User login
Strategic Initiatives for Veterans with Lung Cancer (FULL)
The Veterans Health Administration (VHA) facilitates care for > 7,700 veterans with newly diagnosed lung cancer each year.1 This includes comprehensive clinical evaluations and management that are facilitated through interdisciplinary networks of pulmonologists, radiologists, thoracic surgeons, radiation oncologists, and medical oncologists. Veterans with lung cancer have access to advanced medical technologies at US Department of Veterans Affairs (VA) medical centers (VAMCs), including the latest US Food and Drug Administration (FDA)-approved targeted radiation delivery systems and novel immunotherapies, as well as precision oncology-driven clinical trials.2
Despite access to high-quality care, lung cancer remains the leading cause of cancer-related mortality among VHA enrollees as well as the US population.3 About 15 veterans die of lung cancer each day; most are diagnosed with advanced stage III or stage IV disease. To address this issue, VHA launched 3 new initiatives between 2016 and 2017 to improve outcomes for veterans impacted by lung cancer. The VA Partnership to increase Access to Lung Screening (VA-PALS) is a clinical implementation project to increase access to early detection lung screening scans at 10 VAMCs. The Veterans Affairs Lung cancer surgery Or stereotactic Radiotherapy (VALOR) is a phase 3 randomized trial that investigates the role of stereotactic body radiation therapy (SBRT) as a potential alternative to surgery for veterans with operable stage I non-small cell lung cancer (NSCLC). The VA Radiation Oncology Quality Surveillance program (VA-ROQS) established national expert-derived benchmarks for the quality assurance of lung cancer therapy.
VA-PALS
The central mission of VA-PALS is to reduce lung cancer mortality among veterans at risk by increasing access to low-dose computed tomography (LDCT) lung screening scans.4,5 The program was developed as a public-private partnership to introduce structured lung cancer screening programs at 10 VAMCs to safely manage large cohorts of veterans undergoing annual screening scans. The VA-PALS project brings together pulmonologists, radiologists, thoracic surgeons, radiation oncologists, medical oncologists, and computer scientists who have experience developing open-source electronic health record systems for VHA networks. The project was launched in 2017 after an earlier clinical demonstration project identified substantial variability and challenges with efforts to implement new lung cancer screening programs in the VA.6
Each of the 10 VA-PALS-designated lung cancer screening programs (Atlanta, Georgia; Phoenix, Arizona; Indianapolis, Indiana; Chicago, Illinois; Nashville, Tennessee; Philadelphia, Pennsylvania; St. Louis, Missouri; Denver, Colorado; Milwaukee, Wisconsin; and Cleveland, Ohio) assumes a major responsibility for ordering and evaluating the results of LDCT scans to ensure appropriate follow-up care of veterans with abnormal radiographic findings. Lung cancer screening programs are supported with a full-time navigator (nurse practitioner or physician assistant) who has received training from the VA-PALS project team with direct supervision by a local site director who is a pulmonologist, thoracic surgeon, or medical oncologist. Lung cancer screening programs establish a centralized approach that aims to reduce the burden on primary care providers for remembering to order annual baseline and repeat LDCT scans. The lung screening programs also manage radiographic findings that usually are benign to facilitate appropriate decisions to minimize the risk of unnecessary tests and procedures. Program implementation across VA-PALS sites includes a strong connection among participants through meetings, newsletters, and attendance at conferences to create a collaborative learning network, which has been shown to improve dissemination of best practices across the VHA.7,8
The International Early Lung Cancer Action Program (I-ELCAP), which pioneered the use of LDCT to reduce lung cancer mortality, is a leading partner for VA-PALS.9 This group has > 25 years of experience overcoming many of the obstacles and challenges that new lung cancer screening programs face.10 The I-ELCAP has successfully implemented new lung cancer screening programs at > 70 health care institutions worldwide. Their implementation processes provide continuous oversight for each center. As a result, the I-ELCAP team has developed a large and detailed lung cancer screening registry with > 75,000 patients enrolled globally, comprising a vast database of clinical data that has produced > 270 scientific publications focusing on improving the quality and safety of lung cancer screening.11,12
These reports have helped guide evidence-based recommendations for lung cancer screening in several countries and include standardized processes for patient counseling and smoking cessation, data acquisition and interpretation of LDCT images, and clinical management of abnormal findings to facilitate timely transition from diagnosis to treatment.13-15 The I-ELCAP management system detects 10% abnormal findings in the baseline screening study, which declines to 6% in subsequent years.12 The scientific findings from this approach have provided additional insights into technical CT scanning errors that can affect tumor nodule measurements.16 The vast amount of clinical data and expertise have helped explore genetic markers.17 The I-ELCAP has facilitated cost-effectiveness investigations to determine the value of screening, and their research portfolio includes investigations into the longer-term outcomes after primary treatment for patients with screen-detected lung cancers.18,19
I-ELCAP gifted its comprehensive clinical software management system that has been in use for the above contributions for use in the VHA through an open source agreement without licensing fees. The I-ELCAP software management system was rewritten in MUMPS, the software programming language that is used by the VA Computerized Patient Record System (CPRS). The newly adapted VA-PALS/I-ELCAP system underwent modifications with VHA clinicians’ input, and was successfully installed at the Phoenix VA Health Care System in Arizona, which has assumed a leading role for the VA-PALS project.
The VA-PALS/I-ELCAP clinical management system currently is under review by the VA Office of Information and Technology for broad distribution across the VHA through the VA Enterprise Cloud. Once in use across the VHA, the VA-PALS/I-ELCAP clinical management system will offer a longitudinal central database that can support numerous quality improvement and quality assurance initiatives, as well as innovative research projects. Research opportunities include: (1) large-scale examination of LDCT images with artificial intelligence and machine learning techniques; (2) epidemiologic investigations of environmental and genetic risk factors to better understand the high percentage of veterans diagnosed with lung cancer who were never smokers or had quit many years ago; and (3) multisite clinical trials that explore early detection blood screening tests that are under development.
The VA-PALS project is sponsored by the VHA Office of Rural Health as an enterprise-wide initiative that focuses on reaching rural veterans at risk. The project received additional support through the VA Secretary’s Center for Strategic Partnerships with a $5.8 million grant from the Bristol-Myers Squibb Foundation. The VistA (Veterans Health Information Systems and Technology Architecture) Expertise Network is an additional key partner that helped adapt the VAPALS-ELCAP system for use on VHA networks.
VALOR Trial
The VA Cooperative Studies Program (CSP) #2005 VALOR study is a randomized phase 3 clinical trial that evaluates optimal treatment for participants with operable early-stage NSCLC.20 The trial is sponsored by the CSP, which is responsible for and provides resources for the planning and conduct of large multicenter surgical and clinical trials in VHA.21 The CSP #2005 VALOR study plans to enroll veterans with stage I NSCLC who will be treated with a surgical lobectomy or SBRT according to random assignment. An alternative surgical approach with a segmentectomy is acceptable, although patients in poor health who are only qualify for a wedge resection will not be enrolled. The CSP will follow each participant for at least 5 years to evaluate which treatment, if either, results in a higher overall survival rate. Secondary outcome measures are quality of life, pulmonary function, health state utilities, patterns of failure, and causes of death.
Although the study design of the VALOR trial is relatively straightforward, recruitment of participants to similar randomized trials of surgery vs SBRT for operable stage I NSCLC outside the VA has historically been very difficult. Three earlier phase 3 trials in the Netherlands and US closed prematurely after collectively enrolling only 4% of planned participants. Although a pooled analysis of 2 of these trials demonstrated a statistically significant difference of 95% vs 79% survival in favor of SBRT at a median follow-up of 40 months, the analysis was underpowered because only 58 of the planned 1,380 participants were enrolled.22,23
The CSP #2005 VALOR study team was keenly aware of these past challenges and addressed many of the obstacles to enrollment by optimizing eligibility criteria and follow-up requirements. Enrollment sites were carefully selected after confirming equipoise between the 2 treatments, and study coordinators at each enrollment site were empowered to provide a leading role with recruitment. Multiple communication channels were established for constant contact to disseminate new best practices for recruitment as they were identified. Furthermore, a veteran-centric educational recruitment video, approved by the VA Central Institutional Review Board, was designed to help study participants better understand the purpose of participating in a clinical trial (www.vacsp.research.va.gov/CSP_2005/CSP_2005.asp).
After the first year of recruitment, researchers identified individual clinician and patient preferences as the predominant difficulty with recruitment, which was not easy to address. The CSP #2005 VALOR study team opted to partner directly with the Qualitative Research Integrated within Trials (QuinteT) team in the United Kingdom to adopt its methods to successfully support randomized clinical trials with serious recruitment challenges.24,25 By working directly with the QuinteT director, the CSP #2005 VALOR team made a major revision to the informed consent forms by shifting focus away from disclosing potential harms of research to an informative document that emphasized the purpose of the study. The work with QuinteT also led to the creation of balanced narratives for study teams to use and for potential participants to read. These provide a more consistent message that describes why the study is important and why clinicians are no longer certain that surgery is the optimal treatment for all patients with operable stage I NSCLC.
The VALOR clinical trial, opened in 2017, remains open at only 9 VAMCs. As of early 2020, it has enrolled more participants than all previous phase 3 trials combined. Once completed, the results from CSP #2005 VALOR study will help clinicians and veterans with operable stage I NSCLC better understand the tradeoffs of surgery vs SBRT as an initial treatment option. Plans are under way to expand the scope of the trial and include investigations of pretreatment radiomic signatures and genetic markers from biopsy tissue and blood samples, to better predict when surgery or SBRT might be the best treatment option for an individual patient.
