User login
Estimating Fall Risk in Veterans With Atrial Fibrillation
Atrial fibrillation (AF) is the most common chronic cardiac rhythm disturbance and increases an individual’s risk of stroke 5-fold.1 Anticoagulation therapy reduces the risk of stroke by > 60% in patients with AF.2 The risk of AF increases with age, yet the perceived risk of fall in elderly patients taking warfarin reduces the use of this therapy.3
A single-institution study in 2000 revealed that 49% of veterans with AF were not receiving anticoagulation therapy. In 13% of cases, warfarin was withheld due to the perceived fall risk.4 Some studies of anticoagulation therapy for AF, in keeping with recommendations of the Medicare Health Care Quality Improvement Program National Stroke Project, have excluded patients who are deemed at high risk for falls.5 Although fall risk is being used in both research and clinical settings to determine the safety of prescribing warfarin for AF, how to determine such a patient’s fall risk has not been defined.
Although several rules for predicting falls in community dwellers have been published, none are routinely assessed during a patient’s hospital stay.6 Research shows the Morse Fall Scale (MFS) is a widely used, validated tool for assessing fall risk among hospitalized patients and indicates VA patients to be at high risk for falls.7,8 All patients hospitalized at the John L. McClellan Memorial Veterans Hospital (JLMMVH) in Little Rock, Arkansas, receive a MFS score at admission. If the MFS score is predictive of the postdischarge risk of a veteran with AF falling, the score would assist in determining which patients can be safely discharged while taking anticoagulation therapy.
The present study is a retrospective chart review of all patients with AF discharged from the JLMMVH during 2006 and their subsequent risk of falls requiring acute medical care. Based on CDC data indicating the risk for nonfatal falls by persons aged > 65 years to be more than twice that of younger persons and the established fall risk ranges of the MFS, it was hypothesized that AF patients aged ≥ 65 years with a modified MFS score (MMS) ≥ 55 would be at a significantly greater risk of fall requiring acute medical care following hospital discharge than would those of the same age with lower scores.
Methods
This study was approved by the JLMMVH Institutional Review Board. The electronic medical records (EMRs) of all veterans with a diagnosis of AF discharged from the JLMMVH during 2006 were manually reviewed for study inclusion. The year 2006 was chosen in order to ensure adequate subject follow-up time.
Inclusion criteria consisted of discharge from an acute care unit and the patient’s most recent electrocardiogram (ECG) prior to the index discharge, showing AF or atrial flutter; or the most recent ECG prior to the index discharge, showing a fully paced rhythm consistent with an underlying rhythm of AF and documentation of previously diagnosed chronic AF for which a permanent pacemaker was placed.
Exclusion criteria consisted of discharge due to patient death; transient (persisting < 24 hours) AF associated with an acute medical illness or surgical procedure; index hospitalization representing transfer temporarily from another VAMC for the sole purpose of performing a procedure; hospitalization lasting < 24 hours (not coded as a hospital admission); mechanical heart valve; index admission for a neurosurgical procedure, hemorrhagic stroke, or bleeding esophageal/gastric varices; anticoagulation therapy recommended by the physician at the time of discharge but declined by the patient; incomplete or missing MFS score in the EMR; and lack of follow-up after the index discharge. Temporary transfers from outside facilities were excluded, due to anticipated difficulty in performing follow-up. Individuals for whom anticoagulation therapy was either inappropriate (eg, bleeding varices) or absolutely required (eg, mechanical heart valve) also were excluded.
Data Collection
Each EMR was reviewed, and the following data were abstracted: (1) patient age; (2) date of first hospital discharge during 2006; (3) final MFS score and subscores recorded during the index hospitalization; (4) date of the first fall requiring acute medical evaluation; (5) severe bleeding associated with the fall; (6) date of the subject’s death; and (7) date of the last recorded follow-up. The occurrence of a postdischarge fall and of fall-associated severe bleeding was determined by review of all hospitalizations, clinic visits, emergency department (ED) visits, outside records scanned into the EMR, and visiting nurse reports. The MFS score was converted to a MMS by subtracting points given for the presence of an IV line during the hospitalization, as such a fall risk would end at discharge.
Endpoints
The primary endpoint for the study was the occurrence of a fall following hospital discharge, resulting in evaluation of the subject in an outpatient clinic or ED within 24 hours. The primary comparison was between subjects aged ≥ 65 years with a MMS ≥ 55 and subjects aged ≥ 65 years with a MMS < 55.
