User login
Management of Dyslipidemia in the Elderly
From the Harrison School of Pharmacy, Auburn University, Mobile, AL.
Abstract
- Objective: To summarize the literature relevant to managing dyslipidemia in the elderly and review recommendations for initiating lipid-lowering therapy.
- Methods: Review of the literature.
- Results: Statins are the most commonly utilized medication class for lipid-lowering in the general population, and they are recommended for primary prevention in patients between the ages of 40 to 75 with at least 1 risk factor for cardiovascular disease as well as for any patient needing secondary prevention. In the elderly, statins may be appropriate for both primary and secondary prevention if the benefits outweigh the risks. Based on the available evidence, it is safe to recommend statin therapy to elderly patients who require secondary prevention given the known benefits in reducing cardiovascular morbidity and mortality for patients up to the age of 80. For primary prevention, statin therapy may be beneficial, but one must carefully evaluate for comorbid conditions, life expectancy, concomitant medications, overall health status, frailty, and patient or family preference. Several other classes of lipid-lowering agents exist; however, there is not enough evidence for us to recommend use in the elderly population for cardiovascular risk reduction in either primary or secondary scenarios.
- Conclusion: Although clinical research in the elderly population is limited, evidence supports the use of statins in elderly patients for secondary prevention and in patients up to age 75 for primary prevention; however clinicians must use clinical judgement and take into consideration the patient’s situation regarding comorbidities, polypharmacy, and possible adverse effects. More high-quality evidence is necessary.
Key words: hyperlipidemia; geriatrics; elderly; patient-centered care; statin; cardiovascular disease.
The number of Americans age 65 years and older is projected to more than double, from 46 million today to over 98 million by 2060, and the 65-and-older age group’s share of the total population will rise to nearly 24% [1]. Life expectancy is now predicted to be > 20 years for women at age 65 and > 17 years for men at age 65 in many high-income countries, including the United States [2]. This demographic shift toward an older population will result in a higher burden of coronary heart disease and stroke, with atherosclerotic cardiovascular disease (ASCVD) prevalence and costs projected to increase substantially [3].
Among adults seeking medical care in the United States, roughly 95 million have a total cholesterol (TC) level of ≥ 200 mg/dL or more, and approximately 29 million have a TC > 240 mg/dL [4]. Cholesterol screening is important since most patients suffering from dyslipidemia are asymptomatic. Dyslipidemia is a major risk factor for the development of atherosclerotic disease. Because of the complications associated with dyslipidemia, it is vital that patients are provided with primary and/or secondary prevention strategies to reduce the risk of cardiovascular disease (CVD) and protect high-risk patients from recurring events. A clinical controversy exists surrounding the elderly population, concerning whether or not clinicians should be providing lipid-lowering treatment to this group of individuals for dyslipidemia. The evidence is limited for patients over age 65, and even more so for the very elderly (> 80 years); therefore, it is necessary to review the available evidence to make an appropriate decision when it comes to managing dyslipidemia in the elderly population
Currently, HMG-CoA reductase inhibitors (statins) are the only known class of medications for the treatment of dyslipidemia that will prevent both primary and secondary cardiovascular (CV) events, including death. Statin intensity (Table 1)
Guideline Recommendations
Current guidelines differ in their recommendations for treating dyslipidemia in the elderly population. In 2016, the Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) released updated guidelines for managing dyslipidemia. These guidelines recommend that older patients with established CVD be treated in the same way as younger patients because of the many benefits statin therapy demonstrated in clinical trials. They also suggest that statin therapy be started at a lower doses to achieve goals for primary prevention in the older population. In addition, CVD risk factors (hypertension, diabetes, dyslipidemia, smoking) should be addressed in this population to reduce CVD risk. They also acknowledged that primary prevention may not prolong life in the older adult, but treatment does reduce cardiovascular mortality and statin therapy is recommended to reduce the overall risk of CV morbidity in this population [11]. In contrast, The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines changed the management and treatment of dyslipidemia by highlighting “statin benefit groups” rather than recommending a treat-to-target goal as guidelines had done for many years. ACC/AHA recommends a moderate-intensity statin for patients > 75 years of age for secondary prevention versus the use of a high-intensity statin for patients who are between the ages of 40 and 75 based on the pooled cohort risk equation. In patients over age 75 with no history of CVD, no specific recommendation is available for the use of lipid-lowering therapy at this time [12]. ACC/AHA is expected to publish a new set of guidelines sometime in 2018 and they are projected to utilize lipid-lowering goals in combination with the pooled cohort equation to assess overall risk in patients with dyslipidemia.
The 2015 National Lipid Association (NLA) released “Part 1” guidelines for the management of dyslipidemia and then provided “Part 2” about a year later, which focuses on management for special populations. To summarize, the NLA guidelines recommend that elderly patients between the ages of 65 and 80 receive a high-intensity statin for secondary prevention after special consideration of the potential risks and benefits. In patients over the age of 80, NLA recommends a moderate-intensity statin for secondary prevention. For primary prevention, NLA recommends utilizing the pooled cohort risk equation to analyze patient characteristics, keeping in mind that age is a driving factor for increased risk of CVD and that the actual risk for developing a CV event may be “overestimated” if the patient has no other risk factors other than their age. When evaluating patients between the ages of 65 and 79 for primary prevention, NLA suggests following Part 1 of the guidelines. In Part 1, NLA recommends evaluating the patient’s characteristics and suggests a moderate- or high-intensity statin if the patient is considered “very high risk” or “high risk” and a moderate-intensity statin for patients who are considered “moderate risk”. For patients over the age of 80, they recommend utilizing a moderate- or a low-intensity statin depending on frailty status or if significant comorbidities or polypharmacy exist [13,14].
In 2017, the American Association of Clinical Endocrinologist (AACE) released guidelines for the management of dyslipidemia and CVD prevention. AACE recommends that patients over age 65 be screened for dyslipidemia, and those who have multiple risk factors, other than age, should be considered for treatment with lipid-lowering therapy. AACE focuses on specific target LDL-C levels as treatment goals [15].
In addition to statins, other lipid-lowering therapies are used to treat dyslipidemia. The 2016 American College of Cardiology (ACC) Task Force reported on the use of non-statin therapies for the management of dyslipidemia and prevention of clinical ASCVD [16]. The committee concluded that ezetimibe added to statin therapy, bile acid sequestrants as monotherapy, and niacin as monotherapy all have some benefit for the prevention of clinical ASCVD. These guidelines also discuss the use of PCSK-9 inhibitors and their potential to decrease the risk of clinical ASCVD, but trials are currently ongoing to determine actual benefit. These guidelines address special populations but they do not consider the elderly in their recommendations. Currently, the only special populations included are patients with heart failure, those on hemodialysis, women who are of childbearing age or pregnant, and those with autoimmune diseases [16]. The literature available for each individual medication is discussed in further detail below.
Evidence for Secondary Prevention
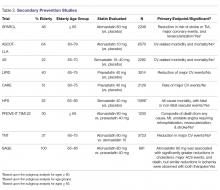
The benefits of statin therapy for secondary prevention in the elderly is more established than it is for primary prevention (Table 2).
The ASCOT–LLA (Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm), published in 2003, evaluated the effect of atorvastatin 10 mg on reducing ASCVD events in moderate-risk patients between 40–79 years of age who had hypertension and normal or slightly elevated LDL-C levels, with at least 2 other risk factors for CVD (age > 55 years was considered a risk factor). The primary outcome was non-fatal MI including silent MI and fatal CHD. A significant reduction was seen in the primary endpoint. Over half of the study population was > 60 years of age, with a mean age of 63 years. In a post-hoc analysis, stroke prevention was found to be similar in patients who were > 70 years of age and those < 70 years of age [19].
One of the first trials to specifically analyze the impact of age on lipid-lowering therapy in secondary ASCVD prevention was the Scandinavian Simvastatin Survival Study (4S), published in 1994. They evaluated the effect of simvastatin 20 mg on CV-related mortality and morbidity in patients 35–70 years of age with hyperlipidemia and a history of angina or acute MI occurring > 6 months of the study starting. The primary outcome was all-cause mortality. The secondary endpoint was time to first major CV event, which included coronary death, non-fatal acute MI, resuscitated cardiac arrest, and silent MI. Simvastatin significantly reduced the primary outcome and CHD-related deaths. A subgroup analysis of the study population > 60 years of age showed that age made no significant impact on primary or secondary outcomes; however, investigators noted that these subgroup analyses had less statistical power than the population as a whole [20].
Published in 1998, the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study evaluated the effects of pravastatin 40 mg daily on CHD-related mortality and overall mortality in patients with hyperlipidemia and clinical ASCVD (previous MI or unstable angina). The primary outcome observed was fatal CHD. Pravastatin significantly reduced the primary outcome, overall mortality, and pre-specified CV events. In a subgroup analysis, age group ( 65, and > 70 years) had no significant impact on the combined outcome of death from CHD and nonfatal MI; however, patients 65 to 70 years of age made up less than half of the study population [21].
The Cholesterol and Recurrent Events (CARE) trial, published in 1996, looked at the effect of pravastatin 40mg therapy for secondary ASCVD prevention following an MI in patients who had average cholesterol levels (defined as TC < 240 mg/dL and LDL-C 115–174 mg/dL). The primary endpoint assessed was time to fatal CHD or nonfatal MI. To meet statistical power they looked at subgroups for a broader outcome of a major coronary event (including fatal CHD, nonfatal MI, bypass surgery, and angioplasty). Pravastatin significantly reduced the primary outcome. The significant reduction in coronary events produced by pravastatin was noted to be significantly greater in women and in patients with higher pretreatment levels of LDL-C, but was not significantly impacted by age group (24–59 vs. 60–75 years) [22].
The Heart Protection Study (HPS), published in 2002, looked at the long-term effects of lowering LDL-C with simvastatin 40 mg in patients 40 to 80 years of age at high risk for mortality due to either vascular or nonvascular causes. The primary outcome assessed was all-cause mortality, with fatal or nonfatal vascular events as co-primary outcomes for subcategory analyses. Simvastatin significantly reduced both primary and co-primary outcomes, but there was no significant difference when they looked at nonvascular mortality between groups. Neither age nor baseline LDL levels were reported to have had a significant impact on outcomes. Over half the population was > 65 years of age, and about one-third of the population was > 70 years of age [23].
The PROVE-IT/TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, published in 2004, compared pravastatin 40 mg (moderate-intensity) to atorvastatin 80 mg (high-intensity) for secondary ASCVD prevention in patients with recent acute coronary syndrome (ACS) 65 years of age and the mean age was 58 years [24].
The TNT (Treating to New Targets) trial, published in 2005, looked at secondary ASCVD prevention in regards to targeting LDL-C levels to < 100 mg/dL or < 70 mg/dL with atorvastatin 10 mg and atorvastatin 80 mg. Patients had stable coronary artery disease (CAD) and baseline LDL-C levels < 130 mg/dL. The primary endpoint was the occurrence of a CV event (CAD mortality, nonfatal MI not related to procedure, resuscitation after cardiac arrest, or fatal or nonfatal stroke). High-intensity atorvastatin (80 mg) significantly reduced the primary outcome. The mean age of the study population was approximately 61 years. The study reported no statistical interaction for age or sex in the primary outcome measure [25].
The Study Assessing Goals in the Elderly (SAGE), published in 2007, evaluated the effects of pravastatin 40 mg (moderate-intensity) vs atorvastatin 80 mg (high-intensity) on secondary ASCVD prevention in patients 65 to 85 years (mean age 72) with stable CHD, LDL-C 100–250 mg/dL, with at least 1 episode of myocardial ischemia with total ischemia duration > 3 minutes. The primary efficacy outcome observed was absolute change in total duration of myocardial ischemia on 48-hour ambulatory electrocardiographic monitoring from baseline to month 12. No significant difference was observed in efficacy between the two groups for the primary endpoint, but the intensive statin therapy group showed greater benefit respective to several secondary outcomes, including major acute CV events and death [26].
