User login
Pelvic pain
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.
Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
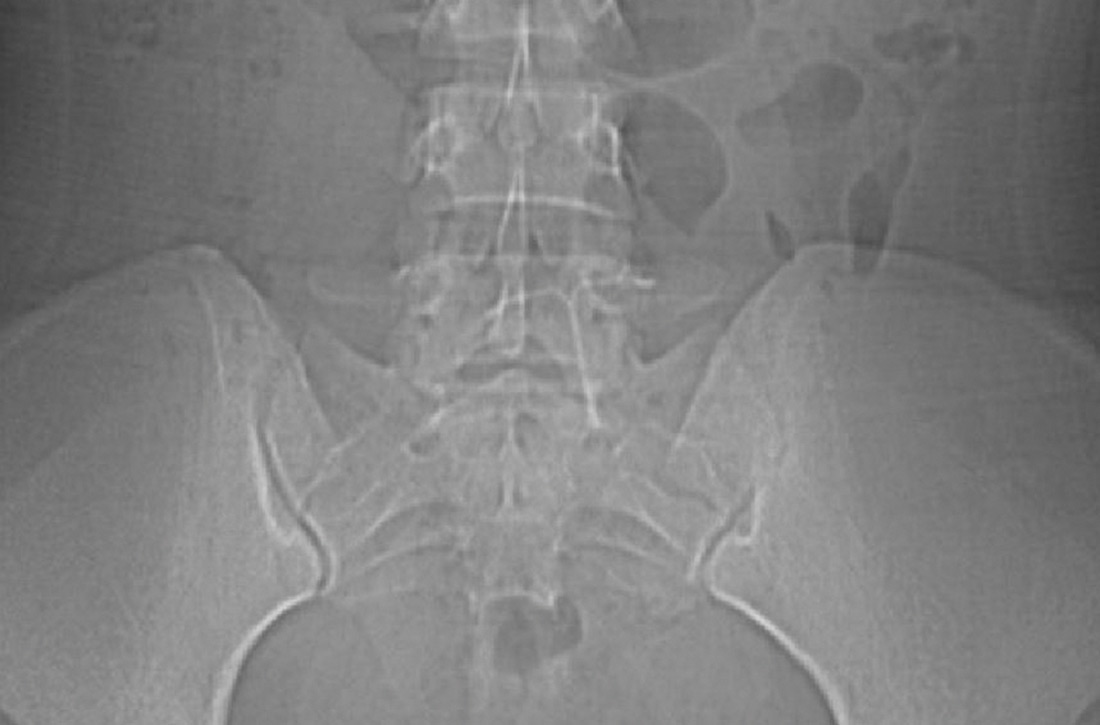
