User login
2017 ACC/AHA hypertension guidelines: Toward tighter control
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
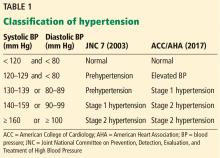
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
Should all patients with significant proteinuria take a renin-angiotensin inhibitor?
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
Most patients with proteinuria benefit from a renin-angiotensin-aldosterone system (RAAS) inhibitor. Exceptions due to adverse effects are discussed below.
WHY RAAS INHIBITORS?
RAAS inhibitors—particularly angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—reduce proteinuria and slow the progression of chronic kidney disease by improving glomerular hemodynamics, restoring the altered glomerular barrier function, and limiting the nonhemodynamic effects of angiotensin II and aldosterone, such as fibrosis and vascular endothelial dysfunction.1 Studies have shown that these protective effects are, at least in part, independent of the reduction in systemic blood pressure.2,3
EVIDENCE FOR USING RAAS INHIBITORS IN PATIENTS WITH PROTEINURIA
In nondiabetic kidney disease, there is strong evidence from the REIN and AASK trials that treatment with ACE inhibitors results in slower decline in glomerular filtration rate (GFR), and this risk reduction is more pronounced in patients with a higher degree of proteinuria.4–6
In type 1 diabetes, treatment with an ACE inhibitor in patients with overt proteinuria was associated with a 50% decrease in the risk of the combined end point of death, dialysis, or renal transplant.7 Patients with moderately increased albuminuria who were treated with an ACE inhibitor also had a reduced incidence of progression to overt proteinuria.8 Angiotensin inhibition may be beneficial even in normotensive patients with type 1 diabetes and persistent moderately increased albuminuria.9,10
In type 2 diabetes, the IDNT and RENAAL trials showed that treatment with an ARB in patients with overt nephropathy was associated with a statistically significant decrease (20% in IDNT, 16% in RENAAL) in the risk of the combined end point of death, end-stage renal disease, or doubling of serum creatinine.11,12 While there are more data for ARBs than for ACE inhibitors in type 2 diabetes, the DETAIL study showed that an ACE inhibitor was at least as effective as an ARB in providing long-term renal protection in type 2 diabetes and moderately increased albuminuria.13
Data are limited on the role of angiotensin inhibition in normotensive patients with type 2 diabetes and persistent moderately increased albuminuria, but consensus opinion suggests treatment with an ACE inhibitor or ARB in these patients if there are no contraindications.
LIMITATIONS
Adverse effects of ACE inhibitors and ARBs include cough (more with ACE inhibitors), angioedema (more with ACE inhibitors), and hyperkalemia.
The use of ARBs in patients with a history of ACE inhibitor-related angioedema has been previously discussed in this Journal.14 Guidelines advocate caution when prescribing ARBs for patients who will benefit from RAAS inhibition and have had ACE inhibitor-related angioedema.15
RAAS inhibitor therapy can cause a modest rise in creatinine due to reduction in intraglomerular pressure. An elevation in creatinine of up to 30% that stabilizes in the first 2 months is not necessarily a reason to discontinue therapy. However, a continued rise in creatinine should prompt evaluation for excessive fall in blood pressure (especially with volume depletion from concomitant diuretic use), possible bilateral renal artery stenosis, or both. There is no level of GFR or serum creatinine at which an ACE inhibitor or ARB is absolutely contraindicated, and this decision should be made on an individual basis in conjunction with a nephrologist.
Risks for hyperkalemia should always be kept in mind at lower GFR levels. It would be prudent to check serum creatinine and potassium levels within the first week or two after starting or intensifying RAAS inhibition in these patients.
CAUTION
Combination therapy with an ACE inhibitor and an ARB was hypothesized to provide more complete RAAS blockade, with the hope of better clinical outcomes. However, this strategy has been questioned with results from three studies—ONTARGET, ALTITUDE, and the VA NEPHRON-D study—all of which showed worse renal outcomes, hypertension, and hyperkalemia with use of dual RAAS blockade.16–20 The combined evidence so far suggests that dual RAAS blockade should not be routinely prescribed.