VA-ROQS
The VA-ROQS was created in 2016 to compare treatment of veterans with lung cancer in the VHA with quality standards recommended by nationally recognized experts in lung cancer care. Partnering with Washington University in St. Louis, Missouri and the American Society for Radiation Oncology, the VHA established a blue-ribbon panel of experts to review clinical trial data and medical literature to provide evidence-based quality metrics for lung cancer therapy. As a result, 26 metrics applicable to each patient’s case were developed, published, and used to assess lung cancer care in each VHA radiation oncology practice.26
By 2019, the resulting data led to a report on 773 lung cancer cases accumulated from all VHA radiation oncology practices. Performance data for each quality metric were compared for each practice within the VHA, which found that VHA practices met > 80% of all 1,278 metrics scored. Quality metrics included those documented within each patient health record and the specific radiation delivery parameters that reflected each health care provider’s treatment. After team investigators visited each center and recorded treatment data, VA-ROQS is now maturing to permit continuous, electronic monitoring of all lung cancer treatment delivered within VHA. As each veteran’s case is planned, the quality of the therapy is monitored, assessed, and reported to the treating physician. Each VHA radiation oncologist will receive up-to-date evaluation of each case compared with these evidence-based quality standards. The quality standards are reviewed by the blue-ribbon panel to keep the process current and valid.
Future of VHA Lung Cancer Care
As VHA continues to prioritize resources to improve and assure optimal outcomes for veterans with lung cancer, it is now looking to create a national network of Lung Cancer Centers of Excellence (LCCE) as described in the VA Budget Submission for fiscal year 2021. If Congress approves funding, LCCEs will soon be developed within the VA regional Veteran Integrated Service Network system to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information, including data on pharmacogenomic profiles. Such a network would create more opportunities to leverage public–private partnerships similar to the VA-PALS project. Creation of LCCEs would help the VA leverage an even stronger learning network to support more research so that all veterans who are impacted by lung cancer have access to personalized care that optimizes safety, quality of life, and overall survival. The lessons learned, networks developed, and partnerships established through VA-PALS, VALOR, and VA-ROQS are instrumental toward achieving these goals.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Dawson GA, Cheuk AV, Lutz S, et al. The availability of advanced radiation oncology technology within the Veterans Health Administration radiation oncology centers. Fed Pract. 2016;33(suppl 4):18S-22S.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
4. National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
5. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT Screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
6. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi:10.1001/jamainternmed.2016.9022
7. Clancy C. Creating World-class care and service for our nation’s finest: how Veterans Health Administration Diffusion of Excellence Initiative Is innovating and transforming Veterans Affairs health care. Perm J. 2019;23:18.301. doi:10.7812/TPP/18.301
8. Elnahal SM, Clancy CM, Shulkin DJ. A framework for disseminating clinical best practices in the VA health system. JAMA. 2017;317(3):255-256. doi:10.1001/jama.2016.18764
9. Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99-105. doi:10.1016/S0140-6736(99)06093-6
10. Mulshine JL, Henschke CI. Lung cancer screening: achieving more by intervening less. Lancet Oncol. 2014;15(12):1284-1285. doi:10.1016/S1470-2045(14)70418-8
11. Henschke CI, Li K, Yip R, Salvatore M, Yankelevitz DF. The importance of the regimen of screening in maximizing the benefit and minimizing the harms. Ann Transl Med. 2016;4(8):153. doi:10.21037/atm.2016.04.06
12. Henschke CI, Yip R, Yankelevitz DF, Smith JP; International Early Lung Cancer Action Program Investigators*. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158(4):246-252. doi:10.7326/0003-4819-158-4-201302190-00004
13. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
14. Henschke CI, Yankelevitz DF, Yip R, et al. Tumor volume measurement error using computed tomography imaging in a phase II clinical trial in lung cancer. J Med Imaging (Bellingham). 2016;3(3):035505. doi:10.1117/1.JMI.3.3.035505
15. Yip R, Henschke CI, Yankelevitz DF, Boffetta P, Smith JP; International Early Lung Cancer Investigators. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24(3):201-208. doi:10.1097/CEJ.0000000000000065
16. Armato SG 3rd, McLennan G, Bidaut L, et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys. 2011;38(2):915-931. doi:10.1118/1.3528204
17. Gill RK, Vazquez MF, Kramer A, et al. The use of genetic markers to identify lung cancer in fine needle aspiration samples. Clin Cancer Res. 2008;14(22):7481-7487. doi:10.1158/1078-0432.CCR-07-5242
18. Pyenson BS, Henschke CI, Yankelevitz DF, Yip R, Dec E. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272-282.
19. Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14(1):37-44. doi:10.12788/jcso.0205
20. Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res. 2016;5(2):183-189. doi:10.21037/tlcr.2016.04.05
21. Bakaeen FG, Reda DJ, Gelijns AC, et al. Department of Veterans Affairs Cooperative Studies Program network of dedicated enrollment sites: implications for surgical trials [published correction appears in JAMA Surg. 2014 Sep;149(9):961]. JAMA Surg. 2014;149(6):507-513. doi:10.1001/jamasurg.2013.4150
22. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials [published correction appears in Lancet Oncol. 2015 Sep;16(9):e427]. Lancet Oncol. 2015;16(6):630-637. doi:10.1016/S1470-2045(15)70168-3
23. Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer. 2017;103:6-10. doi:10.1016/j.lungcan.2016.11.005
24. Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials. 2016;17(1):283. Published 2016 Jun 8. doi:10.1186/s13063-016-1391-4
25. Rooshenas L, Scott LJ, Blazeby JM, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108-120. doi:10.1016/j.jclinepi.2018.10.004
26. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance Program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi:10.1016/j.ijrobp.2019.08.064
The Veterans Health Administration (VHA) facilitates care for > 7,700 veterans with newly diagnosed lung cancer each year.1 This includes comprehensive clinical evaluations and management that are facilitated through interdisciplinary networks of pulmonologists, radiologists, thoracic surgeons, radiation oncologists, and medical oncologists. Veterans with lung cancer have access to advanced medical technologies at US Department of Veterans Affairs (VA) medical centers (VAMCs), including the latest US Food and Drug Administration (FDA)-approved targeted radiation delivery systems and novel immunotherapies, as well as precision oncology-driven clinical trials.2
Despite access to high-quality care, lung cancer remains the leading cause of cancer-related mortality among VHA enrollees as well as the US population.3 About 15 veterans die of lung cancer each day; most are diagnosed with advanced stage III or stage IV disease. To address this issue, VHA launched 3 new initiatives between 2016 and 2017 to improve outcomes for veterans impacted by lung cancer. The VA Partnership to increase Access to Lung Screening (VA-PALS) is a clinical implementation project to increase access to early detection lung screening scans at 10 VAMCs. The Veterans Affairs Lung cancer surgery Or stereotactic Radiotherapy (VALOR) is a phase 3 randomized trial that investigates the role of stereotactic body radiation therapy (SBRT) as a potential alternative to surgery for veterans with operable stage I non-small cell lung cancer (NSCLC). The VA Radiation Oncology Quality Surveillance program (VA-ROQS) established national expert-derived benchmarks for the quality assurance of lung cancer therapy.
VA-PALS
The central mission of VA-PALS is to reduce lung cancer mortality among veterans at risk by increasing access to low-dose computed tomography (LDCT) lung screening scans.4,5 The program was developed as a public-private partnership to introduce structured lung cancer screening programs at 10 VAMCs to safely manage large cohorts of veterans undergoing annual screening scans. The VA-PALS project brings together pulmonologists, radiologists, thoracic surgeons, radiation oncologists, medical oncologists, and computer scientists who have experience developing open-source electronic health record systems for VHA networks. The project was launched in 2017 after an earlier clinical demonstration project identified substantial variability and challenges with efforts to implement new lung cancer screening programs in the VA.6
Each of the 10 VA-PALS-designated lung cancer screening programs (Atlanta, Georgia; Phoenix, Arizona; Indianapolis, Indiana; Chicago, Illinois; Nashville, Tennessee; Philadelphia, Pennsylvania; St. Louis, Missouri; Denver, Colorado; Milwaukee, Wisconsin; and Cleveland, Ohio) assumes a major responsibility for ordering and evaluating the results of LDCT scans to ensure appropriate follow-up care of veterans with abnormal radiographic findings. Lung cancer screening programs are supported with a full-time navigator (nurse practitioner or physician assistant) who has received training from the VA-PALS project team with direct supervision by a local site director who is a pulmonologist, thoracic surgeon, or medical oncologist. Lung cancer screening programs establish a centralized approach that aims to reduce the burden on primary care providers for remembering to order annual baseline and repeat LDCT scans. The lung screening programs also manage radiographic findings that usually are benign to facilitate appropriate decisions to minimize the risk of unnecessary tests and procedures. Program implementation across VA-PALS sites includes a strong connection among participants through meetings, newsletters, and attendance at conferences to create a collaborative learning network, which has been shown to improve dissemination of best practices across the VHA.7,8
The International Early Lung Cancer Action Program (I-ELCAP), which pioneered the use of LDCT to reduce lung cancer mortality, is a leading partner for VA-PALS.9 This group has > 25 years of experience overcoming many of the obstacles and challenges that new lung cancer screening programs face.10 The I-ELCAP has successfully implemented new lung cancer screening programs at > 70 health care institutions worldwide. Their implementation processes provide continuous oversight for each center. As a result, the I-ELCAP team has developed a large and detailed lung cancer screening registry with > 75,000 patients enrolled globally, comprising a vast database of clinical data that has produced > 270 scientific publications focusing on improving the quality and safety of lung cancer screening.11,12
These reports have helped guide evidence-based recommendations for lung cancer screening in several countries and include standardized processes for patient counseling and smoking cessation, data acquisition and interpretation of LDCT images, and clinical management of abnormal findings to facilitate timely transition from diagnosis to treatment.13-15 The I-ELCAP management system detects 10% abnormal findings in the baseline screening study, which declines to 6% in subsequent years.12 The scientific findings from this approach have provided additional insights into technical CT scanning errors that can affect tumor nodule measurements.16 The vast amount of clinical data and expertise have helped explore genetic markers.17 The I-ELCAP has facilitated cost-effectiveness investigations to determine the value of screening, and their research portfolio includes investigations into the longer-term outcomes after primary treatment for patients with screen-detected lung cancers.18,19
I-ELCAP gifted its comprehensive clinical software management system that has been in use for the above contributions for use in the VHA through an open source agreement without licensing fees. The I-ELCAP software management system was rewritten in MUMPS, the software programming language that is used by the VA Computerized Patient Record System (CPRS). The newly adapted VA-PALS/I-ELCAP system underwent modifications with VHA clinicians’ input, and was successfully installed at the Phoenix VA Health Care System in Arizona, which has assumed a leading role for the VA-PALS project.
