User login
Infected with COVID-19: One psychiatrist’s story
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Emil: Coronavirus disease 2019 (COVID-19) wasn’t really on my mind until the first weekend in March, specifically Sunday, March 8, 2020. That weekend had us traveling from Chicago to Berwyn, Pennsylvania to attend the funeral of one of my older cousins. Though we were the only ones from his side at the graveside, his funeral had drawn numerous relatives, none of whom were “socially distanced.”
On our way home, I received an e-mail from a colleague in Brazil who had invited me to speak at a conference in São Paulo. He told me that several of my American colleagues had contacted him and informed him that their universities had banned travel because of COVID. “I’m coming,” I replied. “I don’t think COVID’s going to be a big deal here.” He said COVID wasn’t a “big deal” in Brazil, either. Famous last words.
The next weekend, I left early on Saturday morning to start my call duty at the hospital. After finishing rounds at one hospital and going to the next, I got a text from my wife, Anne, asking “What’s wrong with your people over there? What kind of doctors would take a 65-year-old colleague with a history of asthma, and history of an ICU stay with 10 days on a respirator with acute respiratory distress syndrome 10 years ago, and have him exposed to this lethal virus? Are they trying to kill you?”
It stopped me in my tracks. She was right. A lot had changed in a week. In that single week, it had become clear that COVID was a real threat, and I was vulnerable. I finished my call duty but made it clear to the “powers that be” I was going to stay home and isolate for the next few weeks, until we knew more. I was ahead of the curve, but not by much: within days, Chicago shut down with a “stay-at-home” order.
Anne: When the threat of COVID first became known, I said to family and friends, “If Emil gets this, it’s going to be very, very bad.” After that, we made certain to wear masks and gloves when we went out, which wasn’t often.
Emil: We stayed in for the next 3 months until we moved to Columbus, Ohio for my new position as Vice Chair for Research in the Department of Psychiatry and Behavioral Health at The Ohio State University Wexner Medical Center (OSUMC).
The day after arriving, I went to the emergency dental clinic because of a severe toothache. While they couldn’t save my tooth, I got something in return: COVID. The clinic took more than appropriate precautions, but I was in a very large room, not a private office, with many patients having their teeth drilled and whatever it is dentists do (actually, I do know; my father was a dentist).
Continue to: All was fine until 2 days later...
All was fine until 2 days later, when I began to feel a bit “unwell” on late Friday afternoon. I went out to do some chores the next morning, but soon returned home exhausted. The rest of the weekend was more of the same, and I was surprised at how I just couldn’t get anything done. On Monday, I felt a chill and thought I might have COVID.
The next morning, I went to OSUMC for a COVID test, but by then I already knew the result. The night before, Anne started complaining of a dry cough that would not stop.
Anne: When I realized Emil had COVID, I wrote to a friend, “If he gets bad and has to go to the hospital, or worse … he goes on a ventilator, I may need to be admitted to a psych ward!” I was still upset from the memory of sitting by Emil’s bedside when he was sick, and on a ventilator, 10 years ago, with his doctors talking with me about when, not if, he died.
Emil: My test came back within 8 hours on Tuesday. It was positive, as was the one for Anne the next day. The doctor I spoke to that evening thought I was only having a mild case and that I should just stay isolated. We immediately got a thermometer and a pulse oximeter to follow our symptoms. Anne’s oxygen saturation levels were always above 95%, but mine were lower, and by Friday, 3 days later and 1 week after my first symptoms, they were down to 92% or less. At that point, we both went to the ER at OSUMC.
Anne: We went to different places in the ER to be evaluated. As Emil was being wheeled away in the ER for his evaluation, I ran over for a kiss—with our masks on.
Continue to: As my ER evaluation...
As my ER evaluation was concluding, my doctor said, “I want someone, preferably the same person, to check in on you every day.” I replied I had a friend who is a critical care nurse. He smiled and said, “Excellent.” My friend called every day, and when she didn’t like how I sounded, on some days, she found an excuse to call again.
Emil: I barely recall my ER evaluation, except that I was to be admitted for observation and supplemental oxygen. I accepted this with aplomb, knowing I was in good hands and hoping I’d be home soon.
Anne: Because we were in the same ER, I thought I’d be able to see Emil once they decided to admit him. No. They wouldn’t even let me go to him to get his wallet for safekeeping. Instead, it was brought to me in a hazmat bag. Thus began our forced separation for the next 5 weeks.
Emil: I had to wait hours for a bed and was wheeled up late in the evening to a double room with one other patient, also with COVID, I supposed. While I had an oxygen mask on, we were only separated by a curtain. I had no idea I wouldn’t see Anne for weeks.
Anne: I returned “home” to a house I had spent less than 5 days in. We had barely moved in and it only had a bed, a couch, a TV, and a kitchen chair. I didn’t even know my neighbors to wave at, and … I was in quarantine. No one could come to me. Our eldest daughter was alone near Burlington, Vermont (where she had escaped to from New York City when it was the national epicenter for COVID back in March). Our youngest daughter was alone in Los Angeles, and our son, a newly minted First Lieutenant in the Army, was stationed in Afghanistan. “Good for him,” I thought. He could safely interact with his army buddies. It was so ironic; the one in the war zone was the only one of us who was safe from COVID.
Continue to: I reached out to family and friends...
I reached out to family and friends and asked for prayers. Emil was prayed for by all of our Catholic, Methodist, Jewish, Muslim, and Buddhist friends. As I told him later, he was prayed for from Afghanistan to Alaska. My extended family activated a text chain so all I had to do was reply and everyone on the chain would have the same information. I also received many notes and cards of support from friends and Emil’s family. Many told me how strong I was and how I would be fine. Later, I realized how many of these were from widows who were telling me I would survive bereavement, should that be the outcome.
Emil: The next day, the doctors started me on a 5-day course of the newly “approved” antiviral remdesivir, and the day after that, I received 2 units of convalescent plasma on “compassionate use” from the Mayo Clinic. It didn’t matter. I kept getting worse.
Anne: I received twice-daily updates from the nurses. When the updates were late in coming, I crawled the walls, waiting at least 2 hours before reaching out. One day, the nurse who answered said she couldn’t talk because his nurse was dealing with an emergency with him. I didn’t take a deep breath until his nurse called back to say he was stable. Regardless, he just kept getting sicker and sicker, and I began to fear he would not make it.
Emil: By Day 5, my X-ray showed clear evidence of a bilateral pneumonia (it had appeared “normal” on admission) and I was transferred to a “step-up unit.” The next day, I was transferred to the ICU and placed on a ventilator, in the prone position, for 16 hours a day.
Anne: The day Emil was transferred to the ICU, he told me he was worried about his fate. He called and asked me to stay on the phone with him while waiting to go to the ICU. We were both so weak we couldn’t do more than say “I love you” and listen to the other’s labored breathing. That was our last phone call until he was off the ventilator 10 days later.
Continue to: Emil's reply
Emil: At this point I had no idea what was going on. I was on a ventilator and I was “out.”
Anne: In the meantime, my family made sure I knew they were thinking of us. Every day I woke up with a text from one cousin asking how the night was while my sister checked in every afternoon. They sent flowers and baskets of goodies. Knowing how difficult it was waiting for updates, they sent me a jigsaw puzzle with a thousand pieces. I was surprised at how important that was for binding my anxiety. A friend sent books from my favorite writers.
Despite all this, I was absolutely beside myself the night Emil was placed on the ventilator. I cleaned and scrubbed the house; not that it needed it, I needed it. In the bedroom I saw a bottle under the bed. I retrieved it but couldn’t get up off the floor. I was weak and had tremendous muscle pain each time I moved. I had my phone, so knew I wouldn’t be stranded, but … I didn’t relish the idea of calling 911 and have them break down the front door in their hazmat suits. After more than 30 minutes, and much effort, I was able to get myself up; soon after, I put a house key outside.
When a friend who was taking care of our 2 dogs in Chicago heard that Emil was on the ventilator, she drove through the night to bring them to me so I would have them for solace. She couldn’t even come in the house. She stayed at a nearby hotel and visited with me from outside with masks on waiting for the updates.
Emil: Being an elder lawyer married to a physician, Anne knows a thing or 2 about medicine (because she’s seen a thing or 2 about medicine). She’s even been known to give her elderly clients Mini-Mental State Exams. In addition to talking with members of her support system, Anne was also talking with friends and relatives who are physicians. One exclaimed, “He’s having a cytokine storm!” and said I needed steroids. Another said, yes, that and serious “anti-inflammatory” drugs. At that moment, data supporting the use of steroids or “anti-inflammatories” in COVID hadn’t yet become public. The data on steroids came out early the next week in the Lancet and the data on “anti-inflammatories” was still in process until a few weeks later.
Continue to: Anne was ahead of the curve...
Anne was ahead of the curve and advocated hard for both treatments. At the same time, my OSUMC physicians were considering other options for me. They were checking on my inflammatory status by following my levels of C-reactive protein (CRP) and interleukin-6 (IL-6). On Days 2 and 3, my CRP level was 64 mg/L and my IL-6 level was 32 pg/mL (neither should be higher than 1).
