User login
Deep Soft Tissue Mass of the Knee
The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
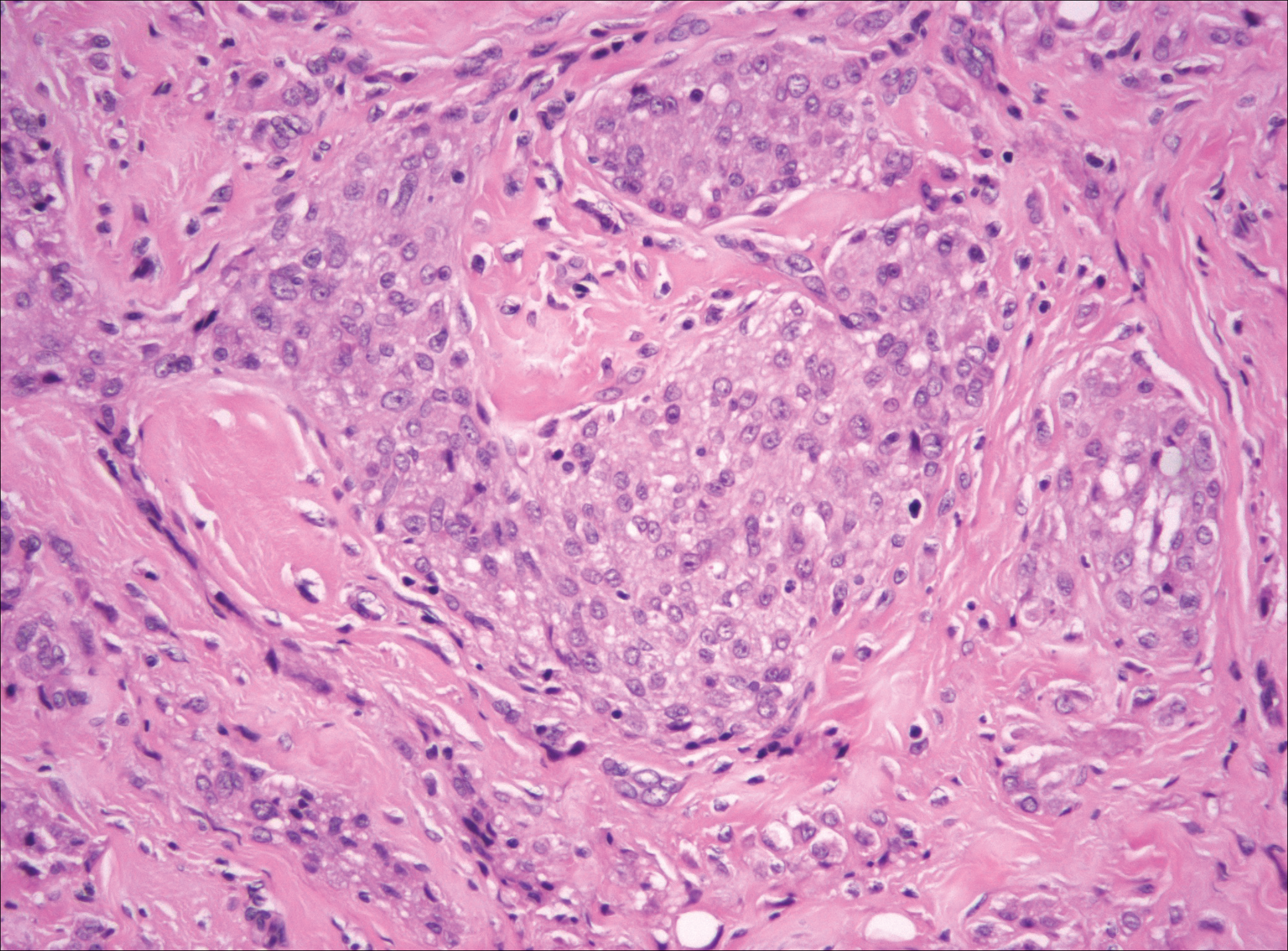
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
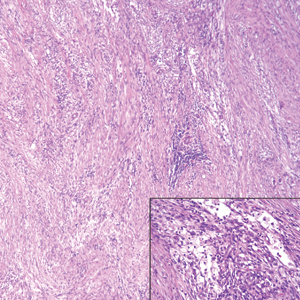
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.
