventable readmissions for these patients, along with the recognition of gaps in quality, ultimately provided the rationale for a medical center‐funded transitional care intervention for uninsured and low‐income publicly insured patients.
Several transitional care improvement programs have shown effectiveness in reducing hospital readmissions,1, 35 but most have been conducted in settings where patients have secure access to outpatient care, and none have focused specifically on uninsured or Medicaid patients. Moreover, the development of these programs requires time and capital. Transitional care programs that have published results, to date, have been funded through government or private foundation grants1, 35; however, broader implementation of transitional care innovations will require financial and intellectual engagement of healthcare institutions themselves.
This report describes development of the Care Transitions Innovation (C‐TraIn), a multicomponent transitional care intervention for uninsured and low‐income publicly insured adults at a large, urban academic medical center, Oregon Health & Science University (OHSU). Because institutional funding and engagement is critical to the sustainability and scalability of similar programs, we also describe our process for gaining institutional support. Our hypothesis is that C‐TraIn can reduce readmissions and emergency department (ED) use at 30 days after hospital discharge, compared with usual care.
METHODS
Engaging Institutional Leaders
Early and continued efforts to engage hospital administrators were integral to our ultimate success in gaining institutional funding and leadership support. Initially, we convened what we called a Health Systems Morbidity and Mortality conference, featuring an uninsured patient who told of his postdischarge experiences and costly, potentially preventable readmission. We invited a broad array of potential stakeholders, including representatives from hospital administration, hospital case managers and social workers, community safety‐net providers, inpatient and outpatient physicians, residents, and medical students. Our patient was previously admitted to OHSU and diagnosed with pneumonia, hypothyroidism, sleep apnea, and depression. At discharge, he was given a list of low‐cost clinics; however, he was unable to arrange follow‐up, could not afford prescriptions, and felt overwhelmed trying to navigate a complex system. Consequently, he received no outpatient healthcare and his illnesses progressed. Unable to stay awake as a long‐haul trucker, he lost his job and subsequently his housing, and was readmitted to the intensive care unit with severe hypercarbic respiratory failure, volume overload, and hypothyroidism. The $130,000 charge for his 19‐day rehospitalization was largely un‐recuperated by the hospital. The case was a stark example of the patient‐safety and financial costs of fragmented care, and the conference was a nidus for further institutional engagement and program development, the key steps of which are described in Table 1.
| Time | Key Step | How Step Was Achieved | Take Home Points |
|---|---|---|---|
| |||
| July 2008July 2009 | 1. Identified key stakeholders | Considered varied stakeholders impacted by transitional care gaps for uninsured and Medicaid patients | Casting a wide net early in the process promoted high level of engagement and allowed self‐identification of some stakeholders |
| 2. Framed problems and opportunities; exposed costs of existing system shortcomings | Educational conference (that we called a Health Systems M&M) fostered a blame‐free environment to explore varied perspectives | Individual patient story made policy issue more accessible to a wide range of stakeholders | |
| Discussion of exposed drivers and costs of misaligned incentives; highlighted inroads to developing a business case for change | |||
| Oct 2008June 2009 | 3. Identified administrative allies and leaders with high bridging capital | Follow‐up with administrator after Health System M&M allowed further identification of key administrative stakeholders | Administrator insight highlighted institutional priorities and strategic plans |
| Ongoing meetings over 9 moto advocate for change, explore support for program development | Key ally within administration facilitated conversation with executive leadership whose support was a critical for program success | ||
| July 2009June 2010 | 4. Framed processes locally with continued involvement from multiple stakeholders | Performed multicomponent needs assessment | Patient assessment included inpatients for ease of survey administration |
| Utilized efforts of student volunteers for low‐budget option | |||
| Existing administrative support aided patient tracking | |||
| Non‐integrated health system and lack of claims data for uninsured limited usefulness of administrative utilization data | |||
| 5. Performed cost analysis to further support the business and quality case | Used OHSU data from needs assessment patient sample to estimate potential costs and savings of saved readmissions and avoided ED visits | Business case highlighted existing costs to OHSU for uncompensated care; program presented a solution to realign incentives and better allocate existing hospital expenditures | |
| Qualitative patient interviews exposed opportunity for quality improvement | Highlighted pilot as an opportunity for institutional learning about transitional care improvements | ||
| 6. Use needs assessment to map intervention | Drew upon local and national health systems expertise through literature review and consultation with local and national program leaders | OHSU's Care Transitions Innovation (C‐TraIn) includes elements aimed at improving access, patient education, care coordination, and systems integration (Table 2) | |
| Matched patient needs to specific elements of program design | |||
Planning the Intervention
Findings from a patient needs assessment and community stakeholder meetingsdescribed belowdirectly informed a multicomponent intervention that includes linkages and payment for medical homes for uninsured patients who lack access to outpatient care, a transitional care nurse whose care bridges inpatient and outpatient settings, inpatient pharmacy consultation, and provision of 30 days of medications at hospital discharge for uninsured patients (Table 2).
| Program Element | Description | Resources per 200 Patients |
|---|---|---|
| ||
| Transitional care RN | Augments patient education and care coordination in the hospital until 30 days after discharge. Tasks include: | 1.0 FTE nurse salary* |
| developing a personal health record with inpatients | ||
| completing a home visit within 72 hr of discharge to focus on medication reconciliation and patient self‐management | ||
| low‐risk patients receive 3 calls and no home visit (see Supporting Information, Appendix 1, in the online version of this article) | ||
| 2 subsequent phone calls to provide additional coaching, identify unmet needs, and close the loop on incomplete financial paperwork | ||
| The nurse provides a warm handoff with clinic staff, assists in scheduling timely posthospital follow‐up, and assures timely transfer of DC summaries. She coordinates posthospital care management with Medicaid case‐workers when available. | ||
| Pharmacy | Consultation: Inpatient pharmacists reconcile and simplify medication regimens, educate patients, and assess adherence barriers. | 0.4 FTE inpatient pharmacist salary |
| Prescription support: For uninsured patients, pharmacists guide MD prescribing towards medications available on the C‐TraIn value‐based formulary, a low‐cost formulary that reflects medications available through $4 plans, a Medicaid formulary, and FQHC on‐site pharmacies. | Estimated $12/prescription; 6.5 prescriptions/patient | |
| Uninsured patients are given 30 days of bridging prescription medications at hospital discharge free of charge. | ||
| Outpatient medical home and specialty care linkages | OHSU has partnered with outpatient clinics on a per‐patient basis to support funding of primary care for uninsured patients who lack a usual source of care. Clinics also provide coordinated care for Medicaid patients without assigned primary care, and have committed to engaging in continuous quality improvement. Clinics include an academic general internal medicine practice, an FQHC specializing in addiction and care for the homeless, and an FQHC that serves a low‐income rural population. | Estimated 8 primary care visits/yr at $205/visit (FQHC reimbursement rate) equates to $1640/ patient/yr. |
| Timely posthospital specialty care related to index admission diagnoses is coordinated through OHSU's outpatient specialty clinics. | ||
| Monthly care coordination meetings | We convene a diverse team of community clinic champions, OHSU inpatient and outpatient pharmacy and nurse representatives, hospital administrative support, and a CareOregon representive. | |
| At each meeting, we review individual patient cases, seek feedback from diverse, and previously siloed, team members, and engage in ongoing quality improvement. | ||
Needs Assessment
We conducted a mixed‐methods needs assessment of consecutive nonelderly adult inpatients (65 years old) admitted to general medicine and cardiology, between July and October 2009, with no insurance, Medicaid, or MedicareMedicaid. Five volunteer medical and pre‐medical students surveyed 116 patients (see Supporting Information survey, Appendix 2, in the online version of this article). Forty patients reported prior admission within the last 6 months. With these participants, we conducted in‐depth semi‐structured interviews assessing self‐perceived transitional care barriers. Investigators drew preliminary themes from the interviews but delayed a scientifically rigorous qualitative analysis, given a compressed timeline in which to meet program development needs. Of the 116 patients surveyed, 22 had MedicareMedicaid. Given that many of these patients discharged to skilled nursing facilities, we focused program development using data from the 94 uninsured and Medicaid patients (Table 3).
| Uninsured (n = 43 patients) | Medicaid (n = 51 patients) | |
|---|---|---|
| ||
| Lack usual source of care (%) | 33.3 | 11.1* |
| Self‐reported 6 mo rehospitalization (%) | 60.0 | 48.6 |
| Average no. Rx prior to hospitalization | 4.4 | 13.8 |
| Barriers to taking meds as prescribed (%) | 42.9 | 21.6* |
| Cost of meds as leading barrier (%) | 30.0 | 2.9* |
| Marginal housing (%) | 40.5 | 32.4 |
| Low health literacy (%) | 41.5 | 41.7 |
| Transportation barrier (%) | 11.9 | 31.4* |
| Comorbid depression (%) | 54.8 | 45.9 |
| Income 30 K (%) | 79.5 | 96.8 |
Finding 1: Thirty‐three percent of uninsured and 11% of Medicaid patients lacked a usual source of care. This was highest among Portland‐area residents (45%). Program element: We forged relationships with 3 outpatient clinics and developed a contractual relationship whereby OHSU pays for medical homes for uninsured patients lacking usual care. Finding 2: Patients were unclear as to how to self‐manage care or who to contact with questions after hospitalization. Program element: Transitional care nurse provides intensive peridischarge education, performs home visits within 3 days of discharge, and serves as a point person for patients during the peridischarge period. Finding 3: Among uninsured patients, cost was the leading barrier to taking medications as prescribed and often led to self‐rationing of medications without provider input. Program element: We developed a low‐cost, value‐based formulary for uninsured patients that parallels partnering clinic formularies, $4 plans, and medication assistance programs. After 30 days of program‐funded medications, patients then get medications through these other sources. Inpatient pharmacists consult on all patients to reconcile medications, identify access and adherence gaps, provide patient education, and communicate across settings. Finding 4: Comorbid depression was common. Program element: We sought partnerships with clinics with integrated mental health services. Finding 5: Over half of patients live in 3 counties surrounding Portland. Program element: We restricted our intervention to patients residing in local counties and included postdischarge home visits in our model. Partnering clinics match patient geographic distribution. Finding 6: Self‐ reported 6‐month readmission (60%) rates exceeded rates estimated by hospital administrative data (18%), supporting qualitative findings that patients seek care at numerous hospitals. Program element: Given that utilization claims data are unavailable for the uninsured, we included phone follow‐up surveys to assess self‐reported utilization 30 days postdischarge. Finding 7: Using administrative data, we estimated that the hospital loses an average of $11,000 per readmission per patient in direct, unremunerated costs. Indirect costs (such as costs of hospital staff) and opportunity costs (of potential revenue from an insured patient occupying the bed) were excluded, thus presenting a conservative estimate of cost savings. Program element: We used local cost data to support the business case and emphasize potential value of an up‐front investment in transitional care.
Defining the Setting
We convened a series of 3 work group meetings with diverse internal and external stakeholders (Table 4) to further define an intervention in the context of local health system realities. Work groups shaped the program in several specific ways. First, community clinic leaders emphasized that limited specialty access is an important barrier when caring for recently hospitalized uninsured and Medicaid patients. They felt expanded postdischarge access to specialists would be important to increase their capacity for recently discharged patients. Thus, we streamlined patients' posthospital specialty access for conditions treated during hospitalization. Second, initially we considered linking with 1 clinic; however, health systems researchers and clinic providers cautioned us, suggesting that partnering with multiple clinics would make our work more broadly applicable. Finally, pharmacists and financial assistance staff revealed that financial assistance forms are often not completed during hospitalization because inpatients lack access to income documentation. This led us to incorporate help with financial paperwork into the postdischarge intervention.
| Clinical staff |
| Hospital medicine physician |
| General internal medicine physician |
| Hospital ward nurse staff |
| Pharmacy (inpatient, outpatient, medication assistance programs) |
| Care management/social work |
| Emergency medicine |
| Health system leadership |
| Hospital administrative leadership |
| Primary care clinic leadership |
| Safety‐net clinic leadership |
| Specialty clinic leadership |
| Hospital business development and strategic planning |
| CareOregon (Medicaid managed care) leadership |
| Other |
| Patients |
| Health systems researchers |
| Clinical informatics |
| Hospital financials (billing, financial screening, admitting) |
Pilot Testing
We conducted pilot testing over 4 weeks, incorporating a Plan‐Do‐Study‐Act approach. For example, our transitional care nurse initially used an intervention guide with a list of steps outlined; however, we quickly discovered that the multiple and varied needs of this patient populationincluding housing, transportation, and foodwere overwhelming and pulled the nurse in many directions. In consultation with our quality improvement experts, we reframed the intervention guide as a checklist to be completed for each patient.