While I don’t recall much before being on the ventilator, I do recall my alarm at seeing my CRP/IL-6 levels go up in real time on alerts from “My Chart” (my CRP/IL-6 levels were 149/123 within 4 days of admission, and reached a high of about 250/190 as I entered the ICU). I knew what those numbers meant. It was surreal; like watching myself die off in the distance, emotionally disconnected from the whole scene.
The decision to give steroids was relatively easy, and I was started on dexamethasone, a very inexpensive steroid, on Day 7 (ICU Day 2). The decision of which “antiinflammatory” to give was more difficult, as OSUMC had over 40 treatment protocols for COVID. Anne suggested 2 drugs based on recommendations from our physician friends—tocilizumab and acalabrutinib— both were on the market for other conditions and very expensive. The first is an IL-6 antagonist, while the second shuts down cytokine production in B cells, an effect also observed in lung tissue. While tocilizumab was not included in any of the OSUMC COVID protocols, acalabrutinib was, and I started on that medication on Day 8 (ICU Day 3).
Anne: My experience being the advocate was different than the first time 10 years before. That time, Emil had a community-acquired pneumonia, with which our doctors had much experience. This time, I was more active because no one had much information about how to deal with COVID and, thus, there was no standard of care. In fact, Emil was only the second patient to receive acalabrutinib at OSUMC; later, we found out that that patient did well.
Emil: The “anti-inflammatory” strategy worked. Within 5 days of starting the 2 drugs, my CRP and IL-6 levels were down to 10 and 5, respectively; a reduction of >95%. As these levels dropped, so did my oxygen requirements.
Continue to: Anne's reply
Anne: Emil was finally on the upswing. I woke up the next morning and, surprisingly, found that my first emotion wasn’t one of terror. His ICU doctor, a real booster for Emil, made it her mission to get him off the ventilator before the end of her ICU service week. She succeeded.
Emil: Five days after coming off the ventilator, I went to a rehab unit for reconditioning and to begin the long process of recovering my strength and stamina.
Most people say to me, “How awful for you! How terrible!” I smile and say, “Yeah, well, I missed all the excitement. It was really much worse for Anne.” I told them that, although you don’t recall anything while on the ventilator, you get retrograde amnesia for the several days prior to artificial ventilation. I have texts on my cell phone, written by me in those first few days, I don’t recall writing. Anne says we had conversations all the way up to my admission to the ICU; I recall none of those. Frankly, that’s for the best.
One thing to highlight is that your brain doesn’t stop working while you’re “out.” I had numerous vivid dreams, or whatever they were, while on the ventilator and after. Many were “bizarre and dark,” others were “dark and bizarre.” A few were amusing— in the end. I recall watching a TV news program segment describing how we donated our 2 little dogs to the Queen of England, who then gave them to her youngest son, Edward. I swear, I actually “saw” this TV program and watched the Queen and her son (and his wife) playing with our dogs. I was so convinced, I asked Anne where our dogs were; with her, of course. No, she assured me, we hadn’t given them to Queen Elizabeth II. Another conversation I swore I had with Anne was one in which she was telling me she was starting the vetting process to be a VP candidate for Joe Biden (Anne had been involved in Chicago politics so … not totally “crazy”). Nevertheless, I was quickly disabused of this one by my eldest daughter, also a lawyer.
Anne: This time, like the last time he was on a ventilator, Emil took a few more days to clear all the drugs keeping him sedated. Last time, his medical center sent his colleague, the Chair of Neurology, to check on him because there was a concern that he wasn’t “clearing” fast enough. This time, I was the one reassuring the doctors and nurses to be “patient.” At the same time, I was disabusing him of his far-fetched idea that he was head of all research at OSUMC and head of the ICU. He told me, “I don’t understand it. Don’t these people know they work for me?” “No,” I told him. “You are a patient there, and you need to behave.” Aside from that, Emil was fairly lucid. As one of his nurses said, “He’s oriented, he’s just wrong!”
Continue to: Emil's reply
Emil: Some people have asked me if this experience has changed my perspective. It could have, but I went through something worse 10 years ago when I was first brought back from the “mostly dead.” After that, I realized the most important things in life are the people you love and the people who love you; the good stuff is “gravy” and everything else isn’t worth spending much time or energy on. The first thing I said to Anne when we were face-to-face, as I entered the rehab facility (with masks on, of course), was “I can’t do this to you again.”
Anne: One of the most inhumane aspects of COVID is that you can’t be with your loved one while they are sick. Last time I spent 10 to 12 hours a day at the bedside. This time I couldn’t be there at all. It was especially hard because I knew from the last time how much my presence meant to him. If I left, he would get agitated. His heart rate would come down by 10 beats when I sat next to him.
When we had our first post-ventilator conversation on Father’s Day, he was surprised I was so excited to talk to him. Somehow, he thought I had abandoned him. What he didn’t know was that I was thinking about getting a job in Housekeeping at the hospital just so I could go see him!
Emil: In the end, I’m now back to baseline and grateful I’m alive. I still have things I want to do professionally and personally, and am appreciative I’ll have more time for those. However, I am appalled at how a serious public health issue has been turned into a political weapon by “science deniers” and that this is continuing to kill our citizens. That’s not a nightmare from when I was ill. It’s the “day-mare” we are living now.
Intermittent explosive disorder: Taming temper tantrums in the volatile, impulsive adult
Mr. P, age 41, has a “problem with anger.” Since age 17, he has had sudden outbursts of screaming and shouting, with occasional minor damage to objects. These outbursts—including episodes of “road rage”—occur once or more per week and almost daily for months at a time.
Mr. P has also had more violent episodes— sometimes every 2 to 3 months—in which he has punched holes in walls, destroyed a computer with a hammer, and assaulted other people with his fists. These events are not premeditated and are typically triggered by Mr. P’s frustration at not being “perfect” or by others breaking what he considers “general rules of conduct.”
The day before his initial visit, while he was stuck in traffic, Mr. P saw a car speeding down the shoulder. Enraged, he pulled in front of the car so that the driver had to slam on the brakes. He jumped out of his car and approached the other driver, shouting obscenities. The other driver locked her door and tried to ignore Mr. P until he returned to his car. Mr. P noted that this episode “ruined” his day because of his lingering anger and irritability.
Intermittent explosive disorder (IED) is more common and complex than was once thought, based on recent evidence. Recurrent, problematic, impulsive aggression is highly comorbid with other psychiatric conditions—including mood and personality disorders—and undermines social relationships and job performance. Typical characteristics of IED are outlined in Table 1.1-3
Table 1
Typical characteristics of intermittent explosive disorder
| Onset in childhood or adolescence (mean age 15), with average duration ±20 years |
| Aggressive outbursts: |
|
| Some episodes may appear without identifiable provocation |
| Male to female ratio 3:1, although some data suggest gender parity |
| Source: Adapted from references 1-3 |
This article offers updated diagnostic criteria and a two-pronged algorithm that can help you diagnose and treat this aggression disorder.
HOW COMMON IS IED?
DSM-IV states that IED is “apparently rare.” This statement is far from surprising, given the limitations of DSM criteria. Surveys of hospitalized patients in the 1980s found that only 1.1% met DSM-III criteria for IED.4 In another study of more than 400 patients seeking treatment for aggression, only 1.8% met DSM-III criteria for IED (although far more would likely have met DSM-IV criteria).5
A more recent survey of 411 psychiatric outpatients6 found that 3.8% met current and 6.2% met lifetime DSM-IV criteria for IED, using the Structured Clinical Interview for DSM-IV Diagnoses (SCID). Reanalysis of a threefold larger data set from the same study site (Coccaro and Zimmerman, unpublished) yielded the same result.
Far from rare. More recently, our findings from a small sample suggested that the community rate of lifetime IED is about 4% by DSM-IV criteria and 5% by research criteria. In the United States, we estimate that the lifetime rate of IED could be 4.5 to 18 million persons using DSM-IV criteria or 6.7 to 22.2 million using IED research criteria. If so, IED is at least as common as other major psychiatric disorders, including schizophrenia or bipolar illness. The ongoing National Comorbidity Study is expected to produce more definitive community data.
PSYCHIATRIC COMORBIDITY
Axis I disorders. IED is highly comorbid with mood, anxiety, and substance use disorders,3,7,8 although no causal relationship has been shown
Mood and substance abuse disorders. IED’s age of onset may precede that of mood and substance use disorders, according to analysis of our unpublished data. If so, comorbid IED may not occur in the context of mood or substance use disorders.
Anxiety disorders. We have noted a similar pattern with IED and anxiety disorders, although phobic anxiety disorders (simple or social phobia) tend to manifest earlier than IED. This suggests that early-onset phobic anxiety might be associated with an increased risk of IED in adolescence or young adulthood.
Bipolar disorder. McElroy9 has suggested a relationship between IED and bipolar disorder. In some samples, as many as one-half of IED patients (56%) have comorbid bipolar disorder when one includes bipolar II and cyclothymia.3 Moreover, some subjects’ aggressive episodes appear to resemble “microdysphoric manic episodes.”9 Other studies,8 however, find a much lower rate (10% or less) of IED comorbidity with bipolar illness.
Bipolar disorder overall may not be highly comorbid with IED, although rates may be higher in specialty clinic samples. In individuals with any kind of bipolar disorder, mood stabilizers— rather than selective serotonin reuptake inhibitors (SSRIs)—are probably the better choice as first-line treatment of IED.9
Axis II disorders. DSM-IV allows IED diagnosis in individuals with borderline or antisocial personality disorder, as long as these cluster B disorders do not better explain the aggressive behavior. How a clinician makes this distinction is not clear; in fact, most clinicians do not diagnose IED in patients with personality disorders, regardless of the clinical picture.