The VA-PALS/I-ELCAP clinical management system currently is under review by the VA Office of Information and Technology for broad distribution across the VHA through the VA Enterprise Cloud. Once in use across the VHA, the VA-PALS/I-ELCAP clinical management system will offer a longitudinal central database that can support numerous quality improvement and quality assurance initiatives, as well as innovative research projects. Research opportunities include: (1) large-scale examination of LDCT images with artificial intelligence and machine learning techniques; (2) epidemiologic investigations of environmental and genetic risk factors to better understand the high percentage of veterans diagnosed with lung cancer who were never smokers or had quit many years ago; and (3) multisite clinical trials that explore early detection blood screening tests that are under development.
The VA-PALS project is sponsored by the VHA Office of Rural Health as an enterprise-wide initiative that focuses on reaching rural veterans at risk. The project received additional support through the VA Secretary’s Center for Strategic Partnerships with a $5.8 million grant from the Bristol-Myers Squibb Foundation. The VistA (Veterans Health Information Systems and Technology Architecture) Expertise Network is an additional key partner that helped adapt the VAPALS-ELCAP system for use on VHA networks.
VALOR Trial
The VA Cooperative Studies Program (CSP) #2005 VALOR study is a randomized phase 3 clinical trial that evaluates optimal treatment for participants with operable early-stage NSCLC.20 The trial is sponsored by the CSP, which is responsible for and provides resources for the planning and conduct of large multicenter surgical and clinical trials in VHA.21 The CSP #2005 VALOR study plans to enroll veterans with stage I NSCLC who will be treated with a surgical lobectomy or SBRT according to random assignment. An alternative surgical approach with a segmentectomy is acceptable, although patients in poor health who are only qualify for a wedge resection will not be enrolled. The CSP will follow each participant for at least 5 years to evaluate which treatment, if either, results in a higher overall survival rate. Secondary outcome measures are quality of life, pulmonary function, health state utilities, patterns of failure, and causes of death.
Although the study design of the VALOR trial is relatively straightforward, recruitment of participants to similar randomized trials of surgery vs SBRT for operable stage I NSCLC outside the VA has historically been very difficult. Three earlier phase 3 trials in the Netherlands and US closed prematurely after collectively enrolling only 4% of planned participants. Although a pooled analysis of 2 of these trials demonstrated a statistically significant difference of 95% vs 79% survival in favor of SBRT at a median follow-up of 40 months, the analysis was underpowered because only 58 of the planned 1,380 participants were enrolled.22,23
The CSP #2005 VALOR study team was keenly aware of these past challenges and addressed many of the obstacles to enrollment by optimizing eligibility criteria and follow-up requirements. Enrollment sites were carefully selected after confirming equipoise between the 2 treatments, and study coordinators at each enrollment site were empowered to provide a leading role with recruitment. Multiple communication channels were established for constant contact to disseminate new best practices for recruitment as they were identified. Furthermore, a veteran-centric educational recruitment video, approved by the VA Central Institutional Review Board, was designed to help study participants better understand the purpose of participating in a clinical trial (www.vacsp.research.va.gov/CSP_2005/CSP_2005.asp).
After the first year of recruitment, researchers identified individual clinician and patient preferences as the predominant difficulty with recruitment, which was not easy to address. The CSP #2005 VALOR study team opted to partner directly with the Qualitative Research Integrated within Trials (QuinteT) team in the United Kingdom to adopt its methods to successfully support randomized clinical trials with serious recruitment challenges.24,25 By working directly with the QuinteT director, the CSP #2005 VALOR team made a major revision to the informed consent forms by shifting focus away from disclosing potential harms of research to an informative document that emphasized the purpose of the study. The work with QuinteT also led to the creation of balanced narratives for study teams to use and for potential participants to read. These provide a more consistent message that describes why the study is important and why clinicians are no longer certain that surgery is the optimal treatment for all patients with operable stage I NSCLC.
The VALOR clinical trial, opened in 2017, remains open at only 9 VAMCs. As of early 2020, it has enrolled more participants than all previous phase 3 trials combined. Once completed, the results from CSP #2005 VALOR study will help clinicians and veterans with operable stage I NSCLC better understand the tradeoffs of surgery vs SBRT as an initial treatment option. Plans are under way to expand the scope of the trial and include investigations of pretreatment radiomic signatures and genetic markers from biopsy tissue and blood samples, to better predict when surgery or SBRT might be the best treatment option for an individual patient.
VA-ROQS
The VA-ROQS was created in 2016 to compare treatment of veterans with lung cancer in the VHA with quality standards recommended by nationally recognized experts in lung cancer care. Partnering with Washington University in St. Louis, Missouri and the American Society for Radiation Oncology, the VHA established a blue-ribbon panel of experts to review clinical trial data and medical literature to provide evidence-based quality metrics for lung cancer therapy. As a result, 26 metrics applicable to each patient’s case were developed, published, and used to assess lung cancer care in each VHA radiation oncology practice.26
By 2019, the resulting data led to a report on 773 lung cancer cases accumulated from all VHA radiation oncology practices. Performance data for each quality metric were compared for each practice within the VHA, which found that VHA practices met > 80% of all 1,278 metrics scored. Quality metrics included those documented within each patient health record and the specific radiation delivery parameters that reflected each health care provider’s treatment. After team investigators visited each center and recorded treatment data, VA-ROQS is now maturing to permit continuous, electronic monitoring of all lung cancer treatment delivered within VHA. As each veteran’s case is planned, the quality of the therapy is monitored, assessed, and reported to the treating physician. Each VHA radiation oncologist will receive up-to-date evaluation of each case compared with these evidence-based quality standards. The quality standards are reviewed by the blue-ribbon panel to keep the process current and valid.
Future of VHA Lung Cancer Care
As VHA continues to prioritize resources to improve and assure optimal outcomes for veterans with lung cancer, it is now looking to create a national network of Lung Cancer Centers of Excellence (LCCE) as described in the VA Budget Submission for fiscal year 2021. If Congress approves funding, LCCEs will soon be developed within the VA regional Veteran Integrated Service Network system to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information, including data on pharmacogenomic profiles. Such a network would create more opportunities to leverage public–private partnerships similar to the VA-PALS project. Creation of LCCEs would help the VA leverage an even stronger learning network to support more research so that all veterans who are impacted by lung cancer have access to personalized care that optimizes safety, quality of life, and overall survival. The lessons learned, networks developed, and partnerships established through VA-PALS, VALOR, and VA-ROQS are instrumental toward achieving these goals.
The Veterans Health Administration (VHA) facilitates care for > 7,700 veterans with newly diagnosed lung cancer each year.1 This includes comprehensive clinical evaluations and management that are facilitated through interdisciplinary networks of pulmonologists, radiologists, thoracic surgeons, radiation oncologists, and medical oncologists. Veterans with lung cancer have access to advanced medical technologies at US Department of Veterans Affairs (VA) medical centers (VAMCs), including the latest US Food and Drug Administration (FDA)-approved targeted radiation delivery systems and novel immunotherapies, as well as precision oncology-driven clinical trials.2
Despite access to high-quality care, lung cancer remains the leading cause of cancer-related mortality among VHA enrollees as well as the US population.3 About 15 veterans die of lung cancer each day; most are diagnosed with advanced stage III or stage IV disease. To address this issue, VHA launched 3 new initiatives between 2016 and 2017 to improve outcomes for veterans impacted by lung cancer. The VA Partnership to increase Access to Lung Screening (VA-PALS) is a clinical implementation project to increase access to early detection lung screening scans at 10 VAMCs. The Veterans Affairs Lung cancer surgery Or stereotactic Radiotherapy (VALOR) is a phase 3 randomized trial that investigates the role of stereotactic body radiation therapy (SBRT) as a potential alternative to surgery for veterans with operable stage I non-small cell lung cancer (NSCLC). The VA Radiation Oncology Quality Surveillance program (VA-ROQS) established national expert-derived benchmarks for the quality assurance of lung cancer therapy.
