User login
Renal denervation to treat resistant hypertension: Guarded optimism
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
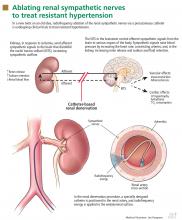
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
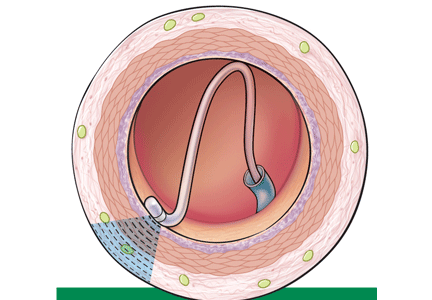
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
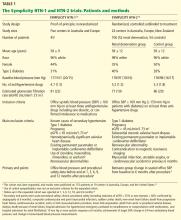
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
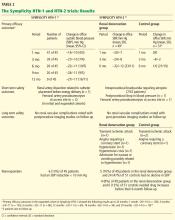
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
KEY POINTS
- Renal sympathetic nerves help regulate volume and blood pressure as they innervate the renal tubules, blood vessels, and juxtaglomerular apparatus. They carry both afferent and efferent signals between the central nervous system and the kidneys.
- Surgical sympathectomy was done in the 1950s for malignant hypertension. It had lasting antihypertensive results but also caused severe procedure-related morbidity. A new percutaneous procedure for selective renal denervation offers the advantage of causing few major procedure-related adverse effects.
- Selective renal denervation decreases norepinephrine spillover and muscle sympathetic nerve activity, evidence that the procedure reduces sympathetic tone.
- The major clinical trials done so far have found that renal denervation lowers blood pressure significantly, and the reduction is sustained for at least 3 years.