User login
Vaccine update: The latest recommendations from ACIP
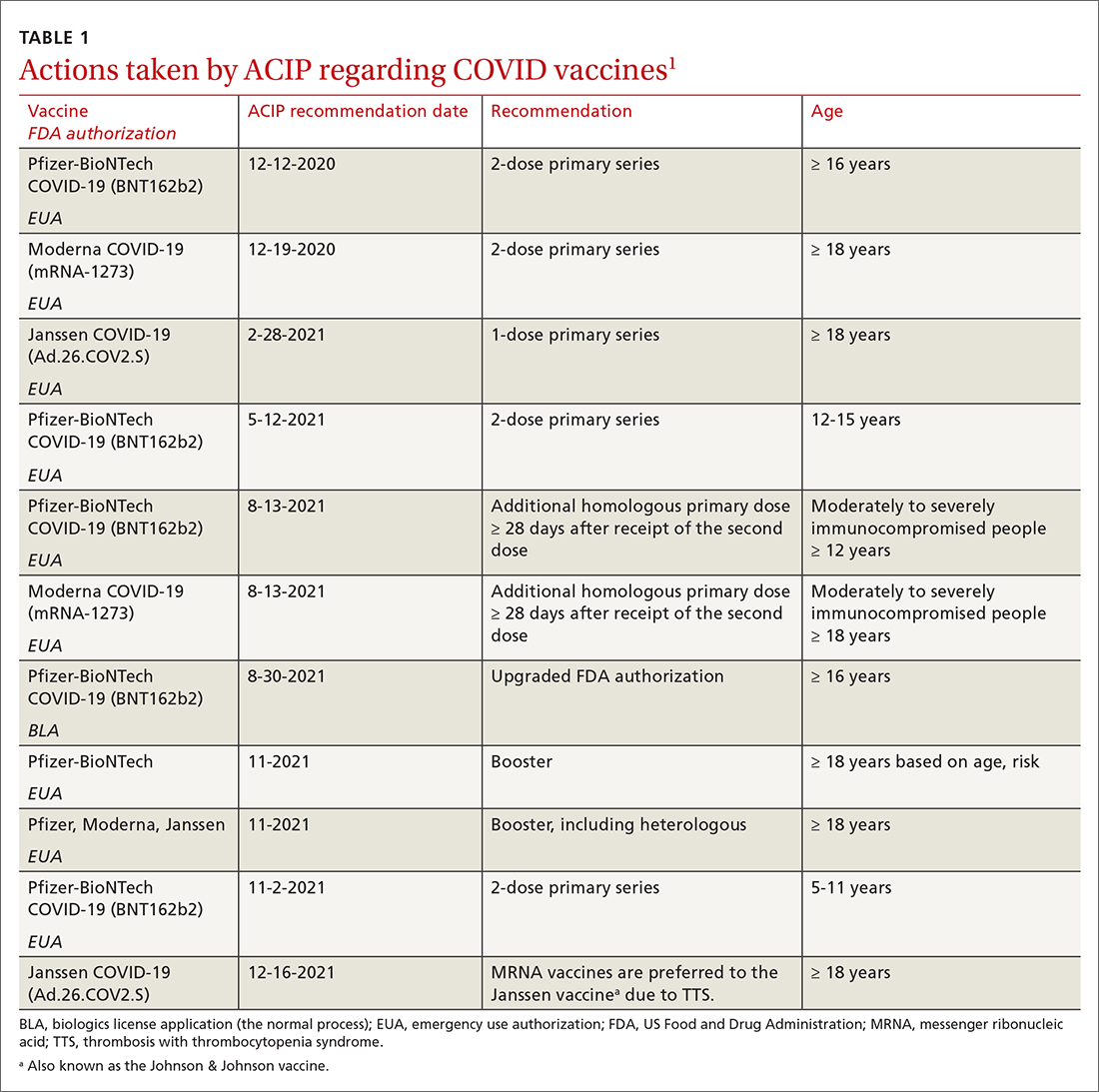
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.
Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
In a typical year, the Advisory Committee on Immunization Practices (ACIP) has three 1.5- to 2-day meetings to make recommendations for the use of new and existing vaccines in the US population. However, 2021 was not a typical year. Last year, ACIP held 17 meetings for a total of 127 hours. Most of these were related to vaccines to prevent COVID-19. There are now 3 COVID-19 vaccines authorized for use in the United States: the 2-dose mRNA-based Pfizer-BioNTech/Comirnaty and Moderna COVID-19 vaccines and the single-dose adenovirus, vector-based Janssen (Johnson & Johnson) COVID-19 vaccine.
TABLE 11 includes the actions taken by the ACIP from late 2020 through 2021 related to COVID-19 vaccines. All of these recommendations except 1 occurred after the US Food and Drug Administration (FDA) approved the product using an emergency use authorization (EUA). The exception is the recommendation for use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for those ages 16 years and older, which was approved under the normal process 8 months after widespread use under an EUA.