A secondary endpoint was the occurrence of severe bleeding associated with a fall. Severe bleeding was defined as fatal bleeding; and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome; and/or bleeding causing a fall in hemoglobin level of ≥ 2 g/dL or leading to transfusion of ≥ 2 units of whole blood or red blood cells.9
Statistical Analysis
An estimated analyzable sample size (df = 1, α = 0.05, and a critical value for χ2 of 3.841) of 180 subjects was based on CDC age-related fall rates, MFS-related fall rates, and published sensitivity and specificity values of the MFS.7,10,11 An estimated exclusion rate of 25% to 30% based on published rates of AF-related hospital mortality; transient (persisting < 24 hours) AF; patients with AF declining recommended anticoagulation therapy; and hospital admissions lasting < 24 hours (coded as observations) yielded a total estimated study sample size of 240 to 257 subjects.
Life-table analysis (time until fall) was performed using the LIFETEST procedure (SAS Institute Inc.; Cary, NC). Subject death and end of follow-up in EMRs were treated as censored events. Comparison of survival curves was accomplished using the log-rank statistic. To generate a user-friendly predictive rule, intervals of 5-year age cutoff values (eg, aged 55, aged 60, aged 65 years) were used for survival comparisons. The MMS is calculated in multiples of 5, hence, all possible score cutoffs were considered in survival comparisons. The 2-sample t test was performed for comparison of mean age and MMS between groups and reported as mean ± SD. A P value < .05 was considered statistically significant. Statistical analysis was performed using SAS Enterprise Guide 5.1.
Results
A search of JCMMVH EMRs yielded 270 patients with a diagnosis of AF discharged from the hospital during 2006. Seventy-seven patients were excluded from analysis for the following reasons: dead at time of discharge, 28; transient (persisting < 24 hours) AF associated with an acute medical illness, 12; referred solely for a procedure, 19; mechanical heart valve, 2; patient declined to take anticoagulation therapy, 2; hemorrhagic stroke, 1; bleeding esophageal varices, 1; lacking MFS documentation, 10; and no postdischarge follow-up documented, 2. All subjects except 1 were male. Both the age and MMS of subjects represented non-normal distributions (Anderson-Darling statistic 1.8, P < .001; and 6.7, P < .005). The median subject age was 74 years; the median MMS was 25.
During the approximately 7-year follow-up period (follow-up range 2-2,545 days), 59 of the 193 subjects (31%) fell. No fall resulted in severe bleeding or death. The mean age of subjects who fell was 73.0 ± 10.3 years compared with 71.6 ± 10.5 years for nonfallers (P = .40). Likewise, the mean MMS for subjects who fell was 34.1 ± 22.3 compared with 30.3 ± 19.9 for nonfallers (P = .24). The mean time until first fall (mean survival) was 725 ± 642 days; whereas the mean length of follow-up for people who did not fall (including those censored due to death) was 1,050 ± 869 days. Subject age and MMS were positively correlated, though weakly (Pearson r = 0.36; Spearman r = 0.37).
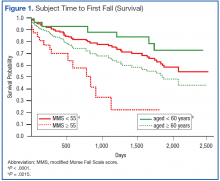
Grouping subjects by MMS alone yielded significantly divergent survival curves only for cutoffs of MMS ≥ 40, ≥ 50, ≥ 55 (log-rank statistic P = .0061, P = .0002, and P < .0001, respectively). Figure 1 (red) shows the difference in survival for MMS ≥ 55 vs MMS < 55, where the mean time to fall was 701 ± 88 days for those with a MMS ≥ 55 compared with 1,628 ± 65 days for MMS < 55.
When age cutoff alone (using 5-year age intervals) was used to construct fall survival curves, only breakpoints of age ≥ 60, ≥ 75, and ≥ 80 years yielded significantly divergent curves (log-rank statistic P = .0215, P = .0264, and P = .011, respectively). Figure 1 (green) shows the difference in survival for subjects aged < 60 years vs aged ≥ 60 years.
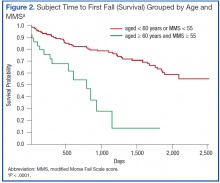
The hypothesized combined cutoff of subjects aged ≥ 65 years and MMS ≥ 55 yielded divergent survival curves (log-rank statistic of P = .0011). However, survival curves based on a cutoff of subjects aged ≥ 60 years and ≥ 55 MMS yielded the most statistically significant separation (logrank statistic P < .0001) (Figure 2). Subjects aged < 60 years or with a MMS < 55 had a mean survival of 1,634 ± 65 days; whereas those aged ≥ 60 years and a MMS ≥ 55 had a mean survival of 668 ± 90 days.
A notable similarity of the survival curves for MMS ≥ 55 vs MMS < 55 compared with those based on a cutoff of subjects aged ≥ 60 years and ≥ 55 MMS is observed in comparing Figures 1 (red) and 2. The log-rank statistic chi-square values are 17.44 and 22.75, respectively, suggesting the separation of subjects by a combination of age and MMS yields a more robust divergence in outcomes than does separation by MMS alone.