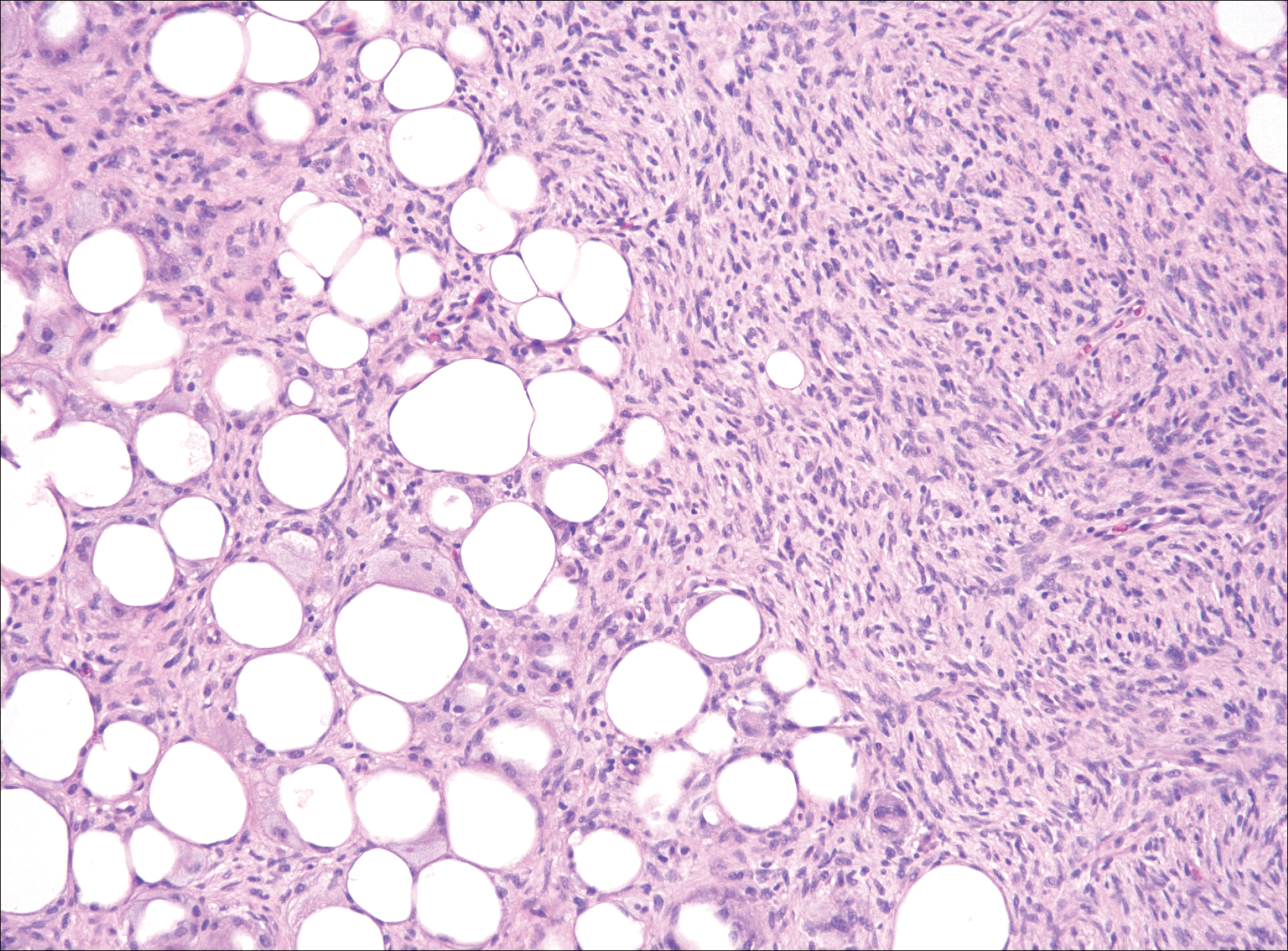
The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15
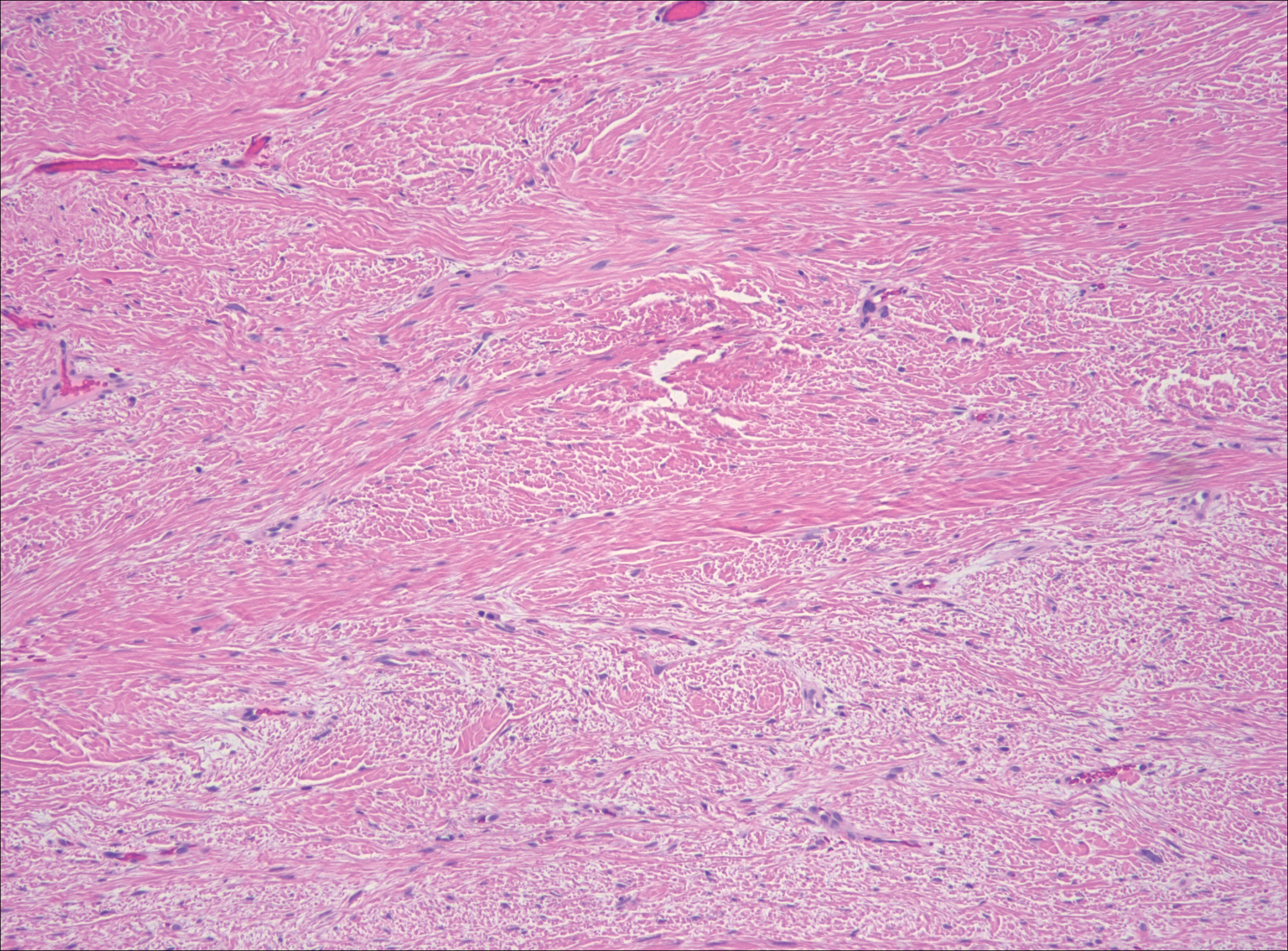
Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16
