User login
In Vivo Measurement of Rotator Cuff Tear Tension: Medial Versus Lateral Footprint Position
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
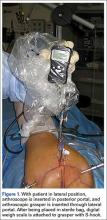
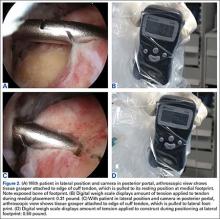
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
Although recent clinical results of arthroscopic rotator cuff repair (RCR) have been encouraging, achieving anatomical healing of full-thickness rotator cuff tears remains a challenge.1-4 Several factors influence rotator cuff healing after repair.1,3-8 Patient-related factors include advanced patient age, tear size, tear chronicity, and amount of fatty infiltration.1,3,5,6,8-10 Tension applied to the repair construct is a significant factor as well.11,12
In the literature, limited consideration has been given to repair tension.13 The majority of studies have focused on other factors, mainly repair technique. Some surgeons advocate use of a double-row repair construct in which the rotator cuff tendon is pulled to the lateral margin of the footprint.14-19 Double-row techniques, which include the transosseous-equivalent (TOE) construct, are biomechanically superior to other repairs.20-26 Another purported benefit of double-row repair is more complete restoration and pressurization of the rotator cuff footprint.21,24,27,28
Rotator cuff tears typically occur near the dysvascular region of the diseased musculotendinous unit, often leaving a stump of tissue attached to the tuberosity and ultimately a shortened tendon.29 In addition, full-thickness tears often retract over time. Meyer and colleagues29 recently demonstrated that this shortening is irreversible. Snyder30 and Sostak and colleagues31 suggested that pulling a shortened, degenerative rotator cuff tendon to the lateral margin of the footprint results in increased tissue tension compared with that produced with a more medially based repair just off the articular margin. In our opinion, the possible increase in tension during a laterally based repair, whether single- or double-row, may place excessive strain on the diseased tissue as well as the surgical construct, potentially contributing to repair failure.
We conducted a study to evaluate the difference, if any, in tension applied to the rotator cuff tendon positioned at the medial versus lateral margin of the footprint during arthroscopic RCR. We hypothesized significantly more tension would be placed on the rotator cuff tendon when positioned at the lateral versus medial footprint.
Methods
After obtaining Institutional Review Board approval for this study, we collected data on a consecutive series of patients who underwent arthroscopic RCR performed by Dr. Getelman at a single institution. Only patients with primary full-thickness tears of the supraspinatus and/or infraspinatus were included. Exclusion criteria included revision rotator cuff surgeries, partial-thickness tears, concurrent subscapularis tears requiring anchor fixation, and any tears that could not be mobilized to the lateral footprint without interval slides or margin convergence. The 20 identified patients constituted the study group.
Demographic factors, including age and preoperative length of symptoms, were recorded after chart review. Magnetic resonance imaging (MRI) was performed for all patients before surgery and was retrospectively reviewed. Dr. Getelman assigned each patient a modified Goutallier score, based on MRI, to assess for fatty infiltration/atrophy.32 Each patient was placed in the lateral decubitus position with the operative arm in balanced suspension at 70° of abduction. Standard glenohumeral and subacromial diagnostic arthroscopy was performed. The rotator cuff tear was gently debrided back to a healthy-appearing margin in preparation for repair. The tear was then measured in the anterior-posterior (A-P) and medial-lateral (M-L) planes using a premeasured, marked suture, as previously described.33 Complete bursal and articular-sided releases were performed to allow for appropriate mobilization of the tendon. The tear was classified as crescent-shaped, U-shaped, or L-shaped.
Viewing from the posterior portal, the surgeon inserted a tissue grasper through the lateral portal. The tendon was grasped at multiple points along its edge, anterior to posterior, and was translated laterally to assess its reducibility; the apex of the tear correlated with the point of maximal excursion and coverage of the footprint. Once confirmed, the rotator cuff tear apex was clamped with a tissue grasper. After placement in a sterile arthroscopic camera sleeve (DeRoyal camera drape with perforated tip), a calibrated digital weigh scale (American Weigh Scales model H22 portable electronic hanging scale, with accuracy of 0.01 lb) was attached to the tissue grasper with an S-hook (Figure 1). The tendon edge was first translated about 3 mm lateral to the articular margin (the medial footprint position), and tension was recorded (Figures 2A, 2B). After a 1-minute relaxation period, the tendon edge was translated to the lateral edge of the rotator cuff footprint (the lateral footprint position), and tension was recorded again (Figures 2C, 2D). A medially based single-row RCR with triple-loaded sutures and bone marrow vents placed in the lateral tuberosity was then completed, regardless of tension, tear size, or tear morphology.31 Typically, 1 anchor was used for every 10 to 15 mm of A-P tear length.