Hepatitis B vaccine now for all nonimmune adults up through 59 years
Since the introduction of hepatitis B (HepB) vaccines in 1980, the incidence of hepatitis B virus (HBV) infections in the United States has been reduced dramatically; there were an estimated 287,000 cases in 19852 and 19,200 in 2014.3 However, the incidence among adults has not declined in recent years and among someage groups has actually increased. Among those ages 40 to 49 years, the rate went from 1.9 per 100,000 in 20114 to 2.7 per 100,000 population in 2019.5 In those ages 50 to 59, there was an increase from 1.1 to 1.6 per 100,000 population over the same period of time.4,5
Recommendations for using HepB vaccine in adults have been based on risk that involves individual behavior, occupation, and medical conditions (TABLE 26). The presence of these risk factors is often unknown to medical professionals, who rarely ask about or document them. And patients can be reluctant to disclose them for fear of being stigmatized. The consequence has been a low rate of vaccination in at-risk adults.

At its November 2021 meeting, ACIP accepted the advice of the Hepatitis Work Group to move to a universal adult recommendation through age 59.7 ACIP believed that the incidence of acute infection in those ages 60 and older was too low to merit a universal recommendation. The new recommendation states that
Multiple HepB vaccine products are available for adults. Two are recombinant-based and require 3 doses: Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck). One is recombinant based and requires only 2 doses: Heplisav-B (Dynavax Technologies). A new product recently approved by the FDA, PREHEVBRIO (VBI Vaccines), is another recombinant 3-dose option that the ACIP will consider early in 2022. HepB and HepA vaccines can also be co-administered with Twinrix (GlaxoSmithKline).
Pneumococcal vaccines: New PCV vaccines alter prescribing choices
The ACIP recommendations for pneumococcal vaccines in adults have been very confusing, involving 2 vaccines: PCV13 (Prevnar13, Pfizer) and PPSV23 (Pneumovax23, Merck). Both PCV13 and PPSV23 given in series were recommended for immunocompromised patients, but only PPSV23 was recommended for those with chronic medical conditions. For those 65 and older, PPSV23 was recommended for all individuals (including those with no chronic or immunocompromising condition), and PCV13 was recommended for those with immunocompromising conditions. Other adults in this older age group could receive PCV13 based on individual risk and shared clinical decision making.8
Continue to: This past year...
This past year, 2 new PCV vaccines were approved by the FDA: PCV15 (Vaxneuvance, Merck) and PCV20 (Prevnar20, Pfizer). While considering these new vaccines, the ACIP re-assessed its entire approval of pneumococcal vaccines. First, they retained the cutoff for universal pneumococcal vaccination at 65 years. For those younger than 65, they combined chronic medical conditions and immunocompromising conditions into a single at-risk group (TABLE 39). They then issued the same recommendation for older adults and those younger than 65 with risks: to receive a PCV vaccine, either PCV15 or PCV20. If they receive PCV15, it should be followed by PPSV23. PPSV23 is not recommended for those who receive PCV20. Therefore,

Recombinant zoster vaccine (RZV) has been licensed and recommended in the United States since 2017 in a 2-dose schedule for adults ages 50 years and older. In the summer of 2021, the FDA expanded the indication for use of RZV to include individuals 18 to 49 years of age who are or will be immunodeficient or immunosuppressed due to known disease or therapy. In October, the ACIP agreed and recommended 2 RZV doses for those 19 years and older in these risk groups (TABLE 410).