Discussion
This retrospective chart review evaluated the utility of a MMS combined with age in predicting the risk of patients with AF experiencing serious falls following hospital discharge. When used alone, the MMS separates those at relatively low and high risk of subsequent falls requiring acute medical care. When combined with the factor of patient age, this separation improves and is most predictive for the group of AF patients aged ≥ 60 years with a MMS of ≥ 55. Half of this group had fallen 668 ± 90 days after discharge; whereas those aged < 60 years or with a MMS < 55 did not reach the point of 50% falling until 1,634 ± 65 days after discharge. Age alone allows a statistically significant differentiation of fall risk, but less so than does the MMS alone or the MMS combined with age.
Assessing fall risk can be as simple as asking whether a patient has fallen during the previous year or has a problem with balance or gait, or it can be as complex as an in-depth investigation of physical, cognitive, pharmacologic, environmental, and social factors.12,13 Beyond the parameters of validity and discrimination power, a predictive tool must be easy to use. Within the VA hospital system, where the MFS is a part of every nursing intake assessment, a MMS can be obtained within seconds from the EMR. This, coupled with the patient’s age, allows the provider to immediately identify those patients with AF who are at high risk for serious falls following hospital discharge.
Strengths and Limitations
A major strength of the present study is the fact that the data accuracy was ensured by individual review of each subject’s EMR. Administrative coding was used only for the initial identification of potential subjects for inclusion. Although 28.5% of potential subjects were excluded from this analysis, > 50% of such exclusions were due to death as the reason for discharge and transient AF associated with an acute medical stressor. Other strengths include the length of follow-up (1,050 ± 869 days, excluding subject deaths) and the generalizability of the subject population. The major weakness of this study is the relatively small sample size and its retrospective methodology.
Summary
The validity of the MFS modified for the postdischarge setting was demonstrated as a readily available tool for identifying patients with AF at high risk of falls following a hospital stay. Such a tool should allow physicians to appropriately prescribe anticoagulation therapy for those patients with AF who are at a lower risk of falls.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation. 2010;121(7):e46-e215.
2. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449-1457.
3. Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J. 2011;161(2):241-246.
4. Bradley BC, Perdue KS, Tisdel KA, Gilligan DM. Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a Veterans Affairs medical center. Am J Cardiol. 2000;85(5):568-572.
5. Bravata DM, Rosenbeck K, Kancir S, Brass LM. The use of warfarin in veterans with atrial fibrillation. BMC Cardiovasc Disord. 2004;4(1):18.
6. Pluijm SM, Smit JH, Tromp EA, et al. A risk profile for identifying community-dwelling elderly with a high risk of recurrent falling: Results of a 3-year prospective study. Osteoporos Int. 2006;17(3):417-425.
7. Schwendimann R, De Geest S, Milisen K. Evaluation of the Morse Fall Scale inhospitalised patients. Age Ageing. 2006;35(3):311-313.
8. Quigley PA, Palacios P, Spehar AM. Veterans’ fall risk profile: A prevalence study. Clin Interv Aging. 2006;1(2):169-173.
9. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694.
10. Centers for Disease Control and Prevention. QuickStats: Rate of nonfatal, medically consulted fall injury episodes, by age group—National Health Interview Survey, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(4):81.
11. Bailey PH, Rietze LL, Moroso S, Szilva N. A description of a process to calibrate the Morse fall scale in a long-term care home. Appl Nurs Res. 2011;24(4):263-268.
12. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49(5):664-672.
13. Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297(1):77-86.
Atrial fibrillation (AF) is the most common chronic cardiac rhythm disturbance and increases an individual’s risk of stroke 5-fold.1 Anticoagulation therapy reduces the risk of stroke by > 60% in patients with AF.2 The risk of AF increases with age, yet the perceived risk of fall in elderly patients taking warfarin reduces the use of this therapy.3
A single-institution study in 2000 revealed that 49% of veterans with AF were not receiving anticoagulation therapy. In 13% of cases, warfarin was withheld due to the perceived fall risk.4 Some studies of anticoagulation therapy for AF, in keeping with recommendations of the Medicare Health Care Quality Improvement Program National Stroke Project, have excluded patients who are deemed at high risk for falls.5 Although fall risk is being used in both research and clinical settings to determine the safety of prescribing warfarin for AF, how to determine such a patient’s fall risk has not been defined.
Although several rules for predicting falls in community dwellers have been published, none are routinely assessed during a patient’s hospital stay.6 Research shows the Morse Fall Scale (MFS) is a widely used, validated tool for assessing fall risk among hospitalized patients and indicates VA patients to be at high risk for falls.7,8 All patients hospitalized at the John L. McClellan Memorial Veterans Hospital (JLMMVH) in Little Rock, Arkansas, receive a MFS score at admission. If the MFS score is predictive of the postdischarge risk of a veteran with AF falling, the score would assist in determining which patients can be safely discharged while taking anticoagulation therapy.