VA-PALS
The central mission of VA-PALS is to reduce lung cancer mortality among veterans at risk by increasing access to low-dose computed tomography (LDCT) lung screening scans.4,5 The program was developed as a public-private partnership to introduce structured lung cancer screening programs at 10 VAMCs to safely manage large cohorts of veterans undergoing annual screening scans. The VA-PALS project brings together pulmonologists, radiologists, thoracic surgeons, radiation oncologists, medical oncologists, and computer scientists who have experience developing open-source electronic health record systems for VHA networks. The project was launched in 2017 after an earlier clinical demonstration project identified substantial variability and challenges with efforts to implement new lung cancer screening programs in the VA.6
Each of the 10 VA-PALS-designated lung cancer screening programs (Atlanta, Georgia; Phoenix, Arizona; Indianapolis, Indiana; Chicago, Illinois; Nashville, Tennessee; Philadelphia, Pennsylvania; St. Louis, Missouri; Denver, Colorado; Milwaukee, Wisconsin; and Cleveland, Ohio) assumes a major responsibility for ordering and evaluating the results of LDCT scans to ensure appropriate follow-up care of veterans with abnormal radiographic findings. Lung cancer screening programs are supported with a full-time navigator (nurse practitioner or physician assistant) who has received training from the VA-PALS project team with direct supervision by a local site director who is a pulmonologist, thoracic surgeon, or medical oncologist. Lung cancer screening programs establish a centralized approach that aims to reduce the burden on primary care providers for remembering to order annual baseline and repeat LDCT scans. The lung screening programs also manage radiographic findings that usually are benign to facilitate appropriate decisions to minimize the risk of unnecessary tests and procedures. Program implementation across VA-PALS sites includes a strong connection among participants through meetings, newsletters, and attendance at conferences to create a collaborative learning network, which has been shown to improve dissemination of best practices across the VHA.7,8
The International Early Lung Cancer Action Program (I-ELCAP), which pioneered the use of LDCT to reduce lung cancer mortality, is a leading partner for VA-PALS.9 This group has > 25 years of experience overcoming many of the obstacles and challenges that new lung cancer screening programs face.10 The I-ELCAP has successfully implemented new lung cancer screening programs at > 70 health care institutions worldwide. Their implementation processes provide continuous oversight for each center. As a result, the I-ELCAP team has developed a large and detailed lung cancer screening registry with > 75,000 patients enrolled globally, comprising a vast database of clinical data that has produced > 270 scientific publications focusing on improving the quality and safety of lung cancer screening.11,12
These reports have helped guide evidence-based recommendations for lung cancer screening in several countries and include standardized processes for patient counseling and smoking cessation, data acquisition and interpretation of LDCT images, and clinical management of abnormal findings to facilitate timely transition from diagnosis to treatment.13-15 The I-ELCAP management system detects 10% abnormal findings in the baseline screening study, which declines to 6% in subsequent years.12 The scientific findings from this approach have provided additional insights into technical CT scanning errors that can affect tumor nodule measurements.16 The vast amount of clinical data and expertise have helped explore genetic markers.17 The I-ELCAP has facilitated cost-effectiveness investigations to determine the value of screening, and their research portfolio includes investigations into the longer-term outcomes after primary treatment for patients with screen-detected lung cancers.18,19
I-ELCAP gifted its comprehensive clinical software management system that has been in use for the above contributions for use in the VHA through an open source agreement without licensing fees. The I-ELCAP software management system was rewritten in MUMPS, the software programming language that is used by the VA Computerized Patient Record System (CPRS). The newly adapted VA-PALS/I-ELCAP system underwent modifications with VHA clinicians’ input, and was successfully installed at the Phoenix VA Health Care System in Arizona, which has assumed a leading role for the VA-PALS project.
The VA-PALS/I-ELCAP clinical management system currently is under review by the VA Office of Information and Technology for broad distribution across the VHA through the VA Enterprise Cloud. Once in use across the VHA, the VA-PALS/I-ELCAP clinical management system will offer a longitudinal central database that can support numerous quality improvement and quality assurance initiatives, as well as innovative research projects. Research opportunities include: (1) large-scale examination of LDCT images with artificial intelligence and machine learning techniques; (2) epidemiologic investigations of environmental and genetic risk factors to better understand the high percentage of veterans diagnosed with lung cancer who were never smokers or had quit many years ago; and (3) multisite clinical trials that explore early detection blood screening tests that are under development.
The VA-PALS project is sponsored by the VHA Office of Rural Health as an enterprise-wide initiative that focuses on reaching rural veterans at risk. The project received additional support through the VA Secretary’s Center for Strategic Partnerships with a $5.8 million grant from the Bristol-Myers Squibb Foundation. The VistA (Veterans Health Information Systems and Technology Architecture) Expertise Network is an additional key partner that helped adapt the VAPALS-ELCAP system for use on VHA networks.
VALOR Trial
The VA Cooperative Studies Program (CSP) #2005 VALOR study is a randomized phase 3 clinical trial that evaluates optimal treatment for participants with operable early-stage NSCLC.20 The trial is sponsored by the CSP, which is responsible for and provides resources for the planning and conduct of large multicenter surgical and clinical trials in VHA.21 The CSP #2005 VALOR study plans to enroll veterans with stage I NSCLC who will be treated with a surgical lobectomy or SBRT according to random assignment. An alternative surgical approach with a segmentectomy is acceptable, although patients in poor health who are only qualify for a wedge resection will not be enrolled. The CSP will follow each participant for at least 5 years to evaluate which treatment, if either, results in a higher overall survival rate. Secondary outcome measures are quality of life, pulmonary function, health state utilities, patterns of failure, and causes of death.
Although the study design of the VALOR trial is relatively straightforward, recruitment of participants to similar randomized trials of surgery vs SBRT for operable stage I NSCLC outside the VA has historically been very difficult. Three earlier phase 3 trials in the Netherlands and US closed prematurely after collectively enrolling only 4% of planned participants. Although a pooled analysis of 2 of these trials demonstrated a statistically significant difference of 95% vs 79% survival in favor of SBRT at a median follow-up of 40 months, the analysis was underpowered because only 58 of the planned 1,380 participants were enrolled.22,23
The CSP #2005 VALOR study team was keenly aware of these past challenges and addressed many of the obstacles to enrollment by optimizing eligibility criteria and follow-up requirements. Enrollment sites were carefully selected after confirming equipoise between the 2 treatments, and study coordinators at each enrollment site were empowered to provide a leading role with recruitment. Multiple communication channels were established for constant contact to disseminate new best practices for recruitment as they were identified. Furthermore, a veteran-centric educational recruitment video, approved by the VA Central Institutional Review Board, was designed to help study participants better understand the purpose of participating in a clinical trial (www.vacsp.research.va.gov/CSP_2005/CSP_2005.asp).
After the first year of recruitment, researchers identified individual clinician and patient preferences as the predominant difficulty with recruitment, which was not easy to address. The CSP #2005 VALOR study team opted to partner directly with the Qualitative Research Integrated within Trials (QuinteT) team in the United Kingdom to adopt its methods to successfully support randomized clinical trials with serious recruitment challenges.24,25 By working directly with the QuinteT director, the CSP #2005 VALOR team made a major revision to the informed consent forms by shifting focus away from disclosing potential harms of research to an informative document that emphasized the purpose of the study. The work with QuinteT also led to the creation of balanced narratives for study teams to use and for potential participants to read. These provide a more consistent message that describes why the study is important and why clinicians are no longer certain that surgery is the optimal treatment for all patients with operable stage I NSCLC.
The VALOR clinical trial, opened in 2017, remains open at only 9 VAMCs. As of early 2020, it has enrolled more participants than all previous phase 3 trials combined. Once completed, the results from CSP #2005 VALOR study will help clinicians and veterans with operable stage I NSCLC better understand the tradeoffs of surgery vs SBRT as an initial treatment option. Plans are under way to expand the scope of the trial and include investigations of pretreatment radiomic signatures and genetic markers from biopsy tissue and blood samples, to better predict when surgery or SBRT might be the best treatment option for an individual patient.
VA-ROQS
The VA-ROQS was created in 2016 to compare treatment of veterans with lung cancer in the VHA with quality standards recommended by nationally recognized experts in lung cancer care. Partnering with Washington University in St. Louis, Missouri and the American Society for Radiation Oncology, the VHA established a blue-ribbon panel of experts to review clinical trial data and medical literature to provide evidence-based quality metrics for lung cancer therapy. As a result, 26 metrics applicable to each patient’s case were developed, published, and used to assess lung cancer care in each VHA radiation oncology practice.26
By 2019, the resulting data led to a report on 773 lung cancer cases accumulated from all VHA radiation oncology practices. Performance data for each quality metric were compared for each practice within the VHA, which found that VHA practices met > 80% of all 1,278 metrics scored. Quality metrics included those documented within each patient health record and the specific radiation delivery parameters that reflected each health care provider’s treatment. After team investigators visited each center and recorded treatment data, VA-ROQS is now maturing to permit continuous, electronic monitoring of all lung cancer treatment delivered within VHA. As each veteran’s case is planned, the quality of the therapy is monitored, assessed, and reported to the treating physician. Each VHA radiation oncologist will receive up-to-date evaluation of each case compared with these evidence-based quality standards. The quality standards are reviewed by the blue-ribbon panel to keep the process current and valid.