SAS software was used for statistical analysis, the Wilcoxon signed rank test for continuous or ordinal data comparisons between paired groups, and the Mann-Whitney test for continuous or ordinal data comparisons between independent, unmatched groups. One-way analysis of variance (ANOVA) was used to compare means among the 3 groups of morphology subtypes. Linear regression was performed to assess the simultaneous relationship between potential predictors (age, sex, length of symptoms, Goutallier score, tear size) and medial or lateral tension, where medial tension was included as an additional potential predictor for lateral tension. Restricted cubic splines were fit to assess linearity. Predictors were retained in multivariate regression using backward variable retention. Because of inadequate sample size, additivity was assumed except for sex. Statistical significance was set at P < .05.
Results
Of the 20 rotator cuff tears evaluated (Table 1), 13 were crescent-shaped, 5 were U-shaped, and 2 were L-shaped. Mean (SD) A-P tear size was 17.7 (5.8) mm, and mean (SD) M-L tear size was 19.1 (8.6) mm. Mean age of the 20 patients (15 men, 5 women) was 57.9 years (range, 44-72 years). Mean (SD) length of symptoms was 12.9 (12.4) months (range, 3-48 months). Mean (SD) modified Goutallier score was 1.4 (0.7; range, 0-3).
Mean (SD) rotator cuff tension for all tears approximated to the medial footprint was 0.41 (0.33) pound, and mean (SD) cuff tension for all tears approximated to the lateral footprint was 2.21 (1.20) pounds—representing a 5.4-fold difference (P < .0001).
No statistically significant differences were detected in the ANOVA comparing tensions at medial and lateral positions among tear morphologic subtypes (all Ps >.05).
Subgroup analysis (Table 2) was performed for smaller (≤20 mm A-P) and larger (>20 mm A-P) tears. For smaller tears, mean (SD) tension was 0.27 (0.24) pound applied with the cuff tendon pulled to the medial footprint and 2.06 (1.06) pounds applied with the tendon pulled to the lateral footprint—a 7.6-fold difference (P < .0018). For larger tears, mean (SD) tension was 0.58 (0.37) pound applied with the tendon pulled to the medial footprint and 2.38 (1.4) pounds applied with the tendon pulled to the lateral footprint—a 4.1-fold difference (P < .005).
A statistically significant difference in tensions was found between small and large cuff tears positioned at the medial footprint (0.27 vs 0.58 lb; P = .0367); no difference was found between groups with the tendon at the lateral footprint (2.06 vs 2.38 lb; P = .284).
Univariate and multivariate analyses were performed using linear regression analysis (Table 3). During univariate analysis for medial footprint position, A-P tear size and Goutallier score both positively correlated with increasing tension; for lateral footprint position, no factors statistically correlated with lateral tension, though there was a positive trend for medial tension and female sex. During multivariate analysis for medial footprint position, only A-P tear size positively correlated with increasing tension; for lateral footprint position, both age (in nonlinear fashion as function of age + age2) and medial tension positively correlated with increasing tension.
Discussion
Our results indicated that significantly more tension is placed on the torn rotator cuff tendon when it is reduced across the footprint from a medial to a more lateral position in vivo. More tension was required for all tears to be reduced to the lateral footprint compared with the medial footprint. As expected, compared with smaller tears, larger tears required significantly more tension in order to be reduced to the medial footprint. Interestingly, no statistical difference was found between tensions required to reduce either small or large tears to the lateral footprint, which suggests that, regardless of tear size, more force must be applied to reduce the torn tendon to the lateral footprint compared with the medial footprint.
Hersche and Gerber34 were the first to report rotator cuff tension measurements in vivo. Although their study did not specifically compare cuff tensions reducing the tear to the medial versus lateral footprint, it did examine tension at displacement of 10 and 20 mm. Tension increased from 27 N to 60 N, correlating with a 2.2-fold difference between the 2 distances. Domb and colleagues35 also compared in vivo rotator cuff tension differences between the medial footprint and the lateral footprint in 4 patients. Mean tension applied to the cuff during reduction to the articular margin was 27 N, or 6 pounds. Mean tension needed to reduce the cuff to the lateral tuberosity was 76 N, or 17 pounds, for a 2.8-fold difference. Tears were not measured but were described as massive and retracted.
