User login
PARP inhibitors: New developments in ovarian cancer treatment
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 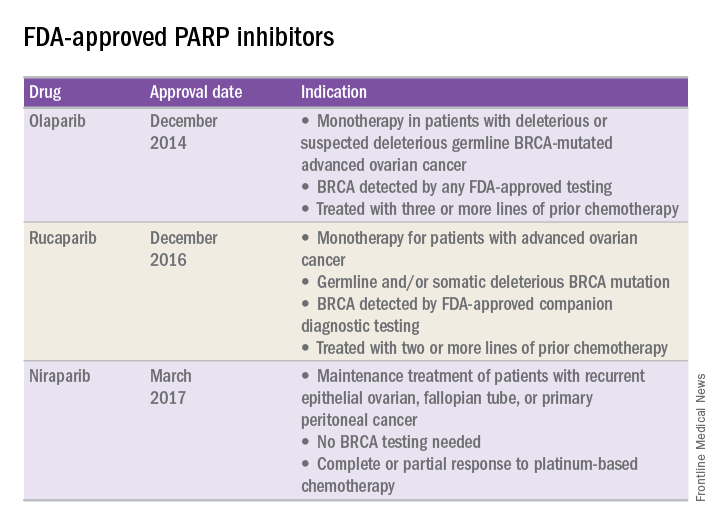
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Optimizing surveillance for gynecologic cancers
Gynecologic cancers contribute to approximately 15% of cancer survivorship care for women. Many patients share surveillance visits between their gynecologic oncologist and their ob.gyn. or primary care physician to capitalize on preexisting relationships and ensure the provision of comprehensive wellness care. Providing high quality surveillance care is challenging because it requires vigilance in the detection of recurrence but also avoidance of unnecessary, costly, and inaccurate testing.
The oncologic benefits for various surveillance guidelines are not well established by prospective studies. However, in updated surveillance recommendations, the Society of Gynecologic Oncology (SGO) takes available data, costs, and benefits into consideration.1 The guidelines, authored by Ritu Salani, MD, provide an excellent resource for understanding appropriate testing and evaluation during surveillance care.
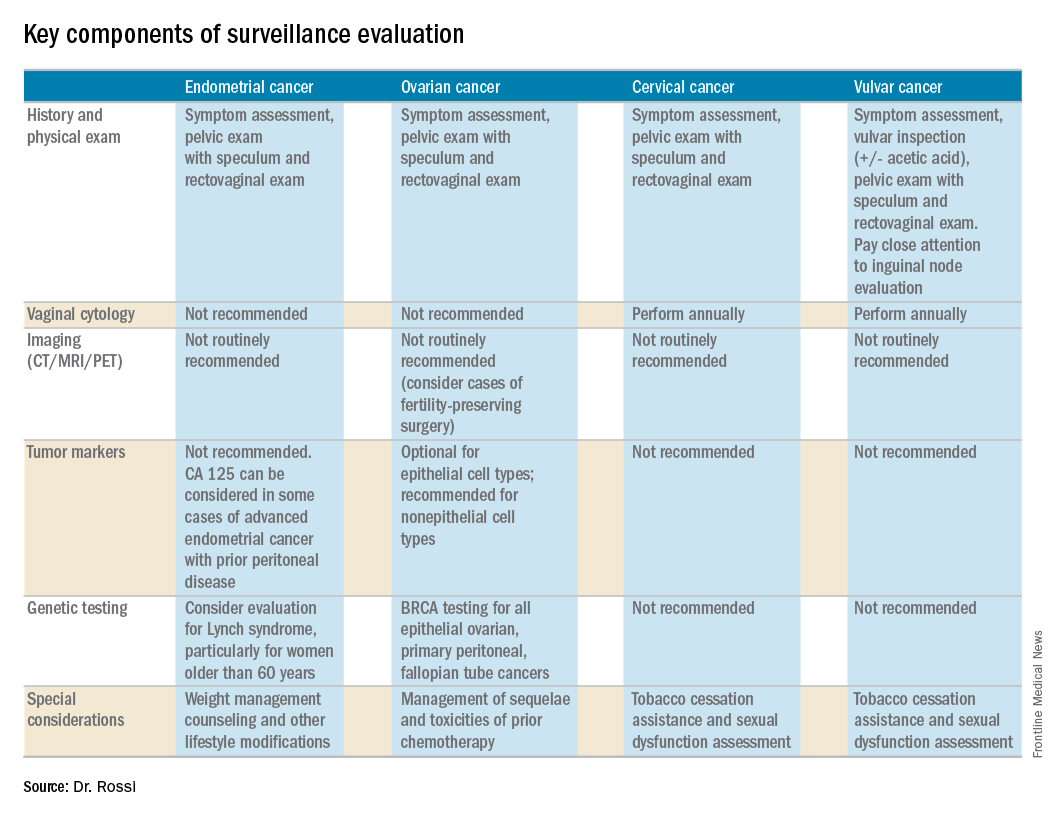
The cornerstone of a surveillance visit is a thorough symptom assessment. Positive reporting of symptoms remains the most sensitive method for detecting recurrences; therefore, patients should be educated and quizzed on common recurrence symptoms. For example, endometrial cancer most commonly recurs in the vagina with symptoms of vaginal bleeding or discharge. Lower extremity swelling can signify pelvic sidewall recurrences and abdominal bloating or pain can signify peritoneal recurrence of ovarian or endometrial cancer.
All women who are undergoing surveillance for gynecologic cancers should receive physical examinations that include a pelvic exam with a speculum and bimanual exam with rectovaginal exam. Many locoregional recurrences are salvageable for most gynecologic cancers, which is not true for most distant recurrences, emphasizing the importance of pelvic examinations.
In addition to surveillance of recurrence, these visits should focus on risk modification – tobacco, obesity, bone demineralization – as well as preventive health strategies, such as vaccinations, nongynecologic cancer screenings, and cardiovascular disease intervention. Clinicians should also ask about sequelae to cancer therapy, such as neuropathy, lymphedema, sexual dysfunction, depression, and fatigue.
Endometrial cancer
Endometrial cancer recurs most commonly among women with a history of advanced stage cancer or early stage disease with high/intermediate risk factors, but all survivors should be evaluated regularly for recurrence. The vagina is the most common site for recurrence. Fortunately, many vaginal recurrences can be cured with salvage therapies.