In summary, while these trials provide evidence that statin therapy is beneficial in a wide range of patients with clinical ASCVD and dyslipidemia, the trial data does not provide definitive guidance for treating elderly patients at this time. Given the small percentage of elderly patients that were included, some of the trial results reporting statistical significance in this age group hold less clinical significance. It appears that high-intensity statin therapy was more likely to effectively prevent clinical ASCVD and death than moderate-intensity statin therapy, but more evidence is needed regarding secondary prevention in patients over age 75.
Evidence for Primary Prevention
The PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) was published in 2002 to assess the efficacy of pravastatin in patients between the ages of 70 and 82 (mean age 75 years) with pre-existing vascular disease (coronary, cerebral, or peripheral) or at an elevated risk (smoking, hypertension, or diabetes). Patients were randomized to receive either placebo or pravastatin 40 mg (a moderate-intensity statin). They found that pravastatin therapy reduced the risk of the composite outcome of CHD-related death, nonfatal MI, and fatal or nonfatal stroke in this elderly population. A post-hoc analysis comparing primary versus secondary prevention groups found no significant differences between these subgroups [7].
Han et al recently conducted a post hoc secondary analysis of older participants (65 years and older) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial–Lipid-Lowering Trial (ALLHAT-LLT). The intervention for ALLHAT-LLT was 40 mg pravastatin. They found no significant differences in all-cause mortality or cardiovascular outcomes between the pravastatin and usual care groups [27]
JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin), published in 2008, examined the efficacy of rosuvastatin vs. placebo in low- to moderate-risk men 50 years and older and women 60 years and older using a composite outcome of MI, unstable angina, stroke, arterial revascularization, or CVD death. Rosuvastatin did significantly decrease the primary endpoint, however it did not reduce the risk of overall death [28]. A subgroup analysis was performed on the elderly (65–75 years) study participants in JUPITER demonstrating a significant risk reduction for the combined CV endpoint and a nonsignificant reduction of all-cause mortality [29].
CARDS (Collaborative Atorvastatin Diabetes Study), published in 2004, looked at statin use for primary prevention in high-risk patients with type 2 diabetes without high LDL-C, but they had to have at least 1 additional risk factor for CVD. The primary outcome was first acute CHD event (myocardial infarction including silent infarction, unstable angina, acute coronary heart disease death, resuscitated cardiac arrest), coronary revascularization procedures, or stroke. Atorvastatin 10 mg, a moderate-intensity statin, significantly decreased occurrence of the primary outcome [30]. A subgroup analysis was performed to evaluate patients specifically between the ages of 65 and 75 and found a similar outcome in the elderly with a significant reduction in first major CV event and stroke [31].
A recent study evaluating primary prevention in patients with an intermediate risk for CVD was the HOPE-3 (Heart Outcomes Prevention Evaluation), published in 2016. Two co-primary outcomes were evaluated: the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, while the second primary outcome also included revascularization, heart failure, and resuscitated cardiac arrest. Rosuvastatin significantly decreased occurrence of both co-primary endpoints. About half of the study populations was over the age of 65 with a median age of 71 [32].
In addition to these trials of primary prevention, summarized in Table 3, a meta-analysis was published in 2013 to assess whether statins reduce all-cause mortality and CV events in elderly people without established CV disease.
As demonstrated by the above studies, it is evident that statins do help reduce the risk of CV events, regardless of statin intensity, but they do not consistently prevent death. However, the trials that did not demonstrate a significant outcome related to death utilized a moderate-intensity statin; if a high-intensity statin was used in those trials, there may have been a benefit [7,27]. More study is needed to evaluate the use of high-intensity statins in the elderly for the prevention of all-cause mortality and CV-related death.
Fortunately, the ongoing STAREE (STAtin Therapy for Reducing Events in the Elderly) study is looking specifically at the impact of statin therapy in adults 70 and older. Patients with a history of CVD or dementia are excluded. Results are set to be released in 2020 [34].
Risks of Using Statins in Older Adults
Statin use has been linked to a number of unwanted adverse effects.
Myalgia
Myalgia is variable but may occur in up to 25% of patients using statin therapy, and elderly patients typically experience more statin-associated myalgia than younger patients [35,36]. Elderly patients are more prone to decreased muscle mass and therefore may be at a higher risk of developing myalgia pain. Elderly patients are also utilizing more medications, leading to the potential for increased drug-drug interactions that could lead to myalgia. Elderly patients may also lose the function of drug metabolizing enzymes responsible for breaking down statin therapy, which may also increase the risk for statin-associated myalgia. One study demonstrated that elderly patients were more likely to discontinue statin therapy due to muscle pain and elderly patients reported more muscle side effects than their younger cohorts [37]. It is important to monitor for muscle pain and weakness in every patient. If they experience any myalgia, it is recommended to either lower the dose or discontinue the statin once it is determined to be statin-related. After myalgia resolves, therapy can be reinitiated at a lower dose or with a different statin if the patient is deemed high-risk. If creatine phosphokinase levels are greater than 10 times above the upper limit of normal, then discontinue the statin and wait for levels to return to normal. Re-initiation may be appropriate, but the the risks and benefits must be weighed. Simvastatin and atorvastatin are associated with higher rates of myalgia while pravastatin and rosuvastatin have the least myalgia pain associated with use [38,39].
Statin Intolerance
Statin intolerance, while not very common, is typically seen more often in special populations such as women, Asian patients, and the elderly. For a patient to be considered intolerant to statins, they need to have documented muscle symptoms or an elevated creatine phosphokinase level. Although not well defined, many clinicians consider improvement of symptoms with statin withdrawal as a diagnosis for statin intolerance. Typically patients are then rechallenged with 1 to 2 other statins and if still unable to tolerate, then different lipid-lowering therapies may be utilized [40]. In the elderly, it is important to rule out other causes for myalgia and monitor for significant drug interactions that may lead to muscle pain, particularly if the patient is requiring secondary prevention with statin therapy, before discontinuation.
Dementia
In 2012, the FDA issued a warning about the potential risk of cognitive impairment with the use of statins, which was based on case reports, not clinical trial data [41]. The NLA guidelines do not recommend baseline cognitive assessments prior to starting therapy and recommend that if patients do report cognitive impairment, other contributing factors and the risk associated with stopping statin therapy must be considered. Statin therapy may be discontinued to assess reversibility of symptoms, and if symptoms resolve, then it may be more beneficial to keep the patient off statin therapy. Clinicians may also consider lowering the dose or switching to another statin if they feel it is necessary for the patient to continue with a statin, particularly if the patient requires secondary prevention. Evidence suggests that statins are not associated with adverse effects on cognition and should not be withheld due to the potential for causing cognitive impairment alone [42]. The prevalence of cognitive impairment increases with age, so it is important for a clinician to rule out age-related processes or other disease states, such as Alzheimer’s, before discontinuation of previously tolerated statin therapy.
Renal Impairment
Kidney function must be evaluated prior to initiation of a statin in an elderly person as well as during the time the patient is taking a statin. Because statins are eliminated via the kidney, and because most elderly patients have decreased kidney function, the potential for drug build-up in the body is higher than in a younger patient and may lead to more adverse effects. Atorvastatin is the only option that does not require dose adjustment. All other statins should be adjusted based upon the level of renal impairment. The results from the SHARP study, published in 2011, showed that the combination of ezetimibe and simvastatin versus placebo significantly reduced ASCVD events in patients with moderate to severe chronic kidney disease, including those receiving dialysis. Specifically, this trial showed a significant reduction of ischemic events and occurrence of arterial revascularization procedures. Although the trial did not show a significant difference in incidence of MI or CHD-related mortality, the trial was not adequately powered to show differences in results among the individual ASCVD events and it is not clear whether the results can guide the use of statin therapy in all patients with chronic kidney disease [43]. Statins may be beneficial in renal insufficiency to lower LDL-C, but more studies are needed to assess CVD outcomes related to statin use in patients with a history of kidney disease [44].
Hepatic Function
Statins have been known to increase liver enzymes and in rare cases lead to liver injury, which typically has led to underutilization of therapy in clinical practice. Risk factors associated with this include preexisting hepatitis, advanced age, chronic alcohol use, and use of concomitant medications that may also cause hepatotoxicity, such as acetaminophen. When a statin-induced hepatic effect is suspected, it is important to first rule out other causes or disease states that may be undiagnosed. If no other cause can be found, clinicians may choose to reduce the statin dose, switch the statin, or discontinue the statin altogether if the risk outweighs the benefit. Additionally, statins do not have to be held in patients who have preexisting hepatic dysfunction if use is clearly indicated because the cardiovascular benefits typically outweigh the risks of causing liver injury. Clinical judgement is still warranted and patients with preexisting liver conditions should be monitored regularly [45].
Cost Considerations
Several studies have demonstrated that statin therapy, in the general population, is economical for both primary and secondary prevention of CVD [46,47]. The 4S study found simvastatin therapy to be cost-effective; for example, the cost per life year gained for a 70-year-old man with high chlesterol was $3800 [48]. In contrast, primary prevention in middle-aged men, based on the West of Scotland trial, averages about $35,000 per year of life gained [46]. In a 2015 study that utilized an established Markov simulation model, researchers studied adults 75 to 94 years and examined the cost-effectiveness of generic statins for primary prevention in this population. The authors estimated treating this population with statins over the next decade would be cost-effective. However, the researchers cautioned that the CV benefits and cost-effectiveness would be offset with even a modest increased risk of cognitive impairments or functional limitations. Statin use was not cost-effective in diabetes patients who did not have elevated LDL-C levels [49].
Non-Statin Therapies
Several other classes of medications are available for the management of hyperlipidemia; however, none of these lipid-lowering therapies have been found to reduce CVD events or mortality in the elderly population.
Ezetimibe
Ezetimibe blocks the absorption of intestinal cholesterol and is typically combined with statin therapy to lower LDL-C. Up until the IMPROVE-IT trial was published in 2015, ezetimibe did not have much use in clinical practice. This landmark trial was a large double-blind study that looked at secondary prevention in patients with ACS, comparing ezetimibe 10 mg and simvastatin 40 mg versus simvastatin 40 mg alone. The authors included patients over the age of 50 (mean age 64) with clinical ASCVD. They found that the addition of ezetimibe to simvastatin did reduce the primary composite outcome (CV mortality, major CV events, or nonfatal stroke) when compared to simvastatin alone [50]. This trial demonstrates clinical benefit with the addition of ezetimibe to statin therapy and adds additional evidence to support a target LDL-C of less than 70 mg/dL; however, the elderly population was not adequately represented in the study to allow extrapolation of these results to older patients.
PCSK-9 Inhibitors
The proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors are a newer class of monoclonal antibodies that were first approved by the US Food and Drug Administration in 2015. Alirocumab and evolocumab, both approved PCSK-9 inhibitors, bind to LDL receptors on the surface of hepatocytes and assist in the internalization of LDL receptors for lysosomal degradation. By inhibiting the binding of PCSK-9 to the LDL receptors, there is an overall increase in LDL receptors available on the cell surface to bind to LDL particles, thereby lowering LDL-C levels. Treatment with these agents are currently considered (in addition to diet and maximally tolerated statin therapy) in adult patients with heterozygous familial hypercholesterolemia or clinical ASCVD requiring further reduction in LDL-C. Two studies were published focusing on the use of PCSK-9 inhibitors: Open-label Study of Long-term Evaluation against LDL Cholesterol (OSLER) and the Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM). Overall, these studies demonstrated a 60% reduction of LDL-C among patients with high CVD risk on maximum-tolerated statin therapy. Furthermore, the ODYSSEY LONG TERM trial did find that the rate of major CVD adverse events was significantly lower with alirocumab added to maximum-tolerated statin therapy, with a hazard ratio of 0.52 [51].
One recent study of evolocumab, named the Further Cardiovascular OUtcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER), enrolled patients between the ages of 40 and 85 with 1 major CV risk factor or 2 minor CV risk factors. The primary endpoint was a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. Evolocumab lowered major CV events by roughly 15% when added to statin therapy in patients who were at high risk for clinical ASCVD. The mean age of the patients in the trial was 63; however, it is unclear how many of the study participants were elderly [52].