RAAS INHIBITION IN PRACTICE
RAAS inhibition should be instituted and continued in patients with proteinuria who are able to tolerate the therapy and do not experience adverse effects as discussed above. Although there is no specific consensus guideline on the frequency of assessment of albumin excretion after diagnosis of albuminuria and institution of RAAS inhibition and blood pressure control in patients with diabetes, periodic surveillance at least once a year is reasonable to assess response to therapy and possible disease progression.21 If there is significant proteinuria or possibility of nondiabetic kidney disease, the patient should be referred to a nephrologist.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
- Taal MW, Brenner BM. Renoprotective benefits of RAS inhibition: from ACEI to angiotensin II antagonists. Kidney Int 2000; 57:1803–1817.
- Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis 2005; 45:281–287.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 1997; 349:1857–1863.
- Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999; 354:359–364.
- Agodoa LY, Appel L, Bakris GL, et al; African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001; 285:2719–2728.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Viberti G, Mogensen CE, Groop LC, Pauls JF. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271:275–279.
- ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med 2001; 134:370–379.
- Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 1997; 349:1787–1792.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Copper ME, de Zeeuw D, et al; RENAAL study investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Barnett AH, Bain SC, Bouter P, et al; Diabetics Exposed to Telmisartan and Enalapril Study Group. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351:1952–1961.
- Sharma P, Nagarajan V. Q: Can an ARB be given to patients who have had angioedema on an ACE inhibitor? Cleve Clin J Med 2013; 80:755–757.
- Kidney Disease Outcomes Quality Initiative (K/DOQI).K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 1):S1–S290.
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Mann JF, Anderson C, Gao P, et al; ONTARGET Investigators. Dual inhibition of the renin-angiotensin system in high-risk diabetes and risk for stroke and other outcomes: results of the ONTARGET trial. J Hypertens 2013; 31:414–421.
- Parving HH, Brenner BM, McMurray JJ, et al; ALTITUDE Investigators. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367:2204–2213.
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369:1892–1903.
- American Diabetes Association. Microvascular complications and foot care. Sec. 9. In: Standards of Medical Care in Diabetes—2015. Diabetes Care 2015;38(suppl 1):S58–S66.
Renal denervation to treat resistant hypertension: Guarded optimism
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
Can a percutaneous catheter-based procedure effectively treat resistant hypertension?
Radiofrequency ablation of the renal sympathetic nerves is undergoing randomized controlled trials in patients who have resistant hypertension and other disorders that involve the sympathetic nervous system. Remarkably, the limited results available so far look good.
This article discusses the physiologic rationale for renal denervation, the evidence from studies in humans of the benefits, risks, and complications of the procedure, upcoming trials, and areas for future research.
DESPITE MANY TREATMENT OPTIONS, RESISTANT HYPERTENSION IS COMMON
Hypertension is a leading reason for visits to physicians in the United States and is associated with increased rates of cardiovascular disease and death.1,2 A variety of antihypertensive agents are available, and the percentage of people with hypertension whose blood pressure is under control has increased over the past 2 decades. Nevertheless, population-based studies show that the control rate remains suboptimal.3 Effective pharmacologic treatment may be limited by inadequate doses or inappropriate combinations of antihypertensive drugs, concurrent use of agents that raise the blood pressure, noncompliance with dietary restrictions, and side effects that result in poor compliance with drug therapy.
Resistant hypertension is defined as failure to achieve goal blood pressure in patients who are adhering to full tolerated doses of an appropriate three-drug regimen that includes a diuretic.1,4,5 If we use these criteria, many patients labelled as having resistant hypertension probably do not truly have it; instead, they are nonadherent to therapy or are on an inadequate or inappropriate regimen. Although the true prevalence of resistant hypertension is not clear, estimates from large clinical trials suggest that about 20% to 30% of hypertensive patients may meet the criteria for it.4 For the subset of patients who have truly resistant hypertension, nonpharmacologic treatments such as renal sympathetic denervation are an intriguing avenue.
