User login
Generalized Pustular Psoriasis Treated With Risankizumab
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
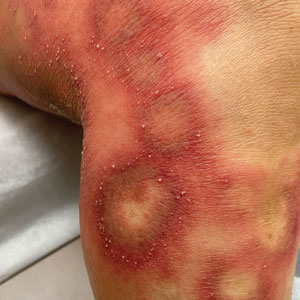
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
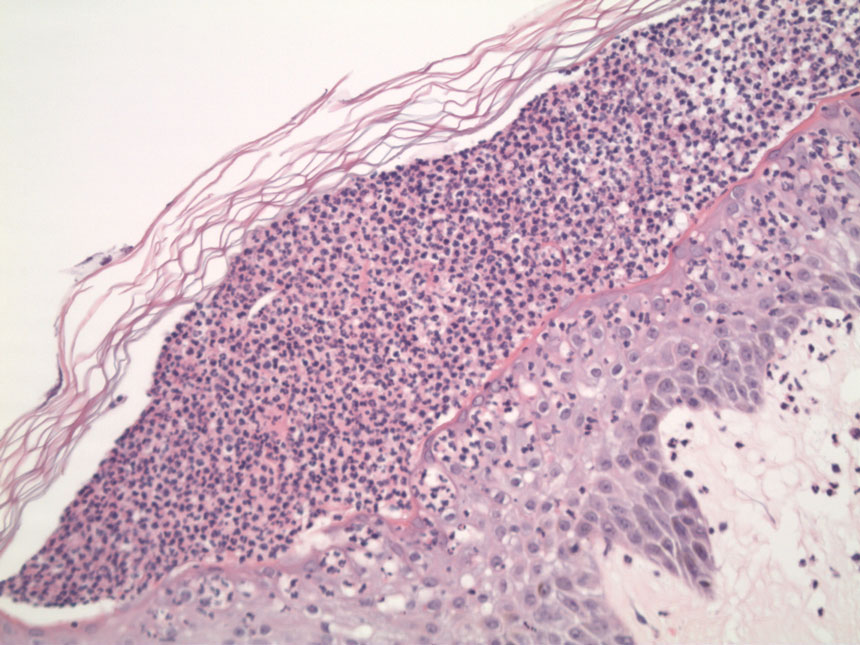
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.
Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.
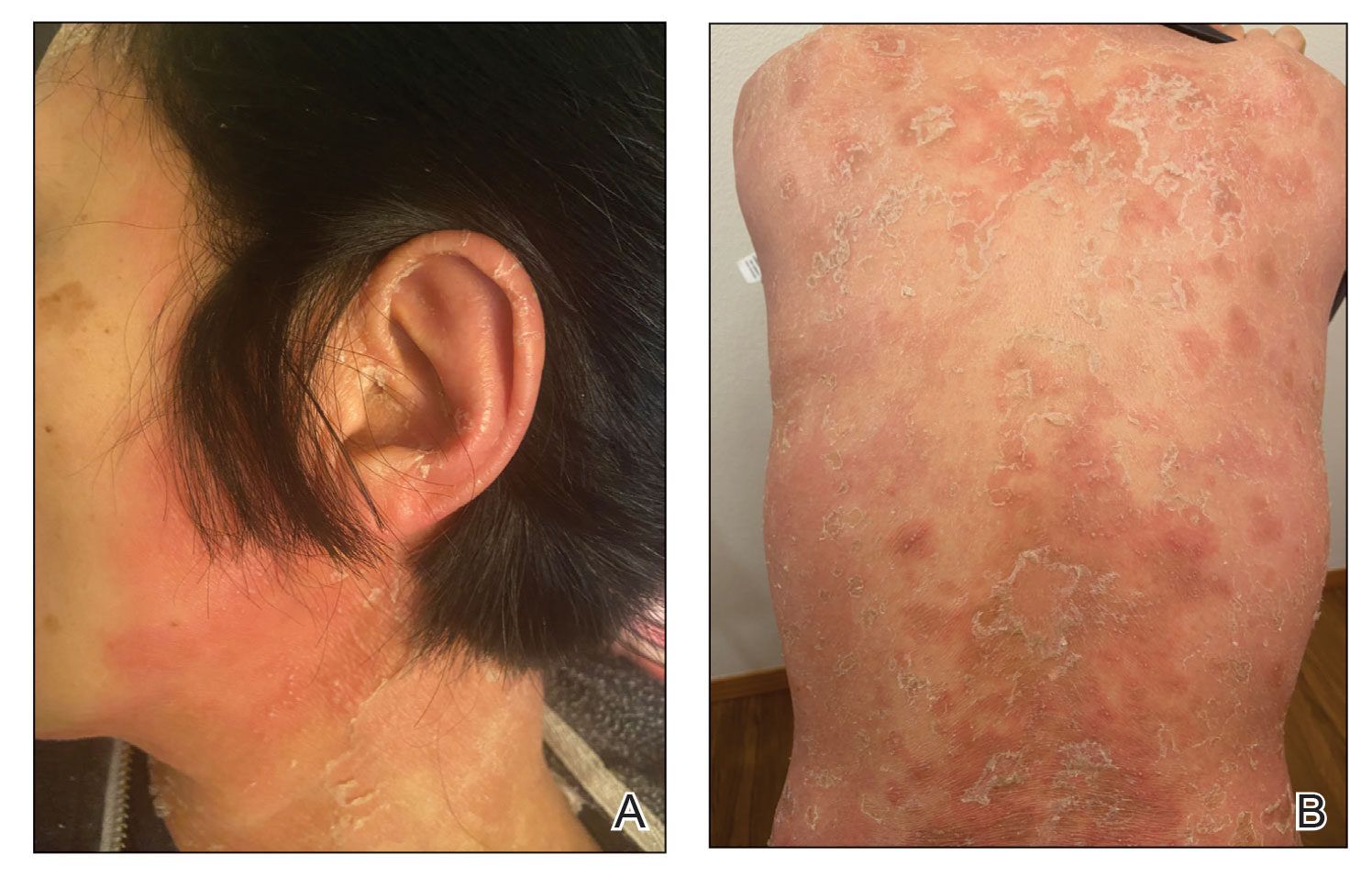
Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.

Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.

Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
To the Editor:
Generalized pustular psoriasis (GPP) is a rare but severe subtype of psoriasis that can present with systemic symptoms and organ failure, sometimes leading to hospitalization and even death.1,2 Due to the rarity of this subtype and GPP being excluded from clinical trials for plaque psoriasis, there is limited information on the optimal treatment of this disease.
More than 20 systemic medications have been described in the literature for treating GPP, including systemic steroids, traditional immunosuppressants, retinoids, and biologics, which often are used in combination; none have been consistently effective.3 Among biologic therapies, the use of tumor necrosis factor α as well as IL-12/23 and IL-17 inhibitors has been reported, with the least amount of experience with IL-17 inhibitors.4
A 53-year-old Korean woman presented to the dermatology clinic for evaluation of a widespread painful rash involving the face, neck, torso, arms, and legs that had been treated intermittently with systemic steroids by her primary care physician for several months before presentation. She had no relevant medical or dermatologic history. She denied taking prescription or over-the-counter medications.
Physical examination revealed the patient was afebrile, but she reported general malaise and chills. She had widespread erythematous, annular, scaly plaques that coalesced into polycyclic plaques studded with nonfollicular-based pustules on the forehead, frontal hairline, neck, chest, abdomen, back, arms, and legs (Figure 1).
![Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively. Initial presentation (day 0 [prior to treatment with risankizumab]). A and B, Scaly plaques coalesced into polycyclic plaques studded with nonfollicular-based pustules on the leg and neck, respectively.](https://cdn.mdedge.com/files/s3fs-public/CT111002096_Fig1_AB.jpg)
Two 4-mm punch biopsies were performed for hematoxylin and eosin staining and direct immunofluorescence. Histopathologic analysis showed prominent subcorneal neutrophilic pustules and spongiform collections of neutrophils in the spinous layer without notable eosinophils (Figure 2). Direct immunofluorescence was negative.

Based on the clinical history, physical examination, histopathology, and unremarkable drug history, a diagnosis of GPP was made. Initially, acitretin 25 mg/d was prescribed, but the patient was unable to start treatment because the cost of the drug was prohibitive. Her condition worsened, and she returned to the clinic 2 days later. Based on knowledge of an ongoing phase 3, open-label study for risankizumab in GPP, a sample of risankizumab 150 mg was administered subcutaneously in this patient. Three days later, most of the pustules on the upper half of the patient’s body had dried up and she began to desquamate from head to toe (Figure 3).The patient developed notable edema of the lower extremities, which required furosemide 20 mg/d andibuprofen 600 mg every 6 hours for symptom relief.

Ten days after the initial dose of risankizumab, the patient continued to steadily improve. All the pustules had dried up and she was already showing signs of re-epithelialization. Edema and pain also had notably improved. She received 2 additional samples of risankizumab 150 mg at weeks 4 and 16, at which point she was able to receive compassionate care through the drug manufacturer’s program. At follow-up 151 days after the initial dose of risankizumab, the patient’s skin was completely clear.