This recommendation was based on the elevated risk of herpes zoster documented in those with immune-suppressing conditions and therapies. In the conditions studied, the incidence in these younger adults exceeded that for older adults, for whom the vaccine is recommended.10 There are many immune conditions and immune-suppressing medications. The ACIP Zoster Work Group did not have efficacy and safety information on the use of RZV in each one of them, even though their recommendation includes them all. Many of these patients are under the care of specialists whose specialty societies had been recommending zoster vaccine for their patients, off label, prior to the FDA authorization.
Rabies vaccine is now available in 2-dose schedule
People who should receive rabies pre-exposure prophylaxis (PrEP) with rabies vaccine include laboratory personnel who work with rabies virus, biologists who work with bats, animal care professionals, wildlife biologists, veterinarians, and travelers who may be at risk of encountering rabid dogs. The recommendation has been for 3 doses of rabies vaccine at 0, 7, and 21-28 days. The ACIP voted at its June 2021 meeting to adopt a 2-dose PrEP schedule of 0 and 7 days.11 This will be especially helpful to travelers who want to complete the recommended doses prior to departure. Those who have sustained risk over time can elect to have a third dose after 21 days and before 3 years, or elect to have titers checked. More detailed clinical advice will be published in the CDC’s Morbidity and Mortality Weekly Report in 2022.
Dengue vaccine: New rec for those 9-16 years
In 2019, the FDA approved the first dengue vaccine for use in the United States for children 9 to 16 years old who had laboratory-confirmed previous dengue virus infection and who were living in an area where dengue is endemic. The CYD-TDV dengue vaccine (Dengvaxia) is a live-attenuated tetravalent vaccine built on a yellow fever vaccine backbone. Its effectiveness is 82% for prevention of symptomatic dengue, 79% for prevention of dengue-associated hospitalizations, and 84% against severe dengue.12
Continue to: Dengue viruses...
Dengue viruses (DENV) are transmitted by Aedes mosquitoes. There are 4 serotypes of dengue, and all 4 appear to be circulating in most endemic countries. Clinical disease varies from a mild febrile illness to severe disease. The most common clinical presentation includes sudden onset of fever, headache, retro-orbital pain, myalgia and arthralgia, abdominal pain, and nausea.
Severe disease includes plasma leakage, shock, respiratory distress, severe bleeding, and organ failure. While severe dengue can occur with a primary infection, a second infection with a different DENV increases the risk of severe dengue. A small increased risk of severe dengue occurs when dengue infection occurs after vaccination in those with no evidence of previous dengue infection. It is felt that the vaccine serves as a primary infection that increases the risk of severe dengue with subsequent infections. This is the reason that the vaccine is recommended only for those with a documented previous dengue infection.
At its June 2021 meeting, the ACIP recommended 3-doses of Dengvaxia, administered at 0, 6, and 12 months, for individuals 9 to 16 years of age who have laboratory confirmation of previous dengue infection and live in endemic areas.12 These areas include the territories and affiliated states of Puerto Rico, American Samoa, US Virgin Islands, Federated States of Micronesia, Republic of Marshall Islands, and the Republic of Palau. Puerto Rico accounts for 85% of the population of these areas and 95% of reported dengue cases.12The reason for the delay between FDA approval and the ACIP recommendation was the need to wait for a readily available, accurate laboratory test to confirm previous dengue infection, which is now available. There are other dengue vaccines in development including 2 live-attenuated, tetravalent vaccine candidates in Phase 3 trials.