IED comorbidity with borderline or antisocial personality disorders varies with the sample. Persons with personality disorders who seek treatment of aggressive behavior are more likely to have comorbid IED (90%) than those not seeking treatment who are outpatients (50%) or in the community (25%).1,7
Individuals with personality disorders and IED score higher in aggression and lower in psychosocial function than do similar individuals without IED,7 indicating that the additional diagnosis is relevant.
Case report continued.
Mr. P’s outbursts have cost him several friendships, including romantic relationships. He has never advanced at work because he is seen as too volatile to supervise subordinates. Though some of Mr. P’s aggressive outbursts have occurred under the influence of alcohol, most are not related to alcohol or drug use. He has no medical problems and no other psychiatric history.
A full diagnostic evaluation uncovers a personality disorder, not otherwise specified (eight scattered traits from obsessive-compulsive personality disorder and from each of the cluster B personality disorders), and no Axis I condition other than intermittent explosive disorder.
PROBLEMS DEFINING IED
Intermittent explosive disorder is the only DSM diagnosis that applies to persons with histories of recurrent, problematic aggression not caused by another mental or physical disorder. Even so, little research on IED is available. DSM criteria for IED are poorly operationalized and have improved only modestly since the diagnosis was first included in DSM-III. In that revision, IED had four criteria.
“A” criteria specified recurrent outbursts of “seriously assaultive or destructive behavior,” but left unanswered important questions such as:
- What behavior crosses the threshold for seriously” assaultive or destructive?
- Does any physical assault qualify, or only those that cause physical injury (or stigmata)?
- How often or within what time must the behavior occur?
The phrase “recurrent acts of aggression” suggested that at least three acts of aggression were required to reach the threshold, but DSM-III provided no guidelines.
“B” criteria stated that the aggression should be out of proportion to the provocation. But how should one judge this criterion, when provocative stimuli sometimes are clearly sufficient to prompt a justifiably aggressive act?
“C” criteria excluded persons who are aggressive or impulsive between ill-defined “aggressive episodes.” This exclusion was especially limiting because individuals with recurrent, problematic, aggressive behaviors generally are impulsive and aggressive between more-severe outbursts. Excluding those who otherwise met diagnostic criteria for IED led to a spuriously low prevalence rate and limited the number of research subjects. DSM-IV eliminated this criterion but made no other notable changes in IED criteria.
“D” criteria in DSM-III and III-R further restricted the number of individuals who could meet this diagnosis:
- In DSM-III, antisocial personality disorder excluded the diagnosis of IED.
- In DSM-III-R, borderline personality disorder was added as an exclusionary factor.
Because of these restrictions, very few clinically valid cases of IED (individuals meeting A and B criteria) could receive an IED diagnosis.10
EVOLVING DIAGNOSTIC CRITERIA
By the early 1990s, DSM diagnostic criteria clearly severely restricted the study of recurrent, problematic aggression, even though research since DSM-III had greatly advanced our understanding of human aggression. For example, data linked impulsive aggression to deficits in central serotonergic function and suggested that agents that enhance serotonergic activity could modify this behavior.
Some investigators proposed research criteria for IED (IED-R) so that individuals with recurrent, problematic, impulsive aggression could be identified and studied. Research criteria first published in 19987 proposed six changes/clarifications in IED diagnostic criteria:
Lower-intensity aggression. The scope of aggressive behavior was expanded to include verbal and indirect physical aggression, provided that these behaviors are associated with distress and/or impairment. Data from double-blind, placebo-controlled trials indicated that these lower-intensity (although usually higher frequency) behaviors respond well to treatment with SSRIs.11,12
Impulsivity. The aggression was specified as impulsive. This change identified individuals with greater liability for deficits in central serotonergic function and excluded individuals with premeditated or criminal aggression.
A minimal frequency of aggression over time was proposed to make the IED diagnosis more reliable and to ensure that persons with only occasional impulsive aggressive outbursts (especially of low severity) were given this diagnosis.
Subjective distress (in the individual) and/or social or occupational dysfunction was proposed so that putatively aggressive individuals are not diagnosed for manifesting behaviors that are not functionally severe.
Diagnostic exclusionary criteria were modified so that individuals with:
- antisocial or borderline personality disorder could be diagnosed with IED if otherwise warranted
- aggressive behaviors confined within major depression episodes could not be diagnosed with IED.
This last change recognized that impulsive, aggressive outbursts could point to major depressive disorder.
When the revised criteria were tested in patients seeking treatment for aggression, those who met IED-R criteria were found to exhibit significantly greater aggression and impulsivity (using validated scales) and lower global functioning than those who did not.7 Statistical adjustments made to account for aggression score differences eliminated the difference in global functioning, which suggested a direct link between aggression and global function in individuals with IED-R.
Two patterns. Later research uncovered at least patterns of aggressive outbursts:
- low intensity at high frequency (such as verbal arguments or door slamming approximately twice weekly)
- high intensity at low frequency (such as physical aggression resulting in injury or destruction of nontrivial property at least three times per year).
Data revealed that 69% of individuals with IED-like histories displayed both aggression patterns, 20% displayed only the high-intensity/low-frequency pattern, and 11% displayed only the low-intensity/high-frequency pattern.
Because further analysis revealed no important differences between these groups in measures of aggression and impulsivity, IED-R criteria were revised to include both patterns in the “A” criteria. This revision integrated the essences of IED-R and DSM criteria into one diagnostic set (Table 2).
INFLUENCE OF HEREDITY
No twin or adoption studies of IED have been performed. However, family history data suggest that IED (or IED-type behavior) is familial. I recently conducted a blinded, controlled, family history study using IED-R criteria and found a significantly elevated risk for IED (p < 0.01) in relatives of persons with a history of IED (26%), compared with non-IED controls (8%). Comorbid conditions did not affect the risk among the IED subjects or their relatives, suggesting that IED is familial and independent of other conditions.13
Nearly all studies of aggression’s biology and treatment have measured aggression as a dimensional variable along a continuous scale from low to high.14 Our studies have allowed us to explore biological and treatment response correlates. In preliminary analyses, we have found that the maximal prolactin response to d-fenfluramine challenge and the number of platelet serotonin transporter binding sites are:
- reduced in subjects meeting research criteria for IED
- inversely correlated with dimensional measures of impulsive aggression.
Table 2
Updated diagnostic criteria for intermittent explosive disorder
| A. Recurrent incidents of aggression manifest as either: |
| 1. Verbal or physical aggression towards other people, animals, or property occurring twice weekly on average for 1 month |
| OR |
| 2. Three episodes involving physical assault against other people or destruction of property over a 1-year period |
| B. The degree of aggressiveness expressed is grossly out of proportion to the provocation or any precipitating psychosocial stressors |
| C. The aggressive behavior is generally not premeditated (ie, is impulsive) and is not committed to achieve a tangible objective (such as money, power, intimidation, etc.) |
| D. The aggressive behavior causes marked distress in the individual or impairs occupational or interpersonal functioning |
| E. The aggressive behavior is not better explained by another mental disorder (such as a major depressive/manic/psychotic disorder, attention-deficit/hyperactivity disorder, general medical condition [head trauma, Alzheimer’s disease], or due to the direct physiologic effects of a substance) |
| Source: Adapted from reference 7 |
Earlier, Virkkunen et al15 reported reduced cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations in persons diagnosed with IED based on DSM-III criteria, compared with persons who were not diagnosed with IED and those who demonstrated nonimpulsive aggression.
TREATING IED
Cognitive therapy. Few double-blind, randomized, placebo-controlled trials of any treatments for IED have been published. Trials using cognitive-behavioral approaches have reduced self-rated anger and its expression in young adults with anger disorders.16 Although many of these subjects may have had IED, it is not known if this approach works in IED.
Table 3
Characteristic behaviors of aggressive individuals*
| Severity | Behaviors |
|---|---|
| Mildly aggressive | Occasional verbal arguments and/or temper tantrums |
| Moderately aggressive | Frequent verbal arguments and temper tantrums (about twice weekly on average), occasional destruction of property, rare or occasional physical assault against others (usually without injury) |
| Highly aggressive | Frequent verbal arguments and temper tantrums (about twice weekly) and/or more than occasional destruction of property or physical assault against others, sometimes with injury |
| * Characteristics given are descriptive and not based on data. | |
Drug therapy. SSRIs. A trial by this author using fluoxetine showed that impulsive aggressive behavior responds to treatment that targets the central serotonergic system.12 Forty subjects with personality disorders and histories of impulsive aggression received fluoxetine, 20 to 60 mg qd, or placebo for 12 weeks. Fluoxetine reduced overt aggression and irritability about 67% more than placebo, as assessed by the Overt Aggression Scale Modified for Outpatients (OAS-M).