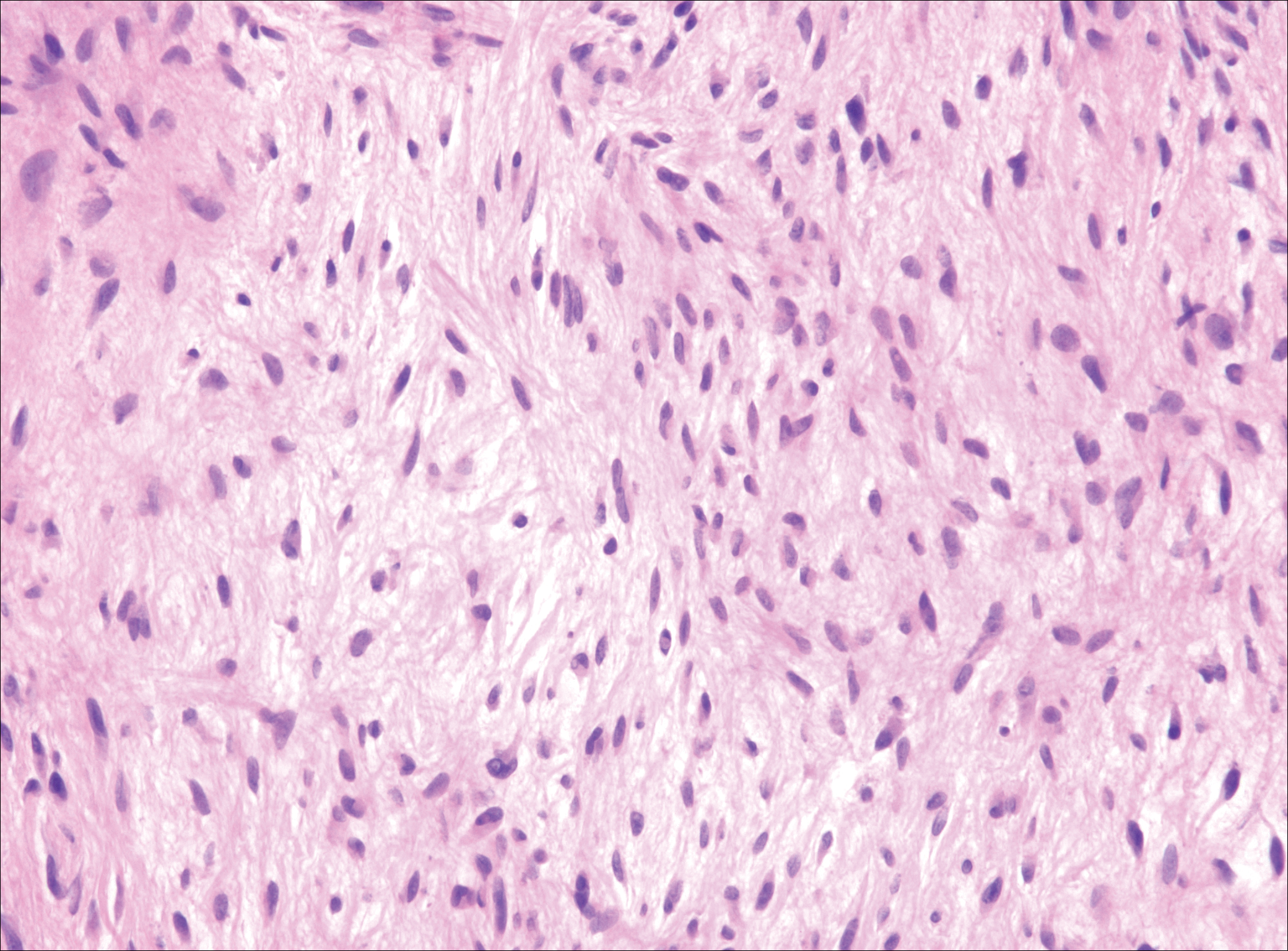
Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.
- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.

The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15

Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16

Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.