The present study is a retrospective chart review of all patients with AF discharged from the JLMMVH during 2006 and their subsequent risk of falls requiring acute medical care. Based on CDC data indicating the risk for nonfatal falls by persons aged > 65 years to be more than twice that of younger persons and the established fall risk ranges of the MFS, it was hypothesized that AF patients aged ≥ 65 years with a modified MFS score (MMS) ≥ 55 would be at a significantly greater risk of fall requiring acute medical care following hospital discharge than would those of the same age with lower scores.
Methods
This study was approved by the JLMMVH Institutional Review Board. The electronic medical records (EMRs) of all veterans with a diagnosis of AF discharged from the JLMMVH during 2006 were manually reviewed for study inclusion. The year 2006 was chosen in order to ensure adequate subject follow-up time.
Inclusion criteria consisted of discharge from an acute care unit and the patient’s most recent electrocardiogram (ECG) prior to the index discharge, showing AF or atrial flutter; or the most recent ECG prior to the index discharge, showing a fully paced rhythm consistent with an underlying rhythm of AF and documentation of previously diagnosed chronic AF for which a permanent pacemaker was placed.
Exclusion criteria consisted of discharge due to patient death; transient (persisting < 24 hours) AF associated with an acute medical illness or surgical procedure; index hospitalization representing transfer temporarily from another VAMC for the sole purpose of performing a procedure; hospitalization lasting < 24 hours (not coded as a hospital admission); mechanical heart valve; index admission for a neurosurgical procedure, hemorrhagic stroke, or bleeding esophageal/gastric varices; anticoagulation therapy recommended by the physician at the time of discharge but declined by the patient; incomplete or missing MFS score in the EMR; and lack of follow-up after the index discharge. Temporary transfers from outside facilities were excluded, due to anticipated difficulty in performing follow-up. Individuals for whom anticoagulation therapy was either inappropriate (eg, bleeding varices) or absolutely required (eg, mechanical heart valve) also were excluded.
Data Collection
Each EMR was reviewed, and the following data were abstracted: (1) patient age; (2) date of first hospital discharge during 2006; (3) final MFS score and subscores recorded during the index hospitalization; (4) date of the first fall requiring acute medical evaluation; (5) severe bleeding associated with the fall; (6) date of the subject’s death; and (7) date of the last recorded follow-up. The occurrence of a postdischarge fall and of fall-associated severe bleeding was determined by review of all hospitalizations, clinic visits, emergency department (ED) visits, outside records scanned into the EMR, and visiting nurse reports. The MFS score was converted to a MMS by subtracting points given for the presence of an IV line during the hospitalization, as such a fall risk would end at discharge.
Endpoints
The primary endpoint for the study was the occurrence of a fall following hospital discharge, resulting in evaluation of the subject in an outpatient clinic or ED within 24 hours. The primary comparison was between subjects aged ≥ 65 years with a MMS ≥ 55 and subjects aged ≥ 65 years with a MMS < 55.
A secondary endpoint was the occurrence of severe bleeding associated with a fall. Severe bleeding was defined as fatal bleeding; and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome; and/or bleeding causing a fall in hemoglobin level of ≥ 2 g/dL or leading to transfusion of ≥ 2 units of whole blood or red blood cells.9
Statistical Analysis
An estimated analyzable sample size (df = 1, α = 0.05, and a critical value for χ2 of 3.841) of 180 subjects was based on CDC age-related fall rates, MFS-related fall rates, and published sensitivity and specificity values of the MFS.7,10,11 An estimated exclusion rate of 25% to 30% based on published rates of AF-related hospital mortality; transient (persisting < 24 hours) AF; patients with AF declining recommended anticoagulation therapy; and hospital admissions lasting < 24 hours (coded as observations) yielded a total estimated study sample size of 240 to 257 subjects.
Life-table analysis (time until fall) was performed using the LIFETEST procedure (SAS Institute Inc.; Cary, NC). Subject death and end of follow-up in EMRs were treated as censored events. Comparison of survival curves was accomplished using the log-rank statistic. To generate a user-friendly predictive rule, intervals of 5-year age cutoff values (eg, aged 55, aged 60, aged 65 years) were used for survival comparisons. The MMS is calculated in multiples of 5, hence, all possible score cutoffs were considered in survival comparisons. The 2-sample t test was performed for comparison of mean age and MMS between groups and reported as mean ± SD. A P value < .05 was considered statistically significant. Statistical analysis was performed using SAS Enterprise Guide 5.1.