Women with the lowest risk for recurrence (stage IA, grades 1 and 2 disease) who did not originally qualify for adjuvant therapy can be followed every 6 months for 2 years and then annually.
Vaginal cytology is no longer recommended for the routine surveillance of endometrial cancer because of its poor sensitivity in detecting recurrence and low positive predictive value (particularly after vaginal radiation).2 Any suspicious lesions identified on speculum examination should be biopsied, rather than sampled with cytologic smear. Routine imaging (with CT or PET/CT) and cancer antigen (CA) 125 tumor marker assessment is not supported unless the initial stage was advanced. These tests should be reserved for confirmation of concerning symptoms or examination findings.
This group of patients has particular survivorship needs with respect to obesity interventions. Obesity is associated with poor prognosis from endometrial cancer, and patients should be counseled about this and offered strategies for weight loss and lifestyle modification. Lynch syndrome testing and colon cancer screening are also an important consideration in this population.
Ovarian cancer
Ovarian cancer recurrence rates are high, and, while salvage therapies are rarely curative, enduring responses may be achieved in some patients, making surveillance visits critical. The SGO recommends surveillance visits every 3 months in the first 2 years, every 4 months in year 3, and then every 6 months for an additional 2 years. At these visits, patients should be queried about symptoms with particular emphasis on peritoneal signs (bloating, distension, gastrointestinal disturbance, and abdominal pain) as most recurrences will be within the peritoneal cavity.
CA 125 tumor marker elevation during the surveillance phase may signal recurrence prior to the development of symptoms but initiating chemotherapy early because of elevations in CA 125 does not improve survival.3 However, in the platinum-sensitive population with a longer disease-free interval, earlier detection of recurrence by CA 125 may identify patients in whom complete secondary cytoreduction is more attainable and is associated with improved survival.4 Therefore, the SGO suggests that CA 125 assessment is optional. The benefits and limitations of earlier detection of recurrence should be discussed with each patient. This recommendation differs for survivors of nonepithelial ovarian cancers (such as germ-cell or sex-cord stromal), in which case the measurement of the appropriate tumor markers (such as alpha-fetoprotein, human chorionic gonadotropin, and inhibin) should be performed routinely as part of surveillance evaluation.
Evidence does not support routine imaging (such as CT or PET). It should be reserved as a confirmatory measure for patients with concerning symptoms, examination findings, or elevations in tumor markers. When ovarian cancer has been treated with fertility-preserving surgery in women of younger reproductive ages, pelvic ultrasounds may be used as part of their surveillance care to monitor retained ovaries and pelvic structures.
BRCA-gene testing should be offered to all women with epithelial ovarian, fallopian tube, and primary peritoneal cancer as it impacts future cancer risk, as well as chemotherapy selection.5
Cervical cancer
In the first 2 years after completing primary treatment for cervical cancer, those at high risk for recurrence (including those who were recommended to adjuvant therapy) should be evaluated every 3 months for 2 years, followed by visits at 6-month intervals for an additional 3 years. Low-risk patients can be followed every 6 months for 2 years, followed by annual visits thereafter.
Pap testing should be performed annually, rather than at each surveillance visit. It should not to detect recurrence – for which it has poor sensitivity and specificity – but rather to detect new HPV-related dysplasia.6
Many patients with cervical cancer have a tobacco use history, placing them at risk for other cancers. Educate patients about the risk and provide cessation assistance.
Vulvar cancer
Prognosis for early stage vulvar cancer is very good; however, local recurrences are common (as much as 40%) in the 10 years following diagnosis.7 It is important to thoroughly inspect the vulva, vagina, and cervix at each surveillance visit. In high-risk patients, examinations should take place every 3 months for the first 2 years after completing primary treatment and every 6 months until 5 years post treatment. Low-risk patients can be followed every 6 months for 2 years and annually thereafter.
Identification and early treatment of dysplasia is important. Careful attention should also be made to palpation of the inguinal nodal regions. One in 10 women will have a late recurrence (greater than 5 years), so vulvar inspections should continue at least annually for the remainder of a woman’s life.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Gynecol Oncol. 2017 Mar 31. doi: 10.1016/j.ygno.2017.03.022.
2. Obstet Gynecol. 2013 Jan;121(1):129-35.
3. Lancet. 2010 Oct 2;376(9747):1155-63.
4. Gynecol Oncol. 2009 Jan;112(1):265-74.
5. Gynecol Oncol. 2015 Jan;136(1):3-7.
6. Obstet Gynecol. 2011 Sep;118(3):548-53.
7. Gynecol Oncol. 2016 Jan;140(1):8-14.
Gynecologic cancers contribute to approximately 15% of cancer survivorship care for women. Many patients share surveillance visits between their gynecologic oncologist and their ob.gyn. or primary care physician to capitalize on preexisting relationships and ensure the provision of comprehensive wellness care. Providing high quality surveillance care is challenging because it requires vigilance in the detection of recurrence but also avoidance of unnecessary, costly, and inaccurate testing.
The oncologic benefits for various surveillance guidelines are not well established by prospective studies. However, in updated surveillance recommendations, the Society of Gynecologic Oncology (SGO) takes available data, costs, and benefits into consideration.1 The guidelines, authored by Ritu Salani, MD, provide an excellent resource for understanding appropriate testing and evaluation during surveillance care.
The cornerstone of a surveillance visit is a thorough symptom assessment. Positive reporting of symptoms remains the most sensitive method for detecting recurrences; therefore, patients should be educated and quizzed on common recurrence symptoms. For example, endometrial cancer most commonly recurs in the vagina with symptoms of vaginal bleeding or discharge. Lower extremity swelling can signify pelvic sidewall recurrences and abdominal bloating or pain can signify peritoneal recurrence of ovarian or endometrial cancer.
All women who are undergoing surveillance for gynecologic cancers should receive physical examinations that include a pelvic exam with a speculum and bimanual exam with rectovaginal exam. Many locoregional recurrences are salvageable for most gynecologic cancers, which is not true for most distant recurrences, emphasizing the importance of pelvic examinations.
In addition to surveillance of recurrence, these visits should focus on risk modification – tobacco, obesity, bone demineralization – as well as preventive health strategies, such as vaccinations, nongynecologic cancer screenings, and cardiovascular disease intervention. Clinicians should also ask about sequelae to cancer therapy, such as neuropathy, lymphedema, sexual dysfunction, depression, and fatigue.