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
1. ACIP. COVID-19 vaccine recommendations. Accessed February 8, 2022. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
2. CDC. Division of viral hepatitis. Disease burden from viral hepatitis A, B, and C in the United States. Accessed February 8 2022. www.cdc.gov/hepatitis/PDFs/disease_burden.pdf
3. CDC. Surveillance for viral hepatitis – United States, 2014. Hepatitis B. Accessed February 8, 2022. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#:~:text=HEPATITIS%20B-,Acute%20Hepatitis%20B,B%20cases%20occurred%20in%202014
4. CDC. Viral hepatitis surveillance: United States, 2011. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2011surveillance/pdfs/2011HepSurveillanceRpt.pdf
5. CDC. Viral hepatitis surveillance report, 2019. Hepatitis B. Accessed February 8, 2022. www.cdc.gov/hepatitis/statistics/2019surveillance/HepB.htm
6. Schillie S, Harris A, Link-Gelles R, et al. Recommendations of the Advisory Committee on Immunization Practices for use of a hepatitis B vaccine with a novel adjuvant. MMWR Morb Mortal Wkly Rep. 2018;67:455-458.
7. CDC. Advisory Committee on Immunization Practices. Meeting recommendations, November 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/index.html
8. Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:1069-1075.
9. Kobayashi M. Considerations for use of PCV15 and PCV20 in U.S. adults. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/05-Pneumococcal-Kobayashi.pdf
10. Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:80-84.
11. CDC. ACIP recommendations. June 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/recommendations.html
12. Paz-Bailey G. Dengue vaccine. Evidence to recommendation framework. Presented to the ACIP June 24, 2021. Accessed February 8, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-Dengue-Paz-Bailey-508.pdf
USPSTF releases updated guidance on asymptomatic A-fib
In January 2022, the US Preventive Services Task Force updated its 2018 statement on screening for atrial fibrillation (AF) in older adults (≥ 50 years).1,2 The supporting evidence review sought to include data on newer screening methods, such as automated blood pressure cuffs, pulse oximeters, and consumer-facing devices (eg, smartphone apps). However, ultimately, the recommendation did not change; it remains an “I” statement, meaning the evidence is insufficient to assess the balance of benefits and harms of screening for AF in asymptomatic adults with no signs or symptoms.1,2
Atrial fibrillation and stroke. AF is common, and the prevalence increases with age: from < 0.2% in those younger than 55 years to about 10% for those ages 85 and older.1,2 AF is a strong risk factor for stroke, and when detected, stroke prevention measures—either restoration of normal rhythm or use of anticoagulants—can be implemented as appropriate.
The available evidence for the effectiveness of stroke prevention comes from patients with AF that was detected because of symptoms or pulse palpation during routine care. It is not known if screening asymptomatic adults using electrocardiography, or newer electronic devices that detect irregular heartbeats, achieves these same benefits—and there is the potential for harm from the use of anticoagulants.