Future of VHA Lung Cancer Care
As VHA continues to prioritize resources to improve and assure optimal outcomes for veterans with lung cancer, it is now looking to create a national network of Lung Cancer Centers of Excellence (LCCE) as described in the VA Budget Submission for fiscal year 2021. If Congress approves funding, LCCEs will soon be developed within the VA regional Veteran Integrated Service Network system to ensure that treatment decisions for veterans with lung cancer are based on all available molecular information, including data on pharmacogenomic profiles. Such a network would create more opportunities to leverage public–private partnerships similar to the VA-PALS project. Creation of LCCEs would help the VA leverage an even stronger learning network to support more research so that all veterans who are impacted by lung cancer have access to personalized care that optimizes safety, quality of life, and overall survival. The lessons learned, networks developed, and partnerships established through VA-PALS, VALOR, and VA-ROQS are instrumental toward achieving these goals.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Dawson GA, Cheuk AV, Lutz S, et al. The availability of advanced radiation oncology technology within the Veterans Health Administration radiation oncology centers. Fed Pract. 2016;33(suppl 4):18S-22S.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
4. National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
5. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT Screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
6. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi:10.1001/jamainternmed.2016.9022
7. Clancy C. Creating World-class care and service for our nation’s finest: how Veterans Health Administration Diffusion of Excellence Initiative Is innovating and transforming Veterans Affairs health care. Perm J. 2019;23:18.301. doi:10.7812/TPP/18.301
8. Elnahal SM, Clancy CM, Shulkin DJ. A framework for disseminating clinical best practices in the VA health system. JAMA. 2017;317(3):255-256. doi:10.1001/jama.2016.18764
9. Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99-105. doi:10.1016/S0140-6736(99)06093-6
10. Mulshine JL, Henschke CI. Lung cancer screening: achieving more by intervening less. Lancet Oncol. 2014;15(12):1284-1285. doi:10.1016/S1470-2045(14)70418-8
11. Henschke CI, Li K, Yip R, Salvatore M, Yankelevitz DF. The importance of the regimen of screening in maximizing the benefit and minimizing the harms. Ann Transl Med. 2016;4(8):153. doi:10.21037/atm.2016.04.06
12. Henschke CI, Yip R, Yankelevitz DF, Smith JP; International Early Lung Cancer Action Program Investigators*. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158(4):246-252. doi:10.7326/0003-4819-158-4-201302190-00004
13. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
14. Henschke CI, Yankelevitz DF, Yip R, et al. Tumor volume measurement error using computed tomography imaging in a phase II clinical trial in lung cancer. J Med Imaging (Bellingham). 2016;3(3):035505. doi:10.1117/1.JMI.3.3.035505
15. Yip R, Henschke CI, Yankelevitz DF, Boffetta P, Smith JP; International Early Lung Cancer Investigators. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24(3):201-208. doi:10.1097/CEJ.0000000000000065
16. Armato SG 3rd, McLennan G, Bidaut L, et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys. 2011;38(2):915-931. doi:10.1118/1.3528204
17. Gill RK, Vazquez MF, Kramer A, et al. The use of genetic markers to identify lung cancer in fine needle aspiration samples. Clin Cancer Res. 2008;14(22):7481-7487. doi:10.1158/1078-0432.CCR-07-5242
18. Pyenson BS, Henschke CI, Yankelevitz DF, Yip R, Dec E. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272-282.
19. Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14(1):37-44. doi:10.12788/jcso.0205
20. Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res. 2016;5(2):183-189. doi:10.21037/tlcr.2016.04.05
21. Bakaeen FG, Reda DJ, Gelijns AC, et al. Department of Veterans Affairs Cooperative Studies Program network of dedicated enrollment sites: implications for surgical trials [published correction appears in JAMA Surg. 2014 Sep;149(9):961]. JAMA Surg. 2014;149(6):507-513. doi:10.1001/jamasurg.2013.4150
22. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials [published correction appears in Lancet Oncol. 2015 Sep;16(9):e427]. Lancet Oncol. 2015;16(6):630-637. doi:10.1016/S1470-2045(15)70168-3
23. Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer. 2017;103:6-10. doi:10.1016/j.lungcan.2016.11.005
24. Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials. 2016;17(1):283. Published 2016 Jun 8. doi:10.1186/s13063-016-1391-4
25. Rooshenas L, Scott LJ, Blazeby JM, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108-120. doi:10.1016/j.jclinepi.2018.10.004
26. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance Program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi:10.1016/j.ijrobp.2019.08.064
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701. doi:10.7205/milmed-d-11-00434
2. Dawson GA, Cheuk AV, Lutz S, et al. The availability of advanced radiation oncology technology within the Veterans Health Administration radiation oncology centers. Fed Pract. 2016;33(suppl 4):18S-22S.
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi:10.3322/caac.21551
4. National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the National Lung Screening Trial. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
5. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT Screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. doi:10.1056/NEJMoa1911793
6. Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the Veterans Health Administration. JAMA Intern Med. 2017;177(3):399-406. doi:10.1001/jamainternmed.2016.9022
7. Clancy C. Creating World-class care and service for our nation’s finest: how Veterans Health Administration Diffusion of Excellence Initiative Is innovating and transforming Veterans Affairs health care. Perm J. 2019;23:18.301. doi:10.7812/TPP/18.301
8. Elnahal SM, Clancy CM, Shulkin DJ. A framework for disseminating clinical best practices in the VA health system. JAMA. 2017;317(3):255-256. doi:10.1001/jama.2016.18764
9. Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;354(9173):99-105. doi:10.1016/S0140-6736(99)06093-6
10. Mulshine JL, Henschke CI. Lung cancer screening: achieving more by intervening less. Lancet Oncol. 2014;15(12):1284-1285. doi:10.1016/S1470-2045(14)70418-8
11. Henschke CI, Li K, Yip R, Salvatore M, Yankelevitz DF. The importance of the regimen of screening in maximizing the benefit and minimizing the harms. Ann Transl Med. 2016;4(8):153. doi:10.21037/atm.2016.04.06
12. Henschke CI, Yip R, Yankelevitz DF, Smith JP; International Early Lung Cancer Action Program Investigators*. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2013;158(4):246-252. doi:10.7326/0003-4819-158-4-201302190-00004
13. Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and perceptions about smoking cessation in the context of lung cancer screening. JAMA Intern Med. 2015;175(9):1530-1537. doi:10.1001/jamainternmed.2015.3558
14. Henschke CI, Yankelevitz DF, Yip R, et al. Tumor volume measurement error using computed tomography imaging in a phase II clinical trial in lung cancer. J Med Imaging (Bellingham). 2016;3(3):035505. doi:10.1117/1.JMI.3.3.035505
15. Yip R, Henschke CI, Yankelevitz DF, Boffetta P, Smith JP; International Early Lung Cancer Investigators. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev. 2015;24(3):201-208. doi:10.1097/CEJ.0000000000000065
16. Armato SG 3rd, McLennan G, Bidaut L, et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): a completed reference database of lung nodules on CT scans. Med Phys. 2011;38(2):915-931. doi:10.1118/1.3528204
17. Gill RK, Vazquez MF, Kramer A, et al. The use of genetic markers to identify lung cancer in fine needle aspiration samples. Clin Cancer Res. 2008;14(22):7481-7487. doi:10.1158/1078-0432.CCR-07-5242
18. Pyenson BS, Henschke CI, Yankelevitz DF, Yip R, Dec E. Offering lung cancer screening to high-risk medicare beneficiaries saves lives and is cost-effective: an actuarial analysis. Am Health Drug Benefits. 2014;7(5):272-282.
19. Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol. 2016;14(1):37-44. doi:10.12788/jcso.0205
20. Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res. 2016;5(2):183-189. doi:10.21037/tlcr.2016.04.05
21. Bakaeen FG, Reda DJ, Gelijns AC, et al. Department of Veterans Affairs Cooperative Studies Program network of dedicated enrollment sites: implications for surgical trials [published correction appears in JAMA Surg. 2014 Sep;149(9):961]. JAMA Surg. 2014;149(6):507-513. doi:10.1001/jamasurg.2013.4150
22. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials [published correction appears in Lancet Oncol. 2015 Sep;16(9):e427]. Lancet Oncol. 2015;16(6):630-637. doi:10.1016/S1470-2045(15)70168-3
23. Samson P, Keogan K, Crabtree T, et al. Interpreting survival data from clinical trials of surgery versus stereotactic body radiation therapy in operable Stage I non-small cell lung cancer patients. Lung Cancer. 2017;103:6-10. doi:10.1016/j.lungcan.2016.11.005
24. Donovan JL, Rooshenas L, Jepson M, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials. 2016;17(1):283. Published 2016 Jun 8. doi:10.1186/s13063-016-1391-4
25. Rooshenas L, Scott LJ, Blazeby JM, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108-120. doi:10.1016/j.jclinepi.2018.10.004
26. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance Program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi:10.1016/j.ijrobp.2019.08.064
Lung Cancer in the VA at a National Level
The Availability of Advanced Radiation Oncology Technology Within VHA Radiation Oncology Centers
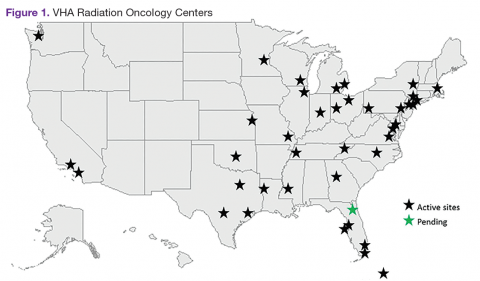
The VHA is the primary care provider for 20.4% of the more than 21.9 million military veterans.1 Surveys report that over a lifetime, an estimated 28.4% of U.S. veterans will receive some measure of their health care from the VHA.2 An estimated 40,000 new cancer cases are diagnosed each year from these veterans, resulting in a minimum of 175,000 veterans receiving cancer care in VHA facilities.3 The 39 VHA facilities currently with onsite radiation oncology practices annually provide radiation therapy to about 20,000 veterans (Figure 1).