Results
A search of JCMMVH EMRs yielded 270 patients with a diagnosis of AF discharged from the hospital during 2006. Seventy-seven patients were excluded from analysis for the following reasons: dead at time of discharge, 28; transient (persisting < 24 hours) AF associated with an acute medical illness, 12; referred solely for a procedure, 19; mechanical heart valve, 2; patient declined to take anticoagulation therapy, 2; hemorrhagic stroke, 1; bleeding esophageal varices, 1; lacking MFS documentation, 10; and no postdischarge follow-up documented, 2. All subjects except 1 were male. Both the age and MMS of subjects represented non-normal distributions (Anderson-Darling statistic 1.8, P < .001; and 6.7, P < .005). The median subject age was 74 years; the median MMS was 25.
During the approximately 7-year follow-up period (follow-up range 2-2,545 days), 59 of the 193 subjects (31%) fell. No fall resulted in severe bleeding or death. The mean age of subjects who fell was 73.0 ± 10.3 years compared with 71.6 ± 10.5 years for nonfallers (P = .40). Likewise, the mean MMS for subjects who fell was 34.1 ± 22.3 compared with 30.3 ± 19.9 for nonfallers (P = .24). The mean time until first fall (mean survival) was 725 ± 642 days; whereas the mean length of follow-up for people who did not fall (including those censored due to death) was 1,050 ± 869 days. Subject age and MMS were positively correlated, though weakly (Pearson r = 0.36; Spearman r = 0.37).
Grouping subjects by MMS alone yielded significantly divergent survival curves only for cutoffs of MMS ≥ 40, ≥ 50, ≥ 55 (log-rank statistic P = .0061, P = .0002, and P < .0001, respectively). Figure 1 (red) shows the difference in survival for MMS ≥ 55 vs MMS < 55, where the mean time to fall was 701 ± 88 days for those with a MMS ≥ 55 compared with 1,628 ± 65 days for MMS < 55.
When age cutoff alone (using 5-year age intervals) was used to construct fall survival curves, only breakpoints of age ≥ 60, ≥ 75, and ≥ 80 years yielded significantly divergent curves (log-rank statistic P = .0215, P = .0264, and P = .011, respectively). Figure 1 (green) shows the difference in survival for subjects aged < 60 years vs aged ≥ 60 years.
The hypothesized combined cutoff of subjects aged ≥ 65 years and MMS ≥ 55 yielded divergent survival curves (log-rank statistic of P = .0011). However, survival curves based on a cutoff of subjects aged ≥ 60 years and ≥ 55 MMS yielded the most statistically significant separation (logrank statistic P < .0001) (Figure 2). Subjects aged < 60 years or with a MMS < 55 had a mean survival of 1,634 ± 65 days; whereas those aged ≥ 60 years and a MMS ≥ 55 had a mean survival of 668 ± 90 days.
A notable similarity of the survival curves for MMS ≥ 55 vs MMS < 55 compared with those based on a cutoff of subjects aged ≥ 60 years and ≥ 55 MMS is observed in comparing Figures 1 (red) and 2. The log-rank statistic chi-square values are 17.44 and 22.75, respectively, suggesting the separation of subjects by a combination of age and MMS yields a more robust divergence in outcomes than does separation by MMS alone.
Discussion
This retrospective chart review evaluated the utility of a MMS combined with age in predicting the risk of patients with AF experiencing serious falls following hospital discharge. When used alone, the MMS separates those at relatively low and high risk of subsequent falls requiring acute medical care. When combined with the factor of patient age, this separation improves and is most predictive for the group of AF patients aged ≥ 60 years with a MMS of ≥ 55. Half of this group had fallen 668 ± 90 days after discharge; whereas those aged < 60 years or with a MMS < 55 did not reach the point of 50% falling until 1,634 ± 65 days after discharge. Age alone allows a statistically significant differentiation of fall risk, but less so than does the MMS alone or the MMS combined with age.
Assessing fall risk can be as simple as asking whether a patient has fallen during the previous year or has a problem with balance or gait, or it can be as complex as an in-depth investigation of physical, cognitive, pharmacologic, environmental, and social factors.12,13 Beyond the parameters of validity and discrimination power, a predictive tool must be easy to use. Within the VA hospital system, where the MFS is a part of every nursing intake assessment, a MMS can be obtained within seconds from the EMR. This, coupled with the patient’s age, allows the provider to immediately identify those patients with AF who are at high risk for serious falls following hospital discharge.
Strengths and Limitations
A major strength of the present study is the fact that the data accuracy was ensured by individual review of each subject’s EMR. Administrative coding was used only for the initial identification of potential subjects for inclusion. Although 28.5% of potential subjects were excluded from this analysis, > 50% of such exclusions were due to death as the reason for discharge and transient AF associated with an acute medical stressor. Other strengths include the length of follow-up (1,050 ± 869 days, excluding subject deaths) and the generalizability of the subject population. The major weakness of this study is the relatively small sample size and its retrospective methodology.
