User login
Epidermal Growth Factor Receptor Inhibitor–Induced Symmetrical Drug-Related Intertriginous and Flexural Exanthema: Should You Discontinue the Offending Agent?
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
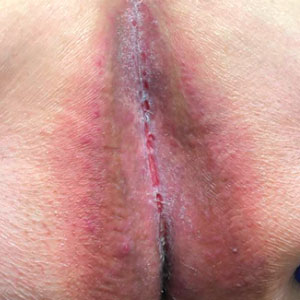
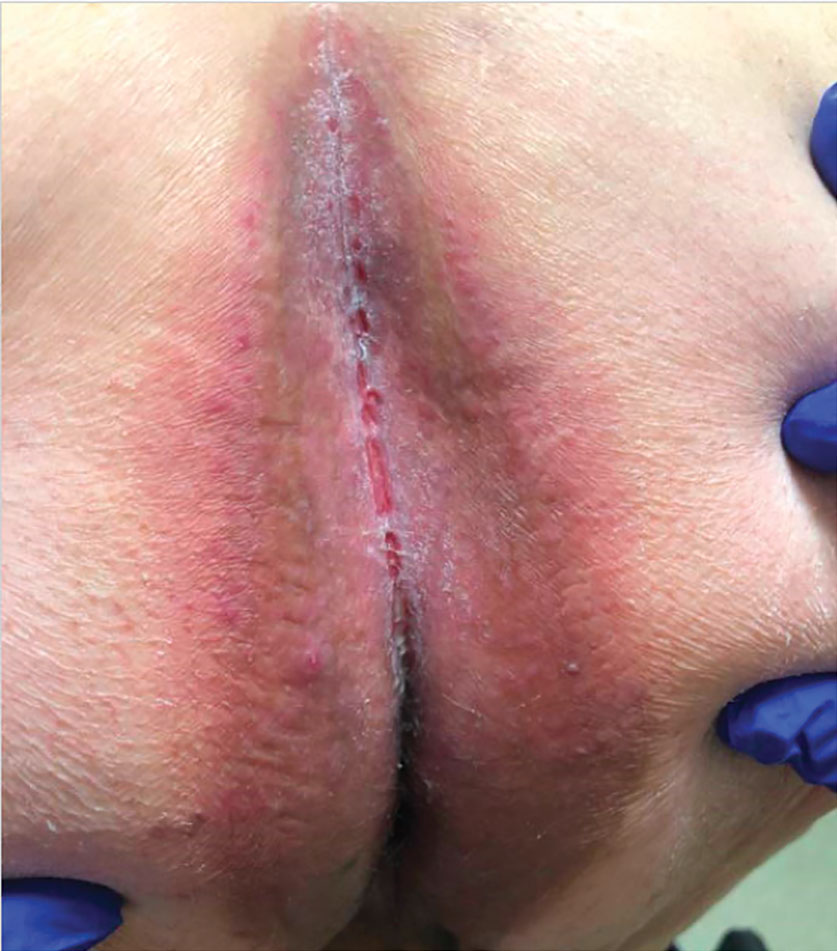
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.
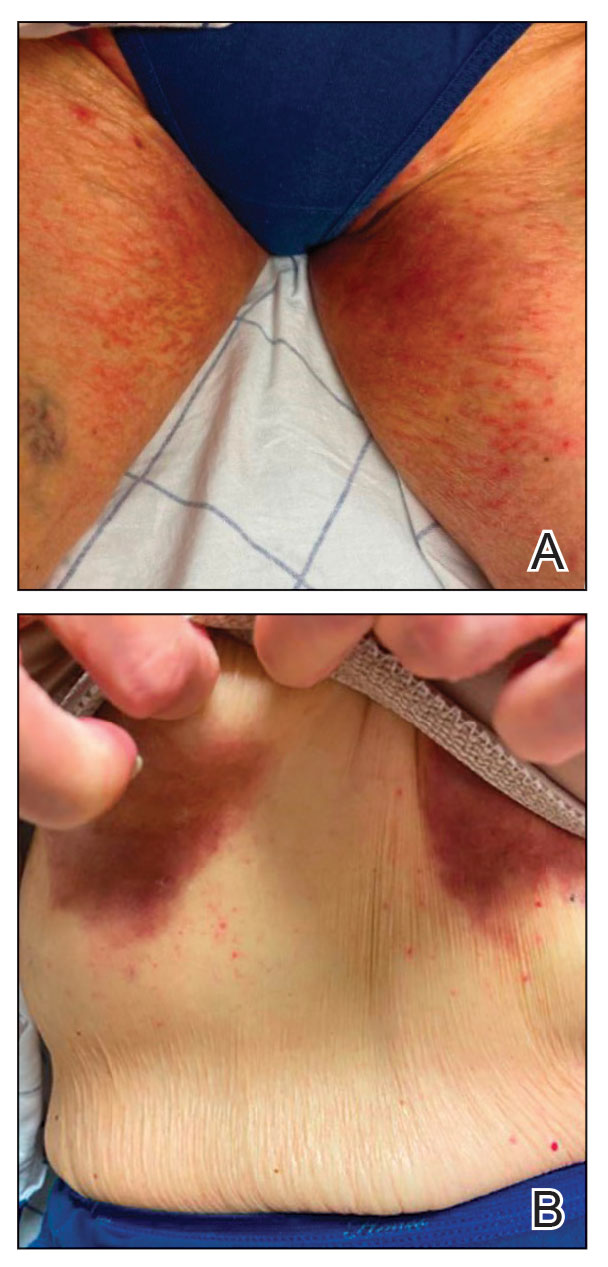
Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.
Comment
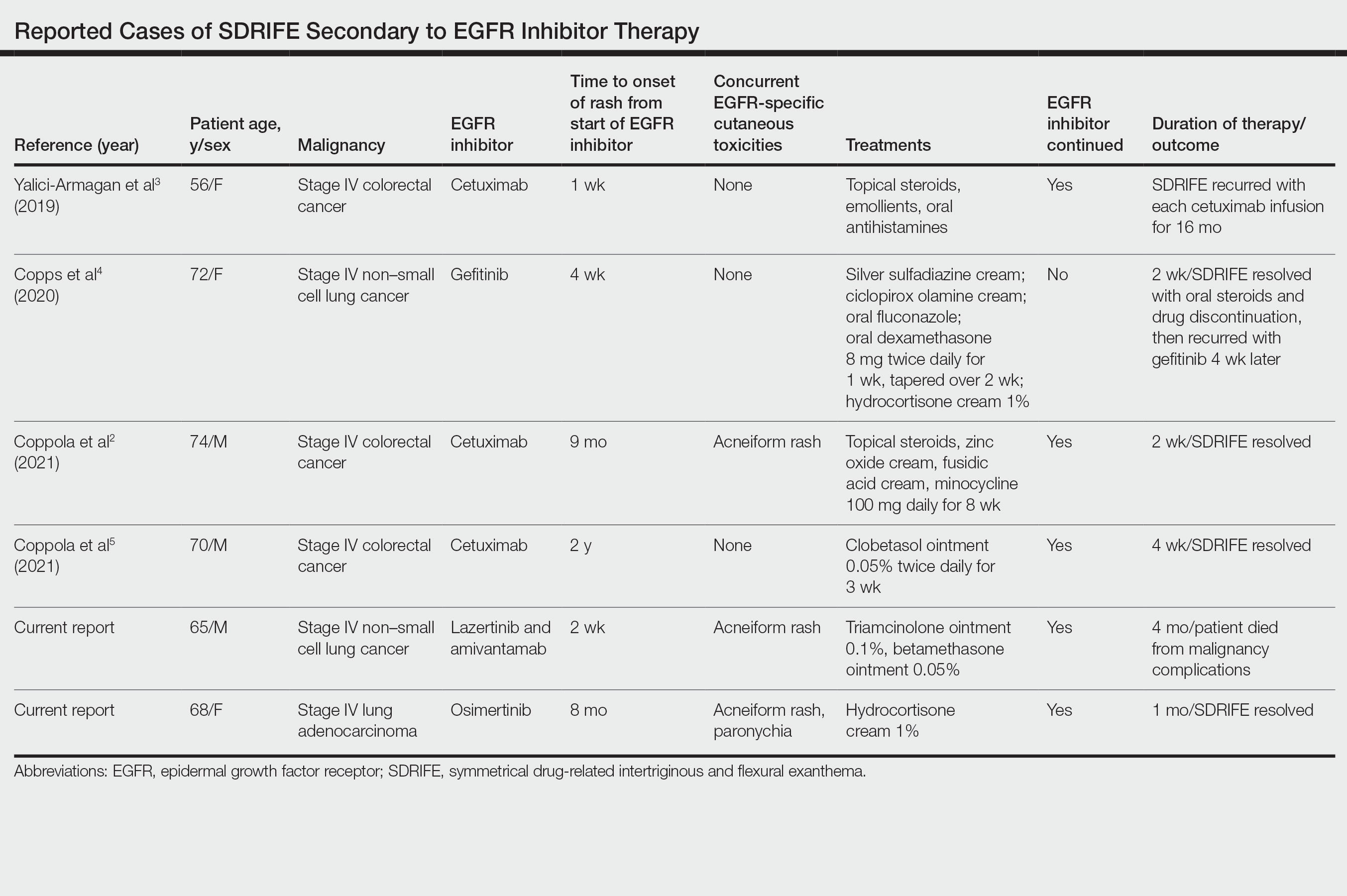
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.
Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.

Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.

Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.

Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.

Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.

Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.

Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
Practice Points
- Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon but increasingly recognized cutaneous adverse event (AE) of epidermal growth factor receptor (EGFR) inhibitors.
- Epidermal growth factor receptor inhibitor–associated SDRIFE may be approached similarly to other EGFR inhibitor–related cutaneous AEs in that it may not require discontinuation of the offending agent.