Pilot testing also underscored the importance of monthly meetings to promote shared learning and create a forum for communication and problem solving across settings. During these meetings, patient case discussions inform continuous quality improvement and promote energy‐sustaining team‐building. Information is then disseminated to each clinic site and arm of the intervention through a designated champion from each group. We also planned to meet monthly with the hospital executive director to balance service and research needs, and engage in rapid‐cycle change throughout our 1‐year demonstration project.
Funding the Program
We talked to others with experience implementing nurse‐led transitional care interventions. Based on these discussions, we anticipated our nurse would be able to see 200 patients over the course of 1 year, and we developed our budget accordingly (Table 2). From our needs assessment, we knew 60% of patients reported at least 1 hospitalization in the 6 months prior. If we assumed that 60% (120) of the 200 patients randomized to our intervention would get readmitted, then a 20% reduction would lead to 24 avoided readmissions and translate into $264,000 in savings for the health system. Even though the hospital would not reap all of these savings, as patients get admitted to other area hospitals, hospital administration acknowledged the value of setting the stage for community‐wide solutions. Moreover, the benefit was felt to extend beyond financial savings to improved quality and institutional learning around transitional care.
PROGRAM EVALUATION
We are conducting a clustered, randomized controlled trial to evaluate C‐TraIn's impact on quality, access, and high‐cost utilization at 30 days after hospital discharge. Results are anticipated in mid‐2012. We chose to perform an analysis clustered by admitting team, because communication between the C‐TraIn nurse, physician team, and pharmacist consult services could introduce secular change effects that could impact the care received by other patients on a given team. There are 5 general medicine resident teams, 1 hospitalist service, and 1 cardiology service, and the physician personnel for each team changes from month to month. Because the cardiology and hospitalist services differ slightly from resident teams, we chose a randomized cross‐over design such that intervention and control teams are redesignated every 3 months. To enhance internal validity, study personnel who enroll patients and administer baseline and 30‐day surveys are blinded to intervention status. We are collecting data on prior utilization, usual source of care, outpatient access, insurance, patient activation,6 functional status,7, 8 self‐rated health,7 health literacy, care transitions education,9 alcohol and substance abuse, and social support.10 Our primary outcome will be self‐reported 30‐day hospital readmission and ED use. We will also evaluate administrative claims data to identify 30‐day OHSU readmission and ED utilization rates. We will assess whether improved access to medications, rates of outpatient follow‐up and time to follow‐up mediate any effect on primary outcomes. Secondary outcomes will include outpatient utilization, patient activation, self‐rated health, and functional status.
Given limited experience with transitional care programs in socioeconomically disadvantaged patients, we are measuring acceptability and feasibility by tracking rates of those declining the intervention, and through semi‐structured interviews at 30 days. We are monitoring fidelity to core elements of the program through chart and checklist reviews, and seeking provider feedback through in‐person meetings with key implementers. To ensure possibility of broader adoption beyond OHSU, we are developing a toolkit that defines core program elements and can be adapted for use in various settings.
DISCUSSION
Using a process of broad stakeholder engagement, exposure of financial incentives, and data‐driven understanding of institutional and population needs, we built consensus and gained institutional financial commitment for implementation of a multicomponent transitional care program for uninsured and Medicaid patients. Our experience is relevant to other hospital systems, and may have particular relevance to academic medical centers, whose tripartite mission of clinical care, research, and education make them a natural place for healthcare reform.11
Several key lessons from our experience may be widely applicable. First, key administrative allies helped us understand institutional priorities and identify key institutional change‐agents. Though initial attempts to gain support were met cautiously, persistent advocacy, development of a strong business case, and support from several administrative allies compelled further leadership support. Second, unlike traditional grant funding cycles, hospital budgets operate in real‐time rapid‐change cycles, necessitating rapid data collection, analysis, and program design. Such demands could potentially threaten the viability of the program itself, or result in premature diffusion of novel practices into disparate populations. Communication with administrative leadership about the value of sound research design within the context of faster‐paced institutional needs was important and allowed time for data‐driven program development and diffusion. Simultaneously, we recognized the need to move quickly, provide regular progress updates, and use existing institutional resources, such as volunteer students and business development office, when possible.
We found that cross‐site hospitalcommunity partnerships are an essential program element. Partnership occurs through a payment agreement and through active engagement in ongoing quality improvement, including clinic representation at monthly team meetings. Clinic partnerships have enabled multidisciplinary cross‐site communication and relationships that facilitate innovation across routinely siloed elements of the system, allowing the team to anticipate and respond to patient problems before they lead to readmissions or poor outcomes. Our experience matches findings from recent program evaluations that found that care coordination attempts are unsuccessful without strong cross‐site linkages.12 These linkages are especially challenging and needed for uninsured and Medicaid patients, given their traditional lack of access and the additional social and financial barriers that influence their care.13
Limitations of our study include: implementation at a single, academic medical center; secular changes (which we mitigate against using randomized trial design); and potential for low power, if readmission rates are lower than anticipated from needs assessment data. Additionally, the need for a willing and invested program champion to coordinate an often messy, complex intervention may limit generalizability.
While transitional care programs continue to proliferate in response to increasingly recognized gaps in a fragmented care system,14, 15 few interventions specifically address the needs of socioeconomically disadvantaged patients. The major study that did5 was conducted in Massachusetts, where many patients received care through a state Free Care program and robust local safety‐net. Others have largely been tested in integrated care settings,1 and target patients who are part of managed care programs.1, 4, 16
To our knowledge, there are no well‐described programs that include explicit purchasing of outpatient medical homes for uninsured patients who would not otherwise have access to care. Our experience shifts the paradigm of the role of hospitals in care for the uninsured and underinsured: instead of a reactive, uncoordinated role, we assert that the hospital's strategic up‐front allocation of resources has a sound business, quality, and ethical foundation. This is especially important, given a new era of payment reform and coordinated care organizations. There is an opportunity to both improve quality for the uninsured and Medicaid patients, control costs, and gain valuable experience that can inform transitional care improvements for broader patient populations. If our study is successful in reducing readmissions, there may be important implications as to how to redefine the hospital's role in outpatient access to care linkages, especially for uninsured and Medicaid patients.
Acknowledgements
The authors acknowledge Char Riley, Dawn Whitney, and Tara Harben of OHSU, as well as volunteer research assistants Amie Leaverton, Molly McClain, Emily Johnson, Travis Geraci, and Claudia Sells.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- ,,,,.Medicaid patients at high risk for frequent hospital admission: real‐time identification and remediable risks.J Urban Health.2009;86(2):230–241.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- ,,,,.The effect of Evercare on hospital use.J Am Geriatr Soc.2003;51(10):1427–1434.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,,.Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers.Health Serv Res.2004;39(4 pt 1):1005–1026.
- The EuroQol Group.EuroQol—a new facility for the measurement of health‐related quality of life.Health Policy.1990;16(3):199–208.
- ,,,,,.Trajectories of life‐space mobility after hospitalization.Ann Intern Med.2009;150(6):372–378.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,,,.Assessing social support: the social support questionnaire.J Pers Soc Psychol.1983;44(1):127–139.
- .Payment reform and the mission of academic medical centers.N Engl J Med.2010;363(19):1784–1786.
- ,,,.Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials.JAMA.2009;301(6):603–618.
- ,,,.Post‐discharge intervention in vulnerable, chronically ill patients.J Hosp Med.2012;7(2):124–130.
- ,,, et al.Discharge planning from hospital to home.Cochrane Database Syst Rev.2010(1):000313.
- .Preventing the rebound: improving care transition in hospital discharge processes.Aust Health Rev.2010;34(4):445–451.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- ,,,.The care transitions intervention: results of a randomized controlled trial.Arch Intern Med.2006;166(17):1822–1828.
- ,,,,.Medicaid patients at high risk for frequent hospital admission: real‐time identification and remediable risks.J Urban Health.2009;86(2):230–241.
- ,,,,,.Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.J Am Geriatr Soc.2004;52(5):675–684.
- ,,,,.The effect of Evercare on hospital use.J Am Geriatr Soc.2003;51(10):1427–1434.
- ,,, et al.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150(3):178–187.
- ,,,.Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers.Health Serv Res.2004;39(4 pt 1):1005–1026.
- The EuroQol Group.EuroQol—a new facility for the measurement of health‐related quality of life.Health Policy.1990;16(3):199–208.
- ,,,,,.Trajectories of life‐space mobility after hospitalization.Ann Intern Med.2009;150(6):372–378.
- ,,.Assessing the quality of preparation for posthospital care from the patient's perspective: the care transitions measure.Med Care.2005;43(3):246–255.
- ,,,.Assessing social support: the social support questionnaire.J Pers Soc Psychol.1983;44(1):127–139.
- .Payment reform and the mission of academic medical centers.N Engl J Med.2010;363(19):1784–1786.
- ,,,.Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials.JAMA.2009;301(6):603–618.
- ,,,.Post‐discharge intervention in vulnerable, chronically ill patients.J Hosp Med.2012;7(2):124–130.
- ,,, et al.Discharge planning from hospital to home.Cochrane Database Syst Rev.2010(1):000313.
- .Preventing the rebound: improving care transition in hospital discharge processes.Aust Health Rev.2010;34(4):445–451.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
Post‐Discharge Intervention
The inpatient to outpatient transition marks an abrupt paradigm shift from intensive, provider‐initiated care to self‐managed care, in which patients are primarily responsible for maintaining day‐to‐day health behaviors, following through with outpatient appointments, and negotiating medications, transport, and equipment needs. Studies indicate that medication nonadherence and medication‐related adverse events are common during the post‐discharge period, and may be related to the discontinuities associated with transitions of care.110
Given the complexity and uncertainty inherent in care transitions for patients with chronic illness, it is not surprising that poorly executed care transitions have been associated with increased risk of rehospitalizations and emergency department (ED) use.11 Patients with chronic illness are at particularly high risk for recurrent hospitalization.1114 System‐wide improvements in chronic illness care have been successful in triaging longitudinally followed, high‐risk outpatients to appropriate higher‐intensity care management interventions.1519 But the post‐discharge setting presents unique challenges for patients with chronic illness, especially those that are socioeconomically disadvantaged. External barriers, such as lack of transportation, lack of monitoring equipment, confusion about arranging follow‐up care, uncertainty about medication regimen changes, and financial constraints, represent important targets for improving care in the transition from inpatient to outpatient settings.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as members transfer between different locations or different levels of care.20 Most successful transitional care interventions have focused on geriatric populations or patients with congestive heart failure enrolled in health maintenance organizations, and have involved intensive nurse case management from the hospital setting through the post‐discharge period.2125 In these studies, trained nurse case managers provided critical support and patient education to improve patients' ability to self‐manage chronic illness.2629
In a resource‐poor Medicaid payment structure, the implementation of intensive care management across a broad population of patients may not be practical; alternative options for intervention dosing and implementation may be needed. Few studies have examined care needs and the impact of transitional care interventions in socioeconomically disadvantaged patients with chronic illness, though a recent study including such a population did find benefit from a pharmacist‐based intervention.30 The purpose of our study was to examine the impact of a low‐cost, post‐discharge needs assessment, as an adjunct to an existing care management program, on the risk of recurrent hospitalization in a clinically diverse cohort of chronically ill Medicaid managed care enrollees.