Although repair tension has long been recognized as a crucial factor in RCR healing, little clinical research has focused on the effects of excess tension. Davidson and Rivenburgh11 prospectively followed the clinical outcomes of 67 consecutive cuff repairs after intraoperative tension measurement and found that high-tension repairs (>8 lb) had significantly lower clinical outcome measures. However, the authors did not report on correlations with radiologic healing and stated, “Functional outcome is inversely proportional to rotator cuff repair tension.” Further study of the in vivo effects of increased tension on clinical and radiologic outcomes is needed.
Several animal studies have been conducted on the effects of tension on RCRs. Gerber and colleagues36 reported that the force needed to produce 1 cm of sheep supraspinatus tendon excursion increased 7-fold, from 6.8 N to 47.8 N, after 40 weeks of tendon tear. Coleman and colleagues37 compared the modulus of elasticity in sheep supraspinatus tendon after 6 weeks and 18 weeks of detachment and reported increases of 60% and 70%, respectively. Gimbel and colleagues38 showed that, in a rat model, “repair tension rapidly increased initially after injury followed by a progressive, but less dramatic, increase with additional time.” Of note, we did not identify any correlation between chronicity of symptoms and the tension needed to reduce the tendon medially or to a more lateral position on the footprint.
In acute tears, the cuff tissue is more compliant and mobile and can be pulled laterally across its anatomical footprint with minimal tension.39 In contrast, cuff tissue in the more commonly encountered chronic tear is less compliant and is not mobile enough to be pulled to the lateral margin of the footprint without added stress.30,34,35 In large, acute tears in which there are minimal tissue degeneration and retraction, a laterally based footprint-restoring technique may be performed with minimal tension. This technique may have advantages over a medially based repair. In the literature, more attention needs to be directed toward the biomechanics and biology of chronic rotator cuff tears, as these are more commonly encountered.
Almost all of the prospective studies that have compared single- and double-row RCR have found no significant differences in MRI healing rates or clinical results at follow-up up to 2 years.14,16,40-45 Detailed analysis of the surgical techniques used in all these studies revealed that the rotator cuff tendons were repaired back to the lateral footprint in both the single- and double-row constructs.14,16,40-45 Although no clinical studies have compared medially and laterally based single-row repairs, our data suggest that medially based repairs have lower tensions and therefore should not be considered equivalent. Sostak and colleagues31 and Murray and colleagues46 have shown that a medially based single-row RCR can achieve excellent clinical and anatomical results, likely partly because of the lower tension applied to the torn cuff tissue.31,46 Studies are needed to compare medially and laterally based repairs, including single- and double-row repairs.
The vast majority of recent research has aimed to counteract construct tension with stronger biomechanical constructs.20-26 Surgeons have also aimed to improve biological healing by pulling the tendon laterally across the footprint to achieve complete footprint coverage, ultimately increasing the surface area for tendon–bone healing. This has led to the development of various double-row repair techniques, in which the cuff tendon is pulled to the lateral margin of its footprint. One row of anchors is placed in the medial aspect of the footprint, while a second is placed in the lateral aspect; the cuff is reduced and compressed to the tuberosity with various suture configurations. The TOE technique was developed to improve pressurization of the cuff tendon across the footprint by linking the 2 rows with bridging sutures. In doing so, however, the potentially deleterious effects of increased tension introduced by pulling the tendon laterally may have been overlooked. Nevertheless, the biomechanics and stress distribution likely differ between single-row repair and TOE repairs, and direct comparisons cannot be made at this time. The medial row of a double-row or TOE construct may stress-shield or “unload” the more lateral tissue. Studies are needed in order to better understand the tension differential and stress distribution of various double-row constructs.
Recognizing tear morphology is crucial in maximizing chances of healing after cuff repair. For example, a crescent-shaped tear is reduced to the tuberosity with direct lateral translation of the apex of the tear, which is also the deepest or most displaced part of the tear. On the other hand, reducing an L- or reverse L-shaped tear to the tuberosity is not as direct; reducing the deepest or most displaced part of the tear would lead to overreduction and overtensioning of the tendon. However, often the exact “elbow” of the tear is not obvious and appears more rounded; therefore, it is crucial for the surgeon to examine the mobility of the torn tendon along its entire length to minimize tension. Study is needed to assess tension along the entire length of the tear for different tear morphologies and sizes.
Although our results showed that increased tension was needed to reduce a torn tendon to its lateral footprint, no study has indicated exactly how much is “too much” tension. As stated earlier, use of stronger biomechanical constructs, including TOE constructs, may overcome the increased tension associated with laterally based repairs. In addition, laterally based repairs, either single- or double-row, may be best suited for tears with lower tension, whereas medially based repairs may be best suited for higher tension tears. It is also possible that the difference in tensions noted in this study is not significant enough to have a clinical impact on choice of construct or on anatomical healing. We need studies that correlate anatomical healing rates with repair tension in order to better guide surgeons on when to use a medially or laterally based repair.