How does this compare to other recommendations? The American Heart Association and the American Stroke Association recommend active screening for AF, by pulse assessment, in those ages 65 years and older.3 This does not differ as much as it appears to from the USPSTF statement. The difference is in terminology: The USPSTF considers pulse assessment part of routine care; the other organizations call it “screening.”
What you should—and shouldn’t—do. The USPSTF states that “Clinicians should use their clinical judgement regarding whether to screen and how to screen for AF.” Any patient with signs or symptoms of AF or who is discovered to have an irregular pulse should be assessed for AF. Those found to have AF should be assessed for their risk of stroke and treated accordingly. However, attempting to find “silent” AF in those who do not have an irregular pulse on exam, by way of any screening devices, has no proven benefit.
1. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-365.
2. USPSTF. Screening for atrial fibrillation: final recommendation statement. Published January 25, 2022. Accessed February 2, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/atrial-fibrillation-screening
3. Meschia JF, Bushnell C, Boden-Albala B, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754-3832. doi: 10.1161/STR.0000000000000046
In January 2022, the US Preventive Services Task Force updated its 2018 statement on screening for atrial fibrillation (AF) in older adults (≥ 50 years).1,2 The supporting evidence review sought to include data on newer screening methods, such as automated blood pressure cuffs, pulse oximeters, and consumer-facing devices (eg, smartphone apps). However, ultimately, the recommendation did not change; it remains an “I” statement, meaning the evidence is insufficient to assess the balance of benefits and harms of screening for AF in asymptomatic adults with no signs or symptoms.1,2
Atrial fibrillation and stroke. AF is common, and the prevalence increases with age: from < 0.2% in those younger than 55 years to about 10% for those ages 85 and older.1,2 AF is a strong risk factor for stroke, and when detected, stroke prevention measures—either restoration of normal rhythm or use of anticoagulants—can be implemented as appropriate.
The available evidence for the effectiveness of stroke prevention comes from patients with AF that was detected because of symptoms or pulse palpation during routine care. It is not known if screening asymptomatic adults using electrocardiography, or newer electronic devices that detect irregular heartbeats, achieves these same benefits—and there is the potential for harm from the use of anticoagulants.
How does this compare to other recommendations? The American Heart Association and the American Stroke Association recommend active screening for AF, by pulse assessment, in those ages 65 years and older.3 This does not differ as much as it appears to from the USPSTF statement. The difference is in terminology: The USPSTF considers pulse assessment part of routine care; the other organizations call it “screening.”
What you should—and shouldn’t—do. The USPSTF states that “Clinicians should use their clinical judgement regarding whether to screen and how to screen for AF.” Any patient with signs or symptoms of AF or who is discovered to have an irregular pulse should be assessed for AF. Those found to have AF should be assessed for their risk of stroke and treated accordingly. However, attempting to find “silent” AF in those who do not have an irregular pulse on exam, by way of any screening devices, has no proven benefit.