SURGICAL SYMPATHETIC DENERVATION: TRIED AND ABANDONED IN THE 1950s
More than a half century ago, a surgical procedure, thoracolumbar sympathectomy (in which sympathetic nerve trunks and splanchnic nerves were removed), was sometimes performed to control blood pressure in patients with malignant hypertension. This was effective but caused debilitating side effects such as postural hypotension, erectile dysfunction, and syncope.
Smithwick and Thompson6 reported that, in 1,266 hypertensive patients who underwent this procedure and 467 medically treated controls, the 5-year mortality rates were 19% and 54%, respectively. Forty-five percent of those who survived the surgery had significantly lower blood pressure afterward, and the antihypertensive effect lasted 10 years or more.
The procedure fell out of favor due to the morbidity associated with this nonselective approach and to the increased availability of drug therapy.
THE SYMPATHETIC NERVOUS SYSTEM IS A DRIVER OF HYPERTENSION
A variety of evidence suggests that hyperactivation of the sympathetic nervous system plays a major role in initiating and maintaining hypertension. For example, drugs that inhibit the sympathetic drive at various levels have a blood-pressure-lowering effect. Further, direct intraneural recordings show a high level of sympathetic nerve activity in the muscles of hypertensive patients, who also have high levels of cardiac and renal norepinephrine “spillover”—ie, the amount of this neurotransmitter that escapes neuronal uptake and local metabolism and spills over into the circulation.7
The kidneys are supplied with postganglionic sympathetic nerve fibers that end in the efferent and afferent renal arterioles, the juxtaglomerular apparatus, and the renal tubular system. Studies in animals and humans have shown that an increase in efferent signals (ie, from the brain to the kidney) leads to renal vasoconstriction and decreased renal blood flow, increased renin release, and sodium retention.8,9 Afferent signals (from the kidney to the central nervous system), which are increased in states of renal ischemia, renal parenchymal injury, and hypoxia, disinhibit the vasomotor center (the nuclei tractus solitarii) in the central nervous system, leading to increased efferent signals to the kidneys, heart, and peripheral blood vessels (Figure 1).10
Enhanced sympathetic activity in patients with hypertension may play a role in subsequent target-organ damage such as left ventricular hypertrophy, congestive heart failure, and progressive renal damage.11
Studies of renal denervation in animals, using surgical and chemical techniques, have further helped to establish the role of renal sympathetic nerves in hypertension.12,13
CATHETER-BASED RENAL DENERVATION
Renal sympathetic nerves run through the adventitia of the renal arteries in a mesh-like pattern.
In the renal denervation procedure, a specially designed catheter is inserted into a femoral artery and advanced into one of the renal arteries. There, radiofrequency energy is applied to the endoluminal surface according to a proprietary algorithm, thereby delivering thermal injury selectively to the renal sympathetic nerves without affecting the abdominal, pelvic, or lower-extremity nerves. The energy delivered is lower than that used for cardiac electrophysiologic procedures.
The nerves are not imaged or mapped before treatment. The procedure is performed on both sides, with four to six sites ablated in a longitudinal and rotational manner in 2-minute treatments at each site, to cover the full circumference (Figure 1).
In the United States, the device (Symplicity Renal Denervation System; Medtronic, Inc, Mountain View, CA) is available only for investigational use.
Below, we briefly review the studies of renal denervation to date. SYMPLICITY HTN-1 Symplicity HTN-1 was a proof-of-principle study in 45 patients with resistant hypertension (Table 1).14,15
Effect on blood pressure. Six months after renal denervation, blood pressure was significantly lower than at baseline (−22/−11 mm Hg, 95% confidence interval [CI] 10/5 mm Hg) in 26 patients available for follow-up. At 12 months, the difference from baseline was −27/−10 mm Hg (95% CI 16/11 mm Hg) in 9 patients available for follow-up (Table 2).14
Evidence of the durability of blood pressure reduction came from an expanded cohort of 153 patients followed for 2 years after denervation.16
Further follow-up data showed a sustained and significant blood pressure reduction through 3 years after denervation (unpublished results presented at the 2012 annual meeting of the American College of Cardiology). Notably, patients who were initially considered to be nonresponders (defined as failure of their blood pressure to go down by at least 10 mm Hg) were all reported to have a clinical response at 36 months.