Unfortunately, the studies discussed above do not represent the elderly population well and the agents have not been studied long-term to determine the effects of continued use beyond 2 years. Long-term outcome studies are currently underway; however, it is unknown at this time whether elderly patients are being considered in these studies. It is known that genetic variation of the PCSK-9 locus does lower LDL-C in the elderly but does not significantly lower their risk of vascular disease [51]. At this time, until further evidence is available, we do not recommend the use of PCSK-9 inhibitors in elderly patients.
Nicotinic Acid
Nicotinic acid (Niacin, Niacin ER), also known as vitamin B3, has been utilized for decades as a vitamin supplement, an anti-wrinkle agent, and is known to have neuroprotective effects. It has also been utilized for dyslipidemia and has had some benefits when used alone to decrease cardiovascular disease [53]. Unfortunately the Coronary Drug Project was completed in the 1980s and did not incorporate patients over the age of 64, therefore making the results difficult to apply to elderly patients today [54]. Other literature has been published in recent years to refute that study, claiming there is no additional benefit to using niacin for cardiovascular protection and these studies have included elderly patients. In the AIM-HIGH trial, published in 2011, approximately 46% of the patients were 65 or older. Patients who were previously taking statin therapy that had known cardiovascular disease were enrolled. Niacin added to simvastatin 40–80 mg lowered LDL-C, triglycerides, and increased HDL-C, but the addition of niacin was not proven to help lower the risk of cardiovascular events [55]. The HPS2-THRIVE study enrolled patients with known cardiovascular disease between the ages of 50 and 80 years and found no benefit in preventing CVD when adding niacin to statin therapy [56]. With its side effect profile, risk for increased glucose intolerance, and lack of evidence to demonstrate benefit for prevention of CV events, we do not recommend niacin for use in the elderly at this time.
Bile Acid Sequestrants
The ATP III guidelines [57] noted that when statins are not sufficient to lower high cholesterol, bile acid sequestrants also known as resins could be added. More recently, the 2016 ACC expert consensus on non-statin therapies for LDL-C lowering [16] stated resins may be considered in select circumstances as a second-line agent for adults with ezetimibe intolerance and with triglycerides
Fibrates
While fibrates (gemfibrozil, fenofibrate, clofibrate) have not been studied to demonstrate a reduction in CVD or CVD mortality in the elderly population, this medication class is beneficial in patients with hypertriglyceridemia to lower triglyceride levels and prevent pancreatitis. Fibrates are recommended for patients with triglyceride levels approaching 500 mg/dL. Fibrates can also increase high-density lipoproteins, which tend to be lower in the elderly population and considered a risk factor for CVD. Gemfibrozil is not recommended in combination with statin therapy due to an increased risk of myalgia. Fenofibrate is the drug of choice, particularly for diabetic patients with very uncontrolled triglyceride levels because it will not affect glucose levels [57]. At this time, we do not recommend the use of fibrates in the elderly population unless they are at risk for developing pancreatitis and have elevated triglyceride levels.
Patient-Centered Care
Evidence-based medicine can aid in making sound clinical decisions for proper patient care; however, treatment plans should consider the individual patient’s perspectives and needs, beliefs, expectations, and goals. In the elderly population, we must also consider factors such as finances, pill-burden, drug-drug interactions, physiological needs, comorbid disease states, and overall life expectancy. In addition, the elderly population is physiologically heterogeneous group and recommendations for therapy need to be individualized. Chronological age does not necessarily correspond to vascular age and risk factors for cardiovascular disease do not predict outcomes as well in the elderly as they do in younger patients. While older patients may view having to take 1 less medication as more important than preventing a heart attack or stroke at the age of 80, it is advisable to discuss all potential outcomes related to morbidity associated with the occurrence of an MI or stroke due to the lack of statin therapy. Additionally, pharmacists can play a vital role in evaluating elderly patients and their medication regimens. Elderly patients should undergo a medication reconciliation at each visit to evaluate drug-drug interactions, side effects, and potentially harmful medication combinations that may lead to increased adverse drug outcomes.
Conclusion
CHD increases with age, and most patients who have a CV event are more likely to die with advancing age. Based on the the limited available evidence, statin therapy is beneficial in the elderly population in reducing overall CV morbidity. We recommend beginning with with a moderate-intensity statin and adjusting accordingly. High-intensity statin therapy appears to be effective for elderly patients for secondary prevention, but clinicians should use clinical judgment and monitor for adverse events, particularly myalgia pain. At this time, we are unable to determine if non-statin therapies for the elderly would be beneficial and do not recommend their use unless the patient is at risk for pancreatitis, in which case a fenofibrate is recommended.
Corresponding author: Nicole A. Slater, PharmD, BCACP, Auburn University, Harrison School of Pharmacy, 650 Clinic Dr., Mobile, AL 36688.
Financial disclosures: None.
1. Fact sheet: Aging in the United States. Accessed at www.prb.org/aging-unitedstates-fact-sheet/.
2. Kontis JE, Bennett CD, Mathers G, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323–35.
3. Odden MD, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e1–e458.
5. Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Amer Col Cardiol 2008;52:1769–81.
6. Stone NJ. Statins in secondary prevention: intensity matters. J Am Coll Cardiol 2017;69: 2707–9.
7. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30.
8. Heart Protection Study Collaborative Group Writers. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
9. Lefevre F, Nishida L. Special report: the efficacy and safety of statins in the elderly. TEC Assessment Program 2007;21
10. Pravastatin benefits elderly patients: results of PROSPER study. Cardiovasc J S Afr 2003;14:48.
11. Catapano AL, Graham I, Backer GD, et al. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058.
12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
13. Jacobson TA, Ito MK, Maki K, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol 2015;9:129-169
14. Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol 2015;9:S1–22.e1.
15. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologist and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23:1–87.
16. Lloyd-Jones DM, Morris PB, Minissian MB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016;68:92–125.
17. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59.
18. Chaturvedi S, Zivin J, Breazna A, et al. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009;72:688–94.
19. Sever PS, Dahior B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian cardiac outcomes trial—lipid lowering arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
20. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9.
21. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin of coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–09.
23. Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
24. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–04.
25. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
26. Deedwania P, Stone PH, Bairey CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation 2007;115:700–7.
27. Han BH, Sutin D, Williamson JD, et al; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177:955–65.
28. Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C Reactive protein. N Engl J Med 2008; 359:2195–207.
29. Glynn RJ, Koenig W, Nordestgaard BG, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann Intern Med 2010;152:488–96.
30. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
31. Neil HA, DeMicco DA, Luo D, et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: collaborative atorvastatin diabetes study (CARDS). Diabetes Care 2006;29:2378–84.
32. Yusuf S, Bosch J, Dagenais G, et al; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31.
33. Savarese G, Gotta AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–99.
34. National Institute of Health. A clinical trial of STAtin therapy for reducing events in the elderly (STAREE). Clinical Trials. https://clinicaltrials.gov/ct2/show/NCT02099123. Accessed June 5, 2018.
35. Gaist D, Rodríquez, LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565–9.
36. Pasternak RC., Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567–72.
37. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15.
38. Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep 2010;12:322–30.
39. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14.
40. Ahmad Z. Statin intolerance. Am J Cardiol 2014;113:1765–71.
41. Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Published February 28, 2012. Accessed June 5, 2018.
42. Gauthier JM, Massicotte A. Statins and their effect on cognition: let’s clear up the confusion. Can Pharm J (Ott) 2015;148:150–55.
43. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92.
44. Vaziri ND, Anzalone DA, Catini J. Statins in chronic didney disease: when and when not to use them. J Fam Pract 2016;65:8 Suppl. www.mdedge.com/jfp/custom/statins-chronic-kidney-disease-when-and-when-not-use-them-1
45. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci 2016;8:23–8.
46. Caro J, Klittich W, McGuire A, et al. The West of Scotland coronary prevention study: Economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315:1577–82.
47. Schectman G, Wolff N, Byrd JC, et al. Physician extenders for cost-effective management of hypercholesterolemia. J Gen Intern Med 1996;11:277–86.
48. Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian simvastatin survival study group. N Engl J Med 1997;336:332–6.
49. Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–41.
50. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97.
51. Polisecki E, Peter I, Robertson M, et al. Genetic variation at the PCSK9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis 2008;200:95–101.
52. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22.
53. Sinthupoom N, Prachayasittikul V, Prachayasittikul S, et al. Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications. Eur Food Res Technol 2015;240: 1–17.
54. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55.
55. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67.
56. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12.
57. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421.
58. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–64.
From the Harrison School of Pharmacy, Auburn University, Mobile, AL.
Abstract
- Objective: To summarize the literature relevant to managing dyslipidemia in the elderly and review recommendations for initiating lipid-lowering therapy.
- Methods: Review of the literature.
- Results: Statins are the most commonly utilized medication class for lipid-lowering in the general population, and they are recommended for primary prevention in patients between the ages of 40 to 75 with at least 1 risk factor for cardiovascular disease as well as for any patient needing secondary prevention. In the elderly, statins may be appropriate for both primary and secondary prevention if the benefits outweigh the risks. Based on the available evidence, it is safe to recommend statin therapy to elderly patients who require secondary prevention given the known benefits in reducing cardiovascular morbidity and mortality for patients up to the age of 80. For primary prevention, statin therapy may be beneficial, but one must carefully evaluate for comorbid conditions, life expectancy, concomitant medications, overall health status, frailty, and patient or family preference. Several other classes of lipid-lowering agents exist; however, there is not enough evidence for us to recommend use in the elderly population for cardiovascular risk reduction in either primary or secondary scenarios.
- Conclusion: Although clinical research in the elderly population is limited, evidence supports the use of statins in elderly patients for secondary prevention and in patients up to age 75 for primary prevention; however clinicians must use clinical judgement and take into consideration the patient’s situation regarding comorbidities, polypharmacy, and possible adverse effects. More high-quality evidence is necessary.
Key words: hyperlipidemia; geriatrics; elderly; patient-centered care; statin; cardiovascular disease.
The number of Americans age 65 years and older is projected to more than double, from 46 million today to over 98 million by 2060, and the 65-and-older age group’s share of the total population will rise to nearly 24% [1]. Life expectancy is now predicted to be > 20 years for women at age 65 and > 17 years for men at age 65 in many high-income countries, including the United States [2]. This demographic shift toward an older population will result in a higher burden of coronary heart disease and stroke, with atherosclerotic cardiovascular disease (ASCVD) prevalence and costs projected to increase substantially [3].
Among adults seeking medical care in the United States, roughly 95 million have a total cholesterol (TC) level of ≥ 200 mg/dL or more, and approximately 29 million have a TC > 240 mg/dL [4]. Cholesterol screening is important since most patients suffering from dyslipidemia are asymptomatic. Dyslipidemia is a major risk factor for the development of atherosclerotic disease. Because of the complications associated with dyslipidemia, it is vital that patients are provided with primary and/or secondary prevention strategies to reduce the risk of cardiovascular disease (CVD) and protect high-risk patients from recurring events. A clinical controversy exists surrounding the elderly population, concerning whether or not clinicians should be providing lipid-lowering treatment to this group of individuals for dyslipidemia. The evidence is limited for patients over age 65, and even more so for the very elderly (> 80 years); therefore, it is necessary to review the available evidence to make an appropriate decision when it comes to managing dyslipidemia in the elderly population
Currently, HMG-CoA reductase inhibitors (statins) are the only known class of medications for the treatment of dyslipidemia that will prevent both primary and secondary cardiovascular (CV) events, including death. Statin intensity (Table 1)
Guideline Recommendations
Current guidelines differ in their recommendations for treating dyslipidemia in the elderly population. In 2016, the Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) released updated guidelines for managing dyslipidemia. These guidelines recommend that older patients with established CVD be treated in the same way as younger patients because of the many benefits statin therapy demonstrated in clinical trials. They also suggest that statin therapy be started at a lower doses to achieve goals for primary prevention in the older population. In addition, CVD risk factors (hypertension, diabetes, dyslipidemia, smoking) should be addressed in this population to reduce CVD risk. They also acknowledged that primary prevention may not prolong life in the older adult, but treatment does reduce cardiovascular mortality and statin therapy is recommended to reduce the overall risk of CV morbidity in this population [11]. In contrast, The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines changed the management and treatment of dyslipidemia by highlighting “statin benefit groups” rather than recommending a treat-to-target goal as guidelines had done for many years. ACC/AHA recommends a moderate-intensity statin for patients > 75 years of age for secondary prevention versus the use of a high-intensity statin for patients who are between the ages of 40 and 75 based on the pooled cohort risk equation. In patients over age 75 with no history of CVD, no specific recommendation is available for the use of lipid-lowering therapy at this time [12]. ACC/AHA is expected to publish a new set of guidelines sometime in 2018 and they are projected to utilize lipid-lowering goals in combination with the pooled cohort equation to assess overall risk in patients with dyslipidemia.