METHODS
Setting
CareOregon is an Oregon‐based, not‐for‐profit, Medicaid managed care organization that administers outpatient and inpatient health benefits for nearly 110,000 members, 5% of whom are dually eligible for Medicaid and Medicare benefits. The CareOregon network includes 950 primary‐care providers, 3000 specialists, 33 hospitals contracted statewide, and 14 public health departments. Approximately 85% of CareOregon's membership lives in the Portland metropolitan area. The remaining 15% are dispersed across mostly rural Oregon counties. CareOregon's membership is both culturally and medically diverse: 55% are female, 21% below age 5 (63% below age 20), and 43% self‐identify as persons of color. Hospitalized patients are generally cared for by inpatient‐based physicians, and an array of primary care and specialty providers based in safety‐net clinics, private practices, and university‐based clinics cares for patients out of the hospital setting. In 2004, CareOregon began CareSupport, a care management program designed to incorporate the principles of the Chronic Care Model31 in which a team‐structured approach is used to improve patient self‐management and coordination of care.
Patients
As part of the authorization and concurrent review activity of CareOregon's Utilization Management unit, hospitalized CareOregon members are identified and a discharge date is entered in the system. CareOregon programmers develop a daily hospital discharge report, which is reviewed each day by medical assistants. Between January and July 2007, this process was used to prospectively identify all CareOregon members over age 35 discharged from 1 of 10 area hospitals. Seven hospitals served as intervention sites, the other 3 as control sites. Patients discharged from intervention hospitals were called at home after discharge and screened for eligibility. Those with one or more chronic illnesses (congestive heart failure, ischemic heart disease, diabetes mellitus, arthritis, depression, chronic obstructive pulmonary disease, and asthma), who consented to participate, were included in the study. The presence of a chronic illness was determined by patient self‐report, and subsequently corroborated by inpatient and outpatient International Classification of Diseases, Ninth Revision (ICD‐9) codes in CareOregon's claims dataset. Patients with one or more of the following were excluded: 1) language other than English; 2) no telephone access; 3) direct, elective hospital admission; 4) admission for <24 hours; 5) primary residence in an extended care facility.
Patients discharged from control hospitals during the study period were included in the control group if they were over age 35, were hospitalized for more than 24 hours, and had one or more chronic illnesses (listed above) as determined by ICD‐9 codes. These patients were identified exclusively through the CareOregon claims database, which includes both inpatient and outpatient diagnostic coding and basic sociodemographic information.
Six of the 10 hospitals from which patients were discharged are large (>300 beds), located in an urban district, and have a robust hospital medicine service. Three of these large, urban hospitals were intervention sites (hospitals 1, 3, and 6), and 3 were control sites (hospitals 2, 4, and 5). All except one of these hospitals (hospital 1) has its own internal medicine residency program. Of the 4 remaining hospitals, all of which were intervention sites, 2 are small (<300 beds) and in an urban district (hospitals 7 and 9), and 2 are small and in rural districts (hospitals 8 and 10).
Intervention
Trained medical assistants conducted a scripted post‐discharge telephone‐based needs assessment and attended to simple interventions. Training included lectures on basic chronic condition management and several months in a peer‐to‐peer learning environment with more experienced medical assistants. Medical assistants were also paired with nurse care managers who provided ongoing training and clinical mentorship. The trained medical assistants called intervention patients within 2‐7 days of hospital discharge. If a patient was not available, a contact number was given and up to 2 additional attempts were made to reach the patient. Medical assistants administered a 35‐item needs assessment survey, which typically took 10‐15 minutes to complete (see Supporting Appendix A in the online version of this article). The survey is based on an existing theoretical framework of healthcare utilization which considers predisposing sociodemographic and healthcare belief variables, enabling resources, and illness level variables.32 Our survey (see Supporting Appendix A in the online version of this article) includes 4 related subdomains: 1) enabling resources (medical home, transportation, housing); 2) psychosocial comorbidities; 3) patient activation33; and 4) past utilization.
The needs assessment was designed to identify issues requiring near‐term resolution, such as the need for follow‐up care, pharmacy access, transportation needs, and medical equipment needs. These identified needs prompted appropriate, immediate, brief‐touch interventions by the medical assistants (eg, arrange transportation, schedule a follow‐up appointment, or provide access telephone numbers).
The needs assessment was also designed to identify members for referral to intensive care management (CareSupport), based on responses to questions about their medical home relationship, prior utilization, self‐management ability, and presence of competing needs. Medical assistants referred patients for intensive care management if they had high‐intensity needs in any one of these domains, or any need in two or more domains. For example, a patient with a history of frequent emergency room visits would be referred for more intensive care management. The CareSupport teama registered nurse specially trained in case management, a behavioral health specialist, and a medical assistantreviewed each referred case. For patients qualifying based on an anticipated ongoing need identified in one or more of the above domains, the CareSupport team constructed an individualized, multifaceted care plan based on disease‐specific guidelines and results of the needs assessment.
The study was approved by the institutional review board of the Oregon Health and Sciences University.
Comparator
Patients in the control cohort received usual care as recommended by discharging and outpatient providers. Patients in the control cohort were not given brief‐touch interventions or referred to CareSupport by study personnel. They could be referred to CareSupport by their outpatient providers.
Analysis
The primary outcome variable was recurrent hospitalization within 60 days to any hospital after index hospitalization discharge. The CareOregon dataset includes inpatient and outpatient claims at any site. Because there may be up to a 3‐month delay in posting claims, we examined the claims dataset 6 months from index hospital discharge for all patients. Sociodemographic, chronic illness comorbidity, and prior utilization data were collected from the CareOregon claims dataset. We used the adjusted clinical group (ACG) score for case‐mix adjustment. The ACG predictive model is an automated risk assessment tool that uses ambulatory diagnoses to identify patients at risk for high inpatient and outpatient utilization in the following year.34, 35 ACG scores range from 0 to 1, with a score of 0.5 corresponding to a 50% chance of high utilization (ie, of being in the top 3% of utilizers) over the following year.
Data for all patients enrolled in the CareSupport care management program are entered and tracked through a separate database, which we accessed to determine whether patients were enrolled in this program during the study period. Information about specific brief‐touch interventions performed was entered narratively by medical assistants.
Baseline characteristics of the 2 groups were compared using t tests or 2 tests, as appropriate. All patients were analyzed according to the group to which they were originally assigned, regardless of subsequent enrollment in CareSupport. We used bivariate analyses to identify covariates associated with the primary outcome. These and other clinically important variables were used to develop a generalized estimated equation model of the impact of the transitional care intervention on risk of rehospitalization within 60 days of discharge, accounting for clustering of patients within hospitals. We included age, hospitalization within the past year, and ACG score as covariates in the final model.
In secondary analyses, we sought to determine whether any association between our transitional care intervention and rehospitalization was mediated by greater use of primary care or care management services. To do this, we used the CareOregon claims dataset to determine primary care utilization for the year following hospital discharge, and we used the CareSupport database to determine whether patients were enrolled in care management. We then repeated our original multivariate model, adding primary care utilization as a covariate, and considered mediation to be present if the addition of primary care utilization substantively attenuated the association between intervention and rehospitalization. We then conducted the same mediation analysis using CareSupport enrollment as a covariate. All analyses were conducted using Stata/SE 9.0 (College Station, TX).
RESULTS
We enrolled 97 intervention and 130 control patients. Follow‐up utilization data were available for all patients. Table 1 compares sociodemographic, utilization, and comorbidity characteristics of the 2 groups, and Table 2 summarizes patient distribution and characteristics of the hospitals from which they were discharged. The control group was significantly older and more racially diverse than the intervention group. On the other hand, the intervention group had a higher burden of illness as suggested by higher ACG scores and a higher rate of hospitalization within the previous year. Most patients had been hospitalized at large, urban hospitals.
| Variable | Intervention (n = 97) | Control (n = 130) |
|---|---|---|
| ||
| Mean age (SE) | 56.3 (1.1) | 60.1 (1.2)* |
| Caucasian race, n (%) | 81 (83.5) | 70 (53.8)* |
| Female, n (%) | 56 (57.7) | 83 (63.8) |
| Mean ACG score (SE) | 0.49 (0.03) | 0.39 (0.03)* |
| Mean hospitalizations in prior year (SE) | 1.97 (0.26) | 1.18 (0.13)* |
| No primary care visit in prior year, n (%) | 8 (8.2) | 19 (14.6) |
| Medicare + Medicaid, n (%) | 40 (41.2) | 47 (36.2) |
| Chronic illness, n (%) | ||
| Diabetes mellitus | 48 (49.5) | 67 (51.5) |
| Depression | 17 (17.7) | 23 (17.9) |
| Congestive heart failure | 29 (29.5) | 45 (34.6) |
| Chronic obstructive pulmonary disease or asthma | 51 (52.6) | 57 (43.8) |
| Hospital No., Intervention or Control | No. of Patients | Hospital Characteristics |
|---|---|---|
| ||
| 1, I | 13 | Large, urban |
| 2, C | 89 | Large, urban |
| 3, I | 26 | Large, urban |
| 4, C | 35 | Large, urban |
| 5, C | 6 | Large, urban |
| 6, I | 30 | Large, urban |
| 7, I | 4 | Small, urban |
| 8, I | 5 | Small, rural |
| 9, I | 7 | Small, urban |
| 10, I | 12 | Small, rural |
Patients in the intervention group had a slightly lower 60‐day rehospitalization rate compared to the control group, but this difference was not statistically significant in unadjusted analyses (Table 3; 23.7% vs 29.2%, P = 0.35). This difference became significant after controlling for ACG score, prior inpatient utilization, and age: adjusted odds ratio (OR) [95% confidence interval (CI)] 0.49 [0.24‐1.00].
| Intervention (n = 97) | Control (n = 130) | Adjusted OR (95% CI) | |
|---|---|---|---|
| |||
| Unadjusted | 23 (23.7%) | 38 (29.2%) | 0.75 (0.41‐1.37) |
| Adjusted, model 1* | 0.49 (0.24‐1.00) | ||
| Model 1 + primary care utilization | 0.49 (0.24‐1.00) | ||
| Model 1 + care management | 0.41 (0.19‐0.88) | ||
Nearly half the intervention patients received one or more brief‐touch interventions (48.5%), and the majority of patients (61.7%) receiving a brief‐touch intervention did not require referral to care management. Table 4 lists examples of brief‐touch interventions received by patients. More patients in the intervention group than in the control group were enrolled in the CareSupport care management program (40.2% vs 14.6%, P < 0.001), and about half (53.8%) of the intervention patients referred for care management did not receive a brief‐touch intervention. Patients enrolled in care management were slightly younger (mean age 56.7 vs 59.1 years, P = 0.16), but had a higher burden of illness (mean ACG score 0.53 vs 0.40, P = 0.006) than those not enrolled in care management.
| Type of Assistance* | n (%) |
|---|---|
| |
| Access information | 13 (13.4) |
| Clinic visit/PCP change | 13 (13.4) |
| Simple self‐management advice | 10 (10.3) |
| Health promotions packet | 6 (6.2) |
| Transportation | 4 (4.1) |
| Tobacco cessation guidance | 4 (4.1) |
| Prescription/pharmacy | 4 (4.1) |
| Flu vaccine promotion | 2 (2.1) |
| Housing/home support | 1 (1.0) |
| Any type of assistance | 47 (48.5) |
More patients in the intervention group compared to control patients had one or more primary care visits within the year after hospital discharge (86.6% vs 72.3%, P = 0.01), and within 60 days after hospital discharge (68.0% vs 58.5%, P = 0.14), though the latter difference did not reach statistical significance. Interestingly, in an exploratory analysis, we found that hospitalization was more likely to introduce discontinuities in longitudinal primary care in control patients than in intervention patients: among patients who had had 3 or more primary care visits in the year prior to hospitalization, control patients were more likely than intervention patients to have 2 or fewer primary care visits during the year following hospitalization (33.8% vs 20.6%, P = 0.03).