Adverse events. In the initial and expanded cohorts combined, one patient suffered a renal artery dissection due to manipulation of the guiding catheter before the radiofrequency energy was delivered, and three patients developed a femoral pseudoaneurysm. No other long-term arterial complications were observed.
Comments. Limitations of this study included a small number of patients, no control group, and a primary outcome of a reduction in office blood pressure rather than in ambulatory blood pressure.
Additionally, although the authors concluded that there was no significant deterioration in renal function during the study period, we should note that in an additional follow-up period in this cohort, 10 patients with available 2-year data had a decrease in estimated glomerular filtration rate (eGFR) of −16.0 mL/min/1.73 m2. In 5 patients who did not have spironolactone (Aldactone) or another diuretic added after the first year of followup, a lesser but significant decrease (−7.8 mL/min/1.73 m2) was noted. The investigators surmised that denervation may enhance diuretic sensitivity, leading to prerenal azotemia in some patients.17
SYMPLICITY HTN-2
The Symplicity HTN-2 trial was a larger, randomized, efficacy study that built on the earlier results, providing additional evidence of therapeutic benefit.15
An international cohort of 106 patients with resistant hypertension, defined as systolic blood pressure of 160 mm Hg or higher (or ≥ 150 mm Hg in patients with type 2 diabetes) despite the use of three or more antihypertensive medications, were randomly assigned to undergo renal denervation with the Symplicity device (n = 52) or to continue their previous treatment with antihypertensive medications alone (n = 54). The primary effectiveness end point was the change in seated office blood pressure from baseline to 6 months (Table 1).
Effect on blood pressure. In the denervation group, at 6 months, office blood pressure had changed by a mean of −32/−12 mm Hg (standard deviation [SD] 23/11 mm Hg) compared with a mean change of 1/0 mm Hg (SD 21/10 mm Hg) in the control group. Fortyone (84%) of the 49 patients who underwent denervation had a decrease in systolic blood pressure of 10 mm Hg or more at 6 months compared with baseline values, while five (10%) had no decline in systolic blood pressure. Nineteen patients had a reduction in systolic pressure to less than 140 mm Hg in the denervation group.
A subset of patients (20 in the denervation group and 25 in the control group) underwent 24-hour ambulatory blood pressure monitoring at 6 months. This showed a similar though less pronounced fall in blood pressure in the denervation group and no change in the controls. A subanalysis that censored all data for patients whose medication was increased during the follow-up period showed a blood pressure reduction of −31/−12 mm Hg (SD 22/11 mm Hg) in the renal denervation group.
Adverse events. Procedure-related adverse events included a single femoral artery pseudoaneurysm, one case of postprocedural hypotension requiring a reduction in antihypertensive medications, and 7 (13%) of 52 patients who experienced intraprocedural bradycardia requiring atropine.
Effect on renal function. No significant difference was noted between groups in the mean change in renal function at 6 months, whether assessed by eGFR, serum creatinine level, or cystatin C level. At 6 months, no patient had a decrease of more than 50% in eGFR, although two patients who underwent renal denervation and three controls had more than a 25% decrease in eGFR.
At 6 months, the urine albumin-to-creatinine ratio had changed by a median of −3 mg/g (range −1,089 to 76) in 38 patients in the treatment group and by 1 mg/g (range −538 to 227) in 37 controls.
Most patients (88%) undergoing renal denervation underwent renal arterial imaging at 6 months, on which a single patient showed possible progression of an underlying atherosclerotic lesion that was unrelated to the procedure and that did not require intervention.
Denervation and the normal stress response. Whether renal denervation negatively affects the body’s physiologic response to stress that is normally mediated by sympathetic nerve activity was addressed in an extended investigation of Symplicity HTN-2 using cardiopulmonary exercise tests at baseline and 3 months after renal denervation.18 In the denervation group, blood pressure during exercise was significantly lower at 3 months than at baseline, but the heart rate increase at different levels of exercise was not affected. Additionally, the resting heart rate was lower and heart rate recovery after exercise improved after the procedure, particularly in patients without diabetes.