All subjects met research criteria for IED. A reanalysis suggests that SSRIs may be most effective in moderately aggressive patients (Table 3),17 whose serotonergic system may be less impaired than that of highly aggressive patients.18
Mood stabilizers. Impulsively aggressive subjects who do not respond to an SSRI may respond to a mood stabilizer.19 An antiaggressive response in IED-like subjects has been reported for lithium,20 carbamazepine,21 and diphenylhydantoin.22
Recently, Hollander et al23 reported greater reduction in overt aggression scores in IED subjects with a DSM cluster B personality disorder who were treated with divalproex, compared with placebo. This study used the same design and outcome measure as our study12 and included subjects who met both DSM-IV and research criteria for IED.
For unknown reasons, divalproex was no more effective than placebo in IED subjects without cluster B personality disorder. More research is needed to uncover predictors of antiaggressive response in IED subjects.
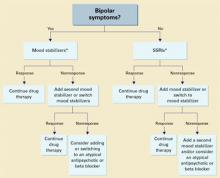
Unipolar vs. bipolar. McElroy9 has suggested using SSRIs (or other antidepressants) as first-line treatment for IED subjects with unipolar affective symptoms and mood stabilizers for those with bipolar affective symptoms. IED subjects without bipolar affective symptoms should be treated first with SSRIs (Algorithm). Preliminary data suggest a role for atypical antipsychotics to treat aggressive behavior in patients with schizophrenia or bipolar disorder, but no empiric data exist.
Beta blockers such as propranolol also may be considered.2 However, beta blockers are more difficult to dose and are associated with more burdensome side effects, compared with SSRIs.
Algorithm Suggested 2-pronged approach for treating intermittent explosive disorder
* With or without an anger management program, which may precede drug interventionThe full effects of antiaggressive treatment with an SSRI (E. Coccaro, unpublished observations) or a mood stabilizer19 may take 3 months to observe12,20,22,23 and tend to disappear soon after treatment is discontinued.
Therefore, an adequate trial of SSRIs or mood stabilizers is no less than 3 months. If improvement is seen, continue drug treatment indefinitely.
Case report continued.
Mr. P was started on an SSRI. His aggressive outbursts decreased in intensity and frequency over 3 months but were not eliminated. After 6 months he dropped out of treatment, but returned 5 weeks later because his aggressive outbursts had resumed their pre-treatment level.
SSRI treatment was restarted, and Mr. P began a 12-week anger management course of relaxation training, cognitive restructuring, and coping skills training. He gained greater control over his aggressive outbursts and continues monthly medication checks and anger management “booster sessions.”
Related resources
- Galovski T, Blanchard EB, Veazey C. Intermittent explosive disorder and other psychiatric comorbidity among court-referred and self-referred aggressive drivers. Behav Res Ther 2002;40:641-51.
- Olvera RL. Intermittent explosive disorder: epidemiology, diagnosis and management. CNS Drugs 2002;16:517-26.
Drug brand names
- Carbamazepine • Tegretol
- Diphenylhydantoin • Dilantin
- Divalproex • Depakote
- Fluoxetine • Prozac
- Lithium • Lithobid
- Propanolol • Inderal
Disclosure
Dr. Coccaro reports that he receives research grants and serves on the speaker’s bureau or as a consultant to Eli Lilly and Co., Abbott Laboratories, GlaxoSmithKline, and Forrest Laboratories.
1. Coccaro EF, Schimdt CA, Samuels JF, et al. Lifetime rates of intermittent explosive disorder in a community sample (abstract). Philadelphia: American Psychiatric Association annual meeting, 2002.
2. Mattes JA. Comparative effectiveness of carbamazepine and propranolol for rage outbursts. J Neuropsychiatry Clin Neurosci 1990;2:159-64.
3. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry 1998;59:203-10.
4. Monopolis S, Lion JR. Problems in the diagnosis of intermittent explosive disorder. Am J Psychiatry 1983;140:1200-2.
5. Zimmerman M, Mattia J, Younken S, Torres M. The prevalence of DSM-IV impulse control disorders in psychiatric outpatients (abstract 265). Washington, DC: American Psychiatric Association annual meeting, 1998.
6. Zimmerman M, Mattia J, Younken S, Torres M. The prevalence of DSM-IV impulse control disorders in psychiatric outpatients (APA new research abstracts #265). Washington, DC: American Psychiatric Publishing, Inc., 1998.
7. Coccaro EF, Kavoussi RJ, Berman ME, Lish JD. Intermittent explosive disorder-revised: development, reliability and validity of research criteria. Compr Psychiatry 1998;39:368-76.
8. Galovski T, Blanchard EB, Veazey C. Intermittent explosive disorder and other psychiatric comorbidity among court-referred and self-referred aggressive drivers. Behav Res Ther 2002;40:641-51.
9. McElroy SL. Recognition and treatment of DSM-IV intermittent explosive disorder. J Clin Psychiatry 1999;60(suppl 15):12-16.
10. Felthous AR, Bryant G, Wingerter CB, Barratt E. The diagnosis of intermittent explosive disorder in violent men. Bull Am Acad Psychiatry Law 1991;19:71-9.
11. Salzman C, Wolfson AN, Schatzberg A, et al. Effect of fluoxetine on anger in symptomatic volunteers with borderline personality disorder. J Clin Psychopharmacology 1995;15:23-9.
12. Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality disordered subjects. Arch Gen Psychiatry 1997;54:1081-8.
13. Coccaro EF. Family history study of intermittent explosive disorder (abstract). Washington, DC: American Psychiatric Association annual meeting, 1999.
14. Coccaro EF, Siever LJ. Pathophysiology and treatment of aggression. In: Davis KL, Charney D, Coyle JT, Nemeroff D (eds). Psychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002;1709-24
15. Virkkunen M, Rawlings R, Tokola R, et al. CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry 1994;51:20-7.
16. Deffenbacher JL. Psychosocial interventions: anger disorders. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker (in press).
17. Lee R, Coccaro EF. Treatment of aggression: serotonergic agents. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker (in press).
18. Coccaro EF, Kavoussi RJ, Hauger RL. Serotonin function and antiaggressive responses to fluoxetine: a pilot study. Biol Psychiatry 1997;42:546-52.
19. Kavoussi RJ, Coccaro EF. Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry 1998;59:676-80.
20. Sheard MH, Marini J, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry 1976;133:1409-13.
21. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder: alprazolam, carbamazepine, trifluroperazine, and tranylcypromine. Arch Gen Psychiatry 1988;45:111-19.
22. Barratt ES, Stanford MS, Felthous AR, Kent TA. The effects of phenytoin on impulsive and premeditated aggression: a controlled study. J Clin Psychopharmacology 1997;17:341-9.
23. Hollander E, Tracy KA, Swann AC, et al. Divalproex sodium is superior to placebo for impulsive aggression in Cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
Mr. P, age 41, has a “problem with anger.” Since age 17, he has had sudden outbursts of screaming and shouting, with occasional minor damage to objects. These outbursts—including episodes of “road rage”—occur once or more per week and almost daily for months at a time.
Mr. P has also had more violent episodes— sometimes every 2 to 3 months—in which he has punched holes in walls, destroyed a computer with a hammer, and assaulted other people with his fists. These events are not premeditated and are typically triggered by Mr. P’s frustration at not being “perfect” or by others breaking what he considers “general rules of conduct.”
The day before his initial visit, while he was stuck in traffic, Mr. P saw a car speeding down the shoulder. Enraged, he pulled in front of the car so that the driver had to slam on the brakes. He jumped out of his car and approached the other driver, shouting obscenities. The other driver locked her door and tried to ignore Mr. P until he returned to his car. Mr. P noted that this episode “ruined” his day because of his lingering anger and irritability.
Intermittent explosive disorder (IED) is more common and complex than was once thought, based on recent evidence. Recurrent, problematic, impulsive aggression is highly comorbid with other psychiatric conditions—including mood and personality disorders—and undermines social relationships and job performance. Typical characteristics of IED are outlined in Table 1.1-3
Table 1
Typical characteristics of intermittent explosive disorder
| Onset in childhood or adolescence (mean age 15), with average duration ±20 years |
| Aggressive outbursts: |
|
| Some episodes may appear without identifiable provocation |
| Male to female ratio 3:1, although some data suggest gender parity |
| Source: Adapted from references 1-3 |
This article offers updated diagnostic criteria and a two-pronged algorithm that can help you diagnose and treat this aggression disorder.
HOW COMMON IS IED?
DSM-IV states that IED is “apparently rare.” This statement is far from surprising, given the limitations of DSM criteria. Surveys of hospitalized patients in the 1980s found that only 1.1% met DSM-III criteria for IED.4 In another study of more than 400 patients seeking treatment for aggression, only 1.8% met DSM-III criteria for IED (although far more would likely have met DSM-IV criteria).5
A more recent survey of 411 psychiatric outpatients6 found that 3.8% met current and 6.2% met lifetime DSM-IV criteria for IED, using the Structured Clinical Interview for DSM-IV Diagnoses (SCID). Reanalysis of a threefold larger data set from the same study site (Coccaro and Zimmerman, unpublished) yielded the same result.
Far from rare. More recently, our findings from a small sample suggested that the community rate of lifetime IED is about 4% by DSM-IV criteria and 5% by research criteria. In the United States, we estimate that the lifetime rate of IED could be 4.5 to 18 million persons using DSM-IV criteria or 6.7 to 22.2 million using IED research criteria. If so, IED is at least as common as other major psychiatric disorders, including schizophrenia or bipolar illness. The ongoing National Comorbidity Study is expected to produce more definitive community data.