Endometrial cancer
Endometrial cancer recurs most commonly among women with a history of advanced stage cancer or early stage disease with high/intermediate risk factors, but all survivors should be evaluated regularly for recurrence. The vagina is the most common site for recurrence. Fortunately, many vaginal recurrences can be cured with salvage therapies.
Women with the lowest risk for recurrence (stage IA, grades 1 and 2 disease) who did not originally qualify for adjuvant therapy can be followed every 6 months for 2 years and then annually.
Vaginal cytology is no longer recommended for the routine surveillance of endometrial cancer because of its poor sensitivity in detecting recurrence and low positive predictive value (particularly after vaginal radiation).2 Any suspicious lesions identified on speculum examination should be biopsied, rather than sampled with cytologic smear. Routine imaging (with CT or PET/CT) and cancer antigen (CA) 125 tumor marker assessment is not supported unless the initial stage was advanced. These tests should be reserved for confirmation of concerning symptoms or examination findings.
This group of patients has particular survivorship needs with respect to obesity interventions. Obesity is associated with poor prognosis from endometrial cancer, and patients should be counseled about this and offered strategies for weight loss and lifestyle modification. Lynch syndrome testing and colon cancer screening are also an important consideration in this population.
Ovarian cancer
Ovarian cancer recurrence rates are high, and, while salvage therapies are rarely curative, enduring responses may be achieved in some patients, making surveillance visits critical. The SGO recommends surveillance visits every 3 months in the first 2 years, every 4 months in year 3, and then every 6 months for an additional 2 years. At these visits, patients should be queried about symptoms with particular emphasis on peritoneal signs (bloating, distension, gastrointestinal disturbance, and abdominal pain) as most recurrences will be within the peritoneal cavity.
CA 125 tumor marker elevation during the surveillance phase may signal recurrence prior to the development of symptoms but initiating chemotherapy early because of elevations in CA 125 does not improve survival.3 However, in the platinum-sensitive population with a longer disease-free interval, earlier detection of recurrence by CA 125 may identify patients in whom complete secondary cytoreduction is more attainable and is associated with improved survival.4 Therefore, the SGO suggests that CA 125 assessment is optional. The benefits and limitations of earlier detection of recurrence should be discussed with each patient. This recommendation differs for survivors of nonepithelial ovarian cancers (such as germ-cell or sex-cord stromal), in which case the measurement of the appropriate tumor markers (such as alpha-fetoprotein, human chorionic gonadotropin, and inhibin) should be performed routinely as part of surveillance evaluation.
Evidence does not support routine imaging (such as CT or PET). It should be reserved as a confirmatory measure for patients with concerning symptoms, examination findings, or elevations in tumor markers. When ovarian cancer has been treated with fertility-preserving surgery in women of younger reproductive ages, pelvic ultrasounds may be used as part of their surveillance care to monitor retained ovaries and pelvic structures.
BRCA-gene testing should be offered to all women with epithelial ovarian, fallopian tube, and primary peritoneal cancer as it impacts future cancer risk, as well as chemotherapy selection.5
Cervical cancer
In the first 2 years after completing primary treatment for cervical cancer, those at high risk for recurrence (including those who were recommended to adjuvant therapy) should be evaluated every 3 months for 2 years, followed by visits at 6-month intervals for an additional 3 years. Low-risk patients can be followed every 6 months for 2 years, followed by annual visits thereafter.
Pap testing should be performed annually, rather than at each surveillance visit. It should not to detect recurrence – for which it has poor sensitivity and specificity – but rather to detect new HPV-related dysplasia.6
Many patients with cervical cancer have a tobacco use history, placing them at risk for other cancers. Educate patients about the risk and provide cessation assistance.
Vulvar cancer
Prognosis for early stage vulvar cancer is very good; however, local recurrences are common (as much as 40%) in the 10 years following diagnosis.7 It is important to thoroughly inspect the vulva, vagina, and cervix at each surveillance visit. In high-risk patients, examinations should take place every 3 months for the first 2 years after completing primary treatment and every 6 months until 5 years post treatment. Low-risk patients can be followed every 6 months for 2 years and annually thereafter.
Identification and early treatment of dysplasia is important. Careful attention should also be made to palpation of the inguinal nodal regions. One in 10 women will have a late recurrence (greater than 5 years), so vulvar inspections should continue at least annually for the remainder of a woman’s life.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Gynecol Oncol. 2017 Mar 31. doi: 10.1016/j.ygno.2017.03.022.
2. Obstet Gynecol. 2013 Jan;121(1):129-35.
3. Lancet. 2010 Oct 2;376(9747):1155-63.
4. Gynecol Oncol. 2009 Jan;112(1):265-74.
5. Gynecol Oncol. 2015 Jan;136(1):3-7.
6. Obstet Gynecol. 2011 Sep;118(3):548-53.
7. Gynecol Oncol. 2016 Jan;140(1):8-14.
Gynecologic cancers contribute to approximately 15% of cancer survivorship care for women. Many patients share surveillance visits between their gynecologic oncologist and their ob.gyn. or primary care physician to capitalize on preexisting relationships and ensure the provision of comprehensive wellness care. Providing high quality surveillance care is challenging because it requires vigilance in the detection of recurrence but also avoidance of unnecessary, costly, and inaccurate testing.
The oncologic benefits for various surveillance guidelines are not well established by prospective studies. However, in updated surveillance recommendations, the Society of Gynecologic Oncology (SGO) takes available data, costs, and benefits into consideration.1 The guidelines, authored by Ritu Salani, MD, provide an excellent resource for understanding appropriate testing and evaluation during surveillance care.
The cornerstone of a surveillance visit is a thorough symptom assessment. Positive reporting of symptoms remains the most sensitive method for detecting recurrences; therefore, patients should be educated and quizzed on common recurrence symptoms. For example, endometrial cancer most commonly recurs in the vagina with symptoms of vaginal bleeding or discharge. Lower extremity swelling can signify pelvic sidewall recurrences and abdominal bloating or pain can signify peritoneal recurrence of ovarian or endometrial cancer.
All women who are undergoing surveillance for gynecologic cancers should receive physical examinations that include a pelvic exam with a speculum and bimanual exam with rectovaginal exam. Many locoregional recurrences are salvageable for most gynecologic cancers, which is not true for most distant recurrences, emphasizing the importance of pelvic examinations.