Nationally, tumor control and toxicity outcomes have each improved over recent decades as advances have occurred in imaging, radiation treatment planning, and equipment for the delivery of radiotherapy.4 The VHA has kept pace with these technological advancements to the point where image-guided radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT), and stereotactic body radiotherapy (SBRT) are widely available at VHA centers. Additionally, all active VHA radiation oncology centers have earned accreditation from the American College of Radiology, while 3 new centers are in the process of gaining accreditation.
When technologies deemed to be medically necessary are not available onsite, these treatments are made available to veterans through referral to other VHA or non-VHA centers. Here, the authors present the results of a survey of VHA-based radiation oncologists to evaluate onsite availability of various radiation technologies.
Methods
The VHA Palliative Radiotherapy Task Force constructed an online survey and sent it to the 82 radiation oncologists practicing at the 38 VHA radiation oncology centers that were active at the time. After emailing the survey,follow-up phone calls were made to maximize response rates. The survey was conducted during the months of May and June of 2014.
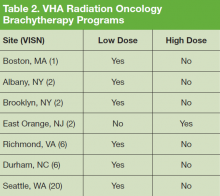
In this survey, all 82 VHA radiation oncologists were queried on the availability of advanced radiation delivery technologies including IGRT, IMRT, and SBRT at their facilities. The authors also surveyed for presence of brachytherapy (BT) programs, stereotactic radiosurgery (SRS), and cone-beam computed tomography (CBCT). Information was collected regarding the extent to which physicians can treat cases requiring SRS and/or SBRT onsite vs through referral to another facility for treatment. These data were gathered from a survey conducted in conjunction with a larger survey on the practice and patterns of care in the treatment of patients with brain metastases within the VHA.5,6 The data presented here apply to radiation therapy in general and are not limited to the treatment of brain metastases.
Results
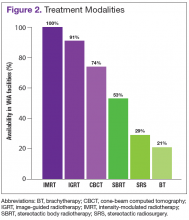
The overall response rate was 76% (62 of 82 radiation oncologists). At the time of the survey, 90% (34 of 38) of active VHA radiation oncology treatment facilities were represented. However as of May 2016, there are 40 active VHA radiation oncology centers. Figure 2 describes the availability of various treatment delivery systems. The data demonstrated 100% availability of IMRT. Respondents reported onsite availability of IGRT at 91%, CBCT at 74%, and SBRT at 53%. Treatment technologies that were not as widely available at VHA facilities with inherent radiation oncology practices included SRS at 29% and BT at 21%. For cases requiring SRS, 69% (40 of 58) of respondents who answered this question indicated that they refer patients to other VHA radiation oncology centers or VHA contracted private entities. This report is limited by the following factors:
- A narrow scope of practices was surveyed. The survey was solely sent to VHA physicians at 38 active VHA radiation oncology centers out of 144 VHA hospitals. Therefore the practices at VHA medical centers without active VHA radiation was not acquired with this survey.
- This survey only addresses availability of these newer treatment technologies, not their actual use, in treating cancers predominant within the VHA.
- Literature comparison in this report is based on current use of these technologies for some of the reports cited, rather than availability as this report reflects. As such, direct comparisons could be misleading.
Discussion
Although the total number of veterans has been decreasing in recent years, the number of veterans enrolling into VHA-related programs has been increasing and is expected to expand increase further in years to come.1,2 It is important for radiation oncologists to keep pace with new technologies to ensure their patients have access to the best possible treatments.
Advances in radiation oncology have allowed radiotherapy to evolve from the 2-dimensional treatments of the 1950s to the 1980s, to more targeted treatments that employ advanced imaging and complex planning. Modern techniques for delivery of radiotherapy are better at confining radiation dose to the tumor volume while minimizing the irradiation of normal structures. The use of cumbersome blocks, wedges, and tissue compensators has given way to treatment with internal collimation techniques such as IMRT, SBRT, and SRS. These techniques rely heavily on image guidance for tumor targeting. Four-dimensional planning and treatment allow radiation oncologists to track tumor and normal tissue motion, thereby increasing the accuracy and precision of radiation treatments.
As is true in the community, IMRT and IGRT are widely available within the VHA. According to a survey by Simpson and colleagues evaluating the use of IGRT in the U.S., 93% of radiation oncologists use IGRT.7 Similarly, the survey presented here demonstrates that 91% of VHA radiation oncologists report availability of IGRT at their centers. All VHA radiation oncologists surveyed report access to IMRT.
Shen’s recent report evaluating radiotherapy patterns of practice from 2002 to 2010 examined volume of payments for treatment delivery by codes for office-based IMRT.8 These authors noted an increase in the usage of IMRT as a percentage of external beam radiotherapy from 2002 to 2010 of 0% to 70%, respectively. They further noted during this period that IGRT use, based on total payments for treatment delivery, increased from 2.1% to 11.1%.
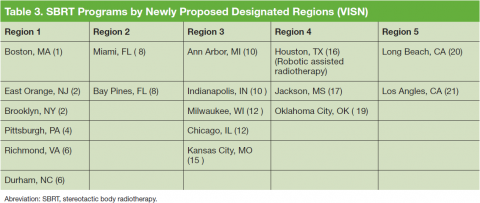
The reported use of onsite SBRT among VHA physicians is slightly less than that of community physicians. A survey study by Pan and colleagues demonstrated that 63.9% of U.S. radiation oncologists use SBRT, while in the survey study presented here, 53% of VHA radiation oncologists reported availability of onsite SBRT.9 Of note, the lack of availability of onsite SBRT at VHA centers does not preclude treatment with SBRT when medically necessary. These cases can be referred to other VHA or community centers with the requisite accreditation credentials. Because of the increasing use of SBRT and related technologies in the treatment of some cancers, an improved availability of SBRT in the future within the VHA will allow for some centers to participate in the Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR) trial, which was approved for open recruitment in 2015.
Although BT and SRS are not as widely available within the VHA as other evaluated technologies such as IGRT and IMRT, their availability mirrors a similar limited availability in the community.10-12 When necessary these services also can be provided for veterans through referral to other VHA or non-VHA centers.
The benefit of charged particle radiotherapy, such as proton beam radiotherapy, is limited to specific cancers.13 This technology is not widely available in the community or within the VHA. Because of a VHA policy currently in place permitting non-VHA care when needed, veterans who require treatment with charged particle radiotherapy are referred to accredited non-VHA radiation oncology centers when indicated.
Conclusion
In this survey, 92% of the VHA radiation oncology centers are accredited by the American College of Radiology. Further, VHA radiation oncologists respondents reported availability of treatment technologies in line with responses of physicians from community based surveys. The majority of VHA radiation oncologists report access to IMRT, IGRT, CBCT, and SBRT. While BT and SRS are not available onsite at the majority of the 40 VHA radiation oncology centers, this mirrors limited availability and use of these technologies in the community as well.
Acknowledgments
This article was based on a presentation at the ASCO Quality Care Symposium (October 17-18, 2014) in Boston, Massachusetts. Dawson GA, Cheuk AV, Jolly S, et al. Advanced radiation oncology technology within the Veterans Health Administration (VHA). J Clin Oncol. 214;32(suppl 30):52.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. National Center for Veterans Analysis and Statistics. 2010 National Survey of Veterans: reported plan to use VA health care in the future. U.S. Department of Veteran Affairs website. http://www.va.gov/vetdata/docs/QuickFacts/2010NSV_Quick_Fact_Final.pdf. Published December 2011. Accessed April 4, 2016.
2. National Center for Veterans Analysis and Statistics. 2010 National Survey Veterans: enrollment and usage of VA benefits and services. U.S. Department of Veteran Affairs website. http://www.va.gov/vedata/docs/quickfacts/Surveys-slideshow.pdf. Published August 15, 2011. Accessed April 4, 2016.
3. Zulling LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Mil Med. 2012;177(6):693-701.
4. International Atomic Energy Agency. Recent developments in the technology of radiation oncology. International Atomic Energy Agency website. https://www.iaea.org/About/Policy/GC/GC55/GC55InfDocuments/English/gc55inf-5-att1_en.pdf. Accessed April 4, 2016.
5. Dawson GA, Jolly S, Fosmire H, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P114) radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: supportive measures (Part 1 of 2 reports). Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain#sthash.fcB6idE7.dpuf. Published April 30, 2015. Accessed April 4, 2016.
6. Cheuk AV, Gutt R, Moghanaki D, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P118) Radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: part 2 clinical treatment patterns. Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain-part-2#sthash.fwW0g1RZ.dpuf. Published April 30, 2015. Accessed April 4, 2016.
7. Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey on the use of image-guided radiotherapy in the United States. Cancer. 2010;116(16):3953–3960.
8. Shen X, Showalter TN, Mishra MV, et al. Radiation oncology services in the modern era: evolving patterns of usage and payments in the office setting for medicare patients from 2000 to 2010. J Oncol Pract. 2014;10(4):e201-e207.
9. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117(19):4566-4572.
10. Mahmood U, Pugh T, Frank S, et al. Declining u se of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13(2):157-162.
11. Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109-e116.
12. Kong FM, Cuneo KC, Wang L, et al. Patterns of practice in radiation therapy for non-small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4(2):e133-e141.
13. Trikalinos TA, Terasawa T, Ip S, Raman G, Lau J. Particle Beam Radiation Therapies for Cancer. Technical Brief, No. 1. Rockville, MD: Agency for Healthcare Research and Quality; 2009.