PSYCHIATRIC COMORBIDITY
Axis I disorders. IED is highly comorbid with mood, anxiety, and substance use disorders,3,7,8 although no causal relationship has been shown
Mood and substance abuse disorders. IED’s age of onset may precede that of mood and substance use disorders, according to analysis of our unpublished data. If so, comorbid IED may not occur in the context of mood or substance use disorders.
Anxiety disorders. We have noted a similar pattern with IED and anxiety disorders, although phobic anxiety disorders (simple or social phobia) tend to manifest earlier than IED. This suggests that early-onset phobic anxiety might be associated with an increased risk of IED in adolescence or young adulthood.
Bipolar disorder. McElroy9 has suggested a relationship between IED and bipolar disorder. In some samples, as many as one-half of IED patients (56%) have comorbid bipolar disorder when one includes bipolar II and cyclothymia.3 Moreover, some subjects’ aggressive episodes appear to resemble “microdysphoric manic episodes.”9 Other studies,8 however, find a much lower rate (10% or less) of IED comorbidity with bipolar illness.
Bipolar disorder overall may not be highly comorbid with IED, although rates may be higher in specialty clinic samples. In individuals with any kind of bipolar disorder, mood stabilizers— rather than selective serotonin reuptake inhibitors (SSRIs)—are probably the better choice as first-line treatment of IED.9
Axis II disorders. DSM-IV allows IED diagnosis in individuals with borderline or antisocial personality disorder, as long as these cluster B disorders do not better explain the aggressive behavior. How a clinician makes this distinction is not clear; in fact, most clinicians do not diagnose IED in patients with personality disorders, regardless of the clinical picture.
IED comorbidity with borderline or antisocial personality disorders varies with the sample. Persons with personality disorders who seek treatment of aggressive behavior are more likely to have comorbid IED (90%) than those not seeking treatment who are outpatients (50%) or in the community (25%).1,7
Individuals with personality disorders and IED score higher in aggression and lower in psychosocial function than do similar individuals without IED,7 indicating that the additional diagnosis is relevant.
Case report continued.
Mr. P’s outbursts have cost him several friendships, including romantic relationships. He has never advanced at work because he is seen as too volatile to supervise subordinates. Though some of Mr. P’s aggressive outbursts have occurred under the influence of alcohol, most are not related to alcohol or drug use. He has no medical problems and no other psychiatric history.
A full diagnostic evaluation uncovers a personality disorder, not otherwise specified (eight scattered traits from obsessive-compulsive personality disorder and from each of the cluster B personality disorders), and no Axis I condition other than intermittent explosive disorder.
PROBLEMS DEFINING IED
Intermittent explosive disorder is the only DSM diagnosis that applies to persons with histories of recurrent, problematic aggression not caused by another mental or physical disorder. Even so, little research on IED is available. DSM criteria for IED are poorly operationalized and have improved only modestly since the diagnosis was first included in DSM-III. In that revision, IED had four criteria.
“A” criteria specified recurrent outbursts of “seriously assaultive or destructive behavior,” but left unanswered important questions such as:
- What behavior crosses the threshold for seriously” assaultive or destructive?
- Does any physical assault qualify, or only those that cause physical injury (or stigmata)?
- How often or within what time must the behavior occur?
The phrase “recurrent acts of aggression” suggested that at least three acts of aggression were required to reach the threshold, but DSM-III provided no guidelines.
“B” criteria stated that the aggression should be out of proportion to the provocation. But how should one judge this criterion, when provocative stimuli sometimes are clearly sufficient to prompt a justifiably aggressive act?
“C” criteria excluded persons who are aggressive or impulsive between ill-defined “aggressive episodes.” This exclusion was especially limiting because individuals with recurrent, problematic, aggressive behaviors generally are impulsive and aggressive between more-severe outbursts. Excluding those who otherwise met diagnostic criteria for IED led to a spuriously low prevalence rate and limited the number of research subjects. DSM-IV eliminated this criterion but made no other notable changes in IED criteria.
“D” criteria in DSM-III and III-R further restricted the number of individuals who could meet this diagnosis:
- In DSM-III, antisocial personality disorder excluded the diagnosis of IED.
- In DSM-III-R, borderline personality disorder was added as an exclusionary factor.
Because of these restrictions, very few clinically valid cases of IED (individuals meeting A and B criteria) could receive an IED diagnosis.10
EVOLVING DIAGNOSTIC CRITERIA
By the early 1990s, DSM diagnostic criteria clearly severely restricted the study of recurrent, problematic aggression, even though research since DSM-III had greatly advanced our understanding of human aggression. For example, data linked impulsive aggression to deficits in central serotonergic function and suggested that agents that enhance serotonergic activity could modify this behavior.
Some investigators proposed research criteria for IED (IED-R) so that individuals with recurrent, problematic, impulsive aggression could be identified and studied. Research criteria first published in 19987 proposed six changes/clarifications in IED diagnostic criteria:
Lower-intensity aggression. The scope of aggressive behavior was expanded to include verbal and indirect physical aggression, provided that these behaviors are associated with distress and/or impairment. Data from double-blind, placebo-controlled trials indicated that these lower-intensity (although usually higher frequency) behaviors respond well to treatment with SSRIs.11,12
Impulsivity. The aggression was specified as impulsive. This change identified individuals with greater liability for deficits in central serotonergic function and excluded individuals with premeditated or criminal aggression.
A minimal frequency of aggression over time was proposed to make the IED diagnosis more reliable and to ensure that persons with only occasional impulsive aggressive outbursts (especially of low severity) were given this diagnosis.
Subjective distress (in the individual) and/or social or occupational dysfunction was proposed so that putatively aggressive individuals are not diagnosed for manifesting behaviors that are not functionally severe.
Diagnostic exclusionary criteria were modified so that individuals with:
- antisocial or borderline personality disorder could be diagnosed with IED if otherwise warranted
- aggressive behaviors confined within major depression episodes could not be diagnosed with IED.
This last change recognized that impulsive, aggressive outbursts could point to major depressive disorder.
When the revised criteria were tested in patients seeking treatment for aggression, those who met IED-R criteria were found to exhibit significantly greater aggression and impulsivity (using validated scales) and lower global functioning than those who did not.7 Statistical adjustments made to account for aggression score differences eliminated the difference in global functioning, which suggested a direct link between aggression and global function in individuals with IED-R.
Two patterns. Later research uncovered at least patterns of aggressive outbursts:
- low intensity at high frequency (such as verbal arguments or door slamming approximately twice weekly)
- high intensity at low frequency (such as physical aggression resulting in injury or destruction of nontrivial property at least three times per year).
Data revealed that 69% of individuals with IED-like histories displayed both aggression patterns, 20% displayed only the high-intensity/low-frequency pattern, and 11% displayed only the low-intensity/high-frequency pattern.
Because further analysis revealed no important differences between these groups in measures of aggression and impulsivity, IED-R criteria were revised to include both patterns in the “A” criteria. This revision integrated the essences of IED-R and DSM criteria into one diagnostic set (Table 2).
INFLUENCE OF HEREDITY
No twin or adoption studies of IED have been performed. However, family history data suggest that IED (or IED-type behavior) is familial. I recently conducted a blinded, controlled, family history study using IED-R criteria and found a significantly elevated risk for IED (p < 0.01) in relatives of persons with a history of IED (26%), compared with non-IED controls (8%). Comorbid conditions did not affect the risk among the IED subjects or their relatives, suggesting that IED is familial and independent of other conditions.13
Nearly all studies of aggression’s biology and treatment have measured aggression as a dimensional variable along a continuous scale from low to high.14 Our studies have allowed us to explore biological and treatment response correlates. In preliminary analyses, we have found that the maximal prolactin response to d-fenfluramine challenge and the number of platelet serotonin transporter binding sites are:
- reduced in subjects meeting research criteria for IED
- inversely correlated with dimensional measures of impulsive aggression.
Table 2
Updated diagnostic criteria for intermittent explosive disorder
| A. Recurrent incidents of aggression manifest as either: |
| 1. Verbal or physical aggression towards other people, animals, or property occurring twice weekly on average for 1 month |
| OR |
| 2. Three episodes involving physical assault against other people or destruction of property over a 1-year period |
| B. The degree of aggressiveness expressed is grossly out of proportion to the provocation or any precipitating psychosocial stressors |
| C. The aggressive behavior is generally not premeditated (ie, is impulsive) and is not committed to achieve a tangible objective (such as money, power, intimidation, etc.) |
| D. The aggressive behavior causes marked distress in the individual or impairs occupational or interpersonal functioning |
| E. The aggressive behavior is not better explained by another mental disorder (such as a major depressive/manic/psychotic disorder, attention-deficit/hyperactivity disorder, general medical condition [head trauma, Alzheimer’s disease], or due to the direct physiologic effects of a substance) |
| Source: Adapted from reference 7 |
Earlier, Virkkunen et al15 reported reduced cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations in persons diagnosed with IED based on DSM-III criteria, compared with persons who were not diagnosed with IED and those who demonstrated nonimpulsive aggression.
TREATING IED
Cognitive therapy. Few double-blind, randomized, placebo-controlled trials of any treatments for IED have been published. Trials using cognitive-behavioral approaches have reduced self-rated anger and its expression in young adults with anger disorders.16 Although many of these subjects may have had IED, it is not known if this approach works in IED.