The Diagnosis: Nodular Fasciitis
The diagnosis of spindle cell tumors can be challenging; however, by using a variety of immunoperoxidase stains and fluorescent in situ hybridization (FISH) testing in conjunction with histology, it often is possible to arrive at a definitive diagnosis. For this case, the histologic features in conjunction with the immunoperoxidase stains and FISH were consistent with a diagnosis of nodular fasciitis.
Nodular fasciitis is a benign, self-limiting, myofibroblastic, soft-tissue proliferation typically found in the subcutaneous tissue.1 It can be found anywhere on the body but most commonly on the upper arms and trunk. It most often is seen in young adults, and many cases have been reported in association with a history of trauma to the area.1,2 It typically measures less than 2 cm in diameter.3 The diagnosis of nodular fasciitis is particularly challenging because it mimics sarcoma, both in presentation and in histologic findings with rapid growth, high mitotic activity, and increased cellularity.1,4-7 In contrast to malignancy, nodular fasciitis has no atypical mitoses and little cytologic atypia.8,9 Rather, it contains plump myofibroblasts loosely arranged in a myxoid or fibrous stroma that also may contain lymphocytes, extravasated erythrocytes, and osteoclastlike giant cells distributed throughout.5,10,11 In this case, lymphocytes, extravasated red blood cells, and myxoid change are present, suggesting the diagnosis of nodular fasciitis. In other cases, however, these features may be much more limited, making the diagnosis more challenging. The spindle cells are arranged in poorly defined short fascicles. The tumor cells do not infiltrate between individual adipocytes. There is no notable cytologic atypia.
Because of the difficulty in making the diagnosis, overtreatment of this benign condition can be a problem, causing increased morbidity.1 Erickson-Johnson et al12 identified the role of an ubiquitin-specific peptidase 6, USP6, gene rearrangement on chromosome 17p13 in 92% (44/48) of cases of nodular fasciitis. The USP6 gene most often is rearranged with the myosin heavy chain 9 gene, MYH9, on chromosome 22q12.3. With this rearrangement, the MYH9 promoter leads to the overexpression of USP6, causing tumor formation.2,13 The use of multiple immunoperoxidase stains can be important in the identification of nodular fasciitis. Nodular fasciitis stains negative for S-100, epithelial membrane antigen, CD34, β-catenin, and cytokeratin, but typically stains positive for smooth muscle actin.9
Although dermatofibrosarcoma protuberans (DFSP) was in the differential diagnosis, these tumors tend to have greater cellularity than nodular fasciitis. In addition, the spindle cells of DFSP typically are arranged in a storiform pattern. Another characteristic feature of DFSP is that the tumor cells will infiltrate between adipose cells creating a lacelike or honeycomblike appearance within the subcutaneous tissue (Figure 1). Immunohistochemistry staining and FISH testing may be useful in making a diagnosis of DFSP. These tumors typically are positive for CD34 by immunoperoxidase staining and demonstrate a translocation t(17;22)(q21;q13) between platelet-derived growth factor subunit B gene, PDGFB, and collagen type I alpha 1 chain gene, COL1A1, by FISH.