Summary
The validity of the MFS modified for the postdischarge setting was demonstrated as a readily available tool for identifying patients with AF at high risk of falls following a hospital stay. Such a tool should allow physicians to appropriately prescribe anticoagulation therapy for those patients with AF who are at a lower risk of falls.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Atrial fibrillation (AF) is the most common chronic cardiac rhythm disturbance and increases an individual’s risk of stroke 5-fold.1 Anticoagulation therapy reduces the risk of stroke by > 60% in patients with AF.2 The risk of AF increases with age, yet the perceived risk of fall in elderly patients taking warfarin reduces the use of this therapy.3
A single-institution study in 2000 revealed that 49% of veterans with AF were not receiving anticoagulation therapy. In 13% of cases, warfarin was withheld due to the perceived fall risk.4 Some studies of anticoagulation therapy for AF, in keeping with recommendations of the Medicare Health Care Quality Improvement Program National Stroke Project, have excluded patients who are deemed at high risk for falls.5 Although fall risk is being used in both research and clinical settings to determine the safety of prescribing warfarin for AF, how to determine such a patient’s fall risk has not been defined.
Although several rules for predicting falls in community dwellers have been published, none are routinely assessed during a patient’s hospital stay.6 Research shows the Morse Fall Scale (MFS) is a widely used, validated tool for assessing fall risk among hospitalized patients and indicates VA patients to be at high risk for falls.7,8 All patients hospitalized at the John L. McClellan Memorial Veterans Hospital (JLMMVH) in Little Rock, Arkansas, receive a MFS score at admission. If the MFS score is predictive of the postdischarge risk of a veteran with AF falling, the score would assist in determining which patients can be safely discharged while taking anticoagulation therapy.
The present study is a retrospective chart review of all patients with AF discharged from the JLMMVH during 2006 and their subsequent risk of falls requiring acute medical care. Based on CDC data indicating the risk for nonfatal falls by persons aged > 65 years to be more than twice that of younger persons and the established fall risk ranges of the MFS, it was hypothesized that AF patients aged ≥ 65 years with a modified MFS score (MMS) ≥ 55 would be at a significantly greater risk of fall requiring acute medical care following hospital discharge than would those of the same age with lower scores.
Methods
This study was approved by the JLMMVH Institutional Review Board. The electronic medical records (EMRs) of all veterans with a diagnosis of AF discharged from the JLMMVH during 2006 were manually reviewed for study inclusion. The year 2006 was chosen in order to ensure adequate subject follow-up time.
Inclusion criteria consisted of discharge from an acute care unit and the patient’s most recent electrocardiogram (ECG) prior to the index discharge, showing AF or atrial flutter; or the most recent ECG prior to the index discharge, showing a fully paced rhythm consistent with an underlying rhythm of AF and documentation of previously diagnosed chronic AF for which a permanent pacemaker was placed.
Exclusion criteria consisted of discharge due to patient death; transient (persisting < 24 hours) AF associated with an acute medical illness or surgical procedure; index hospitalization representing transfer temporarily from another VAMC for the sole purpose of performing a procedure; hospitalization lasting < 24 hours (not coded as a hospital admission); mechanical heart valve; index admission for a neurosurgical procedure, hemorrhagic stroke, or bleeding esophageal/gastric varices; anticoagulation therapy recommended by the physician at the time of discharge but declined by the patient; incomplete or missing MFS score in the EMR; and lack of follow-up after the index discharge. Temporary transfers from outside facilities were excluded, due to anticipated difficulty in performing follow-up. Individuals for whom anticoagulation therapy was either inappropriate (eg, bleeding varices) or absolutely required (eg, mechanical heart valve) also were excluded.
Data Collection
Each EMR was reviewed, and the following data were abstracted: (1) patient age; (2) date of first hospital discharge during 2006; (3) final MFS score and subscores recorded during the index hospitalization; (4) date of the first fall requiring acute medical evaluation; (5) severe bleeding associated with the fall; (6) date of the subject’s death; and (7) date of the last recorded follow-up. The occurrence of a postdischarge fall and of fall-associated severe bleeding was determined by review of all hospitalizations, clinic visits, emergency department (ED) visits, outside records scanned into the EMR, and visiting nurse reports. The MFS score was converted to a MMS by subtracting points given for the presence of an IV line during the hospitalization, as such a fall risk would end at discharge.
Endpoints
The primary endpoint for the study was the occurrence of a fall following hospital discharge, resulting in evaluation of the subject in an outpatient clinic or ED within 24 hours. The primary comparison was between subjects aged ≥ 65 years with a MMS ≥ 55 and subjects aged ≥ 65 years with a MMS < 55.