In addition to surveillance of recurrence, these visits should focus on risk modification – tobacco, obesity, bone demineralization – as well as preventive health strategies, such as vaccinations, nongynecologic cancer screenings, and cardiovascular disease intervention. Clinicians should also ask about sequelae to cancer therapy, such as neuropathy, lymphedema, sexual dysfunction, depression, and fatigue.

Endometrial cancer
Endometrial cancer recurs most commonly among women with a history of advanced stage cancer or early stage disease with high/intermediate risk factors, but all survivors should be evaluated regularly for recurrence. The vagina is the most common site for recurrence. Fortunately, many vaginal recurrences can be cured with salvage therapies.
Women with the lowest risk for recurrence (stage IA, grades 1 and 2 disease) who did not originally qualify for adjuvant therapy can be followed every 6 months for 2 years and then annually.
Vaginal cytology is no longer recommended for the routine surveillance of endometrial cancer because of its poor sensitivity in detecting recurrence and low positive predictive value (particularly after vaginal radiation).2 Any suspicious lesions identified on speculum examination should be biopsied, rather than sampled with cytologic smear. Routine imaging (with CT or PET/CT) and cancer antigen (CA) 125 tumor marker assessment is not supported unless the initial stage was advanced. These tests should be reserved for confirmation of concerning symptoms or examination findings.
This group of patients has particular survivorship needs with respect to obesity interventions. Obesity is associated with poor prognosis from endometrial cancer, and patients should be counseled about this and offered strategies for weight loss and lifestyle modification. Lynch syndrome testing and colon cancer screening are also an important consideration in this population.
Ovarian cancer
Ovarian cancer recurrence rates are high, and, while salvage therapies are rarely curative, enduring responses may be achieved in some patients, making surveillance visits critical. The SGO recommends surveillance visits every 3 months in the first 2 years, every 4 months in year 3, and then every 6 months for an additional 2 years. At these visits, patients should be queried about symptoms with particular emphasis on peritoneal signs (bloating, distension, gastrointestinal disturbance, and abdominal pain) as most recurrences will be within the peritoneal cavity.
CA 125 tumor marker elevation during the surveillance phase may signal recurrence prior to the development of symptoms but initiating chemotherapy early because of elevations in CA 125 does not improve survival.3 However, in the platinum-sensitive population with a longer disease-free interval, earlier detection of recurrence by CA 125 may identify patients in whom complete secondary cytoreduction is more attainable and is associated with improved survival.4 Therefore, the SGO suggests that CA 125 assessment is optional. The benefits and limitations of earlier detection of recurrence should be discussed with each patient. This recommendation differs for survivors of nonepithelial ovarian cancers (such as germ-cell or sex-cord stromal), in which case the measurement of the appropriate tumor markers (such as alpha-fetoprotein, human chorionic gonadotropin, and inhibin) should be performed routinely as part of surveillance evaluation.
Evidence does not support routine imaging (such as CT or PET). It should be reserved as a confirmatory measure for patients with concerning symptoms, examination findings, or elevations in tumor markers. When ovarian cancer has been treated with fertility-preserving surgery in women of younger reproductive ages, pelvic ultrasounds may be used as part of their surveillance care to monitor retained ovaries and pelvic structures.
BRCA-gene testing should be offered to all women with epithelial ovarian, fallopian tube, and primary peritoneal cancer as it impacts future cancer risk, as well as chemotherapy selection.5
Cervical cancer
In the first 2 years after completing primary treatment for cervical cancer, those at high risk for recurrence (including those who were recommended to adjuvant therapy) should be evaluated every 3 months for 2 years, followed by visits at 6-month intervals for an additional 3 years. Low-risk patients can be followed every 6 months for 2 years, followed by annual visits thereafter.
Pap testing should be performed annually, rather than at each surveillance visit. It should not to detect recurrence – for which it has poor sensitivity and specificity – but rather to detect new HPV-related dysplasia.6
Many patients with cervical cancer have a tobacco use history, placing them at risk for other cancers. Educate patients about the risk and provide cessation assistance.
Vulvar cancer
Prognosis for early stage vulvar cancer is very good; however, local recurrences are common (as much as 40%) in the 10 years following diagnosis.7 It is important to thoroughly inspect the vulva, vagina, and cervix at each surveillance visit. In high-risk patients, examinations should take place every 3 months for the first 2 years after completing primary treatment and every 6 months until 5 years post treatment. Low-risk patients can be followed every 6 months for 2 years and annually thereafter.
Identification and early treatment of dysplasia is important. Careful attention should also be made to palpation of the inguinal nodal regions. One in 10 women will have a late recurrence (greater than 5 years), so vulvar inspections should continue at least annually for the remainder of a woman’s life.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reported having no relevant financial disclosures.
References
1. Gynecol Oncol. 2017 Mar 31. doi: 10.1016/j.ygno.2017.03.022.
2. Obstet Gynecol. 2013 Jan;121(1):129-35.
3. Lancet. 2010 Oct 2;376(9747):1155-63.
4. Gynecol Oncol. 2009 Jan;112(1):265-74.
5. Gynecol Oncol. 2015 Jan;136(1):3-7.
6. Obstet Gynecol. 2011 Sep;118(3):548-53.
7. Gynecol Oncol. 2016 Jan;140(1):8-14.
Ovarian cancer screening update
Ovarian cancer remains the most deadly gynecologic malignancy in the United States with more than 14,000 deaths in 2016. Yet, the prevalence remains low with approximately 22,000 cases in 2016. Stage at diagnosis is one of the strongest predictors of overall survival. The 5-year overall survival is more than 90% with stage I disease; this drops to 25% for those with distant metastases. Unfortunately, three-quarters of patients have disease spread beyond the ovary at the time ovarian cancer is clinically identified.
In this update, we will review:
• The fundamentals of ovarian cancer screening.
• How to identify patients who would benefit from surveillance.
• The usefulness of tumor markers.
• The results from recent large ovarian cancer screening trials.
Screening is a critical part of secondary prevention through early disease detection, when patients are asymptomatic and treatment can stop progression. Core principles of a good screening test are that the test is noninvasive, tolerable to the patient, and not costly. The disease should pose a major health threat and be detected at a stage at which intervention can impart a survival advantage. Most critically, the test should be sensitive and specific (i.e., detect disease when it is truly present and rarely be positive in the absence of disease).