The 2015 National Lipid Association (NLA) released “Part 1” guidelines for the management of dyslipidemia and then provided “Part 2” about a year later, which focuses on management for special populations. To summarize, the NLA guidelines recommend that elderly patients between the ages of 65 and 80 receive a high-intensity statin for secondary prevention after special consideration of the potential risks and benefits. In patients over the age of 80, NLA recommends a moderate-intensity statin for secondary prevention. For primary prevention, NLA recommends utilizing the pooled cohort risk equation to analyze patient characteristics, keeping in mind that age is a driving factor for increased risk of CVD and that the actual risk for developing a CV event may be “overestimated” if the patient has no other risk factors other than their age. When evaluating patients between the ages of 65 and 79 for primary prevention, NLA suggests following Part 1 of the guidelines. In Part 1, NLA recommends evaluating the patient’s characteristics and suggests a moderate- or high-intensity statin if the patient is considered “very high risk” or “high risk” and a moderate-intensity statin for patients who are considered “moderate risk”. For patients over the age of 80, they recommend utilizing a moderate- or a low-intensity statin depending on frailty status or if significant comorbidities or polypharmacy exist [13,14].
In 2017, the American Association of Clinical Endocrinologist (AACE) released guidelines for the management of dyslipidemia and CVD prevention. AACE recommends that patients over age 65 be screened for dyslipidemia, and those who have multiple risk factors, other than age, should be considered for treatment with lipid-lowering therapy. AACE focuses on specific target LDL-C levels as treatment goals [15].
In addition to statins, other lipid-lowering therapies are used to treat dyslipidemia. The 2016 American College of Cardiology (ACC) Task Force reported on the use of non-statin therapies for the management of dyslipidemia and prevention of clinical ASCVD [16]. The committee concluded that ezetimibe added to statin therapy, bile acid sequestrants as monotherapy, and niacin as monotherapy all have some benefit for the prevention of clinical ASCVD. These guidelines also discuss the use of PCSK-9 inhibitors and their potential to decrease the risk of clinical ASCVD, but trials are currently ongoing to determine actual benefit. These guidelines address special populations but they do not consider the elderly in their recommendations. Currently, the only special populations included are patients with heart failure, those on hemodialysis, women who are of childbearing age or pregnant, and those with autoimmune diseases [16]. The literature available for each individual medication is discussed in further detail below.
Evidence for Secondary Prevention
The benefits of statin therapy for secondary prevention in the elderly is more established than it is for primary prevention (Table 2).
The ASCOT–LLA (Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm), published in 2003, evaluated the effect of atorvastatin 10 mg on reducing ASCVD events in moderate-risk patients between 40–79 years of age who had hypertension and normal or slightly elevated LDL-C levels, with at least 2 other risk factors for CVD (age > 55 years was considered a risk factor). The primary outcome was non-fatal MI including silent MI and fatal CHD. A significant reduction was seen in the primary endpoint. Over half of the study population was > 60 years of age, with a mean age of 63 years. In a post-hoc analysis, stroke prevention was found to be similar in patients who were > 70 years of age and those < 70 years of age [19].
One of the first trials to specifically analyze the impact of age on lipid-lowering therapy in secondary ASCVD prevention was the Scandinavian Simvastatin Survival Study (4S), published in 1994. They evaluated the effect of simvastatin 20 mg on CV-related mortality and morbidity in patients 35–70 years of age with hyperlipidemia and a history of angina or acute MI occurring > 6 months of the study starting. The primary outcome was all-cause mortality. The secondary endpoint was time to first major CV event, which included coronary death, non-fatal acute MI, resuscitated cardiac arrest, and silent MI. Simvastatin significantly reduced the primary outcome and CHD-related deaths. A subgroup analysis of the study population > 60 years of age showed that age made no significant impact on primary or secondary outcomes; however, investigators noted that these subgroup analyses had less statistical power than the population as a whole [20].
Published in 1998, the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study evaluated the effects of pravastatin 40 mg daily on CHD-related mortality and overall mortality in patients with hyperlipidemia and clinical ASCVD (previous MI or unstable angina). The primary outcome observed was fatal CHD. Pravastatin significantly reduced the primary outcome, overall mortality, and pre-specified CV events. In a subgroup analysis, age group ( 65, and > 70 years) had no significant impact on the combined outcome of death from CHD and nonfatal MI; however, patients 65 to 70 years of age made up less than half of the study population [21].
The Cholesterol and Recurrent Events (CARE) trial, published in 1996, looked at the effect of pravastatin 40mg therapy for secondary ASCVD prevention following an MI in patients who had average cholesterol levels (defined as TC < 240 mg/dL and LDL-C 115–174 mg/dL). The primary endpoint assessed was time to fatal CHD or nonfatal MI. To meet statistical power they looked at subgroups for a broader outcome of a major coronary event (including fatal CHD, nonfatal MI, bypass surgery, and angioplasty). Pravastatin significantly reduced the primary outcome. The significant reduction in coronary events produced by pravastatin was noted to be significantly greater in women and in patients with higher pretreatment levels of LDL-C, but was not significantly impacted by age group (24–59 vs. 60–75 years) [22].
The Heart Protection Study (HPS), published in 2002, looked at the long-term effects of lowering LDL-C with simvastatin 40 mg in patients 40 to 80 years of age at high risk for mortality due to either vascular or nonvascular causes. The primary outcome assessed was all-cause mortality, with fatal or nonfatal vascular events as co-primary outcomes for subcategory analyses. Simvastatin significantly reduced both primary and co-primary outcomes, but there was no significant difference when they looked at nonvascular mortality between groups. Neither age nor baseline LDL levels were reported to have had a significant impact on outcomes. Over half the population was > 65 years of age, and about one-third of the population was > 70 years of age [23].
The PROVE-IT/TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, published in 2004, compared pravastatin 40 mg (moderate-intensity) to atorvastatin 80 mg (high-intensity) for secondary ASCVD prevention in patients with recent acute coronary syndrome (ACS) 65 years of age and the mean age was 58 years [24].
The TNT (Treating to New Targets) trial, published in 2005, looked at secondary ASCVD prevention in regards to targeting LDL-C levels to < 100 mg/dL or < 70 mg/dL with atorvastatin 10 mg and atorvastatin 80 mg. Patients had stable coronary artery disease (CAD) and baseline LDL-C levels < 130 mg/dL. The primary endpoint was the occurrence of a CV event (CAD mortality, nonfatal MI not related to procedure, resuscitation after cardiac arrest, or fatal or nonfatal stroke). High-intensity atorvastatin (80 mg) significantly reduced the primary outcome. The mean age of the study population was approximately 61 years. The study reported no statistical interaction for age or sex in the primary outcome measure [25].
The Study Assessing Goals in the Elderly (SAGE), published in 2007, evaluated the effects of pravastatin 40 mg (moderate-intensity) vs atorvastatin 80 mg (high-intensity) on secondary ASCVD prevention in patients 65 to 85 years (mean age 72) with stable CHD, LDL-C 100–250 mg/dL, with at least 1 episode of myocardial ischemia with total ischemia duration > 3 minutes. The primary efficacy outcome observed was absolute change in total duration of myocardial ischemia on 48-hour ambulatory electrocardiographic monitoring from baseline to month 12. No significant difference was observed in efficacy between the two groups for the primary endpoint, but the intensive statin therapy group showed greater benefit respective to several secondary outcomes, including major acute CV events and death [26].
In summary, while these trials provide evidence that statin therapy is beneficial in a wide range of patients with clinical ASCVD and dyslipidemia, the trial data does not provide definitive guidance for treating elderly patients at this time. Given the small percentage of elderly patients that were included, some of the trial results reporting statistical significance in this age group hold less clinical significance. It appears that high-intensity statin therapy was more likely to effectively prevent clinical ASCVD and death than moderate-intensity statin therapy, but more evidence is needed regarding secondary prevention in patients over age 75.
Evidence for Primary Prevention
The PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) was published in 2002 to assess the efficacy of pravastatin in patients between the ages of 70 and 82 (mean age 75 years) with pre-existing vascular disease (coronary, cerebral, or peripheral) or at an elevated risk (smoking, hypertension, or diabetes). Patients were randomized to receive either placebo or pravastatin 40 mg (a moderate-intensity statin). They found that pravastatin therapy reduced the risk of the composite outcome of CHD-related death, nonfatal MI, and fatal or nonfatal stroke in this elderly population. A post-hoc analysis comparing primary versus secondary prevention groups found no significant differences between these subgroups [7].
Han et al recently conducted a post hoc secondary analysis of older participants (65 years and older) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial–Lipid-Lowering Trial (ALLHAT-LLT). The intervention for ALLHAT-LLT was 40 mg pravastatin. They found no significant differences in all-cause mortality or cardiovascular outcomes between the pravastatin and usual care groups [27]
JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin), published in 2008, examined the efficacy of rosuvastatin vs. placebo in low- to moderate-risk men 50 years and older and women 60 years and older using a composite outcome of MI, unstable angina, stroke, arterial revascularization, or CVD death. Rosuvastatin did significantly decrease the primary endpoint, however it did not reduce the risk of overall death [28]. A subgroup analysis was performed on the elderly (65–75 years) study participants in JUPITER demonstrating a significant risk reduction for the combined CV endpoint and a nonsignificant reduction of all-cause mortality [29].
CARDS (Collaborative Atorvastatin Diabetes Study), published in 2004, looked at statin use for primary prevention in high-risk patients with type 2 diabetes without high LDL-C, but they had to have at least 1 additional risk factor for CVD. The primary outcome was first acute CHD event (myocardial infarction including silent infarction, unstable angina, acute coronary heart disease death, resuscitated cardiac arrest), coronary revascularization procedures, or stroke. Atorvastatin 10 mg, a moderate-intensity statin, significantly decreased occurrence of the primary outcome [30]. A subgroup analysis was performed to evaluate patients specifically between the ages of 65 and 75 and found a similar outcome in the elderly with a significant reduction in first major CV event and stroke [31].
A recent study evaluating primary prevention in patients with an intermediate risk for CVD was the HOPE-3 (Heart Outcomes Prevention Evaluation), published in 2016. Two co-primary outcomes were evaluated: the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, while the second primary outcome also included revascularization, heart failure, and resuscitated cardiac arrest. Rosuvastatin significantly decreased occurrence of both co-primary endpoints. About half of the study populations was over the age of 65 with a median age of 71 [32].
In addition to these trials of primary prevention, summarized in Table 3, a meta-analysis was published in 2013 to assess whether statins reduce all-cause mortality and CV events in elderly people without established CV disease.
As demonstrated by the above studies, it is evident that statins do help reduce the risk of CV events, regardless of statin intensity, but they do not consistently prevent death. However, the trials that did not demonstrate a significant outcome related to death utilized a moderate-intensity statin; if a high-intensity statin was used in those trials, there may have been a benefit [7,27]. More study is needed to evaluate the use of high-intensity statins in the elderly for the prevention of all-cause mortality and CV-related death.
Fortunately, the ongoing STAREE (STAtin Therapy for Reducing Events in the Elderly) study is looking specifically at the impact of statin therapy in adults 70 and older. Patients with a history of CVD or dementia are excluded. Results are set to be released in 2020 [34].
Risks of Using Statins in Older Adults
Statin use has been linked to a number of unwanted adverse effects.