Generalized pustular psoriasis remains a difficult disease to study, given its rarity and unpredictable course. Spesolimab, a humanized anti–IL-36 receptor monoclonal antibody, was recently approved by the US Food and Drug Administration (FDA) for the treatment of GPP.5 In the pivotal trial (ClinicalTrials.gov Identifier NCT03782792),5 an astonishingly high 54% of patients (19/35) given a single dose of intravenous spesolimab reached the primary end point of no pustules at day 7. However, safety concerns, such as serious infections and severe cutaneous adverse reactions, as well as logistical challenges that come with intravenous administration for an acute disease, may prevent widespread adoption by community dermatologists.
Tumor necrosis factor α, IL-17, and IL-23 inhibitors currently are approved for the treatment of GPP in Japan, Thailand, and Taiwan based on small, nonrandomized, open-label studies.6-10 More recently, results from a phase 3, randomized, open-label study to assess the efficacy and safety of 2 different dosing regimens of risankizumab with 8 Japanese patients with GPP were published.11 However, there currently is only a single approved medication for GPP in Europe and the United States. Therefore, additional therapies, particularly those that have already been established in dermatology, would be welcome in treating this disease.
A number of questions still need to be answered regarding treating GPP with risankizumab:
• What is the optimal dose and schedule of this drug? Our patient received the standard 150-mg dose that is FDA approved for moderate to severe plaque psoriasis; would a higher dose, such as the FDA-approved 600-mg dosing used to treat Crohn disease, have led to a more rapid and durable response?12
• For how long should these patients be treated? Will their disease follow the same course as psoriasis vulgaris, requiring long-term, continuous treatment?
• An ongoing 5-year, open-label extension study of spesolimab might eventually answer that question and currently is recruiting participants (NCT03886246).
• Is there a way to predict a priori which patients will be responders? Biomarkers—especially through the use of tape stripping—are promising, but validation studies are still needed.13
• Because 69% (24/35) of enrolled patients in the treatment group of the spesolimab trial did not harbor a mutation of the IL36RN gene, how reliable is mutation status in predicting treatment response?5
Of note, some of these questions also apply to guttate psoriasis, a far more common subtype of psoriasis that also is worth exploring.
Nevertheless, these are exciting times for patients with GPP. What was once considered an obscure orphan disease is the focus of major recent publications3 and phase 3, randomized, placebo-controlled studies.5 We can be cautiously optimistic that in the next few years we will be in a better position to care for patients with GPP.
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
- Shah M, Aboud DM Al, Crane JS, et al. Pustular psoriasis. In. Zeichner J, ed. Acneiform Eruptions in Dermatology: A Differential Diagnosis. 2021:295-307. doi:10.1007/978-1-4614-8344-1_42
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi:10.1056/NEJMra0804595
- Noe MH, Wan MT, Mostaghimi A, et al. Evaluation of a case series of patients with generalized pustular psoriasis in the United States. JAMA Dermatol. 2022;158:73-78. doi:10.1001/jamadermatol.2021.4640
- Miyachi H, Konishi T, Kumazawa R, et al. Treatments and outcomes of generalized pustular psoriasis: a cohort of 1516 patients in a nationwide inpatient database in Japan. J Am Acad Dermatol. 2022;86:1266-1274. doi:10.1016/J.JAAD.2021.06.008
- Bachelez H, Choon S-E, Marrakchi S, et al; . Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288. doi:10.1016/J.JAAD.2011.01.032
- Torii H, Nakagawa H; . Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011;38:321-334. doi:10.1111/J.1346-8138.2010.00971.X
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155. doi:10.1111/JDV.12773
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017. doi:10.1111/1346-8138.13306
- Torii H, Terui T, Matsukawa M, et al. Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol. 2016;43:767-778. doi:10.1111/1346-8138.13214
- Yamanaka K, Okubo Y, Yasuda I, et al. Efficacy and safety of risankizumab in Japanese patients with generalized pustular psoriasis or erythrodermic psoriasis: primary analysis and 180-week follow-up results from the phase 3, multicenter IMMspire study [published online December 13, 2022]. J Dermatol. doi:10.1111/1346-8138.16667
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. doi:10.1016/S0140-6736(22)00467-6
- Hughes AJ, Tawfik SS, Baruah KP, et al. Tape strips in dermatology research. Br J Dermatol. 2021;185:26-35. doi:10.1111/BJD.19760
PRACTICE POINTS
- Generalized pustular psoriasis (GPP) is a potentially life-threatening condition that can be precipitated by systemic steroids.
- Although more than 20 systemic medications have been tried with varying success, there has not been a single US Food and Drug Administration–approved medication for GPP until recently with the approval of spesolimab, an IL-36 receptor inhibitor.
- Risankizumab, a high-affinity humanized monoclonal antibody that targets the p19 subunit of the IL-23 cytokine, also has shown promise in a recent phase 3, open-label study for GPP.