A secondary endpoint was the occurrence of severe bleeding associated with a fall. Severe bleeding was defined as fatal bleeding; and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular with compartment syndrome; and/or bleeding causing a fall in hemoglobin level of ≥ 2 g/dL or leading to transfusion of ≥ 2 units of whole blood or red blood cells.9
Statistical Analysis
An estimated analyzable sample size (df = 1, α = 0.05, and a critical value for χ2 of 3.841) of 180 subjects was based on CDC age-related fall rates, MFS-related fall rates, and published sensitivity and specificity values of the MFS.7,10,11 An estimated exclusion rate of 25% to 30% based on published rates of AF-related hospital mortality; transient (persisting < 24 hours) AF; patients with AF declining recommended anticoagulation therapy; and hospital admissions lasting < 24 hours (coded as observations) yielded a total estimated study sample size of 240 to 257 subjects.
Life-table analysis (time until fall) was performed using the LIFETEST procedure (SAS Institute Inc.; Cary, NC). Subject death and end of follow-up in EMRs were treated as censored events. Comparison of survival curves was accomplished using the log-rank statistic. To generate a user-friendly predictive rule, intervals of 5-year age cutoff values (eg, aged 55, aged 60, aged 65 years) were used for survival comparisons. The MMS is calculated in multiples of 5, hence, all possible score cutoffs were considered in survival comparisons. The 2-sample t test was performed for comparison of mean age and MMS between groups and reported as mean ± SD. A P value < .05 was considered statistically significant. Statistical analysis was performed using SAS Enterprise Guide 5.1.
Results
A search of JCMMVH EMRs yielded 270 patients with a diagnosis of AF discharged from the hospital during 2006. Seventy-seven patients were excluded from analysis for the following reasons: dead at time of discharge, 28; transient (persisting < 24 hours) AF associated with an acute medical illness, 12; referred solely for a procedure, 19; mechanical heart valve, 2; patient declined to take anticoagulation therapy, 2; hemorrhagic stroke, 1; bleeding esophageal varices, 1; lacking MFS documentation, 10; and no postdischarge follow-up documented, 2. All subjects except 1 were male. Both the age and MMS of subjects represented non-normal distributions (Anderson-Darling statistic 1.8, P < .001; and 6.7, P < .005). The median subject age was 74 years; the median MMS was 25.
During the approximately 7-year follow-up period (follow-up range 2-2,545 days), 59 of the 193 subjects (31%) fell. No fall resulted in severe bleeding or death. The mean age of subjects who fell was 73.0 ± 10.3 years compared with 71.6 ± 10.5 years for nonfallers (P = .40). Likewise, the mean MMS for subjects who fell was 34.1 ± 22.3 compared with 30.3 ± 19.9 for nonfallers (P = .24). The mean time until first fall (mean survival) was 725 ± 642 days; whereas the mean length of follow-up for people who did not fall (including those censored due to death) was 1,050 ± 869 days. Subject age and MMS were positively correlated, though weakly (Pearson r = 0.36; Spearman r = 0.37).
Grouping subjects by MMS alone yielded significantly divergent survival curves only for cutoffs of MMS ≥ 40, ≥ 50, ≥ 55 (log-rank statistic P = .0061, P = .0002, and P < .0001, respectively). Figure 1 (red) shows the difference in survival for MMS ≥ 55 vs MMS < 55, where the mean time to fall was 701 ± 88 days for those with a MMS ≥ 55 compared with 1,628 ± 65 days for MMS < 55.
When age cutoff alone (using 5-year age intervals) was used to construct fall survival curves, only breakpoints of age ≥ 60, ≥ 75, and ≥ 80 years yielded significantly divergent curves (log-rank statistic P = .0215, P = .0264, and P = .011, respectively). Figure 1 (green) shows the difference in survival for subjects aged < 60 years vs aged ≥ 60 years.
The hypothesized combined cutoff of subjects aged ≥ 65 years and MMS ≥ 55 yielded divergent survival curves (log-rank statistic of P = .0011). However, survival curves based on a cutoff of subjects aged ≥ 60 years and ≥ 55 MMS yielded the most statistically significant separation (logrank statistic P < .0001) (Figure 2). Subjects aged < 60 years or with a MMS < 55 had a mean survival of 1,634 ± 65 days; whereas those aged ≥ 60 years and a MMS ≥ 55 had a mean survival of 668 ± 90 days.
A notable similarity of the survival curves for MMS ≥ 55 vs MMS < 55 compared with those based on a cutoff of subjects aged ≥ 60 years and ≥ 55 MMS is observed in comparing Figures 1 (red) and 2. The log-rank statistic chi-square values are 17.44 and 22.75, respectively, suggesting the separation of subjects by a combination of age and MMS yields a more robust divergence in outcomes than does separation by MMS alone.