Myalgia
Myalgia is variable but may occur in up to 25% of patients using statin therapy, and elderly patients typically experience more statin-associated myalgia than younger patients [35,36]. Elderly patients are more prone to decreased muscle mass and therefore may be at a higher risk of developing myalgia pain. Elderly patients are also utilizing more medications, leading to the potential for increased drug-drug interactions that could lead to myalgia. Elderly patients may also lose the function of drug metabolizing enzymes responsible for breaking down statin therapy, which may also increase the risk for statin-associated myalgia. One study demonstrated that elderly patients were more likely to discontinue statin therapy due to muscle pain and elderly patients reported more muscle side effects than their younger cohorts [37]. It is important to monitor for muscle pain and weakness in every patient. If they experience any myalgia, it is recommended to either lower the dose or discontinue the statin once it is determined to be statin-related. After myalgia resolves, therapy can be reinitiated at a lower dose or with a different statin if the patient is deemed high-risk. If creatine phosphokinase levels are greater than 10 times above the upper limit of normal, then discontinue the statin and wait for levels to return to normal. Re-initiation may be appropriate, but the the risks and benefits must be weighed. Simvastatin and atorvastatin are associated with higher rates of myalgia while pravastatin and rosuvastatin have the least myalgia pain associated with use [38,39].
Statin Intolerance
Statin intolerance, while not very common, is typically seen more often in special populations such as women, Asian patients, and the elderly. For a patient to be considered intolerant to statins, they need to have documented muscle symptoms or an elevated creatine phosphokinase level. Although not well defined, many clinicians consider improvement of symptoms with statin withdrawal as a diagnosis for statin intolerance. Typically patients are then rechallenged with 1 to 2 other statins and if still unable to tolerate, then different lipid-lowering therapies may be utilized [40]. In the elderly, it is important to rule out other causes for myalgia and monitor for significant drug interactions that may lead to muscle pain, particularly if the patient is requiring secondary prevention with statin therapy, before discontinuation.
Dementia
In 2012, the FDA issued a warning about the potential risk of cognitive impairment with the use of statins, which was based on case reports, not clinical trial data [41]. The NLA guidelines do not recommend baseline cognitive assessments prior to starting therapy and recommend that if patients do report cognitive impairment, other contributing factors and the risk associated with stopping statin therapy must be considered. Statin therapy may be discontinued to assess reversibility of symptoms, and if symptoms resolve, then it may be more beneficial to keep the patient off statin therapy. Clinicians may also consider lowering the dose or switching to another statin if they feel it is necessary for the patient to continue with a statin, particularly if the patient requires secondary prevention. Evidence suggests that statins are not associated with adverse effects on cognition and should not be withheld due to the potential for causing cognitive impairment alone [42]. The prevalence of cognitive impairment increases with age, so it is important for a clinician to rule out age-related processes or other disease states, such as Alzheimer’s, before discontinuation of previously tolerated statin therapy.
Renal Impairment
Kidney function must be evaluated prior to initiation of a statin in an elderly person as well as during the time the patient is taking a statin. Because statins are eliminated via the kidney, and because most elderly patients have decreased kidney function, the potential for drug build-up in the body is higher than in a younger patient and may lead to more adverse effects. Atorvastatin is the only option that does not require dose adjustment. All other statins should be adjusted based upon the level of renal impairment. The results from the SHARP study, published in 2011, showed that the combination of ezetimibe and simvastatin versus placebo significantly reduced ASCVD events in patients with moderate to severe chronic kidney disease, including those receiving dialysis. Specifically, this trial showed a significant reduction of ischemic events and occurrence of arterial revascularization procedures. Although the trial did not show a significant difference in incidence of MI or CHD-related mortality, the trial was not adequately powered to show differences in results among the individual ASCVD events and it is not clear whether the results can guide the use of statin therapy in all patients with chronic kidney disease [43]. Statins may be beneficial in renal insufficiency to lower LDL-C, but more studies are needed to assess CVD outcomes related to statin use in patients with a history of kidney disease [44].
Hepatic Function
Statins have been known to increase liver enzymes and in rare cases lead to liver injury, which typically has led to underutilization of therapy in clinical practice. Risk factors associated with this include preexisting hepatitis, advanced age, chronic alcohol use, and use of concomitant medications that may also cause hepatotoxicity, such as acetaminophen. When a statin-induced hepatic effect is suspected, it is important to first rule out other causes or disease states that may be undiagnosed. If no other cause can be found, clinicians may choose to reduce the statin dose, switch the statin, or discontinue the statin altogether if the risk outweighs the benefit. Additionally, statins do not have to be held in patients who have preexisting hepatic dysfunction if use is clearly indicated because the cardiovascular benefits typically outweigh the risks of causing liver injury. Clinical judgement is still warranted and patients with preexisting liver conditions should be monitored regularly [45].
Cost Considerations
Several studies have demonstrated that statin therapy, in the general population, is economical for both primary and secondary prevention of CVD [46,47]. The 4S study found simvastatin therapy to be cost-effective; for example, the cost per life year gained for a 70-year-old man with high chlesterol was $3800 [48]. In contrast, primary prevention in middle-aged men, based on the West of Scotland trial, averages about $35,000 per year of life gained [46]. In a 2015 study that utilized an established Markov simulation model, researchers studied adults 75 to 94 years and examined the cost-effectiveness of generic statins for primary prevention in this population. The authors estimated treating this population with statins over the next decade would be cost-effective. However, the researchers cautioned that the CV benefits and cost-effectiveness would be offset with even a modest increased risk of cognitive impairments or functional limitations. Statin use was not cost-effective in diabetes patients who did not have elevated LDL-C levels [49].
Non-Statin Therapies
Several other classes of medications are available for the management of hyperlipidemia; however, none of these lipid-lowering therapies have been found to reduce CVD events or mortality in the elderly population.
Ezetimibe
Ezetimibe blocks the absorption of intestinal cholesterol and is typically combined with statin therapy to lower LDL-C. Up until the IMPROVE-IT trial was published in 2015, ezetimibe did not have much use in clinical practice. This landmark trial was a large double-blind study that looked at secondary prevention in patients with ACS, comparing ezetimibe 10 mg and simvastatin 40 mg versus simvastatin 40 mg alone. The authors included patients over the age of 50 (mean age 64) with clinical ASCVD. They found that the addition of ezetimibe to simvastatin did reduce the primary composite outcome (CV mortality, major CV events, or nonfatal stroke) when compared to simvastatin alone [50]. This trial demonstrates clinical benefit with the addition of ezetimibe to statin therapy and adds additional evidence to support a target LDL-C of less than 70 mg/dL; however, the elderly population was not adequately represented in the study to allow extrapolation of these results to older patients.
PCSK-9 Inhibitors
The proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors are a newer class of monoclonal antibodies that were first approved by the US Food and Drug Administration in 2015. Alirocumab and evolocumab, both approved PCSK-9 inhibitors, bind to LDL receptors on the surface of hepatocytes and assist in the internalization of LDL receptors for lysosomal degradation. By inhibiting the binding of PCSK-9 to the LDL receptors, there is an overall increase in LDL receptors available on the cell surface to bind to LDL particles, thereby lowering LDL-C levels. Treatment with these agents are currently considered (in addition to diet and maximally tolerated statin therapy) in adult patients with heterozygous familial hypercholesterolemia or clinical ASCVD requiring further reduction in LDL-C. Two studies were published focusing on the use of PCSK-9 inhibitors: Open-label Study of Long-term Evaluation against LDL Cholesterol (OSLER) and the Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM). Overall, these studies demonstrated a 60% reduction of LDL-C among patients with high CVD risk on maximum-tolerated statin therapy. Furthermore, the ODYSSEY LONG TERM trial did find that the rate of major CVD adverse events was significantly lower with alirocumab added to maximum-tolerated statin therapy, with a hazard ratio of 0.52 [51].
One recent study of evolocumab, named the Further Cardiovascular OUtcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER), enrolled patients between the ages of 40 and 85 with 1 major CV risk factor or 2 minor CV risk factors. The primary endpoint was a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. Evolocumab lowered major CV events by roughly 15% when added to statin therapy in patients who were at high risk for clinical ASCVD. The mean age of the patients in the trial was 63; however, it is unclear how many of the study participants were elderly [52].
Unfortunately, the studies discussed above do not represent the elderly population well and the agents have not been studied long-term to determine the effects of continued use beyond 2 years. Long-term outcome studies are currently underway; however, it is unknown at this time whether elderly patients are being considered in these studies. It is known that genetic variation of the PCSK-9 locus does lower LDL-C in the elderly but does not significantly lower their risk of vascular disease [51]. At this time, until further evidence is available, we do not recommend the use of PCSK-9 inhibitors in elderly patients.
Nicotinic Acid
Nicotinic acid (Niacin, Niacin ER), also known as vitamin B3, has been utilized for decades as a vitamin supplement, an anti-wrinkle agent, and is known to have neuroprotective effects. It has also been utilized for dyslipidemia and has had some benefits when used alone to decrease cardiovascular disease [53]. Unfortunately the Coronary Drug Project was completed in the 1980s and did not incorporate patients over the age of 64, therefore making the results difficult to apply to elderly patients today [54]. Other literature has been published in recent years to refute that study, claiming there is no additional benefit to using niacin for cardiovascular protection and these studies have included elderly patients. In the AIM-HIGH trial, published in 2011, approximately 46% of the patients were 65 or older. Patients who were previously taking statin therapy that had known cardiovascular disease were enrolled. Niacin added to simvastatin 40–80 mg lowered LDL-C, triglycerides, and increased HDL-C, but the addition of niacin was not proven to help lower the risk of cardiovascular events [55]. The HPS2-THRIVE study enrolled patients with known cardiovascular disease between the ages of 50 and 80 years and found no benefit in preventing CVD when adding niacin to statin therapy [56]. With its side effect profile, risk for increased glucose intolerance, and lack of evidence to demonstrate benefit for prevention of CV events, we do not recommend niacin for use in the elderly at this time.
Bile Acid Sequestrants
The ATP III guidelines [57] noted that when statins are not sufficient to lower high cholesterol, bile acid sequestrants also known as resins could be added. More recently, the 2016 ACC expert consensus on non-statin therapies for LDL-C lowering [16] stated resins may be considered in select circumstances as a second-line agent for adults with ezetimibe intolerance and with triglycerides
Fibrates
While fibrates (gemfibrozil, fenofibrate, clofibrate) have not been studied to demonstrate a reduction in CVD or CVD mortality in the elderly population, this medication class is beneficial in patients with hypertriglyceridemia to lower triglyceride levels and prevent pancreatitis. Fibrates are recommended for patients with triglyceride levels approaching 500 mg/dL. Fibrates can also increase high-density lipoproteins, which tend to be lower in the elderly population and considered a risk factor for CVD. Gemfibrozil is not recommended in combination with statin therapy due to an increased risk of myalgia. Fenofibrate is the drug of choice, particularly for diabetic patients with very uncontrolled triglyceride levels because it will not affect glucose levels [57]. At this time, we do not recommend the use of fibrates in the elderly population unless they are at risk for developing pancreatitis and have elevated triglyceride levels.
Patient-Centered Care
Evidence-based medicine can aid in making sound clinical decisions for proper patient care; however, treatment plans should consider the individual patient’s perspectives and needs, beliefs, expectations, and goals. In the elderly population, we must also consider factors such as finances, pill-burden, drug-drug interactions, physiological needs, comorbid disease states, and overall life expectancy. In addition, the elderly population is physiologically heterogeneous group and recommendations for therapy need to be individualized. Chronological age does not necessarily correspond to vascular age and risk factors for cardiovascular disease do not predict outcomes as well in the elderly as they do in younger patients. While older patients may view having to take 1 less medication as more important than preventing a heart attack or stroke at the age of 80, it is advisable to discuss all potential outcomes related to morbidity associated with the occurrence of an MI or stroke due to the lack of statin therapy. Additionally, pharmacists can play a vital role in evaluating elderly patients and their medication regimens. Elderly patients should undergo a medication reconciliation at each visit to evaluate drug-drug interactions, side effects, and potentially harmful medication combinations that may lead to increased adverse drug outcomes.
Conclusion
CHD increases with age, and most patients who have a CV event are more likely to die with advancing age. Based on the the limited available evidence, statin therapy is beneficial in the elderly population in reducing overall CV morbidity. We recommend beginning with with a moderate-intensity statin and adjusting accordingly. High-intensity statin therapy appears to be effective for elderly patients for secondary prevention, but clinicians should use clinical judgment and monitor for adverse events, particularly myalgia pain. At this time, we are unable to determine if non-statin therapies for the elderly would be beneficial and do not recommend their use unless the patient is at risk for pancreatitis, in which case a fenofibrate is recommended.