Comments. The Symplicity HTN-2 trial benefited from a randomized trial design and strict inclusion criteria of treatment resistance, but it still had notable limitations. A pretrial evaluation for causes of secondary hypertension or white-coat hypertension was not explicitly described. The control group did not undergo a sham procedure, and data analyzers were not masked to treatment assignment. Although not analyzed as a primary end point, the use of home-based and 24-hour ambulatory blood pressure assessment—measures important for determining white-coat hypertension—revealed substantial differences in blood pressure changes relative to office measurements. Because nearly all the patients (97%) were white, the generalizability of treatment results to black patients with resistant hypertension may be limited. Isolated diastolic hypertension (defined as diastolic pressure ≥ 90 mm Hg with systolic pressure < 140 mm Hg), which is more common in younger patients, was not studied.
DOES RENAL DENERVATION REDUCE SYMPATHETIC TONE?
A subgroup of 10 patients in the Symplicity HTN-1 trial whose mean 6-month office blood pressure was reduced by 22/12 mm Hg underwent assessment of renal norepinephrine spillover. A substantial (47%) reduction in renal norepinephrine spillover was noted 1 month after the procedure.14
The investigators additionally described a marked reduction in renal norepinephrine spillover from both kidneys in one patient, with a reduction of 48% from the left kidney and 75% from the right kidney 1 month after the procedure. Whole-body norepinephrine spillover in this patient was reduced by 42%. This effect was accompanied by a 50% decrease in plasma renin activity and by an increase in renal plasma flow. Aldosterone levels were not reported.19
Thus, the decrease in renal norepinephrine spillover suggests a reduction of renal efferent activity, and the decrease in total body norepinephrine spillover suggests a reduction in central sympathetic drive via the renal afferent pathway.
Microneurography in this same patient showed a gradual reduction in muscle sympathetic nerve activity to normal levels, from 56 bursts per minute at baseline to 41 at 30 days and 19 at 12 months).19 Decreased renin secretion, via circulating angiotensin II, may affect central sympathetic outflow as well.
Comments. While these findings address some of the underlying mechanisms, the small number of patients in whom these studies were done limits the generalizability of the results. The impact of the procedure on renal hemodynamics will need to be studied, including possible direct effects of the procedure, and whether there are differences in different study populations or differences based on blood pressure levels.
WHICH PATIENTS RESPOND BEST TO THIS PROCEDURE?
Although the Symplicity HTN-2 investigators report some predictors of increased reduction in blood pressure on multivariate analysis, including increased blood pressure at baseline and reduced heart rate at baseline, these are not specific enough to enable patient selection.
Interestingly, results from the expanded cohort of the Symplicity HTN-1 study found that patients on central sympatholytic agents such as clonidine had a greater reduction in blood pressure, although the reason for this is unclear.16 Identifying specific predictors of treatment success at baseline will be essential in future studies.
The earlier Symplicity trials and the ongoing Symplicity HTN-3 trial are in patients who have high blood pressure not responding to three or more antihypertensive drugs. The mean baseline systolic blood pressure in the Symplicity HTN-1 and HTN-2 trials was 178 mm Hg, and patients were taking an average of five antihypertensive drugs (Table 1). It is not known whether denervation will produce similar blood-pressure-lowering results across the spectrum of hypertension severity.
WHAT ARE THE LONG-TERM RESULTS OF DENERVATION?
Enthusiasm for the results from the Symplicity trials is tempered by concerns about the durability of the effects of the procedure, the need for better understanding of the impact of renal denervation on a wide array of pathophysiologic cascades leading to hypertension, and the effect on renal hemodynamics.
Antihypertensive efficacy has been reported to persist up to 2 years after the procedure,16 with recent unpublished data suggesting efficacy up to 3 years, but longer follow-up is needed to address whether these effects are finite.
Although reinnervation of afferent renal nerves has not been described, transplant models have shown anatomic regrowth of efferent nerves; the impact of this efferent reinnervation on blood pressure remains unclear. Experience from renal transplantation also shows that implanted kidneys that are “denervated” can still maintain fluid and electrolyte regulation.