Discussion
This retrospective chart review evaluated the utility of a MMS combined with age in predicting the risk of patients with AF experiencing serious falls following hospital discharge. When used alone, the MMS separates those at relatively low and high risk of subsequent falls requiring acute medical care. When combined with the factor of patient age, this separation improves and is most predictive for the group of AF patients aged ≥ 60 years with a MMS of ≥ 55. Half of this group had fallen 668 ± 90 days after discharge; whereas those aged < 60 years or with a MMS < 55 did not reach the point of 50% falling until 1,634 ± 65 days after discharge. Age alone allows a statistically significant differentiation of fall risk, but less so than does the MMS alone or the MMS combined with age.
Assessing fall risk can be as simple as asking whether a patient has fallen during the previous year or has a problem with balance or gait, or it can be as complex as an in-depth investigation of physical, cognitive, pharmacologic, environmental, and social factors.12,13 Beyond the parameters of validity and discrimination power, a predictive tool must be easy to use. Within the VA hospital system, where the MFS is a part of every nursing intake assessment, a MMS can be obtained within seconds from the EMR. This, coupled with the patient’s age, allows the provider to immediately identify those patients with AF who are at high risk for serious falls following hospital discharge.
Strengths and Limitations
A major strength of the present study is the fact that the data accuracy was ensured by individual review of each subject’s EMR. Administrative coding was used only for the initial identification of potential subjects for inclusion. Although 28.5% of potential subjects were excluded from this analysis, > 50% of such exclusions were due to death as the reason for discharge and transient AF associated with an acute medical stressor. Other strengths include the length of follow-up (1,050 ± 869 days, excluding subject deaths) and the generalizability of the subject population. The major weakness of this study is the relatively small sample size and its retrospective methodology.
Summary
The validity of the MFS modified for the postdischarge setting was demonstrated as a readily available tool for identifying patients with AF at high risk of falls following a hospital stay. Such a tool should allow physicians to appropriately prescribe anticoagulation therapy for those patients with AF who are at a lower risk of falls.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation. 2010;121(7):e46-e215.
2. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449-1457.
3. Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J. 2011;161(2):241-246.
4. Bradley BC, Perdue KS, Tisdel KA, Gilligan DM. Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a Veterans Affairs medical center. Am J Cardiol. 2000;85(5):568-572.
5. Bravata DM, Rosenbeck K, Kancir S, Brass LM. The use of warfarin in veterans with atrial fibrillation. BMC Cardiovasc Disord. 2004;4(1):18.
6. Pluijm SM, Smit JH, Tromp EA, et al. A risk profile for identifying community-dwelling elderly with a high risk of recurrent falling: Results of a 3-year prospective study. Osteoporos Int. 2006;17(3):417-425.
7. Schwendimann R, De Geest S, Milisen K. Evaluation of the Morse Fall Scale inhospitalised patients. Age Ageing. 2006;35(3):311-313.
8. Quigley PA, Palacios P, Spehar AM. Veterans’ fall risk profile: A prevalence study. Clin Interv Aging. 2006;1(2):169-173.
9. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694.
10. Centers for Disease Control and Prevention. QuickStats: Rate of nonfatal, medically consulted fall injury episodes, by age group—National Health Interview Survey, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(4):81.
11. Bailey PH, Rietze LL, Moroso S, Szilva N. A description of a process to calibrate the Morse fall scale in a long-term care home. Appl Nurs Res. 2011;24(4):263-268.
12. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49(5):664-672.
13. Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297(1):77-86.
1. Lloyd-Jones D, Adams RJ, Brown TM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2010 update: A report from the American Heart Association. Circulation. 2010;121(7):e46-e215.
2. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449-1457.
3. Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J. 2011;161(2):241-246.
4. Bradley BC, Perdue KS, Tisdel KA, Gilligan DM. Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a Veterans Affairs medical center. Am J Cardiol. 2000;85(5):568-572.
5. Bravata DM, Rosenbeck K, Kancir S, Brass LM. The use of warfarin in veterans with atrial fibrillation. BMC Cardiovasc Disord. 2004;4(1):18.
6. Pluijm SM, Smit JH, Tromp EA, et al. A risk profile for identifying community-dwelling elderly with a high risk of recurrent falling: Results of a 3-year prospective study. Osteoporos Int. 2006;17(3):417-425.
7. Schwendimann R, De Geest S, Milisen K. Evaluation of the Morse Fall Scale inhospitalised patients. Age Ageing. 2006;35(3):311-313.
8. Quigley PA, Palacios P, Spehar AM. Veterans’ fall risk profile: A prevalence study. Clin Interv Aging. 2006;1(2):169-173.
9. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692-694.
10. Centers for Disease Control and Prevention. QuickStats: Rate of nonfatal, medically consulted fall injury episodes, by age group—National Health Interview Survey, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(4):81.
11. Bailey PH, Rietze LL, Moroso S, Szilva N. A description of a process to calibrate the Morse fall scale in a long-term care home. Appl Nurs Res. 2011;24(4):263-268.
12. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49(5):664-672.
13. Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297(1):77-86.