Screening vs. case finding
A significant distinction should be made between average-risk patients and high-risk patients. Ob.gyns. frequently encounter high-risk patients who would benefit from regular surveillance or case finding (for example, patients with BRCA deleterious mutations or with Lynch syndrome). There are multiple risk factors for ovarian cancer, but the strongest known is family history, which is present in 15% of ovarian cancer patients. Having one relative with ovarian cancer increases the lifetime risk of ovarian cancer up to 5%. When a patient reports having one or more family members with ovarian cancer, it is important to differentiate between a common sporadic presentation and a rare familial cancer syndrome. ACOG Practice Bulletin 103 provides excellent guidance on which patients warrant formal genetic risk assessments by a genetic counselor.2
Tumor markers
During the last 25 years, screening for ovarian cancer in an average-risk population has been evaluated in multiple large prospective studies using serum tumor markers (i.e., CA 125) and ultrasound results.
CA 125 and HE4 tumor markers are frequently elevated in ovarian cancer and have been studied in ovarian cancer screening. However, while having a high sensitivity for detecting disease, they are nonspecific because they are also elevated in numerous benign conditions and therefore have not proven to be a useful screening tool in the average-risk population. There are clinically available tumor marker panels that are not intended for screening. Rather, they clarify the uncertainty of the presurgical adnexal mass evaluation by providing a risk score. High risk scores are generally managed in conjunction with a gynecologic oncology referral.
Multimodal screening
Combined assessment of both ultrasound findings and tumor marker levels shows more promise with respect to prediction of ovarian cancer. However, a systematic review of 25 ovarian cancer screening studies concluded that screening low-risk populations should not be included in clinical practice until randomized trials assessed the effect on mortality and the risk of adverse events. Three large randomized controlled trials have been completed to date.3,4,5
The U.K. Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) results appear promising. However, the revealing analysis was post hoc since the original study design did not take into account the inherent delayed effect in screening studies. While these results may provide a basis for future successful screening for ovarian cancer, confirmatory further analysis is pending, using additional data over a period of the next 3 years.
Ultimately, we are all excited about the possibility of effective screening protocols for ovarian cancer and await completed analyses of UKCTOCS. Until their benefits are confirmed, screening and preventive measures should be limited to those at high risk for ovarian cancer.
References
1. Hippokratia. 2007 Apr;11(2):63-6.
2. Obstet Gynecol. 2009 Apr;113(4):957-66.
3. Am J Obstet Gynecol. 2005 Nov;193(5):1630-9.
4. Int J Gynecol Cancer. 2008 May-Jun;18(3):414-20.
5. Lancet. 2016 Mar 5;387(10022):945-56.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the most deadly gynecologic malignancy in the United States with more than 14,000 deaths in 2016. Yet, the prevalence remains low with approximately 22,000 cases in 2016. Stage at diagnosis is one of the strongest predictors of overall survival. The 5-year overall survival is more than 90% with stage I disease; this drops to 25% for those with distant metastases. Unfortunately, three-quarters of patients have disease spread beyond the ovary at the time ovarian cancer is clinically identified.
In this update, we will review:
• The fundamentals of ovarian cancer screening.
• How to identify patients who would benefit from surveillance.
• The usefulness of tumor markers.
• The results from recent large ovarian cancer screening trials.
Screening is a critical part of secondary prevention through early disease detection, when patients are asymptomatic and treatment can stop progression. Core principles of a good screening test are that the test is noninvasive, tolerable to the patient, and not costly. The disease should pose a major health threat and be detected at a stage at which intervention can impart a survival advantage. Most critically, the test should be sensitive and specific (i.e., detect disease when it is truly present and rarely be positive in the absence of disease).
Screening vs. case finding
A significant distinction should be made between average-risk patients and high-risk patients. Ob.gyns. frequently encounter high-risk patients who would benefit from regular surveillance or case finding (for example, patients with BRCA deleterious mutations or with Lynch syndrome). There are multiple risk factors for ovarian cancer, but the strongest known is family history, which is present in 15% of ovarian cancer patients. Having one relative with ovarian cancer increases the lifetime risk of ovarian cancer up to 5%. When a patient reports having one or more family members with ovarian cancer, it is important to differentiate between a common sporadic presentation and a rare familial cancer syndrome. ACOG Practice Bulletin 103 provides excellent guidance on which patients warrant formal genetic risk assessments by a genetic counselor.2
Tumor markers
During the last 25 years, screening for ovarian cancer in an average-risk population has been evaluated in multiple large prospective studies using serum tumor markers (i.e., CA 125) and ultrasound results.
CA 125 and HE4 tumor markers are frequently elevated in ovarian cancer and have been studied in ovarian cancer screening. However, while having a high sensitivity for detecting disease, they are nonspecific because they are also elevated in numerous benign conditions and therefore have not proven to be a useful screening tool in the average-risk population. There are clinically available tumor marker panels that are not intended for screening. Rather, they clarify the uncertainty of the presurgical adnexal mass evaluation by providing a risk score. High risk scores are generally managed in conjunction with a gynecologic oncology referral.
Multimodal screening
Combined assessment of both ultrasound findings and tumor marker levels shows more promise with respect to prediction of ovarian cancer. However, a systematic review of 25 ovarian cancer screening studies concluded that screening low-risk populations should not be included in clinical practice until randomized trials assessed the effect on mortality and the risk of adverse events. Three large randomized controlled trials have been completed to date.3,4,5
The U.K. Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) results appear promising. However, the revealing analysis was post hoc since the original study design did not take into account the inherent delayed effect in screening studies. While these results may provide a basis for future successful screening for ovarian cancer, confirmatory further analysis is pending, using additional data over a period of the next 3 years.
Ultimately, we are all excited about the possibility of effective screening protocols for ovarian cancer and await completed analyses of UKCTOCS. Until their benefits are confirmed, screening and preventive measures should be limited to those at high risk for ovarian cancer.