Table 3
Characteristic behaviors of aggressive individuals*
| Severity | Behaviors |
|---|---|
| Mildly aggressive | Occasional verbal arguments and/or temper tantrums |
| Moderately aggressive | Frequent verbal arguments and temper tantrums (about twice weekly on average), occasional destruction of property, rare or occasional physical assault against others (usually without injury) |
| Highly aggressive | Frequent verbal arguments and temper tantrums (about twice weekly) and/or more than occasional destruction of property or physical assault against others, sometimes with injury |
| * Characteristics given are descriptive and not based on data. | |
Drug therapy. SSRIs. A trial by this author using fluoxetine showed that impulsive aggressive behavior responds to treatment that targets the central serotonergic system.12 Forty subjects with personality disorders and histories of impulsive aggression received fluoxetine, 20 to 60 mg qd, or placebo for 12 weeks. Fluoxetine reduced overt aggression and irritability about 67% more than placebo, as assessed by the Overt Aggression Scale Modified for Outpatients (OAS-M).
All subjects met research criteria for IED. A reanalysis suggests that SSRIs may be most effective in moderately aggressive patients (Table 3),17 whose serotonergic system may be less impaired than that of highly aggressive patients.18
Mood stabilizers. Impulsively aggressive subjects who do not respond to an SSRI may respond to a mood stabilizer.19 An antiaggressive response in IED-like subjects has been reported for lithium,20 carbamazepine,21 and diphenylhydantoin.22
Recently, Hollander et al23 reported greater reduction in overt aggression scores in IED subjects with a DSM cluster B personality disorder who were treated with divalproex, compared with placebo. This study used the same design and outcome measure as our study12 and included subjects who met both DSM-IV and research criteria for IED.
For unknown reasons, divalproex was no more effective than placebo in IED subjects without cluster B personality disorder. More research is needed to uncover predictors of antiaggressive response in IED subjects.
Unipolar vs. bipolar. McElroy9 has suggested using SSRIs (or other antidepressants) as first-line treatment for IED subjects with unipolar affective symptoms and mood stabilizers for those with bipolar affective symptoms. IED subjects without bipolar affective symptoms should be treated first with SSRIs (Algorithm). Preliminary data suggest a role for atypical antipsychotics to treat aggressive behavior in patients with schizophrenia or bipolar disorder, but no empiric data exist.
Beta blockers such as propranolol also may be considered.2 However, beta blockers are more difficult to dose and are associated with more burdensome side effects, compared with SSRIs.
Algorithm Suggested 2-pronged approach for treating intermittent explosive disorder
* With or without an anger management program, which may precede drug interventionThe full effects of antiaggressive treatment with an SSRI (E. Coccaro, unpublished observations) or a mood stabilizer19 may take 3 months to observe12,20,22,23 and tend to disappear soon after treatment is discontinued.
Therefore, an adequate trial of SSRIs or mood stabilizers is no less than 3 months. If improvement is seen, continue drug treatment indefinitely.
Case report continued.
Mr. P was started on an SSRI. His aggressive outbursts decreased in intensity and frequency over 3 months but were not eliminated. After 6 months he dropped out of treatment, but returned 5 weeks later because his aggressive outbursts had resumed their pre-treatment level.
SSRI treatment was restarted, and Mr. P began a 12-week anger management course of relaxation training, cognitive restructuring, and coping skills training. He gained greater control over his aggressive outbursts and continues monthly medication checks and anger management “booster sessions.”
Related resources
- Galovski T, Blanchard EB, Veazey C. Intermittent explosive disorder and other psychiatric comorbidity among court-referred and self-referred aggressive drivers. Behav Res Ther 2002;40:641-51.
- Olvera RL. Intermittent explosive disorder: epidemiology, diagnosis and management. CNS Drugs 2002;16:517-26.
Drug brand names
- Carbamazepine • Tegretol
- Diphenylhydantoin • Dilantin
- Divalproex • Depakote
- Fluoxetine • Prozac
- Lithium • Lithobid
- Propanolol • Inderal
Disclosure
Dr. Coccaro reports that he receives research grants and serves on the speaker’s bureau or as a consultant to Eli Lilly and Co., Abbott Laboratories, GlaxoSmithKline, and Forrest Laboratories.
Mr. P, age 41, has a “problem with anger.” Since age 17, he has had sudden outbursts of screaming and shouting, with occasional minor damage to objects. These outbursts—including episodes of “road rage”—occur once or more per week and almost daily for months at a time.
Mr. P has also had more violent episodes— sometimes every 2 to 3 months—in which he has punched holes in walls, destroyed a computer with a hammer, and assaulted other people with his fists. These events are not premeditated and are typically triggered by Mr. P’s frustration at not being “perfect” or by others breaking what he considers “general rules of conduct.”
The day before his initial visit, while he was stuck in traffic, Mr. P saw a car speeding down the shoulder. Enraged, he pulled in front of the car so that the driver had to slam on the brakes. He jumped out of his car and approached the other driver, shouting obscenities. The other driver locked her door and tried to ignore Mr. P until he returned to his car. Mr. P noted that this episode “ruined” his day because of his lingering anger and irritability.
Intermittent explosive disorder (IED) is more common and complex than was once thought, based on recent evidence. Recurrent, problematic, impulsive aggression is highly comorbid with other psychiatric conditions—including mood and personality disorders—and undermines social relationships and job performance. Typical characteristics of IED are outlined in Table 1.1-3
Table 1
Typical characteristics of intermittent explosive disorder
| Onset in childhood or adolescence (mean age 15), with average duration ±20 years |
| Aggressive outbursts: |
|
| Some episodes may appear without identifiable provocation |
| Male to female ratio 3:1, although some data suggest gender parity |
| Source: Adapted from references 1-3 |
This article offers updated diagnostic criteria and a two-pronged algorithm that can help you diagnose and treat this aggression disorder.
HOW COMMON IS IED?
DSM-IV states that IED is “apparently rare.” This statement is far from surprising, given the limitations of DSM criteria. Surveys of hospitalized patients in the 1980s found that only 1.1% met DSM-III criteria for IED.4 In another study of more than 400 patients seeking treatment for aggression, only 1.8% met DSM-III criteria for IED (although far more would likely have met DSM-IV criteria).5
A more recent survey of 411 psychiatric outpatients6 found that 3.8% met current and 6.2% met lifetime DSM-IV criteria for IED, using the Structured Clinical Interview for DSM-IV Diagnoses (SCID). Reanalysis of a threefold larger data set from the same study site (Coccaro and Zimmerman, unpublished) yielded the same result.
Far from rare. More recently, our findings from a small sample suggested that the community rate of lifetime IED is about 4% by DSM-IV criteria and 5% by research criteria. In the United States, we estimate that the lifetime rate of IED could be 4.5 to 18 million persons using DSM-IV criteria or 6.7 to 22.2 million using IED research criteria. If so, IED is at least as common as other major psychiatric disorders, including schizophrenia or bipolar illness. The ongoing National Comorbidity Study is expected to produce more definitive community data.
PSYCHIATRIC COMORBIDITY
Axis I disorders. IED is highly comorbid with mood, anxiety, and substance use disorders,3,7,8 although no causal relationship has been shown
Mood and substance abuse disorders. IED’s age of onset may precede that of mood and substance use disorders, according to analysis of our unpublished data. If so, comorbid IED may not occur in the context of mood or substance use disorders.
Anxiety disorders. We have noted a similar pattern with IED and anxiety disorders, although phobic anxiety disorders (simple or social phobia) tend to manifest earlier than IED. This suggests that early-onset phobic anxiety might be associated with an increased risk of IED in adolescence or young adulthood.
Bipolar disorder. McElroy9 has suggested a relationship between IED and bipolar disorder. In some samples, as many as one-half of IED patients (56%) have comorbid bipolar disorder when one includes bipolar II and cyclothymia.3 Moreover, some subjects’ aggressive episodes appear to resemble “microdysphoric manic episodes.”9 Other studies,8 however, find a much lower rate (10% or less) of IED comorbidity with bipolar illness.
Bipolar disorder overall may not be highly comorbid with IED, although rates may be higher in specialty clinic samples. In individuals with any kind of bipolar disorder, mood stabilizers— rather than selective serotonin reuptake inhibitors (SSRIs)—are probably the better choice as first-line treatment of IED.9
Axis II disorders. DSM-IV allows IED diagnosis in individuals with borderline or antisocial personality disorder, as long as these cluster B disorders do not better explain the aggressive behavior. How a clinician makes this distinction is not clear; in fact, most clinicians do not diagnose IED in patients with personality disorders, regardless of the clinical picture.
IED comorbidity with borderline or antisocial personality disorders varies with the sample. Persons with personality disorders who seek treatment of aggressive behavior are more likely to have comorbid IED (90%) than those not seeking treatment who are outpatients (50%) or in the community (25%).1,7
Individuals with personality disorders and IED score higher in aggression and lower in psychosocial function than do similar individuals without IED,7 indicating that the additional diagnosis is relevant.
Case report continued.