The distinction between the fibrous phase of nodular fasciitis and fibromatosis can be challenging. The size of the lesion may be helpful, with most lesions of nodular fasciitis being less than 3 cm, while lesions of fibromatosis have a mean diameter of 7 cm.5,14 Microscopically, both tumors demonstrate a fascicular growth pattern; however, the fascicles in nodular fasciitis tend to be short and irregular compared to the longer fascicles seen in fibromatosis (Figure 2). Immunohistochemistry staining has limited utility with only 56% (14/25) of superficial fibromatoses having positive nuclear staining for β-catenin.15

Low-grade fibromyxoid sarcoma (LGFMS) would be unusual in this clinical scenario. Only 13% to 19% of cases present in patients younger than 18 years (mean age, 33 years).16 In LGFMS there are cytologically bland spindle cells that are typically arranged in a patternless or whorled pattern (Figure 3), though fascicular architecture may be seen. There are alternating areas of fibrous and myxoid stroma. A curvilinear vasculature network and lack of lymphocytes and extravasated red blood cells are histologic features favoring LGFMS over nodular fasciitis. Immunohistochemistry staining and FISH testing can be useful in making the diagnosis of LGFMS. These tumors are characterized by a translocation t(7;16)(q34;p11) involving the fusion in sarcoma, FUS, and cAMP responsive element binding protein 3 like 2, CREB3L2, genes.16 Positive immunohistochemistry staining for MUC4 can be seen in up to 100% of LGFMS and is absent in many other spindle cell tumors.16

Plexiform fibrohistiocytic tumor (PFT) is least likely to be confused with nodular fasciitis. Histologically these tumors are characterized by multiple small nodules arranged in a plexiform pattern (Figure 4). Within the nodules, 3 cell types may be noted: spindle fibroblast-like cells, mononuclear histiocyte-like cells, and osteoclastlike cells.17 Either the spindle cells or the mononuclear cells may predominate in cases of PFT. Immunohistochemistry staining of PFT is nonspecific and there are no molecular/FISH studies that can be used to help confirm the diagnosis.

- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
- Shin C, Low I, Ng D, et al. USP6 gene rearrangement in nodular fasciitis and histological mimics. Histopathology. 2016;69:784-791.
- Kumar E, Patel NR, Demicco EG, et al. Cutaneous nodular fasciitis with genetic analysis: a case series. J Cutan Pathol. 2016;43:1143-1149.
- Nishio J. Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors. Oncol Lett. 2013;5:12-18.
- Lin X, Wang L, Zhang Y, et al. Variable Ki67 proliferative index in 65 cases of nodular fasciitis, compared with fibrosarcoma and fibromatosis. Diagn Pathol. 2013;8:50.
- Goldstein J, Cates J. Differential diagnostic considerations of desmoid-type fibromatosis. Adv Anat Pathol. 2015;22:260-266.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyons, France: IARC Press; 2013.
- Bridge JA, Cushman-Vokoun AM. Molecular diagnostics of soft tissue tumors. Arch Pathol Lab Med. 2011;135:588-601.
- Anzeljc AJ, Oliveira AM, Grossniklaus HE, et al. Nodular fasciitis of the orbit: a case report confirmed by molecular cytogenetic analysis. Ophthalmic Plast Reconstr Surg. 2017;33(3S suppl 1):S152-S155.
- de Paula SA, Cruz AA, de Alencar VM, et al. Nodular fasciitis presenting as a large mass in the upper eyelid. Ophthalmic Plast Reconstr Surg. 2006;22:494-495.
- Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer. 1982;49:1668-1678.
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology. 1984;16:161-166.
- Erickson-Johnson MR, Chou MM, Evers BR, et al. Nodular fasciitis: a novel model of transient neoplasia induced by MYH9-USP6 gene fusion. Lab Invest. 2011;91:1427-1433.
- Amary MF, Ye H, Berisha F, et al. Detection of USP6 gene rearrangement in nodular fasciitis: an important diagnostic tool. Virchows Arch. 2013;463:97-98.
- Wirth L, Klein A, Baur-Melnyk A. Desmoid tumors of the extremity and trunk. a retrospective study of 44 patients. BMC Musculoskelet Disord. 2018;19:2.
- Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cells lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509-514.
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60-67.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138.