References
1. Hippokratia. 2007 Apr;11(2):63-6.
2. Obstet Gynecol. 2009 Apr;113(4):957-66.
3. Am J Obstet Gynecol. 2005 Nov;193(5):1630-9.
4. Int J Gynecol Cancer. 2008 May-Jun;18(3):414-20.
5. Lancet. 2016 Mar 5;387(10022):945-56.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the most deadly gynecologic malignancy in the United States with more than 14,000 deaths in 2016. Yet, the prevalence remains low with approximately 22,000 cases in 2016. Stage at diagnosis is one of the strongest predictors of overall survival. The 5-year overall survival is more than 90% with stage I disease; this drops to 25% for those with distant metastases. Unfortunately, three-quarters of patients have disease spread beyond the ovary at the time ovarian cancer is clinically identified.
In this update, we will review:
• The fundamentals of ovarian cancer screening.
• How to identify patients who would benefit from surveillance.
• The usefulness of tumor markers.
• The results from recent large ovarian cancer screening trials.
Screening is a critical part of secondary prevention through early disease detection, when patients are asymptomatic and treatment can stop progression. Core principles of a good screening test are that the test is noninvasive, tolerable to the patient, and not costly. The disease should pose a major health threat and be detected at a stage at which intervention can impart a survival advantage. Most critically, the test should be sensitive and specific (i.e., detect disease when it is truly present and rarely be positive in the absence of disease).
Screening vs. case finding
A significant distinction should be made between average-risk patients and high-risk patients. Ob.gyns. frequently encounter high-risk patients who would benefit from regular surveillance or case finding (for example, patients with BRCA deleterious mutations or with Lynch syndrome). There are multiple risk factors for ovarian cancer, but the strongest known is family history, which is present in 15% of ovarian cancer patients. Having one relative with ovarian cancer increases the lifetime risk of ovarian cancer up to 5%. When a patient reports having one or more family members with ovarian cancer, it is important to differentiate between a common sporadic presentation and a rare familial cancer syndrome. ACOG Practice Bulletin 103 provides excellent guidance on which patients warrant formal genetic risk assessments by a genetic counselor.2
Tumor markers
During the last 25 years, screening for ovarian cancer in an average-risk population has been evaluated in multiple large prospective studies using serum tumor markers (i.e., CA 125) and ultrasound results.
CA 125 and HE4 tumor markers are frequently elevated in ovarian cancer and have been studied in ovarian cancer screening. However, while having a high sensitivity for detecting disease, they are nonspecific because they are also elevated in numerous benign conditions and therefore have not proven to be a useful screening tool in the average-risk population. There are clinically available tumor marker panels that are not intended for screening. Rather, they clarify the uncertainty of the presurgical adnexal mass evaluation by providing a risk score. High risk scores are generally managed in conjunction with a gynecologic oncology referral.
Multimodal screening
Combined assessment of both ultrasound findings and tumor marker levels shows more promise with respect to prediction of ovarian cancer. However, a systematic review of 25 ovarian cancer screening studies concluded that screening low-risk populations should not be included in clinical practice until randomized trials assessed the effect on mortality and the risk of adverse events. Three large randomized controlled trials have been completed to date.3,4,5
The U.K. Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) results appear promising. However, the revealing analysis was post hoc since the original study design did not take into account the inherent delayed effect in screening studies. While these results may provide a basis for future successful screening for ovarian cancer, confirmatory further analysis is pending, using additional data over a period of the next 3 years.
Ultimately, we are all excited about the possibility of effective screening protocols for ovarian cancer and await completed analyses of UKCTOCS. Until their benefits are confirmed, screening and preventive measures should be limited to those at high risk for ovarian cancer.
References
1. Hippokratia. 2007 Apr;11(2):63-6.
2. Obstet Gynecol. 2009 Apr;113(4):957-66.
3. Am J Obstet Gynecol. 2005 Nov;193(5):1630-9.
4. Int J Gynecol Cancer. 2008 May-Jun;18(3):414-20.
5. Lancet. 2016 Mar 5;387(10022):945-56.
Dr. Pierce is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Sentinel lymph node technique in endometrial cancer, Part 2
As reviewed in Part 1, surgery is indicated for the staging and treatment of endometrial cancer. Lymph node status is one of the most important factors in determining prognosis and the need for adjuvant treatment. The extent of lymph node evaluation is controversial as full lymphadenectomy carries risks, including increased operative time, blood loss, nerve injury, and lymphedema.
Two trials have found no survival benefit from lymphadenectomy for endometrial cancer; however, other evidence suggests that women without known nodal status may be more likely to receive radiotherapy.1,2,3
Given these issues, the sentinel lymph node technique strikes a balance between the risks and benefits of lymph node evaluation in endometrial cancer.
Sentinel lymph nodes (SLN) are the first nodes to drain a tumor site, and thus, are typically the first to demonstrate occult malignancy. The use of the SLN technique as an alternative to complete lymphadenectomy in endometrial cancer has been well described, although its accuracy and the validity of its use are still debated.
The viability of the SLN technique is predicated on the ability to achieve mapping of dye or tracer from the tumor to the first lymph node to drain the tumor. The lymphatic drainage of the endometrium is complex and unlike vulvar or breast cancer, endometrial cancer is less accessible for peritumoral injection. Several injection techniques have been described; cervical injection is the easiest to achieve and has been found to have similar or higher SLN detection than hysteroscopic or fundal injections.4,5
There are a number of techniques for SLN detection, each with unique benefits and risks. Visual identification of blue dye, most frequently isosulfan blue, is the “colorimetric method” and has been used most commonly with cervical injection for endometrial cancer. Injection of isosulfan blue does not require specialized equipment, however visualization in obese patients is inferior.6
Technetium sulfur colloid (Tc) is a radioactive tracer that can be detected by gamma probes. A preoperative lymphoscintigraphy and a handheld gamma probe are used to map lymphatics. This technique has limitations, including the additional time and coordination of procedures, as well as some evidence of poor correlation between lymphoscintigraphy and surgical SLN mapping.7
Indocyanine green (ICG) is a fluorescent dye that has excellent signal penetration and allows for real-time visual identification using near-infrared fluorescence imaging. The bilateral detection rate with ICG appears comparable or better than blue dye.8 Combinations of dye, either ICG plus Tc or Tc plus blue dye, may be also used to increase SLN detection.