Mr. P’s outbursts have cost him several friendships, including romantic relationships. He has never advanced at work because he is seen as too volatile to supervise subordinates. Though some of Mr. P’s aggressive outbursts have occurred under the influence of alcohol, most are not related to alcohol or drug use. He has no medical problems and no other psychiatric history.
A full diagnostic evaluation uncovers a personality disorder, not otherwise specified (eight scattered traits from obsessive-compulsive personality disorder and from each of the cluster B personality disorders), and no Axis I condition other than intermittent explosive disorder.
PROBLEMS DEFINING IED
Intermittent explosive disorder is the only DSM diagnosis that applies to persons with histories of recurrent, problematic aggression not caused by another mental or physical disorder. Even so, little research on IED is available. DSM criteria for IED are poorly operationalized and have improved only modestly since the diagnosis was first included in DSM-III. In that revision, IED had four criteria.
“A” criteria specified recurrent outbursts of “seriously assaultive or destructive behavior,” but left unanswered important questions such as:
- What behavior crosses the threshold for seriously” assaultive or destructive?
- Does any physical assault qualify, or only those that cause physical injury (or stigmata)?
- How often or within what time must the behavior occur?
The phrase “recurrent acts of aggression” suggested that at least three acts of aggression were required to reach the threshold, but DSM-III provided no guidelines.
“B” criteria stated that the aggression should be out of proportion to the provocation. But how should one judge this criterion, when provocative stimuli sometimes are clearly sufficient to prompt a justifiably aggressive act?
“C” criteria excluded persons who are aggressive or impulsive between ill-defined “aggressive episodes.” This exclusion was especially limiting because individuals with recurrent, problematic, aggressive behaviors generally are impulsive and aggressive between more-severe outbursts. Excluding those who otherwise met diagnostic criteria for IED led to a spuriously low prevalence rate and limited the number of research subjects. DSM-IV eliminated this criterion but made no other notable changes in IED criteria.
“D” criteria in DSM-III and III-R further restricted the number of individuals who could meet this diagnosis:
- In DSM-III, antisocial personality disorder excluded the diagnosis of IED.
- In DSM-III-R, borderline personality disorder was added as an exclusionary factor.
Because of these restrictions, very few clinically valid cases of IED (individuals meeting A and B criteria) could receive an IED diagnosis.10
EVOLVING DIAGNOSTIC CRITERIA
By the early 1990s, DSM diagnostic criteria clearly severely restricted the study of recurrent, problematic aggression, even though research since DSM-III had greatly advanced our understanding of human aggression. For example, data linked impulsive aggression to deficits in central serotonergic function and suggested that agents that enhance serotonergic activity could modify this behavior.
Some investigators proposed research criteria for IED (IED-R) so that individuals with recurrent, problematic, impulsive aggression could be identified and studied. Research criteria first published in 19987 proposed six changes/clarifications in IED diagnostic criteria:
Lower-intensity aggression. The scope of aggressive behavior was expanded to include verbal and indirect physical aggression, provided that these behaviors are associated with distress and/or impairment. Data from double-blind, placebo-controlled trials indicated that these lower-intensity (although usually higher frequency) behaviors respond well to treatment with SSRIs.11,12
Impulsivity. The aggression was specified as impulsive. This change identified individuals with greater liability for deficits in central serotonergic function and excluded individuals with premeditated or criminal aggression.
A minimal frequency of aggression over time was proposed to make the IED diagnosis more reliable and to ensure that persons with only occasional impulsive aggressive outbursts (especially of low severity) were given this diagnosis.
Subjective distress (in the individual) and/or social or occupational dysfunction was proposed so that putatively aggressive individuals are not diagnosed for manifesting behaviors that are not functionally severe.
Diagnostic exclusionary criteria were modified so that individuals with:
- antisocial or borderline personality disorder could be diagnosed with IED if otherwise warranted
- aggressive behaviors confined within major depression episodes could not be diagnosed with IED.
This last change recognized that impulsive, aggressive outbursts could point to major depressive disorder.
When the revised criteria were tested in patients seeking treatment for aggression, those who met IED-R criteria were found to exhibit significantly greater aggression and impulsivity (using validated scales) and lower global functioning than those who did not.7 Statistical adjustments made to account for aggression score differences eliminated the difference in global functioning, which suggested a direct link between aggression and global function in individuals with IED-R.
Two patterns. Later research uncovered at least patterns of aggressive outbursts:
- low intensity at high frequency (such as verbal arguments or door slamming approximately twice weekly)
- high intensity at low frequency (such as physical aggression resulting in injury or destruction of nontrivial property at least three times per year).
Data revealed that 69% of individuals with IED-like histories displayed both aggression patterns, 20% displayed only the high-intensity/low-frequency pattern, and 11% displayed only the low-intensity/high-frequency pattern.
Because further analysis revealed no important differences between these groups in measures of aggression and impulsivity, IED-R criteria were revised to include both patterns in the “A” criteria. This revision integrated the essences of IED-R and DSM criteria into one diagnostic set (Table 2).
INFLUENCE OF HEREDITY
No twin or adoption studies of IED have been performed. However, family history data suggest that IED (or IED-type behavior) is familial. I recently conducted a blinded, controlled, family history study using IED-R criteria and found a significantly elevated risk for IED (p < 0.01) in relatives of persons with a history of IED (26%), compared with non-IED controls (8%). Comorbid conditions did not affect the risk among the IED subjects or their relatives, suggesting that IED is familial and independent of other conditions.13
Nearly all studies of aggression’s biology and treatment have measured aggression as a dimensional variable along a continuous scale from low to high.14 Our studies have allowed us to explore biological and treatment response correlates. In preliminary analyses, we have found that the maximal prolactin response to d-fenfluramine challenge and the number of platelet serotonin transporter binding sites are:
- reduced in subjects meeting research criteria for IED
- inversely correlated with dimensional measures of impulsive aggression.
Table 2
Updated diagnostic criteria for intermittent explosive disorder
| A. Recurrent incidents of aggression manifest as either: |
| 1. Verbal or physical aggression towards other people, animals, or property occurring twice weekly on average for 1 month |
| OR |
| 2. Three episodes involving physical assault against other people or destruction of property over a 1-year period |
| B. The degree of aggressiveness expressed is grossly out of proportion to the provocation or any precipitating psychosocial stressors |
| C. The aggressive behavior is generally not premeditated (ie, is impulsive) and is not committed to achieve a tangible objective (such as money, power, intimidation, etc.) |
| D. The aggressive behavior causes marked distress in the individual or impairs occupational or interpersonal functioning |
| E. The aggressive behavior is not better explained by another mental disorder (such as a major depressive/manic/psychotic disorder, attention-deficit/hyperactivity disorder, general medical condition [head trauma, Alzheimer’s disease], or due to the direct physiologic effects of a substance) |
| Source: Adapted from reference 7 |
Earlier, Virkkunen et al15 reported reduced cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations in persons diagnosed with IED based on DSM-III criteria, compared with persons who were not diagnosed with IED and those who demonstrated nonimpulsive aggression.
TREATING IED
Cognitive therapy. Few double-blind, randomized, placebo-controlled trials of any treatments for IED have been published. Trials using cognitive-behavioral approaches have reduced self-rated anger and its expression in young adults with anger disorders.16 Although many of these subjects may have had IED, it is not known if this approach works in IED.
Table 3
Characteristic behaviors of aggressive individuals*
| Severity | Behaviors |
|---|---|
| Mildly aggressive | Occasional verbal arguments and/or temper tantrums |
| Moderately aggressive | Frequent verbal arguments and temper tantrums (about twice weekly on average), occasional destruction of property, rare or occasional physical assault against others (usually without injury) |
| Highly aggressive | Frequent verbal arguments and temper tantrums (about twice weekly) and/or more than occasional destruction of property or physical assault against others, sometimes with injury |
| * Characteristics given are descriptive and not based on data. | |
Drug therapy. SSRIs. A trial by this author using fluoxetine showed that impulsive aggressive behavior responds to treatment that targets the central serotonergic system.12 Forty subjects with personality disorders and histories of impulsive aggression received fluoxetine, 20 to 60 mg qd, or placebo for 12 weeks. Fluoxetine reduced overt aggression and irritability about 67% more than placebo, as assessed by the Overt Aggression Scale Modified for Outpatients (OAS-M).
All subjects met research criteria for IED. A reanalysis suggests that SSRIs may be most effective in moderately aggressive patients (Table 3),17 whose serotonergic system may be less impaired than that of highly aggressive patients.18
Mood stabilizers. Impulsively aggressive subjects who do not respond to an SSRI may respond to a mood stabilizer.19 An antiaggressive response in IED-like subjects has been reported for lithium,20 carbamazepine,21 and diphenylhydantoin.22
Recently, Hollander et al23 reported greater reduction in overt aggression scores in IED subjects with a DSM cluster B personality disorder who were treated with divalproex, compared with placebo. This study used the same design and outcome measure as our study12 and included subjects who met both DSM-IV and research criteria for IED.
For unknown reasons, divalproex was no more effective than placebo in IED subjects without cluster B personality disorder. More research is needed to uncover predictors of antiaggressive response in IED subjects.
Unipolar vs. bipolar. McElroy9 has suggested using SSRIs (or other antidepressants) as first-line treatment for IED subjects with unipolar affective symptoms and mood stabilizers for those with bipolar affective symptoms. IED subjects without bipolar affective symptoms should be treated first with SSRIs (Algorithm). Preliminary data suggest a role for atypical antipsychotics to treat aggressive behavior in patients with schizophrenia or bipolar disorder, but no empiric data exist.