The VHA is the primary care provider for 20.4% of the more than 21.9 million military veterans.1 Surveys report that over a lifetime, an estimated 28.4% of U.S. veterans will receive some measure of their health care from the VHA.2 An estimated 40,000 new cancer cases are diagnosed each year from these veterans, resulting in a minimum of 175,000 veterans receiving cancer care in VHA facilities.3 The 39 VHA facilities currently with onsite radiation oncology practices annually provide radiation therapy to about 20,000 veterans (Figure 1).
Nationally, tumor control and toxicity outcomes have each improved over recent decades as advances have occurred in imaging, radiation treatment planning, and equipment for the delivery of radiotherapy.4 The VHA has kept pace with these technological advancements to the point where image-guided radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT), and stereotactic body radiotherapy (SBRT) are widely available at VHA centers. Additionally, all active VHA radiation oncology centers have earned accreditation from the American College of Radiology, while 3 new centers are in the process of gaining accreditation.
When technologies deemed to be medically necessary are not available onsite, these treatments are made available to veterans through referral to other VHA or non-VHA centers. Here, the authors present the results of a survey of VHA-based radiation oncologists to evaluate onsite availability of various radiation technologies.
Methods
The VHA Palliative Radiotherapy Task Force constructed an online survey and sent it to the 82 radiation oncologists practicing at the 38 VHA radiation oncology centers that were active at the time. After emailing the survey,follow-up phone calls were made to maximize response rates. The survey was conducted during the months of May and June of 2014.
In this survey, all 82 VHA radiation oncologists were queried on the availability of advanced radiation delivery technologies including IGRT, IMRT, and SBRT at their facilities. The authors also surveyed for presence of brachytherapy (BT) programs, stereotactic radiosurgery (SRS), and cone-beam computed tomography (CBCT). Information was collected regarding the extent to which physicians can treat cases requiring SRS and/or SBRT onsite vs through referral to another facility for treatment. These data were gathered from a survey conducted in conjunction with a larger survey on the practice and patterns of care in the treatment of patients with brain metastases within the VHA.5,6 The data presented here apply to radiation therapy in general and are not limited to the treatment of brain metastases.
Results
The overall response rate was 76% (62 of 82 radiation oncologists). At the time of the survey, 90% (34 of 38) of active VHA radiation oncology treatment facilities were represented. However as of May 2016, there are 40 active VHA radiation oncology centers. Figure 2 describes the availability of various treatment delivery systems. The data demonstrated 100% availability of IMRT. Respondents reported onsite availability of IGRT at 91%, CBCT at 74%, and SBRT at 53%. Treatment technologies that were not as widely available at VHA facilities with inherent radiation oncology practices included SRS at 29% and BT at 21%. For cases requiring SRS, 69% (40 of 58) of respondents who answered this question indicated that they refer patients to other VHA radiation oncology centers or VHA contracted private entities. This report is limited by the following factors:
- A narrow scope of practices was surveyed. The survey was solely sent to VHA physicians at 38 active VHA radiation oncology centers out of 144 VHA hospitals. Therefore the practices at VHA medical centers without active VHA radiation was not acquired with this survey.
- This survey only addresses availability of these newer treatment technologies, not their actual use, in treating cancers predominant within the VHA.
- Literature comparison in this report is based on current use of these technologies for some of the reports cited, rather than availability as this report reflects. As such, direct comparisons could be misleading.
Discussion
Although the total number of veterans has been decreasing in recent years, the number of veterans enrolling into VHA-related programs has been increasing and is expected to expand increase further in years to come.1,2 It is important for radiation oncologists to keep pace with new technologies to ensure their patients have access to the best possible treatments.
Advances in radiation oncology have allowed radiotherapy to evolve from the 2-dimensional treatments of the 1950s to the 1980s, to more targeted treatments that employ advanced imaging and complex planning. Modern techniques for delivery of radiotherapy are better at confining radiation dose to the tumor volume while minimizing the irradiation of normal structures. The use of cumbersome blocks, wedges, and tissue compensators has given way to treatment with internal collimation techniques such as IMRT, SBRT, and SRS. These techniques rely heavily on image guidance for tumor targeting. Four-dimensional planning and treatment allow radiation oncologists to track tumor and normal tissue motion, thereby increasing the accuracy and precision of radiation treatments.
As is true in the community, IMRT and IGRT are widely available within the VHA. According to a survey by Simpson and colleagues evaluating the use of IGRT in the U.S., 93% of radiation oncologists use IGRT.7 Similarly, the survey presented here demonstrates that 91% of VHA radiation oncologists report availability of IGRT at their centers. All VHA radiation oncologists surveyed report access to IMRT.
Shen’s recent report evaluating radiotherapy patterns of practice from 2002 to 2010 examined volume of payments for treatment delivery by codes for office-based IMRT.8 These authors noted an increase in the usage of IMRT as a percentage of external beam radiotherapy from 2002 to 2010 of 0% to 70%, respectively. They further noted during this period that IGRT use, based on total payments for treatment delivery, increased from 2.1% to 11.1%.
The reported use of onsite SBRT among VHA physicians is slightly less than that of community physicians. A survey study by Pan and colleagues demonstrated that 63.9% of U.S. radiation oncologists use SBRT, while in the survey study presented here, 53% of VHA radiation oncologists reported availability of onsite SBRT.9 Of note, the lack of availability of onsite SBRT at VHA centers does not preclude treatment with SBRT when medically necessary. These cases can be referred to other VHA or community centers with the requisite accreditation credentials. Because of the increasing use of SBRT and related technologies in the treatment of some cancers, an improved availability of SBRT in the future within the VHA will allow for some centers to participate in the Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR) trial, which was approved for open recruitment in 2015.
Although BT and SRS are not as widely available within the VHA as other evaluated technologies such as IGRT and IMRT, their availability mirrors a similar limited availability in the community.10-12 When necessary these services also can be provided for veterans through referral to other VHA or non-VHA centers.
The benefit of charged particle radiotherapy, such as proton beam radiotherapy, is limited to specific cancers.13 This technology is not widely available in the community or within the VHA. Because of a VHA policy currently in place permitting non-VHA care when needed, veterans who require treatment with charged particle radiotherapy are referred to accredited non-VHA radiation oncology centers when indicated.
Conclusion
In this survey, 92% of the VHA radiation oncology centers are accredited by the American College of Radiology. Further, VHA radiation oncologists respondents reported availability of treatment technologies in line with responses of physicians from community based surveys. The majority of VHA radiation oncologists report access to IMRT, IGRT, CBCT, and SBRT. While BT and SRS are not available onsite at the majority of the 40 VHA radiation oncology centers, this mirrors limited availability and use of these technologies in the community as well.
Acknowledgments
This article was based on a presentation at the ASCO Quality Care Symposium (October 17-18, 2014) in Boston, Massachusetts. Dawson GA, Cheuk AV, Jolly S, et al. Advanced radiation oncology technology within the Veterans Health Administration (VHA). J Clin Oncol. 214;32(suppl 30):52.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
The VHA is the primary care provider for 20.4% of the more than 21.9 million military veterans.1 Surveys report that over a lifetime, an estimated 28.4% of U.S. veterans will receive some measure of their health care from the VHA.2 An estimated 40,000 new cancer cases are diagnosed each year from these veterans, resulting in a minimum of 175,000 veterans receiving cancer care in VHA facilities.3 The 39 VHA facilities currently with onsite radiation oncology practices annually provide radiation therapy to about 20,000 veterans (Figure 1).
Nationally, tumor control and toxicity outcomes have each improved over recent decades as advances have occurred in imaging, radiation treatment planning, and equipment for the delivery of radiotherapy.4 The VHA has kept pace with these technological advancements to the point where image-guided radiotherapy (IGRT), intensity-modulated radiotherapy (IMRT), and stereotactic body radiotherapy (SBRT) are widely available at VHA centers. Additionally, all active VHA radiation oncology centers have earned accreditation from the American College of Radiology, while 3 new centers are in the process of gaining accreditation.
When technologies deemed to be medically necessary are not available onsite, these treatments are made available to veterans through referral to other VHA or non-VHA centers. Here, the authors present the results of a survey of VHA-based radiation oncologists to evaluate onsite availability of various radiation technologies.
Methods
The VHA Palliative Radiotherapy Task Force constructed an online survey and sent it to the 82 radiation oncologists practicing at the 38 VHA radiation oncology centers that were active at the time. After emailing the survey,follow-up phone calls were made to maximize response rates. The survey was conducted during the months of May and June of 2014.
In this survey, all 82 VHA radiation oncologists were queried on the availability of advanced radiation delivery technologies including IGRT, IMRT, and SBRT at their facilities. The authors also surveyed for presence of brachytherapy (BT) programs, stereotactic radiosurgery (SRS), and cone-beam computed tomography (CBCT). Information was collected regarding the extent to which physicians can treat cases requiring SRS and/or SBRT onsite vs through referral to another facility for treatment. These data were gathered from a survey conducted in conjunction with a larger survey on the practice and patterns of care in the treatment of patients with brain metastases within the VHA.5,6 The data presented here apply to radiation therapy in general and are not limited to the treatment of brain metastases.
Results
The overall response rate was 76% (62 of 82 radiation oncologists). At the time of the survey, 90% (34 of 38) of active VHA radiation oncology treatment facilities were represented. However as of May 2016, there are 40 active VHA radiation oncology centers. Figure 2 describes the availability of various treatment delivery systems. The data demonstrated 100% availability of IMRT. Respondents reported onsite availability of IGRT at 91%, CBCT at 74%, and SBRT at 53%. Treatment technologies that were not as widely available at VHA facilities with inherent radiation oncology practices included SRS at 29% and BT at 21%. For cases requiring SRS, 69% (40 of 58) of respondents who answered this question indicated that they refer patients to other VHA radiation oncology centers or VHA contracted private entities. This report is limited by the following factors:
- A narrow scope of practices was surveyed. The survey was solely sent to VHA physicians at 38 active VHA radiation oncology centers out of 144 VHA hospitals. Therefore the practices at VHA medical centers without active VHA radiation was not acquired with this survey.