Our mediation analyses suggested that neither post‐hospitalization primary care utilization within 60 days nor care management services accounted for the lower rate of recurrent hospitalization in the intervention group (Table 3). The addition of post‐hospitalization primary care utilization to the original model did not change the primary effect estimate, while controlling for care management enrollment actually increased the effect size. Finally, there was no difference in readmission rates between those receiving and not receiving brief‐touch interventions (31.8 vs 25.7%, P = 0.92).
DISCUSSION
We found that a simple, telephone‐based, transitional care intervention may be associated with lower 60‐day rehospitalization rates in a cohort of Medicaid managed care patients. We observed a reduced rate of readmissions in the intervention, a difference that became significant after adjustment for important confounders. Implicit in the design of the intervention was a recognition that patients' transitional care needs may vary, from help negotiating the post‐discharge follow‐up care process to more substantial and complex care management support needs. Our study adds to the current body of literature by examining an understudied, socioeconomically disadvantaged population in a resource‐poor health system. Importantly, our study targeted the most intensive intervention to those with the highest anticipated needs based on a simple triage scheme. Such targeted approaches may be especially important in resource‐poor settings.
Although our study was too small to characterize in detail the relative importance of specific elements of our intervention responsible for lower short‐term rehospitalization rates, the study does highlight the diversity of transitional care needs. Patients received logistic support negotiating the health system, preventive health promotion, and patient empowerment through self‐management and information access training. Nearly half the patients received a documented simple telephone‐based intervention, and many of these patients did not require referral for intensive nurse care management. On the other hand, our needs assessment did identify over one‐third of recently discharged patients as having more complex chronic disease management needs requiring assessment for ongoing nurse care management.
We were not able to identify the specific aspects of the intervention responsible for the observed reduction in recurrent hospitalization. Our mediation analysis suggests that triaging patients to a nurse care management program was not responsible for the observed reduction in recurrent hospitalizations. In fact, the analysis suggests these patients may have been more likely to require hospitalization, though our study was too small to allow strong conclusions to be drawn from a subgroup analysis. Past studies have similarly suggested that patients enrolled in care management may simply have a higher burden of disease or may have the need for hospitalization recognized more frequently.36 Readmission rates were also similar between patients who did and did not receive a brief‐touch intervention, possibly suggesting that patients with a higher level of need were appropriately selected to receive assistance.
Although our intervention appeared to increase post‐discharge follow‐up in primary care, this also did not explain the observed reduction in 60‐day rehospitalization rates. Despite differences in post‐discharge outpatient utilization patterns, there were relatively few patients in either group that had no follow‐up, and the lack of effect may simply reflect inadequate power given our small sample size. On the other hand, the lack of association between outpatient utilization and 60‐day rehospitalization rates may reflect a true lack of association between primary care follow‐up and rehospitalization as seen in some studies, though a larger Medicare study did find an association.3638
Improvements in outpatient utilization patterns, as we saw in this study, may be a laudable intermediate outcome benefit despite the lack of association with 60‐day rehospitalization rates in our study. Short‐term rehospitalization rates represent only one outcome and do not capture the expected slow, iterative benefits from chronic illness risk reduction, which may accrue over time, with stable longitudinal primary care and associated outpatient chronic illness care systems' innovations.15, 31, 39, 40
Recent studies of transitional care interventions in publicly insured adults have produced mixed results. An evaluation of Medicare demonstration projects found largely negative results, but did find 2 successful programs in which the highest‐risk patients seemed to benefit most, a finding that supports the importance of assessing risk and appropriately dosing interventions.41 Another recent study in a socioeconomically disadvantaged population suggests the utility of an alternate transitional care approach centered on a pharmacy‐based intervention.30
Ours is essentially a test‐of‐concept study, with several important limitations, which should temper widespread application of these results and suggest the need for further study. The sample size of our study was limited, and this, coupled with a slightly lower‐than‐expected event rate, limits our ability to detect potentially important effects. Our study was not a randomized trial, and we cannot discount the possibility that our results reflect the effect of residual or unmeasured confounders, especially those factors such as patient volume and care quality related to the discharging hospitals themselves. We attempted to minimize the effects of such confounders by balancing the types of hospitals included in each group, and by accounting for clustering by hospital in our statistical analysis. Important differences in baseline characteristics between the 2 groups also raise the possibility of residual confounding despite multivariate adjustment. However, the intervention group generally carried a higher burden of illness which would, if anything, have biased results towards the null. The pragmatic study design necessitated an intervention that was defined broadly and left much to the discretion of the staff delivering the intervention, rather than adherence to a strictly defined protocol. We believe this approach allows evaluation of systems innovations within limited‐resource settings, but we acknowledge the challenges this presents in applying study results to other settings. Finally, only approximately 1 in 4 intervention patients were successfully contacted and completed the post‐discharge survey within 1 week. The relatively low rate of successful telephone contact underscores the difficulty of implementing transitional care interventions dependent on post‐discharge contact in a socioeconomically disadvantaged population with unstable telephone access. Because only successfully contacted patients were included in the intervention group, selection bias is a potential issue, though again, most baseline discrepancies between the 2 groups suggest that intervention patients were more complex.
Transitions of care in uninsured and publicly insured nonelderly adults should be studied in greater depth. Outpatient access to care deficiencies may be compounded in these groups, especially as states face widespread budget crises. Future studies should examine the effects of inpatient to outpatient linkages for such patients. Also, studies should assess the impact of transitional care interventions on self‐management, quality of care, and intermediate health outcomes in the outpatient setting after hospital discharge. Future research should taxonomize the range of transitional care needs by qualitatively evaluating subgroups of patients and delineating challenges faced by each group. For example, the post‐discharge needs of marginally housed patients may be unique and could inform the development of interventions specifically targeted to this group.
In summary, we found that a simple, brief‐touch intervention and needs assessment in the post‐discharge period may be associated with reduced recurrent hospitalization rates in a cohort of chronically ill Medicaid managed care patients with diverse post‐discharge care needs, though the exact mechanisms responsible for the observed improvements are unclear. Future studies should evaluate transitional care interventions targeted to needs in a larger group of chronically ill patients.
Acknowledgements
The authors thank Drs Jennifer Malcom and Lauren Robertson for their help in collecting the data for this project.
- ,,.The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly.Arch Intern Med.1990;150(4):841–845.
- ,,,,.Drug‐associated hospital admissions in older medical patients.J Am Geriatr Soc.1988;36(12):1092–1098.
- ,,.Medication adherence in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.2001;35(5):539–545.
- ,,,.Corticosteroid use after hospital discharge among high‐risk adults with asthma.Am J Respir Crit Care Med.2004;170(12):1281–1285.
- ,,,.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.J Am Coll Cardiol.2002;40(9):1589–1595.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital [see comment].Ann Intern Med.2003;138(3):161–167.
- ,,,,,.Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long‐term care facilities.Arch Intern Med.2004;164(5):545–550.
- ,,.The adverse effects of hospitalization on drug regimens.Arch Intern Med.1991;151(8):1562–1564.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting [see comment].J Gen Intern Med.2003;18(8):646–651.
- ,,,.Posthospital care transitions: patterns, complications, and risk identification.Health Serv Res.2004;39(5):1449–1465.
- ,.Diagnosing diabetes and preventing rehospitalizations: the urban diabetes study.Med Care.2006;44(3):292–296.
- ,,,,,.Predictors of rehospitalization and death after a severe exacerbation of COPD [see comment].Chest.2007;132(6):1748–1755.
- ,,,.Multiple hospitalizations for patients with diabetes.Diabetes Care.2003;26(5):1421–1426.
- ,,,,,.Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review.Diabetes Care.2001;24(10):1821–1833.
- ,,,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies.Rockville, MD:Agency for Healthcare Research and Quality, US Department of Health and Human Services;2004.
- ,,,.Clinical service organisation for heart failure [systematic review].Cochrane Database Syst Rev.2005;2:CD002752.
- ,,,.Group based training for self‐management strategies in people with type 2 diabetes mellitus [systematic review].Cochrane Database Syst Rev.2005;2:CD003417.
- ,,,,,.Proactive case management of high‐risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial.Am J Manag Care.2005;11(4):253–260.
- ,;for the American Geriatrics Society Health Care Systems. Improving the quality of transitional care for persons with complex care needs.J Am Geriatr Soc.2003;51(4):556–557.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Effects of a home‐based intervention among patients with congestive heart failure discharged from acute hospital care.Arch Intern Med.1998;158(10):1067–1072.
- ,,,.Prolonged beneficial effects of a home‐based intervention on unplanned readmissions and mortality among patients with congestive heart failure.Arch Intern Med.1999;159(3):257–261.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention.J Am Geriatr Soc.2004;52(11):1817–1825.
- ,,.Self‐management interventions for chronic illness.Lancet.2004;364(9444):1523–1537.
- ,,.Effectiveness of self‐management training in type 2 diabetes: a systematic review of randomized controlled trials.Diabetes Care.2001;24(3):561–587.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- ,,,,.Collaborative management of chronic illness.Ann Intern Med.1997;127(12):1097–1102.
- ,,,.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150:178–187.
- ,,.Improving primary care for patients with chronic illness.JAMA.2002;288(14):1775–1779.
- ,.Societal and individual determinants of medical care utilization in the United States.Milbank Q.2005;83(4):1–28.
- ,,,.Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers.Health Serv Res.2004;39(4 pt 1):1005–1026.
- ,,.Adjusted clinical groups: predictive accuracy for Medicaid enrollees in three states.Health Care Financ Rev.2002;24(1):43–61.
- ,,,.Ambulatory care groups: a categorization of diagnoses for research and management.Health Serv Res.1991;26(1):53–74.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–1447.
- ,,,.Randomized controlled trial of emergency department interventions to improve primary care follow‐up for patients with acute asthma.Chest.2006;129(2):257–265.
- ,,,.Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure.JAMA.2010;303(17):1716–1722.
- Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34).UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352(9131):854–865.
- ,,,,,.Effect of improved glycemic control on health care costs and utilization.JAMA.2001;285(2):182–189.
- ,,,.Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials.JAMA.2009;301(6):603–618.
The inpatient to outpatient transition marks an abrupt paradigm shift from intensive, provider‐initiated care to self‐managed care, in which patients are primarily responsible for maintaining day‐to‐day health behaviors, following through with outpatient appointments, and negotiating medications, transport, and equipment needs. Studies indicate that medication nonadherence and medication‐related adverse events are common during the post‐discharge period, and may be related to the discontinuities associated with transitions of care.110
Given the complexity and uncertainty inherent in care transitions for patients with chronic illness, it is not surprising that poorly executed care transitions have been associated with increased risk of rehospitalizations and emergency department (ED) use.11 Patients with chronic illness are at particularly high risk for recurrent hospitalization.1114 System‐wide improvements in chronic illness care have been successful in triaging longitudinally followed, high‐risk outpatients to appropriate higher‐intensity care management interventions.1519 But the post‐discharge setting presents unique challenges for patients with chronic illness, especially those that are socioeconomically disadvantaged. External barriers, such as lack of transportation, lack of monitoring equipment, confusion about arranging follow‐up care, uncertainty about medication regimen changes, and financial constraints, represent important targets for improving care in the transition from inpatient to outpatient settings.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as members transfer between different locations or different levels of care.20 Most successful transitional care interventions have focused on geriatric populations or patients with congestive heart failure enrolled in health maintenance organizations, and have involved intensive nurse case management from the hospital setting through the post‐discharge period.2125 In these studies, trained nurse case managers provided critical support and patient education to improve patients' ability to self‐manage chronic illness.2629
In a resource‐poor Medicaid payment structure, the implementation of intensive care management across a broad population of patients may not be practical; alternative options for intervention dosing and implementation may be needed. Few studies have examined care needs and the impact of transitional care interventions in socioeconomically disadvantaged patients with chronic illness, though a recent study including such a population did find benefit from a pharmacist‐based intervention.30 The purpose of our study was to examine the impact of a low‐cost, post‐discharge needs assessment, as an adjunct to an existing care management program, on the risk of recurrent hospitalization in a clinically diverse cohort of chronically ill Medicaid managed care enrollees.