Follow-up renal imaging in the Symplicity trials did not indicate renal artery stenosis at the sites of denervation in patients who underwent the procedure. Animal studies using the Symplicity catheter system showed renal nerve injury as evidenced by nerve fibrosis and thickened epineurium and perineurium, but no significant smooth muscle hyperplasia, arterial stenosis, or thrombosis by angiography or histology at 6 months.20
WHAT ARE THE RISKS?
Adverse effects that were noted in the short term are detailed under discussion of the trials and in Table 2.
Long-term adverse events in the Symplicity HTN-2 trial that required hospitalization were reported in five patients in the denervation group and three patients in the control group (Table 2). These included transient ischemic attacks, hypertensive crises, hypotensive episodes, angina, and nausea.
Renal function was maintained for the duration of both trials, and details regarding eGFR change have been described above under the discussion of the trials.
Diffuse visceral pain at the time of the procedure is reported as an expected occurrence, managed with intravenous analgesic medications.
DOES SYMPATHETIC DENERVATION HAVE A ROLE IN OTHER CONDITIONS?
Interestingly, other sympathetically driven diseases, such as diabetes mellitus and polycystic ovary syndrome, may prove to be targets for this therapy in the future.21
Mahfoud et al22 conducted a pilot study in 37 patients with resistant hypertension undergoing renal denervation and 13 control patients. Fasting glucose levels declined from 118 ± 3.4 mg/dL to 108 ± 3.8 mg/dL after 3 months in the intervention group (P = .039), compared with no change in the control group. Insulin and C-peptide levels were also lower in the intervention group. The reported improvement in glucose metabolism and insulin sensitivity suggests that the beneficial effects of this procedure may extend beyond blood pressure reduction.
Brandt et al23 reported regression of left ventricular hypertrophy and significantly improved cardiac functional parameters, including increase in ejection fraction and improved diastolic dysfunction, in a study of 46 patients who underwent renal denervation. This findings suggests a potential beneficial effect on cardiac remodeling.
Witkowski et al24 reported lowering of blood pressure in 10 patients with refractory hypertension and obstructive sleep apnea who underwent renal denervation, which was accompanied by improvement of sleep apnea severity.
Ukena et al25 reported reduction in ventricular tachyarrhythmias in two patients with congestive heart failure who had therapy-resistant electrical storm.
A recent pilot study in 15 patients with stage 3 and 4 chronic kidney disease (mean eGFR 31 mL/min/1.73 m2) showed significantly improved office blood pressure control up to 1 year, restoration of nocturnal dipping on 24-hour monitoring, as well as a nonsignificant trend towards increased hemoglobin levels and decreased proteinuria. No additional deterioration of renal function was reported in these patients (2 patients had renal function assessed up to 1 year).26
Thus, the benefits of this procedure may extend to other diseases that have a common underlying thread of elevated sympathetic activity, by targeting the “sympathorenal” axis.27
GUARDED OPTIMISM AND FUTURE DIRECTIONS
Given the well-known cardiovascular risks and health care costs associated with uncontrolled hypertension and the continued challenge that physicians face in managing it, novel therapies such as renal denervation may provide an adjunct to existing pharmacologic approaches.
While there is certainly cause for guarded optimism, especially with the striking blood pressure-lowering results seen in trials so far, it should be kept in mind that the mechanisms leading to the hypertensive response are complex and multifactorial, and further understanding of this therapy with long-term follow-up is needed. A comparison study with spironolactone, which is increasingly being used to treat resistant hypertension (in the absence of a diagnosis of primary aldosteronism)28,29 would help to further establish the role of this procedure.