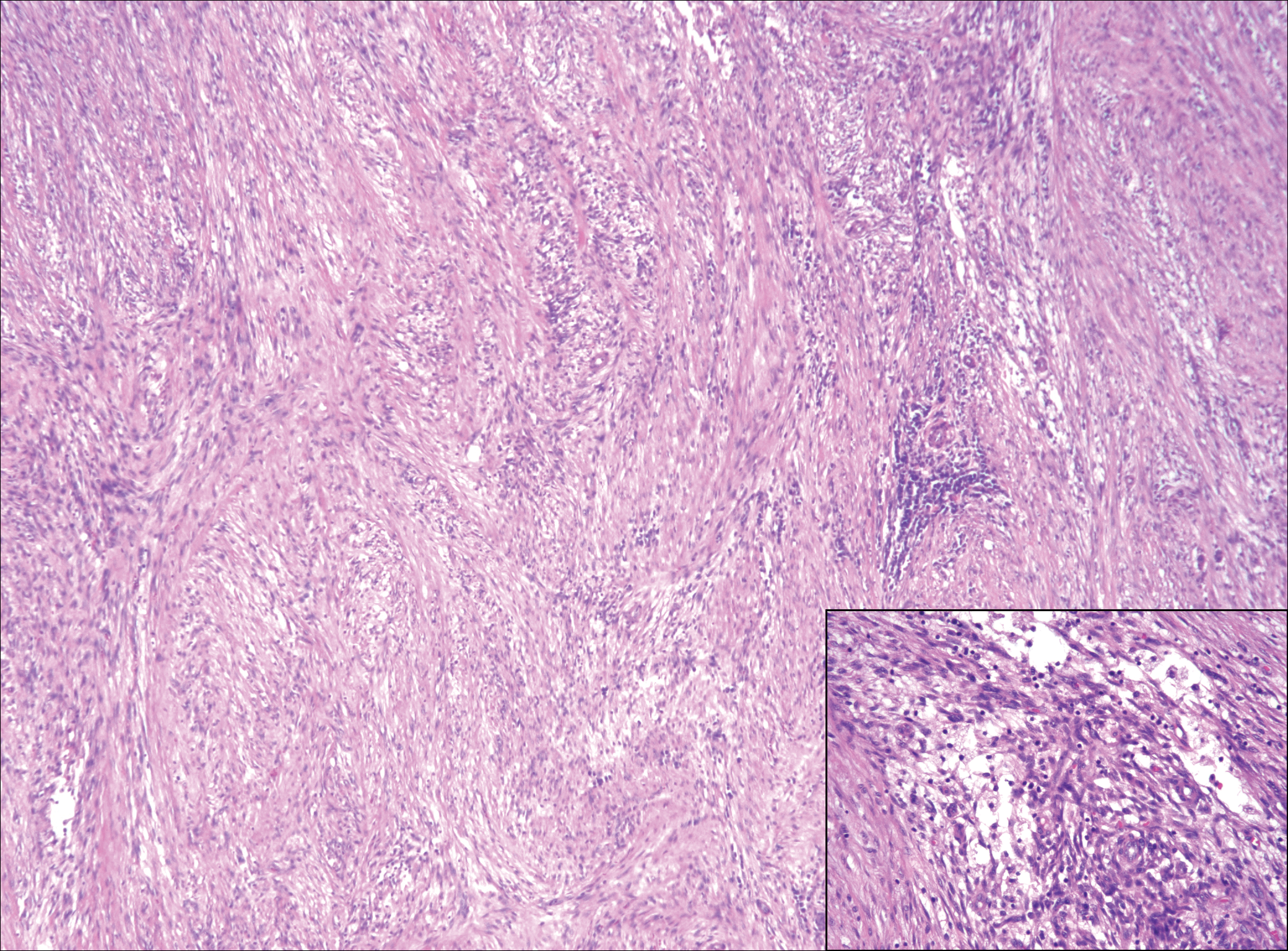
A 16-year-old adolescent girl presented with a bump over the left posterior knee of 1 month's duration. Her medical history was unremarkable. She denied recent trauma or injury to the area. On physical examination there was a visible and palpable tense nontender mass the size of an egg over the left posterior knee. Magnetic resonance imaging showed a lobulated mass-like focus of T2 hyperintensity centered at the subcutaneous tissues and superficial myofascial plane of the gastrocnemius on the posterior knee. Complete excision of the lesion was performed and demonstrated a 2.6.2 ×2.9.2 ×2.1-cm mass within subcutaneous adipose tissue. There was no microscopic involvement of skeletal muscle. Immunohistochemistry staining of the tumor was performed that was positive for smooth muscle actin and negative for desmin, S-100, CD34, pan-cytokeratin, and β-catenin. Fluorescent in situ hybridization testing demonstrated rearrangement of the ubiquitin-specific peptidase 6 gene, USP6, locus (17p13).