Corresponding author: Nicole A. Slater, PharmD, BCACP, Auburn University, Harrison School of Pharmacy, 650 Clinic Dr., Mobile, AL 36688.
Financial disclosures: None.
From the Harrison School of Pharmacy, Auburn University, Mobile, AL.
Abstract
- Objective: To summarize the literature relevant to managing dyslipidemia in the elderly and review recommendations for initiating lipid-lowering therapy.
- Methods: Review of the literature.
- Results: Statins are the most commonly utilized medication class for lipid-lowering in the general population, and they are recommended for primary prevention in patients between the ages of 40 to 75 with at least 1 risk factor for cardiovascular disease as well as for any patient needing secondary prevention. In the elderly, statins may be appropriate for both primary and secondary prevention if the benefits outweigh the risks. Based on the available evidence, it is safe to recommend statin therapy to elderly patients who require secondary prevention given the known benefits in reducing cardiovascular morbidity and mortality for patients up to the age of 80. For primary prevention, statin therapy may be beneficial, but one must carefully evaluate for comorbid conditions, life expectancy, concomitant medications, overall health status, frailty, and patient or family preference. Several other classes of lipid-lowering agents exist; however, there is not enough evidence for us to recommend use in the elderly population for cardiovascular risk reduction in either primary or secondary scenarios.
- Conclusion: Although clinical research in the elderly population is limited, evidence supports the use of statins in elderly patients for secondary prevention and in patients up to age 75 for primary prevention; however clinicians must use clinical judgement and take into consideration the patient’s situation regarding comorbidities, polypharmacy, and possible adverse effects. More high-quality evidence is necessary.
Key words: hyperlipidemia; geriatrics; elderly; patient-centered care; statin; cardiovascular disease.
The number of Americans age 65 years and older is projected to more than double, from 46 million today to over 98 million by 2060, and the 65-and-older age group’s share of the total population will rise to nearly 24% [1]. Life expectancy is now predicted to be > 20 years for women at age 65 and > 17 years for men at age 65 in many high-income countries, including the United States [2]. This demographic shift toward an older population will result in a higher burden of coronary heart disease and stroke, with atherosclerotic cardiovascular disease (ASCVD) prevalence and costs projected to increase substantially [3].
Among adults seeking medical care in the United States, roughly 95 million have a total cholesterol (TC) level of ≥ 200 mg/dL or more, and approximately 29 million have a TC > 240 mg/dL [4]. Cholesterol screening is important since most patients suffering from dyslipidemia are asymptomatic. Dyslipidemia is a major risk factor for the development of atherosclerotic disease. Because of the complications associated with dyslipidemia, it is vital that patients are provided with primary and/or secondary prevention strategies to reduce the risk of cardiovascular disease (CVD) and protect high-risk patients from recurring events. A clinical controversy exists surrounding the elderly population, concerning whether or not clinicians should be providing lipid-lowering treatment to this group of individuals for dyslipidemia. The evidence is limited for patients over age 65, and even more so for the very elderly (> 80 years); therefore, it is necessary to review the available evidence to make an appropriate decision when it comes to managing dyslipidemia in the elderly population
Currently, HMG-CoA reductase inhibitors (statins) are the only known class of medications for the treatment of dyslipidemia that will prevent both primary and secondary cardiovascular (CV) events, including death. Statin intensity (Table 1)
Guideline Recommendations
Current guidelines differ in their recommendations for treating dyslipidemia in the elderly population. In 2016, the Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) released updated guidelines for managing dyslipidemia. These guidelines recommend that older patients with established CVD be treated in the same way as younger patients because of the many benefits statin therapy demonstrated in clinical trials. They also suggest that statin therapy be started at a lower doses to achieve goals for primary prevention in the older population. In addition, CVD risk factors (hypertension, diabetes, dyslipidemia, smoking) should be addressed in this population to reduce CVD risk. They also acknowledged that primary prevention may not prolong life in the older adult, but treatment does reduce cardiovascular mortality and statin therapy is recommended to reduce the overall risk of CV morbidity in this population [11]. In contrast, The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines changed the management and treatment of dyslipidemia by highlighting “statin benefit groups” rather than recommending a treat-to-target goal as guidelines had done for many years. ACC/AHA recommends a moderate-intensity statin for patients > 75 years of age for secondary prevention versus the use of a high-intensity statin for patients who are between the ages of 40 and 75 based on the pooled cohort risk equation. In patients over age 75 with no history of CVD, no specific recommendation is available for the use of lipid-lowering therapy at this time [12]. ACC/AHA is expected to publish a new set of guidelines sometime in 2018 and they are projected to utilize lipid-lowering goals in combination with the pooled cohort equation to assess overall risk in patients with dyslipidemia.
The 2015 National Lipid Association (NLA) released “Part 1” guidelines for the management of dyslipidemia and then provided “Part 2” about a year later, which focuses on management for special populations. To summarize, the NLA guidelines recommend that elderly patients between the ages of 65 and 80 receive a high-intensity statin for secondary prevention after special consideration of the potential risks and benefits. In patients over the age of 80, NLA recommends a moderate-intensity statin for secondary prevention. For primary prevention, NLA recommends utilizing the pooled cohort risk equation to analyze patient characteristics, keeping in mind that age is a driving factor for increased risk of CVD and that the actual risk for developing a CV event may be “overestimated” if the patient has no other risk factors other than their age. When evaluating patients between the ages of 65 and 79 for primary prevention, NLA suggests following Part 1 of the guidelines. In Part 1, NLA recommends evaluating the patient’s characteristics and suggests a moderate- or high-intensity statin if the patient is considered “very high risk” or “high risk” and a moderate-intensity statin for patients who are considered “moderate risk”. For patients over the age of 80, they recommend utilizing a moderate- or a low-intensity statin depending on frailty status or if significant comorbidities or polypharmacy exist [13,14].
In 2017, the American Association of Clinical Endocrinologist (AACE) released guidelines for the management of dyslipidemia and CVD prevention. AACE recommends that patients over age 65 be screened for dyslipidemia, and those who have multiple risk factors, other than age, should be considered for treatment with lipid-lowering therapy. AACE focuses on specific target LDL-C levels as treatment goals [15].
In addition to statins, other lipid-lowering therapies are used to treat dyslipidemia. The 2016 American College of Cardiology (ACC) Task Force reported on the use of non-statin therapies for the management of dyslipidemia and prevention of clinical ASCVD [16]. The committee concluded that ezetimibe added to statin therapy, bile acid sequestrants as monotherapy, and niacin as monotherapy all have some benefit for the prevention of clinical ASCVD. These guidelines also discuss the use of PCSK-9 inhibitors and their potential to decrease the risk of clinical ASCVD, but trials are currently ongoing to determine actual benefit. These guidelines address special populations but they do not consider the elderly in their recommendations. Currently, the only special populations included are patients with heart failure, those on hemodialysis, women who are of childbearing age or pregnant, and those with autoimmune diseases [16]. The literature available for each individual medication is discussed in further detail below.
Evidence for Secondary Prevention
The benefits of statin therapy for secondary prevention in the elderly is more established than it is for primary prevention (Table 2).
The ASCOT–LLA (Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm), published in 2003, evaluated the effect of atorvastatin 10 mg on reducing ASCVD events in moderate-risk patients between 40–79 years of age who had hypertension and normal or slightly elevated LDL-C levels, with at least 2 other risk factors for CVD (age > 55 years was considered a risk factor). The primary outcome was non-fatal MI including silent MI and fatal CHD. A significant reduction was seen in the primary endpoint. Over half of the study population was > 60 years of age, with a mean age of 63 years. In a post-hoc analysis, stroke prevention was found to be similar in patients who were > 70 years of age and those < 70 years of age [19].
One of the first trials to specifically analyze the impact of age on lipid-lowering therapy in secondary ASCVD prevention was the Scandinavian Simvastatin Survival Study (4S), published in 1994. They evaluated the effect of simvastatin 20 mg on CV-related mortality and morbidity in patients 35–70 years of age with hyperlipidemia and a history of angina or acute MI occurring > 6 months of the study starting. The primary outcome was all-cause mortality. The secondary endpoint was time to first major CV event, which included coronary death, non-fatal acute MI, resuscitated cardiac arrest, and silent MI. Simvastatin significantly reduced the primary outcome and CHD-related deaths. A subgroup analysis of the study population > 60 years of age showed that age made no significant impact on primary or secondary outcomes; however, investigators noted that these subgroup analyses had less statistical power than the population as a whole [20].
Published in 1998, the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study evaluated the effects of pravastatin 40 mg daily on CHD-related mortality and overall mortality in patients with hyperlipidemia and clinical ASCVD (previous MI or unstable angina). The primary outcome observed was fatal CHD. Pravastatin significantly reduced the primary outcome, overall mortality, and pre-specified CV events. In a subgroup analysis, age group ( 65, and > 70 years) had no significant impact on the combined outcome of death from CHD and nonfatal MI; however, patients 65 to 70 years of age made up less than half of the study population [21].
The Cholesterol and Recurrent Events (CARE) trial, published in 1996, looked at the effect of pravastatin 40mg therapy for secondary ASCVD prevention following an MI in patients who had average cholesterol levels (defined as TC < 240 mg/dL and LDL-C 115–174 mg/dL). The primary endpoint assessed was time to fatal CHD or nonfatal MI. To meet statistical power they looked at subgroups for a broader outcome of a major coronary event (including fatal CHD, nonfatal MI, bypass surgery, and angioplasty). Pravastatin significantly reduced the primary outcome. The significant reduction in coronary events produced by pravastatin was noted to be significantly greater in women and in patients with higher pretreatment levels of LDL-C, but was not significantly impacted by age group (24–59 vs. 60–75 years) [22].
The Heart Protection Study (HPS), published in 2002, looked at the long-term effects of lowering LDL-C with simvastatin 40 mg in patients 40 to 80 years of age at high risk for mortality due to either vascular or nonvascular causes. The primary outcome assessed was all-cause mortality, with fatal or nonfatal vascular events as co-primary outcomes for subcategory analyses. Simvastatin significantly reduced both primary and co-primary outcomes, but there was no significant difference when they looked at nonvascular mortality between groups. Neither age nor baseline LDL levels were reported to have had a significant impact on outcomes. Over half the population was > 65 years of age, and about one-third of the population was > 70 years of age [23].
The PROVE-IT/TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, published in 2004, compared pravastatin 40 mg (moderate-intensity) to atorvastatin 80 mg (high-intensity) for secondary ASCVD prevention in patients with recent acute coronary syndrome (ACS) 65 years of age and the mean age was 58 years [24].
The TNT (Treating to New Targets) trial, published in 2005, looked at secondary ASCVD prevention in regards to targeting LDL-C levels to < 100 mg/dL or < 70 mg/dL with atorvastatin 10 mg and atorvastatin 80 mg. Patients had stable coronary artery disease (CAD) and baseline LDL-C levels < 130 mg/dL. The primary endpoint was the occurrence of a CV event (CAD mortality, nonfatal MI not related to procedure, resuscitation after cardiac arrest, or fatal or nonfatal stroke). High-intensity atorvastatin (80 mg) significantly reduced the primary outcome. The mean age of the study population was approximately 61 years. The study reported no statistical interaction for age or sex in the primary outcome measure [25].
The Study Assessing Goals in the Elderly (SAGE), published in 2007, evaluated the effects of pravastatin 40 mg (moderate-intensity) vs atorvastatin 80 mg (high-intensity) on secondary ASCVD prevention in patients 65 to 85 years (mean age 72) with stable CHD, LDL-C 100–250 mg/dL, with at least 1 episode of myocardial ischemia with total ischemia duration > 3 minutes. The primary efficacy outcome observed was absolute change in total duration of myocardial ischemia on 48-hour ambulatory electrocardiographic monitoring from baseline to month 12. No significant difference was observed in efficacy between the two groups for the primary endpoint, but the intensive statin therapy group showed greater benefit respective to several secondary outcomes, including major acute CV events and death [26].