METHODS
Setting
CareOregon is an Oregon‐based, not‐for‐profit, Medicaid managed care organization that administers outpatient and inpatient health benefits for nearly 110,000 members, 5% of whom are dually eligible for Medicaid and Medicare benefits. The CareOregon network includes 950 primary‐care providers, 3000 specialists, 33 hospitals contracted statewide, and 14 public health departments. Approximately 85% of CareOregon's membership lives in the Portland metropolitan area. The remaining 15% are dispersed across mostly rural Oregon counties. CareOregon's membership is both culturally and medically diverse: 55% are female, 21% below age 5 (63% below age 20), and 43% self‐identify as persons of color. Hospitalized patients are generally cared for by inpatient‐based physicians, and an array of primary care and specialty providers based in safety‐net clinics, private practices, and university‐based clinics cares for patients out of the hospital setting. In 2004, CareOregon began CareSupport, a care management program designed to incorporate the principles of the Chronic Care Model31 in which a team‐structured approach is used to improve patient self‐management and coordination of care.
Patients
As part of the authorization and concurrent review activity of CareOregon's Utilization Management unit, hospitalized CareOregon members are identified and a discharge date is entered in the system. CareOregon programmers develop a daily hospital discharge report, which is reviewed each day by medical assistants. Between January and July 2007, this process was used to prospectively identify all CareOregon members over age 35 discharged from 1 of 10 area hospitals. Seven hospitals served as intervention sites, the other 3 as control sites. Patients discharged from intervention hospitals were called at home after discharge and screened for eligibility. Those with one or more chronic illnesses (congestive heart failure, ischemic heart disease, diabetes mellitus, arthritis, depression, chronic obstructive pulmonary disease, and asthma), who consented to participate, were included in the study. The presence of a chronic illness was determined by patient self‐report, and subsequently corroborated by inpatient and outpatient International Classification of Diseases, Ninth Revision (ICD‐9) codes in CareOregon's claims dataset. Patients with one or more of the following were excluded: 1) language other than English; 2) no telephone access; 3) direct, elective hospital admission; 4) admission for <24 hours; 5) primary residence in an extended care facility.
Patients discharged from control hospitals during the study period were included in the control group if they were over age 35, were hospitalized for more than 24 hours, and had one or more chronic illnesses (listed above) as determined by ICD‐9 codes. These patients were identified exclusively through the CareOregon claims database, which includes both inpatient and outpatient diagnostic coding and basic sociodemographic information.
Six of the 10 hospitals from which patients were discharged are large (>300 beds), located in an urban district, and have a robust hospital medicine service. Three of these large, urban hospitals were intervention sites (hospitals 1, 3, and 6), and 3 were control sites (hospitals 2, 4, and 5). All except one of these hospitals (hospital 1) has its own internal medicine residency program. Of the 4 remaining hospitals, all of which were intervention sites, 2 are small (<300 beds) and in an urban district (hospitals 7 and 9), and 2 are small and in rural districts (hospitals 8 and 10).
Intervention
Trained medical assistants conducted a scripted post‐discharge telephone‐based needs assessment and attended to simple interventions. Training included lectures on basic chronic condition management and several months in a peer‐to‐peer learning environment with more experienced medical assistants. Medical assistants were also paired with nurse care managers who provided ongoing training and clinical mentorship. The trained medical assistants called intervention patients within 2‐7 days of hospital discharge. If a patient was not available, a contact number was given and up to 2 additional attempts were made to reach the patient. Medical assistants administered a 35‐item needs assessment survey, which typically took 10‐15 minutes to complete (see Supporting Appendix A in the online version of this article). The survey is based on an existing theoretical framework of healthcare utilization which considers predisposing sociodemographic and healthcare belief variables, enabling resources, and illness level variables.32 Our survey (see Supporting Appendix A in the online version of this article) includes 4 related subdomains: 1) enabling resources (medical home, transportation, housing); 2) psychosocial comorbidities; 3) patient activation33; and 4) past utilization.
The needs assessment was designed to identify issues requiring near‐term resolution, such as the need for follow‐up care, pharmacy access, transportation needs, and medical equipment needs. These identified needs prompted appropriate, immediate, brief‐touch interventions by the medical assistants (eg, arrange transportation, schedule a follow‐up appointment, or provide access telephone numbers).
The needs assessment was also designed to identify members for referral to intensive care management (CareSupport), based on responses to questions about their medical home relationship, prior utilization, self‐management ability, and presence of competing needs. Medical assistants referred patients for intensive care management if they had high‐intensity needs in any one of these domains, or any need in two or more domains. For example, a patient with a history of frequent emergency room visits would be referred for more intensive care management. The CareSupport teama registered nurse specially trained in case management, a behavioral health specialist, and a medical assistantreviewed each referred case. For patients qualifying based on an anticipated ongoing need identified in one or more of the above domains, the CareSupport team constructed an individualized, multifaceted care plan based on disease‐specific guidelines and results of the needs assessment.
The study was approved by the institutional review board of the Oregon Health and Sciences University.
Comparator
Patients in the control cohort received usual care as recommended by discharging and outpatient providers. Patients in the control cohort were not given brief‐touch interventions or referred to CareSupport by study personnel. They could be referred to CareSupport by their outpatient providers.
Analysis
The primary outcome variable was recurrent hospitalization within 60 days to any hospital after index hospitalization discharge. The CareOregon dataset includes inpatient and outpatient claims at any site. Because there may be up to a 3‐month delay in posting claims, we examined the claims dataset 6 months from index hospital discharge for all patients. Sociodemographic, chronic illness comorbidity, and prior utilization data were collected from the CareOregon claims dataset. We used the adjusted clinical group (ACG) score for case‐mix adjustment. The ACG predictive model is an automated risk assessment tool that uses ambulatory diagnoses to identify patients at risk for high inpatient and outpatient utilization in the following year.34, 35 ACG scores range from 0 to 1, with a score of 0.5 corresponding to a 50% chance of high utilization (ie, of being in the top 3% of utilizers) over the following year.
Data for all patients enrolled in the CareSupport care management program are entered and tracked through a separate database, which we accessed to determine whether patients were enrolled in this program during the study period. Information about specific brief‐touch interventions performed was entered narratively by medical assistants.
Baseline characteristics of the 2 groups were compared using t tests or 2 tests, as appropriate. All patients were analyzed according to the group to which they were originally assigned, regardless of subsequent enrollment in CareSupport. We used bivariate analyses to identify covariates associated with the primary outcome. These and other clinically important variables were used to develop a generalized estimated equation model of the impact of the transitional care intervention on risk of rehospitalization within 60 days of discharge, accounting for clustering of patients within hospitals. We included age, hospitalization within the past year, and ACG score as covariates in the final model.
In secondary analyses, we sought to determine whether any association between our transitional care intervention and rehospitalization was mediated by greater use of primary care or care management services. To do this, we used the CareOregon claims dataset to determine primary care utilization for the year following hospital discharge, and we used the CareSupport database to determine whether patients were enrolled in care management. We then repeated our original multivariate model, adding primary care utilization as a covariate, and considered mediation to be present if the addition of primary care utilization substantively attenuated the association between intervention and rehospitalization. We then conducted the same mediation analysis using CareSupport enrollment as a covariate. All analyses were conducted using Stata/SE 9.0 (College Station, TX).
RESULTS
We enrolled 97 intervention and 130 control patients. Follow‐up utilization data were available for all patients. Table 1 compares sociodemographic, utilization, and comorbidity characteristics of the 2 groups, and Table 2 summarizes patient distribution and characteristics of the hospitals from which they were discharged. The control group was significantly older and more racially diverse than the intervention group. On the other hand, the intervention group had a higher burden of illness as suggested by higher ACG scores and a higher rate of hospitalization within the previous year. Most patients had been hospitalized at large, urban hospitals.
| Variable | Intervention (n = 97) | Control (n = 130) |
|---|---|---|
| ||
| Mean age (SE) | 56.3 (1.1) | 60.1 (1.2)* |
| Caucasian race, n (%) | 81 (83.5) | 70 (53.8)* |
| Female, n (%) | 56 (57.7) | 83 (63.8) |
| Mean ACG score (SE) | 0.49 (0.03) | 0.39 (0.03)* |
| Mean hospitalizations in prior year (SE) | 1.97 (0.26) | 1.18 (0.13)* |
| No primary care visit in prior year, n (%) | 8 (8.2) | 19 (14.6) |
| Medicare + Medicaid, n (%) | 40 (41.2) | 47 (36.2) |
| Chronic illness, n (%) | ||
| Diabetes mellitus | 48 (49.5) | 67 (51.5) |
| Depression | 17 (17.7) | 23 (17.9) |
| Congestive heart failure | 29 (29.5) | 45 (34.6) |
| Chronic obstructive pulmonary disease or asthma | 51 (52.6) | 57 (43.8) |
| Hospital No., Intervention or Control | No. of Patients | Hospital Characteristics |
|---|---|---|
| ||
| 1, I | 13 | Large, urban |
| 2, C | 89 | Large, urban |
| 3, I | 26 | Large, urban |
| 4, C | 35 | Large, urban |
| 5, C | 6 | Large, urban |
| 6, I | 30 | Large, urban |
| 7, I | 4 | Small, urban |
| 8, I | 5 | Small, rural |
| 9, I | 7 | Small, urban |
| 10, I | 12 | Small, rural |
Patients in the intervention group had a slightly lower 60‐day rehospitalization rate compared to the control group, but this difference was not statistically significant in unadjusted analyses (Table 3; 23.7% vs 29.2%, P = 0.35). This difference became significant after controlling for ACG score, prior inpatient utilization, and age: adjusted odds ratio (OR) [95% confidence interval (CI)] 0.49 [0.24‐1.00].
| Intervention (n = 97) | Control (n = 130) | Adjusted OR (95% CI) | |
|---|---|---|---|
| |||
| Unadjusted | 23 (23.7%) | 38 (29.2%) | 0.75 (0.41‐1.37) |
| Adjusted, model 1* | 0.49 (0.24‐1.00) | ||
| Model 1 + primary care utilization | 0.49 (0.24‐1.00) | ||
| Model 1 + care management | 0.41 (0.19‐0.88) | ||
Nearly half the intervention patients received one or more brief‐touch interventions (48.5%), and the majority of patients (61.7%) receiving a brief‐touch intervention did not require referral to care management. Table 4 lists examples of brief‐touch interventions received by patients. More patients in the intervention group than in the control group were enrolled in the CareSupport care management program (40.2% vs 14.6%, P < 0.001), and about half (53.8%) of the intervention patients referred for care management did not receive a brief‐touch intervention. Patients enrolled in care management were slightly younger (mean age 56.7 vs 59.1 years, P = 0.16), but had a higher burden of illness (mean ACG score 0.53 vs 0.40, P = 0.006) than those not enrolled in care management.
| Type of Assistance* | n (%) |
|---|---|
| |
| Access information | 13 (13.4) |
| Clinic visit/PCP change | 13 (13.4) |
| Simple self‐management advice | 10 (10.3) |
| Health promotions packet | 6 (6.2) |
| Transportation | 4 (4.1) |
| Tobacco cessation guidance | 4 (4.1) |
| Prescription/pharmacy | 4 (4.1) |
| Flu vaccine promotion | 2 (2.1) |
| Housing/home support | 1 (1.0) |
| Any type of assistance | 47 (48.5) |
More patients in the intervention group compared to control patients had one or more primary care visits within the year after hospital discharge (86.6% vs 72.3%, P = 0.01), and within 60 days after hospital discharge (68.0% vs 58.5%, P = 0.14), though the latter difference did not reach statistical significance. Interestingly, in an exploratory analysis, we found that hospitalization was more likely to introduce discontinuities in longitudinal primary care in control patients than in intervention patients: among patients who had had 3 or more primary care visits in the year prior to hospitalization, control patients were more likely than intervention patients to have 2 or fewer primary care visits during the year following hospitalization (33.8% vs 20.6%, P = 0.03).