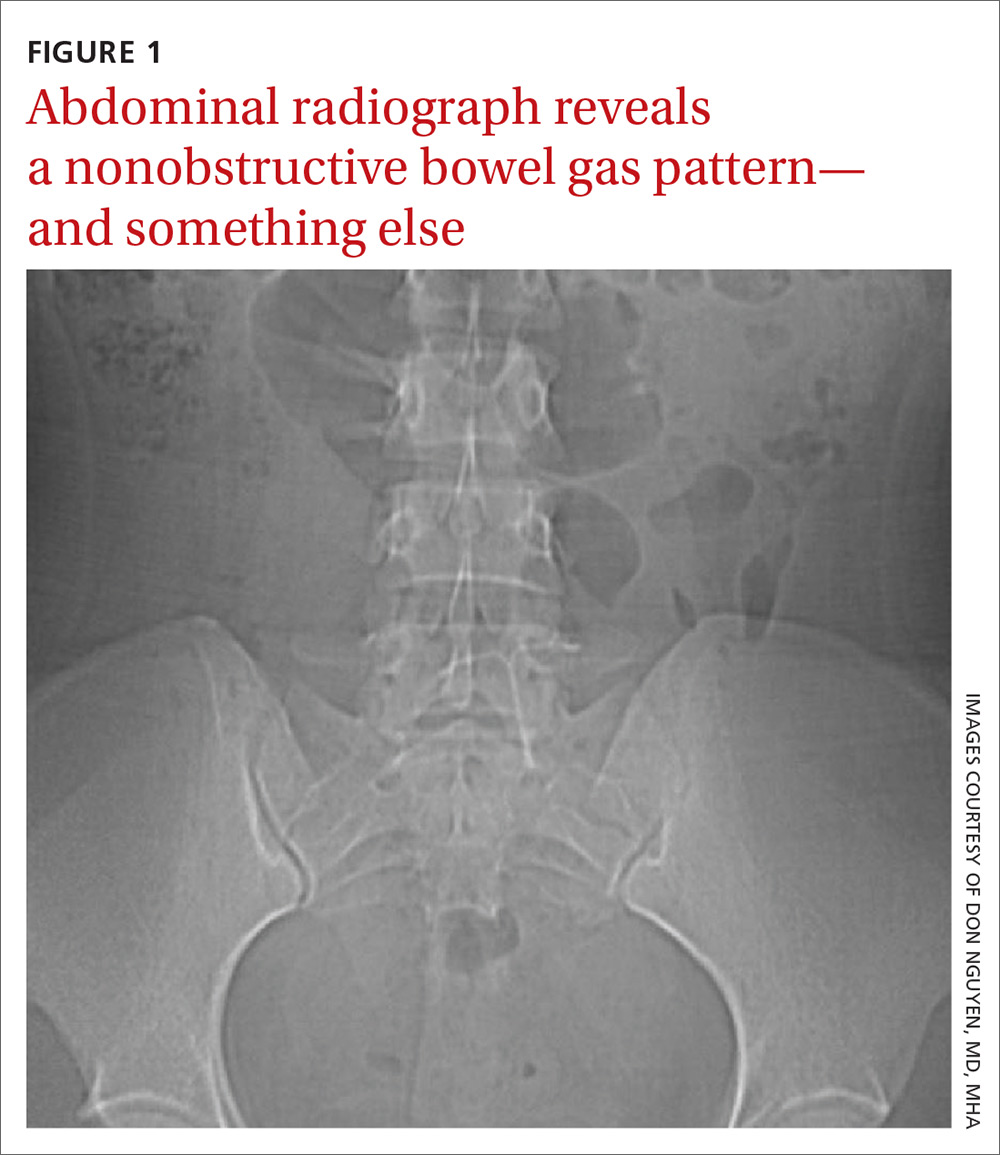
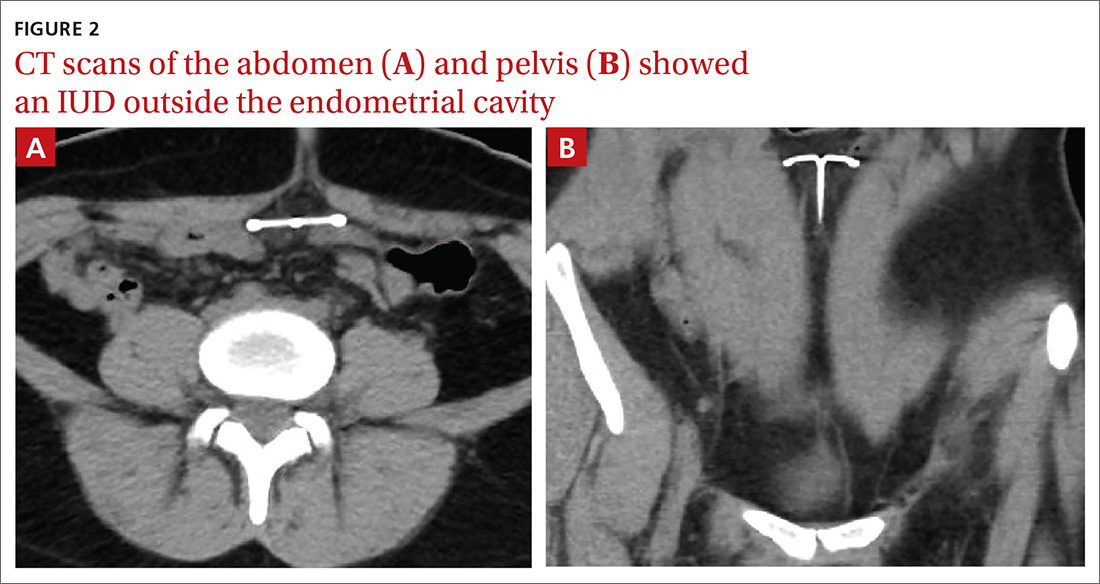
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.

Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
A 34-year-old woman with no significant past medical history presented as a new patient to our family medicine clinic with 2 weeks of intermittent lower abdominal and pelvic pain. She was sexually active with 1 partner and denied abnormal vaginal discharge or bleeding. She mentioned she’d had an intrauterine contraceptive device (IUD) placed a few weeks ago. The patient was afebrile, and her pelvic examination was unremarkable.
Physical examination showed mild tenderness to palpation over the lower abdomen without rebound tenderness or guarding. A complete metabolic panel revealed no significant abnormalities, and her human chorionic gonadotropin levels were normal.

Findings from the physical exam and her clinical history prompted the need for imaging. An abdominal radiograph (FIGURE 1) and noncontrast computed tomography (FIGURES 2A and 2B) were subsequently ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Intra-abdominal IUD migration
The abdominal radiograph revealed a nonobstructive bowel gas pattern with an IUD overlaying the central lower abdomen and pelvis at the L5-S1 level (FIGURE 1). Computed tomography (CT) of her abdomen and pelvis showed that the IUD was outside the endometrial cavity (FIGURES 2A and 2B). There was no evidence of pneumoperitoneum or bowel perforation. Based on the work-up and imaging, the patient’s pain was due to intra-abdominal IUD malpositioning.
Diagnostic criteria for IUD malpositioning include device migration into 1 of several locations, such as the lower uterine segment or cervix. IUD malpositioning can involve the rotation or protrusion of the device into or through the myometrium. On imaging, a well-positioned IUD should have a straight stem contained within the endometrial cavity, with the arms of the IUD extending laterally at the uterine fundus.
For our patient, an abdominal radiograph showed that her IUD was superiorly displaced outside the expected region of the endometrial cavity. CT helped to confirm this.
Complications with IUDs are few
Using an IUD is an increasingly popular method of contraception because it is effective and generally well tolerated, with minimal adverse effects or complications. In a multicenter retrospective chart review of 2138 patients who had IUDs, Aoun et al found that serious complications included pelvic inflammatory disease (2%), IUD expulsion (6%), and pregnancy (1%).1 In a retrospective cohort study examining complications among 90,489 women with IUDs, Berenson et al found ectopic pregnancy and uterine perforation affected < 1%.2
A less serious complication is IUD malpositioning. Although it does seem to occur more often than other, more serious complications, the exact incidence is unknown. In a retrospective case-control study, Braaten et al reported the rate for IUD malpositioning was 10.4% among 182 women.3 Malpositioned IUDs may be more likely to occur in those with suspected adenomyosis.3 In a study by de Kroon et al, the estimated prevalence rate for an abnormal IUD position ranged from 4% to 7.7% among 195 patients.4
Continue to: The clinical presentation of IUD migration
The clinical presentation of IUD migration
Identification of a malpositioned IUD is needed to avoid the possible increased risk for uterine perforation, IUD expulsion, or pregnancy.5
IUDs that have perforated the uterus float freely in the pelvis or abdomen and can result in injury to adjacent structures as well as peritonitis, fistulas, and hemorrhage.5-7 In addition, adhesion formation over the IUD can lead to intestinal obstruction, infertility, and chronic pain.6
Common symptoms of IUD malpositioning include abdominal or pelvic pain and abnormal bleeding, although many patients may be asymptomatic.8 In a retrospective study of 167 patients with IUDs who underwent pelvic ultrasound, 28 patients were found to have an IUD in an abnormal position.8 Rates of bleeding and pain were higher in patients with malpositioned IUDs (35.7% and 39.3%, respectively) than in those with a normally positioned IUD (15.1% and 19.4%, respectively).8
The differential Dx includes endometriosis and fibroids
IUD malpositioning can be distinguished from other diagnoses that cause pelvic pain and have similar presentations—including endometriosis, ectopic pregnancy, and fibroids—through imaging study findings, clinical history, and presentation.
Other conditions that may need to be ruled out include pelvic inflammatory disease, acute appendicitis, and ovarian cysts.9 A thorough history and physical examination can help rule out these conditions by organ system, and laboratory and imaging studies can help to confirm the diagnosis.
Continue to: Which imaging tool to use, and when
Which imaging tool to use, and when
Assessment of intrauterine contraception placement requires evaluation of the uterine cavity; gynecologic examination alone is not sufficient to fully evaluate for IUD position. Certain imaging studies are particularly helpful for revealing possible IUD migration.