In summary, while these trials provide evidence that statin therapy is beneficial in a wide range of patients with clinical ASCVD and dyslipidemia, the trial data does not provide definitive guidance for treating elderly patients at this time. Given the small percentage of elderly patients that were included, some of the trial results reporting statistical significance in this age group hold less clinical significance. It appears that high-intensity statin therapy was more likely to effectively prevent clinical ASCVD and death than moderate-intensity statin therapy, but more evidence is needed regarding secondary prevention in patients over age 75.
Evidence for Primary Prevention
The PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) was published in 2002 to assess the efficacy of pravastatin in patients between the ages of 70 and 82 (mean age 75 years) with pre-existing vascular disease (coronary, cerebral, or peripheral) or at an elevated risk (smoking, hypertension, or diabetes). Patients were randomized to receive either placebo or pravastatin 40 mg (a moderate-intensity statin). They found that pravastatin therapy reduced the risk of the composite outcome of CHD-related death, nonfatal MI, and fatal or nonfatal stroke in this elderly population. A post-hoc analysis comparing primary versus secondary prevention groups found no significant differences between these subgroups [7].
Han et al recently conducted a post hoc secondary analysis of older participants (65 years and older) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial–Lipid-Lowering Trial (ALLHAT-LLT). The intervention for ALLHAT-LLT was 40 mg pravastatin. They found no significant differences in all-cause mortality or cardiovascular outcomes between the pravastatin and usual care groups [27]
JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin), published in 2008, examined the efficacy of rosuvastatin vs. placebo in low- to moderate-risk men 50 years and older and women 60 years and older using a composite outcome of MI, unstable angina, stroke, arterial revascularization, or CVD death. Rosuvastatin did significantly decrease the primary endpoint, however it did not reduce the risk of overall death [28]. A subgroup analysis was performed on the elderly (65–75 years) study participants in JUPITER demonstrating a significant risk reduction for the combined CV endpoint and a nonsignificant reduction of all-cause mortality [29].
CARDS (Collaborative Atorvastatin Diabetes Study), published in 2004, looked at statin use for primary prevention in high-risk patients with type 2 diabetes without high LDL-C, but they had to have at least 1 additional risk factor for CVD. The primary outcome was first acute CHD event (myocardial infarction including silent infarction, unstable angina, acute coronary heart disease death, resuscitated cardiac arrest), coronary revascularization procedures, or stroke. Atorvastatin 10 mg, a moderate-intensity statin, significantly decreased occurrence of the primary outcome [30]. A subgroup analysis was performed to evaluate patients specifically between the ages of 65 and 75 and found a similar outcome in the elderly with a significant reduction in first major CV event and stroke [31].
A recent study evaluating primary prevention in patients with an intermediate risk for CVD was the HOPE-3 (Heart Outcomes Prevention Evaluation), published in 2016. Two co-primary outcomes were evaluated: the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, while the second primary outcome also included revascularization, heart failure, and resuscitated cardiac arrest. Rosuvastatin significantly decreased occurrence of both co-primary endpoints. About half of the study populations was over the age of 65 with a median age of 71 [32].
In addition to these trials of primary prevention, summarized in Table 3, a meta-analysis was published in 2013 to assess whether statins reduce all-cause mortality and CV events in elderly people without established CV disease.
As demonstrated by the above studies, it is evident that statins do help reduce the risk of CV events, regardless of statin intensity, but they do not consistently prevent death. However, the trials that did not demonstrate a significant outcome related to death utilized a moderate-intensity statin; if a high-intensity statin was used in those trials, there may have been a benefit [7,27]. More study is needed to evaluate the use of high-intensity statins in the elderly for the prevention of all-cause mortality and CV-related death.
Fortunately, the ongoing STAREE (STAtin Therapy for Reducing Events in the Elderly) study is looking specifically at the impact of statin therapy in adults 70 and older. Patients with a history of CVD or dementia are excluded. Results are set to be released in 2020 [34].
Risks of Using Statins in Older Adults
Statin use has been linked to a number of unwanted adverse effects.
Myalgia
Myalgia is variable but may occur in up to 25% of patients using statin therapy, and elderly patients typically experience more statin-associated myalgia than younger patients [35,36]. Elderly patients are more prone to decreased muscle mass and therefore may be at a higher risk of developing myalgia pain. Elderly patients are also utilizing more medications, leading to the potential for increased drug-drug interactions that could lead to myalgia. Elderly patients may also lose the function of drug metabolizing enzymes responsible for breaking down statin therapy, which may also increase the risk for statin-associated myalgia. One study demonstrated that elderly patients were more likely to discontinue statin therapy due to muscle pain and elderly patients reported more muscle side effects than their younger cohorts [37]. It is important to monitor for muscle pain and weakness in every patient. If they experience any myalgia, it is recommended to either lower the dose or discontinue the statin once it is determined to be statin-related. After myalgia resolves, therapy can be reinitiated at a lower dose or with a different statin if the patient is deemed high-risk. If creatine phosphokinase levels are greater than 10 times above the upper limit of normal, then discontinue the statin and wait for levels to return to normal. Re-initiation may be appropriate, but the the risks and benefits must be weighed. Simvastatin and atorvastatin are associated with higher rates of myalgia while pravastatin and rosuvastatin have the least myalgia pain associated with use [38,39].
Statin Intolerance
Statin intolerance, while not very common, is typically seen more often in special populations such as women, Asian patients, and the elderly. For a patient to be considered intolerant to statins, they need to have documented muscle symptoms or an elevated creatine phosphokinase level. Although not well defined, many clinicians consider improvement of symptoms with statin withdrawal as a diagnosis for statin intolerance. Typically patients are then rechallenged with 1 to 2 other statins and if still unable to tolerate, then different lipid-lowering therapies may be utilized [40]. In the elderly, it is important to rule out other causes for myalgia and monitor for significant drug interactions that may lead to muscle pain, particularly if the patient is requiring secondary prevention with statin therapy, before discontinuation.
Dementia
In 2012, the FDA issued a warning about the potential risk of cognitive impairment with the use of statins, which was based on case reports, not clinical trial data [41]. The NLA guidelines do not recommend baseline cognitive assessments prior to starting therapy and recommend that if patients do report cognitive impairment, other contributing factors and the risk associated with stopping statin therapy must be considered. Statin therapy may be discontinued to assess reversibility of symptoms, and if symptoms resolve, then it may be more beneficial to keep the patient off statin therapy. Clinicians may also consider lowering the dose or switching to another statin if they feel it is necessary for the patient to continue with a statin, particularly if the patient requires secondary prevention. Evidence suggests that statins are not associated with adverse effects on cognition and should not be withheld due to the potential for causing cognitive impairment alone [42]. The prevalence of cognitive impairment increases with age, so it is important for a clinician to rule out age-related processes or other disease states, such as Alzheimer’s, before discontinuation of previously tolerated statin therapy.
Renal Impairment
Kidney function must be evaluated prior to initiation of a statin in an elderly person as well as during the time the patient is taking a statin. Because statins are eliminated via the kidney, and because most elderly patients have decreased kidney function, the potential for drug build-up in the body is higher than in a younger patient and may lead to more adverse effects. Atorvastatin is the only option that does not require dose adjustment. All other statins should be adjusted based upon the level of renal impairment. The results from the SHARP study, published in 2011, showed that the combination of ezetimibe and simvastatin versus placebo significantly reduced ASCVD events in patients with moderate to severe chronic kidney disease, including those receiving dialysis. Specifically, this trial showed a significant reduction of ischemic events and occurrence of arterial revascularization procedures. Although the trial did not show a significant difference in incidence of MI or CHD-related mortality, the trial was not adequately powered to show differences in results among the individual ASCVD events and it is not clear whether the results can guide the use of statin therapy in all patients with chronic kidney disease [43]. Statins may be beneficial in renal insufficiency to lower LDL-C, but more studies are needed to assess CVD outcomes related to statin use in patients with a history of kidney disease [44].
Hepatic Function
Statins have been known to increase liver enzymes and in rare cases lead to liver injury, which typically has led to underutilization of therapy in clinical practice. Risk factors associated with this include preexisting hepatitis, advanced age, chronic alcohol use, and use of concomitant medications that may also cause hepatotoxicity, such as acetaminophen. When a statin-induced hepatic effect is suspected, it is important to first rule out other causes or disease states that may be undiagnosed. If no other cause can be found, clinicians may choose to reduce the statin dose, switch the statin, or discontinue the statin altogether if the risk outweighs the benefit. Additionally, statins do not have to be held in patients who have preexisting hepatic dysfunction if use is clearly indicated because the cardiovascular benefits typically outweigh the risks of causing liver injury. Clinical judgement is still warranted and patients with preexisting liver conditions should be monitored regularly [45].
Cost Considerations
Several studies have demonstrated that statin therapy, in the general population, is economical for both primary and secondary prevention of CVD [46,47]. The 4S study found simvastatin therapy to be cost-effective; for example, the cost per life year gained for a 70-year-old man with high chlesterol was $3800 [48]. In contrast, primary prevention in middle-aged men, based on the West of Scotland trial, averages about $35,000 per year of life gained [46]. In a 2015 study that utilized an established Markov simulation model, researchers studied adults 75 to 94 years and examined the cost-effectiveness of generic statins for primary prevention in this population. The authors estimated treating this population with statins over the next decade would be cost-effective. However, the researchers cautioned that the CV benefits and cost-effectiveness would be offset with even a modest increased risk of cognitive impairments or functional limitations. Statin use was not cost-effective in diabetes patients who did not have elevated LDL-C levels [49].
Non-Statin Therapies
Several other classes of medications are available for the management of hyperlipidemia; however, none of these lipid-lowering therapies have been found to reduce CVD events or mortality in the elderly population.
Ezetimibe
Ezetimibe blocks the absorption of intestinal cholesterol and is typically combined with statin therapy to lower LDL-C. Up until the IMPROVE-IT trial was published in 2015, ezetimibe did not have much use in clinical practice. This landmark trial was a large double-blind study that looked at secondary prevention in patients with ACS, comparing ezetimibe 10 mg and simvastatin 40 mg versus simvastatin 40 mg alone. The authors included patients over the age of 50 (mean age 64) with clinical ASCVD. They found that the addition of ezetimibe to simvastatin did reduce the primary composite outcome (CV mortality, major CV events, or nonfatal stroke) when compared to simvastatin alone [50]. This trial demonstrates clinical benefit with the addition of ezetimibe to statin therapy and adds additional evidence to support a target LDL-C of less than 70 mg/dL; however, the elderly population was not adequately represented in the study to allow extrapolation of these results to older patients.
PCSK-9 Inhibitors
The proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors are a newer class of monoclonal antibodies that were first approved by the US Food and Drug Administration in 2015. Alirocumab and evolocumab, both approved PCSK-9 inhibitors, bind to LDL receptors on the surface of hepatocytes and assist in the internalization of LDL receptors for lysosomal degradation. By inhibiting the binding of PCSK-9 to the LDL receptors, there is an overall increase in LDL receptors available on the cell surface to bind to LDL particles, thereby lowering LDL-C levels. Treatment with these agents are currently considered (in addition to diet and maximally tolerated statin therapy) in adult patients with heterozygous familial hypercholesterolemia or clinical ASCVD requiring further reduction in LDL-C. Two studies were published focusing on the use of PCSK-9 inhibitors: Open-label Study of Long-term Evaluation against LDL Cholesterol (OSLER) and the Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM). Overall, these studies demonstrated a 60% reduction of LDL-C among patients with high CVD risk on maximum-tolerated statin therapy. Furthermore, the ODYSSEY LONG TERM trial did find that the rate of major CVD adverse events was significantly lower with alirocumab added to maximum-tolerated statin therapy, with a hazard ratio of 0.52 [51].
One recent study of evolocumab, named the Further Cardiovascular OUtcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER), enrolled patients between the ages of 40 and 85 with 1 major CV risk factor or 2 minor CV risk factors. The primary endpoint was a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. Evolocumab lowered major CV events by roughly 15% when added to statin therapy in patients who were at high risk for clinical ASCVD. The mean age of the patients in the trial was 63; however, it is unclear how many of the study participants were elderly [52].