Our mediation analyses suggested that neither post‐hospitalization primary care utilization within 60 days nor care management services accounted for the lower rate of recurrent hospitalization in the intervention group (Table 3). The addition of post‐hospitalization primary care utilization to the original model did not change the primary effect estimate, while controlling for care management enrollment actually increased the effect size. Finally, there was no difference in readmission rates between those receiving and not receiving brief‐touch interventions (31.8 vs 25.7%, P = 0.92).
DISCUSSION
We found that a simple, telephone‐based, transitional care intervention may be associated with lower 60‐day rehospitalization rates in a cohort of Medicaid managed care patients. We observed a reduced rate of readmissions in the intervention, a difference that became significant after adjustment for important confounders. Implicit in the design of the intervention was a recognition that patients' transitional care needs may vary, from help negotiating the post‐discharge follow‐up care process to more substantial and complex care management support needs. Our study adds to the current body of literature by examining an understudied, socioeconomically disadvantaged population in a resource‐poor health system. Importantly, our study targeted the most intensive intervention to those with the highest anticipated needs based on a simple triage scheme. Such targeted approaches may be especially important in resource‐poor settings.
Although our study was too small to characterize in detail the relative importance of specific elements of our intervention responsible for lower short‐term rehospitalization rates, the study does highlight the diversity of transitional care needs. Patients received logistic support negotiating the health system, preventive health promotion, and patient empowerment through self‐management and information access training. Nearly half the patients received a documented simple telephone‐based intervention, and many of these patients did not require referral for intensive nurse care management. On the other hand, our needs assessment did identify over one‐third of recently discharged patients as having more complex chronic disease management needs requiring assessment for ongoing nurse care management.
We were not able to identify the specific aspects of the intervention responsible for the observed reduction in recurrent hospitalization. Our mediation analysis suggests that triaging patients to a nurse care management program was not responsible for the observed reduction in recurrent hospitalizations. In fact, the analysis suggests these patients may have been more likely to require hospitalization, though our study was too small to allow strong conclusions to be drawn from a subgroup analysis. Past studies have similarly suggested that patients enrolled in care management may simply have a higher burden of disease or may have the need for hospitalization recognized more frequently.36 Readmission rates were also similar between patients who did and did not receive a brief‐touch intervention, possibly suggesting that patients with a higher level of need were appropriately selected to receive assistance.
Although our intervention appeared to increase post‐discharge follow‐up in primary care, this also did not explain the observed reduction in 60‐day rehospitalization rates. Despite differences in post‐discharge outpatient utilization patterns, there were relatively few patients in either group that had no follow‐up, and the lack of effect may simply reflect inadequate power given our small sample size. On the other hand, the lack of association between outpatient utilization and 60‐day rehospitalization rates may reflect a true lack of association between primary care follow‐up and rehospitalization as seen in some studies, though a larger Medicare study did find an association.3638
Improvements in outpatient utilization patterns, as we saw in this study, may be a laudable intermediate outcome benefit despite the lack of association with 60‐day rehospitalization rates in our study. Short‐term rehospitalization rates represent only one outcome and do not capture the expected slow, iterative benefits from chronic illness risk reduction, which may accrue over time, with stable longitudinal primary care and associated outpatient chronic illness care systems' innovations.15, 31, 39, 40
Recent studies of transitional care interventions in publicly insured adults have produced mixed results. An evaluation of Medicare demonstration projects found largely negative results, but did find 2 successful programs in which the highest‐risk patients seemed to benefit most, a finding that supports the importance of assessing risk and appropriately dosing interventions.41 Another recent study in a socioeconomically disadvantaged population suggests the utility of an alternate transitional care approach centered on a pharmacy‐based intervention.30
Ours is essentially a test‐of‐concept study, with several important limitations, which should temper widespread application of these results and suggest the need for further study. The sample size of our study was limited, and this, coupled with a slightly lower‐than‐expected event rate, limits our ability to detect potentially important effects. Our study was not a randomized trial, and we cannot discount the possibility that our results reflect the effect of residual or unmeasured confounders, especially those factors such as patient volume and care quality related to the discharging hospitals themselves. We attempted to minimize the effects of such confounders by balancing the types of hospitals included in each group, and by accounting for clustering by hospital in our statistical analysis. Important differences in baseline characteristics between the 2 groups also raise the possibility of residual confounding despite multivariate adjustment. However, the intervention group generally carried a higher burden of illness which would, if anything, have biased results towards the null. The pragmatic study design necessitated an intervention that was defined broadly and left much to the discretion of the staff delivering the intervention, rather than adherence to a strictly defined protocol. We believe this approach allows evaluation of systems innovations within limited‐resource settings, but we acknowledge the challenges this presents in applying study results to other settings. Finally, only approximately 1 in 4 intervention patients were successfully contacted and completed the post‐discharge survey within 1 week. The relatively low rate of successful telephone contact underscores the difficulty of implementing transitional care interventions dependent on post‐discharge contact in a socioeconomically disadvantaged population with unstable telephone access. Because only successfully contacted patients were included in the intervention group, selection bias is a potential issue, though again, most baseline discrepancies between the 2 groups suggest that intervention patients were more complex.
Transitions of care in uninsured and publicly insured nonelderly adults should be studied in greater depth. Outpatient access to care deficiencies may be compounded in these groups, especially as states face widespread budget crises. Future studies should examine the effects of inpatient to outpatient linkages for such patients. Also, studies should assess the impact of transitional care interventions on self‐management, quality of care, and intermediate health outcomes in the outpatient setting after hospital discharge. Future research should taxonomize the range of transitional care needs by qualitatively evaluating subgroups of patients and delineating challenges faced by each group. For example, the post‐discharge needs of marginally housed patients may be unique and could inform the development of interventions specifically targeted to this group.
In summary, we found that a simple, brief‐touch intervention and needs assessment in the post‐discharge period may be associated with reduced recurrent hospitalization rates in a cohort of chronically ill Medicaid managed care patients with diverse post‐discharge care needs, though the exact mechanisms responsible for the observed improvements are unclear. Future studies should evaluate transitional care interventions targeted to needs in a larger group of chronically ill patients.
Acknowledgements
The authors thank Drs Jennifer Malcom and Lauren Robertson for their help in collecting the data for this project.
The inpatient to outpatient transition marks an abrupt paradigm shift from intensive, provider‐initiated care to self‐managed care, in which patients are primarily responsible for maintaining day‐to‐day health behaviors, following through with outpatient appointments, and negotiating medications, transport, and equipment needs. Studies indicate that medication nonadherence and medication‐related adverse events are common during the post‐discharge period, and may be related to the discontinuities associated with transitions of care.110
Given the complexity and uncertainty inherent in care transitions for patients with chronic illness, it is not surprising that poorly executed care transitions have been associated with increased risk of rehospitalizations and emergency department (ED) use.11 Patients with chronic illness are at particularly high risk for recurrent hospitalization.1114 System‐wide improvements in chronic illness care have been successful in triaging longitudinally followed, high‐risk outpatients to appropriate higher‐intensity care management interventions.1519 But the post‐discharge setting presents unique challenges for patients with chronic illness, especially those that are socioeconomically disadvantaged. External barriers, such as lack of transportation, lack of monitoring equipment, confusion about arranging follow‐up care, uncertainty about medication regimen changes, and financial constraints, represent important targets for improving care in the transition from inpatient to outpatient settings.
Transitional care has been defined as a set of actions designed to ensure the coordination and continuity of healthcare as members transfer between different locations or different levels of care.20 Most successful transitional care interventions have focused on geriatric populations or patients with congestive heart failure enrolled in health maintenance organizations, and have involved intensive nurse case management from the hospital setting through the post‐discharge period.2125 In these studies, trained nurse case managers provided critical support and patient education to improve patients' ability to self‐manage chronic illness.2629
In a resource‐poor Medicaid payment structure, the implementation of intensive care management across a broad population of patients may not be practical; alternative options for intervention dosing and implementation may be needed. Few studies have examined care needs and the impact of transitional care interventions in socioeconomically disadvantaged patients with chronic illness, though a recent study including such a population did find benefit from a pharmacist‐based intervention.30 The purpose of our study was to examine the impact of a low‐cost, post‐discharge needs assessment, as an adjunct to an existing care management program, on the risk of recurrent hospitalization in a clinically diverse cohort of chronically ill Medicaid managed care enrollees.
METHODS
Setting
CareOregon is an Oregon‐based, not‐for‐profit, Medicaid managed care organization that administers outpatient and inpatient health benefits for nearly 110,000 members, 5% of whom are dually eligible for Medicaid and Medicare benefits. The CareOregon network includes 950 primary‐care providers, 3000 specialists, 33 hospitals contracted statewide, and 14 public health departments. Approximately 85% of CareOregon's membership lives in the Portland metropolitan area. The remaining 15% are dispersed across mostly rural Oregon counties. CareOregon's membership is both culturally and medically diverse: 55% are female, 21% below age 5 (63% below age 20), and 43% self‐identify as persons of color. Hospitalized patients are generally cared for by inpatient‐based physicians, and an array of primary care and specialty providers based in safety‐net clinics, private practices, and university‐based clinics cares for patients out of the hospital setting. In 2004, CareOregon began CareSupport, a care management program designed to incorporate the principles of the Chronic Care Model31 in which a team‐structured approach is used to improve patient self‐management and coordination of care.
Patients
As part of the authorization and concurrent review activity of CareOregon's Utilization Management unit, hospitalized CareOregon members are identified and a discharge date is entered in the system. CareOregon programmers develop a daily hospital discharge report, which is reviewed each day by medical assistants. Between January and July 2007, this process was used to prospectively identify all CareOregon members over age 35 discharged from 1 of 10 area hospitals. Seven hospitals served as intervention sites, the other 3 as control sites. Patients discharged from intervention hospitals were called at home after discharge and screened for eligibility. Those with one or more chronic illnesses (congestive heart failure, ischemic heart disease, diabetes mellitus, arthritis, depression, chronic obstructive pulmonary disease, and asthma), who consented to participate, were included in the study. The presence of a chronic illness was determined by patient self‐report, and subsequently corroborated by inpatient and outpatient International Classification of Diseases, Ninth Revision (ICD‐9) codes in CareOregon's claims dataset. Patients with one or more of the following were excluded: 1) language other than English; 2) no telephone access; 3) direct, elective hospital admission; 4) admission for <24 hours; 5) primary residence in an extended care facility.
Patients discharged from control hospitals during the study period were included in the control group if they were over age 35, were hospitalized for more than 24 hours, and had one or more chronic illnesses (listed above) as determined by ICD‐9 codes. These patients were identified exclusively through the CareOregon claims database, which includes both inpatient and outpatient diagnostic coding and basic sociodemographic information.
Six of the 10 hospitals from which patients were discharged are large (>300 beds), located in an urban district, and have a robust hospital medicine service. Three of these large, urban hospitals were intervention sites (hospitals 1, 3, and 6), and 3 were control sites (hospitals 2, 4, and 5). All except one of these hospitals (hospital 1) has its own internal medicine residency program. Of the 4 remaining hospitals, all of which were intervention sites, 2 are small (<300 beds) and in an urban district (hospitals 7 and 9), and 2 are small and in rural districts (hospitals 8 and 10).