- This survey only addresses availability of these newer treatment technologies, not their actual use, in treating cancers predominant within the VHA.
- Literature comparison in this report is based on current use of these technologies for some of the reports cited, rather than availability as this report reflects. As such, direct comparisons could be misleading.
Discussion
Although the total number of veterans has been decreasing in recent years, the number of veterans enrolling into VHA-related programs has been increasing and is expected to expand increase further in years to come.1,2 It is important for radiation oncologists to keep pace with new technologies to ensure their patients have access to the best possible treatments.
Advances in radiation oncology have allowed radiotherapy to evolve from the 2-dimensional treatments of the 1950s to the 1980s, to more targeted treatments that employ advanced imaging and complex planning. Modern techniques for delivery of radiotherapy are better at confining radiation dose to the tumor volume while minimizing the irradiation of normal structures. The use of cumbersome blocks, wedges, and tissue compensators has given way to treatment with internal collimation techniques such as IMRT, SBRT, and SRS. These techniques rely heavily on image guidance for tumor targeting. Four-dimensional planning and treatment allow radiation oncologists to track tumor and normal tissue motion, thereby increasing the accuracy and precision of radiation treatments.
As is true in the community, IMRT and IGRT are widely available within the VHA. According to a survey by Simpson and colleagues evaluating the use of IGRT in the U.S., 93% of radiation oncologists use IGRT.7 Similarly, the survey presented here demonstrates that 91% of VHA radiation oncologists report availability of IGRT at their centers. All VHA radiation oncologists surveyed report access to IMRT.
Shen’s recent report evaluating radiotherapy patterns of practice from 2002 to 2010 examined volume of payments for treatment delivery by codes for office-based IMRT.8 These authors noted an increase in the usage of IMRT as a percentage of external beam radiotherapy from 2002 to 2010 of 0% to 70%, respectively. They further noted during this period that IGRT use, based on total payments for treatment delivery, increased from 2.1% to 11.1%.
The reported use of onsite SBRT among VHA physicians is slightly less than that of community physicians. A survey study by Pan and colleagues demonstrated that 63.9% of U.S. radiation oncologists use SBRT, while in the survey study presented here, 53% of VHA radiation oncologists reported availability of onsite SBRT.9 Of note, the lack of availability of onsite SBRT at VHA centers does not preclude treatment with SBRT when medically necessary. These cases can be referred to other VHA or community centers with the requisite accreditation credentials. Because of the increasing use of SBRT and related technologies in the treatment of some cancers, an improved availability of SBRT in the future within the VHA will allow for some centers to participate in the Veterans Affairs Lung Cancer Surgery or Stereotactic Radiotherapy (VALOR) trial, which was approved for open recruitment in 2015.
Although BT and SRS are not as widely available within the VHA as other evaluated technologies such as IGRT and IMRT, their availability mirrors a similar limited availability in the community.10-12 When necessary these services also can be provided for veterans through referral to other VHA or non-VHA centers.
The benefit of charged particle radiotherapy, such as proton beam radiotherapy, is limited to specific cancers.13 This technology is not widely available in the community or within the VHA. Because of a VHA policy currently in place permitting non-VHA care when needed, veterans who require treatment with charged particle radiotherapy are referred to accredited non-VHA radiation oncology centers when indicated.
Conclusion
In this survey, 92% of the VHA radiation oncology centers are accredited by the American College of Radiology. Further, VHA radiation oncologists respondents reported availability of treatment technologies in line with responses of physicians from community based surveys. The majority of VHA radiation oncologists report access to IMRT, IGRT, CBCT, and SBRT. While BT and SRS are not available onsite at the majority of the 40 VHA radiation oncology centers, this mirrors limited availability and use of these technologies in the community as well.
Acknowledgments
This article was based on a presentation at the ASCO Quality Care Symposium (October 17-18, 2014) in Boston, Massachusetts. Dawson GA, Cheuk AV, Jolly S, et al. Advanced radiation oncology technology within the Veterans Health Administration (VHA). J Clin Oncol. 214;32(suppl 30):52.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. National Center for Veterans Analysis and Statistics. 2010 National Survey of Veterans: reported plan to use VA health care in the future. U.S. Department of Veteran Affairs website. http://www.va.gov/vetdata/docs/QuickFacts/2010NSV_Quick_Fact_Final.pdf. Published December 2011. Accessed April 4, 2016.
2. National Center for Veterans Analysis and Statistics. 2010 National Survey Veterans: enrollment and usage of VA benefits and services. U.S. Department of Veteran Affairs website. http://www.va.gov/vedata/docs/quickfacts/Surveys-slideshow.pdf. Published August 15, 2011. Accessed April 4, 2016.
3. Zulling LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Mil Med. 2012;177(6):693-701.
4. International Atomic Energy Agency. Recent developments in the technology of radiation oncology. International Atomic Energy Agency website. https://www.iaea.org/About/Policy/GC/GC55/GC55InfDocuments/English/gc55inf-5-att1_en.pdf. Accessed April 4, 2016.
5. Dawson GA, Jolly S, Fosmire H, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P114) radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: supportive measures (Part 1 of 2 reports). Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain#sthash.fcB6idE7.dpuf. Published April 30, 2015. Accessed April 4, 2016.
6. Cheuk AV, Gutt R, Moghanaki D, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P118) Radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: part 2 clinical treatment patterns. Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain-part-2#sthash.fwW0g1RZ.dpuf. Published April 30, 2015. Accessed April 4, 2016.
7. Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey on the use of image-guided radiotherapy in the United States. Cancer. 2010;116(16):3953–3960.
8. Shen X, Showalter TN, Mishra MV, et al. Radiation oncology services in the modern era: evolving patterns of usage and payments in the office setting for medicare patients from 2000 to 2010. J Oncol Pract. 2014;10(4):e201-e207.
9. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117(19):4566-4572.
10. Mahmood U, Pugh T, Frank S, et al. Declining u se of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13(2):157-162.
11. Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109-e116.
12. Kong FM, Cuneo KC, Wang L, et al. Patterns of practice in radiation therapy for non-small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4(2):e133-e141.
13. Trikalinos TA, Terasawa T, Ip S, Raman G, Lau J. Particle Beam Radiation Therapies for Cancer. Technical Brief, No. 1. Rockville, MD: Agency for Healthcare Research and Quality; 2009.
1. National Center for Veterans Analysis and Statistics. 2010 National Survey of Veterans: reported plan to use VA health care in the future. U.S. Department of Veteran Affairs website. http://www.va.gov/vetdata/docs/QuickFacts/2010NSV_Quick_Fact_Final.pdf. Published December 2011. Accessed April 4, 2016.
2. National Center for Veterans Analysis and Statistics. 2010 National Survey Veterans: enrollment and usage of VA benefits and services. U.S. Department of Veteran Affairs website. http://www.va.gov/vedata/docs/quickfacts/Surveys-slideshow.pdf. Published August 15, 2011. Accessed April 4, 2016.
3. Zulling LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Mil Med. 2012;177(6):693-701.
4. International Atomic Energy Agency. Recent developments in the technology of radiation oncology. International Atomic Energy Agency website. https://www.iaea.org/About/Policy/GC/GC55/GC55InfDocuments/English/gc55inf-5-att1_en.pdf. Accessed April 4, 2016.
5. Dawson GA, Jolly S, Fosmire H, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P114) radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: supportive measures (Part 1 of 2 reports). Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain#sthash.fcB6idE7.dpuf. Published April 30, 2015. Accessed April 4, 2016.
6. Cheuk AV, Gutt R, Moghanaki D, et al; US Veterans Healthcare Administration National Palliative Radiotherapy Task Force. (P118) Radiotherapeutic care within the Veterans Health Administration of US veterans with metastatic cancer to the brain: part 2 clinical treatment patterns. Cancer Network website. http://www.cancernetwork.com/ars-2015/radiotherapeutic-care-within-veterans-health-administration-us-veterans-metastatic-cancer-brain-part-2#sthash.fwW0g1RZ.dpuf. Published April 30, 2015. Accessed April 4, 2016.
7. Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey on the use of image-guided radiotherapy in the United States. Cancer. 2010;116(16):3953–3960.
8. Shen X, Showalter TN, Mishra MV, et al. Radiation oncology services in the modern era: evolving patterns of usage and payments in the office setting for medicare patients from 2000 to 2010. J Oncol Pract. 2014;10(4):e201-e207.
9. Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117(19):4566-4572.
10. Mahmood U, Pugh T, Frank S, et al. Declining u se of brachytherapy for the treatment of prostate cancer. Brachytherapy. 2014;13(2):157-162.
11. Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85(2):e109-e116.
12. Kong FM, Cuneo KC, Wang L, et al. Patterns of practice in radiation therapy for non-small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4(2):e133-e141.
13. Trikalinos TA, Terasawa T, Ip S, Raman G, Lau J. Particle Beam Radiation Therapies for Cancer. Technical Brief, No. 1. Rockville, MD: Agency for Healthcare Research and Quality; 2009.
Consensus Statement Supporting the Recommendation for Single-Fraction Palliative Radiotherapy for Uncomplicated, Painful Bone Metastases
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.