Other possible effects of increased tension associated with laterally based repairs, including beneficial effects, must be considered as well. Viscoelastic properties of human rotator cuff tendon may dissipate increased tension over time through a variety of mechanisms. Stress relaxation, gap formation, creep, and the hysteresis effect, all associated with cyclical loading in the early healing period, may lead to dissipation of force over time.47,48 These more complex biomechanical properties of RCR constructs are yet to be clearly defined.
This study had several weaknesses. Its data represent a static measurement of time-zero rotator cuff tension, which greatly simplifies the biomechanics of the torn rotator cuff and repair construct as well as changes that occur with healing. During cuff repair, forces typically are distributed through several fixation points in stepwise process and are not focused on a single point of tissue with a grasper. Therefore, the findings of this study may not directly correlate with medially versus laterally based repairs in vivo. Furthermore, as this is a time-zero measurement, we could not determine whether the tension differential between the 2 repair positions remained static over time. Current literature suggests that muscle atrophy, fatty infiltration, and loss of elasticity of the musculotendinous unit are relatively irreversible.35,37,49 In addition, determining the precise apex of a cuff tear can be difficult, so error may have been introduced during this process. Last, although placement of the cuff tissue at the medial or lateral footprint position was based on visual estimation by an experienced and skilled arthroscopist, error may have been introduced based on this imprecise technique.
Conclusion
This study demonstrated a significant, 5.4-fold increase in in vivo time-zero rotator cuff tension with the tendon edge reduced to the lateral footprint rather than the medial footprint.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
1. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87(6):1229-1240.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219-224.
3. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg. 2009;18(1):13-20.
4. Huijsmans PE, Pritchard MP, Berghs BM, van Rooyen KS, Wallace AL, de Beer JF. Arthroscopic rotator cuff repair with double-row fixation. J Bone Joint Surg Am. 2007;89(6):1248-1257.
5. Gulotta LV, Nho SJ, Dodson CC, Adler RS, Altchek DW, MacGillivray JD; HSS Arthroscopic Rotator Cuff Registry. Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part II—prognostic factors for clinical and radiographic outcomes. J Shoulder Elbow Surg. 2011;20(6):941-946.
6. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg. 2009;1(2):96-104.
7. Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719-728.
8. Oh JH, Kim SH, Ji HM, Jo KH, Bin SW, Gong HS. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy. 2009;25(1):30-39.
9. Tashjian RZ, Hollins AM, Kim HM, et al. Factors affecting healing rates after arthroscopic double-row rotator cuff repair. Am J Sports Med. 2010;38(12):2435-2442.
10. Burkhart SS, Lo IK. Arthroscopic rotator cuff repair. J Am Acad Orthop Surg. 2006;14(6):333-346.
11. Davidson PA, Rivenburgh DW. Rotator cuff repair tension as a determinant of functional outcome. J Shoulder Elbow Surg. 2000;9(6):502-506.
12. Goutallier D, Postel JM, Van Driessche S, Godefroy D, Radier C. Tension-free cuff repairs with excision of macroscopic tendon lesions and muscular advancement: results in a prospective series with limited fatty muscular degeneration. J Shoulder Elbow Surg. 2006;15(2):164-172.
13. Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-568.
14. Ma HL, Chiang ER, Wu HT, et al. Clinical outcome and imaging of arthroscopic single-row and double-row rotator cuff repair: a prospective randomized trial. Arthroscopy. 2012;28(1):16-24.
15. Mihata T, Watanabe C, Fukunishi K, et al. Functional and structural outcomes of single-row versus double-row versus combined double-row and suture-bridge repair for rotator cuff tears. Am J Sports Med. 2011;39(10):2091-2098.
16. Koh KH, Kang KC, Lim TK, Shon MS, Yoo JC. Prospective randomized clinical trial of single- versus double-row suture anchor repair in 2- to 4-cm rotator cuff tears: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(4):453-462.
17. Voigt C, Bosse C, Vosshenrich R, Schulz AP, Lill H. Arthroscopic supraspinatus tendon repair with suture-bridging technique: functional outcome and magnetic resonance imaging. Am J Sports Med. 2010;38(5):983-991.
18. Lafosse L, Brzoska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 pt 2):275-286.
19. Park JY, Lhee SH, Choi JH, Park HK, Yu JW, Seo JB. Comparison of the clinical outcomes of single- and double-row repairs in rotator cuff tears. Am J Sports Med. 2008;36(7):1310-1316.