Intervention
Trained medical assistants conducted a scripted post‐discharge telephone‐based needs assessment and attended to simple interventions. Training included lectures on basic chronic condition management and several months in a peer‐to‐peer learning environment with more experienced medical assistants. Medical assistants were also paired with nurse care managers who provided ongoing training and clinical mentorship. The trained medical assistants called intervention patients within 2‐7 days of hospital discharge. If a patient was not available, a contact number was given and up to 2 additional attempts were made to reach the patient. Medical assistants administered a 35‐item needs assessment survey, which typically took 10‐15 minutes to complete (see Supporting Appendix A in the online version of this article). The survey is based on an existing theoretical framework of healthcare utilization which considers predisposing sociodemographic and healthcare belief variables, enabling resources, and illness level variables.32 Our survey (see Supporting Appendix A in the online version of this article) includes 4 related subdomains: 1) enabling resources (medical home, transportation, housing); 2) psychosocial comorbidities; 3) patient activation33; and 4) past utilization.
The needs assessment was designed to identify issues requiring near‐term resolution, such as the need for follow‐up care, pharmacy access, transportation needs, and medical equipment needs. These identified needs prompted appropriate, immediate, brief‐touch interventions by the medical assistants (eg, arrange transportation, schedule a follow‐up appointment, or provide access telephone numbers).
The needs assessment was also designed to identify members for referral to intensive care management (CareSupport), based on responses to questions about their medical home relationship, prior utilization, self‐management ability, and presence of competing needs. Medical assistants referred patients for intensive care management if they had high‐intensity needs in any one of these domains, or any need in two or more domains. For example, a patient with a history of frequent emergency room visits would be referred for more intensive care management. The CareSupport teama registered nurse specially trained in case management, a behavioral health specialist, and a medical assistantreviewed each referred case. For patients qualifying based on an anticipated ongoing need identified in one or more of the above domains, the CareSupport team constructed an individualized, multifaceted care plan based on disease‐specific guidelines and results of the needs assessment.
The study was approved by the institutional review board of the Oregon Health and Sciences University.
Comparator
Patients in the control cohort received usual care as recommended by discharging and outpatient providers. Patients in the control cohort were not given brief‐touch interventions or referred to CareSupport by study personnel. They could be referred to CareSupport by their outpatient providers.
Analysis
The primary outcome variable was recurrent hospitalization within 60 days to any hospital after index hospitalization discharge. The CareOregon dataset includes inpatient and outpatient claims at any site. Because there may be up to a 3‐month delay in posting claims, we examined the claims dataset 6 months from index hospital discharge for all patients. Sociodemographic, chronic illness comorbidity, and prior utilization data were collected from the CareOregon claims dataset. We used the adjusted clinical group (ACG) score for case‐mix adjustment. The ACG predictive model is an automated risk assessment tool that uses ambulatory diagnoses to identify patients at risk for high inpatient and outpatient utilization in the following year.34, 35 ACG scores range from 0 to 1, with a score of 0.5 corresponding to a 50% chance of high utilization (ie, of being in the top 3% of utilizers) over the following year.
Data for all patients enrolled in the CareSupport care management program are entered and tracked through a separate database, which we accessed to determine whether patients were enrolled in this program during the study period. Information about specific brief‐touch interventions performed was entered narratively by medical assistants.
Baseline characteristics of the 2 groups were compared using t tests or 2 tests, as appropriate. All patients were analyzed according to the group to which they were originally assigned, regardless of subsequent enrollment in CareSupport. We used bivariate analyses to identify covariates associated with the primary outcome. These and other clinically important variables were used to develop a generalized estimated equation model of the impact of the transitional care intervention on risk of rehospitalization within 60 days of discharge, accounting for clustering of patients within hospitals. We included age, hospitalization within the past year, and ACG score as covariates in the final model.
In secondary analyses, we sought to determine whether any association between our transitional care intervention and rehospitalization was mediated by greater use of primary care or care management services. To do this, we used the CareOregon claims dataset to determine primary care utilization for the year following hospital discharge, and we used the CareSupport database to determine whether patients were enrolled in care management. We then repeated our original multivariate model, adding primary care utilization as a covariate, and considered mediation to be present if the addition of primary care utilization substantively attenuated the association between intervention and rehospitalization. We then conducted the same mediation analysis using CareSupport enrollment as a covariate. All analyses were conducted using Stata/SE 9.0 (College Station, TX).
RESULTS
We enrolled 97 intervention and 130 control patients. Follow‐up utilization data were available for all patients. Table 1 compares sociodemographic, utilization, and comorbidity characteristics of the 2 groups, and Table 2 summarizes patient distribution and characteristics of the hospitals from which they were discharged. The control group was significantly older and more racially diverse than the intervention group. On the other hand, the intervention group had a higher burden of illness as suggested by higher ACG scores and a higher rate of hospitalization within the previous year. Most patients had been hospitalized at large, urban hospitals.
| Variable | Intervention (n = 97) | Control (n = 130) |
|---|---|---|
| ||
| Mean age (SE) | 56.3 (1.1) | 60.1 (1.2)* |
| Caucasian race, n (%) | 81 (83.5) | 70 (53.8)* |
| Female, n (%) | 56 (57.7) | 83 (63.8) |
| Mean ACG score (SE) | 0.49 (0.03) | 0.39 (0.03)* |
| Mean hospitalizations in prior year (SE) | 1.97 (0.26) | 1.18 (0.13)* |
| No primary care visit in prior year, n (%) | 8 (8.2) | 19 (14.6) |
| Medicare + Medicaid, n (%) | 40 (41.2) | 47 (36.2) |
| Chronic illness, n (%) | ||
| Diabetes mellitus | 48 (49.5) | 67 (51.5) |
| Depression | 17 (17.7) | 23 (17.9) |
| Congestive heart failure | 29 (29.5) | 45 (34.6) |
| Chronic obstructive pulmonary disease or asthma | 51 (52.6) | 57 (43.8) |
| Hospital No., Intervention or Control | No. of Patients | Hospital Characteristics |
|---|---|---|
| ||
| 1, I | 13 | Large, urban |
| 2, C | 89 | Large, urban |
| 3, I | 26 | Large, urban |
| 4, C | 35 | Large, urban |
| 5, C | 6 | Large, urban |
| 6, I | 30 | Large, urban |
| 7, I | 4 | Small, urban |
| 8, I | 5 | Small, rural |
| 9, I | 7 | Small, urban |
| 10, I | 12 | Small, rural |
Patients in the intervention group had a slightly lower 60‐day rehospitalization rate compared to the control group, but this difference was not statistically significant in unadjusted analyses (Table 3; 23.7% vs 29.2%, P = 0.35). This difference became significant after controlling for ACG score, prior inpatient utilization, and age: adjusted odds ratio (OR) [95% confidence interval (CI)] 0.49 [0.24‐1.00].
| Intervention (n = 97) | Control (n = 130) | Adjusted OR (95% CI) | |
|---|---|---|---|
| |||
| Unadjusted | 23 (23.7%) | 38 (29.2%) | 0.75 (0.41‐1.37) |
| Adjusted, model 1* | 0.49 (0.24‐1.00) | ||
| Model 1 + primary care utilization | 0.49 (0.24‐1.00) | ||
| Model 1 + care management | 0.41 (0.19‐0.88) | ||
Nearly half the intervention patients received one or more brief‐touch interventions (48.5%), and the majority of patients (61.7%) receiving a brief‐touch intervention did not require referral to care management. Table 4 lists examples of brief‐touch interventions received by patients. More patients in the intervention group than in the control group were enrolled in the CareSupport care management program (40.2% vs 14.6%, P < 0.001), and about half (53.8%) of the intervention patients referred for care management did not receive a brief‐touch intervention. Patients enrolled in care management were slightly younger (mean age 56.7 vs 59.1 years, P = 0.16), but had a higher burden of illness (mean ACG score 0.53 vs 0.40, P = 0.006) than those not enrolled in care management.
| Type of Assistance* | n (%) |
|---|---|
| |
| Access information | 13 (13.4) |
| Clinic visit/PCP change | 13 (13.4) |
| Simple self‐management advice | 10 (10.3) |
| Health promotions packet | 6 (6.2) |
| Transportation | 4 (4.1) |
| Tobacco cessation guidance | 4 (4.1) |
| Prescription/pharmacy | 4 (4.1) |
| Flu vaccine promotion | 2 (2.1) |
| Housing/home support | 1 (1.0) |
| Any type of assistance | 47 (48.5) |
More patients in the intervention group compared to control patients had one or more primary care visits within the year after hospital discharge (86.6% vs 72.3%, P = 0.01), and within 60 days after hospital discharge (68.0% vs 58.5%, P = 0.14), though the latter difference did not reach statistical significance. Interestingly, in an exploratory analysis, we found that hospitalization was more likely to introduce discontinuities in longitudinal primary care in control patients than in intervention patients: among patients who had had 3 or more primary care visits in the year prior to hospitalization, control patients were more likely than intervention patients to have 2 or fewer primary care visits during the year following hospitalization (33.8% vs 20.6%, P = 0.03).
Our mediation analyses suggested that neither post‐hospitalization primary care utilization within 60 days nor care management services accounted for the lower rate of recurrent hospitalization in the intervention group (Table 3). The addition of post‐hospitalization primary care utilization to the original model did not change the primary effect estimate, while controlling for care management enrollment actually increased the effect size. Finally, there was no difference in readmission rates between those receiving and not receiving brief‐touch interventions (31.8 vs 25.7%, P = 0.92).
DISCUSSION
We found that a simple, telephone‐based, transitional care intervention may be associated with lower 60‐day rehospitalization rates in a cohort of Medicaid managed care patients. We observed a reduced rate of readmissions in the intervention, a difference that became significant after adjustment for important confounders. Implicit in the design of the intervention was a recognition that patients' transitional care needs may vary, from help negotiating the post‐discharge follow‐up care process to more substantial and complex care management support needs. Our study adds to the current body of literature by examining an understudied, socioeconomically disadvantaged population in a resource‐poor health system. Importantly, our study targeted the most intensive intervention to those with the highest anticipated needs based on a simple triage scheme. Such targeted approaches may be especially important in resource‐poor settings.
Although our study was too small to characterize in detail the relative importance of specific elements of our intervention responsible for lower short‐term rehospitalization rates, the study does highlight the diversity of transitional care needs. Patients received logistic support negotiating the health system, preventive health promotion, and patient empowerment through self‐management and information access training. Nearly half the patients received a documented simple telephone‐based intervention, and many of these patients did not require referral for intensive nurse care management. On the other hand, our needs assessment did identify over one‐third of recently discharged patients as having more complex chronic disease management needs requiring assessment for ongoing nurse care management.
We were not able to identify the specific aspects of the intervention responsible for the observed reduction in recurrent hospitalization. Our mediation analysis suggests that triaging patients to a nurse care management program was not responsible for the observed reduction in recurrent hospitalizations. In fact, the analysis suggests these patients may have been more likely to require hospitalization, though our study was too small to allow strong conclusions to be drawn from a subgroup analysis. Past studies have similarly suggested that patients enrolled in care management may simply have a higher burden of disease or may have the need for hospitalization recognized more frequently.36 Readmission rates were also similar between patients who did and did not receive a brief‐touch intervention, possibly suggesting that patients with a higher level of need were appropriately selected to receive assistance.