Ultrasound—a widely available, radiation-free modality—is the first-line imaging tool for evaluation of an IUD’s position.10 In addition, ultrasound can provide effective evaluation of other pelvic structures, which is helpful in identifying or eliminating other causes of pain or abnormal bleeding.
Conventional radiography. If the IUD is not visualized on ultrasound, the American College of Obstetricians and Gynecologists (ACOG) recommends radiography to determine if the IUD has been expelled or has migrated to an extra-uterine position.6
CT may be best suited for the evaluation of more severe complications of IUD malpositioning, including visceral perforation, abscess formation, or bowel obstruction. CT should be considered if the patient’s clinical presentation is suspicious for a more serious intra-abdominal pathology.
Management depends on the IUD’s position
For patients whose IUD has an uncertain position or nonvisualized intravaginal strings, ACOG’s first-line recommendations include ruling out pregnancy, using an alternative method for contraception, and ordering pelvic ultrasonography.6 ACOG recommendations for the management of IUD malpositioning depend on the device’s location and the patient’s symptomatology.
Continue to: Management of low-lying IUDs
Management of low-lying IUDs is complex. An IUD that is malpositioned in the cervix is considered partially expelled and should be completely removed.6 For asymptomatic patients with an IUD located in the lower uterine segment and above the internal cervical os, there should be strong consideration given to leaving the IUD in place because removal is associated with higher rates of pregnancy given the low rates of initiation of effective contraception following removal.6
IUD malpositioning in the peritoneal cavity requires surgical intervention. Although ACOG’s first-line recommendation is laparoscopic intervention, laparotomy can be considered if laparoscopy does not result in the removal of the IUD or the patient has more severe complications (sepsis or bowel perforation).6 At the time of IUD removal, the clinician should also discuss and/or prescribe interim contraception.
Treatment for our patient included uncomplicated laparoscopic surgical removal of the intra-abdominal IUD. The patient’s symptoms went away following the procedure, and she was subsequently switched to an oral contraceptive.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
1. Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
2. Berenson AB, Tan A, Hirth JM, et al. Complications and continuation of intrauterine device use among commercially insured teenagers. Obstet Gynecol. 2013;121:951-958.
3. Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
4. de Kroon CD, van Houwelingen JC, Trimbos JB, et al. The value of transvaginal ultrasound to monitor the position of an intrauterine device after insertion. A technology assessment study. Hum Reprod. 2003;18:2323-2327.
5. Thonneau P, Almont T, de La Rochebrochard E, et al. Risk factors for IUD failure: results of a large multicentre case-control study. Hum Reprod. 2006;21:2612-2616.
6. ACOG Committee on Gynecologic Practice. Committee Opinion No 672: clinical challenges of long-acting reversible contraceptive methods. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2016;128:e69-e77.
7. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
8. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
9. Bhavasr AK, Felner EJ, Shorma T. Common questions about the evaluation of acute pelvic pain. Am Fam Physician. 2016;93:41-48.
10. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389-1401.
Right hip and pelvic pain
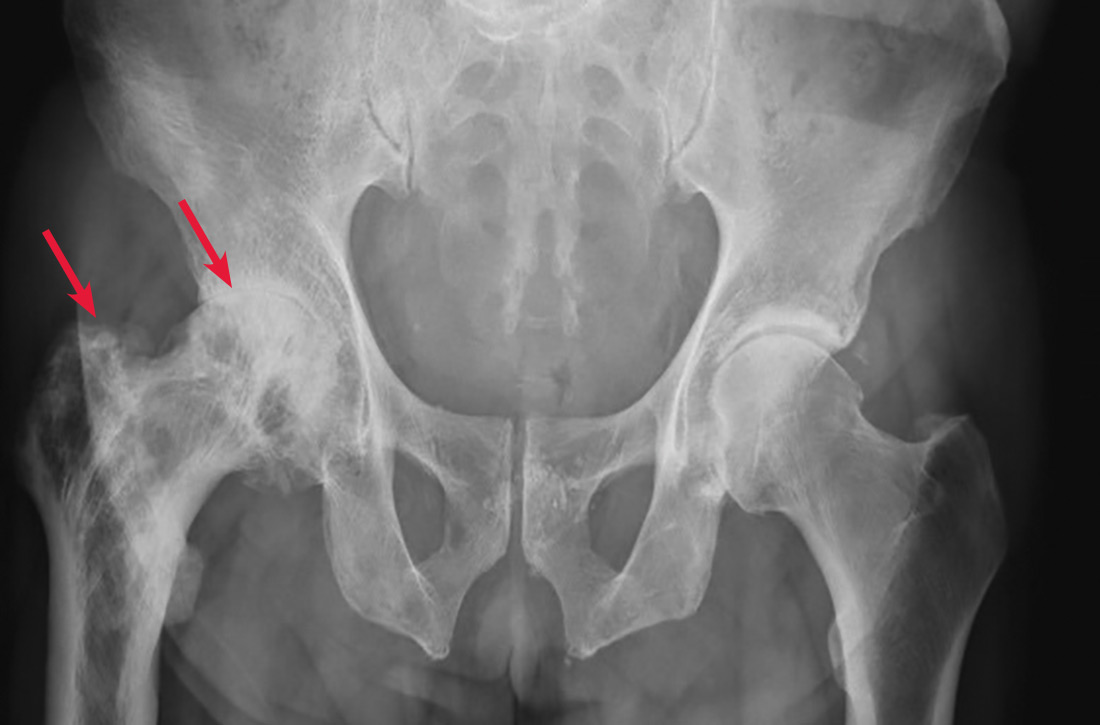
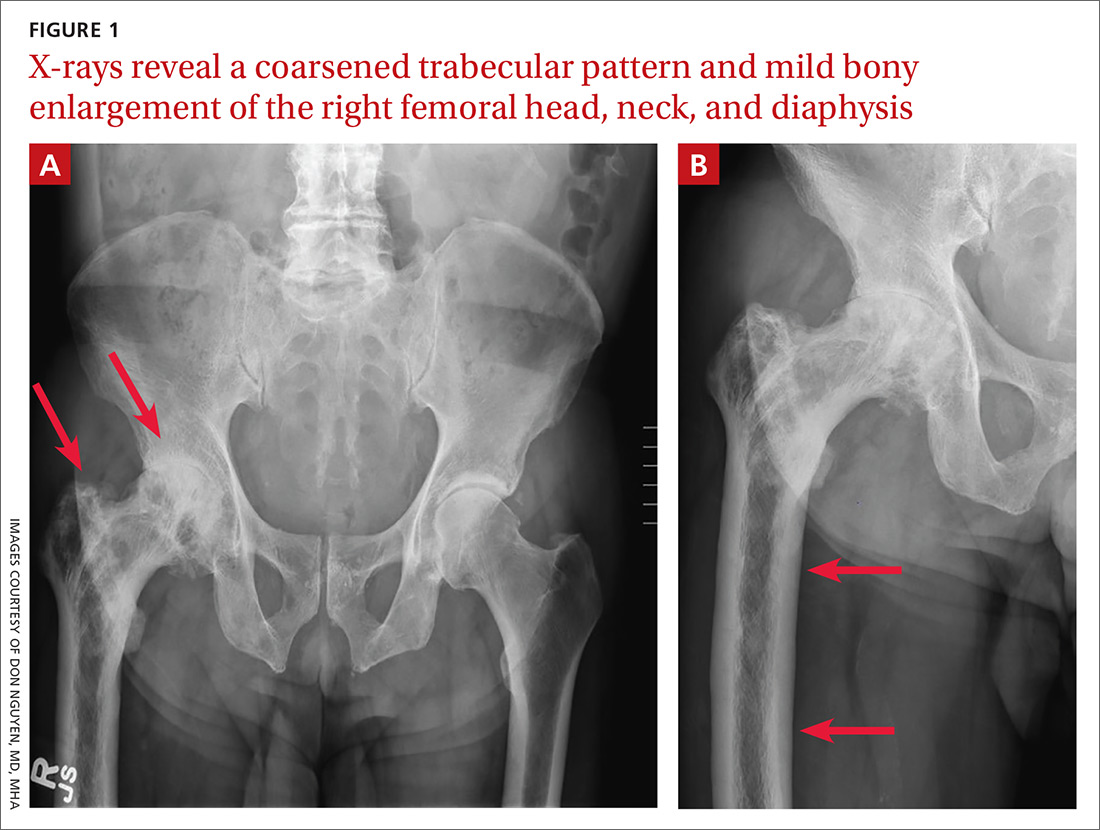
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
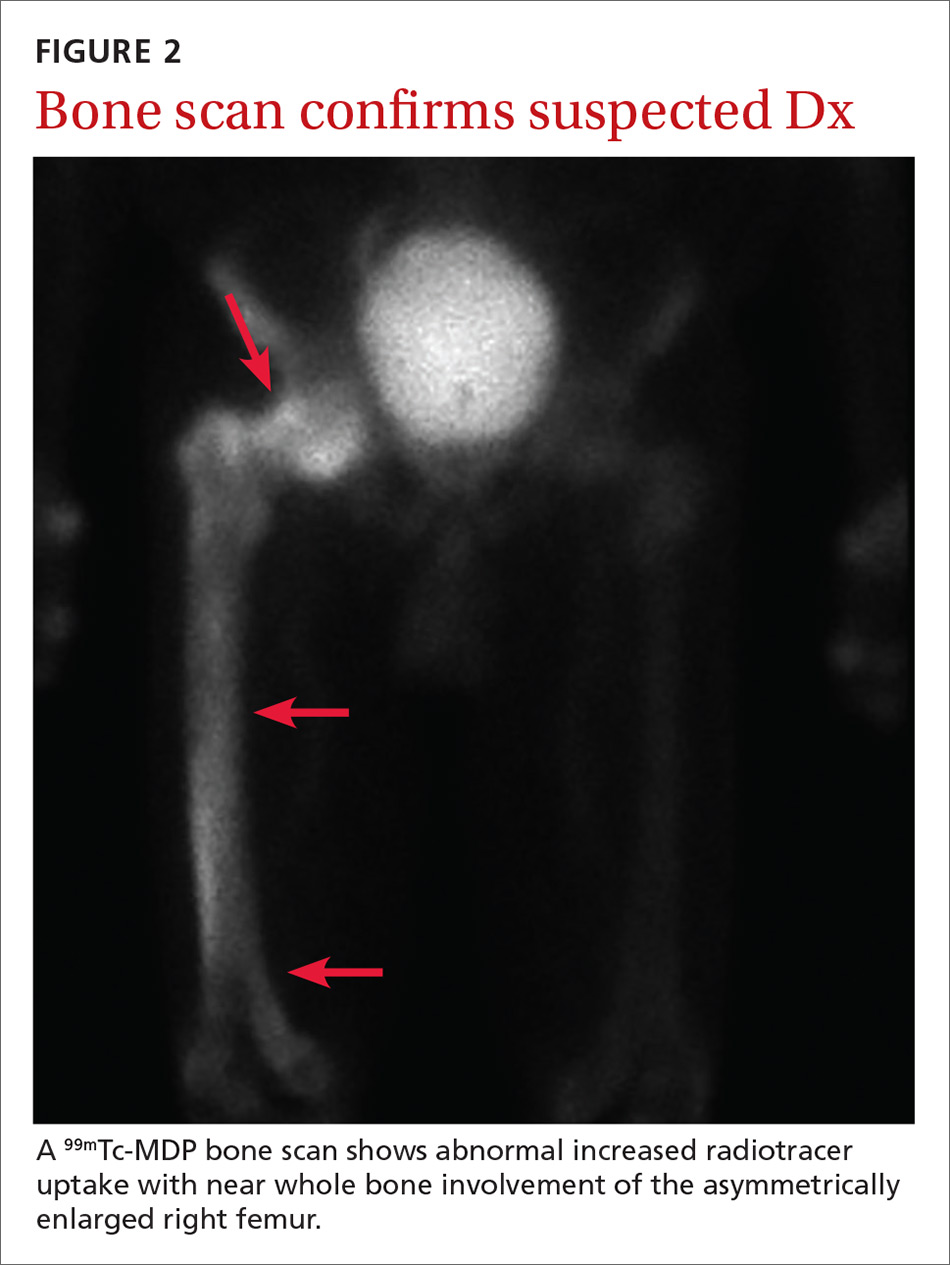
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.
Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; [email protected]
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.

Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; [email protected]
A 65-year-old man with a history of remote colon cancer, peptic ulcer disease, gastroesophageal reflux disease (GERD), and bilateral knee replacements presented with right groin and hip pain of more than a year’s duration. The patient described his hip pain as aching and said that it had worsened over the previous 6 months, interfering with his sleep. He said the pain worsened following activity, and it briefly felt better following an intra-articular corticosteroid injection into his right hip. The patient denied recent trauma or fracture and said he had no scalp pain, hearing loss, or spinal tenderness. Physical examination showed limited range of motion of the right hip and mild tenderness to palpation. Laboratory values were within normal limits. X-rays of the pelvis (Figure 1A) and right hip (Figure 1B) were ordered.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Paget disease of bone
Based on the patient’s clinical history and initial imaging studies, which showed characteristic trabecular thickening with bony enlargement of the right femur, we suspected that he had Paget disease of bone. This was confirmed on subsequent whole-body 99mTc-MDP bone scan (Figure 2), which revealed corresponding diffuse increased radiotracer uptake of the right femur. There was no scintigraphic evidence of osseous involvement of the skull, spine, or pelvis.

Epidemiology/incidence. Paget disease, also known as osteitis deformans, is fairly common in the aging population, with a prevalence ranging from 2% to almost 10%.1,2 Although onset before age 40 is rare, the diagnosis should be considered in younger patients, given the high prevalence. There is a slight male predominance, and the disease is more common in the United Kingdom and Western Europe, as well as in countries settled by European immigrants.3
Both genetic and environmental causes are believed to contribute to the pathogenesis of Paget disease. Mutations in the gene encoding sequestosome 1 (SQSTM1) can be seen in the autosomal dominant familial type (25%-50% of these cases), as well as in sporadic cases.4 Environmental influence has also been postulated as a possible cause, with a viral etiology (eg, chronic measles infection) being the most cited.5
Most patients will be asymptomatic
Paget disease can affect any bone in the body, although the skull, spine, pelvis, and long bones of the lower extremity are the most commonly affected sites.2 Most patients with Paget disease are asymptomatic. When symptoms are present, they either result from direct involvement of the bone or are secondary to bone overgrowth and deformity.
Direct involvement manifests as deep, constant bone pain that is worse at night. Symptoms related to bone overgrowth and deformity include spinal stenosis and related neurologic abnormalities, increased skull size, hearing loss (impingement of cranial nerve VIII), pathologic fracture (most commonly of the femur), and deformity such as protrusio acetabuli or femoral or tibial bowing.6 High-output heart failure and abnormalities in calcium and phosphate balance are uncommon but do occur.
Continue to: Degeneration into osteosarcoma...
Degeneration into osteosarcoma is a rare but almost invariably fatal complication of Paget disease, with an incidence of 0.2% to 1%.7 It clinically manifests as increased bone pain that is poorly responsive to medical therapy, local swelling, and pathologic fracture.8
Radiography is key to the work-up
The diagnosis of Paget disease is primarily radiographic. Early in the disease process, lytic lesions with thinning of the cortex will be noted. Later in the disease, there will be a mixed lytic/sclerotic phase, in which enlargement of the bone, a thickened cortex, and coarsened trabeculae are observed.
Characteristic radiographic findings. Focal lytic lesions in the skull are known as osteoporosis circumscripta. In the sclerotic phase, there is a thickening of the calvaria (termed “cotton wool”). Lesions involving the long bones will begin at the proximal or distal subchondral region and progress toward the diaphysis, with a sharp oblique delineation between involved bone and normal bone; this is described as “blade of grass” or “flame-shaped.”9
Within the pelvis, there will be cortical thickening and sclerosis with enlargement of the iliac wing. Within the spine, there will be enlarged vertebrae with a thickened sclerotic border, resulting in a “picture frame” appearance. Later in the disease, the sclerosis will involve the entire vertebrae (termed “ivory vertebra”).10
Additional testing options include magnetic resonance imaging (MRI), bone scintigraphy, laboratory testing, and biopsy.
Continue to: MRI is recommended...
MRI is recommended when degeneration into osteosarcoma is present—indicated by permeative lesions with cortical breakthrough and a soft-tissue mass. MRI is helpful to further characterize the lesion. Absence of the normal fatty marrow on T1-weighted images would be concerning for tumor involvement.
Bone scintigraphy is used to determine the extent of disease. It will show increased uptake when the lesions are active.