20. Kim DH, ElAttrache NS, Tibone JE, et al. Biomechanical comparison of a single-row versus double-row suture anchor technique for rotator cuff repair. Am J Sports Med. 2006;34(3):407-414.
21. Mazzocca AD, Bollier MJ, Ciminiello AM, et al. Biomechanical evaluation of arthroscopic rotator cuff repairs over time. Arthroscopy. 2010;26(5):592-599.
22. Grimberg J, Diop A, Kalra K, Charousset C, Duranthon LD, Maurel N. In vitro biomechanical comparison of three different types of single- and double-row arthroscopic rotator cuff repairs: analysis of continuous bone–tendon contact pressure and surface during different simulated joint positions. J Shoulder Elbow Surg. 2010;19(2):236-243.
23. Nelson CO, Sileo MJ, Grossman MG, Serra-Hsu F. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: a biomechanical and surface area comparison. Arthroscopy. 2008;24(8):941-948.
24. Park MC, ElAttrache NS, Tibone JE, Ahmad CS, Jun BJ, Lee TQ. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461-468.
25. Park MC, Tibone JE, ElAttrache NS, Ahmad CS, Jun BJ, Lee TQ. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469-476.
26. Ma CB, Comerford L, Wilson J, Puttlitz CM. Biomechanical evaluation of arthroscopic rotator cuff repairs: double-row compared with single-row fixation. J Bone Joint Surg Am. 2006;88(2):403-410.
27. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
28. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon–bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869-1874.
29. Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606-610.
30. Snyder SJ. Single vs. double row suture anchor fixation rotator cuff repair. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; March 8, 2007; San Francisco, CA.
31. Sostak JP, Bahk MS, Getelman MH, Wong IH, Snyder SJ, Burns JP. Arthroscopic single row rotator cuff repair using the “SCOI row”: structural and clinical outcomes. Paper presented at: American Academy of Orthopedic Surgeons Annual Meeting; February 7-11, 2012; San Francisco, CA.
32. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599-605.
33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24(4):403-409.
34. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998;7(4):393-396.
35. Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQ, ElAttrache NS. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(suppl 4):35-39.
36. Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86(9):1973-1982.
37. Coleman SH, Fealy S, Ehteshami JR, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am. 2003;85(12):2391-2402.
38. Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004;(426):258-265.
39. Mannava S, Plate JF, Whitlock PW, et al. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011;93(18):1702-1711.
40. Burks RT, Crim J, Brown N, Fink B, Greis PE. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37(4):674-682.
41. Grasso A, Milano G, Salvatore M, Falcone G, Deriu L, Fabbriciani C. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25(1):4-12.
42. Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35(8):1254-1260.
43. Carbonel I, Martinez AA, Calvo A, Ripalda J, Herrera A. Single-row versus double-row arthroscopic repair in the treatment of rotator cuff tears: a prospective randomized clinical study. Int Orthop. 2012;36(9):1877-1883.
44. Lapner PL, Sabri E, Rakhra K, et al. A multicenter randomized controlled trial comparing single-row with double-row fixation in arthroscopic rotator cuff repair. J Bone Joint Surg Am. 2012;94(14):1249-1257.
45. Gartsman GM, Drake G, Edwards TB, et al. Ultrasound evaluation of arthroscopic full-thickness supraspinatus rotator cuff repair: single-row versus double-row suture bridge (transosseous equivalent) fixation. Results of a prospective, randomized study. J Shoulder Elbow Surg. 2013;22(11):1480-1487.
46. Murray TF Jr, Lajtai G, Mileski RM, Snyder SJ. Arthroscopic repair of medium to large full-thickness rotator cuff tears: outcome at 2- to 6-year follow-up. J Shoulder Elbow Surg. 2002;11(1):19-24.
47. Szczesny SE, Peloquin JM, Cortes DH, Kadlowec JA, Soslowsky LJ, Elliott DM. Biaxial tensile testing and constitutive modeling of human supraspinatus tendon. J Biomech Eng. 2012;134(2):021004.
48. Chaudhury S, Holland C, Vollrath F, Carr AJ. Comparing normal and torn rotator cuff tendons using dynamic shear analysis. J Bone Joint Surg Br. 2011;93(7):942-948.
49. Meyer DC, Hoppeler H, von Rechenberg B, Gerber C. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res. 2004;22(5):1004-1007.
Anterior Hip Capsuloligamentous Reconstruction for Recurrent Instability After Hip Arthroscopy
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.