Although our intervention appeared to increase post‐discharge follow‐up in primary care, this also did not explain the observed reduction in 60‐day rehospitalization rates. Despite differences in post‐discharge outpatient utilization patterns, there were relatively few patients in either group that had no follow‐up, and the lack of effect may simply reflect inadequate power given our small sample size. On the other hand, the lack of association between outpatient utilization and 60‐day rehospitalization rates may reflect a true lack of association between primary care follow‐up and rehospitalization as seen in some studies, though a larger Medicare study did find an association.3638
Improvements in outpatient utilization patterns, as we saw in this study, may be a laudable intermediate outcome benefit despite the lack of association with 60‐day rehospitalization rates in our study. Short‐term rehospitalization rates represent only one outcome and do not capture the expected slow, iterative benefits from chronic illness risk reduction, which may accrue over time, with stable longitudinal primary care and associated outpatient chronic illness care systems' innovations.15, 31, 39, 40
Recent studies of transitional care interventions in publicly insured adults have produced mixed results. An evaluation of Medicare demonstration projects found largely negative results, but did find 2 successful programs in which the highest‐risk patients seemed to benefit most, a finding that supports the importance of assessing risk and appropriately dosing interventions.41 Another recent study in a socioeconomically disadvantaged population suggests the utility of an alternate transitional care approach centered on a pharmacy‐based intervention.30
Ours is essentially a test‐of‐concept study, with several important limitations, which should temper widespread application of these results and suggest the need for further study. The sample size of our study was limited, and this, coupled with a slightly lower‐than‐expected event rate, limits our ability to detect potentially important effects. Our study was not a randomized trial, and we cannot discount the possibility that our results reflect the effect of residual or unmeasured confounders, especially those factors such as patient volume and care quality related to the discharging hospitals themselves. We attempted to minimize the effects of such confounders by balancing the types of hospitals included in each group, and by accounting for clustering by hospital in our statistical analysis. Important differences in baseline characteristics between the 2 groups also raise the possibility of residual confounding despite multivariate adjustment. However, the intervention group generally carried a higher burden of illness which would, if anything, have biased results towards the null. The pragmatic study design necessitated an intervention that was defined broadly and left much to the discretion of the staff delivering the intervention, rather than adherence to a strictly defined protocol. We believe this approach allows evaluation of systems innovations within limited‐resource settings, but we acknowledge the challenges this presents in applying study results to other settings. Finally, only approximately 1 in 4 intervention patients were successfully contacted and completed the post‐discharge survey within 1 week. The relatively low rate of successful telephone contact underscores the difficulty of implementing transitional care interventions dependent on post‐discharge contact in a socioeconomically disadvantaged population with unstable telephone access. Because only successfully contacted patients were included in the intervention group, selection bias is a potential issue, though again, most baseline discrepancies between the 2 groups suggest that intervention patients were more complex.
Transitions of care in uninsured and publicly insured nonelderly adults should be studied in greater depth. Outpatient access to care deficiencies may be compounded in these groups, especially as states face widespread budget crises. Future studies should examine the effects of inpatient to outpatient linkages for such patients. Also, studies should assess the impact of transitional care interventions on self‐management, quality of care, and intermediate health outcomes in the outpatient setting after hospital discharge. Future research should taxonomize the range of transitional care needs by qualitatively evaluating subgroups of patients and delineating challenges faced by each group. For example, the post‐discharge needs of marginally housed patients may be unique and could inform the development of interventions specifically targeted to this group.
In summary, we found that a simple, brief‐touch intervention and needs assessment in the post‐discharge period may be associated with reduced recurrent hospitalization rates in a cohort of chronically ill Medicaid managed care patients with diverse post‐discharge care needs, though the exact mechanisms responsible for the observed improvements are unclear. Future studies should evaluate transitional care interventions targeted to needs in a larger group of chronically ill patients.
Acknowledgements
The authors thank Drs Jennifer Malcom and Lauren Robertson for their help in collecting the data for this project.
- ,,.The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly.Arch Intern Med.1990;150(4):841–845.
- ,,,,.Drug‐associated hospital admissions in older medical patients.J Am Geriatr Soc.1988;36(12):1092–1098.
- ,,.Medication adherence in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.2001;35(5):539–545.
- ,,,.Corticosteroid use after hospital discharge among high‐risk adults with asthma.Am J Respir Crit Care Med.2004;170(12):1281–1285.
- ,,,.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.J Am Coll Cardiol.2002;40(9):1589–1595.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital [see comment].Ann Intern Med.2003;138(3):161–167.
- ,,,,,.Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long‐term care facilities.Arch Intern Med.2004;164(5):545–550.
- ,,.The adverse effects of hospitalization on drug regimens.Arch Intern Med.1991;151(8):1562–1564.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting [see comment].J Gen Intern Med.2003;18(8):646–651.
- ,,,.Posthospital care transitions: patterns, complications, and risk identification.Health Serv Res.2004;39(5):1449–1465.
- ,.Diagnosing diabetes and preventing rehospitalizations: the urban diabetes study.Med Care.2006;44(3):292–296.
- ,,,,,.Predictors of rehospitalization and death after a severe exacerbation of COPD [see comment].Chest.2007;132(6):1748–1755.
- ,,,.Multiple hospitalizations for patients with diabetes.Diabetes Care.2003;26(5):1421–1426.
- ,,,,,.Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review.Diabetes Care.2001;24(10):1821–1833.
- ,,,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies.Rockville, MD:Agency for Healthcare Research and Quality, US Department of Health and Human Services;2004.
- ,,,.Clinical service organisation for heart failure [systematic review].Cochrane Database Syst Rev.2005;2:CD002752.
- ,,,.Group based training for self‐management strategies in people with type 2 diabetes mellitus [systematic review].Cochrane Database Syst Rev.2005;2:CD003417.
- ,,,,,.Proactive case management of high‐risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial.Am J Manag Care.2005;11(4):253–260.
- ,;for the American Geriatrics Society Health Care Systems. Improving the quality of transitional care for persons with complex care needs.J Am Geriatr Soc.2003;51(4):556–557.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Effects of a home‐based intervention among patients with congestive heart failure discharged from acute hospital care.Arch Intern Med.1998;158(10):1067–1072.
- ,,,.Prolonged beneficial effects of a home‐based intervention on unplanned readmissions and mortality among patients with congestive heart failure.Arch Intern Med.1999;159(3):257–261.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention.J Am Geriatr Soc.2004;52(11):1817–1825.
- ,,.Self‐management interventions for chronic illness.Lancet.2004;364(9444):1523–1537.
- ,,.Effectiveness of self‐management training in type 2 diabetes: a systematic review of randomized controlled trials.Diabetes Care.2001;24(3):561–587.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- ,,,,.Collaborative management of chronic illness.Ann Intern Med.1997;127(12):1097–1102.
- ,,,.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150:178–187.
- ,,.Improving primary care for patients with chronic illness.JAMA.2002;288(14):1775–1779.
- ,.Societal and individual determinants of medical care utilization in the United States.Milbank Q.2005;83(4):1–28.
- ,,,.Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers.Health Serv Res.2004;39(4 pt 1):1005–1026.
- ,,.Adjusted clinical groups: predictive accuracy for Medicaid enrollees in three states.Health Care Financ Rev.2002;24(1):43–61.
- ,,,.Ambulatory care groups: a categorization of diagnoses for research and management.Health Serv Res.1991;26(1):53–74.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–1447.
- ,,,.Randomized controlled trial of emergency department interventions to improve primary care follow‐up for patients with acute asthma.Chest.2006;129(2):257–265.
- ,,,.Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure.JAMA.2010;303(17):1716–1722.
- Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34).UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352(9131):854–865.
- ,,,,,.Effect of improved glycemic control on health care costs and utilization.JAMA.2001;285(2):182–189.
- ,,,.Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials.JAMA.2009;301(6):603–618.
- ,,.The role of medication noncompliance and adverse drug reactions in hospitalizations of the elderly.Arch Intern Med.1990;150(4):841–845.
- ,,,,.Drug‐associated hospital admissions in older medical patients.J Am Geriatr Soc.1988;36(12):1092–1098.
- ,,.Medication adherence in elderly patients receiving home health services following hospital discharge.Ann Pharmacother.2001;35(5):539–545.
- ,,,.Corticosteroid use after hospital discharge among high‐risk adults with asthma.Am J Respir Crit Care Med.2004;170(12):1281–1285.
- ,,,.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.J Am Coll Cardiol.2002;40(9):1589–1595.
- ,,,,.The incidence and severity of adverse events affecting patients after discharge from the hospital [see comment].Ann Intern Med.2003;138(3):161–167.
- ,,,,,.Adverse events due to discontinuations in drug use and dose changes in patients transferred between acute and long‐term care facilities.Arch Intern Med.2004;164(5):545–550.
- ,,.The adverse effects of hospitalization on drug regimens.Arch Intern Med.1991;151(8):1562–1564.
- ,,,.Posthospital medication discrepancies: prevalence and contributing factors.Arch Intern Med.2005;165(16):1842–1847.
- ,,,.Medical errors related to discontinuity of care from an inpatient to an outpatient setting [see comment].J Gen Intern Med.2003;18(8):646–651.
- ,,,.Posthospital care transitions: patterns, complications, and risk identification.Health Serv Res.2004;39(5):1449–1465.
- ,.Diagnosing diabetes and preventing rehospitalizations: the urban diabetes study.Med Care.2006;44(3):292–296.
- ,,,,,.Predictors of rehospitalization and death after a severe exacerbation of COPD [see comment].Chest.2007;132(6):1748–1755.
- ,,,.Multiple hospitalizations for patients with diabetes.Diabetes Care.2003;26(5):1421–1426.
- ,,,,,.Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review.Diabetes Care.2001;24(10):1821–1833.
- ,,,,,.Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies.Rockville, MD:Agency for Healthcare Research and Quality, US Department of Health and Human Services;2004.
- ,,,.Clinical service organisation for heart failure [systematic review].Cochrane Database Syst Rev.2005;2:CD002752.
- ,,,.Group based training for self‐management strategies in people with type 2 diabetes mellitus [systematic review].Cochrane Database Syst Rev.2005;2:CD003417.
- ,,,,,.Proactive case management of high‐risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial.Am J Manag Care.2005;11(4):253–260.
- ,;for the American Geriatrics Society Health Care Systems. Improving the quality of transitional care for persons with complex care needs.J Am Geriatr Soc.2003;51(4):556–557.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Effects of a home‐based intervention among patients with congestive heart failure discharged from acute hospital care.Arch Intern Med.1998;158(10):1067–1072.
- ,,,.Prolonged beneficial effects of a home‐based intervention on unplanned readmissions and mortality among patients with congestive heart failure.Arch Intern Med.1999;159(3):257–261.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281(7):613–620.
- ,,,,,.Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention.J Am Geriatr Soc.2004;52(11):1817–1825.
- ,,.Self‐management interventions for chronic illness.Lancet.2004;364(9444):1523–1537.
- ,,.Effectiveness of self‐management training in type 2 diabetes: a systematic review of randomized controlled trials.Diabetes Care.2001;24(3):561–587.
- ,,,.Patient self‐management of chronic disease in primary care.JAMA.2002;288(19):2469–2475.
- ,,,,.Collaborative management of chronic illness.Ann Intern Med.1997;127(12):1097–1102.
- ,,,.A reengineered hospital discharge program to decrease rehospitalization: a randomized trial.Ann Intern Med.2009;150:178–187.
- ,,.Improving primary care for patients with chronic illness.JAMA.2002;288(14):1775–1779.
- ,.Societal and individual determinants of medical care utilization in the United States.Milbank Q.2005;83(4):1–28.
- ,,,.Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers.Health Serv Res.2004;39(4 pt 1):1005–1026.
- ,,.Adjusted clinical groups: predictive accuracy for Medicaid enrollees in three states.Health Care Financ Rev.2002;24(1):43–61.
- ,,,.Ambulatory care groups: a categorization of diagnoses for research and management.Health Serv Res.1991;26(1):53–74.
- ,,.Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission.N Engl J Med.1996;334(22):1441–1447.
- ,,,.Randomized controlled trial of emergency department interventions to improve primary care follow‐up for patients with acute asthma.Chest.2006;129(2):257–265.
- ,,,.Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure.JAMA.2010;303(17):1716–1722.
- Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34).UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352(9131):854–865.
- ,,,,,.Effect of improved glycemic control on health care costs and utilization.JAMA.2001;285(2):182–189.
- ,,,.Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials.JAMA.2009;301(6):603–618.
Copyright © 2011 Society of Hospital Medicine