Beta blockers such as propranolol also may be considered.2 However, beta blockers are more difficult to dose and are associated with more burdensome side effects, compared with SSRIs.
Algorithm Suggested 2-pronged approach for treating intermittent explosive disorder
* With or without an anger management program, which may precede drug interventionThe full effects of antiaggressive treatment with an SSRI (E. Coccaro, unpublished observations) or a mood stabilizer19 may take 3 months to observe12,20,22,23 and tend to disappear soon after treatment is discontinued.
Therefore, an adequate trial of SSRIs or mood stabilizers is no less than 3 months. If improvement is seen, continue drug treatment indefinitely.
Case report continued.
Mr. P was started on an SSRI. His aggressive outbursts decreased in intensity and frequency over 3 months but were not eliminated. After 6 months he dropped out of treatment, but returned 5 weeks later because his aggressive outbursts had resumed their pre-treatment level.
SSRI treatment was restarted, and Mr. P began a 12-week anger management course of relaxation training, cognitive restructuring, and coping skills training. He gained greater control over his aggressive outbursts and continues monthly medication checks and anger management “booster sessions.”
Related resources
- Galovski T, Blanchard EB, Veazey C. Intermittent explosive disorder and other psychiatric comorbidity among court-referred and self-referred aggressive drivers. Behav Res Ther 2002;40:641-51.
- Olvera RL. Intermittent explosive disorder: epidemiology, diagnosis and management. CNS Drugs 2002;16:517-26.
Drug brand names
- Carbamazepine • Tegretol
- Diphenylhydantoin • Dilantin
- Divalproex • Depakote
- Fluoxetine • Prozac
- Lithium • Lithobid
- Propanolol • Inderal
Disclosure
Dr. Coccaro reports that he receives research grants and serves on the speaker’s bureau or as a consultant to Eli Lilly and Co., Abbott Laboratories, GlaxoSmithKline, and Forrest Laboratories.
1. Coccaro EF, Schimdt CA, Samuels JF, et al. Lifetime rates of intermittent explosive disorder in a community sample (abstract). Philadelphia: American Psychiatric Association annual meeting, 2002.
2. Mattes JA. Comparative effectiveness of carbamazepine and propranolol for rage outbursts. J Neuropsychiatry Clin Neurosci 1990;2:159-64.
3. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry 1998;59:203-10.
4. Monopolis S, Lion JR. Problems in the diagnosis of intermittent explosive disorder. Am J Psychiatry 1983;140:1200-2.
5. Zimmerman M, Mattia J, Younken S, Torres M. The prevalence of DSM-IV impulse control disorders in psychiatric outpatients (abstract 265). Washington, DC: American Psychiatric Association annual meeting, 1998.
6. Zimmerman M, Mattia J, Younken S, Torres M. The prevalence of DSM-IV impulse control disorders in psychiatric outpatients (APA new research abstracts #265). Washington, DC: American Psychiatric Publishing, Inc., 1998.
7. Coccaro EF, Kavoussi RJ, Berman ME, Lish JD. Intermittent explosive disorder-revised: development, reliability and validity of research criteria. Compr Psychiatry 1998;39:368-76.
8. Galovski T, Blanchard EB, Veazey C. Intermittent explosive disorder and other psychiatric comorbidity among court-referred and self-referred aggressive drivers. Behav Res Ther 2002;40:641-51.
9. McElroy SL. Recognition and treatment of DSM-IV intermittent explosive disorder. J Clin Psychiatry 1999;60(suppl 15):12-16.
10. Felthous AR, Bryant G, Wingerter CB, Barratt E. The diagnosis of intermittent explosive disorder in violent men. Bull Am Acad Psychiatry Law 1991;19:71-9.
11. Salzman C, Wolfson AN, Schatzberg A, et al. Effect of fluoxetine on anger in symptomatic volunteers with borderline personality disorder. J Clin Psychopharmacology 1995;15:23-9.
12. Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality disordered subjects. Arch Gen Psychiatry 1997;54:1081-8.
13. Coccaro EF. Family history study of intermittent explosive disorder (abstract). Washington, DC: American Psychiatric Association annual meeting, 1999.
14. Coccaro EF, Siever LJ. Pathophysiology and treatment of aggression. In: Davis KL, Charney D, Coyle JT, Nemeroff D (eds). Psychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002;1709-24
15. Virkkunen M, Rawlings R, Tokola R, et al. CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry 1994;51:20-7.
16. Deffenbacher JL. Psychosocial interventions: anger disorders. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker (in press).
17. Lee R, Coccaro EF. Treatment of aggression: serotonergic agents. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker (in press).
18. Coccaro EF, Kavoussi RJ, Hauger RL. Serotonin function and antiaggressive responses to fluoxetine: a pilot study. Biol Psychiatry 1997;42:546-52.
19. Kavoussi RJ, Coccaro EF. Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry 1998;59:676-80.
20. Sheard MH, Marini J, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry 1976;133:1409-13.
21. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder: alprazolam, carbamazepine, trifluroperazine, and tranylcypromine. Arch Gen Psychiatry 1988;45:111-19.
22. Barratt ES, Stanford MS, Felthous AR, Kent TA. The effects of phenytoin on impulsive and premeditated aggression: a controlled study. J Clin Psychopharmacology 1997;17:341-9.
23. Hollander E, Tracy KA, Swann AC, et al. Divalproex sodium is superior to placebo for impulsive aggression in Cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
1. Coccaro EF, Schimdt CA, Samuels JF, et al. Lifetime rates of intermittent explosive disorder in a community sample (abstract). Philadelphia: American Psychiatric Association annual meeting, 2002.
2. Mattes JA. Comparative effectiveness of carbamazepine and propranolol for rage outbursts. J Neuropsychiatry Clin Neurosci 1990;2:159-64.
3. McElroy SL, Soutullo CA, Beckman DA, et al. DSM-IV intermittent explosive disorder: a report of 27 cases. J Clin Psychiatry 1998;59:203-10.
4. Monopolis S, Lion JR. Problems in the diagnosis of intermittent explosive disorder. Am J Psychiatry 1983;140:1200-2.
5. Zimmerman M, Mattia J, Younken S, Torres M. The prevalence of DSM-IV impulse control disorders in psychiatric outpatients (abstract 265). Washington, DC: American Psychiatric Association annual meeting, 1998.
6. Zimmerman M, Mattia J, Younken S, Torres M. The prevalence of DSM-IV impulse control disorders in psychiatric outpatients (APA new research abstracts #265). Washington, DC: American Psychiatric Publishing, Inc., 1998.
7. Coccaro EF, Kavoussi RJ, Berman ME, Lish JD. Intermittent explosive disorder-revised: development, reliability and validity of research criteria. Compr Psychiatry 1998;39:368-76.
8. Galovski T, Blanchard EB, Veazey C. Intermittent explosive disorder and other psychiatric comorbidity among court-referred and self-referred aggressive drivers. Behav Res Ther 2002;40:641-51.
9. McElroy SL. Recognition and treatment of DSM-IV intermittent explosive disorder. J Clin Psychiatry 1999;60(suppl 15):12-16.
10. Felthous AR, Bryant G, Wingerter CB, Barratt E. The diagnosis of intermittent explosive disorder in violent men. Bull Am Acad Psychiatry Law 1991;19:71-9.
11. Salzman C, Wolfson AN, Schatzberg A, et al. Effect of fluoxetine on anger in symptomatic volunteers with borderline personality disorder. J Clin Psychopharmacology 1995;15:23-9.
12. Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality disordered subjects. Arch Gen Psychiatry 1997;54:1081-8.
13. Coccaro EF. Family history study of intermittent explosive disorder (abstract). Washington, DC: American Psychiatric Association annual meeting, 1999.
14. Coccaro EF, Siever LJ. Pathophysiology and treatment of aggression. In: Davis KL, Charney D, Coyle JT, Nemeroff D (eds). Psychopharmacology: the fifth generation of progress. Philadelphia: Lippincott Williams & Wilkins, 2002;1709-24
15. Virkkunen M, Rawlings R, Tokola R, et al. CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry 1994;51:20-7.
16. Deffenbacher JL. Psychosocial interventions: anger disorders. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker (in press).
17. Lee R, Coccaro EF. Treatment of aggression: serotonergic agents. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker (in press).
18. Coccaro EF, Kavoussi RJ, Hauger RL. Serotonin function and antiaggressive responses to fluoxetine: a pilot study. Biol Psychiatry 1997;42:546-52.
19. Kavoussi RJ, Coccaro EF. Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry 1998;59:676-80.
20. Sheard MH, Marini J, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry 1976;133:1409-13.
21. Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder: alprazolam, carbamazepine, trifluroperazine, and tranylcypromine. Arch Gen Psychiatry 1988;45:111-19.
22. Barratt ES, Stanford MS, Felthous AR, Kent TA. The effects of phenytoin on impulsive and premeditated aggression: a controlled study. J Clin Psychopharmacology 1997;17:341-9.
23. Hollander E, Tracy KA, Swann AC, et al. Divalproex sodium is superior to placebo for impulsive aggression in Cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.