In January 2022, the US Preventive Services Task Force updated its 2018 statement on screening for atrial fibrillation (AF) in older adults (≥ 50 years).1,2 The supporting evidence review sought to include data on newer screening methods, such as automated blood pressure cuffs, pulse oximeters, and consumer-facing devices (eg, smartphone apps). However, ultimately, the recommendation did not change; it remains an “I” statement, meaning the evidence is insufficient to assess the balance of benefits and harms of screening for AF in asymptomatic adults with no signs or symptoms.1,2
Atrial fibrillation and stroke. AF is common, and the prevalence increases with age: from < 0.2% in those younger than 55 years to about 10% for those ages 85 and older.1,2 AF is a strong risk factor for stroke, and when detected, stroke prevention measures—either restoration of normal rhythm or use of anticoagulants—can be implemented as appropriate.
The available evidence for the effectiveness of stroke prevention comes from patients with AF that was detected because of symptoms or pulse palpation during routine care. It is not known if screening asymptomatic adults using electrocardiography, or newer electronic devices that detect irregular heartbeats, achieves these same benefits—and there is the potential for harm from the use of anticoagulants.
How does this compare to other recommendations? The American Heart Association and the American Stroke Association recommend active screening for AF, by pulse assessment, in those ages 65 years and older.3 This does not differ as much as it appears to from the USPSTF statement. The difference is in terminology: The USPSTF considers pulse assessment part of routine care; the other organizations call it “screening.”
What you should—and shouldn’t—do. The USPSTF states that “Clinicians should use their clinical judgement regarding whether to screen and how to screen for AF.” Any patient with signs or symptoms of AF or who is discovered to have an irregular pulse should be assessed for AF. Those found to have AF should be assessed for their risk of stroke and treated accordingly. However, attempting to find “silent” AF in those who do not have an irregular pulse on exam, by way of any screening devices, has no proven benefit.
1. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-365.
2. USPSTF. Screening for atrial fibrillation: final recommendation statement. Published January 25, 2022. Accessed February 2, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/atrial-fibrillation-screening
3. Meschia JF, Bushnell C, Boden-Albala B, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754-3832. doi: 10.1161/STR.0000000000000046
1. USPSTF; Davidson KW, Barry MJ, Mangione CM, et al. Screening for atrial fibrillation: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:360-365.
2. USPSTF. Screening for atrial fibrillation: final recommendation statement. Published January 25, 2022. Accessed February 2, 2022. https://uspreventiveservicestaskforce.org/uspstf/recommendation/atrial-fibrillation-screening
3. Meschia JF, Bushnell C, Boden-Albala B, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754-3832. doi: 10.1161/STR.0000000000000046
How to help adults meet dietary recommendations
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