The accuracy of the SLN technique is the cornerstone to its success. In a prospective multicenter study – Senti-Endo – patients with early-stage disease underwent pelvic SLN assessment with cervical injection of a combination of dyes followed by systematic pelvic node dissection. The overall negative predictive value was 97% with three patients who had positive lymph nodes that were not detected, all of whom had a type 2 endometrial cancer.9
With the uptake of the SLN technique, many institutions have protocols surrounding the technique to ensure appropriate SLN detection and evaluation. Physicians using this technique should adhere to protocols supported by National Comprehensive Cancer Network guidelines, taking care to remove any suspicious lymph nodes and perform a full side-specific lymphadenectomy if bilateral mapping is not achieved.
The extent of lymphadenectomy and application of the SLN technique in high-risk endometrial cancer remains controversial. These patients are at higher risk for unsuccessful mapping and isolated para-aortic metastasis. Retrospective series have suggested equivalent oncologic outcomes for women with high-grade cancers who have been staged by SLN biopsy, compared with selective or complete lymphadenectomy.10,11
We await the results of a large prospective trial in which patients undergo comprehensive lymphadenectomy in addition to SLN biopsy to assess the accuracy of the technique (NCT01673022).
Pathologic evaluation of SLNs is frequently done with ultrastaging, which describes additional sectioning and staining of the node. This technique frequently identifies isolated tumor cells and micrometastasis (collectively called low-volume disease) in addition to macrometastasis. The clinical and prognostic significance of low-volume disease is unknown and additional investigation is urgently needed to determine appropriate adjuvant therapy and follow-up for these patients.
The SLN technique is an acceptable approach to assess clinical stage I endometrial cancer. Physicians should consider adding the SLN biopsy to their routine staging techniques prior to exclusively adopting the new technique. They should take care to adhere to SLN algorithms and monitor outcomes.
References
1. J Natl Cancer Inst. 2008;100(23):1707-16.
2. Lancet. 2009 Jan;373(9658):125-36.
3. Am J Obstet Gynecol. 2011 Dec;205(6):562.e1–9.
4. Gynecol Oncol. 2013 Nov;131(2):299-303.
5. Int J Gynecol Cancer. 2013 Nov;23(9):1704-11.
6. Gynecol Oncol 2014 Aug;134(2):281-6.
7. Gynecol Oncol. 2009 Feb;112(2):348-352.
8. Gynecol Oncol. 2014 May;133(2):274-7.
9. Lancet Oncol. 2011 May;12(5):469-76.
10. Ann Surg Oncol. 2016 Jan;23(1):196-202.
11. Gynecol Oncol. 2016 Mar;140(3):394-9.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Sullivan is a clinical fellow in the division of gynecologic oncology at UNC, Chapel Hill. Dr. Rossi and Dr. Sullivan reported having no relevant financial disclosures.
As reviewed in Part 1, surgery is indicated for the staging and treatment of endometrial cancer. Lymph node status is one of the most important factors in determining prognosis and the need for adjuvant treatment. The extent of lymph node evaluation is controversial as full lymphadenectomy carries risks, including increased operative time, blood loss, nerve injury, and lymphedema.
Two trials have found no survival benefit from lymphadenectomy for endometrial cancer; however, other evidence suggests that women without known nodal status may be more likely to receive radiotherapy.1,2,3
Given these issues, the sentinel lymph node technique strikes a balance between the risks and benefits of lymph node evaluation in endometrial cancer.
Sentinel lymph nodes (SLN) are the first nodes to drain a tumor site, and thus, are typically the first to demonstrate occult malignancy. The use of the SLN technique as an alternative to complete lymphadenectomy in endometrial cancer has been well described, although its accuracy and the validity of its use are still debated.
The viability of the SLN technique is predicated on the ability to achieve mapping of dye or tracer from the tumor to the first lymph node to drain the tumor. The lymphatic drainage of the endometrium is complex and unlike vulvar or breast cancer, endometrial cancer is less accessible for peritumoral injection. Several injection techniques have been described; cervical injection is the easiest to achieve and has been found to have similar or higher SLN detection than hysteroscopic or fundal injections.4,5
There are a number of techniques for SLN detection, each with unique benefits and risks. Visual identification of blue dye, most frequently isosulfan blue, is the “colorimetric method” and has been used most commonly with cervical injection for endometrial cancer. Injection of isosulfan blue does not require specialized equipment, however visualization in obese patients is inferior.6
Technetium sulfur colloid (Tc) is a radioactive tracer that can be detected by gamma probes. A preoperative lymphoscintigraphy and a handheld gamma probe are used to map lymphatics. This technique has limitations, including the additional time and coordination of procedures, as well as some evidence of poor correlation between lymphoscintigraphy and surgical SLN mapping.7
Indocyanine green (ICG) is a fluorescent dye that has excellent signal penetration and allows for real-time visual identification using near-infrared fluorescence imaging. The bilateral detection rate with ICG appears comparable or better than blue dye.8 Combinations of dye, either ICG plus Tc or Tc plus blue dye, may be also used to increase SLN detection.
The accuracy of the SLN technique is the cornerstone to its success. In a prospective multicenter study – Senti-Endo – patients with early-stage disease underwent pelvic SLN assessment with cervical injection of a combination of dyes followed by systematic pelvic node dissection. The overall negative predictive value was 97% with three patients who had positive lymph nodes that were not detected, all of whom had a type 2 endometrial cancer.9
With the uptake of the SLN technique, many institutions have protocols surrounding the technique to ensure appropriate SLN detection and evaluation. Physicians using this technique should adhere to protocols supported by National Comprehensive Cancer Network guidelines, taking care to remove any suspicious lymph nodes and perform a full side-specific lymphadenectomy if bilateral mapping is not achieved.
The extent of lymphadenectomy and application of the SLN technique in high-risk endometrial cancer remains controversial. These patients are at higher risk for unsuccessful mapping and isolated para-aortic metastasis. Retrospective series have suggested equivalent oncologic outcomes for women with high-grade cancers who have been staged by SLN biopsy, compared with selective or complete lymphadenectomy.10,11
We await the results of a large prospective trial in which patients undergo comprehensive lymphadenectomy in addition to SLN biopsy to assess the accuracy of the technique (NCT01673022).
Pathologic evaluation of SLNs is frequently done with ultrastaging, which describes additional sectioning and staining of the node. This technique frequently identifies isolated tumor cells and micrometastasis (collectively called low-volume disease) in addition to macrometastasis. The clinical and prognostic significance of low-volume disease is unknown and additional investigation is urgently needed to determine appropriate adjuvant therapy and follow-up for these patients.