Laboratory testing. Serum alkaline phosphatase (sAP) is frequently elevated in patients with Paget disease (normal range, 20-140 IU/L) and reflects the extent and activity of disease. However, this correlation is not always reliable; it depends on monostotic vs polyostotic involvement, as well as which bones are involved. For example, sAP levels may be markedly elevated when the skull is involved but normal when other bones are involved.11 In patients with elevated sAP, serum calcium and 25-hydroxyvitamin D measurements should be obtained in anticipation of bisphosphonate treatment.
Biopsy. If the radiographic findings are typical for Paget disease, bone biopsy is not indicated. However, the main competing diagnosis to consider is malignancy; in atypical cases when imaging is unable to elucidate an underlying tumor, biopsy would be warranted.
Differentiating Paget disease from sclerotic metastasis is important. In metastasis, there will be no trabecular coarsening or enlargement of the bone.
Continue to: Bisphosphonates are a Tx mainstay
Bisphosphonates are a Tx mainstay
Indications for treatment include symptomatic or asymptomatic disease with any of the following: elevated sAP with pagetic changes at sites where complications could occur; sAP more than 2 to 4 times the upper limit of normal; normal sAP with abnormal bone scintigraphy at a site where complications could occur; planned surgery at an active pagetic site; and hypercalcemia in association with immobilization in patients with polyostotic disease.
Newer generation nitrogen-containing bisphosphonates are the mainstay of treatment; they ease pain, slow bone turnover, and promote deposition of normal lamellar bone, which over time will normalize sAP levels.12 The most frequently used and studied bisphosphonates include oral alendronate, oral risedronate, and intravenous zoledronic acid.13
Prior to treatment initiation, the patient should have documented normal serum levels of calcium, phosphorus, and 25-hydroxyvitamin D, and these levels should be monitored throughout the first year of treatment. All patients should receive supplemental vitamin D and calcium to avoid hypocalcemia. sAP should be measured at 3 to 6 months to assess the initial response to therapy. Once the levels equilibrate, sAP can be measured once or twice a year to asses bone activity.14
Our patient was referred to Endocrinology for management of Paget disease of his right hip and femur. Lab values, including sAP and liver function test results, were normal. The patient was prescribed a zoledronic acid infusion (Reclast). At 4-week follow-up, the patient reported moderate relief of bone pain and improved sleep.
CORRESPONDENCE
Don Nguyen, MD, MHA, Brigham and Women’s Hospital, Department of Radiology, 75 Francis Street, Boston, MA 02115; [email protected]
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.
1. Altman RD, Bloch DA, Hochberg MC, et al. Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. 2000;15:461-465.
2. Singer F. Paget’s disease of bone. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2000.
3. Merashli M, Jawad A. Paget’s disease of bone among various ethnic groups. Sultan Qaboos Univ Med J. 2015;15:E22-E26.
4. Hocking LJ, Lucas GJ, Daroszewska A, et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. 2002;11:2735-2739.
5. Reddy SV, Kurihara N, Menaa C, et al. Osteoclasts formed by measles virus-infected osteoclast precursors from hCD46 transgenic mice express characteristics of pagetic osteoclasts. Endocrinology. 2001;142:2898-2905.
6. Moore TE, King AR, Kathol MH, et al. Sarcoma in Paget disease of bone: clinical, radiologic, and pathologic features in 22 cases. AJR Am J Roentgenol. 1991;156:1199-1203.
7. van Staa TP, Selby P, Leufkens HG, et al. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465-471.
8. Hansen MF, Seton M, Merchant A. Osteosarcoma in Paget’s disease of bone. J Bone Miner Res. 2006;21(suppl 2):P58-P63.
9. Wittenberg K. The blade of grass sign. Radiology. 2001;221:199-200.
10. Dennis JM. The solitary dense vertebral body. Radiology. 1961;77:618-621.
11. Seton M. Paget’s disease of bone. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. 4th ed. Philadelphia, PA: Mosby (Elsevier); 2008:2003.
12. Reid IR, Nicholson GC, Weinstein RS, et al. Biochemical and radiologic improvement in Paget’s disease of bone treated with alendronate: a randomized, placebo-controlled trial. Am J Med. 1996;101:341-348.
13. Siris ES, Lyles KW, Singer FR, et al. Medical management of Paget’s disease of bone: indications for treatment and review of current therapies. J Bone Miner Res. 2006;21(suppl 2):P94-P98.
14. Alvarez L, Peris P, Guañabens N, et al. Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone: proposed intervals for monitoring treatment. Rheumatology (Oxford). 2004;43:869-874.