Dietary guidelines provide scientifically sound and practical advice that, if followed by every person, would probably result in less obesity, type 2 diabetes, cardiovascular disease, cancer, and bone loss. But few US adults meet these recommendations, according to a recent report in the CDC’s Morbidity and Mortality Weekly Report (MMWR).1
Data from the 2019 Behavioral Risk Factor Surveillance system indicate that only 12.3% of US adults consumed the recommended amount of fruit and 10% the recommended amount of vegetables (more on that shortly). Women were more likely than men to meet the requirements for fruit (14.5% vs 10.1%) and vegetable (12.4% vs 7.6%) intake. The vegetable recommendation was more likely to be met by those in higher income households than those in the lowest income categories (12.2% vs 6.8%).1
Just what’s recommended? The most recent dietary guidelines from the Department of Agriculture suggest that adults should consume 1.5 to 2 cup-equivalents of fruits and 2 to 3 cup-equivalents of vegetables each day.2 What is a cup-equivalent? Examples include: 1 cup of a raw, or cooked, vegetable or fruit; 1 cup of fruit juice; 2 cups of leafy salad greens; or 1/2 cup of a dried fruit or vegetable. Additional recommendations are that added sugar constitute < 10% of calories per day, saturated fat < 10% of calories per day, and sodium < 2300 mg per day.
Simplify the message to this … There’s an easy message for clinicians to provide to patients: Consume 2 cups of fruit and 2 to 3 cups of vegetables per day; increase intake of whole grains, seafood, nuts, and seeds; choose fat-free and low-fat dairy products; and avoid sugary beverages and foods. But as we know, recommending that patients do something and actually having them do it are often 2 different things. So how can we tip the scales in a healthier direction?
Advise patients not to go it alone. The US Preventive Services Task Force recommends intensive behavioral interventions to alter eating habits. These interventions include individual or group counseling sessions over extended periods (eg, 6 hours of contact time over 6 to 18 months), including some 1-on-1 time with a specially trained professional, such as a primary care physician, nurse, registered dietitian, or nutritionist. The good news is that, for those with cardiovascular risk factors (dyslipidemia, elevated blood pressure, type 2 diabetes, and hypertension), this is a level “B” recommendation—meaning these interventions should be covered by commercial health insurance with no out-of-pocket cost to patients.3
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
1. Lee SH, Moore LV, Park S, et al. Adults meeting fruit and vegetable intake recommendations—United States, 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1-9. Accessed January 18, 2022. www.cdc.gov/mmwr/volumes/71/wr/mm7101a1.htm
2. USDA. Dietary guidelines for Americans 2020-2025. Ninth ed. Accessed January 18, 2022. www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf
3. USPSTF. Healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling interventions. Final recommendation statement. Published November 24, 2020. Accessed January 18, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd
Why mRNA COVID vaccines are preferred (and why patients should be reassured)
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
2021 CDC guidelines on sexually transmitted infections
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
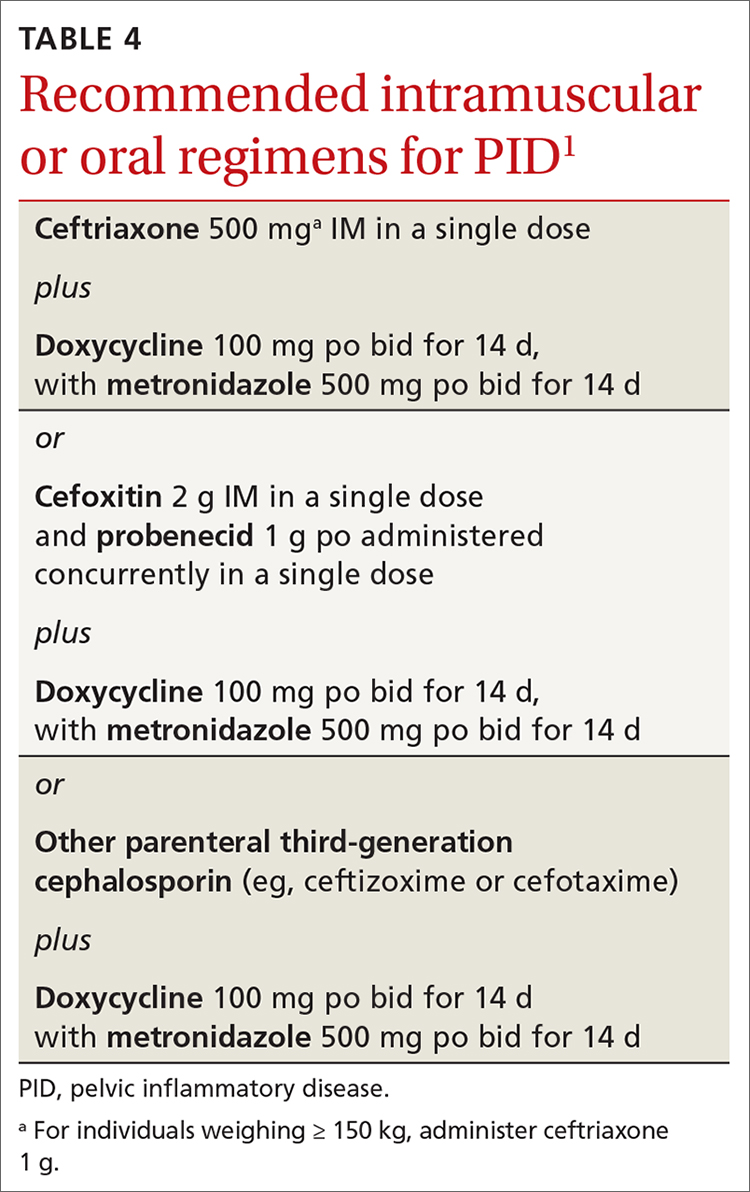
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
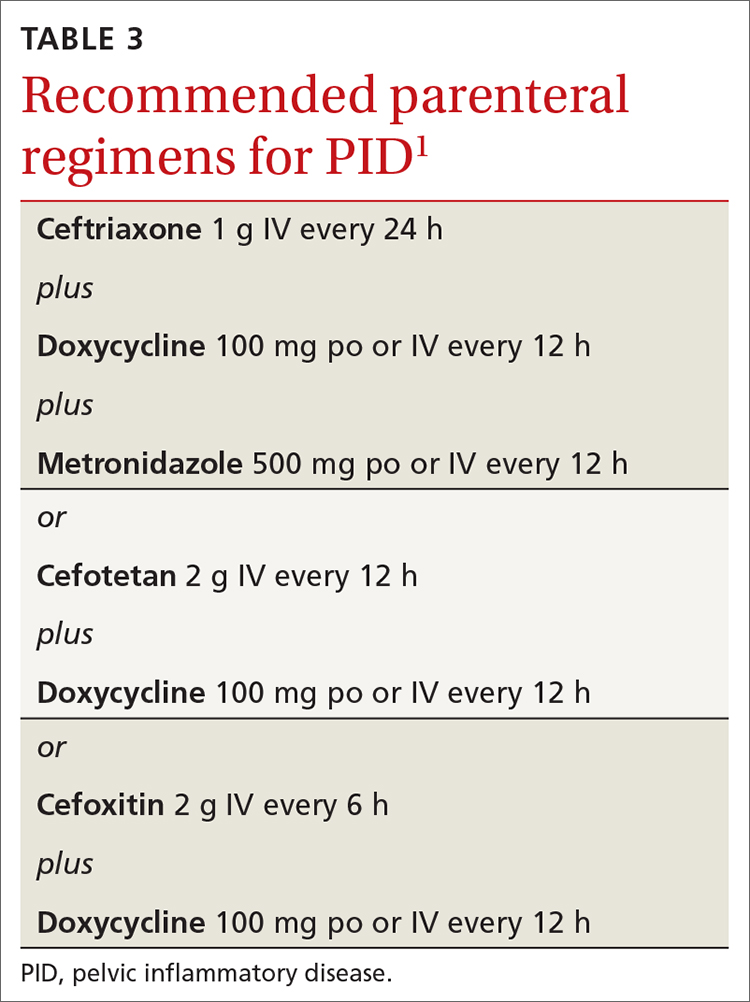
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.