The SLN technique is an acceptable approach to assess clinical stage I endometrial cancer. Physicians should consider adding the SLN biopsy to their routine staging techniques prior to exclusively adopting the new technique. They should take care to adhere to SLN algorithms and monitor outcomes.
References
1. J Natl Cancer Inst. 2008;100(23):1707-16.
2. Lancet. 2009 Jan;373(9658):125-36.
3. Am J Obstet Gynecol. 2011 Dec;205(6):562.e1–9.
4. Gynecol Oncol. 2013 Nov;131(2):299-303.
5. Int J Gynecol Cancer. 2013 Nov;23(9):1704-11.
6. Gynecol Oncol 2014 Aug;134(2):281-6.
7. Gynecol Oncol. 2009 Feb;112(2):348-352.
8. Gynecol Oncol. 2014 May;133(2):274-7.
9. Lancet Oncol. 2011 May;12(5):469-76.
10. Ann Surg Oncol. 2016 Jan;23(1):196-202.
11. Gynecol Oncol. 2016 Mar;140(3):394-9.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Sullivan is a clinical fellow in the division of gynecologic oncology at UNC, Chapel Hill. Dr. Rossi and Dr. Sullivan reported having no relevant financial disclosures.
As reviewed in Part 1, surgery is indicated for the staging and treatment of endometrial cancer. Lymph node status is one of the most important factors in determining prognosis and the need for adjuvant treatment. The extent of lymph node evaluation is controversial as full lymphadenectomy carries risks, including increased operative time, blood loss, nerve injury, and lymphedema.
Two trials have found no survival benefit from lymphadenectomy for endometrial cancer; however, other evidence suggests that women without known nodal status may be more likely to receive radiotherapy.1,2,3
Given these issues, the sentinel lymph node technique strikes a balance between the risks and benefits of lymph node evaluation in endometrial cancer.
Sentinel lymph nodes (SLN) are the first nodes to drain a tumor site, and thus, are typically the first to demonstrate occult malignancy. The use of the SLN technique as an alternative to complete lymphadenectomy in endometrial cancer has been well described, although its accuracy and the validity of its use are still debated.
The viability of the SLN technique is predicated on the ability to achieve mapping of dye or tracer from the tumor to the first lymph node to drain the tumor. The lymphatic drainage of the endometrium is complex and unlike vulvar or breast cancer, endometrial cancer is less accessible for peritumoral injection. Several injection techniques have been described; cervical injection is the easiest to achieve and has been found to have similar or higher SLN detection than hysteroscopic or fundal injections.4,5
There are a number of techniques for SLN detection, each with unique benefits and risks. Visual identification of blue dye, most frequently isosulfan blue, is the “colorimetric method” and has been used most commonly with cervical injection for endometrial cancer. Injection of isosulfan blue does not require specialized equipment, however visualization in obese patients is inferior.6
Technetium sulfur colloid (Tc) is a radioactive tracer that can be detected by gamma probes. A preoperative lymphoscintigraphy and a handheld gamma probe are used to map lymphatics. This technique has limitations, including the additional time and coordination of procedures, as well as some evidence of poor correlation between lymphoscintigraphy and surgical SLN mapping.7
Indocyanine green (ICG) is a fluorescent dye that has excellent signal penetration and allows for real-time visual identification using near-infrared fluorescence imaging. The bilateral detection rate with ICG appears comparable or better than blue dye.8 Combinations of dye, either ICG plus Tc or Tc plus blue dye, may be also used to increase SLN detection.
The accuracy of the SLN technique is the cornerstone to its success. In a prospective multicenter study – Senti-Endo – patients with early-stage disease underwent pelvic SLN assessment with cervical injection of a combination of dyes followed by systematic pelvic node dissection. The overall negative predictive value was 97% with three patients who had positive lymph nodes that were not detected, all of whom had a type 2 endometrial cancer.9
With the uptake of the SLN technique, many institutions have protocols surrounding the technique to ensure appropriate SLN detection and evaluation. Physicians using this technique should adhere to protocols supported by National Comprehensive Cancer Network guidelines, taking care to remove any suspicious lymph nodes and perform a full side-specific lymphadenectomy if bilateral mapping is not achieved.
The extent of lymphadenectomy and application of the SLN technique in high-risk endometrial cancer remains controversial. These patients are at higher risk for unsuccessful mapping and isolated para-aortic metastasis. Retrospective series have suggested equivalent oncologic outcomes for women with high-grade cancers who have been staged by SLN biopsy, compared with selective or complete lymphadenectomy.10,11
We await the results of a large prospective trial in which patients undergo comprehensive lymphadenectomy in addition to SLN biopsy to assess the accuracy of the technique (NCT01673022).
Pathologic evaluation of SLNs is frequently done with ultrastaging, which describes additional sectioning and staining of the node. This technique frequently identifies isolated tumor cells and micrometastasis (collectively called low-volume disease) in addition to macrometastasis. The clinical and prognostic significance of low-volume disease is unknown and additional investigation is urgently needed to determine appropriate adjuvant therapy and follow-up for these patients.
The SLN technique is an acceptable approach to assess clinical stage I endometrial cancer. Physicians should consider adding the SLN biopsy to their routine staging techniques prior to exclusively adopting the new technique. They should take care to adhere to SLN algorithms and monitor outcomes.
References
1. J Natl Cancer Inst. 2008;100(23):1707-16.
2. Lancet. 2009 Jan;373(9658):125-36.
3. Am J Obstet Gynecol. 2011 Dec;205(6):562.e1–9.
4. Gynecol Oncol. 2013 Nov;131(2):299-303.
5. Int J Gynecol Cancer. 2013 Nov;23(9):1704-11.
6. Gynecol Oncol 2014 Aug;134(2):281-6.
7. Gynecol Oncol. 2009 Feb;112(2):348-352.
8. Gynecol Oncol. 2014 May;133(2):274-7.
9. Lancet Oncol. 2011 May;12(5):469-76.
10. Ann Surg Oncol. 2016 Jan;23(1):196-202.
11. Gynecol Oncol. 2016 Mar;140(3):394-9.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Sullivan is a clinical fellow in the division of gynecologic oncology at UNC, Chapel Hill. Dr. Rossi and Dr. Sullivan reported having no relevant financial disclosures.