Studies of carotid baroreceptor stimulation via an implantable device have shown sustained reduction in blood pressure in patients with resistant hypertension. A study comparing this technique with renal denervation for efficacy and safety end points could be considered in the future.30,31
The planned Symplicity HTN-3 study in the United States will be the largest trial to date, with a targeted randomization of more than 500 patients using strict enrollment criteria, including the use of maximally tolerated doses of diuretics and more focus on the use of ambulatory blood pressure monitoring and on the blinding of participants. This study will help further analysis of this technology in a more diverse population.32,33
Future studies should be designed to clarify pathophysiologic mechanisms, patient selection criteria, effects on target organ damage, and efficacy in patients with chronic kidney disease, obesity, congestive heart failure, and in less severe forms of hypertension.
A CALL FOR PARTICIPANTS IN A CLINICAL TRIAL
The Departments of Cardiology and Nephrology and Hypertension at Cleveland Clinic are currently enrolling patients in the Symplicity HTN-3 trial. For more information, please contact George Thomas, MD ([email protected]), or Mehdi Shishehbor, DO, MPH ([email protected]), or visit www.symplifybptrial.com.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13( 169) 2011. http://www.cdc.gov/nchs/data/series/sr_13/sr13_169.pdf. Accessed April 24, 2012.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension 2011; 57:1076–1080.
- Calhoun DA, Jones D, Textor S, et al; American Heart Association Professional Education Committee. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008; 117:e510–e526.
- Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. J Am Med Assoc 1953; 152:1501–1504.
- Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension 2009; 54:1195–1201.
- Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation 1977; 56:691–698.
- Kon V. Neural control of renal circulation. Miner Electrolyte Metab 1989; 15:33–43.
- Campese VM. Neurogenic factors and hypertension in renal disease. Kidney Int Suppl 2000; 75:S2–S6.
- Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–728.
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 2004; 287:H695–H703.
- Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983; 245:F1–F14.
- Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009; 373:1275–1281.
- Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M; Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatmentresistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010; 376:1903–1909.
- Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 2011; 57:911–917.
- Petidis K, Anyfanti P, Doumas M. Renal sympathetic denervation: renal function concerns. Hypertension 2011; 58:e19; author replye20.
- Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 2011; 58:1176–1182.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension (letter). N Engl J Med 2009; 361:932–934.
- Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100:1095–1101.
- Schlaich MP, Straznicky N, Grima M, et al. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens 2011; 29:991–996.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation 2011; 123:1940–1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 2012; 59:901–909.
- Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 2011; 58:559–565.
- Ukena C, Bauer A, Mahfoud F, et al. Renal sympathetic denervation for treatment of electrical storm: first-inman experience. Clin Res Cardiol 2012; 101:63–67.
- Herring D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 2012; May 17[Epub ahead of print]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, Krum H. Sympatho-renal axis in chronic disease. Clin Res Cardiol 2011; 100:1049–1057.
- Chapman N, Dobson J, Wilson S, et al; Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930.
- Papademetriou V, Doumas M, Faselis C, et al. Carotid baroreceptor stimulation for the treatment of resistant hypertension. Int J Hypertens 2011; 2011:964394.
- Ng MM, Sica DA, Frishman WH. Rheos: an implantable carotid sinus stimulation device for the nonpharmacologic treatment of resistant hypertension. Cardiol Rev 2011; 19:52–57.
- US National Institutes of Health. Renal denervation in patients with uncontrolled hypertension (SYMPLICITY HTN-3). http://www.clinicaltrials.gov/ct2/show/NCT01418261. Accessed June 7, 2012.
- Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the Symplicity HTN-3 trial. Clin Cardiol 2012 May 9. [Epub ahead of print]
KEY POINTS
- Renal sympathetic nerves help regulate volume and blood pressure as they innervate the renal tubules, blood vessels, and juxtaglomerular apparatus. They carry both afferent and efferent signals between the central nervous system and the kidneys.
- Surgical sympathectomy was done in the 1950s for malignant hypertension. It had lasting antihypertensive results but also caused severe procedure-related morbidity. A new percutaneous procedure for selective renal denervation offers the advantage of causing few major procedure-related adverse effects.
- Selective renal denervation decreases norepinephrine spillover and muscle sympathetic nerve activity, evidence that the procedure reduces sympathetic tone.
- The major clinical trials done so far have found that renal denervation lowers blood pressure significantly, and the reduction is sustained for at least 3 years.