To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.

Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.

To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.

Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
ACIP simplifies adult vaccinations for HepB and pneumonia
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
REFERENCES
- Weng MK. Universal adult hepatitis B vaccinations: work group considerations. Presented to the Advisory Committee on Immunization Practices on November 3, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-11-2-3/02-HepWG-weng-508.pdf
- Kovayashi M. Considerations for age-based and risk-based use of PCV15 and PCV20 among US adults and proposed policy options. Presented to the Advisory Committee on Immunization Practices on October 20, 2021. Accessed November 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/02-Pneumococcal-Kobayashi-508.pdf
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbid Mortal Wkly Rep. 2019;68:1069-1075.
Beyond the headlines: A closer look at the USPSTF draft recs on aspirin
REFERENCES
- US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medication. Published October 12, 2021. Accessed October 25, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication
- National Center for Health Statistics. Figure 4. Number of deaths, percentage of total deaths, and age-adjusted death rates for the 10 leading causes of death in 2019: United States, 2018 and 2019. In: Data Brief 395: Mortality in the United States 2019. Published December 2020. Accessed October 25, 2021. www.cdc.gov/nchs/data/databriefs/db395-tables-508.pdf
- American College of Cardiology/American Heart Association. Heart risk calculator. Updated November 12, 2017. Accessed October 25, 2021. www.cvriskcalculator.com/
REFERENCES
- US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medication. Published October 12, 2021. Accessed October 25, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication
- National Center for Health Statistics. Figure 4. Number of deaths, percentage of total deaths, and age-adjusted death rates for the 10 leading causes of death in 2019: United States, 2018 and 2019. In: Data Brief 395: Mortality in the United States 2019. Published December 2020. Accessed October 25, 2021. www.cdc.gov/nchs/data/databriefs/db395-tables-508.pdf
- American College of Cardiology/American Heart Association. Heart risk calculator. Updated November 12, 2017. Accessed October 25, 2021. www.cvriskcalculator.com/
REFERENCES
- US Preventive Services Task Force. Aspirin use to prevent cardiovascular disease: preventive medication. Published October 12, 2021. Accessed October 25, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/draft-evidence-review/aspirin-use-to-prevent-cardiovascular-disease-preventive-medication
- National Center for Health Statistics. Figure 4. Number of deaths, percentage of total deaths, and age-adjusted death rates for the 10 leading causes of death in 2019: United States, 2018 and 2019. In: Data Brief 395: Mortality in the United States 2019. Published December 2020. Accessed October 25, 2021. www.cdc.gov/nchs/data/databriefs/db395-tables-508.pdf
- American College of Cardiology/American Heart Association. Heart risk calculator. Updated November 12, 2017. Accessed October 25, 2021. www.cvriskcalculator.com/
Influenza vaccine update, 2021-22
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.
Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1
In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.
The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4
Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.

Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1

In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.

The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4

Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.

Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1

In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.

The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4

Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
USPSTF updates diabetes recs, lowers screening age
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
Hypertension in adults: USPSTF reaffirms this screening protocol
REFERENCES
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
- US Preventive Services Task Force. Screening for hypertension in adults: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
REFERENCES
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
- US Preventive Services Task Force. Screening for hypertension in adults: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987
REFERENCES
- Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-e248.
- US Preventive Services Task Force. Screening for hypertension in adults: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650-1656. doi: 10.1001/jama.2021.4987