Unfortunately, the studies discussed above do not represent the elderly population well and the agents have not been studied long-term to determine the effects of continued use beyond 2 years. Long-term outcome studies are currently underway; however, it is unknown at this time whether elderly patients are being considered in these studies. It is known that genetic variation of the PCSK-9 locus does lower LDL-C in the elderly but does not significantly lower their risk of vascular disease [51]. At this time, until further evidence is available, we do not recommend the use of PCSK-9 inhibitors in elderly patients.
Nicotinic Acid
Nicotinic acid (Niacin, Niacin ER), also known as vitamin B3, has been utilized for decades as a vitamin supplement, an anti-wrinkle agent, and is known to have neuroprotective effects. It has also been utilized for dyslipidemia and has had some benefits when used alone to decrease cardiovascular disease [53]. Unfortunately the Coronary Drug Project was completed in the 1980s and did not incorporate patients over the age of 64, therefore making the results difficult to apply to elderly patients today [54]. Other literature has been published in recent years to refute that study, claiming there is no additional benefit to using niacin for cardiovascular protection and these studies have included elderly patients. In the AIM-HIGH trial, published in 2011, approximately 46% of the patients were 65 or older. Patients who were previously taking statin therapy that had known cardiovascular disease were enrolled. Niacin added to simvastatin 40–80 mg lowered LDL-C, triglycerides, and increased HDL-C, but the addition of niacin was not proven to help lower the risk of cardiovascular events [55]. The HPS2-THRIVE study enrolled patients with known cardiovascular disease between the ages of 50 and 80 years and found no benefit in preventing CVD when adding niacin to statin therapy [56]. With its side effect profile, risk for increased glucose intolerance, and lack of evidence to demonstrate benefit for prevention of CV events, we do not recommend niacin for use in the elderly at this time.
Bile Acid Sequestrants
The ATP III guidelines [57] noted that when statins are not sufficient to lower high cholesterol, bile acid sequestrants also known as resins could be added. More recently, the 2016 ACC expert consensus on non-statin therapies for LDL-C lowering [16] stated resins may be considered in select circumstances as a second-line agent for adults with ezetimibe intolerance and with triglycerides
Fibrates
While fibrates (gemfibrozil, fenofibrate, clofibrate) have not been studied to demonstrate a reduction in CVD or CVD mortality in the elderly population, this medication class is beneficial in patients with hypertriglyceridemia to lower triglyceride levels and prevent pancreatitis. Fibrates are recommended for patients with triglyceride levels approaching 500 mg/dL. Fibrates can also increase high-density lipoproteins, which tend to be lower in the elderly population and considered a risk factor for CVD. Gemfibrozil is not recommended in combination with statin therapy due to an increased risk of myalgia. Fenofibrate is the drug of choice, particularly for diabetic patients with very uncontrolled triglyceride levels because it will not affect glucose levels [57]. At this time, we do not recommend the use of fibrates in the elderly population unless they are at risk for developing pancreatitis and have elevated triglyceride levels.
Patient-Centered Care
Evidence-based medicine can aid in making sound clinical decisions for proper patient care; however, treatment plans should consider the individual patient’s perspectives and needs, beliefs, expectations, and goals. In the elderly population, we must also consider factors such as finances, pill-burden, drug-drug interactions, physiological needs, comorbid disease states, and overall life expectancy. In addition, the elderly population is physiologically heterogeneous group and recommendations for therapy need to be individualized. Chronological age does not necessarily correspond to vascular age and risk factors for cardiovascular disease do not predict outcomes as well in the elderly as they do in younger patients. While older patients may view having to take 1 less medication as more important than preventing a heart attack or stroke at the age of 80, it is advisable to discuss all potential outcomes related to morbidity associated with the occurrence of an MI or stroke due to the lack of statin therapy. Additionally, pharmacists can play a vital role in evaluating elderly patients and their medication regimens. Elderly patients should undergo a medication reconciliation at each visit to evaluate drug-drug interactions, side effects, and potentially harmful medication combinations that may lead to increased adverse drug outcomes.
Conclusion
CHD increases with age, and most patients who have a CV event are more likely to die with advancing age. Based on the the limited available evidence, statin therapy is beneficial in the elderly population in reducing overall CV morbidity. We recommend beginning with with a moderate-intensity statin and adjusting accordingly. High-intensity statin therapy appears to be effective for elderly patients for secondary prevention, but clinicians should use clinical judgment and monitor for adverse events, particularly myalgia pain. At this time, we are unable to determine if non-statin therapies for the elderly would be beneficial and do not recommend their use unless the patient is at risk for pancreatitis, in which case a fenofibrate is recommended.
Corresponding author: Nicole A. Slater, PharmD, BCACP, Auburn University, Harrison School of Pharmacy, 650 Clinic Dr., Mobile, AL 36688.
Financial disclosures: None.
1. Fact sheet: Aging in the United States. Accessed at www.prb.org/aging-unitedstates-fact-sheet/.
2. Kontis JE, Bennett CD, Mathers G, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323–35.
3. Odden MD, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e1–e458.
5. Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Amer Col Cardiol 2008;52:1769–81.
6. Stone NJ. Statins in secondary prevention: intensity matters. J Am Coll Cardiol 2017;69: 2707–9.
7. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30.
8. Heart Protection Study Collaborative Group Writers. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
9. Lefevre F, Nishida L. Special report: the efficacy and safety of statins in the elderly. TEC Assessment Program 2007;21
10. Pravastatin benefits elderly patients: results of PROSPER study. Cardiovasc J S Afr 2003;14:48.
11. Catapano AL, Graham I, Backer GD, et al. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058.
12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
13. Jacobson TA, Ito MK, Maki K, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol 2015;9:129-169
14. Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol 2015;9:S1–22.e1.
15. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologist and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23:1–87.
16. Lloyd-Jones DM, Morris PB, Minissian MB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016;68:92–125.
17. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59.
18. Chaturvedi S, Zivin J, Breazna A, et al. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009;72:688–94.
19. Sever PS, Dahior B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian cardiac outcomes trial—lipid lowering arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
20. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9.
21. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin of coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–09.
23. Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
24. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–04.
25. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
26. Deedwania P, Stone PH, Bairey CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation 2007;115:700–7.
27. Han BH, Sutin D, Williamson JD, et al; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177:955–65.
28. Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C Reactive protein. N Engl J Med 2008; 359:2195–207.
29. Glynn RJ, Koenig W, Nordestgaard BG, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann Intern Med 2010;152:488–96.
30. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
31. Neil HA, DeMicco DA, Luo D, et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: collaborative atorvastatin diabetes study (CARDS). Diabetes Care 2006;29:2378–84.
32. Yusuf S, Bosch J, Dagenais G, et al; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31.
33. Savarese G, Gotta AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–99.
34. National Institute of Health. A clinical trial of STAtin therapy for reducing events in the elderly (STAREE). Clinical Trials. https://clinicaltrials.gov/ct2/show/NCT02099123. Accessed June 5, 2018.
35. Gaist D, Rodríquez, LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565–9.
36. Pasternak RC., Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567–72.
37. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15.
38. Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep 2010;12:322–30.
39. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14.
40. Ahmad Z. Statin intolerance. Am J Cardiol 2014;113:1765–71.
41. Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Published February 28, 2012. Accessed June 5, 2018.
42. Gauthier JM, Massicotte A. Statins and their effect on cognition: let’s clear up the confusion. Can Pharm J (Ott) 2015;148:150–55.
43. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92.
44. Vaziri ND, Anzalone DA, Catini J. Statins in chronic didney disease: when and when not to use them. J Fam Pract 2016;65:8 Suppl. www.mdedge.com/jfp/custom/statins-chronic-kidney-disease-when-and-when-not-use-them-1
45. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci 2016;8:23–8.
46. Caro J, Klittich W, McGuire A, et al. The West of Scotland coronary prevention study: Economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315:1577–82.
47. Schectman G, Wolff N, Byrd JC, et al. Physician extenders for cost-effective management of hypercholesterolemia. J Gen Intern Med 1996;11:277–86.
48. Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian simvastatin survival study group. N Engl J Med 1997;336:332–6.
49. Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–41.
50. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97.
51. Polisecki E, Peter I, Robertson M, et al. Genetic variation at the PCSK9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis 2008;200:95–101.
52. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22.
53. Sinthupoom N, Prachayasittikul V, Prachayasittikul S, et al. Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications. Eur Food Res Technol 2015;240: 1–17.
54. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55.
55. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67.
56. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12.
57. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421.
58. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–64.
1. Fact sheet: Aging in the United States. Accessed at www.prb.org/aging-unitedstates-fact-sheet/.
2. Kontis JE, Bennett CD, Mathers G, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323–35.
3. Odden MD, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e1–e458.
5. Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Amer Col Cardiol 2008;52:1769–81.
6. Stone NJ. Statins in secondary prevention: intensity matters. J Am Coll Cardiol 2017;69: 2707–9.
7. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30.
8. Heart Protection Study Collaborative Group Writers. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
9. Lefevre F, Nishida L. Special report: the efficacy and safety of statins in the elderly. TEC Assessment Program 2007;21
10. Pravastatin benefits elderly patients: results of PROSPER study. Cardiovasc J S Afr 2003;14:48.
11. Catapano AL, Graham I, Backer GD, et al. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058.
12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
13. Jacobson TA, Ito MK, Maki K, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol 2015;9:129-169
14. Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol 2015;9:S1–22.e1.
15. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologist and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23:1–87.
16. Lloyd-Jones DM, Morris PB, Minissian MB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016;68:92–125.
17. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59.
18. Chaturvedi S, Zivin J, Breazna A, et al. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009;72:688–94.
19. Sever PS, Dahior B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian cardiac outcomes trial—lipid lowering arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
20. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9.
21. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin of coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–09.
23. Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
24. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–04.
25. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
26. Deedwania P, Stone PH, Bairey CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation 2007;115:700–7.
27. Han BH, Sutin D, Williamson JD, et al; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177:955–65.
28. Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C Reactive protein. N Engl J Med 2008; 359:2195–207.
29. Glynn RJ, Koenig W, Nordestgaard BG, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann Intern Med 2010;152:488–96.
30. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
31. Neil HA, DeMicco DA, Luo D, et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: collaborative atorvastatin diabetes study (CARDS). Diabetes Care 2006;29:2378–84.
32. Yusuf S, Bosch J, Dagenais G, et al; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31.
33. Savarese G, Gotta AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–99.
34. National Institute of Health. A clinical trial of STAtin therapy for reducing events in the elderly (STAREE). Clinical Trials. https://clinicaltrials.gov/ct2/show/NCT02099123. Accessed June 5, 2018.
35. Gaist D, Rodríquez, LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565–9.
36. Pasternak RC., Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567–72.
37. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15.
38. Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep 2010;12:322–30.
39. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14.
40. Ahmad Z. Statin intolerance. Am J Cardiol 2014;113:1765–71.
41. Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Published February 28, 2012. Accessed June 5, 2018.
42. Gauthier JM, Massicotte A. Statins and their effect on cognition: let’s clear up the confusion. Can Pharm J (Ott) 2015;148:150–55.
43. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92.
44. Vaziri ND, Anzalone DA, Catini J. Statins in chronic didney disease: when and when not to use them. J Fam Pract 2016;65:8 Suppl. www.mdedge.com/jfp/custom/statins-chronic-kidney-disease-when-and-when-not-use-them-1
45. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci 2016;8:23–8.
46. Caro J, Klittich W, McGuire A, et al. The West of Scotland coronary prevention study: Economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315:1577–82.
47. Schectman G, Wolff N, Byrd JC, et al. Physician extenders for cost-effective management of hypercholesterolemia. J Gen Intern Med 1996;11:277–86.
48. Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian simvastatin survival study group. N Engl J Med 1997;336:332–6.
49. Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–41.
50. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97.
51. Polisecki E, Peter I, Robertson M, et al. Genetic variation at the PCSK9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis 2008;200:95–101.
52. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22.
53. Sinthupoom N, Prachayasittikul V, Prachayasittikul S, et al. Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications. Eur Food Res Technol 2015;240: 1–17.
54. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55.
55. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67.
56. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12.
57. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421.
58. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–64.